Abstract
Osteoporosis treatment rates are declining, even among those with past fractures. Novel, low-cost approaches are needed to improve osteoporosis care. We conducted a parallel group, controlled, randomized clinical trial evaluating a behavioral intervention for improving osteoporosis medication use. A total of 2,684 women with self-reported fracture history after age 45 not using osteoporosis therapy from U.S. Global Longitudinal study of Osteoporosis in Women (GLOW) sites were randomized 1:1 to receive a multi-modal, tailored, direct-to-patient, video intervention vs. usual care. The primary study outcome was self-report of osteoporosis medication use at 6-months. Other outcomes included calcium and vitamin D supplementation, bone mineral density (BMD) testing, readiness for behavioral change, and barriers to treatment. In intent-to-treat analyses there were no significant differences between groups (intervention vs control) in osteoporosis medication use (11.7% vs 11.4%, p=0.8), calcium supplementation (31.8% vs 32.6%, p=0.7), vitamin D intake (41.3% vs. 41.9%, p=0.8) or BMD testing (61.8% vs 57.1%, p=0.2). In the intervention group, fewer women were in the pre-contemplative stage of behavior change, more women reported seeing their primary care provider, had concerns regarding osteonecrosis of the jaw, and difficulty in taking/remembering to take osteoporosis medications. We found differences in BMD testing among the subgroup of women with no prior osteoporosis treatment, those who provided contact information, and those with no past BMD testing. In per protocol analyses, women with appreciable exposure to the online intervention (N=257) were more likely to start non-bisphosphonates (OR=2.70 [1.26, 5.79]) compared to usual care group. While our intervention did not increase the use of osteoporosis therapy at 6-months, it increased non-bisphosphonate medication use and BMD testing in select subgroups, shifted participant’s readiness for behavior change, and altered perceptions of barriers to osteoporosis treatment. Achieving changes in osteoporosis care using patient activation approaches alone is challenging.
Trial Registration
clinicaltrials.gov identifier: NCT01907269
Despite medications that lower fracture risk at some sites by more than 50% (1–4) and guidelines endorsing the need for treatment following a fracture,(5) many fragility fracture patients fail to receive osteoporosis treatment,(2, 6) and do not associate their fracture with osteoporosis.(7) Some women consider osteoporosis as benign, do not associate fractures with osteoporosis and view osteoporosis as a natural process of aging. (8) As a result, there is a growing “osteoporosis care gap”.(9, 10) Perceived barriers to osteoporosis treatment include concerns about medication side effects, polypharmacy, and limited drug efficacy.(11–16) Addressing patient barriers through interventions designed to overcome and modify patient perceptions may improve osteoporosis care.(16)
Multifaceted interventions may increase the diagnosis and treatment of osteoporosis.(17–20) Many studies have attempted to improve osteoporosis care through physician-targeted interventions with limited success.(21–28) Those that have evaluated direct-to-patient interventions(29–37) include post-fracture care coordination(36, 37) and patient activation promoting better participation in osteoporosis care.(29–35) The latter have shown somewhat favorable results for osteoporosis outcomes including patient knowledge,(33–35) calcium intake,(30) physical activity,(31) and bone mineral density (BMD) testing.(29) However, most of the low-cost, effective, and generalizable direct-to-patient interventions aimed at improving care have largely been limited to other chronic conditions, such as hypertension(38) or HIV medication adherence.(39)
In an attempt to improve rates of osteoporosis treatment among a high-risk population who previously reported a fracture but currently were not using osteoporosis therapies, we designed a multi-modal, patient-centered, tailored, video-based behavioral intervention, to encourage patients to seek osteoporosis diagnosis and treatment.(16) Our intervention was implemented and evaluated in the Activating Patients at Risk for OsteoPorOsiS (APROPOS) study, targeting participants within the Global Longitudinal study of Osteoporosis in Women (GLOW) cohort.(16)
Methods
Study Design and Participants
The APROPOS study was a parallel, controlled, randomized clinical trial, in which participants received either usual care alone (control group), or in combination with a multi-modal, patient-tailored, behavioral intervention (intervention group).(16) APROPOS was nested within the GLOW cohort,(40) an international, prospective, observational study of women 55+ years of age. The participants in APROPOS were enrolled from 7 U.S. GLOW sites (Birmingham, AL; Los Angeles, CA; Worcester, MA; New York, NY; Cincinnati, OH; Pittsburgh, PA; Seattle, WA). Human subject protocols and consent procedures were reviewed and approved by each site’s Institutional Review Board.
GLOW participants were preliminarily eligible for APROPOS if on one of the five GLOW surveys they self-reported a fracture after age 45. In September 2013, we mailed baseline questionnaires to 4,928 preliminarily eligible GLOW participants; 3,226 (64%) completed baseline surveys, of which 2,684 women, who did not report currently using osteoporosis medication (the second eligibility criteria) besides estrogen, formed the APROPOS study population, and were randomized.
Randomization
We performed stratified randomization by site, self-reported history of osteoporosis treatment, whether contact information was supplied, and whether barriers to osteoporosis treatment were disclosed on the APROPOS baseline survey.(16) In each stratum, patients were randomly assigned to control and intervention groups in a 1:1 ratio using computer-generated lists of random numbers. The allocation of patients was made by a statistician (DTR) without knowledge of the participants’ details. Study investigators were not blinded to the intervention assignment.
Intervention
Development of the behavioral intervention employed in APROPOS has been previously described.(16) Our personalized, direct-to-patient intervention included video vignettes was grounded in the principles of narrative communication (“storytelling”),(41, 42) and guided by the constructs of the Information, Motivation and Behavioral skills model.(43) These vignettes contained stories developed from actual osteoporosis patients’ experiences portrayed by actresses of patient-identified race/ethnicity. The videos were tailored according to participant’s reported race/ethnicity and perceived barriers ranked by participants (e.g., general fears of medications, preference for alternative therapies, concerns about long-term adverse events),(12) or readiness for behavior change, or osteoporosis treatment history. The videos utilized in APROPOS are available at: https://www.youtube.com/channel/UCH3RCRlNvr5B7iuw9tOqQBg/playlists?view_as=public.
The intervention materials were emailed as a hyperlink to a personalized webpage and also mailed in a DVD format. The intervention included three components: (1) an introduction video explaining the reason for receiving the materials, (2) personalized videos addressing barriers to osteoporosis therapy or presenting general osteoporosis information (for those who did not rank barriers to treatment), and (3) a video on “How to communicate with your doctor about bone health” to encourage discussions between participants and their health care provider.(44) The duration of the video intervention program ranged between approximately 5 and 15 minutes. The intervention also included follow-up telephone calls and interactive voice response system (IVR) reminders to view the videos for participants that had not logged on to the intervention online. In the subgroup who viewed the intervention online, we defined appreciable exposure to the intervention as a participant logging on and viewing at least 20 seconds (e.g., the duration of 2 testimonials in the introductory video duration) of their personalized intervention.
Baseline Data Collection
A baseline questionnaire(40, 45) evaluated use of osteoporosis prescription medication and dietary supplements, fracture and general health history, perceived ability to communicate with health care providers about bone health,(46) health literacy,(47) and items from the Patients’ Views about Osteoporosis and Use of Therapy scale.(12) Respondents ranked up to three of the nine potentially modifiable barriers to osteoporosis treatment, which were defined using qualitative methods involving nominal groups and expert opinion.(16) We also assessed participants’ readiness for behavior change using a modified form of the Weinstein Precaution Adoption Process Model (PAPM).(48) We defined pre-contemplative participants as those that had no intent of initiating osteoporosis treatment and self-classified in the unaware and unengaged stages of PAPM.(16) Contemplative participants, defined by the undecided, decided not to act, and decided to act stages of PAPM, were those individuals considering their decision about starting treatment for osteoporosis.(49)
Outcomes and Follow-up
The primary study outcome was self-report of current osteoporosis medication use including: a) bisphosphonate (risedronate, alendronate, ibandronate, zoledronic acid), and b) non-bisphosphonate (raloxifene, teriparatide, calcitonin, denosumab) medications at 6-months. Secondary outcomes were self-reported initiation of calcium and/or vitamin D supplementation, and receipt of BMD testing at 6-months. The follow-up surveys also collected data on exploratory outcomes: barriers to osteoporosis treatment, discussion with health care provider regarding bone health, engagement with intervention components, comorbid medical conditions, fracture history, items from the Patients’ Views about Osteoporosis and Use of Therapy scale.(12) In addition, for participants who accessed the intervention online, we objectively assessed their intervention viewing duration. We also examined osteoporosis care (osteoporosis medication use, BMD testing) at 18-months to account for possible delay in healthcare access.
Statistical Analyses
Assuming that 10% of participants in the control arm would start osteoporosis medication during the study period, we determined that a sample size of 1,342 per group would provide greater than 80% power to detect a 4% absolute difference between the randomized groups in the proportion of new osteoporosis medication use, using a two-sided test at alpha 0.05. We imputed missing data based on available baseline APROPOS and previous GLOW surveys, assuming data missing at random. In intent-to-treat analyses we compared primary, secondary, and exploratory outcomes between the randomized groups at 6- and 18-months. In per protocol analyses we compared participants who had appreciable intervention exposure online to the control group for the following outcomes: self-report of osteoporosis treatment, BMD testing, stage of behavioral change and barriers to treatment. We examined the data for the heterogeneity of treatment effects by an aggregate of likely fragility fractures (any fracture except skull, hands, feet, fingers, toes), sites for major osteoporotic fractures (hip, spine, wrist, humerus), perceived fracture risk, treatment barriers, prior osteoporosis medication use, supplied phone number or email address, and readiness for behavior change.
We used means and standard deviations (SD) to describe continuous variables and proportions for categorical variables. Chi square tests and multivariable logistic regression were used to compare outcomes between the control and intervention, or appreciable exposure to the intervention groups, respectively. We report odds ratios (OR) and adjusted OR (aOR) as results of these analyses. In multivariable logistic regression models we included as covariates those baseline variables, which were found at p<0.10 to be associated with both appreciable intervention exposure and the outcomes considered in the analyses. These covariates included the following characteristics: age, race, education, health literacy, email/phone number being provided, self-rated risk of fracture, prior osteoporosis treatment, BMD testing, and general health. The criterion for statistical significance was p<0.05. No multiple comparison adjustment was performed. All analyses were conducted in SAS (v9.3, Enterprise Guide v4.3, Cary, NC).
Results
Characteristics of the Participants at Study Baseline
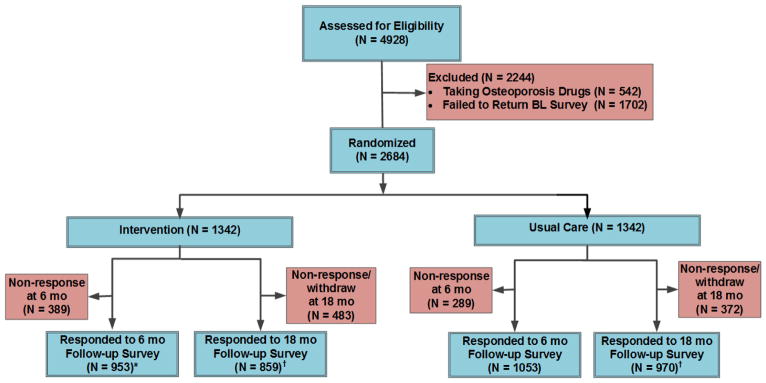
As seen in the CONSORT (Consolidated Standards of Reporting Trials) diagram for the study (Figure 1), we randomized the 2,684 women recruited (September 2013) in the APROPOS study and performed survey follow-up at 6 (May-June 2015) and 18 (June 2016) months following our intervention deployment. Socio-demographic, clinical and osteoporosis-related characteristics of the participants by group assignment, including those who had appreciable intervention online exposure (N=257 [19.2%]) are presented in Table 1. Overall, the study participants were predominately Caucasian, had mean ages in mid-seventies, were college educated (76.7%), in good or excellent health (84.6%), and were using vitamin D or calcium supplementation. Fewer than 10% of women had sustained a fracture in the 12 months prior to randomization. There were no significant differences in socio-demographic characteristics between intervention or control group (Table 1).
Figure 1.
APROPOS Study Design CONSORT. *Chi-square p < 0.05 for the comparison between response rates in the intervention vs control group at 6-months. †Chi-square p < 0.01 for the comparison between response rates in the intervention vs control group at 18-months. BL, baseline; OP, osteoporosis; Rx, prescription.
Table 1.
APROPOS Participant Baseline Characteristics by Treatment Group and Subgroup of Treatment Groups with Appreciable Intervention Online Exposure†
| Characteristic | Control N = 1342 |
Intervention N = 1342 |
Appreciable Exposure N = 257 |
|---|---|---|---|
| Age, years, mean (SD)*** | 74.9 (7.9) | 74.9 (8) | 73.2 (6.9) |
| Race/Ethnicity* | |||
| Non-Hispanic Caucasian | 1239 (92.3%) | 1247 (92.9%) | 246 (95.7%) |
| Black | 52 (3.9%) | 46 (3.4%) | 1 (0.4%) |
| Hispanic | 21 (1.6%) | 25 (1.9%) | 3 (1.2%) |
| Asian | 13 (1.0%) | 13 (1.0%) | 4 (1.6%) |
| Other/Not specified | 10 (0.8%) | 3 (0.2%) | 3 (1.2%) |
| Education*** | |||
| Some high school or less | 53 (4.0%) | 40 (3.0%) | 2 (0.8%) |
| High school graduate | 271 (20.3%) | 255 (19.2%) | 23 (9.1%) |
| Some college or more | 1007 (75.7%) | 1030 (77.8%) | 228 (90.1%) |
| Fracture history | |||
| Wrist | 384 (28.6%) | 382 (28.4%) | 78 (30.4%) |
| Vertebral | 106 (7.9%) | 105 (7.8%) | 15 (5.8%) |
| Hip | 84 (6.3%) | 79 (5.9%) | 15 (5.8%) |
| Fracture history within the previous 12 months | |||
| Yes | 123 (9.4%) | 96 (7.3%) | 24 (9.5%) |
| General health | |||
| Excellent, very good, or good* | 1107 (84.2%) | 1113 (85.0%) | 229 (89.8%) |
| Comorbidities | |||
| Depression | 276 (21.3%) | 272 (21.2%) | 52 (21.2%) |
| Dementia | 20 (1.6%) | 14 (1.1%) | 3 (1.2%) |
| Risk of fracture | |||
| Lower than average | 504 (38.3%) | 542 (41.8%) | 115 (46.0%) |
| Average | 524 (39.8%) | 501 (38.6%) | 83 (33.2%) |
| Higher than average | 288 (21.9%) | 255 (19.7%) | 52 (20.8%) |
| Osteoporosis prescription treatment | |||
| Prior treatment (including estrogen)** | 815 (64.0%) | 826 (64.8%) | 182 (72.8%) |
| Other medications, current | |||
| Vitamin D | 1062 (82.8%) | 1080 (84.1%) | 216 (86.1%) |
| Calcium supplement | 867 (68.7%) | 863 (68.6%) | 178 (71.2%) |
| Phone and email provided | |||
| Phone** | 892 (66.5%) | 899 (67.0%) | 194 (75.5%) |
| Email*** | 657 (49.0%) | 641 (57.5%) | 207 (80.5%) |
| Readiness for osteoporosis behavior change | |||
| Pre-contemplative | 806 (72.0%) | 822 (72.2%) | 169 (72.8%) |
| Contemplative | 270 (24.1%) | 274 (24.1%) | 53 (22.8%) |
| Health literacy | |||
| Adequate*** | 1119 (86.1%) | 1144 (87.5%) | 238 (93.7%) |
| Bone mineral density testing* | |||
| No | 204 (15.6%) | 186 (14.3%) | 24 (9.6%) |
| Yes in the last 12 months | 284 (21.8%) | 277 (21.3%) | 60 (24.0%) |
| Yes, more than 12 months ago | 818 (62.6%) | 835 (64.3%) | 166 (66.4%) |
Appreciable exposure to the intervention online (defined as at least 20 seconds of viewing time); All comparisons examine appreciable exposure versus control:
p<0.05,
p<0.01,
p<0.0001.
Compared with women assigned to the control group, those who had appreciable exposure to the intervention materials were more likely to be Caucasian, supply contact information, report good or excellent general health, have adequate health literacy, and have previously used osteoporosis medications (Table 1).
Intent-to-treat Analysis of the Outcomes
A total of 1,123 (83.7%) women in the intervention group and 1,079 (80.4%) in the control group reported seeing their primary health care provider in the 6-months following intervention deployment (OR=1.25, 95% CI [1.21, 1.30]) (Table 2), and 583 (43.4%) vs. 573 (42.7%) women reported talking with a health care provider about bone health in the intervention vs. control group, respectively (p=0.74).
Table 2.
Rates and Odds Ratios (95% CI) of Receipt of New Osteoporosis Treatments, Testing, Barriers to Treatment, and Stage of Behavioral Change at 6-Month Post Intervention (Intent-to-treat).
| Outcomes | Control N=1342 |
Intervention N=1342 |
Odds Ratio (95% CI) |
|---|---|---|---|
| Primary Outcomes | |||
| Any osteoporosis treatment | 153 (11.4%) | 157 (11.7%) | 1.01 (0.89, 1.16) |
| Bisphosphonate | 132 (10.0%) | 129 (9.8%) | 0.99 (0.86, 1.15) |
| Non-bisphosphonate | 32 (2.6%) | 36 (2.9%) | 1.06 (0.83, 1.37) |
| Secondary Outcomes | |||
| Vitamin D supplements | 562 (41.9%) | 554 (41.3%) | 0.99 (0.90, 1.08) |
| Calcium supplements | 437 (32.6%) | 427 (31.8%) | 0.98 (0.90, 1.08) |
| Bone mineral density testing | 268 (57.1%) | 290 (61.8%) | 1.10 (0.95, 1.28) |
| Self-reported barriers to osteoporosis treatment | |||
| Medication interactions | 310 (23.1%) | 331 (24.6%) | 1.04 (0.94, 1.16) |
| Gastrointestinal problems | 328 (24.4%) | 377 (28.1%) | 1.10 (0.99, 1.22) |
| Osteonecrosis of the jaw* | 331 (24.6%) | 387 (28.9%) | 1.11 (1.01, 1.23) |
| Atypical fractures | 329 (24.5%) | 355 (26.5%) | 1.05 (0.95, 1.16) |
| Preference for natural remedies | 339 (25.2%) | 371 (27.6%) | 1.06 (0.96, 1.18) |
| Medication inefficacy | 281 (20.9%) | 297 (22.1%) | 1.04 (0.93, 1.15) |
| Bone density not improving or had fracture | 150 (11.2%) | 153 (11.4%) | 1.01 (0.90, 1.15) |
| Difficulty taking or remembering* | 242 (18.1%) | 295 (22.0%) | 1.13 (1.01, 1.26) |
| Dentist recommendation | 278 (20.7%) | 299 (22.3%) | 1.05 (0.94, 1.17) |
| Readiness for osteoporosis treatment behavior change | |||
| Pre-contemplative* | 923 (68.8%) | 860 (64.1%) | 0.90 (0.82, 0.99) |
| Contemplative* | 329 (24.5%) | 389 (29.0%) | 1.12 (1.01, 1.24) |
| Discussion with health care provider about bone health | 573 (42.7%) | 583 (43.4%) | 1.03 (1.00, 1.06) |
| Visit with primary care provider*** | 1079 (80.4%) | 1123 (83.7%) | 1.25 (1.21, 1.30) |
p < 0.05,
p<0.001
For the primary outcome, 157 (11.7%) and 153 (11.4%) women self-reported use of osteoporosis prescription medication in the intervention and control groups, respectively (p=0.83) (Table 2). Similarly, we observed no significant differences by study group in the secondary outcomes (starting calcium; starting vitamin D; and BMD testing). Osteoporosis care rates were not different between the intervention and the control groups at 18 months: 131 (11.5%) women in the intervention group and 136 (10.5%) women in the control group started osteoporosis medications (p=0.47); and 530 (82.4%) women in the intervention group and 522 (78.7%) reported having DXA scan one year after the 6-months survey (p=0.09).
For exploratory outcomes, we found that our behavioral intervention influenced participants’ readiness for behavior change at 6-months, with a significantly lower proportion of participants in the pre-contemplative stage in the intervention compared to control group (860 [64.1%] vs. 923 [68.8%], OR=0.90 [0.82, 0.99]) (Table 2). In addition, compared with the control group, at 6-months participants in the intervention group were slightly more likely to report being concerned about treatment barriers that included osteonecrosis of the jaw (387 [28.9%] vs 331 [24.6%], OR=1.11 [1.01, 1.23]) and difficulty in taking/remembering to take osteoporosis medications (295 [22.0%] vs 242 [18.1%], OR=1.13 [1.01, 1.26]).
Analyses of Heterogeneity of Treatment Effects (Subgroups)
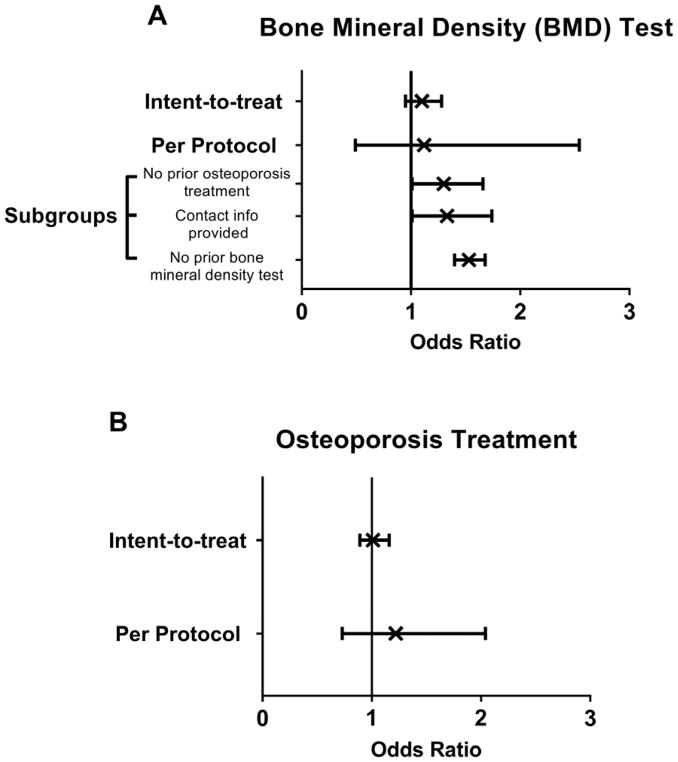
We found significant differences in self-reported BMD testing among the subgroup of women with no prior osteoporosis treatment (OR=1.30 [1.01, 1.66]), among those who provided contact information (OR=1.33 [1.01, 1.74]), and among those who did not report past BMD testing (OR=1.53 [1.40, 1.68]) (Figure 2A). There were no significant intervention effects for primary and secondary outcomes among the subgroup of women with past fragility fractures or major osteoporotic fractures. Additionally, there were no significant intervention effects for self-reported receipt of osteoporosis medication, initiation of calcium or vitamin D, or for BMD testing in subgroups of women with self-reported barriers to osteoporosis treatment or history of past osteoporosis treatment. Subgroups defined by general health rating, perceived fracture risk, readiness to behavior change, visit with primary care provider, discussion with physician about bone health, and contact information availability did not exhibit differential intervention effects.
Figure 2.
Odds Ratio (x) and 95% CI (bands) of Uptake of Osteoporosis Diagnosis Testing by Bone Mineral Density Test (A) and Osteoporosis Treatment (B) Based on Type of Analysis (Intent-to-treat vs Per protocol) at 6-Months. Intent-to-treat was defined as comparison of treatment groups including all patients as originally randomized. Per protocol was defined as comparison of appreciable exposure to the intervention group with the control group.
Per Protocol Analyses
Follow-up surveys at 6-months were completed by 2,006 (74.7%) women. Compared to the control group, at 6-months a significantly greater proportion of women in the intervention group failed to complete the follow-up survey (29.0% vs 21.5%, p= 0.0001).
Figure 1S in the Supplemental Materials presents the distribution of the duration of interaction with the intervention materials. Among those who watched for at least 20 seconds the mean (SD) duration of exposure to the educational materials was 389 (374) seconds, with a median viewing time of 346 seconds. The proportion of self-reported osteoporosis treatment was similar between those with appreciable exposure to the online intervention compared with the control group (aOR=1.22 [0.73, 2.04]) at 6-months (Figure 2B). However, women with appreciable exposure to the intervention were more likely than those in the control group to report starting non-bisphosphonate osteoporosis medications (11 [4.5%] vs 23 [1.8%], OR=2.70[1.26, 5.79]) (Table 3), which remained significant at 18-months (12 [6.5%] vs 24 [2.7%], OR=2.54 [1.22, 5.29]). Of note, nine of the women who took non-bisphosphonate drugs at 18-months in the intervention group were also taking them at 6-months suggesting persistence on osteoporosis therapy. Compared to the control group, those with appreciable exposure to intervention materials were more likely to report being concerned about osteonecrosis of the jaw and to be in the contemplative stage of behavioral change (Table 3). Those who watched longer than the median viewing time (see Figure S1) had similar outcomes as those who we defined earlier as having appreciable exposure to the intervention.
Table 3.
Rates, Odds Ratios (95% CI) and Adjusted Odds Ratios (95% CI) for New Osteoporosis Treatment, Testing, Stage of Behavioral Change, and Barriers Among the Subgroup with Appreciable Intervention Online Exposure (N=257) Referent to All Control Women (N=1,342) at 6-Months.
| Outcomes | Control (N=1342) | Appreciable Exposure (N=257) | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Treatment | ||||
| Any osteoporosis medication | 91 (6.8%) | 21 (8.2%) | 1.22 (0.75, 2.01) | 1.22 (0.73, 2.04)† |
| Bisphosphonate | 71 (5.4%) | 11 (4.5%) | 0.82 (0.43, 1.57) | 0.80 (0.41, 1.54) ‡ |
| Non-bisphosphonate** | 23 (1.8%) | 11 (4.5%) | 2.54 (1.23, 5.27) | 2.70 (1.26, 5.79)§ |
| Bone mineral density testing | 141 (46.5%) | 31 (62.0%) | 1.87 (1.01, 3.46) | 1.12 (0.49, 2.54) || |
| Discussion with health care provider about bone health | 706 (53.9%) | 141 (56.4%) | 1.11 (0.84, 1.46) | 1.01 (0.74, 1.39)¶ |
| Readiness for osteoporosis treatment behavior change | ||||
| Contemplative * | 175 (13.0%) | 49 (19.1%) | 1.57 (1.11, 2.23) | 1.48 (1.01, 2.15)†† |
| Self-reported barriers to osteoporosis treatment | ||||
| Any barriers | 369 (27.5%) | 90 (35.0%) | 1.42 (1.07, 1.89) | 1.27 (0.94,1.72)‡‡ |
| Osteonecrosis of the jaw as a barrier* | 217 (16.2%) | 60 (23.4%) | 1.58 (1.14, 2.18) | 1.52 (1.08, 2.13)§§ |
p < 0.05,
p < 0.01 for adjusted models
Adjusted by baseline characteristics: general health, self-rated risk of fracture, prior osteoporosis treatment, and bone mineral density (BMD) testing
Adjusted by baseline characteristics: self-rated risk of fracture, prior osteoporosis treatment, and BMD testing
Adjusted by baseline characteristics: self-rated risk of fracture, prior osteoporosis treatment, and BMD testing
Adjusted by baseline characteristics: age, race, education, self-rated risk of fracture, prior osteoporosis treatment, email being provided, health literacy, and BMD testing
Adjusted by baseline characteristics: age, self-rated risk of fracture, prior osteoporosis treatment, phone provided, and BMD testing
Adjusted by baseline characteristics: education, self-rated risk of fracture, prior osteoporosis treatment, and BMD testing
Adjusted by baseline characteristics: education, self-rated risk of fracture, prior osteoporosis treatment, health literacy, and BMD testing
Adjusted by age, education, self-rated risk of fracture, prior osteoporosis treatment, health literacy and BMD testing
Discussion
The APROPOS study tested the effectiveness of a multi-modal tailored, patient-directed, behavioral intervention to improve the rates of osteoporosis treatment in women at high risk for future fracture. Among more than 2,500 participants, there were no significant differences in rates of self-reported initiation of osteoporosis treatment, or BMD testing between groups at 6- and 18-months. However, among women who had appreciable exposure to the intervention, we found an increase in non-bisphosphonate medication use. Women with no prior osteoporosis treatment, as well as those who supplied an email address and phone number, were more likely to report BMD testing at 6-months. Taken together our findings in specific subgroups and per protocol analyses indicate that our intervention approach may have potential to improve osteoporosis care and that further refinement of our strategy is needed. We observed that the rate of osteoporosis treatment initiation was ~11% in APROPOS, similar to low rates of osteoporosis pharmacotherapy initiation in high-risk women nationally.(50, 51) This low rate of treatment initiation emphasizes the sizable challenges in encouraging women who have already experienced a fracture to initiate osteoporosis treatment. Using self-reported fracture history to ascertain past fractures may be subject to recall bias and may misclassify future fracture risk, which potentially represents a limitation to our study. However, self-reported fracture history is a relatively well-accepted and validated approach to fracture ascertainment, which has been used in past large epidemiological studies. (52–54) In addition, for some women that participated in our study, osteoporosis medications might not have been indicated because they lacked sufficient prior history of a fragility fracture.
Influencing a person’s behavior to initiate treatment through a behavioral intervention is a multi-stage process. The behavioral intervention we employed in APROPOS addressed the early steps of this pathway from lack of awareness to action, steps that encompass the construct of readiness for behavior change.(55, 56) We observed that women in the intervention group were less likely to be pre-contemplative compared to those in the control group, a finding suggesting that they had transitioned to a decision making-stage and were considering whether to pursue osteoporosis treatment and/or testing. Despite the significant effect of the APROPOS intervention on participants’ readiness for behavioral change, a large proportion of women in the intervention group remained pre-contemplative. A potential explanation may be the belief held by some APROPOS women about their low risk for future fracture, as previously noted in the GLOW cohort(57, 58) and by others.(59, 60) The perception of a low personal susceptibility to future fracture, even among women who have previously fractured, is concordant with reluctance to acknowledge personal susceptibility to health problems(61) (e.g. osteoporosis). This may have acted as a barrier to engaging in osteoporosis management within APROPOS. A possible explanation for the low rate of osteoporosis medication initiation in our study may stem from participants having difficulty trusting the content of the video vignettes included in the intervention, since these educational materials were not endorsed by a source familiar to the patient (e.g. their treating physician).
Timing of the intervention with regard to a previous fracture may influence the relevance of adopting osteoporosis treatment. Consonant with the concept of a “teachable moment,” persons may be more receptive to adopting behavior change(62) when engaged by an intervention shortly after a fracture rather than months/years later. In APROPOS, a very low proportion of participants reported a fracture in the 12-months prior to the intervention deployment; thus, the intervention may have reached the participants at a suboptimal time. In another study, patients who received advice to discuss osteoporosis with their physician immediately after hip fracture repair were more likely to receive appropriate therapeutic intervention.(63) In addition, a meta-analysis of osteoporosis interventions demonstrated that more intensive interventions involving care coordination for secondary fracture prevention can impact both BMD testing and treatment initiation.(64) However, these more intensive and costly approaches may work within select settings such as managed health care (36, 37) or in countries offering socialized medicine, but have limited generalizability to all care environments.
We developed our highly personalized intervention taking into account each participant’s own health. Interestingly, the intervention paradoxically increased participants’ knowledge/concerns of barriers to osteoporosis treatment, with more women in the intervention group reporting fears of osteonecrosis of the jaw and/or difficulty in taking/remembering to take osteoporosis medications. Osteonecrosis of the jaw concern was an important barrier to treatment reported in GLOW and other studies, leading us to develop educational content to overcome potential concerns about this very rare event. Our findings suggest that materials addressing treatment barriers could inadvertently increase anxiety (i.e., nocebo effect) and lead to less treatment initiation. This finding is similar to the observation that media attention to high-consequence, low-probability adverse effects may raise the patient’s knowledge, risk perceptions, and reporting, and thus result in non-adherence.(65–69)
The success of a behavioral intervention is influenced by the participants’ engagement. Despite efforts to encourage its uptake, just under 30 percent of participants accessed the intervention online,(16) and the proportion of participants who interacted appreciably with the intervention was even lower. Considering that the participants were required to self-initiate the personalized video program, engagement with the online intervention in APROPOS was relatively high compared to other studies.(70, 71) However, implementing an online intervention in a population with an average age of 74 years might have contributed to its relatively low uptake, because older age groups are generally thought to be less technology inclined. We partially mitigated this issue by also mailing DVD players and DVDs containing the intervention materials.
For our approach to be successful, once individuals decide to take action they need to effectively communicate their preferences to health care providers. Initiating such communication may be a difficult conversation and intervention group participants could not directly control whether their physician prescribed osteoporosis treatment or testing. Our results are concordant with others showing that patient activation interventions alone, without offering direct engagement in care,(29, 72) may not improve the rates of osteoporosis testing or treatment initiation.(73–75) Therefore, despite the limited success(21–28) in improving osteoporosis care through physician-targeted interventions, combining tailored, patient-directed interventions with physician-targeted interventions could prove to be a fruitful strategy in advancing osteoporosis care.
In APROPOS, we tried to create a highly tailored intervention by identifying modifiable barriers to osteoporosis treatment, but some participants failed to list any treatment barriers or ranked several barriers equally.(16) This demonstrates the difficulty of consistently capturing treatment barrier information, which is essential to developing an intervention individually tailored, as we intended. Because ~ 65% of the patients received previous osteoporosis treatments, it is possible that prior treatment may have influenced the patient perception of need to take future osteoporosis medications In addition, many salient characteristics of optimal tailoring for osteoporosis behavior change may be unknown.
In conclusion, achieving changes in osteoporosis care using patient activation approaches is challenging. While our personalized, multi-modal behavioral intervention did not increase the use of osteoporosis therapy at 6-months, it increased non-bisphosphonate medication use and BMD testing in select subgroups, shifted participant’s readiness for behavior change, and altered perceptions of common barriers to osteoporosis treatment within exploratory analyses, suggesting future research with similarly designed interventions may merit study when applied to select populations.
Supplementary Material
Acknowledgments
Funding/Support
This work was supported by R01 AR060240 (Saag) from the NIAMS/NIH. Dr. Danila is supported by a K23 AR062100 award from NIAMS/NIH. Dr. Wright is supported by grant number K12HS023009 from the Agency for Healthcare Research and Quality.
FAA holds a research contract from Portola and serves as a consultant to Millennium Pharmaceuticals. SLG holds funding from Amgen and Lilly and serves on the advisory board for Merck. AZL serves on the advisory boards for Amgen, Pfizer, and Semonix. SLS receives research funding from Amgen and Lilly, serves as a consultant for Amgen and as a speaker for Lilly and Amgen. ESS serves as a consultant for Amgen and Radius. NBW has stock options, royalties, is company owner and patent owner for OsteoDynamics and has received honoraria for lectures from Amgen and Shire, consulting fees from AbbVie, Amgen, Janssen, Merck, Radius, and Sanofi, and receives research support through Shire. JRC receives consulting and research grants from Amgen. NCW serves as a consultant for Pfizer and receives funding from Amgen. KGS serves as a consultant for Amgen, Merck, and Radius and receives funding from Amgen and Merck.
Abbreviations
- BMD
bone mineral density
- GI
gastrointestinal
- GLOW
Global Longitudinal Study of Osteoporosis in Women
- IMB
information, motivation, behavioral skills model
- IVR
interactive voice-response
- NG
nominal group
- NHANES
National Health and Nutrition Examination Study
- PAPM
Precaution Adoption Process Model
- ONJ
osteonecrosis of the jaw
Footnotes
Role of Sponsor: The NIAMS/NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or, decision to submit the manuscript for publication.
References
- 1.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 2.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England journal of medicine. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 4.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Quality Assurance. [Accessed on October 28, 2010];HEDIS & Quality Measurement. Available at http://www.ncqa.org/tabid/59/Default.aspx.
- 6.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Giangregorio L, Papaioannou A, Thabane L, deBeer J, Cranney A, Dolovich L, et al. Do patients perceive a link between a fragility fracture and osteoporosis? BMC Musculoskelet Disord. 2008;9:38. doi: 10.1186/1471-2474-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alami S, Hervouet L, Poiraudeau S, Briot K, Roux C. Barriers to Effective Postmenopausal Osteoporosis Treatment: A Qualitative Study of Patients’ and Practitioners’ Views. PLoS One. 2016;11(6):e0158365. doi: 10.1371/journal.pone.0158365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. J Bone Miner Res. 2016;31(8):1485–7. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 10.Miller PD. Underdiagnosis and Undertreatment of Osteoporosis: The Battle to Be Won. J Clin Endocrinol Metab. 2016;101(3):852–9. doi: 10.1210/jc.2015-3156. [DOI] [PubMed] [Google Scholar]
- 11.McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect. 2011;14(3):307–20. doi: 10.1111/j.1369-7625.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yood RA, Mazor KM, Andrade SE, Emani S, Chan W, Kahler KH. Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med. 2008;23(11):1815–21. doi: 10.1007/s11606-008-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan KN, Milchak JL, Heilmann RM, Billups SJ, Delate T. Evaluation of primary nonadherence to oral bisphosphonate therapy. J Am Geriatr Soc. 2013;61(11):2046–7. doi: 10.1111/jgs.12521. [DOI] [PubMed] [Google Scholar]
- 14.Gadkari AS, McHorney CA. Medication nonfulfillment rates and reasons: narrative systematic review. Curr Med Res Opin. 2010;26(3):683–705. doi: 10.1185/03007990903550586. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Brenneman SK, Sazonov V, Modi A. Reasons for not initiating osteoporosis therapy among a managed care population. Patient Prefer Adherence. 2015;9:821–30. doi: 10.2147/PPA.S81963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danila MI, Outman RC, Rahn EJ, Mudano AS, Thomas TF, Redden DT, et al. A multi-modal intervention for Activating Patients at Risk for Osteoporosis (APROPOS): Rationale, design, and uptake of online study intervention material. Contemp Clin Trials Commun. 2016;4:14–24. doi: 10.1016/j.conctc.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldstein AC, Schneider J, Smith DH, Vollmer WM, Rix M, Glauber H, et al. Harnessing stakeholder perspectives to improve the care of osteoporosis after a fracture. Osteoporos Int. 2008;19(11):1527–40. doi: 10.1007/s00198-008-0605-3. [DOI] [PubMed] [Google Scholar]
- 18.Laliberte MC, Perreault S, Jouini G, Shea BJ, Lalonde L. Effectiveness of interventions to improve the detection and treatment of osteoporosis in primary care settings: a systematic review and meta-analysis. Osteoporos Int. 2011;22(11):2743–68. doi: 10.1007/s00198-011-1557-6. [DOI] [PubMed] [Google Scholar]
- 19.van Boven JF, Stuurman-Bieze AG, Hiddink EG, Postma MJ, Vegter S. Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. J Manag Care Spec Pharm. 2014;20(8):786–92. doi: 10.18553/jmcp.2014.20.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuurman-Bieze AG, Hiddink EG, van Boven JF, Vegter S. Proactive pharmaceutical care interventions decrease patients’ nonadherence to osteoporosis medication. Osteoporos Int. 2014;25(6):1807–12. doi: 10.1007/s00198-014-2659-8. [DOI] [PubMed] [Google Scholar]
- 21.Majumdar SR, McAlister FA, Johnson JA, Bellerose D, Siminoski K, Hanley DA, et al. Interventions to increase osteoporosis treatment in patients with ‘incidentally’ detected vertebral fractures. Am J Med. 2012;125(9):929–36. doi: 10.1016/j.amjmed.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Ciaschini PM, Straus SE, Dolovich LR, Goeree RA, Leung KM, Woods CR, et al. Community based intervention to optimize osteoporosis management: randomized controlled trial. BMC Geriatr. 2010;10:60. doi: 10.1186/1471-2318-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–75. doi: 10.1503/cmaj.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumdar SR, Johnson JA, Lier DA, Russell AS, Hanley DA, Blitz S, et al. Persistence, reproducibility, and cost-effectiveness of an intervention to improve the quality of osteoporosis care after a fracture of the wrist: results of a controlled trial. Osteoporos Int. 2007;18(3):261–70. doi: 10.1007/s00198-006-0248-1. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, Morrish DW, et al. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann Intern Med. 2004;141(5):366–73. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- 26.Leslie WD, LaBine L, Klassen P, Dreilich D, Caetano PA. Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–6. doi: 10.1503/cmaj.111158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Outman RC, Curtis JR, Locher JL, Allison JJ, Saag KG, Kilgore ML. Improving osteoporosis care in high-risk home health patients through a high-intensity intervention. Contemp Clin Trials. 2012;33(1):206–12. doi: 10.1016/j.cct.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cranney A, Lam M, Ruhland L, Brison R, Godwin M, Harrison MM, et al. A multifaceted intervention to improve treatment of osteoporosis in postmenopausal women with wrist fractures: a cluster randomized trial. Osteoporos Int. 2008;19(12):1733–40. doi: 10.1007/s00198-008-0669-0. [DOI] [PubMed] [Google Scholar]
- 29.Warriner AH, Outman RC, Feldstein AC, Roblin DW, Allison JJ, Curtis JR, et al. Effect of self-referral on bone mineral density testing and osteoporosis treatment. Med Care. 2014;52(8):743–50. doi: 10.1097/MLR.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLeod KM, McCann SE, Horvath PJ, Wactawski-Wende J. Predictors of change in calcium intake in postmenopausal women after osteoporosis screening. J Nutr. 2007;137(8):1968–73. doi: 10.1093/jn/137.8.1968. [DOI] [PubMed] [Google Scholar]
- 31.Winzenberg T, Oldenburg B, Frendin S, De Wit L, Riley M, Jones G. The effect on behavior and bone mineral density of individualized bone mineral density feedback and educational interventions in premenopausal women: a randomized controlled trial [ NCT00273260] BMC Public Health. 2006;6:12. doi: 10.1186/1471-2458-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolinsky FD, Lou Y, Edmonds SW, Hall SF, Jones MP, Wright NC, et al. Activating Patients With a Tailored Bone Density Test Results Letter and Educational Brochure: the PAADRN Randomized Controlled Trial. J Clin Densitom. 2016 doi: 10.1016/j.jocd.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingwell E, Prior JC, Ratner PA, Kennedy SM. Direct-to-participant feedback and awareness of bone mineral density testing results in a population-based sample of mid-aged Canadians. Osteoporos Int. 2010;21(2):307–19. doi: 10.1007/s00198-009-0966-2. [DOI] [PubMed] [Google Scholar]
- 34.Campbell MK, Torgerson DJ, Thomas RE, McClure JD, Reid DM. Direct disclosure of bone density results to patients: effect on knowledge of osteoporosis risk and anxiety level. Osteoporos Int. 1998;8(6):584–90. doi: 10.1007/s001980050103. [DOI] [PubMed] [Google Scholar]
- 35.Wu F, Laslett LL, Wills K, Oldenburg B, Jones G, Winzenberg T. Effects of individualized bone density feedback and educational interventions on osteoporosis knowledge and self-efficacy: a 12-yr prospective study. J Clin Densitom. 2014;17(4):466–72. doi: 10.1016/j.jocd.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011;22(3):457–60. doi: 10.1007/s00198-011-1712-0. [DOI] [PubMed] [Google Scholar]
- 37.Newman ED. Perspectives on pre-fracture intervention strategies: the Geisinger Health System Osteoporosis Program. Osteoporos Int. 2011;22(Suppl 3):451–5. doi: 10.1007/s00198-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 38.Smith DH, Kramer JM, Perrin N, Platt R, Roblin DW, Lane K, et al. A randomized trial of direct-to-patient communication to enhance adherence to beta-blocker therapy following myocardial infarction. Arch Intern Med. 2008;168(5):477–83. doi: 10.1001/archinternmed.2007.132. [DOI] [PubMed] [Google Scholar]
- 39.Ownby RL, Waldrop-Valverde D, Caballero J, Jacobs RJ. Baseline medication adherence and response to an electronically delivered health literacy intervention targeting adherence. Neurobehav HIV Med. 2012;4:113–21. doi: 10.2147/NBHIV.S36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooven FH, Adachi JD, Adami S, Boonen S, Compston J, Cooper C, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int. 2009;20(7):1107–16. doi: 10.1007/s00198-009-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinyard LJ, Kreuter MW. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ Behav. 2007;34(5):777–92. doi: 10.1177/1090198106291963. [DOI] [PubMed] [Google Scholar]
- 42.Slater MD, Rouner D. Entertainment-education and elaboration likelihood: Understanding the processing of narrative persuasion. Communcation Theory. 2002;12(2):173–91. [Google Scholar]
- 43.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111(3):455–74. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 44.Tran AN, Haidet P, Street RL, Jr, O’Malley KJ, Martin F, Ashton CM. Empowering communication: a community-based intervention for patients. Patient Educ Couns. 2004;52(1):113–21. doi: 10.1016/s0738-3991(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 45.Watts NB. Insights from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Nat Rev Endocrinol. 2014;10(7):412–22. doi: 10.1038/nrendo.2014.55. [DOI] [PubMed] [Google Scholar]
- 46.Ashton CM, Holt CL, Wray NP. A patient self-assessment tool to measure communication behaviors during doctor visits about hypertension. Patient Educ Couns. 2010;81(2):275–314. doi: 10.1016/j.pec.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7(4):355. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 49.Mauck K, Cuddihy M, Trousdale R, Pond G, Pankratz V, Melton L., III The decision to accept treatment for osteoporosis following hip fracture: exploring the woman’s perspective using a stage-of-change model. Osteoporos Int. 2002;13(7):560–4. doi: 10.1007/s001980200073. [DOI] [PubMed] [Google Scholar]
- 50.Gleason LJ, Menzies IB, Mendelson DA, Kates SL, Friedman SM. Diagnosis and Treatment of Osteoporosis in High-Risk Patients Prior to Hip Fracture. Geriatric Orthopaedic Surgery & Rehabilitation. 2012;3(2):79–83. doi: 10.1177/2151458512454878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jennings LA, Auerbach AD, Maselli J, Pekow PS, Lindenauer PK, Lee SJ. Missed opportunities for osteoporosis treatment in patients hospitalized for hip fracture. J Am Geriatr Soc. 2010;58(4):650–7. doi: 10.1111/j.1532-5415.2010.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, et al. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135(5):490–9. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 53.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. The accuracy of self-reported fractures in older people. J Clin Epidemiol. 2002;55(5):452–7. doi: 10.1016/s0895-4356(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 54.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein ND, Rothman AJ, Sutton SR. Stage theories of health behavior: conceptual and methodological issues. Health Psychol. 1998;17(3):290. doi: 10.1037//0278-6133.17.3.290. [DOI] [PubMed] [Google Scholar]
- 56.Weinstein ND, Sandman PM, Blalock SJ. The Precaution Adoption Process Model [Google Scholar]
- 57.Gregson CL, Dennison EM, Compston JE, Adami S, Adachi JD, Anderson FA, Jr, et al. Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int. 2014;25(1):85–95. doi: 10.1007/s00198-013-2438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siris ES, Gehlbach S, Adachi JD, Boonen S, Chapurlat RD, Compston JE, et al. Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW) Osteoporos Int. 2011;22(1):27–35. doi: 10.1007/s00198-010-1211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grover ML, Edwards FD, Chang YH, Cook CB, Behrens MC, Dueck AC. Fracture risk perception study: patient self-perceptions of bone health often disagree with calculated fracture risk. Womens Health Issues. 2014;24(1):e69–75. doi: 10.1016/j.whi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Cline RR, Farley JF, Hansen RA, Schommer JC. Osteoporosis beliefs and antiresorptive medication use. Maturitas. 2005;50(3):196–208. doi: 10.1016/j.maturitas.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Weinstein ND. Unrealistic optimism about susceptibility to health problems: conclusions from a community-wide sample. J Behav Med. 1987;10(5):481–500. doi: 10.1007/BF00846146. [DOI] [PubMed] [Google Scholar]
- 62.Nahum-Shani I, Hekler EB, Spruijt-Metz D. Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychol. 2015;34(Suppl):1209–19. doi: 10.1037/hea0000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner MJ, Brophy RH, Demetrakopoulos D, Koob J, Hong R, Rana A, et al. Interventions to improve osteoporosis treatment following hip fracture. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(1):3–7. doi: 10.2106/JBJS.D.02289. [DOI] [PubMed] [Google Scholar]
- 64.Ganda K, Puech M, Chen J, Speerin R, Bleasel J, Center J, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24(2):393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 65.Donovan JL, Blake DR. Patient non-compliance: deviance or reasoned decision-making? Soc Sci Med. 1992;34(5):507–13. doi: 10.1016/0277-9536(92)90206-6. [DOI] [PubMed] [Google Scholar]
- 66.Cole RE. Clinical strategies to address patients’ concerns in osteoporosis management with bisphosphonates. Postgrad Med. 2011;123(2):131–44. doi: 10.3810/pgm.2011.03.2271. [DOI] [PubMed] [Google Scholar]
- 67.Eberth JM, Kline KN, Moskowitz DA, Montealegre JR, Scheurer ME. The role of media and the Internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health. 2014;54(3):289–95. doi: 10.1016/j.jadohealth.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freed GL, Katz SL, Clark SJ. Safety of vaccinations. Miss America, the media, and public health. JAMA. 1996;276(23):1869–72. doi: 10.1001/jama.276.23.1869. [DOI] [PubMed] [Google Scholar]
- 69.Ball LK, Evans G, Bostrom A. Risky business: challenges in vaccine risk communication. Pediatrics. 1998;101(3 Pt 1):453–8. doi: 10.1542/peds.101.3.453. [DOI] [PubMed] [Google Scholar]
- 70.Warriner AH, Outman RC, Allison JJ, Curtis JR, Markward NJ, Redden DT, et al. An internet-based controlled trial aimed to improve osteoporosis prevention among chronic glucocorticoid users. J Rheumatol. 2015;42(8):1478–83. doi: 10.3899/jrheum.141238. [DOI] [PubMed] [Google Scholar]
- 71.Bennett AT, Patel DA, Carlos RC, Zochowski MK, Pennewell SM, Chi AM, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health. 2015;24(11):950–7. doi: 10.1089/jwh.2015.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warriner AH, Outman RC, Kitchin E, Chen L, Morgan S, Saag KG, et al. A randomized trial of a mailed intervention and self-scheduling to improve osteoporosis screening in postmenopausal women. J Bone Miner Res. 2012;27(12):2603–10. doi: 10.1002/jbmr.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon DH, Finkelstein JS, Polinski JM, Arnold M, Licari A, Cabral D, et al. A randomized controlled trial of mailed osteoporosis education to older adults. Osteoporos Int. 2006;17(5):760–7. doi: 10.1007/s00198-005-0049-y. [DOI] [PubMed] [Google Scholar]
- 74.Cram P, Wolinsky FD, Lou Y, Edmonds SW, Hall SF, Roblin DW, et al. Patient-activation and guideline-concordant pharmacological treatment after bone density testing: the PAADRN randomized controlled trial. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bessette L, Davison KS, Jean S, Roy S, Ste-Marie LG, Brown JP. The impact of two educational interventions on osteoporosis diagnosis and treatment after fragility fracture: a population-based randomized controlled trial. Osteoporos Int. 2011;22(12):2963–72. doi: 10.1007/s00198-011-1533-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.