Abstract
Patient: Male, 57
Final Diagnosis: Pseudoepitheliomatous hyperplasia
Symptoms: Dysphonia
Medication: —
Clinical Procedure: Progressive speudoepitheliomaous hyperplasia spreaded
Specialty: Oncology
Objective:
Unusual clinical course
Background:
Pseudoepitheliomatous hyperplasia (PEH) is a reactive epithelial proliferation occurring secondary to infection, neoplasm, injury, and inflammation. The histopathological characteristics of PEH may lead to it being confused with well-differentiated squamous cell carcinoma (SCC).
Case Report:
We present here the case of a 57-year-old male patient, who was diabetic and a smoker, who presented with dysphonia. Although nasal endoscopy suggested SCC, morphological and immunophenotypical study of biopsy tissue ruled out malignancy.
Conclusions:
As the prognosis worsened, the patient required several urgent surgical interventions due to bleeding abscesses and dyspnea. A total laryngectomy was performed.
MeSH Keywords: Abscess, Hyperplasia, Laryngectomy
Background
Pseudoepitheliomatous hyperplasia (PEH) is a reactive epithelial proliferation, suggesting the release of cytokines produced by inflammatory or tumorous cells. It is also known as carcinomatoid hyperplasia and pseudocarcinomatous hyperplasia [1–3]. There is currently no consensus regarding the immunohistochemical markers that could help to differentiate the condition definitively.
We may distinguish PEH from squamous cell carcinoma (SCC) morphologically by cellular irregularities, the presence of mitosis, and vascular, lymphatic, or perineural invasion: The absence of these signs suggests PEH; their presence suggests SCC [3]. PEH may occur in response to a multitude of stimuli, including bacterial, viral, or fungal infection [1,2]. Correct diagnosis and treatment may be obtained by means of extensive, deep excision biopsy [3–5]. At times, due to the similarity with SCC, multiple biopsies are required to avoid unnecessary aggressive surgery [4].
Case Report
We present here the case of a 57-year-old male patient with diabetes. The patient was a smoker of 80 cigarettes a day and a horse breeder by profession. He attended an ENT (ear, nose and throat) consultation in May 2015 for dysphonia. Rhinofibroscopy revealed a whitish image all through the right vocal fold, anterior commissure, and anterior third of the contralateral.
Surgical biopsy found mild reactive dysplasia, with hyperplasia and keratosis in the anterior third of both vocal folds. Ki67 immunostaining (cell proliferation) went no further than the basal epithelial level (within normal values). In view of the clinical-pathological discrepancy, a further biopsy was performed, with similar results (Figure 1).
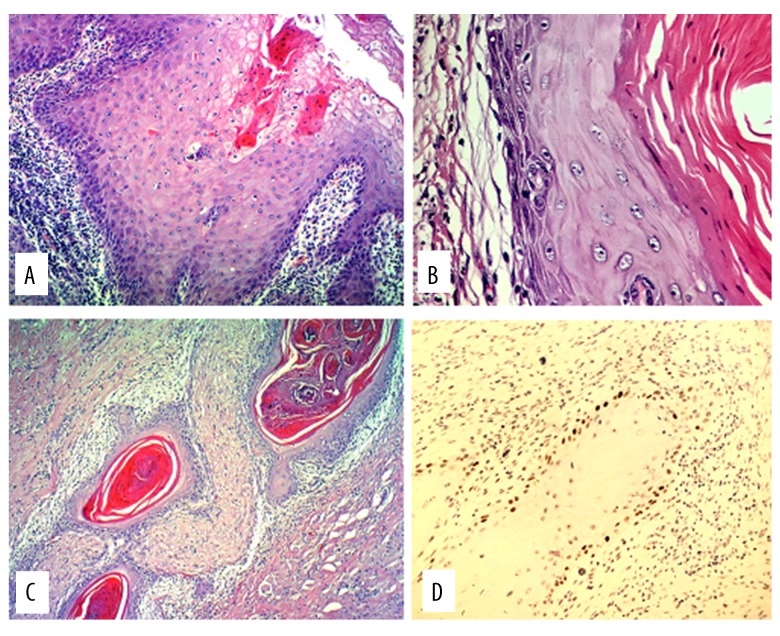
Figure 1.
Hematoxylin and eosin (H&E) stain (10×). Pseudoepitheliomatous hyperplasia with intraepithelial micro-abscesses (A). Squamous cyst wall (H&E 40×) with reactive/reparative dysplasia in base layer (B). No perineural invasion. Squamous cysts surround nerve fibers without infiltrating them (C). P53 in coating of deep squamous cyst, remaining at base layer (D).
In July 2015, the patient required a tracheotomy due to severe dyspnea and stridor. Nasal endoscopy revealed severe edema of the arytenoid cartilage, occluding the glottis. Multiple laryngeal biopsies were taken. The right vocal fold presented mild dysplasia; other samples had PEH. A few days later, a peristomal abscess appeared, requiring surgical draining. The culture returned positive for Streptococcus anginosus. The biopsy returned negative for malignancy. The patient was admitted, improving with IV piperacillin-tazobactam.
In November 2016, a peristomal abscess required surgical draining. A culture returned positive for pseudomonas, anaerobic bacteria, and morganella; biopsies continued to rule out malignancy. The patient was discharged after 25 days, with oral metronidazole plus ciprofloxacin treatment. We never removed the tracheotomy because the glottis remained closed.
Computerized tomography (CT) scan and magnetic resonance imaging evidenced no adenomegaly with criteria for cervical adenopathy (Figure 2). A positron emission tomography (PET)-CT scan was also performed to rule out extra-laryngeal anomalies.
Figure 2.
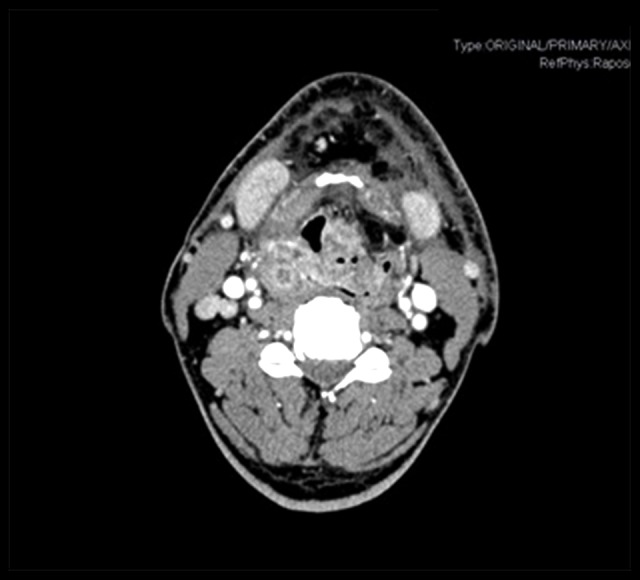
Cervical computerized tomography. Mass occupying larynx. Adenomegaly not having the characteristics of cervical adenopathy.
In February 2017, because of a frozen (lack of functionality) larynx, a total laryngectomy was performed with reconstruction by means of a pectoralis major flap. Due to the PEH including the jugular vein and lymph nodes, a bilateral cervical dissection was performed also (although CT scan evidenced no adenomegaly). Two Anatomical Pathology Unit consultations concluded a diagnosis of reactive mild dysplasia, PEH, abundant fistulized squamous cell nests and abscesses surrounded by scar tissue, without vascular lymphatic or perineural invasion or other signs of malignancy (Figure 3).
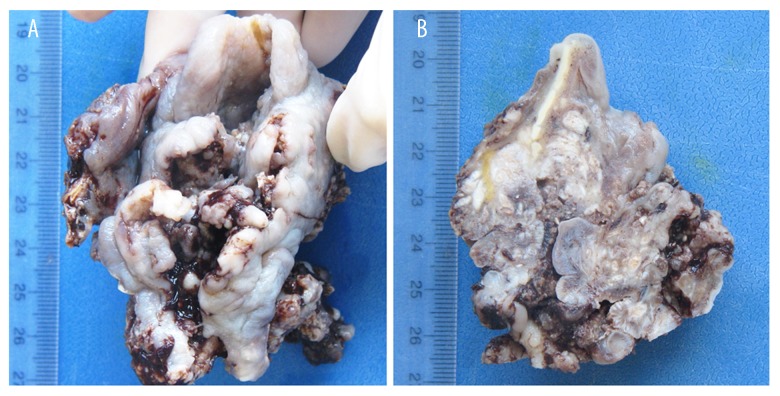
Figure 3.
Complete piece of totally destructured larynx (A). Transversal section of larynx. The white spots are the many abscesses (B).
Discussion
We conducted a bibliography search in PubMed, finding 341 articles relative to PEH. We then specified articles dealing with PEH of the larynx, finding only 23.
The benign nature of PEH means that most cases are resolved by means of excision biopsy, although grafts or flaps are occasionally needed to reconstruct major tissue defects [3,5,6]. On occasions, due to the different underlying causes, a biopsy is not sufficient, and etiological treatment is required, depending on each of the pathologies mentioned in the introduction [1–3]. The most frequent etiology found in cases of laryngeal PEH was granular cell tumors (11 articles), followed by mycosis (7 articles), verrucous carcinoma (2 articles) and microbacteria (1 article). The remaining 2 articles referred to epithelial changes in the larynx and to oncofetal protein IMP3 (a marker for SCC).
The clinical case in question was in response to several unfavorable circumstances: The process was set off by the initial mild dysplasia; the patient’s profession (horse breeder) laid him open to continuous contact with bacteria and because of his diabetes, infections cured more slowly. Additionally, his tracheotomy favored the entry of germs through the stoma, forming peristomal abscesses and granulomas. We were also told that from September 2016, he failed to comply correctly with his therapy, due to personal reasons, this being the cause of his sudden deterioration.
Medical literature provides evidence of a certain similarity with SCC [1,2,7–9] which may occasionally lead to the use of unnecessary aggressive treatments. In this case, nasal endoscopy showed extensive leukoplakia of the right vocal fold, anterior commissure, and anterior third of the left vocal fold. Wenig et al. maintained that leukoplakia is not indicative of underlying dysplastic or malignant lesions, and that there are no defined criteria regarding transformation of dysplasia to SCC [9].
We did not initially perform aggressive surgery due to the absence of a conclusive anatomical-pathological diagnosis of SCC or other malignant lesions. The patient was evaluated using I-Scan-Imaging (ISI). The images showed pattern II (b1–2), with elongated, arborescent vessels, without visible intraepithelial loops in either of the vocal folds and no spiked images. We found articles in the literature on the use of narrow band imaging (similar to ISI) to differentiate hyperplastic from malignant lesions [10–13]. A search in PubMed identified 6 articles on ISI, although none referring to the larynx.
Having ruled out malignancy, we performed conservative treatment over 20 months, using antibiotics and local excision of lesions by means of transoral laser surgery. The literature refers to therapeutic alternatives, including diode laser, KTP laser, photodynamic therapy and microdebriders [5,14,15].
Fifteen days before operating, the patient suffered cardiorespiratory arrest in the ENT office because of bleeding and a severe episode of dyspnea; the patient required cardiopulmonary reanimation. The PEH was spreading around the supraglottic and subglottic, and the patient had a frozen larynx, so we decided to perform a total laryngectomy.
We have 2 reasons for publishing this paper: first, the rarity of the case, and second, the unlikely evolution of a benign lesion to a state where it requires a response as aggressive as total laryngectomy.
Conclusions
Laryngeal PEH is a benign condition. However, as it is infrequent, the literature offers little help on the optimal treatment of cases in which the etiology is unclear. The spread of PEH, the frozen larynx, and the severe episodes of abscesses were the reason for such aggressive treatment.
Footnotes
Conflict of interest
None.
References:
- 1.Narayan V, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: A review. J Int Oral Health. 2015;7(9):148–52. [PMC free article] [PubMed] [Google Scholar]
- 2.El-Khoury J, Kibbi AG, Abbas O. Mucocutaneous pseudoepitheliomatous hyperplasia: A eeview. Am J Dermatopathol. 2012;34(2):165–75. doi: 10.1097/DAD.0b013e31821816ab. [DOI] [PubMed] [Google Scholar]
- 3.Sarangarajan R, Vaishnavi VK, Sivadas G, et al. Pseudoepitheliomatous hyperplasia: Relevance in oral pathology. J Int Oral Health. 2015;7(7):132–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: A review. Am J Dermatopathol. 2011;33(2):112–22. doi: 10.1097/DAD.0b013e3181fcfb47. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Jiao B, Long H. Pseudoepitheliomatous hyperplasia treated by photodynamic therapy with variable irradiation dose and concentration of photosensitizer. Photomed Laser Surg. 2011;29(2):127–30. doi: 10.1089/pho.2009.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Chakrabarti PR, Agrawal D, Somanath S. Pseudoepitheliomatous hyperplasia: A clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7(4):232–34. doi: 10.4103/0974-2077.150787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: The importance of clinicopathologic correlation. JAAD Case Rep. 2017;3(2):151–54. doi: 10.1016/j.jdcr.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira JC, Oton-Leite AF, Guidi R, Mendonça EF. Granular cell tumor mimicking a squamous cell carcinoma of the tongue: A case report. BMC Res Notes. 2017;10(1):14. doi: 10.1186/s13104-016-2325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenig BM. Squamous cell carcinoma of the upper aerodigestive tract: Precursors and problematic variants. Modern Pathol. 2002;15(3):229–54. doi: 10.1038/modpathol.3880520. [DOI] [PubMed] [Google Scholar]
- 10.Yagishita A, Fujii S, Yano T, Kaneko K. Endoscopic findings using narrow-band imaging to distinguish between basal cell hyperplasia and carcinoma of the pharynx. Cancer Sci. 2014;105(7):857–61. doi: 10.1111/cas.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SW, Lee YS, Chang LC, et al. Diagnostic significance of narrow-band imaging for detecting high-grade dysplasia, carcinoma in situ, and carcinoma in oral leukoplakia. Laryngoscope. 2012;122(12):2754–61. doi: 10.1002/lary.23629. [DOI] [PubMed] [Google Scholar]
- 12.Staníková L, Šatanková J, Kučová H, et al. The role of narrow-band imaging (NBI) endoscopy in optical biopsy of vocal cord leukoplakia. Eur Arch Otorhinolaryngol. 2017;274(1):355–59. doi: 10.1007/s00405-016-4244-6. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Liu J, Song F, Zhang S. The clinical diagnostic value of target biopsy using narrow-band imaging endoscopy and accurate laryngeal carcinoma pathologic specimen acquisition. Clin Otolaryngol. 2017;42(1):38–45. doi: 10.1111/coa.12654. [DOI] [PubMed] [Google Scholar]
- 14.Jeng JY, Tomblinson CM, Ocal IT, et al. Laryngeal cryptococcosis: Literature review and guidelines for laser ablation of fungal lesions. Laryngoscope. 2015;126:1625–29. doi: 10.1002/lary.25749. [DOI] [PubMed] [Google Scholar]
- 15.Sarin V, Bhardwaj B, Gill JS, Singh B. Pseudoepitheliomatous hyperplasia of tongue treated by microdebrider shaver. Pakistan Journal of Otolaryngology. 2014;30:26–28. [Google Scholar]