Abstract
Diabetes prevention is a public health priority. Vitamin D supplementation may help prevent the development of diabetes in persons at increased risk. We performed a meta-analysis of controlled clinical trials that assessed glycemic outcome measures among adults at risk for type 2 diabetes, including prediabetes, overweight, or obesity. We searched PUBMED/ MEDLINE, CINAHL, and Google Scholar databases for trials published prior to April 2017. Placebo-controlled clinical trials with random allocation to vitamin D with or without calcium supplementation were selected. Data collection included country, study design, inclusion criteria, sample size, form, and dose of vitamin D, supplementation interval, control group, duration, participant characteristics, comorbidities, baseline and follow-up serum 25-hydroxyvitamin D [25(OH)D] concentration, and available outcome measures [glycosylated hemoglobin (HbA1c), fasting plasma glucose, plasma glucose after 2-hour oral glucose tolerance test, and homeostatic model assessment of insulin resistance (HOMA-IR)]. Data synthesis was conducted using random-effect models (PROSPERO registration no. CRD42017055326). Twenty-eight trials, representing 3848 participants, met the eligibility criteria. Compared with the control group, vitamin D supplementation significantly reduced HbA1c level by –0.48% (95% CI, –0.79 to –0.18), fasting plasma glucose level by –0.46 mmol/L (95% CI, –0.74 to –0.19), and HOMA-IR level by –0.39 (95% CI, –0.68 to –0.11). Subgroup analysis revealed that the effects of vitamin D supplementation on different glycemic measures were influenced by age, calcium coadministration, vitamin D deficiency, serum 25(OH)D level after supplementation, and duration of supplementation. Vitamin D supplementation and improved vitamin D status improved glycemic measures and insulin sensitivity and may be useful as part of a preventive strategy for type 2 diabetes.
Keywords: 25-hydroxyvitamin D, cholecalciferol, diabetes, hemoglobin A1c, prediabetes, vitamin D
A meta-analysis was conducted of 28 randomized clinical trials studying glycemic control in prediabetics. Higher serum 25(OH)D levels lowered different glycemic measures.
Every 3 minutes, a Canadian is diagnosed with type 2 diabetes or prediabetes [1]. Currently, more than 5.7 million Canadians have prediabetes [1]. Prediabetes refers to impaired fasting glucose or impaired glucose tolerance, with fasting blood glucose levels above normal but not elevated enough to be diagnosed as type 2 diabetes mellitus [2]. People with prediabetes are at a 50% higher risk of developing type 2 diabetes [3, 4]. Yet, even if these people at high risk do not progress to type 2 diabetes, prediabetics are still prone to some of the long-term complications associated with diabetes, such as heart disease, stroke, and nerve damage [5]. The pathogenesis of prediabetes involves the development of insulin resistance and later impaired insulin secretion [6], often associated with chronic inflammation, an indicator in the development of type 2 diabetes [7, 8].
In parallel with the increased prevalence of prediabetes, there has been an increasing trend in the prevalence of vitamin D deficiency [9, 10]. Individuals with prediabetes commonly have lower serum 25-hydroxyvitamin D [25(OH)D] concentrations than those with normal glucose control [11–14]. Further, the risk of developing diabetes is much greater for prediabetics who are vitamin D deficient [15, 16]. Low vitamin D status in patients with prediabetes can predict future macrovascular complications by contributing to blood pressure dysregulation, renin-angiotensin activity impairments, endothelial dysfunction, and chronic inflammation [17–19]. Calcium might be beneficial for decreasing the risk of diabetes through its effect on insulin release and its indirect effect on weight loss [20–24]. Given this background, as well as its safety, reasonable price, and accessibility [25], vitamin D supplementation during the prediabetic stage, either taken alone or in combination with calcium, has the potential to halt the progression to diabetes has been investigated [21, 26, 27].
Several probable mechanisms of action may explain a possible role for vitamin D to help improve glucose metabolism, including its anti-inflammatory and immunomodulatory effects [28], the induction of insulin secretion by pancreatic β-cells [29], its indirect effect on regulating calcium concentration in pancreatic β-cells, and subsequent insulin secretion [30]. Further, vitamin D receptors are expressed on various insulin-dependent tissues (including the liver, skeletal muscle, and adipose tissue), suggesting a role for vitamin D in glucose utilization and insulin sensitivity [31]. The influence of vitamin D on genes regulating cellular proliferation, differentiation, and apoptosis in metabolic pathways may also play a role [32, 33]. Moreover, in vitamin D–deficient individuals, moderate elevation of parathyroid hormone (PTH) may impair insulin release from pancreatic β-cells [34, 35].
Observational studies, several randomized controlled trials (RCTs), and meta-analyses [36, 37] have been conducted to investigate the effect of vitamin D supplementation on glycemic measures and insulin sensitivity in prediabetes populations. However, no decisive conclusions can yet be drawn from these existing reports [38, 39]. Some of the conflicting results from these studies are due to limitations, including studies not primarily designed for glycemic outcomes, relatively small sample sizes, limited reports on serum 25(OH)D concentrations, inappropriate or infrequent doses of vitamin D (monthly or large bolus doses), and relatively short duration of supplementation that does not account for physiology (i.e., shorter than the turnover of HbA1c) [40–43]. To address these issues, we undertook a systematic review and meta-analysis of RCTs to delineate the impact of vitamin D supplementation on glycemic control and insulin resistance in prediabetic or overweight/obese adult populations. We restricted our analysis to clinical trials with daily or weekly supplementation, which provides a sustained serum 25(OH)D concentration over time, intervention periods of greater than 2 months or long enough for HbA1c turnover, a measurable impact of vitamin D supplementation on 25(OH)D concentrations, meaning the dose was high enough to impact vitamin D status, and the measurement of different parameters associated with prediabetes [HbA1c, FPG, HOMA-IR, and plasma glucose after 2-hour oral glucose tolerance test (2HPG)].
1. Materials and Methods
A. Literature Search Strategy
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines [44]. The study protocol was registered with the International Prospective Register of Systematic Reviews, PROSPERO (registration no. CRD42017055326). The primary outcome of interest was to systematically review the effect of vitamin D supplementation on glycemic control and insulin resistance as measured by HbA1c, FPG, fasting HOMA-IR, and 2HPG in adult populations with prediabetes, overweight, or obesity. We also evaluated the impact of coadministration of calcium with vitamin D, obesity, serum 25(OH)D status at the beginning and the end of intervention, and the duration of vitamin D supplementation on the abovementioned glycemic measures.
Search terms included [vitamin D; vitamin D3; cholecalciferol; 25(OH)D] AND/OR [prediabetes; insulin resistance; at risk for diabetes; hyperglycemia; HbA1c; glucose] in the title and/or abstract. Studies published in English and those conducted on adults (age ≥18 years old) were selected. Calcium was permitted as a combined supplement with vitamin D or as a supplement provided to both treated and control groups.
B. Data Sources
We searched multiple databases including PUBMED/Medline (Medical Literature Analyses and Retrieval System Online), Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane library, and Google Scholar (as the gray literature suggested by the Cochrane guideline). The reference lists of all articles that met the selection criteria and systematic reviews already published were also hand searched by two reviewers (N.M. and M.M.). Databases were searched for studies published from January 1999 until April 2017.
C. Study Selection
Two reviewers (N.M. and M.M.) selected the studies for the systematic review, which were approved by a third reviewer (S.M.K.). Studies were included in this systematic review and meta-analysis of RCTs if: (1) they were placebo-controlled trials; (2) the population included persons with prediabetes, impaired fasting glucose, impaired glucose tolerance, overweight, or obese; (3) were conducted in adults (≥18 years); (4) intervention was a minimum of 2 months (defined as the minimum time required to assess changes in glucose blood markers) [45]; (5) supplementation was provided on a daily, biweekly, or weekly basis; and (6) reported baseline and end-of-study measures for serum 25(OH)D concentrations and HbA1c or FPG or HOMA-IR or 2HPG, or providing delta values (changes over time).
Studies were excluded if: (1) they were not placebo controlled; (2) the population included subjects with type 2 diabetes, end-stage renal disease, or gestational diabetes or were conducted in children/adolescents; (3) the duration of the trial was less than 2 months; (4) supplementation was provided less frequently than each week (e.g., monthly basis or as a single large bolus dose); (5) serum 25(OH)D concentrations were not reported at baseline and end of study; (6) they were studies in which the mean change or SD of the outcome measures was not reported; or (7) the study had an observational, case control, cross-sectional, or cohort design, or the publication was a narrative review, comment, opinion piece, methodological report, editorial, letter, or conference abstract.
D. Data Extraction and Management
The full-text of each publication that met the inclusion criteria was screened to determine eligibility (N.M. and M.M.). Any disagreement between the researchers regarding the eligibility of particular studies was resolved through discussion with a third reviewer (S.M.K. or H.V.). Following assessment of methodological quality of the trials by the first reviewer (N.M.), data were extracted using a data extraction form and the most important results from each study were summarized by N.M. and M.M. The extracted data were approved by the third and fourth researchers (H.V. and S.M.K.). Data extracted from each study included first author, reference, year of publication, country of study, study design, inclusion criteria, sample size, form of vitamin D, vitamin D dose and supplementation interval, control group, duration of supplementation, participants’ characteristics [sex (n, % male), age, weight, body mass index (BMI)], cosupplementation with calcium, comorbidities, baseline and follow-up serum 25(OH)D concentration (nmol/L), and outcome measures. Any necessary calculations on data were conducted by the first reviewer (N.M.) and checked by the second reviewer (H.V.).
E. Quality Assessment
Study quality and the risk of bias in the eligible RCTs were systematically assessed using the Cochrane criteria checklist [46]. The items used for the assessment of each study were: (1) adequacy of random sequence generation; (2) allocation concealment; (3) sample size and power of study; (4) quality of blinding (both participants and personnel); (5) intention-to-treat analysis (incomplete data); (6) compatibility of groups; (7) clear definition of inclusion and exclusion criteria; and (8) the description of withdrawal and dropout. A judgment of “adequate” (√) indicated low risk of bias, whereas “inadequate” (–) indicated a high risk of bias, taking into account the recommendations of the Cochrane Handbook. We labeled uncertain or unknown risk of bias as “unclear.” Quality assessment was performed by one reviewer (N.M.) and approved by the other reviewers (S.M.K. and H.V.).
F. Statistical Analysis and Data Synthesis
To calculate the effect size, we followed the Cochrane Handbook and used the mean change from baseline to the end of the intervention in the levels and SD of the outcome measures for both control and intervention groups [46]. A meta-analysis was conducted to combine the individual study results. Meta-analysis of RCTs was conducted using Comprehensive Meta-Analysis version 3 software (Biostat 2014, Englewood, NJ) [47]. A P value <0.05 was considered to be statistically significant. We based the meta-analysis on calculating net changes from baseline to the endpoint, when the mean and SDs of the changes were reported, as: [(measure at endpoint in the treatment group – measure at baseline in the treatment group) – (measure at endpoint in the control group – measure at baseline in the control group)]. Effect sizes were expressed as the between-group weighted (standardized) mean difference and 95% CI.
Serum 25(OH)D levels were collated in nmol/L, and we used a multiplication factor of 2.496 to convert 25(OH)D levels respectively from ng/mL to nmol/L [48]. Plasma glucose levels (FPG and 2HPG) were collated in mmol/L, and a multiplication factor of 0.0555 was used to convert glucose levels respectively from mg/dL to mmol/L as appropriate [49].
Data were analyzed using a random-effects model (DerSimonian-Laird method) and the generic inverse variance method to compensate for the heterogeneity of studies due to the broad demographic characteristics of populations being studied [47, 49, 50]. Heterogeneity was assessed using the I2 index with values ≥50% determining the use of the random-effects model. The effect size was determined using weighted mean difference with a 95% CI. For treatment effect, a negative value represents a reduction in the outcome in the intervention group relative to the change in the placebo group. When more than one dose of vitamin D was examined in the same study, data from the highest dose was compared with placebo. Studies with more than two intervention groups (e.g., vitamin D alone and in combination with calcium) were used in subgroup analysis as multiple studies and both compared with the placebo.
We conducted a sensitivity analysis using the leave-one-out method (removing one study each time and repeating the analysis). This analysis allowed us to determine the impact of each study on the overall effect size [51].
G. Publication Bias
We explored publication bias using a visual inspection of Begg’s funnel plot asymmetry, supplemented with Egger’s weighted regression tests [49, 52]. Funnel plots were derived separately from changes in FPG, HbA1c, HOMA-IR, 2HPG, and 25(OH)D depicted as weighted mean difference vs its standard error (SE). This step was followed by adjusting the analysis for the effect of publication bias using the Duval and Tweedie “trim and fill” method [53].
H. Subgroup Analysis
In addition to running a sensitivity analysis and using random-effect models, we addressed heterogeneity using subgroup analyses. Subgroup analyses were performed for the following: (1) population was comprised of individuals with prediabetes vs overweight or obese (prediabetes was diagnosed by having HbA1c measured in the range of 5.8% to 6.4%, and any participant with BMI ≥25 kg/m2 was considered overweight/obese); (2) whether calcium was used in combination with vitamin D; (3) age group (≤45 years vs >45 years); (4) vitamin D deficiency at baseline [serum 25(OH)D level <50 nmol/L vs ≥50 nmol/L]; (5) serum 25(OH)D level at follow-up; (6) body weight status (overweight and obese with BMI ≥25 kg/m2 vs BMI <25 kg/m2); and (7) duration of the intervention (<6 months vs ≥6 months).
For each outcome, the effect size for subgroups (two subgroups) was calculated as the weighted mean difference between treatment and placebo groups using Comprehensive Meta-Analysis software (Biostat 2014). Then a between-subgroup comparison for each outcome was conducted with a simple t test, and the P values were adjusted by Bonferroni correction. Subgroup analyses were determined a priori according to established cutoff points such as vitamin D deficiency, defined as a serum 25(OH)D level of less than 50 nmol/L, or based on the distribution of study populations such as median for age or serum 25(OH)D levels at follow-up.
2. Results
A. Search Results and Study Selection Process
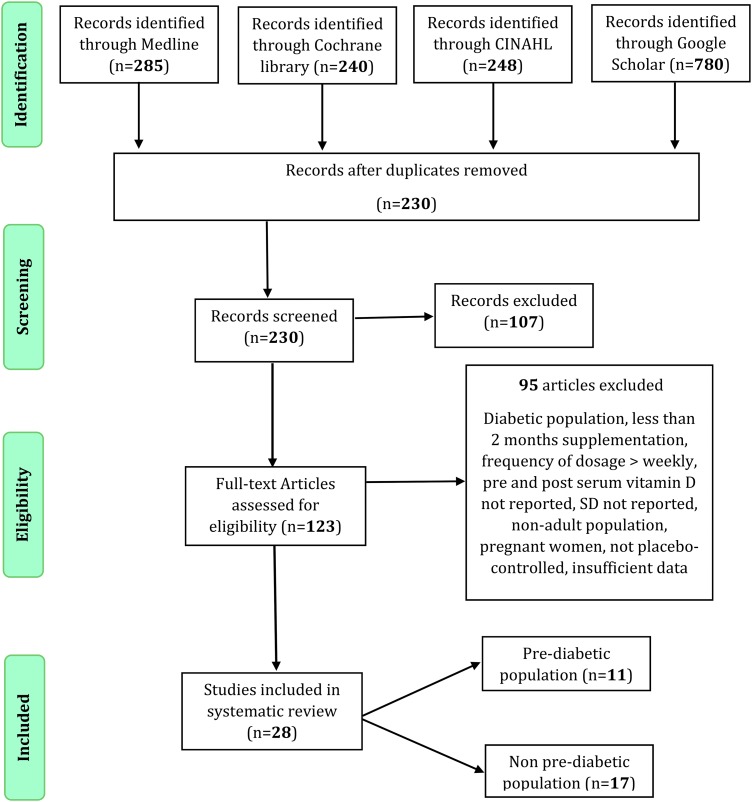
We identified a total of 1553 citations using the search keywords. After removing duplicates, 230 records remained. After screening via titles and abstracts, 123 articles remained for further evaluation. We excluded 95 articles for the following reasons: the population included persons with diabetes, children, or adolescents, or occurred during pregnancy; the duration of supplementation was less than 2 months; supplementation was provided on a monthly basis or as a large bolus dose; and studies were not placebo controlled. Studies with insufficient information, after unsuccessful attempt to obtain the information through communication with the authors, were excluded [54–59]. Twenty-eight RCTs met our eligibility criteria and were included in the meta-analysis. Details of the search process and study selection are illustrated in Fig. 1.
Figure 1.
Study selection flow diagram. PRISMA flow diagram of search results following study section procedure assessing vitamin D supplementation and glycemic control among RCTs of adult population.
B. Risk of Bias Assessment
There was no risk of selection bias because all included studies were reported to be randomized and the allocation was sufficiently concealed. There was a lack of information on blinding of patients and personnel (n = 2) and blinding of outcome assessment (n = 2). However, all evaluated studies had a low risk of bias according to random allocation concealment, comparability of intervention groups, clear definition of inclusion/exclusion criteria, and the description of dropout/withdrawal. Bias due to attrition was a concern in one trial [60]. Intention-to-treat analyses were conducted in 15 studies. An overview of the quality of bias assessment for each study is presented in Table 1.
Table 1.
Quality Assessment of Included Studies
| Authors | Quality of Random Allocation Concealment | Sample Size Large Enough to Detect Difference | Intention to Treat | Blinding Patients/ Personnel | Blinding Outcome Assessment | Comparability of Groups | Clear Definition of Inclusion Exclusion Criteria | Description of Withdrawal and Dropout |
|---|---|---|---|---|---|---|---|---|
| Barengolts et al. 2015 | √ | — | √ | √ | √ | √ | √ | √ |
| Davidson et al. 2013 | √ | √ | √ | √ | √ | √ | √ | √ |
| Oosterwerff et al. 2014 | √ | √ | √ | √ | √ | √ | √ | √ |
| Mitri et al. 2011 | √ | U | √ | √ | √ | √ | √ | √ |
| Harris et al. 2012 | √ | √ | U | U | U | √ | √ | √ |
| Tuomainen et al. 2015 | √ | √ | — | √ | √ | √ | √ | √ |
| Sollid et al. 2014 | √ | √ | √ | √ | √ | √ | √ | √ |
| Dutta et al. 2014 | √ | U | U | — | — | √ | √ | √ |
| Forouhi et al. 2016 | √ | √ | √ | √ | √ | √ | √ | √ |
| Jorde et al. 2016 | √ | √ | √ | U | U | √ | √ | √ |
| Moreira-Lucas et al. 2016 | √ | — | √ | √ | √ | √ | √ | √ |
| Salehpour et al. 2013 | √ | U | U | √ | √ | √ | √ | √ |
| Pittas et al. 2007 | √ | U | U | √ | √ | √ | √ | √ |
| Ramly et al. 2014 | √ | √ | √ | √ | √ | √ | √ | √ |
| Carrillo et al. 2013 | √ | √ | U | √ | √ | √ | √ | — |
| Wamberg et al. 2013 | √ | √ | √ | √ | √ | √ | √ | √ |
| Zittermann et al. 2009 | √ | U | U | √ | √ | √ | √ | √ |
| Gepner et al. 2012 | √ | √ | U | √ | √ | √ | √ | √ |
| Grimnes et al. 2011 | √ | √ | √ | √ | √ | √ | √ | √ |
| Wood et al. 2012 | √ | √ | √ | √ | √ | √ | √ | √ |
| Asemi et al. 2015 | √ | √ | √ | √ | √ | √ | √ | √ |
| Sun et al. 2016 | √ | √ | U | √ | √ | √ | √ | √ |
| Sharifi et al. 2014 | √ | √ | U | √ | √ | √ | √ | √ |
| Mousa et al. 2017 | √ | √ | — | √ | √ | √ | √ | √ |
| Osati et al. 2016 | √ | U | U | √ | √ | √ | √ | √ |
| Grubler et al. 2016 | √ | √ | √ | √ | √ | √ | √ | √ |
| Lorvand Amiri et al. 2016 | √ | √ | U | √ | √ | √ | √ | √ |
| Vahedpoor et al. 2017 | √ | √ | √ | √ | √ | √ | √ | √ |
Abbreviations: √, adequate; —, inadequate; U, unclear.
C. Characteristics of the Included Studies
The characteristics of the included studies are provided in Table 2. Included studies were published between 2007 and 2017 from different countries, including the United States (seven studies), Norway (three studies), Iran (six studies), and one study each from Canada, the Netherlands, Japan, Finland, Scotland, India, Malaysia, the United Kingdom, Australia, Austria, Denmark, and Germany. The sample size varied from 23 [60] to 511 [61]. Participants in six studies were females only [62–66] and males only in one study [67]. The mean age of participants ranged from 26 years [60] to 71 years [21]. The duration of follow-up across studies ranged from 2 months [64] to 5 years [68], with a median follow-up of 22 weeks [interquartile range (IQR): 14 to 48 weeks].
Table 2.
Characteristics of Included Studies
| Study | Place of Study | n | Study Population | Mean Age | % Male | Vitamin D µg (IU) | Other Treatments | Control Group | Duration of Trial | Outcomes Measured | Treated Group |
Control Group |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline 25(OH)D (nmol/L) Cases | Mean Change (nmol/L) Cases | Baseline 25(OH)D (nmol/L) Controls | Mean Change (nmol/L) Controls | |||||||||||
| Barengolts et al. 2015 | US | 173 | Prediabetes and hypovitaminosis D (12–73 nmol/L) overweight | 60 | 100 | 10 µg/d (400 IU/d) | Placebo | 12 mo | HbA1ca | 36 | 84 | 35 | 50 | |
| Davidson et al. 2013 | US | 109 | Prediabetes and 25(OH)D <75, obese and HTN | 55 | NA | Mean 2221 µg/wk (88,865 IU/wk) adjusted based on BMI and baseline D | Placebo | 12 mo | HbA1ca, FPG, 2HPG, HOMA-IR | 55 | 107 | 55 | 55 | |
| Oosterwerff et al. 2014 | Netherlands | 130 | Prediabetes and vitamin D deficiency (<50) and obese | 42 | 61 | 30 µg/d (1200 IU/d) | 500 mg/d CaCO3 | Placebo plus CaCO3 | 16 wk | HbA1c, HOMA-IR, 2HPGa | 25 | 35 | 22 | 23 |
| Mitri et al. 2011 | US | 92 | Prediabetes and overweight | 57 | 49 | 50 µg/d (2000 IU/d) | Placebo | 16 wk | HbA1ca, 2HPGa, FPGa | 61 | 25 | 60 | −18 | |
| Harris et al. 2012 | US | 89 | Prediabetes and obese | 55 | 35 | 100 µg/d (4000 IU/d) | Calcium 600 mg/d | Placebo | 12 wk | HbA1c, HOMA-IRa, FPGa, 2HPG | 40 | 41 | 38 | −1 |
| Tuomainen et al. 2015 | Finland | 73 | Prediabetes and vitamin D deficiency (<75) and overweight | 70 | NA | First arm: 40 µg/d (1600 IU/d); second arm: 80 µg/d (3200 IU/d) | Placebo | 5 mo | HbA1c, FPG, 2HPGa, HOMA-IR | 57 | 45 (80 µg/d) 28 (40 mg/d) | 57 | 4 | |
| Sollid et al. 2014 | Norway | 511 | Prediabetes and obese | 50 | 61 | 500 µg/wk (20,000 IU/wk) | Placebo | 1 y | HbA1c, FPGa, 2HPG, HOMA-IR | 60 | 46 | 61 | 3 | |
| Dutta et al. 2014 | India | 136 | Prediabetes and 25(OH)D <75 overweight | 55 | NA | A; 1500 µg/wk (60,000 IU/wk) for 8 wk then monthly | Calcium 500mg/d C; vitamin D–sufficient group got Ca | Placebo | 1 y | HbA1c, FPGa, 2HPGa, HOMA-IR | 42 | 47 (A) | 95 | −73 (C) |
| Forouhi et al. 2016 | UK | 340 | Prediabetes | 52 | NA | 83 µg/d (3300 IU/d) D3 | Placebo | 4 mo | HbA1c | 46 | 40 | 46 | 0 | |
| Jorde et al. 2016 | Norway | 226 | Prediabetes | 62 | 64 | 500 µg/wk (20,000 IU/wk) | Placebo | 5 y | HbA1c, FPG, 2HPG, HOMA-IRa | 60 | 62 | 61 | 6 | |
| Moreira-Lucas et al. 2016 | Canada | 63 | Prediabetes and vitamin D deficient | 46 | 52 | 700 µg/wk (28,000 IU/wk) | Placebo | 24 wk | FPG, HbA1c, HOMA-IRa | 48 | 51 | 48 | −2 | |
| Salehpour et al. 2013 | Iran | 77 | Premenopausal women and obese | 34 | 0 | 25 µg/d (1000 IU/d) | Placebo | 12 wk | HbA1ca, FPGa, 2HPG, HOMA-IR | 37 | 38 | 47 | 4 | |
| Pittas et al. 2007 | US | 92 | Overweight | 71 | 52 | 18 µg/d (700 IU/d) | Calcium 500 mg/d | Placebo | 3 y | HOMA-IRa, FPGa | 71 | 31 | 81 | −8 |
| Ramly et al. 2014 | Malaysia | 171 | Premenopausal women and D deficient (<50) | 50 | 0 | 1250 µg/wk (50,000 IU/wk) for 2 mo then monthly | Placebo | 12 mo | FPGa, HOMA-IRa | 30 | 56 | 30 | 6 | |
| Carrillo et al. 2013 | US | 23 | Overweight and obese | 26 | 48 | 100 µg/d (4000 IU/d) | Placebo | 12 wk | FPG, 2HPG, HOMA-IR | 48 | 36 | 45 | 14 | |
| Wamberg et al. 2013 | Denmark | 52 | Obese and D deficient (<50) | 34 | 29 | 175 µg/d (7000 IU/d) | Placebo | 26 wk | FPGa, HOMA-IR | 34 | 76 | 34 | 13 | |
| Zittermann et al. 2009 | Germany | 165 | Overweight and average 25(OH)D = 30 | 44 | 33 | 83 µg/d (3332 IU/d) | Placebo | 12 mo | HbA1c, FPG | 30 | 56 | 30 | 12 | |
| Asemi et al. 2015 | Iran | 104 | Women with PCOS and vitamin D deficiency (<50) and obese | 29 | 0 | 1250 µg/wk (50,000 IU/wk) | Plus Ca placebo | Calcium placebo + vitamin D placebo | 2 mo | FPG, HOMA-IRa | 29 | 30 | 35 | 1 |
| Gepner et al. 2012 | US | 109 | Postmenopausal women and 25 ≤ 25(OH)D ≤ 150 | 64 | 0 | 63 µg/d (2500 IU/d) | Placebo | 4 mo | FPG | 76 | 39 | 81 | −0.5 | |
| Grimnes et al. 2011 | Norway | 94 | Vitamin D deficient | 53 | 52 | 500 µg biweekly (20,000 IU biweekly) | Placebo | 6 mo | FPGa, HbA1c, HOMA-IR | 42 | 100 | 39 | 3 | |
| Wood et al. 2012 | Scotland | 305 | Postmenopausal women | 65 | 0 | 25 µg/d (1000 IU/d) or 10 µg/d (400 IU/d) | Placebo | 12 mo | FPG, HOMA-IR | 32 | 43 (25 µg/d) 33 (10 mg/d) | 36 | −3 | |
| Sun et al. 2016 | Japan | 81 | Healthy | 45 | 6 | 11 µg/d (420 IU/d) | Placebo | 1 y | FPGa, HOMA-IR, HbA1ca | 33 | 28 | 32 | −1 | |
| Sharifi et al. 2014 | Iran | 53 | Adults with NAFLD | 42 | 49 | 1250 µg biweekly (50,000 IU/ biweekly) | Placebo | 4 mo | FPG | 37 | 46 | 44 | 6 | |
| Mousa et al. 2017 | Australia | 54 | Overweight and obese | 30 | 65 | 2500 µg (100,000 IU bolus) then 100 µg/d (4000 IU/d) | Placebo | 4 mo | FPG | 31 | 57 | 34 | 2 | |
| Osati et al. 2016 | Iran | 210 | Vitamin D deficient | 38 | 23 | 1250 µg/wk (50,000 IU/wk) | Placebo | 2 mo | FPG, HOMA-IRa | 34 | 35 | 35 | 2 | |
| Grubler et al. 2016 | Austria | 185 | People with arterial HTN | 60 | 53 | 70 µg/d (2800 IU/d) | Placebo | 2 mo | FPG, HbA1ca | 54 | 11 | 51 | 0 | |
| Lorvand Amiri et al. 2016 | Iran | 73 | Patients with NAFLD | 42 | 62 | 25 µg/d (1000 IU/d) | Hypocaloric diet | Placebo | 3 mo | FPG, HOMA-IRa | 25 | 43 | 25 | 2 |
| Vahedpoor et al. 2017 | Iran | 58 | Patients with cervical intraepithelial neoplasia | 37 | NA | 1250 µg biweekly (50,000 IU biweekly) | Placebo | 6 mo | FPGa, HOMA-IRa | 27 | 40 | 28 | −2 | |
Abbreviations: HTN, hypertension; NA, not available; NAFLD, non-alcoholic fatty liver disease; PCOS, polycystic ovary syndrome.
Significant difference between intervention and placebo groups (P < 0.05).
Oral daily doses of vitamin D3 ranged from 10.5 µg/d (420 IU/d) [69] to 175 µg/d (7000 IU/d) [70], with 10 studies providing daily doses over 100 µg/d (4000 IU/d) [60, 63, 64, 70–76]. In 12 studies, vitamin D supplementation was provided on a weekly basis with a dose range of 500 to 2222 µg/wk (20,000 to 88,880 IU/wk), roughly equivalent to a daily dose of 72 to 318 µg (2860 to 12,700 IU/d) [43, 61, 63, 64, 68, 71, 73–78]. The average serum 25(OH)D concentrations at baseline varied from 25 nmol/L [79, 80] to 76 nmol/L [65], with a median of 38 nmol/L (IQR: 31 to 54 nmol/L) in vitamin D–supplemented groups and 41 nmol/L (IQR: 34 to 56 nmol/L) in the placebo groups. Thirteen studies recruited subjects who were vitamin D deficient [serum 25(OH)D <50 nmol/L] or insufficient [serum 25(OH)D <75 nmol/L] at the beginning of the trial [42, 43, 63, 64, 67, 70, 71, 73, 74, 76, 79–81]. Cosupplementation of vitamin D and calcium occurred in four studies, which assessed vitamin D plus calcium against placebo [21, 72, 73, 79]. A determination of prediabetes was an inclusion criterion in 11 RCTs [15, 61, 67, 68, 71–74, 79, 81, 82]. Being overweight or obese (BMI ≥25 kg/m2) was one of the inclusion criteria in 15 RCTs [21, 42, 60–62, 64, 67, 70–73, 75, 79, 81, 82].
There were 26 studies with acceptable methodological quality and low dropout rate (<20%). These studies included a large number of participants in the intervention groups (n = 1924), an average vitamin D supplementation dose of 88 µg/d (3500 IU/d), and a median of 22 weeks (around 6 months) for the length of supplementation, a duration long enough to detect changes in measured outcomes. Each of these features improved the statistical strength of this study. Analyzed together, these data provide a diverse population.
D. Pooled Estimate of the Effect of Vitamin D on Serum 25(OH)D Level
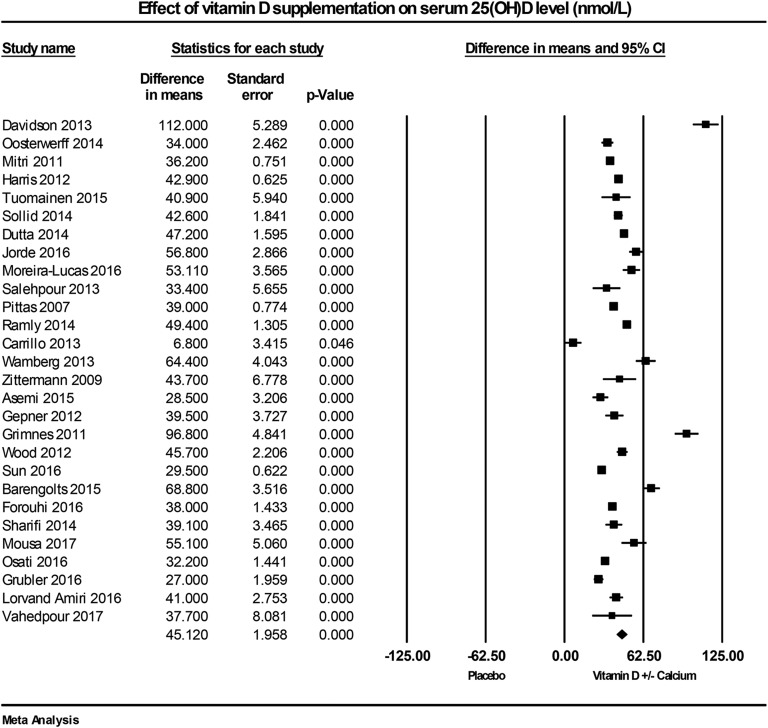
All included trials measured the effect of vitamin D supplementation on serum 25(OH)D concentrations. All trials showed a significant increase in serum 25(OH)D levels in vitamin D–supplemented groups, with the overall median serum 25(OH)D concentration ≥86 nmol/L at follow-up (mean ± SD: 91 ± 25 nmol/L), compared with the placebo group (49 ± 20 nmol/L). In seven studies [64, 69, 76, 78–80, 83], follow-up serum 25(OH)D concentrations were below 86 nmol/L, which may be related to low supplementation dose and/or high BMI. The substantial increase of serum 25(OH)D concentration in two studies [43, 71] was likely related to the high dose of supplementation [300 µg/d (12,000 IU/d) and 143 µg/d (5700 IU/d)]. Overall, serum 25(OH)D concentrations in the treated arms significantly improved by 45.1 nmol/L (95% CI: 41.3 to 48.9; P < 0.001, I2 = 97.4%; Fig. 2).
Figure 2.
Forest plot of mean change from baseline in serum 25(OH)D concentrations (nmol/L) between vitamin D supplementation and control.
E. Pooled Estimate of the Effect of Vitamin D on Glycemic Control
E-1. HbA1c
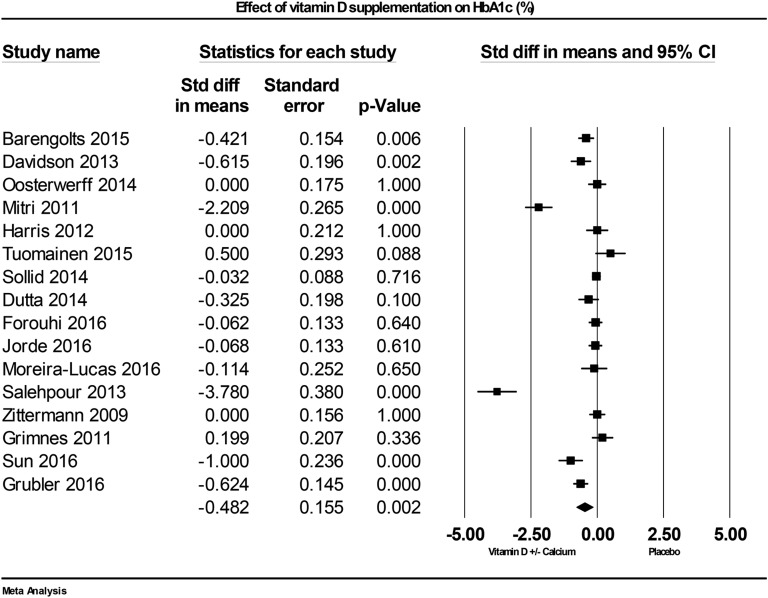
Sixteen studies examined HbA1c as an outcome. Of the individual studies, seven reported reduced HbA1c with vitamin D supplementation [62, 67, 69, 71, 73, 82, 83], and nine reported null results [15, 42, 43, 61, 68, 72, 74, 79, 81]. Vitamin D supplementation and improved vitamin D status reduced HbA1c significantly, compared with placebo, across all studies by –0.48% (95% CI: –0.79 to –0.18; P = 0.002, I2 = 92.1%; Fig. 3).
Figure 3.
Forest plot of mean change from baseline in HbA1c (%) between vitamin D supplementation and control.
E-2. FPG
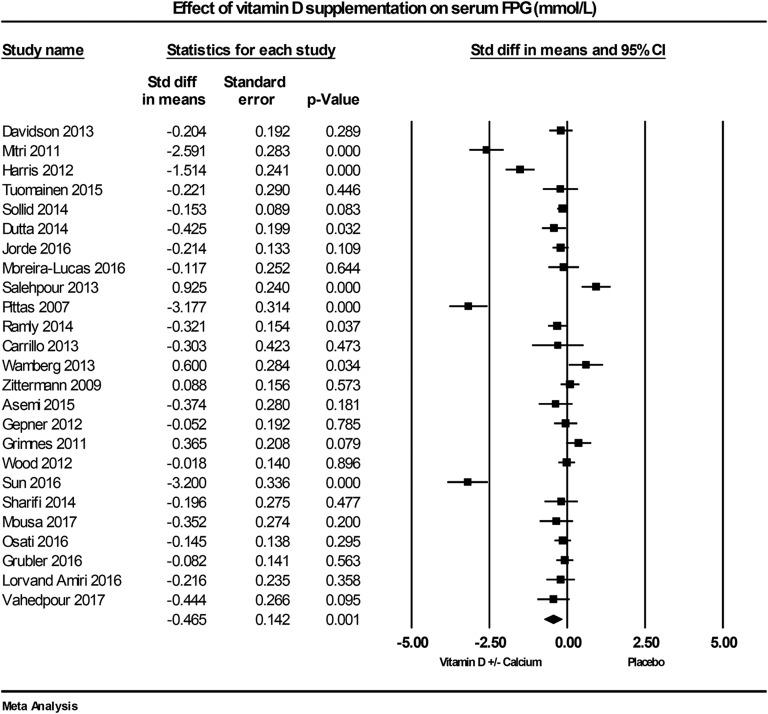
The effect of vitamin D on FPG was reported in 25 RCTs. Of these studies, eight reported reduced FPG with vitamin D supplementation [21, 61, 63, 69, 72, 73, 78, 82], four found an increase [42, 43, 62, 70], and 13 studies reported no change in FPG at follow-up [60, 64–66, 68, 71, 74–77, 80, 81, 83]. The combined data show vitamin D supplementation reduced FPG by –0.46 mmol/L (95% CI: –0.74 to –0.19; P = 0.001, I2 = 92.4%; Fig. 4).
Figure 4.
Forest plot of mean change from baseline in FPG (mmol/L) between vitamin D supplementation and control.
F. Pooled Estimate of the Effect of Vitamin D on Insulin Resistance and Glucose Tolerance
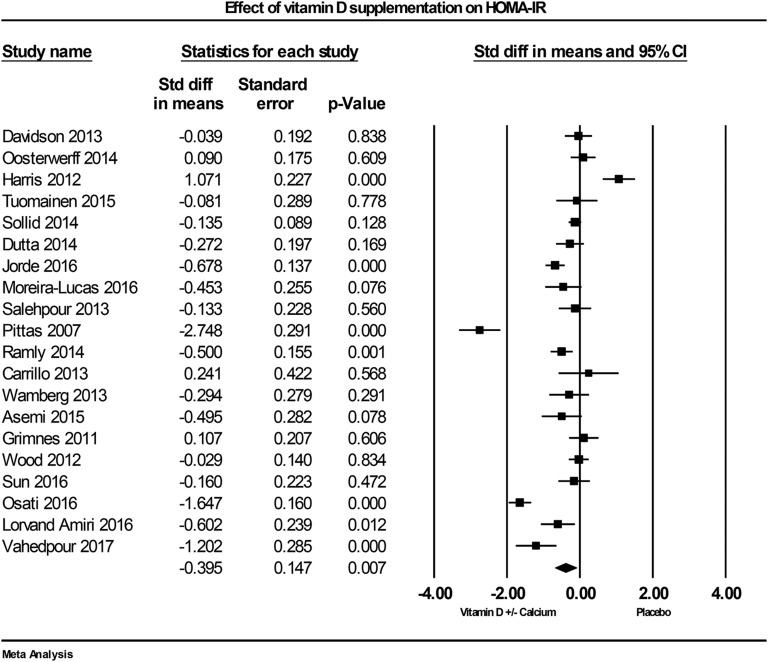
F-1. Fasting HOMA-IR
The influence of vitamin D supplementation on insulin resistance (using HOMA-IR) was evaluated in 20 studies. In the individual studies, eight reported a lowering effect of vitamin D on HOMA-IR [21, 63, 64, 68, 74, 76, 78, 80], one study found an increase [72], and 11 found no effect in the vitamin D–supplemented group [43, 60–62, 66, 69–71, 73, 79, 81]. Vitamin D supplementation was found to reduce HOMA-IR across all studies by –0.39 (95% CI: –0.68 to –0.11; P = 0.007, I2 = 91.3%; Fig. 5).
Figure 5.
Forest plot of mean change from baseline in HOMA-IR between vitamin D supplementation and control.
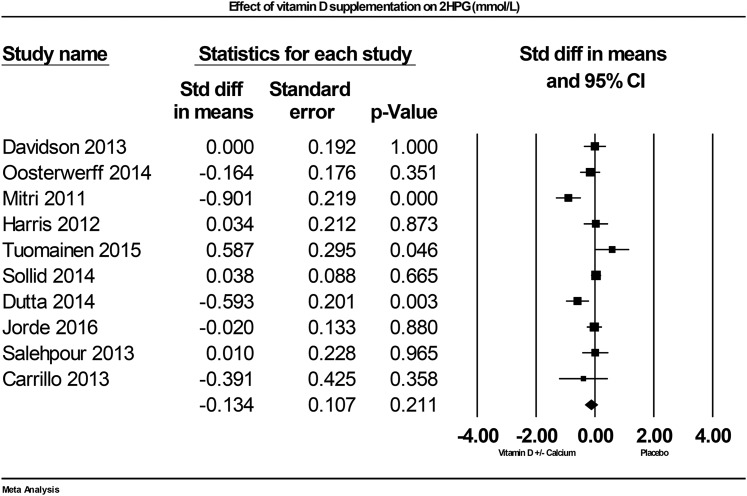
F-2. 2HPG
Ten studies contributed data on the effect of vitamin D supplementation on 2HPG. Of these studies, two reported positive effects of vitamin D supplementation [73, 82], one reported negative effects [81], and seven reported null results [60–62, 68, 71, 72, 79]. Combined data demonstrate that vitamin D supplementation did not significantly effect 2HPG, but a nonsignificant trend was found for decreased 2HPG by –0.13 mmol/L (95% CI: –0.34 to 0.08; P = 0.2, I2 = 69.1%; Fig. 6).
Figure 6.
Forest plot of mean change from baseline in plasma glucose after 2HPG (mmol/L) between vitamin D supplementation and control.
F-3. Sensitivity analysis
In the leave-one-out sensitivity analyses, the pooled effect estimates remained similar across all studies for HOMA-IR, 2HPG, and serum 25(OH)D concentration. These results confirm that the significant difference between the studied groups reflects the overall effect of all included studies. For HbA1c, after excluding one study [62], the effect size decreased from –0.48% (P = 0.002, I2 = 92%) to –0.31% (P = 0.01, I2 = 86%), and the effect of vitamin D supplementation on HbA1c was significant. For FPG, after excluding the study by Sun et al. [69], the effect size decreased from –0.46 mmol/L (P = 0.001, I2 = 92%) to –0.36 mmol/L (P = 0.005, I2 = 90%), and after excluding the study by Pittas et al. [21], the effect size decreased to –0.36 mmol/L (P = 0.004, I2 = 89%). In both situations, the effect of vitamin D supplementation on reduced FPG remained significant.
F-4. Publication bias
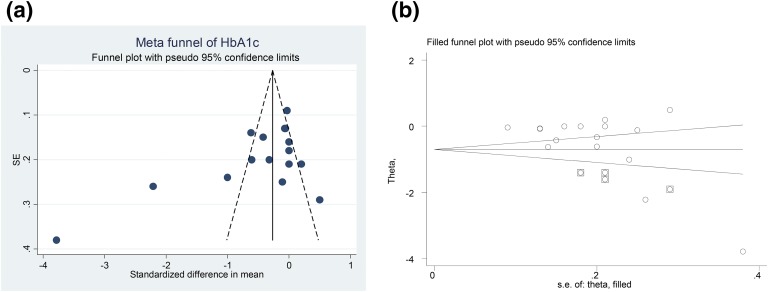
For HbA1c, visual inspection of funnel plot asymmetry demonstrated a potential publication bias for the comparison of HbA1c percentage between vitamin D–administered groups and placebo groups [Fig. 7(a)]. The presence of a publication bias also was suggested by Egger’s linear regression (intercept = –5.13, SE = 2.33; 95% CI = –10.15 to –0.12, t = –2.2, two-tailed P = 0.04). After adjusting the effect size for potential publication bias using the “trim and fill” method, four potentially missing studies were imputed in the funnel plot and the effect size increased from –0.48% (95% CI: –0.79 to –0.18) to –0.71% (95% CI: –1.02 to –0.39) [Fig. 7(b)].
Figure 7.
(a) Funnel plot of SE by standardized mean difference for HbA1c, detailing publication bias in the studies selected for analyses. Closed circles represent observed published studies. (b) “Trim and fill” method to impute for potentially missing studies for HbA1c. Four potentially missing studies were imputed in funnel plot. Closed circles represent observed published studies. Squares with circle inside represent imputed studies.
For FPG, the funnel plot was asymmetric [Supplemental Fig. 1(a)] though Egger’s linear regression (intercept = –3.74, SE = 1.88; 95% CI = –7.64 to 0.15, t = –1.99, two-tailed P = 0.059) did not indicate a potential bias. Using the “trim and fill” correction method, the effect size was adjusted for potential publication bias and six potentially missing studies were imputed in the funnel plot. The effect size increased from –0.46 mmol/L (95% CI: –0.74 to 0.19) to –0.72 mmol/L (95% CI: –1.02 to –0.42) [Supplemental Fig. 1(b)].
For HOMA-IR, funnel plot asymmetry indicated a potential publication bias in determining the effect size of vitamin D supplementation on HOMA-IR changes, compared with placebo group [Supplemental Fig. 2(a)]. The presence of a publication bias was not confirmed by Egger’s linear regression (intercept = –1.09, SE = 2.18; 95% CI = –5.67 to 3.49, t = –0.5, two-tailed P = 0.6). Using the “trim and fill” method to adjust the effect size for potential publication bias, five potentially missing studies were imputed in the funnel plot and the effect size increased from –0.39 (95% CI: –0.68 to –0.11) to –0.62 (95% CI: –0.92 to –0.32) [Supplemental Fig. 2(b)].
Visually inspected funnel plot symmetry did not indicate any potential publication bias for the comparison of 2HPG levels between the vitamin D–supplemented group and placebo groups [Supplemental Fig. 3(a)]. Moreover, the Egger’s linear regression (intercept = –1.19, SE = 1.48; 95% CI: –4.6 to 2.2, t = –0.81, two-tailed P = 0.4) did not detect any publication bias. Using the “trim and fill” correction and adjusting the effect size for potential publication bias, no potentially missing study was imputed in the funnel plot and the effect size remained the same (effect size: –0.13 mmol/L, 95% CI: –0.34 to 0.08) [Supplemental Fig. 3(b)].
For serum 25(OH)D levels, the funnel plot was asymmetric [Supplemental Fig. 4(a)] and Egger’s linear regression (intercept = 3.61, SE = 1.76; 95% CI = –0.005 to 7.23, t = 2.05, two-tailed P = 0.05) indicated a potential bias. Using the “trim and fill” correction method, the effect size was adjusted for potential publication bias and no potentially missing studies were imputed in the funnel plot. The effect size remained the same at 45.1 nmol/L (95% CI: 41.3 to 48.9) [Supplemental Fig. 4(b)].
G. Subgroup Analysis
G-1. Effect of population characteristics
We conducted a subgroup analysis to examine the effect of vitamin D supplementation in prediabetes in comparison with populations that were overweight/obese but not prediabetic (Table 3). The lowering effect was observed in both groups with no significant difference in the change of HbA1c and FPG between prediabetics and overweight/obese participants. Both HbA1c and HOMA-IR showed a greater reduction over time among overweight/obese individuals compared with prediabetics (HbA1c: –0.98 ± 0.45 vs –0.29 ± 0.14, P = 0.1; HOMA-IR: –0.62 ± 0.23 vs –0.07 ± 0.16, P = 0.05). There were not enough studies to perform subgroup analyses on 2HPG.
Table 3.
Meta-analysis and Subgroup Analysis of Primary and Secondary Outcomes
| Subgroup Analysis | No. of Study | No. of Subjects |
Standardized Mean Difference (95% CI) | P Value | Between-Group P Valuea | |
|---|---|---|---|---|---|---|
| Vitamin D | Placebo | |||||
| Study population | ||||||
| HbA1c | ||||||
| Prediabetes | 11 | 892 | 880 | −0.29 ± 0.14 (–0.57 to –0.01) | 0.04 | 0.1 |
| Overweight/obese (not prediabetes) | 5 | 310 | 307 | −0.98 ± 0.45 (–1.87 to –0.10) | 0.02 | |
| FPG | ||||||
| Prediabetes | 8 | 628 | 615 | −0.65 ± 0.23 (–1.11 to –0.19) | 0.005 | 0.2 |
| Overweight/obese (not prediabetes) | 17 | 883 | 885 | −0.38 ± 0.19 (–0.74 to –0.01) | 0.04 | |
| HOMA-IR | ||||||
| Prediabetes | 8 | 647 | 634 | −0.07 ± 0.16 (–0.39 to 0.24) | 0.6 | 0.05 |
| Overweight/obese (not prediabetes) | 12 | 591 | 596 | −0.62 ± 0.23 (–1.07 to –0.18) | 0.006 | |
| Concomitant use of calcium | ||||||
| HbA1c | ||||||
| D vs placebo | 14 | 1082 | 1073 | −0.53 ± 0.18 (–0.88 to –0.19) | 0.002 | 0.2 |
| D + Ca vs placebo | 3 | 166 | 160 | −1.05 ± 0.74 (–2.51 to 0.42) | 0.1 | |
| FPG | ||||||
| D vs placebo | 20 | 1296 | 1286 | −0.18 ± 0.1 (–0.389 to 0.032) | 0.09 | 0.002 |
| D + Ca vs placebo | 5 | 215 | 214 | −1.67 ± 0.5 (–2.68 to –0.66) | <0.001 | |
| HOMA-IR | ||||||
| D vs placebo | 15 | 1004 | 997 | −0.38 ± 0.1 (–0.65 to –0.12) | 0.004 | 0.4 |
| D + Ca vs placebo | 5 | 234 | 233 | −0.46 ± 0.5 (–1.51 to 0.6) | 0.4 | |
| 2HPG | ||||||
| D vs placebo | 6 | 501 | 494 | 0.03 ± 0.06 (–0.09 to 0.16) | 0.6 | 0.02 |
| D + Ca vs placebo | 4 | 209 | 206 | −0.54 ± 0.3 (–1.15 to 0.07) | 0.08 | |
| Age groups | ||||||
| HbA1c | ||||||
| ≤45 y | 4 | 226 | 227 | −1.15 ± 0.6 (–2.33 to 0.04) | 0.06 | 0.05 |
| >45 y | 12 | 976 | 960 | −0.30 ± 0.1 (–0.57 to –0.03) | 0.02 | |
| FPG | ||||||
| ≤45 y | 11 | 449 | 449 | −0.31 ± 0.24 (–0.78 to 0.17) | 0.2 | 0.2 |
| >45 y | 14 | 1062 | 1051 | −0.58 ± 0.20 (–0.94 to –0.23) | 0.001 | |
| HOMA-IR | ||||||
| ≤45 y | 9 | 377 | 379 | −0.48 ± 0.24 (–0.96 to –0.008) | 0.04 | 0.3 |
| >45 y | 11 | 861 | 851 | −0.32 ± 0.19 (–0.69 to 0.04) | 0.08 | |
| 2HPG | ||||||
| ≤45 y | 3 | 114 | 116 | −0.13 ± 0.13 (–0.39 to 0.13) | 0.3 | 0.4 |
| >45 y | 7 | 596 | 584 | −0.13 ± 0.14 (–0.41 to 0.14) | 0.3 | |
| Obesity | ||||||
| HbA1c | ||||||
| Obese | 11 | 853 | 845 | −0.63 ± 0.21 (–1.05 to –0.20) | 0.004 | 0.2 |
| Normal BMI | 5 | 349 | 342 | −0.19 ± 0.17 (–0.51 to –0.14) | 0.2 | |
| FPG | ||||||
| Obese | 17 | 1006 | 1002 | −0.48 ± 0.18 (–0.84 to –0.12) | 0.009 | 0.4 |
| Normal BMI | 8 | 505 | 498 | −0.44 ± 0.24 (–0.91 to 0.03) | 0.06 | |
| HOMA-IR | ||||||
| Obese | 14 | 816 | 812 | −0.44 ± 0.22 (–0.87 to –0.02) | 0.04 | 0.3 |
| Normal BMI | 6 | 422 | 418 | −0.29 ± 0.14 (–0.56 to –0.03) | 0.03 | |
| 25(OH)D level at baseline | ||||||
| HbA1c | ||||||
| <50 nmol/L | 7 | 368 | 353 | −0.14 ± 0.13 (–0.40 to 0.12) | 0.2 | 0.04 |
| ≥50 nmol/L | 9 | 694 | 693 | −0.79 ± 0.25 (–1.27 to –0.30) | 0.001 | |
| FPG | ||||||
| <50 nmol/L | 9 | 456 | 447 | −0.11 ± 0.10 (–0.31 to 0.08) | 0.2 | 0.05 |
| ≥50 nmol/L | 16 | 692 | 693 | −0.69 ± 0.21 (–1.10 to –0.27) | 0.001 | |
| HOMA-IR | ||||||
| <50 nmol/L | 10 | 521 | 512 | −0.36 ± 0.19 (–0.75 to 0.02) | 0.06 | 0.4 |
| ≥50 nmol/L | 10 | 717 | 718 | −0.43 ± 0.23 (–0.88 to 0.02) | 0.06 | |
| 2HPG | ||||||
| <50 nmol/L | 4 | 200 | 191 | −0.08 ± 0.21 (–0.47 to 0.33) | 0.7 | 0.2 |
| ≥50 nmol/L | 6 | 461 | 458 | −0.16 ± 0.14 (–0.43 to 0.11) | 0.2 | |
| Serum 25(OH)D level at follow-up | ||||||
| HbA1c | ||||||
| <86 nmol/L | 4 | 208 | 211 | −0.22 ± 0.20 (–0.66 to 0.22) | 0.3 | 0.05 |
| ≥86 nmol/L | 10 | 891 | 873 | −0.33 ± 0.1 (–0.62 to –0.04) | 0.02 | |
| FPG | ||||||
| <86 nmol/L | 10 | 558 | 562 | −0.37 ± 0.23 (–0.83 to 0.08) | 0.08 | 0.05 |
| ≥86 nmol/L | 14 | 821 | 813 | −0.46 ± 0.19 (–0.82 to –0.09) | 0.01 | |
| HOMA-IR | ||||||
| <86 nmol/L | 10 | 496 | 501 | −0.29 ± 0.25 (–0.80 to 0.21) | 0.2 | 0.1 |
| ≥86 nmol/L | 10 | 742 | 729 | −0.49 ± 0.18 (–0.84 to –0.13) | 0.007 | |
| 2HPG | ||||||
| <86 nmol/L | 4 | 157 | 162 | −0.08 ± 0.11 (–0.30 to 0.14) | 0.5 | 0.4 |
| ≥86 nmol/L | 6 | 553 | 538 | −0.16 ± 0.16 (–0.47 to 0.16) | 0.3 | |
| Duration of supplementation | ||||||
| HbA1c | ||||||
| <6 months | 8 | 461 | 464 | −0.75 ± 0.33 (–1.41 to –0.08) | 0.02 | 0.1 |
| ≥6 mo | 8 | 741 | 723 | −0.25 ± 0.11 (–0.48 to –0.03) | 0.02 | |
| FPG | ||||||
| <6 mo | 14 | 598 | 597 | −0.32 ± 0.19 (–0.71 to 0.06) | 0.09 | 0.1 |
| ≥6 mo | 10 | 913 | 903 | −0.64 ± 0.22 (–1.07 to –0.21) | 0.003 | |
| HOMA-IR | ||||||
| <6 mo | 10 | 407 | 410 | −0.24 ± 0.27 (–0.77 to 0.29) | 0.3 | 0.1 |
| ≥6 mo | 10 | 831 | 820 | −0.53 ± 0.18 (–0.88 to –0.17) | 0.004 | |
| 2HPG | ||||||
| <6 mo | 6 | 227 | 232 | −0.14 ± 0.19 (–0.52 to 0.24) | 0.4 | 0.4 |
| ≥6 mo | 4 | 483 | 468 | −0.11 ± 0.12 (–0.34 to 0.13) | 0.3 | |
P value represents within-group comparison. Subgroup analysis was not done for 2HPG and obesity (one study in obese subgroup). Independent t test for between-groups comparison.
P values were adjusted by Bonferroni correction.
G-2. Effect of combined vitamin D and calcium supplementation
Subgroup analysis was performed to determine if concomitant calcium supplementation influenced the effects of vitamin D (Table 3). There was no significant difference in the change of HbA1c and HOMA-IR when calcium was provided in combination with vitamin D compared with vitamin D alone (HbA1c: –1.05 ± 0.74 vs –0.53 ± 0.18, P = 0.2; HOMA-IR: –0.46 ± 0.5 vs –0.38 ± 0.1, P = 0.4), although coadministration of calcium showed greater reduction in HbA1c. However, FPG (–1.67 ± 0.5 vs –0.18 ± 0.1, P = 0.002) and 2HPG (–0.54 ± 0.3 vs 0.03 ± 0.06, P = 0.02) showed a greater reduction when vitamin D was provided in combination with calcium. Overall, combining calcium with vitamin D improved its effect on glycemic control.
G-3. Influence of age on the effect of vitamin D
Subgroup analysis was conducted to determine if age influenced outcomes by comparing studies in which mean participant age in each study was less or greater than 45 years (Table 3). HbA1c showed greater improvement in populations with a mean age younger than 45 years in comparison with older populations (–1.15 ± 0.6 vs –0.30 ± 0.1, P = 0.05). Greater reduction in FPG for populations older than 45 years was not statistically significant (–0.58 ± 0.20 vs –0.31 ± 0.24, P = 0.2). Changes in HOMA-IR and 2HPG did not differ significantly between the two compared age groups.
G-4. Influence of obesity on the effect of vitamin D
We compared the effect of vitamin D supplementation on glycemic measures between studies conducted in overweight/obese and nonobese populations (Table 3). There was no significant difference in the change of HbA1c, FPG, and HOMA-IR between obese and nonobese populations. We were not able to compare 2HPG as it was reported in only one study with a nonobese population in comparison with nine studies on obese populations.
G-5. Effect of baseline vitamin D status
Participants with vitamin D deficiency, mean serum 25(OH)D concentration <50 nmol/L, at the beginning of the intervention were compared with those who had mean serum 25(OH)D concentration ≥50 nmol/L (Table 3). Greater reductions were found within HbA1c and FPG levels when baseline mean serum 25(OH)D concentration was ≥50 nmol/L, whereas the lowering effect was significantly less in the subgroup with baseline mean 25(OH)D <50 nmol/L (HbA1c: –0.79 ± 0.25 vs –0.14 ± 0.13, P = 0.04; FPG: –0.69 ± 0.21 vs –0.11 ± 0.10, P = 0.05). The lowering effect of vitamin D on HOMA-IR and 2HPG did not differ based on serum 25(OH)D status at baseline.
G-6. Effect of serum 25(OH)D concentration at follow-up
Subgroup analysis was carried out based on the concentration of serum 25(OH)D achieved at follow-up, either below or above the median level (86 nmol/L) (Table 3). Vitamin D supplementation significantly decreased HbA1c (P = 0.05), FPG (P = 0.05), and HOMA-IR (P = 0.1) to a greater extent when serum 25(OH)D concentration achieved was above 86 nmol/L. In agreement with these results, we also found dose-response effects for all four parameters. With increased vitamin D supplementation dose, there was a greater reduction in HbA1c (y = –2926.2x + 2741.3, R2 = 0.06), FPG (y = –2686.4x + 3414.3, r2 = 0.045), HOMA-IR (y = 876.75x + 3437.5, r2 = 0.031), and 2HPG (y = –1779x + 3461.3, r2 = 0.153).
G-7. Effect of length of intervention
We compared the effects of vitamin D in studies of short and long duration (<6 months vs ≥6 months, respectively) (Table 3). For HbA1c, we found that vitamin D supplementation for less than 6 months provided a larger effect size on HbA1c in comparison with long durations (–0.75 ± 0.33 vs –0.25 ± 0.11, P = 0.1). However, both FPG and HOMA-IR showed a greater but nonsignificant reduction with supplementation greater than 6 months compared with shorter durations (FPG: –0.64 ± 0.22 vs –0.32 ± 0.19, P = 0.1; HOMA-IR: –0.53 ± 0.18 vs –0.24 ± 0.27, P = 0.1). The duration of supplementation did not affect the reduction in 2HPG. There were three studies included in our meta-analysis with intervention durations of 2 months [64, 76, 83] that found a significant reduction in HbA1c [83], a decreased trend in FPG [64, 76, 83], and significant reduction in HOMA-IR [64, 76] following vitamin D supplementation. Despite a shorter duration of intervention, compared with other trials, these studies provided higher vitamin D supplementation doses (2800 to 7100 IU/d) and included vitamin D–deficient populations at baseline.
3. Discussion
Interest in the protective effects of vitamin D supplementation against the progression of diabetes has heightened in recent years, and the current analysis underscores some of these benefits. We evaluated 28 studies combined, including 11 centered on prediabetics and 16 on populations at high risk, and found significant effects of vitamin D supplementation on insulin resistance and hyperglycemia. Vitamin D supplementation and increased serum 25(OH)D concentrations improved insulin sensitivity (decreased HOMA-IR), glucose metabolism, and glycemic control (reduced HbA1c and FPG). Our findings suggest that a serum 25(OH)D concentration above 86 nmol/L can improve measures of glucose metabolism and response to insulin in prediabetics. Serum 25(OH)D concentrations above 86 nmol/L were achieved with an average vitamin D supplementation of 88 µg/d (3500 IU/d) or more, taking body weight into account; Ekwaru et al. [84] previously showed that obese individuals often require two to three times the dose of vitamin D that an individual with a normal BMI requires to achieve the same 25(OH)D response. The greatest benefits were found in populations most at risk for early disease. We also found improvements in those considered vitamin D sufficient when they started vitamin D supplementation.
Subgroup analyses further demonstrated that both FPG and 2-hour postprandial plasma glucose reductions were enhanced with vitamin D taken in combination with calcium supplements, as were insulin resistance and fasting HOMA-IR. Longer duration of supplementation seemed to be more effective at reducing HOMA-IR than those shorter than 6 months. We used fasting HOMA-IR, which detects liver insulin resistance and shows inability of insulin in liver to lower glucose concentration. This is different from the fed-state HOMA-IR representing peripheral insulin resistance or failure of glucose uptake in muscle tissues [85]. Other studies have similarly shown that the combination of vitamin D with calcium may help improve glucose metabolism and significantly lower the risk of diabetes [21, 24, 73] by facilitating glucose transportation into different organs and regulating insulin receptor genes and insulin secretion [86, 87]. Subgroup analyses additionally found that individuals who are obese but not prediabetic and younger than 45 years and/or those considered to be vitamin D sufficient at baseline had the largest improvements. The duration of treatment did not influence the short-term measures of response.
These results are consistent with previous reports that have demonstrated that vitamin D supplementation and the highest quartile of serum 25(OH)D concentration, compared with the lowest quartile, reduced the risk of cardiometabolic disorders, including diabetes, metabolic syndrome, and cardiovascular diseases [21, 82, 88]. Moreover, our results support the findings that individuals whose 25(OH)D concentrations increased by at least 50 nmol/L had a 26% reduction in the risk of HbA1c elevation (≥5.8%) [89]. The findings of the current study are not in agreement with all previous reviews [36–38, 90, 91]; however, it should be noted that our methodology properly accounted for the biology of vitamin D and glucose metabolism. For instance, only studies that were sufficiently long enough to detect changes in HbA1c, a 2- to 3-month measure of glucose control, and long enough for 25(OH)D concentrations to reach a plateau, for individuals across the range of BMIs, were included. We also included various blood measurements used in the diagnosis of diabetes.
There is growing biological and mechanistic evidence that support a role for low vitamin D status as a contributing factor in the development of type 2 diabetes. Reduction in insulin secretion, for instance, has been linked to vitamin D deficiency, and optimization of serum 25(OH)D concentrations following vitamin D supplementation has been shown to restore insulin secretion and improve glucose metabolism, glucose tolerance, and insulin sensitivity [30, 92, 93]. Also, vitamin D indirectly stimulates insulin secretion by altering calcium flux through cell membranes to normalize extracellular calcium [21]. Evidence suggests that elevated PTH may be associated with an increased risk of glucose intolerance and diabetes [94, 95], and the concomitant use of calcium with vitamin D decreases PTH levels significantly [96]. Other potential mechanisms demonstrated in vitro and in animal models include improving insulin action through stimulating the expression of the insulin receptor, enhancing insulin responsiveness for glucose transport, gene polymorphism, and the influence of vitamin D on gene expression of metabolic pathways [31, 33], and improving systemic inflammation by a direct effect on cytokines [21].
The current study has several limitations. The trials included in this systematic review were heterogeneous with respect to the outcomes measured, supplemental vitamin D dose and whether it was given in combination with calcium, the length of the intervention, and the definition of a high-risk population. We used a random-effects model in a meta-analysis to overcome these limitations, but this model can have poor error estimations and, with heterogeneous data such as in the current meta-analysis, might default to an arithmetic mean (equal weights). Some of the studies had comparatively small populations (10 to 30 participants per intervention group) that in isolation are hard to extrapolate, but taken together lend support to the larger trials. Most studies did not describe dietary intake, latitude, season, or sun exposure contributing to vitamin D synthesis. Also, although it is difficult to interpret results based solely on vitamin D supplementation, we included only studies that measured serum 25(OH)D to account for outside factors as well as different doses provided and different responses to supplementation based on BMI. We examined the effect of serum 25(OH)D concentration in addition to vitamin D dose to overcome some of these limitations.
This review is based on a recent and up-to-date literature search representing the available data on the effect of vitamin D supplementation on glycemic control in prediabetics and a high-risk population. We included only placebo-controlled RCTs with appropriate methodological qualities and the least probable chance of bias or dropout and studies that measured several outcome measures of glucose control and insulin response: HbA1c, FPG, HOMA-IR, and 2HPG. We ensured that the studies included provided vitamin D supplements as daily or weekly doses to reduce variation introduced by the differences in response in vitamin D metabolism and only those that measured 25(OH)D concentrations. Most of the included studies had been designed for glycemic outcomes and covered a diverse population that supports the generalizability of the results.
4. Conclusion
Vitamin D deficiency and type 2 diabetes are escalating health problems worldwide. The results presented here provide promising evidence that vitamin D supplementation improves glycemic control and attenuates insulin resistance in prediabetics or individuals at high risk of developing diabetes. The amount of vitamin D most likely to produce a response is ≥88 µg/d (3500 IU/d), a level that is close to the current upper level of intake recommended by Health Canada, the US Institute of Medicine, and the European Food Safety Authority. Even though the effect size for vitamin D is not comparable to the effect of metformin or other glycemic control medications, over a long period of time and when administered at the population level, vitamin D supplementation may offer an affordable, safe, and accessible preventive measure.
Supplementary Material
Acknowledgments
We thank Ken Fyie for helping to prepare funnel plots and Brian Rankin for reviewing this paper.
Financial Support: This work was supported by a The World Academy of Sciences studentship of the Chinese Academy of Sciences (to M.M.).
Author Contributions: Formulating research questions: N.M., S.M.K., and H.V.; designing the study: N.M., S.M.K., and H.V.; carrying the study out: N.M. and M.M.; analyzing the data: N.M. and M.M.; writing the article: N.M. and S.M.K.; and final review and approval: N.M., S.M.K., H.V., and M.M.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- 2HPG
plasma glucose after 2-hour oral glucose tolerance test
- BMI
body mass index
- FPG
fasting plasma glucose
- HbA1c
glycosylated hemoglobin
- HOMA-IR
homeostatic model assessment of insulin resistance
- IQR
interquartile range
- PTH
parathyroid hormone
- RCT
randomized controlled trial
- SE
standard error
References and Notes
- 1. Canadian Diabetes Association Diabetes statistics in Canada. Available at: www.diabetes.ca/about-diabetes/types-of-diabetes. Accessed 18 May 2017.
- 2. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care Suppl 2006;29(1):S43–S48. [PubMed] [Google Scholar]
- 3. American Diabetes Association Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buysschaert M, Bergman M. Definition of prediabetes. Med Clin North Am. 2011;95(2):289–297, vii. [DOI] [PubMed] [Google Scholar]
- 5. Mancini GBJCA, Cheng AY, Connelly K, Fitchett D, Goldenberg R, Goodman SG, Leiter LA, Lonn E, Paty B, Poirier P, Stone J, Thompson D, Yale JF. Diabetes for cardiologists: practical issues in diagnosis and management. Can J Cardiol. 2017;33(3):366–377. [DOI] [PubMed] [Google Scholar]
- 6. Nathan DMDM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B; American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. [DOI] [PubMed] [Google Scholar]
- 7. Haffner SMH. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol. 2003;92(4, 4A):18J–26J. [DOI] [PubMed] [Google Scholar]
- 8. Festa A, Hanley AJ, Tracy RP, D’Agostino R Jr, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108(15):1822–1830. [DOI] [PubMed] [Google Scholar]
- 9. Dong JYZW, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5(9):3551–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng R, Li Y, Li G, Li Z, Zhang Y, Li Q, Sun C. Lower serum 25(OH)D concentrations in type 1 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2015;108(3):e71–e75. [DOI] [PubMed] [Google Scholar]
- 11. Gupta AKBM, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care. 2011;34(3):658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin D levels and prediabetes among subjects free of diabetes. Diabetes Care. 2011;34(5):1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giorelli Gde V, Matos LN, Saado A, Soibelman VL, Dias CB. No association between 25-hydroxyvitamin D levels and prediabetes in Brazilian patients. A cross-sectional study. Sao Paulo Med J. 2015;133(2):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poomthavorn P, Saowan S, Mahachoklertwattana P, Chailurkit L, Khlairit P. Vitamin D status and glucose homeostasis in obese children and adolescents living in the tropics. Int J Obes. 2012;36(4):491–495. [DOI] [PubMed] [Google Scholar]
- 15. Forouhi NGMR, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, Rickard AP, Boucher BJ, Chowdhury TA, Griffiths CJ, Greenwald SE, Griffin SJ, Hitman GA. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2016;18(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55(6):1668–1678. [DOI] [PubMed] [Google Scholar]
- 17. Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–724. [DOI] [PubMed] [Google Scholar]
- 18. Sugden JADJ, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–325. [DOI] [PubMed] [Google Scholar]
- 19. Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care. 2011;34(5):1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mears D. Regulation of insulin secretion in islets of Langerhans by Ca(2+)channels. J Membr Biol. 2004;200(2):57–66. [DOI] [PubMed] [Google Scholar]
- 21. Pittas AGHS, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–986. [DOI] [PubMed] [Google Scholar]
- 22. Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromsø study. Eur J Nutr. 2010;49(7):401–407. [DOI] [PubMed] [Google Scholar]
- 23. Jacqmain M, Doucet E, Després JP, Bouchard C, Tremblay A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 2003;77(6):1448–1452. [DOI] [PubMed] [Google Scholar]
- 24. Pittas AGLJ, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimball SMM, Mirhosseini N, Holick MF. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermatoendocrinol. 2017;9(1):e1300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jehle S, Lardi A, Felix B, Hulter HN, Stettler C, Krapf R. Effect of large doses of parenteral vitamin D on glycaemic control and calcium/phosphate metabolism in patients with stable type 2 diabetes mellitus: a randomised, placebo-controlled, prospective pilot study. Swiss Med Wkly. 2014;144:w13942. [DOI] [PubMed] [Google Scholar]
- 27. Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, Salekzamani S, Zahedirad M. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. 2011;93(4):764–771. [DOI] [PubMed] [Google Scholar]
- 28. Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48(6):1038–1050. [DOI] [PubMed] [Google Scholar]
- 29. Pitocco D, Crinò A, Di Stasio E, Manfrini S, Guglielmi C, Spera S, Anguissola GB, Visalli N, Suraci C, Matteoli MC, Patera IP, Cavallo MG, Bizzarri C, Pozzilli P; IMDIAB Group . The effects of calcitriol and nicotinamide on residual pancreatic β-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet Med. 2006;23(8):920–923. [DOI] [PubMed] [Google Scholar]
- 30. Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012:634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–687. [DOI] [PubMed] [Google Scholar]
- 33. Malecki MTKT, Klupa T, Wolkow P, Bochenski J, Wanic K, Sieradzki J. Association study of the vitamin D: 1alpha-hydroxylase (CYP1alpha) gene and type 2 diabetes mellitus in a Polish population. Diabetes Metab. 2003;29(2 Pt 1):119–124. [DOI] [PubMed] [Google Scholar]
- 34. Fadda GZAM, Akmal M, Lipson LG, Massry SG. Direct effect of parathyroid hormone on insulin secretion from pancreatic islets. Am J Physiol. 1990;258(6 Pt 1):E975–E984. [DOI] [PubMed] [Google Scholar]
- 35. Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seida JCM, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poolsup N, Suksomboon N, Plordplong N. Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: a systematic review and meta-analysis. Diabet Med. 2016;33(3):290–299. [DOI] [PubMed] [Google Scholar]
- 38. Nigil Haroon N, Anton A, John J, Mittal M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. J Diabetes Metab Disord. 2015;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol. 2016;175:177–189. [DOI] [PubMed] [Google Scholar]
- 40. Witham MDD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–2119. [DOI] [PubMed] [Google Scholar]
- 41. Patel P, Poretsky L, Liao E. Lack of effect of subtherapeutic vitamin D treatment on glycemic and lipid parameters in type 2 diabetes: a pilot prospective randomized trial. J Diabetes. 2010;2(1):36–40. [DOI] [PubMed] [Google Scholar]
- 42. Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–1327. [DOI] [PubMed] [Google Scholar]
- 43. Grimnes G, Figenschau Y, Almås B, Jorde R, Vitamin D. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes. 2011;60(11):2748–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. [DOI] [PubMed] [Google Scholar]
- 45. Rohlfing CLW, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275–278. [DOI] [PubMed] [Google Scholar]
- 46.Higgins JPT G, Green SE. Cochrane Handbook for Systematic Reviews of Interventions. London, England: The Cochrane Collaboration; 2008.
- 47.Borenstein M H, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis version 2. Englewood Cliffs, NJ: Biostat Inc; 2005.
- 48.Vieth R. The pharmacology of vitamin d, including fortification strategies. In: Feldman D, Pike JW, Gloreieux FH eds. Vitamin D. 2nd ed. Cambridge, MA: Elsevier Academic Press; 2005:995–1018.
- 49. Mazidi MK, Karimi E, Rezaie P, Ferns GA. Treatment with glp1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. 2017;31(7):1237–1242. [DOI] [PubMed] [Google Scholar]
- 50. Sutton AJA, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. West Sussex, England: John Wiley and Sons. [Google Scholar]
- 51. Ferretti G, Bacchetti T, Sahebkar A. Effect of statin therapy on paraoxonase-1 status: a systematic review and meta-analysis of 25 clinical trials. Prog Lipid Res. 2015;60:50–73. [DOI] [PubMed] [Google Scholar]
- 52. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 54. de Boer IH T, Tinker LF, Connelly S, Curb D, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, Weiss NS; Women’s Health Initiative Investigators . Calcium plus vitamin d supplementation and the risk of incident diabetes in the women's health initiative diabetes care. Diabetes Care. 2008;31(4):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Major GCAF, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85(1):54–59. [DOI] [PubMed] [Google Scholar]
- 56. Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, Jean S, Ebeling PR. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and β-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One. 2014;9(10):e109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, Ma S, Amarasekara S. Calcium plus vitamin D3 supplementation facilitated fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J. 2013;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60(3):870–874. [DOI] [PubMed] [Google Scholar]
- 59. Muldowney S, Lucey AJ, Hill TR, Seamans KM, Taylor N, Wallace JMW, Horigan G, Barnes MS, Bonham MP, Duffy EM, Strain JJ, Cashman KD, Kiely M. Incremental cholecalciferol supplementation up to 15 μg/d throughout winter at 51-55° N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. J Nutr. 2012;142(8):1519–1525. [DOI] [PubMed] [Google Scholar]
- 60. Carrillo AEF, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, Teegarden D. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr. 2013;32(3):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sollid STH, Hutchinson MY, Fuskevåg OM, Figenschau Y, Joakimsen RM, Schirmer H, Njølstad I, Svartberg J, Kamycheva E, Jorde R. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014;37(8):2123–2131. [DOI] [PubMed] [Google Scholar]
- 62. Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Amiri F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double-blind, randomized, placebo-controlled clinical trial. Diabet Med. 2013;30(12):1477–1481. [DOI] [PubMed] [Google Scholar]
- 63. Ramly M, Ming MF, Chinna K, Suboh S, Pendek R. Effect of vitamin D supplementation on cardiometabolic risks and health-related quality of life among urban premenopausal women in a tropical country--a randomized controlled trial. PLoS One. 2014;9(10):e110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015A;34(4):586–592. [DOI] [PubMed] [Google Scholar]
- 65. Gepner AD R, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin d supplementation on cardiovascular disease risk. PLoS One. 2012;7(5):e36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wood ADS, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97(10):3557–3568. [DOI] [PubMed] [Google Scholar]
- 67. Barengolts E, Manickam B, Eisenberg Y, Akbar A, Kukreja S, Ciubotaru I. Effect of high-dose vitamin d repletion on glycemic control in African American men with prediabetes and hypovitaminosis D. Endocr Pract. 2015;21(6):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. 2016;101(4):1647–1655. [DOI] [PubMed] [Google Scholar]
- 69. Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Higuchi M. Vitamin D supplementation reduces insulin resistance in Japanese adults: a secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutr Res. 2016;36(10):1121–1129. [DOI] [PubMed] [Google Scholar]
- 70. Wamberg L, Kampmann U, Stødkilde-Jørgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24(7):644–649. [DOI] [PubMed] [Google Scholar]
- 71. Davidson MBD, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36(2):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harris SSP, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14(9):789–794. [DOI] [PubMed] [Google Scholar]
- 73. Dutta D, Mondal SA, Choudhuri S, Maisnam I, Hasanoor Reza AH, Bhattacharya B, Chowdhury S, Mukhopadhyay S. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103(3):e18–e23. [DOI] [PubMed] [Google Scholar]
- 74. Moreira-Lucas TSD, Duncan AM, Rabasa-Lhoret R, Vieth R, Gibbs AL, Badawi A, Wolever TM. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes Metab. 2017;19(1):133–141. [DOI] [PubMed] [Google Scholar]
- 75. Mousa A, Naderpoor N, de Courten MPJ, Teede H, Kellow N, Walker K, Scragg R, de Courten B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. Am J Clin Nutr. 2017;105(6):1372–1381. [DOI] [PubMed] [Google Scholar]
- 76. Osati S, Homayounfar R, Hajifaraji M. Metabolic effects of vitamin D supplementation in vitamin D deficient patients (a double-blind clinical trial). Diabetes Metab Syndr. 2016;10(2, Suppl 1):S7–S10. [DOI] [PubMed] [Google Scholar]
- 77. Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70–80. [DOI] [PubMed] [Google Scholar]
- 78. Vahedpoor Z, Jamilian M, Bahmani F, Aghadavod E, Karamali M, Kashanian M, Asemi Z. Effects of long-term vitamin D supplementation on regression and metabolic status of cervical intraepithelial neoplasia: a randomized, double-blind, placebo-controlled trial. Horm Cancer. 2017;8(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oosterwerff MME, Eekhoff EMW, Van Schoor NM, Boeke AJP, Nanayakkara P, Meijnen R, Knol DL, Kramer MHH, Lips P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: a randomized placebo-controlled trial. Am J Clin Nutr. 2014;100(1):152–160. [DOI] [PubMed] [Google Scholar]
- 80. Lorvand Amiri H, Agah S, Mousavi SN, Hosseini AF, Shidfar F. Regression of non-alcoholic fatty liver by vitamin D supplement: a double-blind randomized controlled clinical trial. Arch Iran Med. 2016;19(9):631–638. [PubMed] [Google Scholar]
- 81. Tuomainen TP V, Virtanen JK, Voutilainen S, Nurmi T, Mursu J, de Mello VDF, Schwab U, Hakumaki M, Pulkki K, Uusitupa M. Glucose metabolism effects of vitamin d in prediabetes: the vitdmet randomized placebo-controlled supplementation study. J Diabetes Res. 2015;2015:672653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94(2):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grübler MRG, Gaksch M, Kienreich K, Verheyen N, Schmid J, Ó Hartaigh B, Richtig G, Scharnagl H, Meinitzer A, Fahrleitner-Pammer A, März W, Tomaschitz A, Pilz S. Effects of vitamin D supplementation on glycated haemoglobin and fasting glucose levels in hypertensive patients: a randomized controlled trial. Diabetes Obes Metab. 2016;18(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 84. Ekwaru JPZ, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9(11):e111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. DeFronzo RAT, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct. 2002;20(3):227–232. [DOI] [PubMed] [Google Scholar]
- 87. Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63(2):275–278. [DOI] [PubMed] [Google Scholar]
- 88. Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65(3):225–236. [DOI] [PubMed] [Google Scholar]
- 89. Munasinghe LLM, Mastroeni MF, Mastroeni SSBS, Loehr SA, Ekwaru JP, Veugelers PJ. The association of serum 25-hydroxyvitamin D concentrations and elevated glycated hemoglobin values: a longitudinal study of non-diabetic participants of a preventive health program. Nutrients. 2017;9(7):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jamka M, Woźniewicz M, Jeszka J, Mardas M, Bogdański P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep. 2015;5(1):16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zuk A, Fitzpatrick T, Rosella LC. Effect of vitamin D3 supplementation on inflammatory markers and glycemic measures among overweight or obese adults: a systematic review of randomized controlled trials. PLoS One. 2016;11(4):e0154215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119(1):84–90. [DOI] [PubMed] [Google Scholar]
- 93. von Hurst PRS, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103(4):549–555. [DOI] [PubMed] [Google Scholar]
- 94. Procopio M, Magro G, Cesario F, Piovesan A, Pia A, Molineri N, Borretta G. The oral glucose tolerance test reveals a high frequency of both impaired glucose tolerance and undiagnosed type 2 diabetes mellitus in primary hyperparathyroidism. Diabet Med. 2002;19(11):958–961. [DOI] [PubMed] [Google Scholar]
- 95. Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30(6):1549–1555. [DOI] [PubMed] [Google Scholar]
- 96. Grant AMA, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA; RECORD Trial Group . Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.