Abstract
BACKGROUND
Infection and inflammation of the reproductive tract are significant causes of male factor infertility. Ascending infections caused by sexually transmitted bacteria or urinary tract pathogens represent the most frequent aetiology of epididymo-orchitis, but viral, haematogenous dissemination is also a contributory factor. Limitations in adequate diagnosis and therapy reflect an obvious need for further understanding of human epididymal and testicular immunopathologies and their contribution to infertility. A major obstacle for advancing our knowledge is the limited access to suitable tissue samples. Similarly, the key events in the inflammatory or autoimmune pathologies affecting human male fertility are poorly amenable to close examination. Moreover, the disease processes generally have occurred long before the patient attends the clinic for fertility assessment. In this regard, data obtained from experimental animal models and respective comparative analyses have shown promise to overcome these restrictions in humans.
OBJECTIVE AND RATIONALE
This narrative review will focus on male fertility disturbances caused by infection and inflammation, and the usefulness of the most frequently applied animal models to study these conditions.
SEARCH METHODS
An extensive search in Medline database was performed without restrictions until January 2018 using the following search terms: ‘infection’ and/or ‘inflammation’ and ‘testis’ and/or ‘epididymis’, ‘infection’ and/or ‘inflammation’ and ‘male genital tract’, ‘male infertility’, ‘orchitis’, ‘epididymitis’, ‘experimental autoimmune’ and ‘orchitis’ or ‘epididymitis’ or ‘epididymo-orchitis’, antisperm antibodies’, ‘vasectomy’. In addition to that, reference lists of primary and review articles were reviewed for additional publications independently by each author. Selected articles were verified by each two separate authors and discrepancies discussed within the team.
OUTCOMES
There is clear evidence that models mimicking testicular and/or epididymal inflammation and infection have been instructive in a better understanding of the mechanisms of disease initiation and progression. In this regard, rodent models of acute bacterial epididymitis best reflect the clinical situation in terms of mimicking the infection pathway, pathogens selected and the damage, such as fibrotic transformation, observed. Similarly, animal models of acute testicular and epididymal inflammation using lipopolysaccharides show impairment of reproduction, endocrine function and histological tissue architecture, also seen in men. Autoimmune responses can be studied in models of experimental autoimmune orchitis (EAO) and vasectomy. In particular, the early stages of EAO development showing inflammatory responses in the form of peritubular lymphocytic infiltrates, thickening of the lamina propria of affected tubules, production of autoantibodies against testicular antigens or secretion of pro-inflammatory mediators, replicate observations in testicular sperm extraction samples of patients with ‘mixed atrophy’ of spermatogenesis. Vasectomy, in the form of sperm antibodies and chronic inflammation, can also be studied in animal models, providing valuable insights into the human response.
WIDER IMPLICATIONS
This is the first comprehensive review of rodent models of both infectious and autoimmune disease of testis/epididymis, and their clinical implications, i.e. their importance in understanding male infertility related to infectious and non-infectious/autoimmune disease of the reproductive organs.
Keywords: infection, inflammation, male infertility, orchitis, epididymitis or epididymo-orchitis, experimental autoimmune orchitis or epididymo-orchitis, rodent or animal model, vasectomy
Introduction
Infection and inflammation of the male reproductive tract are significant, and potentially curable, causes of male factor infertility (Rowe et al., 2000; Weidner et al., 2013). The defined clinical entities comprise urethritis, prostatitis, seminal vesiculitis, epididymitis and orchitis (Krieger, 1984; Weidner et al., 1999). In this regard, ascending, canalicular infections by sexually transmitted bacteria or common uropathogens represent the most frequent cause of inflammatory conditions within the male genital tract (Table I). Orchitis or epididymo-orchitis may also evolve as a complication of systemic, predominantly viral, infections due to haematogenous dissemination of the pathogen (Mikuz and Damjanov, 1982; Dejucq and Jegou, 2001). Moreover, non-infectious, sterile causes of inflammation, such as those caused by environmental threats and autoimmune reactions, need to be considered (Chan and Schlegel, 2002a, 2002b; Schuppe and Meinhardt, 2005) (Table I).
Table I.
Classification of human epididymitis and orchitis according to etiological factors and pathomechanisms.
| Etiology | Main factors | Patho-mechanism | Clinical manifestation |
|---|---|---|---|
| Microorganisms |
|
Ascending, canalicular infection | Epididymitis/Epididymo-orchitis |
| Mycobacterium tuberculosis, M. leprae, Treponema pallidum, Brucella spp. | Canalicular and/or haematogenous infection |
|
|
|
Haematogenous infection | Orchitis | |
| Adenovirus, Enterovirus | Epididymitis | ||
|
Ascending, canalicular infection | Epididymitis | |
|
Epididymitis | ||
| Schistosoma spp., Filariasis | |||
| Chemical noxae | Drugs (e.g. Amiodarone); heavy metals (e.g. mercury compounds) | ? |
|
| Physical factors | Genital trauma, vasectomy | Obstruction | Chronic Epididymitis |
| Unknown | Systemic disease Morbus Behcet, systemic lupus erythematosus, Schönlein-Henoch purpura and other vasculitic disorders | Autoimmune inflammation |
|
| ‘Idiopathic’ | Autoimmune inflammation? |
|
Principally, two different clinical situations can be distinguished according to the acuity of the disease. In patients suffering acute, symptomatic inflammatory conditions of reproductive organs, fertility-related problems are initially of a secondary nature, but may gain importance during follow-up. Conversely, male partners seeking clinical consultation to conceive a child seldom have obvious clinical symptoms. According to World Health Organization recommendations, diagnosis among these patients is consequently entirely based on the combination of impaired semen quality with additional criteria from the medical history, physical examination and the analysis of urine and/or ejaculate (Rowe et al., 2000; Schuppe et al., 2017). These criteria include a history of epididymitis or sexually transmitted disease, thickened or tender epididymis, elevated numbers of peroxidase-positive white blood cells in the ejaculate, culture with significant growth of pathogenic bacteria and/or abnormal biochemistry of the seminal plasma with pathological levels of inflammatory markers or elevated reactive oxygen species (Rowe et al., 2000). For these rather unspecific criteria the diagnostic term ‘male accessory gland infection’ (MAGI) has been coined (Comhaire et al., 1980). Its wide definition also encompasses epididymitis and lesions along the excurrent ducts (Weidner et al., 1999; Dohle et al., 2005) and therefore organs that are not anatomically considered as accessory sex glands. Moreover, the MAGI classification does not allow compartment-specific differential diagnosis of infectious versus non-infectious inflammatory disorders (Haidl et al., 2008; Weidner et al., 2008). In particular, testicular inflammation is likely to be neglected as an underlying cause of male infertility (Schuppe et al., 2008). In asymptomatic patients, subacute or chronic inflammatory reactions in the testis can be diagnosed only by invasive biopsy.
Available epidemiological studies mainly refer to MAGI and, thus, focus on the excurrent ducts. Prevalence rates for male infertility attributable to infection range from 6 to 15% in reports from andrological outpatient clinics (Comhaire et al., 1987; Hellwig, 2008; Tüttelmann and Nieschlag, 2010; Olesen et al., 2017; Punab et al., 2017). There are, however, striking geographical variations, with prevalence rates up to 30% in regions with limited access to medical care (Ekwere, 1995; Ahmed et al., 2010; Eke et al., 2011). These observations have been linked to sexually transmitted infections (STI) and inadequate treatment, leading to secondary male and couple infertility (Bayasgalan et al., 2004; Lunenfeld and Van Steirteghem, 2004; Mascarenhas et al., 2012). However, despite obvious clinical evidence linking infectious epididymitis and epididymo-orchitis to male infertility, consistent epidemiological data are scarce (Ness et al., 1997; Ochsendorf, 2008).
Due to the inconsistent use of definitions and diagnostic shortcomings, the overall impact of genital tract infection and inflammatory conditions on male reproductive health and fertility is a matter of controversy (Schuppe et al., 2017). Crucially, the course of the disease (acute versus chronic), the affected organ and, in case of infections, the type of pathogen has to be taken into account. Moreover, fertility may be disturbed at different levels, comprising deterioration of sperm function and integrity, dysfunction of the accessory glands, obstruction of the epididymal duct, and impairment of spermatogenesis and/or steroidogenesis. It is unambiguous that sequelae of testicular or epididymal inflammation are of major concern even in ‘low-grade’ disease, whereas the impact of prostatitis and urethritis on semen parameters is considered to be limited (Wolff, 1995; Weidner et al., 1999; Haidl et al., 2008; Schuppe et al., 2008). In this complex situation, the topic of infection and inflammation is either underestimated or even neglected in current concepts of male reproductive impairment and respective guidelines on diagnosis and therapy (Barratt et al., 2017; Tournaye et al., 2017a, 2017b; Jungwirth et al., 2018).
There is an obvious need for deeper insight into testicular and epididymal immunopathologies and their contribution to couple infertility. Advancement in the investigation of immunopathological mechanisms involved in human testicular and epididymal inflammation is, however, hindered by restricted access to tissue samples (Chakradhar, 2018). Here, comparative analyses of experimental animal models can overcome these limitations. Unravelling the complex mechanisms underlying the pathogenesis of infection and inflammation in the male genital tract, as well as dissecting their impact on fertility-related parameters, is a pre-requisite for the development of innovative diagnostic tools and evidence-based therapeutic strategies. As an example, there is increasing support from experimental animal models for the view that the mechanisms underlying infectious disease and inflammatory conditions in the male genital tract are interrelated with autoimmune phenomena (Hedger, 2011a). Moreover, mouse bacterial epididymitis models point to the importance of the magnitude of the host response to infection in causing damage (Michel et al., 2016) prompting us to assess the value of anti-inflammatory or immuno-modulatory therapy in addition to standard antibiotic treatment.
Thus, immune-based male factor infertility should be considered in a broader context, beyond the formation of antisperm autoantibodies, as it is commonly defined. Although not established as clinical entities in andrology, this concept includes the characterization of autoimmune orchitis and epididymitis in man. Therefore, in this review we aim to compare observations made in the clinic with data from animal models to evaluate their suitability and limitations, not only to enhance our principal understanding but also to advance clinical diagnosis and treatment of immune-based male factor infertility. Inflammation due to genital trauma or chemical noxae (Table I), low-grade inflammation associated with systemic diseases, such as metabolic syndrome and diabetes, as well as the immunopathology of testicular neoplasia are beyond the scope of this review.
Methods
This narrative review summarizes different primary studies from which conclusions were drawn to present a holistic interpretation contributed by the reviewers’ own experience and existing concepts and models from the literature. The outcome is of a qualitative rather than a quantitative meaning and aims to critically evaluate and comprehend the existing data towards a better understanding of the commonalities and diversities that exist in the literature around this research topic. The authors performed an extensive search in Medline database without restrictions until January 2018. Relevant literature was identified by the following search terms: ‘infection’ and/or ‘inflammation’ and ‘testis’ and/or ‘epididymis’, ‘infection’ and/or ‘inflammation’ and ‘male genital tract’, ‘male infertility’, ‘orchitis’, ‘epididymitis’, ‘experimental autoimmune’ and ‘orchitis’ or ‘epididymitis’ or ‘epididymo-orchitis’, antisperm antibodies’, ‘vasectomy’. In addition to that, reference lists of primary and review articles were reviewed for additional publications independently by each author. Selected articles were verified by each two separate authors and discrepancies discussed within the team.
The primary focus of this study is to understand the relevance of models of infectious and autoimmune epididymo-orchitis to the clinic. Other organs of the male reproductive tract (e.g. prostate), influences of obesity, hormonal imbalances or environmental threats other than pathogens were not covered.
The testicular and epididymal immune environment
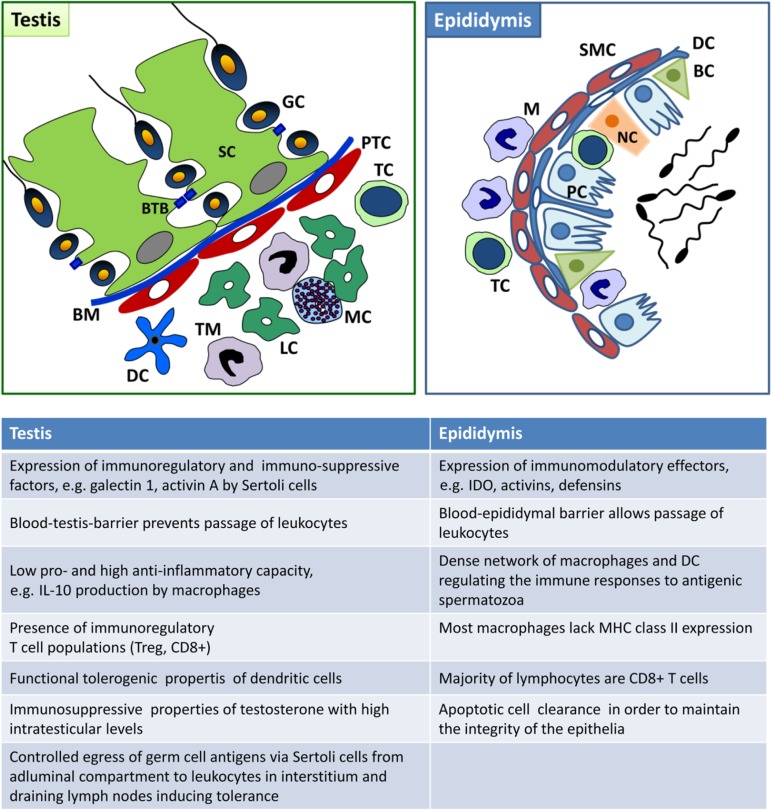
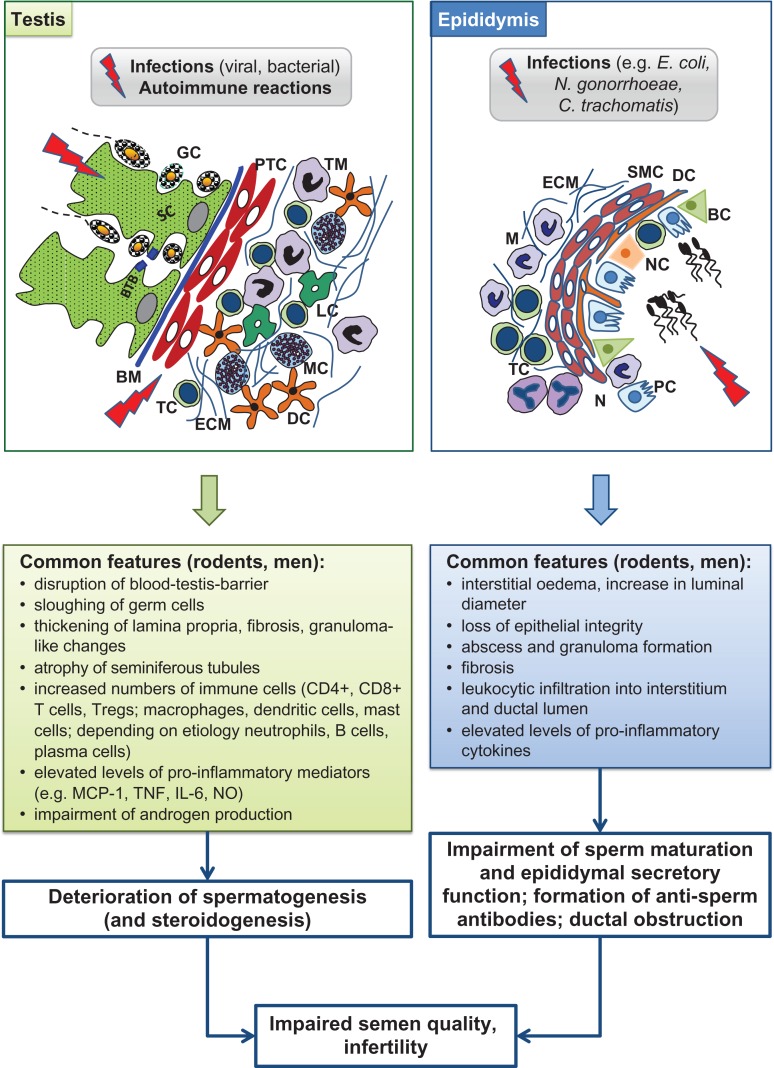
The immune system of the testis and epididymis differ in a number of aspects. Firstly, although immune cells (macrophages close to the wall of the seminiferous tubules) can be in close proximity to spermatogonia, the basement membrane prevents direct physical contact with developing germ cells, whilst leucocytes are observed in the epididymal lumen next to spermatozoa without any barrier in between. Moreover, little evidence exists for extended allograft survival, a hallmark of immune privilege (see below), in the epididymis in contrast to the testis. In support, pro-inflammatory stimuli, such as those caused by bacterial infection, are considerably greater in the epididymis than in the testis (Hedger, 2011a). In rodent orchitis, neutrophils are rather rarely found (in contrast to human), whilst they represent the most frequent leucocyte subset in epididymitis in men and rodents (Mikuz and Damjanov, 1982; Schuppe et al., 2008; Michel et al., 2015). B cells are virtually absent from the normal human and rodent testis and epididymis (Flickinger et al., 1997; Serre and Robaire, 1999; Hedger, 2011a; Klein et al., 2016). Details about the similarities and differences of the testicular and epididymal immune system in rodents and men can be found in Fig. 1 and Table II.
Figure 1.
Immune environment of the normal adult testis and epididymis. BC, basal cell; BM, basement membrane; BTB, blood–testis barrier; DC, dendritic cell; GC, germ cell; LC, Leydig cell; M, macrophage; MC, mast cell; NC, narrow and clear cell; PC, principal cell; PTC, peritubular cell; SC, Sertoli cell; SMC, smooth muscle cell; TC, T cell; TM, testicular macrophage; IL, interleukin; MHC, major histocompatibility complex; IDO, indoleamine 2,3-dioxygenase.
Table II.
Presence of immune cells in the normal testis and epididymis of adult mouse and human.
| Immune cells | Common markers | Testis | Epididymis | ||
|---|---|---|---|---|---|
| Mousea | Human | Mouse | Human | ||
|
|
+++ | +++ | + | + |
| Dendritic cells | CD11c, CD209, MHC class II, CD80, CD86 | + | (+) | ++b | ++ |
| T cells | CD3, CD4, CD8, Foxp3 | + | + | + | + |
| B cells | CD19, CD 20, B220 (CD45R) | + | (+) | +/− | ? |
| Natural killer cells | CD56, CD161 (NK1.1) | + | (+) | ? | ? |
| Mast cells |
|
(+) | + | + | + |
aConcerning the overall occurrence of immune cell subpopulations, comparable results exist for the rat; btubular wall.
(+) Very few.
+++ Abundant.
MHC, major histocompatibility complex.
The structure and immune privilege of the testis
The male gonad is principally separated into two compartments, i.e. the interstitial compartment, where steroidogenic Leydig cells produce androgens, and the seminiferous epithelium, where spermatogenesis occurs. The interstitial compartment also contains leucocytes, fibrocytes as well as blood and lymph vessels. The seminiferous tubules consist of a tubular structure that is framed by the myoid peritubular cells, whose contractions move the immotile spermatozoa intraluminally towards the rete testis and then the epididymis. In the seminiferous epithelium the columnar somatic Sertoli cells form deep invaginations, in which the developing germ cells are embedded to receive physical and nutrient support. Spermatozoa develop from diploid spermatogonia, which mitotically divide until some differentiate and enter meiosis to give rise to tetraploid primary spermatocytes. After meiosis, haploid spherical spermatids originate, which differentiate to elongated spermatids that are finally released in the lumen as highly specialized spermatozoa.
With the principal organization of the testis similar in experimental rodents and men, some differences are evident. Whilst in rodents the peritubular cells consist of only one single layer, in men they are multiconcentric and can harbour leucocytes and capillaries. In men, connective tissue septae originating from the organ capsule (tunica albuginea) separate the interstitial space, a means not evident in rodents. In men, spermatogenesis is also much less ‘efficient’ than in mouse or rat as defined by daily sperm production in relation to testis weight (Johnson et al., 2000).
Immune privileged sites are places in the body where foreign antigens are tolerated without evoking detrimental inflammatory immune responses. The testis was first identified as an immune-privileged organ when histo-incompatible allo- and xenografts transplanted into the testis were shown to survive indefinitely (Bobzien et al., 1983; Head et al., 1983).
In the testis, the auto-antigenic germ cells, which arise in puberty after the establishment of self-tolerance, are protected by multiple, complementary mechanisms that include:
- The blood–testis barrier: The Sertoli cells that besides providing structural and nutritional support to the germ cells, also control access of immune cells and immune effector molecules via the blood–testis barrier (BTB). The BTB consists of highly specialized inter-Sertoli tight, gap and adherens junctions. With the formation of the BTB, neoantigens on meiotic and haploid germ cells are sequestered from the basal part of the seminiferous epithelium and the testicular interstitium and thus direct access to the leucocytes, which reside exclusively in the interstitium (Fig. 1). Of note, a recent study proposes that antigens of male germ cells sequestered behind the BTB are phagocytosed in the apical part of Sertoli cells, pass as cargo through the cells and egress basally, thus circumventing the BTB by intracellular transport. Egressed antigens then cause and maintain systemic tolerance in a regulatory T (Treg) cell dependent mechanism (Tung et al., 2017). Indeed, transient depletion of Treg from normal mice led to spontaneous EAO and production of antibodies that selectively target the egressed meiotic germ cell Ag such as lactate dehydrogenase 3. This new finding indicates that meiotic and postmeiotic sperm antigens are not completely sequestered. This infers that the local regulation in the testis, also operates to maintain systemic tolerance for the non-sequestered sperm antigens. The presence of tolerogenic macrophages in testis is an example.
- The expression of immunoregulatory and immunosuppressive factors by the testicular somatic cells, particularly Sertoli cells, peritubular cells, Leydig cells and testicular macrophages, thereby creating an immune privileged environment. As an example, Sertoli cells have several immunosuppressive properties, such as the production of galectin-1 and other immunoregulatory molecules (Kaur et al., 2014; Gao et al., 2016). Under inflammatory conditions, Sertoli cells release anti-inflammatory cytokines and molecules like activin A, which may counterbalance excessive immune responses (Hedger and Winnall, 2012). It is believed that peritubular cells are also involved in the maintenance of the testicular immune environment (Schuppe and Meinhardt, 2005), as they also express immune mediators, including activin A and Toll-like receptors (TLR) (de Winter et al., 1993; Albrecht et al., 2005; Muller et al., 2005; Mayer et al., 2016). Clearly, their role in testicular immunity and inflammatory responses warrants further study.
- The phenotype of the intratesticular immune cells: examples are the anti-inflammatory/immunoregulatory M2 phenotype of resident testicular macrophages and the functionally tolerogenic characteristics of testicular dendritic cells (Rival et al., 2007; Mossadegh-Keller et al., 2017; Wang et al., 2017) (Table II). Amongst the leucocyte population, macrophages comprise the most abundant immune cells in the testis in most mammals including men and rodents (Hedger, 1997; Bhushan and Meinhardt, 2017). The immunosuppressive phenotype of macrophages is indicated, amongst others, by the expression of the M2 surface marker CD163 and production of the anti-inflammatory cytokine interleukin (IL) 10 (Wang et al., 2017). Under inflammatory conditions, the production of pro-inflammatory mediators, such as tumour necrosis factor (TNF), IL-1, IL-6, monocyte chemotactic protein-1 (MCP-1) and nitric oxide (NO) is dampened, whilst IL-10 secretion increases (O’Bryan et al., 2000; Bhushan et al., 2011, 2015; Winnall et al., 2011b). The maturation state of dendritic cells is regarded as a control point for the induction of peripheral tolerance or autoimmunity. Assessing the levels of antigen-presentation molecules, such as major histocompatibility complex class II antigens (MHC II), co-stimulatory molecules, such as CD80 and CD86, and chemokines acting via the C–C chemokine receptor type 7 (CCR7) indicates that testicular dendritic cells are tolerogenic under normal conditions (Rival et al., 2007, 2008). In addition to testicular macrophages and dendritic cells, several immunoregulatory T cell subpopulations, such as suppressor CD8+ cells, natural killer (NK) cells and CD4+ Foxp3+ regulatory T cells (Treg) are also present in the normal rat and human testis (Mukasa et al., 1995; Tompkins et al., 1998; Schuppe et al., 2008; Jacobo et al., 2009; Duan et al., 2011; Klein et al., 2016). In particular, Treg cells are thought to inhibit antigen specific T cell responses in the adult testis, at least in rodents (De Cesaris et al., 1992; Fijak et al., 2011, 2015; Tung et al., 2017).
The structure and immune environment of the epididymis
The epididymis is a tightly coiled single tubule that connects to the testis via the efferent ducts. The epididymis comprises three distinct regions: the caput (head), which receives the spermatozoa from the efferent ducts, the corpus (body) and the cauda (tail), where sperm are stored and pass to the vas deferens. The epididymal stroma is also divided into distinct morphological segments by connective tissue septa (Stammler et al., 2015). The epididymal duct is formed by a pseudo-stratified epithelium surrounded by a peritubular layer of smooth muscle cells that progressively increases in thickness from the caput to cauda. In strong contrast to the BTB, the blood–epididymis barrier between epididymal epithelial cells is permissive to the passage of leucocytes. Consequently, intraepithelial macrophages and T cells (‘halo cells’) and even intraluminal leucocytes, mainly macrophages, are a frequent observation (Nashan et al., 1989; Pollanen and Cooper, 1994; Jahnukainen et al., 1995; Yakirevich et al., 2002; Hedger, 2011a; Michel et al., 2015) (Fig. 1). Macrophages and dendritic cells are the main leucocyte population in the normal mouse epididymis (Hedger, 2011a) (Table II). Dendritic cells show a regional distribution pattern with cells most prominent in the basal part of the epithelium and peritubular zone of the caput. Here, slim protrusions pass through the epithelial cells and at least partly reach the lumen (Da Silva et al., 2011) (Fig. 1). In the cauda, dendritic cells are much less frequent, have a flat morphology and do not seem to project extensions to the lumen. Numbers and morphology of the dendritic cells in the caput epididymis indicate a possible role in the regulation of systemic self-tolerance towards the neoantigens of spermatozoa (Da Silva et al., 2011). Whether this indeed holds true and involves Treg cells, as principally indicated by Wheeler et al. (2011) in a vasectomy model, remains to be elucidated. Overall, it needs to be noted that the relative contribution of the epididymis (beside the testis) to self-tolerance requires additional studies to address many open questions, such as the role of caput dendritic cells, the blood–epididymis barrier, the role of intraluminal leucocytes and regional differences in immune cell subpopulations to name only a few. All need to be addressed with appropriate methods to answer this fundamental query.
Infectious epididymitis, epididymo-orchitis and orchitis
Clinical features of bacterial epididymitis and epididymo-orchitis
Epididymitis is a common condition in males presenting with acute uni- or bilateral scrotal pain and swelling (Lorenzo et al., 2016). Incidence ranges from 250 to 650 per 100 000 males each year (Nickel et al., 2005; Nicholson et al., 2010). The inflammation may spread to the corresponding testis as ‘epididymo-orchitis’, especially when adequate therapy is delayed. In patients with isolated epididymitis without concomitant orchitis, hydrocele and scrotal wall induration, palpation is sufficient for diagnosis (Eickhoff et al., 1999; Smith et al., 2013). Additional ultrasound is recommended in complicated cases, for follow-up investigations, as well as to exclude testicular torsion in young men (Mevorach et al., 1986; Banyra and Shulyak, 2012; Pilatz et al., 2013). Chronic epididymitis is defined as 3 months or longer history of symptoms of discomfort/pain in the epididymis (Nickel et al., 2002).
In the majority of cases, epididymitis is of infectious origin, with bacterial ascension from the urethra to the epididymis being of principal importance (Pilatz et al., 2015b) (Table I and Fig. 2). Notably, the pathogen spectrum largely depends on the applied diagnostics and the patient cohort investigated. Studies from military hospitals or venereal disease centres suggested dichotomous categories, with STIs in patients < 35 years and classical pathogens causing urinary tract infections in older patients (Harnisch et al., 1977; Berger et al., 1987; Osegbe, 1991). Recently, however, it was demonstrated in 251 patients presenting to the emergency department that, although STIs are more common in younger patients, there is no strict age-specific differential incidence (Pilatz et al., 2015b). In addition, geographic differences can be encountered when comparing the aetiology between industrial and developing countries (Osegbe, 1991; Hoosen et al., 1993).
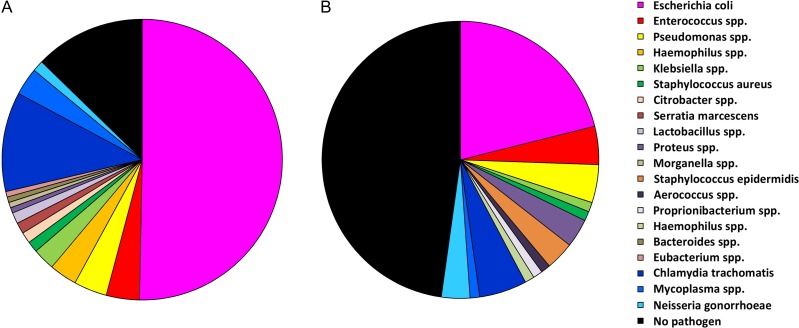
Figure 2.
Pathogen spectrum in patients with acute epididymitis. (A) In patients without antimicrobial pretreatment (n = 157) bacterial pathogens can be identified in 88% of cases. (B) In patients with antimicrobial pretreatment (n = 90) a pathogen detection is only possible in ~54% of cases.
A pooled analysis of 14 studies (1978–1999), including 758 patients and considering STIs and common uropathogens, revealed a pathogen detection rate of 69.8% (Michel et al., 2015). Using modern microbiological methods (culture, PCR, 16S rDNA analysis), we recently showed an improved detection rate of 88% in antibiotic-naïve patients (Pilatz et al., 2015b). Comparable to other urinary tract infections, such as prostatitis and cystitis, Escherichia coli is the dominating pathogen (Fig. 2A). As antimicrobial pretreatment largely decreases the microbiological detection rate (Fig. 2B), microbiological diagnostics should be performed before starting antibiotic therapy (Grant et al., 1987; Lee et al., 1989; Osegbe, 1991; Garthwaite et al., 2007; Pilatz et al., 2015b). Since bacterial ascension is the major route of infection, bacterial analysis in urine/urethra is of utmost importance. Current international guidelines recommend diagnostics on STIs as well as urine culture for classical uropathogens (Workowski and Bolan, 2015; Bonkat et al., 2018).
Despite epididymitis occurring frequently in patients of reproductive age (Wolin, 1971; Berger et al., 1979; Kristensen and Scheibel, 1984; Weidner et al., 1990; Osegbe, 1991; Pilatz et al., 2015b), a systematic review identified only five studies investigating the impact of acute epididymitis on semen parameters (Rusz et al., 2012). Unfortunately, these early reports on a total of 211 patients (Dietz, 1960; Tozzo, 1968; Ludwig and Haselberger, 1977; Weidner et al., 1990; Osegbe, 1991) are very heterogeneous regarding investigation time points and methods of semen analysis (Rusz et al., 2012). Nevertheless, the collective analysis indicates profound deterioration of semen quality (sperm concentration, motility, morphology) together with pronounced leukocytospermia in the acute phase of the disease. After therapy, recovery was reported 3–6 months later. Data are compromised by the fact that some studies used antimicrobial therapies inadequate for Chlamydia trachomatis. Nevertheless, out of the 211 patients evaluated, 10% were reported with azoospermia and a further 30% with oligozoospermia, indicating 40% with post-inflammatory subfertility at least (Rusz et al., 2012).
Accordingly, it is a matter of major concern that the course of epididymitis remains unpredictable despite adequate antimicrobial therapy. After 3 months, ~20% of patients still have an epididymal infiltrate on palpation or ultrasound (Weidner et al., 1990; Eickhoff et al., 1999; Pilatz et al., 2015b). Moreover, given the fact that up to 60% of all cases involve the testis as well (Desai et al., 1986; Kaver et al., 1990; Pilatz et al., 2013), a direct or indirect negative impact on spermatogenesis can be hypothesized. Indeed, two studies report testicular damage and subsequent infertility after acute unilateral epididymitis (Dietz, 1960; Osegbe, 1991). Whereas the histopathology of acute bacterial epididymo-orchitis is characterized by oedema and massive infiltration of predominantly neutrophils into both the interstitial compartment and seminiferous tubules (Mikuz and Damjanov, 1982; Schuppe and Bergmann, 2013) (Fig. 3A), testicular biopsy specimens obtained from two patients during follow-up confirmed the development of severe hypospermatogenesis with seminiferous tubules showing ‘aspermatogenesis’ (loss of the adluminal compartment), thickened lamina propria, and interstitial fibrosis in both ipsi- and contralateral testes (Osegbe, 1991) (Table III). Increased FSH levels support the histopathological findings of testicular failure. On the other hand, a recent study on 90 patients suffering unilateral epididymitis showed no reduction in testicular volume after the acute phase compared with the healthy contralateral side (Pilatz et al., 2013). Thus, in addition to loss of testicular function, inflammatory obstruction of the epididymal duct has to be considered as an underlying cause of persistent oligo- or azoospermia (Fig. 4A).
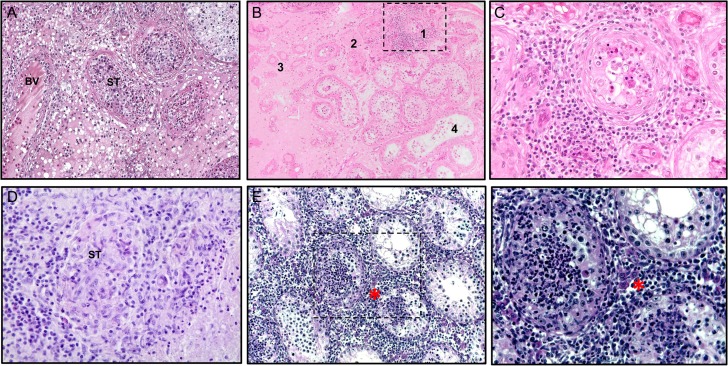
Figure 3.
Histopathology of human orchitis of different etiology and mouse experimental autoimmune orchitis. (A) Human testis: acute bacterial orchitis (epididymo-orchitis) with massive infiltration of both the interstitium and seminiferous tubules (ST) with inflammatory cells, including numerous neutrophils. The architecture of affected ST is largely disrupted, whereas adjacent ST show hypospermatogenesis; interstitial edema and enlarged venous blood vessel (BV) (Periodic acid–Schiff stain, objective ×10). (B) Sequelae of mumps orchitis with persistent focal inflammation in human testis: Dense peritubular lymphocytic infiltrate involving the lamina propria as well as adjacent blood vessels (1), tubular atrophy resulting in complete hyalinization (‘tubular shadows’; 2, 3), and interstitial fibrosis (3). The adjacent seminiferous tubules show hypospermatogenesis; note the ‘flattened’ epithelium with a complete loss of the adluminal compartment in some tubules (4); (hematoxylin–eosin staining, objective ×10). (C) Higher magnification of area 1 in (B); note the characteristic meshwork pattern of the affected lamina propria; the germinal epithelium is largely disrupted, with only a few germ cells remaining (hematoxylin–eosin stain, objective ×40). (D) Human testis: subacute granulomatous orchitis with residual structures of ST containing inflammatory cells (hematoxylin and eosin stain, objective ×40). (E) Characteristic histopathology of mouse experimental autoimmune orchitis (EAO) showing destruction of testicular morphology with reduced size of ST, loss of germ cells and presence of dense peritubular and interstitial inflammatory infiltrates (marked by asterisk; hematoxylin stain, objective ×20). (F) Mouse EAO, higher magnification (hematoxylin stain, objective ×40) of selected area in (E). (A–D) From Schuppe and Bergmann (2013); reprinted with permission of Springer Nature (License number: 4282971349118).
Table III.
Characteristics of pathological changes found in animal models of infectious, inflammatory and autoimmune male factor infertility and their occurrence in respective human disorders.
| Pathology | Animal models | Human disease | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial epididymitis / epididymo-orchitis | Systemic viral disease | LPS-induced inflammation | EAO | Vasectomy | Bacterial epididymitis / epididymo-orchitis | Systemic infection (i.e. viral disease) | Testicular inflammatory lesions in infertile males | ||
| Semen quality | Impaired sperm parameters/azoospermia | + | nd | nd | + | + | + | + | + |
| Leukocytospermia | nd | nd | nd | nd | – | + | – | – | |
| Detection of pathogens | Epididymis (caput) | + | nd | – | – | – | + | + | – |
| Testis | (+) | + | – | – | – | + | + | – | |
| Epididymal pathology/immunopathology | Presence of leucocytic infiltrates | + | nd | nd | + | + | + | nd | nd |
| Accumulation of collagen fibres/fibrosis | + | nd | nd | nd | + | + | nd | nd | |
| Granuloma formation | + | nd | nd | + | + | (+) | – | – | |
| Testicular pathology | Disruption of spermatogenesis/germ cell death | + | + | + | + | + | + | + | + |
| Disruption of tight junctions | – | + | nd | + | nd | + | + | + | |
| Thickened lamina propria of seminiferous tubules | – | + | nd | + | nd | + | + | + | |
| Accumulation of collagen fibres | nd | nd | nd | + | nd | + | + | + | |
| Disruption of steroidogenesis | nd | + | + | + | nd | (+) | + | – | |
| Testicular immunopathology | Presence of lymphocytic infiltrates | + | + | – | + | (+) | + | + | + |
| Increased number of TH17+ T cells and their cytokines | nd | nd | nd | + | nd | nd | nd | + | |
| Increased numbers of macrophages/dendritic cells | + | + | + | + | nd | + | + | (+) | |
| Elevated levels of pro-inflammatory cytokines | + | + | + | + | nd | nd | nd | + | |
| Formation of immune complexes | nd | nd | nd | + | + | nd | nd | (+) | |
| HMGB1 release | + | nd | nd | + | nd | nd | nd | + | |
| Systemic immunopathology | Autoantibodies against haploid germ cells | nd | nd | nd | + | + | (+) | – | (+) |
LPS, lipopolysaccharide; EAO, experimental autoimmune orchitis; nd, not determined; HMGB1, high mobility group protein B1; TH17, T-helper 17 cells.
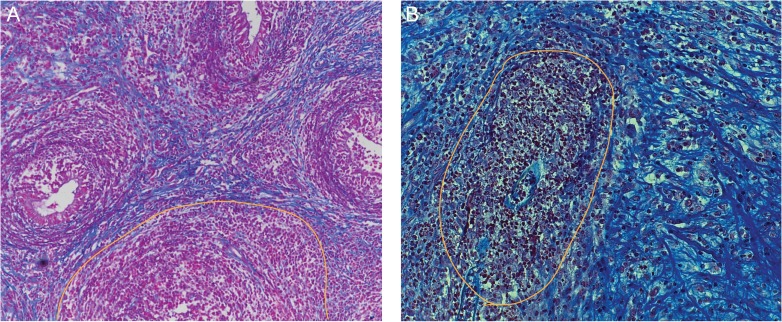
Figure 4.
Histopathology of human ‘chronic’ epididymitis and a mouse epididymitis model. Seven days post infection with uropathogenic E. coli (UPEC) fibrotic transformation, epithelial degeneration and ductal obstruction (yellow line) are visible in mice (B) comparable to the histopathology observed in ‘chronic’ epididymitis in men (A) (azan staining; from Michel et al. (2016)). Reprinted with permissions from Wiley and Sons (license number: 3973511270642).
Animal models of bacterial epididymo-orchitis
Taking biopsies from acute bacterial epididymitis is contraindicated to avoid the risk of uncontrolled spread of the pathogens by the puncture and irreversible damage (i.e. obstruction) of the organ. Hence, human epididymitis samples, which may be used to study the detailed assessment of morphological changes and inflammatory responses, are rarely available. As surrogates the careful design and conduct of appropriate animal studies is warranted. Of particular value are models that mimic the clinical situation, e.g. bacterial infection that is allowed to ascend at least 2–3 days, which corresponds to the average time after infection when men usually report to the clinic with symptoms. Ideally, an animal model should allow for assessment of both acute and chronic impact on the epididymis and the testis and involve a relevant causative pathogen. In this regard, E. coli (Lucchetta et al., 1983; Nielsen, 1987; Hackett et al., 1988; Vieler et al., 1993; Tanaka et al., 1995; Kaya et al., 2006; Demir et al., 2007; Bhushan et al., 2008; Fei et al., 2012) and Chlamydia trachomatis (Moller and Mardh, 1980; Jantos et al., 1989, 1992) have been selected preferentially as model microbes for rodent epididymitis studies, because they represent the most frequently isolated bacterial pathogens in the clinic. The number of bacteria, usually determined by colony forming units (CFU), injected in epididymitis studies in animals ranges from 4 × 104 (Lang et al., 2013, 2014; Cao et al., 2014) to 2 × 107 (Fei et al., 2012) in mice and from 105 (Vieler et al., 1993; Kaya et al., 2006; Biswas et al., 2015) to 108 (Tanaka et al., 1995) CFU in rats. The duration of infection in these studies varies from several hours (Vieler et al., 1993; Tanaka et al., 1995; Kaya et al., 2006) to several days (Kuzan et al., 1989; Bhushan et al., 2008; Turner et al., 2011; Fei et al., 2012; Cao et al., 2014) or even months (Lucchetta et al., 1983; Hackett et al., 1988; Pilatz et al., 2015a). While the bacteria were directly injected into the epididymis (Kuzan et al., 1989; Fei et al., 2012; Cao et al., 2014) or the testis (See et al., 1990), in some studies injection into the vas deferens was performed to model the route of infection in men. In contrast to the situation in human, the latter were often performed in combination with vasoligation proximal of the injection site to ensure unidirectional distribution towards the epididymis and avoid urinary tract involvement.
While the majority of epididymitis patients clinically present with unilateral infection, the contralateral, uninfected side may not be considered normal or free of inflammatory signs (Ludwig et al., 2002). Hence, it cannot serve as an entirely healthy control in experimental models.
Intraductal E. coli epididymitis model
Taking all these aspects into consideration, we designed a rodent model of bacterial epididymitis, in which uropathogenic E. coli (UPEC strain CFT073) were bilaterally injected into the vas deferens of mice (Lang et al., 2013, 2014; Stammler et al., 2015; Khosravi et al., 2016) or rats (Bhushan et al., 2008; Lu et al., 2013; Biswas et al., 2015). Tissues were analysed 3 or 7 days after the infection. In order to delineate the spectrum of pathogens found in men, non-pathogenic commensal E. coli strains (NPEC strain 470) were included. The resulting retrograde ascent of the bacteria induced an infection and inflammation of the epididymis initially in the cauda epididymis and in the proximal epididymis several days later. After 3 days of infection, bacteria were confined to the ductal lumen of the cauda epididymis in mice (Stammler et al., 2015). Later, at 7 days post-infection, pathogens were reaching the caput epididymis and the testis (Biswas et al., 2015; Michel et al., 2016). Similar observations have been made in rat models of unilateral E. coli-induced epididymitis (Lucchetta et al., 1983; Tanaka et al., 1995; Kaya et al., 2006; Demir et al., 2007; Pilatz et al., 2015a).
Initial histopathological changes were primarily observed in the cauda epididymis, with accumulation of collagen fibres, flattening of the epithelium and increase in luminal diameter, oedema, abscess formation and leucocyte infiltration in the interstitium (Fig. 4B and Table III). Furthermore, with the disruption of tight junctions and loss of stereocilia, the integrity of the epithelium was compromised in mice (Lang et al., 2013; Stammler et al., 2015). Following the proximal progression of the infection and the disruption of segmental boundaries, the tissue damage and fibrosis became severe, and collagen deposits of collagen I and fibronectin were detected throughout the cauda and in the more distal segments of the corpus (Stammler et al., 2015; Michel et al., 2016) (Fig. 4B and Table III). Beside a longer exposure to the pathogen, the cauda appeared to be principally more sensitive to fibrotic transformation, as was indicated by in vitro organ culture models (Michel et al., 2016).
Immune cell infiltration of the epididymis of rat occurred as early as 24h post-infection (Tanaka et al., 1995) and by 3 days post-infection, leucocytic infiltration of the interstitial space (Hackett et al., 1988; Tanaka et al., 1995; Kaya et al., 2006; Lang et al., 2013), and in some cases in the ductal lumen as well (Ludwig et al., 1997, 2002; Kaya et al., 2006), was observed. Concomitantly, a surge in pro-inflammatory cytokine levels was observed following E. coli-induced epididymitis (Turner et al., 2011). In the mouse model, infection with NPEC induced a rise in cytokine levels that was even higher than with UPEC, but did not cause damage comparable to that observed after UPEC infection (Lang et al., 2014). Blunting the immune response by deletion of Myd88, an adaptor protein in TLR signalling, reduced tissue damage substantially in UPEC-induced epididymitis in mice. In conclusion, severe histopathological damage and epididymal duct obstruction seem to depend on both the presence of certain E. coli UPEC virulence factors and the magnitude of the inflammatory response, whilst one factor alone results in less dramatic histological alterations (Lang et al., 2014; Michel et al., 2016).
Chlamydia trachomatis epididymitis models
Two rodent models, in mouse (Kuzan et al., 1989) and in rat (Jantos et al., 1992), have been designed to investigate the effects of C. trachomatis-induced epididymitis on the epididymis and testis. In the mouse, injection of the pathogen into the epididymis resulted in initial swelling of the tissue and detection of the bacteria both within epithelial cells and the ductal lumen, as well as immune cell infiltration and flattening of the epithelium. Intravasal injection of bacteria in the rat likewise caused epididymal swelling, cellular infiltration, spermatic granulomas, epithelial disruption and fibrosis in the epididymis. While the pathogens could be recovered from the epididymis up to 90 days post infection, chlamydial antigens were also found in the testes (Jantos et al., 1992).
Direct random injection of both C. trachomatis and E. coli elicits a response comparably milder than in intraductally induced epididymitis, although different time points and numbers of bacteria injected made the exact comparison difficult (Kuzan et al., 1989; Greskovich et al., 1993; Fei et al., 2012; Cao et al., 2014).
Linking animal models of local bacterial infection to the clinic
Amongst all animal models related to infectious and/or inflammatory diseases of the testis and epididymis, the acute bacterial epididymitis models is the closest to the clinical situation (Table III and Fig. 5). Relevant pathogens, canalicular infection pathways, time course, damage observed and consequences for fertility can be readily mimicked in vivo or even in epididymal organ culture models.
Figure 5.
Lessons learned from animal models of testicular and epididymal infection and inflammation. BC, basal cell; BM, basement membrane; BTB, blood–testis barrier; DC, dendritic cell; ECM, extracellular matrix; GC, germ cell; IL, interleukin; LC, Leydig cell; M, macrophage; MC, mast cell; MCP, monocyte chemotactic protein; N, neutrophils; NC, narrow and clear cell; NO, nitric oxide; PC, principal cell; PTC, peritubular cell; SC, Sertoli cell; SMC, smooth muscle cell; TC, T cell; TM, testicular macrophage; TNF, tumor necrosis factor; Treg, regulatory T cell.
Both experimental animal and human data indicate that, in chronic epididymitis, luminal ascent of bacteria is strictly gated with infection-associated tissue damage mostly in the distal cauda segment (Stammler et al., 2015). Consistent with this concept, microbiological screening of testicular tissue obtained from patients with obstructive or non-obstructive azoospermia remained completely negative, despite down-stream detection of STI in some cases (Sripada et al., 2010). On the other hand, the clinical course of epididymitis remains unpredictable despite adequate antimicrobial therapy.
Long-term sequelae seem to be associated with infection by certain microbial strains. As an example, epididymitis elicited by E. coli strains expressing the virulence factor α-haemolysin (such as CFT073) did not result in recovery of initial low sperm counts in mice. This is similar to the clinical observation, that men infected with α-haemolysin-negative E. coli strains recovered from initially low sperm counts after 3 months, whilst this was not the case when α-haemolysin-positive E. coli pathovars were found (Lang et al., 2013). This highlights the role of bacterial virulence factors in the final outcome of genital tract infections.
In addition to the quantitative reduction of semen quality, recent investigations on the sperm proteome in patients following acute epididymitis indicate several differentially expressed sperm proteins. Of those, many have been described in other patient cohorts suffering subfertility, epididymal dysfunction or inflammation of the urogenital tract (Pilatz et al., 2014a). Beside a change in the composition of proteins also the glycome of spermatozoa in E. coli-associated epididymitis was altered as documented by a substantial reduction of sialic acid residues bound to the surface of spermatozoa in men and mice. Mechanistically, α-haemolysin as a pore-forming toxin allowed Ca2+ to enter the cell, thereby eliciting the acrosome reaction liberating stored sialidases. Premature acrosome reaction incapacitates spermatozoa for normal fertilization in both rodents and men (Khosravi et al., 2016). The value of animal models though is emphasized by the fact that hyposialylation was also observed on the epididymal epithelial cells in UPEC epididymitis in mice, an examination not possible under clinical circumstances where surgical intervention in acute epididymitis is rarely indicated (Pilatz et al., 2015b). Of note, removal of sialic acid residues from host cells represents a means for bacteria to manipulate the host’s innate immune response. Animal data point to an anti-viral rather than anti-bacterial response, which could lead to subsequent sterile autoimmunity and ongoing tissue damage once pathogens are removed following antimicrobial therapy. Moreover, sialidase/neuraminidase inhibitors are currently being tested in clinical trials or already in use to treat influenza and sepsis beside other inflammatory diseases (McLaughlin et al., 2015), marking their possible use as adjuvant therapy in epididymitis to preserve fertility.
Similarly, Myd88−/− mice that are characterized by a strongly dampened pro-inflammatory innate immune reaction against invading gram-negative bacteria such as E. coli show substantially less histopathological alteration and no indication of obstructions of the epididymal duct 7 days post-infection in contrast to wildtype. These data from mouse models point to a possible value of an adjuvant immuno-modulatory therapy in cases, where epididymitis has been associated with certain bacterial strains, such as UPEC, known to elicit permanent impairment to fertility (Michel et al., 2016).
The need to consider adjuvant anti-inflammatory treatment is stressed by the fact that in a rat model of E. coli-associated epididymitis damage was evident in the testis that was not prevented by initial fluoroquinolone therapy. Long-term studies up to 6 months after intraductal infection followed by fluoroquinolone treatment documented progressive disruption of testicular architecture (Pilatz et al., 2015a). Although cytokine levels were not measured at 6 months, the principal sensitivity of spermatogenesis to elevated cytokine levels may warrant early anti-inflammatory intervention to maintain fertility. In light of similarities between the pathology seen in bacterial epididymo-orchitis in rodent models and men, evaluating the putative use of adjuvant neuraminidase inhibitor or anti-inflammatory treatment appears to be needed to predict any suitability for the clinic.
A disadvantage of the acute bacterial epididymo-orchitis model represents the ligation of the vas deferens, put in place to prevent a retrograde dissemination of pathogens to the urethra and bladder causing cystitis and possibly sepsis as a co-morbidity. Using vasectomy, it was shown that fibrosis and hypospermatogenesis became evident simply by ligation, albeit only after 12 months (Wheeler et al., 2011). Our data indicate that milder damage of the epididymis, including fibrosis and some interstitial leucocytic infiltration, occurs as early as 7 days post-ligation (and sham injection). This requires a careful differentiation of the pathology and inclusion of further control groups to assess what damage is elicited by the ligation of the vas alone and what is derived from the infection.
Human orchitis and epididymo-orchitis associated with systemic infection
Orchitis may evolve as a complication of systemic, predominantly viral, infections due to haematogenous dissemination of the pathogen (Mikuz and Damjanov, 1982; Dejucq and Jegou, 2001). Whereas the prevalence of bacterial epididymo-orchitis may be estimated from reports on acute epididymitis, consistent epidemiological data concerning the incidence of de novo inflammatory conditions primarily affecting the testis in the general male population are not available (Schuppe et al., 2008, 2017). Despite convincing clinical and pathological evidence that this type of orchitis can lead to disruption of spermatogenesis and steroidogenesis, data on fertility-related sequelae are scarce (Table III).
The classical example of viral orchitis is associated with mumps and typically develops 3–10 days after the onset of parotitis (Beard et al., 1977; Weidner and Krause, 1998). Orchitis is the most common complication of mumps in pubertal and post-pubertal males, with a prevalence of 5–37% and bilateral disease reported in 16–65% of cases (Wesselhoeft, 1920; Beard et al., 1977; Nickel and Plumb, 1986). Although local mumps outbreaks have been reported in inadequately vaccinated populations, orchitis is now relatively rare in post-pubertal men in countries with modern public health practices (Tae et al., 2012; Patel et al., 2017; Willocks et al., 2017).
Studies report that ~50% of the affected testes undergo some degree of atrophy, but are rather heterogeneous with regard to patient cohorts, definition of ‘atrophy’, and follow-up periods (Pilatz et al., 2016). The analysis of testicular biopsies 1 year after mumps orchitis revealed total atrophy of seminiferous tubules in 38% and partial atrophy in 16% of affected testes, even when patients were treated with interferon-α2B during the acute phase of the disease (Yeniyol et al., 2000). Hence, patients suffering mumps orchitis are at risk of developing spermatogenic failure, although data from the pre-vaccination era indicate that the frequency of persistent azoospermia might be as low as 5% (Werner, 1950).
Histopathologically, viral orchitis is characterized by multifocal perivascular as well as peri- and intratubular infiltrates with neutrophils, lymphocytes, plasma cells and macrophages. Affected seminiferous tubules show degeneration of the germinal epithelium sparing few spermatogonia and the Sertoli cells; concomitant thickening of the lamina propria may result in complete hyalinization and fibrosis of the tubules (Mikuz and Damjanov, 1982) (Fig. 3B and C). This pattern of tubular damage has also been described as ‘mixed atrophy’ (Sigg and Hedinger, 1981; Bergmann, 2006). Notably, persistent chronic inflammatory reactions following acute orchitis are characterized by focal or multifocal peritubular lymphocytic infiltrates (Mikuz and Damjanov, 1982; Schuppe and Bergmann, 2013) (Fig. 3C and Table III). Leydig cells in the interstitial compartment show little evidence of damage in most viral orchitis patients.
Less commonly, a range of viral infections other than mumps may be complicated by inflammatory lesions in the testis. These include Coxsackie virus types, Epstein-Barr, influenza and HIV (Dejucq and Jegou, 2001). In early autopsy studies, inflammatory infiltrates were observed in testes of patients with late-stage HIV infection (Chabon et al., 1987). Though clinically overt orchitis is not evolving, persistence of viral DNA in testicular tissue and impairment of semen quality under effective retroviral therapy have recently been reported (van Leeuwen et al., 2008; Pilatz et al., 2014b; Jenabian et al., 2016). In a case series of men who died of a coronavirus infection causing severe acute respiratory syndrome, both, disruption of spermatogenesis and testicular inflammation were observed in the testes (Xu et al., 2006). Most recently, persistence of Zika virus (ZIKV) in the male genital tract has been reported (Paz-Bailey et al., 2017). However, there are no published data on clinical manifestations of orchitis or epididymo-orchitis available (Epelboin et al., 2017).
A predominantly granulomatous, chronic orchitis occurs as a manifestation of tuberculosis, syphilis, lepromatous leprosy, or brucellosis (Mikuz and Damjanov, 1982; Schuppe et al., 2008; Schuppe and Bergmann, 2013) (Fig. 3D). In pre-pubertal boys, epididymo-orchitis may complicate bacterial infections, such as pneumonia, by haematogenous dissemination of the pathogen (Greenfield, 1986).
Models mimicking systemic infection and inflammation
Systemic inflammation due to infection or even non-infectious illnesses has an inhibitory effect on spermatogenesis and steroidogenesis (Woolf et al., 1985; Andrade-Rocha, 2013). Typically, these responses have been attributed to the detrimental effects of fever, leading to an increase in intratesticular temperature, or vascular disturbances. However, studies from animal models suggest that inflammation itself also has a direct effect on testicular function and fertility (see below). Reports on the effect of low-grade inflammation associated with systemic diseases, such as metabolic syndrome and diabetes, as well as immuno-editing associated with testicular neoplasia have recently been summarized elsewhere and are not reflected in this review (Loveland et al., 2017; Maresch et al., 2017).
Animal models of systemic viral disease
There have been a small number of studies in animals of the effects of viral infections on testis function. Crucially, it is necessary to distinguish between systemic viral infections (for example, influenza and mononucleosis) that can indirectly interfere with male reproduction, and viral infections of the male tract itself (mumps, HIV, ZIKV). The detrimental effects of systemic viral infections may be principally exerted through elevated inflammatory responses, fever, vascular disturbances, immune cell activation and blood-borne inflammatory mediators, including cytokines and the anti-viral interferons, which can have inhibitory effects on spermatogenesis and steroidogenesis (Fig. 5 and Table III) (Hedger, 2011a; Satie et al., 2011). Animal models of viral infections of the male tract itself include mumps virus, cytomegalovirus and herpes simplex virus infections in mice (Tebourbi et al., 2001; Malolina et al., 2016; Wu et al., 2016), Sendai virus infection in rats (Melaine et al., 2003), Myxoma virus infection in rabbits (Fountain et al., 1997) and simian immunodeficiency virus infection in monkeys (Shehu-Xhilaga et al., 2005; Houzet et al., 2014; Winnall et al., 2015). In these various studies, infection was frequently associated with leucocytic infiltration (T cells, macrophages), an increase in local production of interferons and pro-inflammatory mediators, disruption of the seminiferous epithelium and primary Leydig cell failure with reduced testosterone levels. Similar to observations in corresponding mouse models (Wu et al., 2016), deterioration of testicular androgen production has been observed in severe cases of bilateral mumps orchitis (Fig. 5 and Table III) (Adamopoulos et al., 1978).
Most recently, Govero et al. (2016) delineated ZIKV infection of the testis and epididymis in mice using a mouse-adapted African strain. The infection of germ cells and Sertoli cells caused deterioration of spermatogenesis resulting in complete germ cell loss, reflected by decreased levels of serum inhibin B. Testicular damage seems to be mediated by both the infection itself and the host’s adaptive immune response, while leucocytes entered the seminiferous epithelium only in the most severe cases (Govero et al., 2016). Of note, the prostate or seminal vesicles were unaffected and innate immune responses were found in Leydig, Sertoli and epididymal epithelial cells, but not in peritubular cells and spermatogonia, exposing these cells as particularly vulnerable for ZIKV infection and as possible repositories for ZIKV (Ma et al., 2017). Although Zika viral load in semen, impaired semen quality and sexual transmission have been reported (D’Ortenzio et al., 2016; Epelboin et al., 2017; Joguet et al., 2017; Paz-Bailey et al., 2017), it remains to be elucidated how murine testicular disease translates to the clinic (Meinhardt, 2017).
In general, studies using these specific infections, however, are complicated by the high degree of species specificity among the viruses and their hosts. Critically, different viruses target different cell types, and even the affected cells, their susceptibility to infective tropism and the pattern and intensity of production of cytokines and interferons by specific testicular cells vary significantly from species to species (Le Goffic et al., 2002; Dejucq-Rainsford and Jegou, 2004; Roulet et al., 2006; Le Tortorec et al., 2008; Wu et al., 2016). Consequently, viral infections within the male reproductive tract have widely variable effects on male reproductive function in different models. In general, the pathology is associated with the distinct local effects of the infection itself, and it is difficult to distinguish more universal effects that may be attributable to inflammation alone. Leydig cells in the interstitial compartment show little evidence of damage in most viral orchitis patients, whilst this is the case in mumps virus infected mouse (Wu et al., 2016). Moreover, different tropism of viruses for human and mouse make the use of either mouse-adapted forms of viruses (e.g. ZIKV) or a replacement by a different virus (e.g. Sendai for rat) necessary as surrogates. This limits somewhat the utility of studies using specific viruses as models for human disease, with the result that animal models involving inflammation without infection are generally more amenable to the study of the role of inflammation in human disease.
Lipopolysaccharide-induced inflammation models
Lipopolysaccharide (LPS) is a component of the cell wall of gram negative bacteria, such as E. coli, and stimulates inflammation and innate immunity by activation of TLR 4 (Beutler, 2000). For many years, LPS has been used to investigate the effects of systemic inflammation, without the complication of infection, in numerous animal models. Intraperitoneal or intravenous injection of LPS in various animal species, particularly rats and mice, exerts predominantly inhibitory effects on Leydig cell steroidogenesis at the testicular and at the hypothalamic–pituitary level, and may also involve peripheral responses to inflammation, such as corticosteroid production (O’Bryan et al., 2000; Gow et al., 2001; Diemer et al., 2003). Moreover, it is increasingly evident that inflammation has direct effects on the somatic (Leydig and Sertoli) cells in the testis and epididymis (epithelial and stromal cells), and their ability to support spermatogenesis and sperm maturation (Hedger, 2011b). Notably, LPS does not induce fever in rats or mice, and the effects of LPS on spermatogenesis in the rat do not replicate the well-characterized effects of either elevated temperature or vascular disturbance on spermatogenesis and steroidogenesis. This has led to the proposition that elevation of cytokines and other inflammatory and antimicrobial mediators may be a major cause of disruption in these animal models, and hence possibly also in human patients. Crucial to this proposition is the observation that the somatic cells of the testis and epididymis themselves express pattern recognition receptors, including TLR4 and viral sensors such as TLR3, and produce inflammatory mediators and interferons in response to stimulation by their ligands (Dejucq et al., 1998; Rodrigues et al., 2008; Winnall et al., 2011a). In fact, evidence suggests that these inflammatory signalling pathways are involved in regulation of normal physiological process in the testis, in addition to mediating defence against infection (Hedger, 2011b). Nonetheless, excessive activation of inflammation and production of inflammatory cytokines, eicosanoids and reactive oxygen species by the somatic cells, as well as by the circulating and resident peripheral leucocytes, disrupts testicular and epididymal function, because they also have direct inhibitory effects on the activity of the somatic cells and spermatogenic cells in these tissues (Hedger, 2011a).
Non-infectious inflammation and autoimmune disease of the testis and epididymis
Non-infectious inflammation of the human testis and epididymis
Autoimmune disorders of the human testis and epididymis have been documented (Chan and Schlegel, 2002a; Silva et al., 2014). Patients suffering autoimmune polyendocrinopathy syndrome 1 due to inactivating mutations of the AIRE gene develop testicular failure and sperm autoantibodies in association with multi-organ autoimmune disease in 30% of cases (Kisand and Peterson, 2011). Moreover, systemic autoimmune disorders, such as lupus erythematosus and different forms of systemic vasculitis including Behcet’s disease, may involve blood vessels of the testis, epididymis, and excurrent ducts, thus resulting in deleterious local inflammatory disease (Nistal and Paniagua, 1997; Silva et al., 2014). Granulomatous orchitis mimicking testicular cancer may occur as a chronic, painless disease in elderly men (Mikuz and Damjanov, 1982). The aetiology of this rare inflammatory disorder is unknown, but germ cell-specific autoimmunity has been discussed as an underlying mechanism. Moreover, manifestation of sarcoidosis as a sterile granulomatous disease was shown in the testis and epididymis (Hedinger, 1991). ‘Post-traumatic’, chronic inflammatory reactions have been observed after herniotomy in both ipsi- and contralateral testes and interpreted as autoimmune orchitis (Hofmann and von Zezschwitz, 1977; Suominen, 1995). An elevated risk of testicular pain, interpreted as ‘orchitis/epididymitis’, has also been reported after hernia repair and vasectomy (Hawn et al., 2006; Goldacre et al., 2007; Horovitz et al., 2012). Notably, pre-existent testicular disorders of either intrinsic or unknown origin may be accompanied by inflammation (Table III, Table IV). In testes from adult men who have undergone orchiectomy due to cryptorchidism, focal inflammatory infiltrates containing mainly T cells and related tubular damage in 44% of the specimens have been found (Nistal et al., 2002). Finally, both acute and chronic inflammatory conditions of the testis and/or epididymis caused by drugs or other chemical compounds have to be considered (Schuppe et al., 2008; Hedger, 2011a; Pilatz et al., 2015b) (Table I). Human autoimmune orchitis or epididymitis, however, have been underestimated as clinical entities and are not established in clinical andrology. From a rheumatologist’s point of view, Silva et al. (2014) proposed a concept of autoimmune orchitis primarily based on the detection of membrane-bound antisperm antibodies (ASA) in semen. This phenomenon, however, is not necessarily reflecting breakdown of the testicular immune privilege, but rather related to immunopathological changes in the epididymis (see below; Fig. 5). Although hampered by the very limited access to biopsy material, delineating human autoimmune orchitis requires tissue-based analyses.
Table IV.
Testicular inflammatory reactions in infertile men: correlations between clinical findings, degree of damage of the seminiferous epithelium and the prevalence of peritubular lymphocytic infiltrates.
| Testicular disorders | Obstruction | Unknown etiology | Congenital/early acquired disorder | Sertoli-cell-only syndrome# | Inflammatory reaction+ |
|---|---|---|---|---|---|
| n = 17 | n = 106 | n = 77 | n = 27 | n = 33 | |
| Total testicular volume (ml) | 40.7 ± 5.0 | 35.4 ± 8.4 | 31.8 ± 7.8 | 26.8 ± 7.0 | 33.3 ± 8.2 |
| Serum FSH (IU/l) | 4.0 ± 2.3 | 6.7 ± 3.4 | 7.0 ± 4.8 | 13.5 ± 5.7 | 4.3 ± 5.2 |
| Mean Johnsen score§ | 8.6 ± 0.3 | 7.2 ± 1.6 | 6.2 ± 1.9 | 2.3 ± 0.8 | 6.3 ± 2.2 |
| Prevalence of peritubular lymphocytic infiltrates (%)$ | 11.8 | 19.8 | 31.2 | 51.6 | 84.9 |
*Retrospective analysis of testicular biopsies obtained from 260 asymptomatic men undergoing diagnostic work-up for infertility; data are mean values ± SD; modified from Schuppe et al. (2001).
§Modified according to de Kretser and Holstein (1976).
$Focal or multifocal; with or without perivascular infiltrates (cell density ranging from scattered to extensive).
#Heterogeneous subgroup, comprising both congenital and acquired forms.
+Considered as ‘primary’ pathology in the testis, in contrast to concomitant (‘secondary’) inflammatory reactions in the other subgroups.
Considering non-infectious inflammation of the human testis, it should be mentioned, that seminoma is almost invariably associated with extensive inflammatory infiltrates, suggesting immune activation induced by the neoplastic process (Hvarness et al., 2013; Klein et al., 2016). Lymphocytic infiltrates are also observed around seminiferous tubules containing testicular germ cell (TGC) neoplasia in situ (cells or in the contralateral testis accompanying unilateral neoplasia (Jahnukainen et al., 1995; Bols et al., 2000; Klein et al., 2016).
Inflammatory lesions of unknown origin in testes of infertile men
In early studies dealing with testicular biopsies obtained from infertile men, inflammatory infiltrates have been reported in 4.8–16.6% of cases (Hofmann and Kuvert, 1979; Suominen and Soderstrom, 1982; Jahnukainen et al., 1995). A systematic re-examination of tissue specimens obtained from asymptomatic patients with impaired fertility, i.e. non-obstructive azoospermia, showed immune cell infiltrates in the interstitial compartment in ~30% of cases (Schuppe et al., 2001) (Table IV). The infiltrates, graded as sparse to dense, mainly comprised lymphocytes and showed a peritubular localization distributed in a focal or multifocal pattern. In addition, the degree of lymphocytic infiltration was correlated with characteristic signs of tubular damage, such as partial or complete loss of germinal epithelium, thickening of the lamina propria and complete tubular fibrosis (Schuppe and Bergmann, 2013) (Fig. 3B and C). Despite the patchy distribution of the lesions, testicular inflammatory reactions are associated with significantly reduced testicular volume and score counts for spermatogenesis, when inflammation represents the primary disorder (Table III). Serum FSH levels are not markedly increased in these cases compared to patients with testicular obstruction and preserved spermatogenesis. In patients with other testicular disorders, the occurrence of peritubular lymphocytic infiltrates is closely correlated with the degree of tubular damage, i.e. impairment of spermatogenesis. With regard to the high overall prevalence of inflammatory lesions, induction of deleterious immune responses in the testis is probably not restricted to infectious agents, but a wide spectrum of etiological factors should be considered (Schuppe and Meinhardt, 2005) (Table I).
Formation of ASA and male infertility
Among men referred for infertility treatment, 4–6% are diagnosed with membrane-bound ASA (Alexander and Anderson, 1979; Mazumdar and Levine, 1998; Chamley and Clarke, 2007; Tüttelmann and Nieschlag, 2010). However, the association of ASA formation with male genital tract infection/inflammation remains a matter of ongoing debate. One prospective study investigated ASA in patients suffering epididymitis, during acute disease as well as after 3 years, and showed increased serum ASA titres in 7/26 patients (Ingerslev et al., 1986). On the other hand, in patients with primary infertility, significantly increased levels of ASA in blood and semen were associated with a history of epididymitis/orchitis (Tchiokadze and Galdava, 2015). In contrast, there is little evidence for a close relationship between the detection of ASA in semen and MAGI (Marconi et al., 2009; Francavilla and Barbonetti, 2017).
Although ASA development could be suspected as a sequela of testicular inflammatory reactions, such as Mumps orchitis, available studies did not reveal a significantly increased prevalence of positive ASA titres in these patients after more than 1 year after diagnosis, except in idiopathic granulomatous orchitis (Shulman et al., 1992; Kalaydjiev et al., 2002).
Animal models of autoimmune-based testicular inflammation
Experimental autoimmune orchitis
Experimental autoimmune orchitis (EAO) serves as a model of autoimmune-based chronic testicular inflammation leading to germ cell apoptosis and to severe damage of spermatogenesis and eventual infertility (Table III) (Tung et al., 1987b; Suescun et al., 1994; Tung, 1995; Tung and Teuscher, 1995; Naito et al., 2012b). The disease has been induced in many species, including guinea pigs and rabbits, whilst rats and mice have received the most attention (Freund et al., 1953; Andrada et al., 1969; Tung et al., 1970; Tung and Woodroffe, 1978; Pelletier et al., 1981; Doncel et al., 1989; Zhou et al., 1989; Itoh et al., 1991b). Classical EAO in rodents is induced by active immunization with syngeneic testicular homogenate (TH) in incomplete or complete Freund’s adjuvant (CFA) followed by injection of inactivated Bordetella pertussis (Bp) bacteria or Bp toxin (Sato et al., 1981; Kohno et al., 1983; Doncel et al., 1989) (Supplementary Table SI). The inflammation first appears in the seminiferous tubules and rete testis, and affects the cauda epididymis and vas deferens as well (Kohno et al., 1983). Macrophages, lymphocytes, eosinophils and neutrophils invade the testis and form clusters around the seminiferous tubules (and also inside the seminiferous tubules in mice), produce elevated levels of pro-inflammatory mediators and lead to spermatogenic disruption and, eventually, loss of the adluminal compartment of the seminiferous epithelium (aspermatogenesis) (Fig. 3 and Table III). Moreover, impairment of adherens and gap junction proteins in the seminiferous tubules contributes to germ cell sloughing (Table III) (Perez et al., 2011, 2012, 2014). Germ cell apoptosis in EAO is mediated by the involvement of Fas/FasL, TNF/TNF receptor 1, IL-6/IL-6 receptor and the Bax/Bcl-2 (BCL2-associated X/B-cell lymphoma 2) system (Theas et al., 2003, 2006; Rival et al., 2006b).
Later stages of the disease are characterized by disruption of the BTB, extensive necrosis and fibrosis of seminiferous tubules (Doncel et al., 1989; Lustig et al., 1993; Tung and Teuscher, 1995; Perez et al., 2012; Nicolas et al., 2017a) (Fig. 3E and F). In severe forms of the disease, granuloma formation has been observed (Fig. 3 and Table III).
Another model of EAO can be elicited by subcutaneous immunization with syngeneic viable TGC without adjuvants in susceptible A/J and C3H/He mouse strains (Sakamoto et al., 1985; Itoh et al., 1991a) (Supplementary Table SI). In classical EAO, an autoimmune response is generated against antigens of haploid germ cells, spermatogonia, Sertoli cells, Leydig cells and the basal lamina of the seminiferous tubules, causing complete loss of germ cells (Sato et al., 1981; Lustig et al., 1982; Adekunle et al., 1987; Tung et al., 1987b; Yule et al., 1988; Teuscher et al., 1994; Fijak et al., 2005) while in TGC-elicited EAO autoimmunity is induced only against antigens of haploid germ cells (Itoh et al., 1994; Qu et al., 2010; Hirai et al., 2013; Terayama et al., 2016). In contrast to classically induced EAO, in TGC-elicited orchitis the seminiferous tubules are not depleted of all germ cells and the inflammation does not affect the epididymis and vas deferens (Tung et al., 1987b; Naito et al., 2012a).
The differences in the development, course and severity of EAO between classical and TGC-induced disease models point to a significant influence of microbial components present in adjuvants and B. pertussis on inflammatory responses in the testis and epididymis. The use of CFA and Bp bacteria, in combination with TH, to induce EAO evokes more severe autoimmune reactions compared to the TGC-induced disease (Musha et al., 2013) (Supplementary Table SI). Adjuvants are generally employed to enhance the inflammatory response during induction of organ-specific autoimmunity, e.g. autoimmune encephalomyelitis (EAE), uveitis or arthritis (Billiau and Matthys, 2001). New data indicate that the effects are specific, as the susceptibility to the induction of EAE and EAO in mice is associated with a locus controlling Bordetella pertussis-induced histamine sensitization (Bphs) identified as histamine receptor H1, an autoimmune disease-associated locus (Sudweeks et al., 1993; Ma et al., 2002). Furthermore, a locus Orch3 located on chromosome 11 and controlling dominant resistance to autoimmune orchitis was identified as kinesin family member 1C (del Rio et al., 2012). Notably, immunogenetically autoimmune orchitis, epididymitis and vasitis seem to be distinct lesions (Roper et al., 1998).
Spontaneous experimental orchitis
In addition, unique EAO models can be produced by experimental manipulation of systemic immune regulation, as in day 3 thymectomy (Taguchi and Nishizuka, 1987; Tung et al., 1987a), mice with deletions of the tolerance-regulating gene Aire (Anderson et al., 2002) and mice with Treg cell depletion (Tung et al., 2017). Several reports have shown spontaneous occurrence of orchitis in mink (Tung et al., 1981), dog (Fritz et al., 1976) and brown Norway rat (Furbeth et al., 1989). Notably, rats that are transgenic for human-β2-microglobulin and HLA subtype B27, a genetic locus strongly associated with ankylosing spondylitis, spontaneously develop epididymo-orchitis. In fact epididymo-orchitis is preceding arthritis in this model (Taurog et al., 2012). EAO can be also transferred to naïve recipients by adoptive transfer of lymphocytes from lymph nodes or spleens of EAO mice (Mahi-Brown et al., 1987; Itoh et al., 1992).
Several studies have uncovered the potential aetiology of spontaneous EAO, and provided insight into the nature of systemic tolerance for the relevant pathogenic antigens (Tung and Lu, 1991; Samy et al., 2006). Because some meiotic germ cell antigens can egress the normal seminiferous tubule, and they are protected by Treg in normal mice, the concept of complete antigen sequestration is no longer valid (Tung et al., 2017). Finally, other studies have revealed the influence of non-immune mechanisms on EAO development. For example, abnormal hypothalamic–pituitary axis function predisposes the mink to EAO (Tung et al., 1981). Defects in hypothalamic function may affect Sertoli cell barrier integrity (Xia et al., 2009) and orchitis in the mink can be rescued by treatment with hCG to stimulate Leydig cell function (Tung et al., 1984). Similarly, defective Sertoli cell barrier properties and spontaneous EAO have been reported in mice with Sertoli cell-specific deletion of the androgen receptor (Meng et al., 2011).
Immunopathology of EAO
As shown by adoptive transfer experiments, CD4+ T cells play a crucial role in the induction of EAO (Mahi-Brown et al., 1987). Analysis of testicular inflammatory infiltrates revealed increased numbers of several T cell subsets, macrophages, dendritic cells (DC) and mast cells in EAO in the rat (Fig. 3 and Table III). During the onset of rat EAO, a dramatic increase in CD4+ and CD8+ T effector cell numbers producing pro-inflammatory cytokines (TNF, interferon-γ, IL-17), which are commonly associated with inflammatory and autoimmune responses, was observed (Table III). In contrast, in the chronic phase of the disease, the CD8+ T cell subset was predominant, suggesting its involvement in the progression of the inflammatory process (Guazzone et al., 2009). Interestingly, in our mouse model of EAO, highly elevated numbers of CD4+ T cells, while reduced numbers of CD8+ T cells were detected, confirmed by a higher ratio of CD4+/CD8+ T cells in the testis. Moreover, a new population of double positive CD4+CD8+ T cells was identified in mouse EAO testis, previously identified in different organs with autoimmune disorders (Nicolas et al., 2017a). Although, the increased accumulation of various immunoregulatory T cell subtypes, such as CD4+CD25+Foxp3+, CD4+Foxp3+ and CD8+Foxp3+ T cells, has been reported in chronically inflamed rat testes, these cells were not able to suppress inflammatory responses generated by the effector T cells during the onset of EAO (Guazzone et al., 2009; Fijak et al., 2011). Interestingly, supplementation of the reduced testosterone levels in EAO animals caused an expansion of Treg cells leading to increased representation of these cells within the CD4+ T cell subset, while simultaneously inhibiting the synthesis of pro-inflammatory mediators MCP-1, TNF and anti-inflammatory IL-10 (Fijak et al., 2011). Further studies confirmed a direct influence of testosterone on the expansion of Treg cells mediated by interaction of the androgen receptor with the transcription factor Foxp3, which is the master regulator of Treg cell function (Fijak et al., 2011, 2015; Walecki et al., 2015).
Mast cells are crucial effector cells, not only for the development of allergic and parasitic diseases but also in the development of autoimmunity (Benoist and Mathis, 2002). In the rat model of EAO, mast cell numbers were significantly upregulated, widely distributed throughout the interstitium and partially degranulated (Fig. 5) (Iosub et al., 2006). Mast cell tryptase activates proteinase-activated receptor-2 (PAR-2), which is expressed on macrophages, peritubular cells and spermatids in normal testis. In orchitis the expression of PAR-2 was increased. In vitro activation of PAR-2 on peritubular cells by tryptase led to expression of inflammatory mediators, MCP-1, cyclooxygenase-2 and transforming growth factor-β2 (Iosub et al., 2006). These data suggest that PAR-2 activation elicited on peritubular cells by mast cell tryptase contributes to acute testicular inflammation.
Along with T cells and mast cells, antigen presenting cells (APC), such as macrophages and dendritic cells, possess a decisive function during the development of EAO (Table III). The presentation of self-antigens by APC to T and B cells is crucial in the initiation and maintenance of tolerance or autoimmunity. In the rat model of EAO, the number of testicular macrophages and dendritic cells was significantly increased during the course of the disease (Fig. 5 and Table III) (Rival et al., 2008, 2006a; Guazzone et al., 2011). Macrophages in EAO testes were intricately involved in the production of the inflammatory mediators TNF, IL-6, MCP-1 and NO (Guazzone et al., 2003; Suescun et al., 2003; Rival et al., 2006b; Jarazo-Dietrich et al., 2012). Our analysis of purified DC from EAO rat testes demonstrated significantly upregulated expression of the chemokine receptor CCR7, which is responsible for the migration of DC to the draining lymph nodes (Rival et al., 2007). Moreover, the expression of IL-10 and IL-12p35 transcripts was detectable only in DC from inflamed testes, pointing to a mature immunogenic state before imminent migration to the lymph nodes. Interestingly, the expression levels of co-stimulatory molecules (CD80, CD86) and MHC II were similar in EAO and control testis (Rival et al., 2007). Further analysis of dendritic cells in testicular draining lymph nodes from EAO rats showed similar findings suggesting that the DC in draining lymph nodes from rats with orchitis are mature, present antigens to T cells and stimulate an autoimmune response against testicular antigens, thus causing immunological disturbances of the testis (Guazzone et al., 2011). A pathogenic role of macrophages and DC in EAO development was additionally confirmed by in vivo depletion of these cells in rats with EAO, using clodronate-containing liposomes, leading to significantly decreased disease incidence and severity (Rival et al., 2008). The involvement of TLR2 and TLR4 in mediating EAO was also indicated by the reduced disease susceptibility in transgenic Tlr2−/− or Tlr4−/− mice (Liu et al., 2015).
Chemokines, chemokine receptors and adhesion molecules are implicated in the recruitment, trafficking and activation of leucocytes to the site of inflammation in EAO. Upregulation of cell adhesion molecules (CD31, CD44, CD106), in conjunction with increased levels of chemokines (MCP-1, macrophage inflammatory proteins 1α and 1β) and chemokine receptors (CCR2, CCR5), contribute to the formation of a chemotactic gradient within the testis, causing the leucocyte infiltration that is characteristic of EAO histopathology (Figs 3 and 5, Table III) (Guazzone et al., 2012, 2003, 2005). Besides cytokines and chemokines, other pro-inflammatory molecules, such as high mobility group box protein 1 (HMGB1), are involved in the regulation of inflammatory reactions in rat and human testis (Table III). Elevated levels of HMGB1 have been reported in the late phase of rat EAO. Moreover, HMGB1 was translocated from the nuclei to the cytoplasm and extracellular space in testicular cells in EAO. Blockade of HMGB1 release by ethyl pyruvate in EAO rats animals reduced disease progression and spermatogenic damage (Aslani et al., 2015). Furthermore, involvement of galectin-1, activins and inhibin in the development of testicular immunopathology is also documented (Suescun et al., 2001; Perez et al., 2015; Lei et al., 2017; Nicolas et al., 2017a, 2017b).
Linking autoimmune orchitis models to human disease
The histopathology of post-infectious or non-infectious human orchitis, as well as focal inflammatory lesions encountered in testicular biopsies from infertile patients with post-infectious testicular failure or ‘mixed atrophy’ of spermatogenesis of unknown origin, intriguingly resemble those developing in rodent EAO (Suominen and Soderstrom, 1982; Schuppe et al., 2008) (Fig. 3B, C and E, F; Tables III and IV). The predominantly peritubular localization of lymphocytes and characteristic morphological changes of the seminiferous tubules such as ‘aspermatogenesis’ support the concept that concomitant activation of autoreactive T cells is involved in inflammatory disorders of the human testis (Table III and Fig. 3). In early clinical experiments, delayed-type hypersensitivity reactions to sonicates prepared from human spermatozoa could be elicited in patients with mumps orchitis (Andrada et al., 1977). Moreover, immunization with testis homogenate in CFA performed before orchidectomy for treatment of prostate carcinoma led to testicular lesions characteristic of EAO in two of four patients tested (Mancini et al., 1965). Testicular biopsies revealed focal interstitial infiltrates with mononuclear cells, thickening of the lamina propria, and depopulation of the seminiferous epithelium. Comparable to rodent models, progressive tubular atrophy eventually results in a Sertoli cell-only syndrome and/or complete hyalinization of seminiferous tubules (Schuppe et al., 2008; Naito et al., 2012b; Aslani et al., 2015).
In line with data from EAO models, the infiltrating immune cells in focal inflammatory lesions in testes of infertile men are predominantly activated CD4+ and CD8+ T cells, which are accompanied by increased numbers of non-resident CD68+ macrophages and mast cells (el-Demiry et al., 1987; Duan et al., 2011; Schuppe and Bergmann, 2013; Klein et al., 2016) (Fig. 5). For non-resident CD68+ macrophages and mast cells, a shift from the interstitium to the seminiferous tubules was also reported for other testicular pathologies such as ‘mixed atrophy’ and has been associated with tissue remodelling and fibrotic changes (Meineke et al., 2000; Frungieri et al., 2002a; Nicolas et al., 2017a). Similar to rat EAO, increased numbers of mast cells expressing tryptase and PAR-2 were found in human testicular fibrosis (Meineke et al., 2000; Frungieri et al., 2002b). Moreover, there is circumstantial evidence that DC are involved in inflammatory disorders of the human testis (Wang and Duan, 2016).
Identification of similar putative auto-antigens involved in the autoimmune attack in rat and human inflamed testes underlines the essential significance of results obtained from animal models. Autoantibodies against heat shock protein (Hsp) 60 and Hsp70, disulphide isomerase ER-60, alpha-1-anti-trypsin, heterogeneous nuclear ribonucleoprotein H1, sperm outer dense fibre major protein 2, and phosphoglycerate kinase 1 were identified in sera from EAO rats (Fijak et al., 2005). Significantly, elevated titres of autoantibodies against disulphide isomerase ER-60 could also be detected in sera from infertile azoospermic patients with histologically confirmed low-grade testicular inflammation (Fijak et al., 2014). Accordingly, determination of ER-60 autoantibody titres in serum could be a novel non-invasive marker. As focal inflammatory lesions of unknown aetiology, usually diagnosed using testicular biopsies, are much more frequent than isolated orchitis, non-invasive methods for diagnosis of early inflammatory events in the testis are needed. In this regard, markers such as ER-60 autoantibody titres originally found in EAO and later confirmed to be potentially valuable for the diagnosis of asymptomatic testicular inflammation resulting in male fertility disturbances in men as well, are currently being tested on a broader scale for the diagnosis of asymptomatic testicular inflammation causing male fertility disturbances. Moreover, the development of reliable assays for quantitative determination of serum autoantibodies directed to cell membrane and internal antigens of spermatozoa as reliable markers of an autoimmune state is critical. Participation of autoantibodies in the development of EAO in mice was supported by the formation of immune complexes of IgG and complement C3 localized outside of the seminiferous tubules and in the thickened tubular basement membrane (Kohno et al., 1983; Yule et al., 1988). Persistence of immunoglobulin and complement deposits in conjunction with a thickened basement membrane have also been described in testis samples from infertile men with spermatogenic disturbances (Jadot-Van De Casseye et al., 1980; Salomon et al., 1982; Lehmann et al., 1987).
The EAO models offer an adequate in vivo system to study the complexity of interactions of testicular cell types (germ cells, somatic cells, immune cells) in context of the endocrine environment, which can heavily influence the immune response (Figs 3, 4 and Table III). Particularly the early stages of EAO development closely reflect the lesions seen in focal inflammatory infiltrates that are frequently observed in testicular biopsies of patients with ‘mixed atrophy’ of spermatogenesis. This is also the stage where experimental therapies, such as new biologicals modulating cytokine action, can be explored. The development of EAO in rodents, with progressively later stages of tubular atrophy, strong immune cell infiltration, hyalinization and loss of germ cells leading to Sertoli cell-only syndrome mirrors only a minority of cases found in men. Although the structure of the immune system in mice and human is similar, some discrepancies in both innate and adaptive immunity response are observed (reviewed in (Mestas and Hughes, 2004). Therefore, it is important to consider the possibility that the pathological reactions occurring in a mouse testis may not reflect precisely the mechanisms playing a role in a human testis.
Disadvantages of the rodent EAO model in relation to the common forms of human focal orchitis include its deteriorating progressive nature, an observation rarely made in men. Moreover, the rodent model is elicited using germ cell antigens in the form of testicular homogenates together with adjuvants to break tolerance or isolated native germ cells, whilst in human the cause of the focal inflammatory damage is completely unknown. In fact, in men it is even unclear if the damage observed is possibly a consequence of autoimmunity at all or rather a reflection of a different primary cause with only secondary involvement of the immune system. Therefore, a caution in the interpretation of data obtained from rodent models should be warranted.
Immunopathological sequelae of vasectomy
Induction of autoantibodies against spermatozoa is a frequent complication of vasectomy in man and animals (Bigazzi, 1981; Adams and Wald, 2009; Lustig et al., 2014). Vasectomy in men produces autoantibodies to sperm antigens at a prevalence of 60–70% at 5–6 months after vasectomy (Adams and Wald, 2009). Whether the autoimmunity to sperm antigens can also trigger epididymal pathology remains unknown as epididymal biopsy is not indicated, but this issue may gain relevance in cases of re-fertilization by vasovasostomy (Francavilla and Barbonetti, 2017). Furthermore, a possible autoimmune basis for focal orchitis seen in some vasectomized men and, more frequently, in patients with azoospermia due to other causes (Table IV) has not been delineated in detail. In this context, animal models of EAO have been used to provide insights into the mechanisms of initiation, progression and timing of autoimmune reactions of the testis, and its genetic control (Wheeler et al., 2011). A recent study focused on the first 10 weeks post-vasectomy, using unilateral vasectomized inbred mice (Wheeler et al., 2011; Rival et al., 2013). Epithelial cell apoptosis and necrosis occurred in the cauda epididymis within 24 h, followed in 80% of these mice by sperm leakage and granuloma formation. Most epididymal granulomata in this mouse model were microscopic in size and as such may evade detection by palpation in vasectomized men. Nevertheless, an increased epididymal size after vasectomy is well known (Cho et al., 2011) and an epididymal head diameter >10.25 mm suggests obstruction (Pezzella et al., 2014). Nonetheless, timing of detection of the autoimmune response is relevant, as all the sequelae in vasectomized mice are preventable by surgical resection of the testis and epididymis on the ipsilateral (vasectomized) side within the first 3 weeks after surgery (Wheeler et al., 2011). The finding raises the question of whether a short immunosuppression regime around the time of vasectomy may reduce this early response and reduce the development of harmful late responses to vasectomy.
It was long assumed that the first contact of the immune system with neoantigens on meiotic and postmeiotic cells in the male occurs in the epididymis, as evidenced by the presence of intraluminal leucocytes next to spermatozoa and possibly extensions of DC reaching the lumen, at least in the caput epididymis (Da Silva et al., 2011). Hence, mechanisms must be in place to prevent autoimmunity. A shift in our understanding of the mechanism of local testicular immune privilege and systemic tolerance to meiotic and postmeiotic germ cell antigens was recently derived by two studies (Wheeler et al., 2011; Tung et al., 2017). Obviously, a differentiation between antigens that are sequestered or non-sequestered from the immune system by the BTB exist. The physical barrier of the BTB can be by-passed by antigens from meiotic or postmeiotic germ cells, such as lactate dehydrogenase 3 (LDH3), via phagocytosis of residual bodies, subsequent cargo transfer to the basis of Sertoli cells and egress to the interstitial space, where they get in contact with local immune cells and can be further transported as processed peptides to draining lymph nodes (non-sequestered antigens). In contrast, other antigens of meiotic and postmeiotic germ cells, such as zonadhesin (Zan), do not egress the seminiferous tubules (Tung et al., 2017). To support this conclusion, it was found that mice with Treg cell depletion alone spontaneously produced antibodies against LDH3 but not against Zan. On the other hand, following vasectomy, where all sperm antigens are released from the injured epididymal ducts, the mice produced antibodies to Zan but not to LDH3 (Wheeler et al., 2011). This indicates, that the immune protection for meiotic and postmeiotic germ cell antigens hinges on the following mechanism: systemic tolerance continuously maintained by egressed (non-sequestered) antigens finally reaching peripheral lymphoid organs to stimulate antigen-specific tolerance involving Treg cells, and local mechanisms including the BTB that protect sequestered antigens such as Zan and control damage to germ cells in orchitis (Wheeler et al., 2011; Tung et al., 2017).
Treg cells strongly influence the autoimmune responses to meiotic and postmeiotic germ cell antigens in vasectomized mice. In this regard, unilateral vasectomy, which strongly exposes spermatozoal neoantigens to the local immune system, rendered mice unresponsive to later induction of EAO using standard injection of testicular homogenates, as it promotes tolerance by induction of testis antigen specific Treg cells within 7 days (Rival et al., 2013). It is therefore not surprising that Treg cell depletion concomitant to unilateral vasectomy resulted in the development of autoimmune orchitis. Of note, the autoantibodies elicited in this model were directed only to a restricted number of meiotic and postmeiotic germ cell neoantigens, with Zan located in the sperm acrosome as a prominent target (Wheeler et al., 2011). Obviously, Treg cell responses only manifest when sperm granuloma are formed in vasectomy. Then the sequestered sperm antigens egress the damaged epididymal epithelium and can stimulate a Treg cell response that causes the initial tolerance state.
The results also raise a much broader clinical question, i.e. whether the state of persistent tolerance to germ cell neoantigens in vasectomized mice can be extrapolated to the response to the molecules known as cancer/testis antigens that are expressed as human cancer antigens (Simpson et al., 2005). In this regard, it is relevant to examine if the post-vasectomy tolerogenic response could interfere with: immune surveillance against nascent tumour development in some cancers in vasectomized men; the strength of tumour immunity that may impact clinical outcome; and/or the immunogenicity of male germ cell neoantigens as a tumour vaccine. The need for further study is underlined by the observed higher rate of tumour development in long-term vasectomized mice (Anderson et al., 1983). Early reports on an increased tumour incidence among vasectomized men (Mettlin et al., 1990; Rosenberg et al., 1990; Eisenberg et al., 2015), however, have not been confirmed, in contrast to an increased overall risk of cancer, including testicular tumours, in infertile patients (Eisenberg et al., 2015). Interestingly, in a database analysis comparing 23 988 males with previous vasectomy to a reference cohort of 146 040 males, the incidence of immune-related diseases was not significantly different between both groups after a mean follow-up of 13 years (Goldacre et al., 2007). In addition, for cancer vaccine development, the sequestered sperm antigens should be more efficacious than the non-sequestered, and tolerogenic sperm antigens.
Despite the early tolerance response, 70–90% of the vasectomized mice have low titres of antisperm antibodies 6–7 months later, consistent with clinical observations (Rival et al., 2013).
It is critical to emphasize that the most serious observable sequelae of vasectomy in mice, by far, is the severe interstitial fibrosis in the epididymis, and the severe degree of hypospermatogenesis at 12 months post-vasectomy (Wheeler et al., 2011; Rival et al., 2013). In contrast to the acute bacterial epididymitis model mentioned above (Michel et al., 2016), these changes are not an immunological sequel. They are caused by the vasoligation per se as they are confined to the ipsilateral epididymis and testis of the unilaterally vasectomized mice (Rival et al., 2013). The severity of fibrosis suggests that the change is irreversible in mice. A critical investigation on these changes in vasectomized men with epididymal complaints or desire for re-fertilization surgery by vasovasostomy may be informative.
This advancement of understanding at the testicular level points to much needed research on the possible involvement of the epididymis, where solid evidence is mostly lacking.
Conclusions and future perspectives
In summary, infection and inflammation both represent relevant entities in male factor infertility (Fig. 5). In this regard, bacterial epididymitis and epididymo-orchitis represent the most frequent aetiology of diseases related to the epididymis and are reasonably well reflected by the corresponding animal rodent models in terms of possible application of pathovars relevant for human epididymitis, course of disease, and histopathology observed. Generally, beside many striking parallels caution in extrapolating rodent data to the human are derived from obvious differences in innate and adaptive immune responses between human and rodents, particularly those directed against pathogenic microorganisms. In human blood defence, it seems that strategies against pathogens dominate, while in mouse tolerance against pathogens is more pronounced (Zschaler et al., 2014). In the human neutrophils are particularly abundant in the blood (50–70%), whereas in the mouse there is a preponderance of lymphocytes (75–90%). Further differences have been reported for TLRs, cytokines and their receptors as well as T cell subsets, to name only a few examples (Zschaler et al., 2014). Such differences need to be considered when using rodents as surrogates for human. New humanized mouse models may overcome some of these obstacles. As an example, the human lymphocyte compartment has at least been partially reconstituted in mice by transferring human hematopoietic cells. Moreover, organoids from human foetal liver (from which leucocyte progenitors arise) or thymus have been transplanted to mice enabling the study of human pathogen infection and immune control (Ramer et al., 2011). Although not applied yet in testicular or epididymal research, humanized mouse models can serve as tools to examine immune control and combat of infection together with new clinical treatment regimens, such as biological, as possible means to preserve fertility in men.
In sterile inflammatory damage of the testis, which is mostly focal and patchy in nature in men, EAO elicited in mouse and rat is the most prominent model. Although similar in the early phase of EAO (30–50 days post induction), its progressively deteriorating nature differs after longer observation periods (>80 days) substantially from most biopsy observations. Currently, inflammatory lesions in the testis of asymptomatic infertile men are only detected with biopsy assessment (Table III). Evidence suggests that more frequently ‘silent’, low-grade human autoimmune orchitis—so far ill-defined as ‘idiopathic’ male infertility—would be found if better non-invasive diagnostic methods of the disease were available. The putative use of detection of ER-60 as an autoantigen has been derived from animal experiments and was confirmed by a pilot study using a small cohort of well characterized patients (Fijak et al., 2005, 2014). However, final confirmation of use as a non-invasive diagnostic, sparing biopsies, is still pending. Together with a lack of information in the literature, biopsy assessment—at least currently—thus, remains the method of choice for the detection of inflammation-associated damage in the human testis.
Although clinical and basic science research has provided a great amount of information many important aspects still need to be elucidated. The many questions raised in this review will hopefully guide future combined clinical and basic science research to better address the diagnosis and treatment of immunological and infection-related infertility in men.
Supplementary Material
Acknowledgements
The authors thank Dr Karen Wheeler and Dr Claudia Rival for their seminal contributions to this review.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors’ roles
A.M., H.-C.S. and M.F. were involved in the conception and design of this review. All authors contributed to the drafting of the article. A.M., H.-C.S., M.F. and M.H. were responsible for the final editing and approval of the article.
Funding
NIH (Grant RO1 AI 41236) and a grant of the Deutsche Forschungsgemeinschaft (DFG, BH93/1-1). The support of the DFG and Monash University to the International Research Training Group between Justus Liebig University of Giessen and Monash University, Melbourne (GRK 1871/1–1871/2) on ‘Molecular pathogenesis on male reproductive disorders’ is gratefully acknowledged. The support of the Victorian Government’s Operational Infrastructure Support Program to the Hudson Institute is also acknowledged. M.F., A.P., H.-C.S. and A.M. were supported by the LOEWE focus group ‘MIBIE’ (Male Infertility during Infection & Inflammation), an excellence initiative of the German State Government of Hessen.
Conflict of interest
The authors declare that no competing interests exist.
References
- Adamopoulos DA, Lawrence DM, Vassilopoulos P, Contoyiannis PA, Swyer GI. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br Med J 1978;1:1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CE, Wald M. Risks and complications of vasectomy. Urol Clin North Am 2009;36:331–336. [DOI] [PubMed] [Google Scholar]
- Adekunle AO, Hickey WF, Smith SM, Tung KS, Teuscher C. Experimental allergic orchitis in mice: IV. Preliminary characterization of the major murine testis specific aspermatogenic autoantigen(s). J Reprod Immunol 1987;12:49–62. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Bello A, Mbibu NH, Maitama HY, Kalayi GD. Epidemiological and aetiological factors of male infertility in northern Nigeria. Niger J Clin Pract 2010;13:205–209. [PubMed] [Google Scholar]
- Albrecht M, Frungieri MB, Gonzalez-Calvar S, Meineke V, Kohn FM, Mayerhofer A. Evidence for a histaminergic system in the human testis. Fertil Steril 2005;83:1060–1063. [DOI] [PubMed] [Google Scholar]
- Alexander NJ, Anderson DJ. Vasectomy: consequences of autoimmunity to sperm antigens. Fertil Steril 1979;32:253–260. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Alexander NJ, Fulgham DL, Palotay JL. Spontaneous tumors in long-term—vasectomized mice. Increased incidence and association with antisperm immunity. Am J Pathol 1983;111:129–139. [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C et al. . Projection of an immunological self shadow within the thymus by the aire protein. Science 2002;298:1395–1401. [DOI] [PubMed] [Google Scholar]
- Andrada JA, Andrada EC, Witebsky E. Experimental autoallergic orchitis in rhesus monkeys. Proc Soc Exp Biol Med 1969;130:1106–1113. [DOI] [PubMed] [Google Scholar]
- Andrada JA, von der Walde F, Hoschoian JC, Comini E, Mancini E. Immunological studies in patients with mumps orchitis. Andrologia 1977;9:207–215. [DOI] [PubMed] [Google Scholar]
- Andrade-Rocha FT. Temporary impairment of semen quality following recent acute fever. Ann Clin Lab Sci 2013;43:94–97. [PubMed] [Google Scholar]
- Aslani F, Schuppe HC, Guazzone VA, Bhushan S, Wahle E, Lochnit G, Lustig L, Meinhardt A, Fijak M. Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis. Hum Reprod 2015;30:417–431. [DOI] [PubMed] [Google Scholar]
- Banyra O, Shulyak A. Acute epididymo-orchitis: staging and treatment. Cent Eur J Urol 2012;65:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt CLR, Bjorndahl L, De Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, Oates RD, van der Poel S St, John B, Sigman M et al. . The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update 2017;23:660–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayasgalan G, Naranbat D, Radnaabazar J, Lhagvasuren T, Rowe PJ. Male infertility: risk factors in Mongolian men. Asian J Androl 2004;6:305–311. [PubMed] [Google Scholar]
- Beard CM, Benson RC Jr., Kelalis PP, Elveback LR, Kurland LT. The incidence and outcome of mumps orchitis in Rochester, Minnesota, 1935 to 1974. Mayo Clin Proc 1977;52:3–7. [PubMed] [Google Scholar]
- Benoist C, Mathis D. Mast cells in autoimmune disease. Nature 2002;420:875–878. [DOI] [PubMed] [Google Scholar]
- Berger RE, Alexander ER, Harnisch JP, Paulsen CA, Monda GD, Ansell J, Holmes KK. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J Urol 1979;121:750–754. [DOI] [PubMed] [Google Scholar]
- Berger RE, Kessler D, Holmes KK. Etiology and manifestations of epididymitis in young men: correlations with sexual orientation. J Infect Dis 1987;155:1341–1343. [DOI] [PubMed] [Google Scholar]
- Bergmann M. Evaluation of testicular biopsy samples from the clinical perspective In: Schill WB, Comhaire FH, Hargreave TB (eds).. Andrology for the Clinician. Heidelberg: Springer, 2006;454–461. [Google Scholar]
- Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol 2000;12:20–26. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Hossain H, Lu Y, Geisler A, Tchatalbachev S, Mikulski Z, Schuler G, Klug J, Pilatz A, Wagenlehner F et al. . Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: implications for testicular immune privilege. PLoS One 2011;6:e28452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan S, Meinhardt A. The macrophages in testis function. J Reprod Immunol 2017;119:107–112. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Tchatalbachev S, Klug J, Fijak M, Pineau C, Chakraborty T, Meinhardt A. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J Immunol 2008;180:5537–5547. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Tchatalbachev S, Lu Y, Frohlich S, Fijak M, Vijayan V, Chakraborty T, Meinhardt A. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J Immunol 2015;194:5455–5464. [DOI] [PubMed] [Google Scholar]
- Bigazzi P. Immunologic effects of vasectomy in men and experimental animals. Prog Clin Biol Res 1981;70:461–476. [PubMed] [Google Scholar]
- Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol 2001;70:849–860. [PubMed] [Google Scholar]
- Biswas B, Bhushan S, Rajesh A, Suraj SK, Lu Y, Meinhardt A, Yenugu S. Uropathogenic Escherichia coli (UPEC) induced antimicrobial gene expression in the male reproductive tract of rat: evaluation of the potential of Defensin 21 to limit infection. Andrology 2015;3:368–375. [DOI] [PubMed] [Google Scholar]
- Bobzien B, Yasunami Y, Majercik M, Lacy PE, Davie JM. Intratesticular transplants of islet xenografts (rat to mouse). Diabetes 1983;32:213–216. [DOI] [PubMed] [Google Scholar]
- Bols B, Jensen L, Jensen A, Braendstrup O. Immunopathology of in situ seminoma. Int J Exp Pathol 2000;81:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkat G, Pickard R, Bartoletti R, Cai T, Bruyere F, Geerlings SE, Köves B, Wagenlehner F. EAU Guidelines on Urological Infections. Arnhem, The Netherlands: EAU Guidelines Office, 2018. [Google Scholar]
- Cao W, Chen W, Liang X, Zhou J, Wei C, Cui S, Liu J. All-trans-retinoic acid ameliorates the inflammation by inducing transforming growth factor beta 1 and interleukin 10 in mouse epididymitis. Am J Reprod Immunol 2014;71:312–321. [DOI] [PubMed] [Google Scholar]
- Chabon AB, Stenger RJ, Grabstald H. Histopathology of testis in acquired immune deficiency syndrome. Urology 1987;29:658–663. [DOI] [PubMed] [Google Scholar]
- Chakradhar S. Puzzling over privilege: how the immune system protects—and fails—the testes. Nat Med 2018;24:2–5. [DOI] [PubMed] [Google Scholar]
- Chamley LW, Clarke GN. Antisperm antibodies and conception. Semin Immunopathol 2007;29:169–184. [DOI] [PubMed] [Google Scholar]
- Chan PT, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part I. J Androl 2002. a;23:453–460. [PubMed] [Google Scholar]
- Chan PT, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part II. J Androl 2002. b;23:461–469. [PubMed] [Google Scholar]
- Cho SH, Min SK, Lee ST. Associations of ultrasonographic features with scrotal pain after vasectomy. Korean J Urol 2011;52:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhaire F, De Kretser D, Farley T, Rowe P. Towards more objectivity in diagnosis and management of male infertility. Int J Androl 1987;10:1–53. [Google Scholar]
- Comhaire F, Verschraegen G, Vermeulen L. Diagnosis of accessory gland infection and its possible role in male infertility. Int J Androl 1980;3:32–45. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011;141:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G, Stefanini M, Ziparo E. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: biological effects on lymphocytes. Biochem Biophys Res Commun 1992;186:1639–1646. [DOI] [PubMed] [Google Scholar]
- de Winter JP, Vanderstichele HM, Timmerman MA, Blok LJ, Themmen AP, de Jong FH. Activin is produced by rat Sertoli cells in vitro and can act as an autocrine regulator of Sertoli cell function. Endocrinology 1993;132:975–982. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Lienard MO, Jegou B. Interferons and interferon-induced antiviral proteins in the testis. J Reprod Immunol 1998;41:291–300. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev 2001;65:208–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejucq-Rainsford N, Jegou B. Viruses in semen and male genital tissues—consequences for the reproductive system and therapeutic perspectives. Curr Pharm Des 2004;10:557–575. [DOI] [PubMed] [Google Scholar]
- del Rio R, McAllister RD, Meeker ND, Wall EH, Bond JP, Kyttaris VC, Tsokos GC, Tung KS, Teuscher C. Identification of Orch3, a locus controlling dominant resistance to autoimmune orchitis, as kinesin family member 1C. PLoS Genet 2012;8:e1003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir A, Turker P, Onol FF, Sirvanci S, Findik A, Tarcan T. Effect of experimentally induced Escherichia coli epididymo-orchitis and ciprofloxacin treatment on rat spermatogenesis. Int J Urol 2007;14:268–272. [DOI] [PubMed] [Google Scholar]
- Desai KM, Gingell JC, Haworth JM. Fate of the testis following epididymitis: a clinical and ultrasound study. J R Soc Med 1986;79:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003;144:2882–2891. [DOI] [PubMed] [Google Scholar]
- Dietz O. [The change in the degree of fertility during the course of acute nonspecific epididymitis. (Contribution to the pathogenesis of primary inhibition of spermiogenesis)]. Arch Med Infant 1960;211:160–166. [PubMed] [Google Scholar]
- Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W. EAU guidelines on male infertility. Eur Urol 2005;48:703–711. [DOI] [PubMed] [Google Scholar]
- Doncel GF, Di Paola JA, Lustig L. Sequential study of the histopathology and cellular and humoral immune response during the development of an autoimmune orchitis in Wistar rats. Am J Reprod Immunol 1989;20:44–51. [DOI] [PubMed] [Google Scholar]
- Duan YG, Yu CF, Novak N, Bieber T, Zhu CH, Schuppe HC, Haidl G, Allam JP. Immunodeviation towards a Th17 immune response associated with testicular damage in azoospermic men. Int J Androl 2011;34:e536–e545. [DOI] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of sexual transmission of Zika virus. N Engl J Med 2016;374:2195–2198. [DOI] [PubMed] [Google Scholar]
- Eickhoff JH, Frimodt-Moller N, Walter S, Frimodt-Moller C. A double-blind, randomized, controlled multicentre study to compare the efficacy of ciprofloxacin with pivampicillin as oral therapy for epididymitis in men over 40 years of age. BJU Int 1999;84:827–834. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol 2015;193:1596–1601. [DOI] [PubMed] [Google Scholar]
- Eke AC, Okafor CI, Ezebialu IU. Male infertility management in a Nigerian tertiary hospital. Int J Gynaecol Obstet 2011;114:85–86. [DOI] [PubMed] [Google Scholar]
- Ekwere PD. Immunological infertility among Nigerian men: incidence of circulating antisperm auto-antibodies and some clinical observations: a preliminary report. Br J Urol 1995;76:366–370. [DOI] [PubMed] [Google Scholar]
- el-Demiry MI, Hargreave TB, Busuttil A, Elton R, James K, Chisholm GD. Immunocompetent cells in human testis in health and disease. Fertil Steril 1987;48:470–479. [DOI] [PubMed] [Google Scholar]
- Epelboin S, Dulioust E, Epelboin L, Benachi A, Merlet F, Patrat C. Zika virus and reproduction: facts, questions and current management. Hum Reprod Update 2017;23:629–645. [DOI] [PubMed] [Google Scholar]
- Fei Z, Hu S, Xiao L, Zhou J, Diao H, Yu H, Fang S, Wang Y, Wan Y, Wang W et al. . mBin1b transgenic mice show enhanced resistance to epididymal infection by bacteria challenge. Genes Immun 2012;13:445–451. [DOI] [PubMed] [Google Scholar]
- Fijak M, Iosub R, Schneider E, Linder M, Respondek K, Klug J, Meinhardt A. Identification of immunodominant autoantigens in rat autoimmune orchitis. J Pathol 2005;207:127–138. [DOI] [PubMed] [Google Scholar]
- Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol 2011;186:5162–5172. [DOI] [PubMed] [Google Scholar]
- Fijak M, Zeller T, Huys T, Klug J, Wahle E, Linder M, Haidl G, Allam JP, Pilatz A, Weidner W et al. . Autoantibodies against protein disulfide isomerase ER-60 are a diagnostic marker for low-grade testicular inflammation. Hum Reprod 2014;29:2382–2392. [DOI] [PubMed] [Google Scholar]
- Fijak M, Damm LJ, Wenzel JP, Aslani F, Walecki M, Wahle E, Eisel F, Bhushan S, Hackstein H, Baal N et al. . Influence of testosterone on inflammatory response in testicular cells and expression of transcription factor Foxp3 in T cells. Am J Reprod Immunol 2015;74:12–25. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Bush LA, Howards SS, Herr JC. Distribution of leukocytes in the epithelium and interstitium of four regions of the Lewis rat epididymis. Anat Rec 1997;248:380–390. [DOI] [PubMed] [Google Scholar]
- Fountain S, Holland MK, Hinds LA, Janssens PA, Kerr PJ. Interstitial orchitis with impaired steroidogenesis and spermatogenesis in the testes of rabbits infected with an attenuated strain of myxoma virus. J Reprod Fertil 1997;110:161–169. [DOI] [PubMed] [Google Scholar]
- Francavilla F, Barbonetti A. Male autoimmune infertility In: Krause W, Naz RK (eds).. Immune Infertility. Impact of Immune Reactions on Human Fertility. Cham, Switzerland: Springer, 2017;187–196. [Google Scholar]
- Freund J, Lipton MM, Thompson GE. Aspermatogenesis in the guinea pig induced by testicular tissue and adjuvants. J Exp Med 1953;97:711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz TE, Lombard SA, Tyler SA, Norris WP. Pathology and familial incidence of orchitis and its relation to thyroiditis in a closed beagle colony. Exp Mol Pathol 1976;24:142–158. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Calandra RS, Lustig L, Meineke V, Köhn FM, Vogt HJ, Mayerhofer A. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil Steril 2002. a;78:298–306. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: possible relevance to human fibrotic disorders. Proc Natl Acad Sci USA 2002. b;99:15072–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbeth C, Hubner G, Thoenes GH. Spontaneous immune complex orchitis in brown Norway rats. Virchows Arch B Cell Pathol Incl Mol Pathol 1989;57:37–45. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang X, Wang Y, Han F, Cai W, Zhao B, Li Y, Han S, Wu X, Hu D. Murine Sertoli cells promote the development of tolerogenic dendritic cells: a pivotal role of galectin-1. Immunology 2016;148:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite MA, Johnson G, Lloyd S, Eardley I. The implementation of European Association of Urology guidelines in the management of acute epididymo-orchitis. Ann R Coll Surg Engl 2007;89:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Immune-related disease before and after vasectomy: an epidemiological database study. Hum Reprod 2007;22:1273–1278. [DOI] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V et al. . Zika virus infection damages the testes in mice. Nature 2016;540:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow RM, O’Bryan MK, Canny BJ, Ooi GT, Hedger MP. Differential effects of dexamethasone treatment on lipopolysaccharide-induced testicular inflammation and reproductive hormone inhibition in adult rats. J Endocrinol 2001;168:193–201. [DOI] [PubMed] [Google Scholar]
- Grant JB, Costello CB, Sequeira PJ, Blacklock NJ. The role of Chlamydia trachomatis in epididymitis. Br J Urol 1987;60:355–359. [DOI] [PubMed] [Google Scholar]
- Greenfield SP. Type B Hemophilus influenzae epididymo-orchitis in the prepubertal boy. J Urol 1986;136:1311–1313. [DOI] [PubMed] [Google Scholar]
- Greskovich F, Mathur S, Nyberg LM Jr., Collins BS. Effect of early antibiotic treatment on the formation of sperm antibodies in experimentally induced epididymitis. Arch Androl 1993;30:183–191. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Rival C, Denduchis B, Lustig L. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in experimental autoimmune orchitis. J Reprod Immunol 2003;60:143–157. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Denduchis B, Lustig L. Involvement of CD44 in leukocyte recruitment to the rat testis in experimental autoimmune orchitis. Reproduction 2005;129:603–609. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech 2009;72:620–628. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Hollwegs S, Mardirosian M, Jacobo P, Hackstein H, Wygrecka M, Schneider E, Meinhardt A, Lustig L, Fijak M. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int J Androl 2011;34:276–289. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Jacobo P, Denduchis B, Lustig L. Expression of cell adhesion molecules, chemokines and chemokine receptors involved in leukocyte traffic in rats undergoing autoimmune orchitis. Reproduction 2012;143:651–662. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Huang TW, Berger RE. Experimental Escherichia coli epididymitis in rabbits. Urology 1988;32:236–240. [DOI] [PubMed] [Google Scholar]
- Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia 2008;40:92–96. [DOI] [PubMed] [Google Scholar]
- Harnisch JP, Berger RE, Alexander ER, Monda G, Holmes KK. Aetiology of acute epididymitis. Lancet 1977;1:819–821. [DOI] [PubMed] [Google Scholar]
- Hawn MT, Itani KM, Giobbie-Hurder A, McCarthy M Jr., Jonasson O, Neumayer LA. Patient-reported outcomes after inguinal herniorrhaphy. Surgery 2006;140:198–205. [DOI] [PubMed] [Google Scholar]
- Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation 1983;36:423–431. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod 1997;2:38–47. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl 2011. a;32:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation—a perspective. J Reprod Immunol 2011. b;88:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Winnall WR. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol Cell Endocrinol 2012;359:30–42. [DOI] [PubMed] [Google Scholar]
- Hedinger C. Pathologie des Hodens. Berlin: Springer, 1991. [Google Scholar]
- Hellwig B. Reproduktionsmedizinische epidemiologische Untersuchungen auf der Basis eines Integrierten Andrologischen Informationsmanagementsystems 2008. Justus-Liebig-Universität.
- Hirai S, Naito M, Terayama H, Hatayama N, Qu N, Musha M, Itoh M. Serum autoantibodies in mice immunized with syngeneic testicular germ cells alone. Am J Reprod Immunol 2013;70:509–517. [DOI] [PubMed] [Google Scholar]
- Hofmann N, Kuvert E. Chronic, nonpathogen-related orchitis. Z Hautkr 1979;54:173–180. [PubMed] [Google Scholar]
- Hofmann N, von Zezschwitz HC. Hodenatrophie und Schädigung des kontralateralen Hodengewebes nach Leistenbruchoperationen. Der Urologe B 1977;17:43–48. [Google Scholar]
- Hoosen AA, O’Farrell N, van den Ende J. Microbiology of acute epididymitis in a developing community. Genitourin Med 1993;69:361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz D, Tjong V, Domes T, Lo K, Grober ED, Jarvi K. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol 2012;187:613–617. [DOI] [PubMed] [Google Scholar]
- Houzet L, Matusali G, Dejucq-Rainsford N. Origins of HIV-infected leukocytes and virions in semen. J Infect Dis 2014;210:S622–S630. [DOI] [PubMed] [Google Scholar]
- Hvarness T, Nielsen JE, Almstrup K, Skakkebaek NE, Rajpert-De Meyts E, Claesson MH. Phenotypic characterisation of immune cell infiltrates in testicular germ cell neoplasia. J Reprod Immunol 2013;100:135–145. [DOI] [PubMed] [Google Scholar]
- Ingerslev HJ, Walter S, Andersen JT, Brandenhoff P, Eldrup J, Geerdsen JP, Scheibel J, Tromholt N, Jensen HM, Hjort T. A prospective study of antisperm antibody development in acute epididymitis. J Urol 1986;136:162–164. [DOI] [PubMed] [Google Scholar]
- Iosub R, Klug J, Fijak M, Schneider E, Frohlich S, Blumbach K, Wennemuth G, Sommerhoff CP, Steinhoff M, Meinhardt A. Development of testicular inflammation in the rat involves activation of proteinase-activated receptor-2. J Pathol 2006;208:686–698. [DOI] [PubMed] [Google Scholar]
- Itoh M, Hiramine C, Hojo K. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. I. Immunological and histological studies. Clin Exp Immunol 1991. a;83:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Hiramine C, Tokunaga Y, Mukasa A, Hojo K. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. II. Immunohistochemical findings of fully-developed inflammatory lesion. Autoimmunity 1991. b;10:89–97. [DOI] [PubMed] [Google Scholar]
- Itoh M, Hiramine C, Mukasa A, Tokunaga Y, Fukui Y, Takeuchi Y, Hojo K. Establishment of an experimental model of autoimmune epididymo-orchitis induced by the transfer of a T-cell line in mice. Int J Androl 1992;15:170–181. [PubMed] [Google Scholar]
- Itoh M, Miki T, Takeuchi Y, Miyake M, De Rooij DG. Immunohistological localization of autoantigens detected by serum autoantibodies from mice with experimental autoimmune orchitis without using adjuvants. Arch Androl 1994;32:45–52. [DOI] [PubMed] [Google Scholar]
- Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4(+) and CD8(+) effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol 2009;81:44–45. [DOI] [PubMed] [Google Scholar]
- Jadot-Van De Casseye M, De Bled G, Gepts W, Schoysman R. An immunohistochemical study for testicular biopsies in cases of male infertility. Andrologia 1980;12:122–129. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Jorgensen N, Pollanen P, Giwercman A, Skakkebaek NE. Incidence of testicular mononuclear cell infiltrates in normal human males and in patients with germ cell neoplasia. Int J Androl 1995;18:313–320. [DOI] [PubMed] [Google Scholar]
- Jantos C, Baumgartner W, Durchfeld B, Schiefer HG. Experimental epididymitis due to Chlamydia trachomatis in rats. Infect Immun 1992;60:2324–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantos C, Krauss H, Altmannsberger M, Thiele D, Weidner W, Schiefer HG. Experimental chlamydial epididymitis. Urol Int 1989;44:279–283. [DOI] [PubMed] [Google Scholar]
- Jarazo-Dietrich S, Jacobo P, Perez CV, Guazzone VA, Lustig L, Theas MS. Up regulation of nitric oxide synthase-nitric oxide system in the testis of rats undergoing autoimmune orchitis. Immunobiology 2012;217:778–787. [DOI] [PubMed] [Google Scholar]
- Jenabian MA, Costiniuk CT, Mehraj V, Ghazawi FM, Fromentin R, Brousseau J, Brassard P, Belanger M, Ancuta P, Bendayan R et al. . Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS 2016;30:2777–2786. [DOI] [PubMed] [Google Scholar]
- Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, Guyomard S, Prisant N, Lamarre P, Dejucq-Rainsford N et al. . Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017;17:1200–1208. [DOI] [PubMed] [Google Scholar]
- Johnson L, Varner DD, Roberts ME, Smith TL, Keillor GE, Scrutchfield WL. Efficiency of spermatogenesis: a comparative approach. Anim Reprod Sci 2000;60–61:471–480. [DOI] [PubMed] [Google Scholar]
- Jungwirth A, Diemer T, Kopa Z, Krausz C, Mihas S, Tournaye H. EAU Guidelines on Male Infertility. Arnhem, The Netherlands: EAU Guidelines Office, 2018. [Google Scholar]
- Kalaydjiev S, Dimitrova D, Nenova M, Peneva S, Dikov I, Nakov L. Serum sperm antibodies are not elevated after mumps orchitis. Fertil Steril 2002;77:76–82. [DOI] [PubMed] [Google Scholar]
- Kaur G, Thompson LA, Dufour JM. Sertoli cells—immunological sentinels of spermatogenesis. Semin Cell Dev Biol 2014;30:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaver I, Matzkin H, Braf ZF. Epididymo-orchitis: a retrospective study of 121 patients. J Fam Pract 1990;30:548–552. [PubMed] [Google Scholar]
- Kaya M, Boleken ME, Zeyrek F, Ozardali I, Kanmaz T, Erel O, Yucesan S. Oxidative and antioxidative status in the testes of rats with acute epididymitis. Urol Int 2006;76:353–358. [DOI] [PubMed] [Google Scholar]
- Khosravi F, Michel V, Galuska CE, Bhushan S, Christian P, Schuppe HC, Pilatz A, Galuska SP, Meinhardt A. Desialylation of spermatozoa and epithelial cell glycocalyx is a consequence of bacterial infection of the epididymis. J Biol Chem 2016;291:17717–17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann N Y Acad Sci 2011;1246:77–91. [DOI] [PubMed] [Google Scholar]
- Klein B, Haggeney T, Fietz D, Indumathy S, Loveland KL, Hedger M, Kliesch S, Weidner W, Bergmann M, Schuppe HC. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum Reprod 2016;31:2192–2202. [DOI] [PubMed] [Google Scholar]
- Kohno S, Munoz JA, Williams TM, Teuscher C, Bernard CC, Tung KS. Immunopathology of murine experimental allergic orchitis. J Immunol 1983;130:2675–2682. [PubMed] [Google Scholar]
- Krieger JN. Epididymitis, orchitis, and related conditions. Sex Transm Dis 1984;11:173–181. [DOI] [PubMed] [Google Scholar]
- Kristensen JK, Scheibel JH. Etiology of acute epididymitis presenting in a venereal disease clinic. Sex Transm Dis 1984;11:32–33. [DOI] [PubMed] [Google Scholar]
- Kuzan FB, Patton DL, Allen SM, Kuo CC. A proposed mouse model for acute epididymitis provoked by genital serovar E, Chlamydia trachomatis. Biol Reprod 1989;40:165–172. [DOI] [PubMed] [Google Scholar]
- Lang T, Dechant M, Sanchez V, Wistuba J, Boiani M, Pilatz A, Stammler A, Middendorff R, Schuler G, Bhushan S et al. . Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E. coli-associated epididymitis. Biol Reprod 2013;89:59. [DOI] [PubMed] [Google Scholar]
- Lang T, Hudemann C, Tchatalbachev S, Stammler A, Michel V, Aslani F, Bhushan S, Chakraborty T, Renz H, Meinhardt A. Uropathogenic Escherichia coli modulates innate immunity to suppress Th1-mediated inflammatory responses during infectious epididymitis. Infect Immun 2014;82:1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Mouchel T, Aubry F, Patard JJ, Ruffault A, Jegou B, Samson M. Production of the chemokines monocyte chemotactic protein-1, regulated on activation normal T cell expressed and secreted protein, growth-related oncogene, and interferon-gamma-inducible protein-10 is induced by the Sendai virus in human and rat testicular cells. Endocrinology 2002;143:1434–1440. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A, Le Grand R, Denis H, Satie AP, Mannioui K, Roques P, Maillard A, Daniels S, Jegou B, Dejucq-Rainsford N. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One 2008;3:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Thirumoorthy T, Lim KB, Sng EH. Epidemiology of acute epididymo-orchitis in Singapore. Ann Acad Med Singapore 1989;18:320–323. [PubMed] [Google Scholar]
- Lei T, Moos S, Klug J, Aslani F, Bhushan S, Wahle E, Fröhlich S, Meinhardt A, Fijak M. Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling. Sci Rep 2018;8:3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Temminck B, Da Rugna D, Leibundgut B, Sulmoni A, Muller H. Role of immunological factors in male infertility. Immunohistochemical and serological evidence. Lab Invest 1987;57:21–28. [PubMed] [Google Scholar]
- Liu Z, Zhao S, Chen Q, Yan K, Liu P, Li N, Cheng CY, Lee WM, Han D. Roles of Toll-like receptors 2 and 4 in mediating experimental autoimmune orchitis induction in mice. Biol Reprod 2015;92:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo L, Rogel R, Sanchez-Gonzalez JV, Perez-Ardavin J, Moreno E, Lujan S, Broseta E, Boronat F. Evaluation of adult acute scrotum in the ER: clinical characteristics, diagnosis, management and costs. Urology 2016;94:36–41. [DOI] [PubMed] [Google Scholar]
- Loveland KL, Klein B, Pueschl D, Indumathy S, Bergmann M, Loveland BE, Hedger MP, Schuppe HC. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front Endocrinol (Lausanne) 2017;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bhushan S, Tchatalbachev S, Marconi M, Bergmann M, Weidner W, Chakraborty T, Meinhardt A. Necrosis is the dominant cell death pathway in uropathogenic escherichia coli elicited epididymo-orchitis and is responsible for damage of rat testis. PLoS One 2013;8:e52919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchetta R, Clavert A, Meyer JM, Bollack C. Acute experimental E. coli epididymitis in the rat and its consequences on spermatogenesis. Urol Res 1983;11:117–120. [DOI] [PubMed] [Google Scholar]
- Ludwig G, Haselberger J. [Epididymitis and fertility. Treatment results in acute unspecific epididymitis]. Fortschr Med 1977;95:397–399. [PubMed] [Google Scholar]
- Ludwig M, Jantos CA, Wolf S, Bergmann M, Failing K, Schiefer HG, Weidner W. Tissue penetration of sparfloxacin in a rat model of experimental Escherichia coli epididymitis. Infection 1997;25:178–184. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Johannes S, Bergmann M, Failing K, Schiefer HG, Weidner W. Experimental Escherichia coli epididymitis in rats: a model to assess the outcome of antibiotic treatment. BJU Int 2002;90:933–938. [DOI] [PubMed] [Google Scholar]
- Lunenfeld B, Van Steirteghem A. Infertility in the third millennium: implications for the individual, family and society: condensed meeting report from the Bertarelli Foundation’s second global conference. Hum Reprod Update 2004;10:317–326. [DOI] [PubMed] [Google Scholar]
- Lustig L, Lourtau L, Perez R, Doncel GF. Phenotypic characterization of lymphocytic cell infiltrates into the testes of rats undergoing autoimmune orchitis. Int J Androl 1993;16:279–284. [DOI] [PubMed] [Google Scholar]
- Lustig L, Rival C, Tung KSK. Autoimmune Orchitis and Autoimmune Oophoritis. San Diego, USA: Elsevier Inc, 2014. [Google Scholar]
- Lustig L, Satz ML, Sztein MB, Denduchis B. Antigens of the basement membranes of the seminiferous tubules induce autoimmunity in Wistar rats. J Reprod Immunol 1982;4:79–90. [DOI] [PubMed] [Google Scholar]
- Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science 2002;297:620–623. [DOI] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X et al. . Zika virus causes testis damage and leads to male infertility in mice. Cell 2017;168:542. [DOI] [PubMed] [Google Scholar]
- Mahi-Brown CA, Yule TD, Tung KS. Adoptive transfer of murine autoimmune orchitis to naive recipients with immune lymphocytes. Cell Immunol 1987;106:408–419. [DOI] [PubMed] [Google Scholar]
- Malolina EA, Kulibin AY, Kushch AA. Neonatal testicular cell transplantation restores murine spermatogenesis damaged in the course of herpes simplex virus-induced orchitis. Reprod Fertil Dev 2016;28:757–764. [DOI] [PubMed] [Google Scholar]
- Mancini RE, Andrada JA, Saraceni D, Bachmann AE, Lavieri JC, Nemirovsky M. Immunological and testicular response in man sensitized with human testicular homogenate. J Clin Endocrinol Metab 1965;25:859–875. [DOI] [PubMed] [Google Scholar]
- Marconi M, Pilatz A, Wagenlehner F, Diemer T, Weidner W. Are antisperm antibodies really associated with proven chronic inflammatory and infectious diseases of the male reproductive tract? Eur Urol 2009;56:708–715. [DOI] [PubMed] [Google Scholar]
- Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update 2018;24:86–105. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Adam M, Glashauser L, Dietrich K, Schwarzer JU, Kohn FM, Strauss L, Welter H, Poutanen M, Mayerhofer A. Sterile inflammation as a factor in human male infertility: involvement of Toll like receptor 2, biglycan and peritubular cells. Sci Rep 2016;6:37128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar S, Levine AS. Antisperm antibodies: etiology, pathogenesis, diagnosis, and treatment. Fertil Steril 1998;70:799–810. [DOI] [PubMed] [Google Scholar]
- McLaughlin MM, Skoglund EW, Ison MG. Peramivir: an intravenous neuraminidase inhibitor. Expert Opin Pharmacother 2015;16:1889–1900. [DOI] [PubMed] [Google Scholar]
- Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 2000;74:239–244. [DOI] [PubMed] [Google Scholar]
- Meinhardt A. Infection: a new threat on the horizon—Zika virus and male fertility. Nat Rev Urol 2017;14:135–136. [DOI] [PubMed] [Google Scholar]
- Melaine N, Ruffault A, Dejucq-Rainsford N, Jegou B. Experimental inoculation of the adult rat testis with Sendai virus: effect on testicular morphology and leukocyte population. Hum Reprod 2003;18:1574–1579. [DOI] [PubMed] [Google Scholar]
- Meng J, Greenlee AR, Taub CJ, Braun RE. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol Reprod 2011;85:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–2738. [DOI] [PubMed] [Google Scholar]
- Mettlin C, Natarajan N, Huben R. Vasectomy and prostate cancer risk. Am J Epidemiol 1990;132:1056–1061. discussion 1062-1055. [DOI] [PubMed] [Google Scholar]
- Mevorach RA, Lerner RM, Dvoretsky PM, Rabinowitz R. Testicular abscess: diagnosis by ultrasonography. J Urol 1986;136:1213–1216. [DOI] [PubMed] [Google Scholar]
- Michel V, Pilatz A, Hedger MP, Meinhardt A. Epididymitis: revelations at the convergence of clinical and basic sciences. Asian J Androl 2015;17:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Duan Y, Stoschek E, Bhushan S, Middendorff R, Young JM, Loveland KL, Kretser DM, Hedger MP, Meinhardt A. Uropathogenic Escherichia coli causes fibrotic remodelling of the epididymis. J Pathol 2016;240:15–24. [DOI] [PubMed] [Google Scholar]
- Mikuz G, Damjanov I. Inflammation of the testis, epididymis, peritesticular membranes, and scrotum. Pathol Annu 1982;17:101–128. [PubMed] [Google Scholar]
- Moller BR, Mardh PA. Experimental epididymitis and urethritis in grivet monkeys provoked by Chlamydia trachomatis. Fertil Steril 1980;34:275–279. [DOI] [PubMed] [Google Scholar]
- Mossadegh-Keller N, Gentek R, Gimenez G, Bigot S, Mailfert S, Sieweke MH. Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med 2017;214:2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa A, Hiromatsu K, Matsuzaki G, O’Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol 1995;155:2047–2056. [PubMed] [Google Scholar]
- Muller R, Klug J, Rodewald M, Meinhardt A. Macrophage migration inhibitory factor suppresses transforming growth factor-beta2 secretion in cultured rat testicular peritubular cells. Reprod Fertil Dev 2005;17:435–438. [DOI] [PubMed] [Google Scholar]
- Musha M, Hirai S, Naito M, Terayama H, Qu N, Hatayama N, Itoh M. The effects of adjuvants on autoimmune responses against testicular antigens in mice. J Reprod Dev 2013;59:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Hirai S, Terayama H, Qu N, Kuerban M, Musha M, Kitaoka M, Ogawa Y, Itoh M. Postinflammation stage of autoimmune orchitis induced by immunization with syngeneic testicular germ cells alone in mice. Med Mol Morphol 2012. a;45:35–44. [DOI] [PubMed] [Google Scholar]
- Naito M, Terayama H, Hirai S, Qu N, Lustig L, Itoh M. Experimental autoimmune orchitis as a model of immunological male infertility. Med Mol Morphol 2012. b;45:185–189. [DOI] [PubMed] [Google Scholar]
- Nashan D, Malorny U, Sorg C, Cooper T, Nieschlag E. Immuno-competent cells in the murine epididymis. Int J Androl 1989;12:85–94. [DOI] [PubMed] [Google Scholar]
- Ness RB, Markovic N, Carlson CL, Coughlin MT. Do men become infertile after having sexually transmitted urethritis? An epidemiologic examination. Fertil Steril 1997;68:205–213. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Rait G, Murray-Thomas T, Hughes G, Mercer CH, Cassell J. Management of epididymo-orchitis in primary care: results from a large UK primary care database. Br J Gen Pract 2010;60:e407–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel WR, Plumb RT. Orchitis In: Walsh PC, Gittes RF, Perlmutter AD, Stamey TA (eds).. Campbell’s Urology; 1986 Philadelphia: WBSaunders Co, 1986;975–978. [Google Scholar]
- Nickel JC, Siemens DR, Nickel KR, Downey J. The patient with chronic epididymitis: characterization of an enigmatic syndrome. J Urol 2002;167:1701–1704. [PubMed] [Google Scholar]
- Nickel JC, Teichman JM, Gregoire M, Clark J, Downey J. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE Study. Urology 2005;66:935–940. [DOI] [PubMed] [Google Scholar]
- Nicolas N, Michel V, Bhushan S, Wahle E, Hayward S, Ludlow H, de Kretser DM, Loveland KL, Schuppe HC, Meinhardt A et al. . Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo-orchitis in mice. Sci Rep 2017. a;7:42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas N, Muir JA, Hayward S, Chen JL, Stanton PG, Gregorevic P, de Kretser DM, Loveland KL, Bhushan S, Meinhardt A et al. . Induction of experimental autoimmune orchitis in mice: responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction 2017. b;154:193–205. [DOI] [PubMed] [Google Scholar]
- Nielsen OS. An experimental study of the treatment of bacterial epididymitis. Scand J Urol Nephrol Suppl 1987;104:115–117. [PubMed] [Google Scholar]
- Nistal M, Paniagua R. Non-neoplastic diseases of the testis In: Eble JN (ed).. Urologic Surgical Pathology. St. Louis: Mosby, 1997;458–565. [Google Scholar]
- Nistal M, Riestra ML, Paniagua R. Focal orchitis in undescended testes: discussion of pathogenetic mechanisms of tubular atrophy. Arch Pathol Lab Med 2002;126:64–69. [DOI] [PubMed] [Google Scholar]
- Ochsendorf FR. Sexually transmitted infections: impact on male fertility. Andrologia 2008;40:72–75. [DOI] [PubMed] [Google Scholar]
- Olesen IA, Andersson AM, Aksglaede L, Skakkebaek NE, Rajpert-de Meyts E, Joergensen N, Juul A. Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility. Fertil Steril 2017; 107, 74–82.e77. [DOI] [PubMed] [Google Scholar]
- Osegbe DN. Testicular function after unilateral bacterial epididymo-orchitis. Eur Urol 1991;19:204–208. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP. Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 2000;63:1285–1293. [DOI] [PubMed] [Google Scholar]
- Patel LN, Arciuolo RJ, Fu J, Giancotti FR, Zucker JR, Rakeman JL, Rosen JB. Mumps outbreak among a highly vaccinated university community-New York City, January–April 2014. Clin Infect Dis 2017;64:408–412. [DOI] [PubMed] [Google Scholar]
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG et al. . Persistence of Zika virus in body fluids—preliminary report. N Engl J Med 2017. doi:10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier RM, Nemirovsky MS, Calvert R, Hugon JS. Effects of immunization with Freund’s complete adjuvant and isologous spermatozoa on the seminiferous epithelium and blood-testis barrier in guinea pigs. Anat Rec 1981;199:197–211. [DOI] [PubMed] [Google Scholar]
- Perez CV, Gomez LG, Gualdoni GS, Lustig L, Rabinovich GA, Guazzone VA. Dual roles of endogenous and exogenous galectin-1 in the control of testicular immunopathology. Sci Rep 2015;5:12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CV, Pellizzari EH, Cigorraga SB, Galardo MN, Naito M, Lustig L, Jacobo PV. IL17A impairs blood-testis barrier integrity and induces testicular inflammation. Cell Tissue Res 2014;358:885–898. [DOI] [PubMed] [Google Scholar]
- Perez C, Sobarzo C, Jacobo P, Jarazo Dietrich S, Theas M, Denduchis B, Lustig L. Impaired expression and distribution of adherens and gap junction proteins in the seminiferous tubules of rats undergoing autoimmune orchitis. Int J Androl 2011;34:e566–e577. [DOI] [PubMed] [Google Scholar]
- Perez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B, Lustig L. Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol Reprod 2012;87:122. [DOI] [PubMed] [Google Scholar]
- Pezzella A, Barbonetti A, D’Andrea S, Necozione S, Micillo A, Di Gregorio A, Francavilla F, Francavilla S. Ultrasonographic caput epididymis diameter is reduced in non-obstructive azoospermia compared with normozoospermia but is not predictive for successful sperm retrieval after TESE. Hum Reprod 2014;29:1368–1374. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Wagenlehner F, Bschleipfer T, Schuppe HC, Diemer T, Linn T, Weidner W, Altinkilic B. Acute epididymitis in ultrasound: results of a prospective study with baseline and follow-up investigations in 134 patients. Eur J Radiol 2013;82:e762–e768. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Lochnit G, Karnati S, Paradowska-Dogan A, Lang T, Schultheiss D, Schuppe HC, Hossain H, Baumgart-Vogt E, Weidner W et al. . Acute epididymitis induces alterations in sperm protein composition. Fertil Steril 2014. a;101:1609–1617. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Discher T, Lochnit G, Wolf J, Schuppe HC, Schuttler CG, Hossain H, Weidner W, Lohmeyer J, Diemer T. Semen quality in HIV patients under stable antiretroviral therapy is impaired compared to WHO 2010 reference values and on sperm proteome level. AIDS 2014. b;28:875–880. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Ceylan I, Schuppe HC, Ludwig M, Fijak M, Chakraborty T, Weidner W, Bergmann M, Wagenlehner F. Experimental Escherichia coli epididymitis in rats: assessment of testicular involvement in a long-term follow-up. Andrologia 2015. a;47:160–167. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Hossain H, Kaiser R, Mankertz A, Schuttler CG, Domann E, Schuppe HC, Chakraborty T, Weidner W, Wagenlehner F. Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur Urol 2015. b;68:428–435. [DOI] [PubMed] [Google Scholar]
- Pilatz A, Boecker M, Schuppe HC, Diemer T, Wagenlehner F. Infection and infertility. Urologe A 2016;55:883–889. [DOI] [PubMed] [Google Scholar]
- Pollanen P, Cooper TG. Immunology of the testicular excurrent ducts. J Reprod Immunol 1994;26:167–216. [DOI] [PubMed] [Google Scholar]
- Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, Korrovits P, Laan M. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod 2017;32:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N, Naito M, Terayama H, Hirai S, Li J, Ogawa Y, Kitaoka M, Itoh M. Developmental ontogeny of autoantigens associated with localized autoimmunity in murine testis and epididymis. J Reprod Immunol 2010;87:45–51. [DOI] [PubMed] [Google Scholar]
- Ramer PC, Chijioke O, Meixlsperger S, Leung CS, Munz C. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol 2011;89:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, Fijak M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res 2006. a;324:311–318. [DOI] [PubMed] [Google Scholar]
- Rival C, Theas MS, Guazzone VA, Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol 2006. b;70:43–58. [DOI] [PubMed] [Google Scholar]
- Rival C, Guazzone VA, von Wulffen W, Hackstein H, Schneider E, Lustig L, Meinhardt A, Fijak M. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod 2007;13:853–861. [DOI] [PubMed] [Google Scholar]
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, Lustig L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol 2008;215:108–117. [DOI] [PubMed] [Google Scholar]
- Rival C, Wheeler K, Jeffrey S, Qiao H, Luu B, Tewalt EF, Engelhard VH, Tardif S, Hardy D, del Rio R et al. . Regulatory T cells and vasectomy. J Reprod Immunol 2013;100:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A, Queiroz DB, Honda L, Silva EJ, Hall SH, Avellar MC. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod 2008;79:1135–1147. [DOI] [PubMed] [Google Scholar]
- Roper RJ, Doerge RW, Call SB, Tung KS, Hickey WF, Teuscher C. Autoimmune orchitis, epididymitis, and vasitis are immunogenetically distinct lesions. Am J Pathol 1998;152:1337–1345. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, Shapiro S. Vasectomy and the risk of prostate cancer. Am J Epidemiol 1990;132:1051–1055. discussion1062-1055. [DOI] [PubMed] [Google Scholar]
- Roulet V, Satie AP, Ruffault A, Le Tortorec A, Denis H, Guist’hau O, Patard JJ, Rioux-Leclerq N, Gicquel J, Jegou B et al. . Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am J Pathol 2006;169:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PJ, Comhaire F, Hargreave TB, Mahmoud A. WHO Manual for the Standarized Investigation, Diagnosis and Management of the Infertile Male. Cambridge: Cambridge University Press, 2000. [Google Scholar]
- Rusz A, Pilatz A, Wagenlehner F, Linn T, Diemer T, Schuppe HC, Lohmeyer J, Hossain H, Weidner W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol 2012;30:23–30. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Himeno K, Sanui H, Yoshida S, Nomoto K. Experimental allergic orchitis in mice. I. A new model induced by immunization without adjuvants. Clin Immunol Immunopathol 1985;37:360–368. [DOI] [PubMed] [Google Scholar]
- Salomon F, Saremaslani P, Jakob M, Hedinger CE. Immune complex orchitis in infertile men. Immunoelectron microscopy of abnormal basement membrane structures. Lab Invest 1982;47:555–567. [PubMed] [Google Scholar]
- Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KS. The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol Rev 2006;212:170–184. [DOI] [PubMed] [Google Scholar]
- Satie AP, Mazaud-Guittot S, Seif I, Mahe D, He Z, Jouve G, Jegou B, Dejucq-Rainsford N. Excess type I interferon signaling in the mouse seminiferous tubules leads to germ cell loss and sterility. J Biol Chem 2011;286:23280–23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Hirokawa K, Hatakeyama S. Experimental allergic orchitis in mice. Histopathological and Immunological studies. Virchows Arch A Pathol Anat Histol 1981;392:147–158. [DOI] [PubMed] [Google Scholar]
- Schuppe HC, Bergmann M. Inflammatory conditions of the testis. In: Jezek D (ed). Atlas of the human testis. London: Springer, 2013, 113–121. [Google Scholar]
- Schuppe HC, Meinhardt A. Immune privilege and inflammation of the testis. Chem Immunol Allergy 2005;88:1–14. [DOI] [PubMed] [Google Scholar]
- Schuppe HC, Meinhardt A, Allam JP, Bergmann M, Weidner W, Haidl G. Chronic orchitis: a neglected cause of male infertility? Andrologia 2008;40:84–91. [DOI] [PubMed] [Google Scholar]
- Schuppe HC, Neumann NJ, Scheffzyk A, Schock-Skasa G, Hofmann N, Schill WB. Inflammatory reactions in testicular biopsies of infertile men. Andrologia 2001;33:327–328. [Google Scholar]
- Schuppe HC, Pilatz A, Hossain H, Diemer T, Wagenlehner F, Weidner W. Urogenital infection as a risk factor for male infertility. Dtsch Arztebl Int 2017;114:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See WA, Taylor TO, Mack LA, Tartaglione TA, Opheim KE, Berger RE. Bacterial epididymitis in the rat: a model for assessing the impact of acute inflammation on epididymal antibiotic penetration. J Urol 1990;144:780–784. [DOI] [PubMed] [Google Scholar]
- Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod 1999;61:705–714. [DOI] [PubMed] [Google Scholar]
- Shehu-Xhilaga M, de Kretser D, Dejucq-Rainsford N, Hedger M. Standing in the way of eradication: HIV-1 infection and treatment in the male genital tract. Curr HIV Res 2005;3:345–359. [DOI] [PubMed] [Google Scholar]
- Shulman A, Shohat B, Gillis D, Yavetz H, Homonnai ZT, Paz G. Mumps orchitis among soldiers: frequency, effect on sperm quality, and sperm antibodies. Fertil Steril 1992;57:1344–1346. [DOI] [PubMed] [Google Scholar]
- Sigg C, Hedinger C. Quantitative and ultrastructural study of germinal epithelium in testicular biopsies with ‘mixed atrophy’. Andrologia 1981;13:412–424. [DOI] [PubMed] [Google Scholar]
- Silva CA, Cocuzza M, Carvalho JF, Bonfa E. Diagnosis and classification of autoimmune orchitis. Autoimmun Rev 2014;13:431–434. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005;5:615–625. [DOI] [PubMed] [Google Scholar]
- Smith RP, Tracy CR, Kavoussi PK, Witmer MT, Costabile RA. The impact of color Doppler ultrasound on treatment patterns of epididymitis in a university-based healthcare system. Indian J Urol 2013;29:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada S, Amezaga MR, Hamilton M, McKenzie H, Templeton A, Bhattacharya S. Absence of chlamydial deoxyribonucleic acid from testicular and epididymal samples from men with obstructive azoospermia. Fertil Steril 2010;93:833–836. [DOI] [PubMed] [Google Scholar]
- Stammler A, Hau T, Bhushan S, Meinhardt A, Jonigk D, Lippmann T, Pilatz A, Schneider-Huther I, Middendorff R. Epididymitis: ascending infection restricted by segmental boundaries. Hum Reprod 2015;30:1557–1565. [DOI] [PubMed] [Google Scholar]
- Sudweeks JD, Todd JA, Blankenhorn EP, Wardell BB, Woodward SR, Meeker ND, Estes SS, Teuscher C. Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci USA 1993;90:3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suescun MO, Calandra RS, Lustig L. Alterations of testicular function after induced autoimmune orchitis in rats. J Androl 1994;15:442–448. [PubMed] [Google Scholar]
- Suescun MO, Lustig L, Calandra RS, Groome NP, Campo S. Correlation between inhibin secretion and damage of seminiferous tubules in a model of experimental autoimmune orchitis. J Endocrinol 2001;170:113–120. [DOI] [PubMed] [Google Scholar]
- Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L. Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats. Biol Reprod 2003;68:2114–2121. [DOI] [PubMed] [Google Scholar]
- Suominen JJ. Sympathetic auto-immune orchitis. Andrologia 1995;27:213–216. [PubMed] [Google Scholar]
- Suominen J, Soderstrom KO. Lymphocyte infiltration in human testicular biopsies. Int J Androl 1982;5:461–466. [DOI] [PubMed] [Google Scholar]
- Tae BS, Ham BK, Kim JH, Park JY, Bae JH. Clinical features of mumps orchitis in vaccinated postpubertal males: a single-center series of 62 patients. Korean J Urol 2012;53:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med 1987;165:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Fujisawa M, Arakawa S, Kamidono S. Local expression of cytokine messenger RNA in rat model of Escherichia coli epididymitis. J Urol 1995;154:2179–2184. [PubMed] [Google Scholar]
- Taurog JD, Rival C, van Duivenvoorde LM, Satumtira N, Dorris ML, Sun M, Shelton JM, Richardson JA, Hamra FK, Hammer RE et al. . Autoimmune epididymoorchitis is essential to the pathogenesis of male-specific spondylarthritis in HLA-B27-transgenic rats. Arthritis Rheum 2012;64:2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchiokadze S, Galdava G. Clinical and Anamnestic Characteristics of Development of Antisperm Immunity in Infertile Men. Georgian Med News 2015;18–22. [PubMed] [Google Scholar]
- Tebourbi L, Courtot AM, Duchateau R, Loeuillet A, Testart J, Cerutti I. Experimental inoculation of male mice with murine cytomegalovirus and effect on offspring. Hum Reprod 2001;16:2041–2049. [DOI] [PubMed] [Google Scholar]
- Terayama H, Hirai S, Naito M, Qu N, Katagiri C, Nagahori K, Hayashi S, Sasaki H, Moriya S, Hiramoto M et al. . Specific autoantigens identified by sera obtained from mice that are immunized with testicular germ cells alone. Sci Rep 2016;6:35599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C, Meeker ND, Livingstone KD, Sudweeks JD, Griffith JS, Wardell BB, Hickey WF. Experimental allergic orchitis in mice. VII. Preliminary characterization of the aspermatogenic autoantigens responsible for eliciting actively and passively induced disease. J Reprod Immunol 1994;26:233–249. [DOI] [PubMed] [Google Scholar]
- Theas MS, Rival C, Dietrich SJ, Guazzone VA, Lustig L. Death receptor and mitochondrial pathways are involved in germ cell apoptosis in an experimental model of autoimmune orchitis. Hum Reprod 2006;21:1734–1742. [DOI] [PubMed] [Google Scholar]
- Theas S, Rival C, Lustig L. Germ cell apoptosis in autoimmune orchitis: involvement of the Fas-FasL system. Am J Reprod Immunol 2003;50:166–176. [DOI] [PubMed] [Google Scholar]
- Tompkins AB, Hutchinson P, de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor beta on lymphocyte subsets in vitro. Biol Reprod 1998;58:943–951. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Krausz C, Oates RD. Concepts in diagnosis and therapy for male reproductive impairment. Lancet Diabetes Endocrinol 2017. a;5:554–564. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol 2017. b;5:544–553. [DOI] [PubMed] [Google Scholar]
- Tozzo PJ. Semen analysis in unilateral epididymitis. N Y State J Med 1968;68:2769–2770. [PubMed] [Google Scholar]
- Tung KS, Unanue ER, Dixon FJ. The immunopathology of experimental allergic orchitis. Am J Pathol 1970;60:313–328. [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Woodroffe AJ. Immunopathology of experimental allergic orchitis in the rabbit. J Immunol 1978;120:320–328. [PubMed] [Google Scholar]
- Tung KS, Ellis L, Teuscher C, Meng A, Blaustein JC, Kohno S, Howell R. The black mink (Mustela vison). A natural model of immunologic male infertility. J Exp Med 1981;154:1016–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Ellis LE, Childs GV, Dufau M. The dark mink: a model of male infertility. Endocrinology 1984;114:922–929. [DOI] [PubMed] [Google Scholar]
- Tung KS, Smith S, Matzner P, Kasai K, Oliver J, Feuchter F, Anderson RE. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Autoantibodies. Am J Pathol 1987. a;126:303–314. [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Yule TD, Mahi-Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol 1987. b;138:752–759. [PubMed] [Google Scholar]
- Tung KS, Lu CY. Immunologic basis of reproductive failure. Monogr Pathol 1991;33:308–333. [PubMed] [Google Scholar]
- Tung KS. Elucidation of autoimmune disease mechanism based on testicular and ovarian autoimmune disease models. Horm Metab Res 1995;27:539–543. [DOI] [PubMed] [Google Scholar]
- Tung KS, Teuscher C. Mechanisms of autoimmune disease in the testis and ovary. Hum Reprod Update 1995;1:35–50. [DOI] [PubMed] [Google Scholar]
- Tung KS, Harakal J, Qiao H, Rival C, Li JC, Paul AG, Wheeler K, Pramoonjago P, Grafer CM, Sun W et al. . Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J Clin Invest 2017;127:1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TT, Mammen T, Kavoussi P, Lysiak JJ, Costabile RA. Cytokine responses to E. coli-induced epididymitis in the rat: blockade by vasectomy. Urology 2011;77:e1509–e1514. [DOI] [PubMed] [Google Scholar]
- Tüttelmann F, Nieschlag E. Classification of andrological disorders. In: Nieschlag E, Behre HM, Nieschlag S (eds). Andrology Male Reproductive Health and Dysfunction. Heidelberg: Springer, 2010, 87–92. [Google Scholar]
- van Leeuwen E, Wit FW, Repping S, Eeftinck Schattenkerk JK, Reiss P, van der Veen F, Prins JM. Effects of antiretroviral therapy on semen quality. AIDS 2008;22:637–642. [DOI] [PubMed] [Google Scholar]
- Vieler E, Jantos C, Schmidts HL, Weidner W, Schiefer HG. Comparative efficacies of ofloxacin, cefotaxime, and doxycycline for treatment of experimental epididymitis due to Escherichia coli in rats. Antimicrob Agents Chemother 1993;37:846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walecki M, Eisel F, Klug J, Baal N, Paradowska-Dogan A, Wahle E, Hackstein H, Meinhardt A, Fijak M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell 2015;26:2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Duan YG. The role of dendritic cells in male reproductive tract. Am J Reprod Immunol 2016;76:186–192. [DOI] [PubMed] [Google Scholar]
- Wang M, Fijak M, Hossain H, Markmann M, Nusing RM, Lochnit G, Hartmann MF, Wudy SA, Zhang L, Gu H et al. . Characterization of the micro-environment of the testis that shapes the phenotype and function of testicular macrophages. J Immunol 2017;198:4327–4340. [DOI] [PubMed] [Google Scholar]
- Weidner W, Garbe C, Weissbach L, Harbrecht J, Kleinschmidt K, Schiefer HG, Friedrich HJ. [Initial therapy of acute unilateral epididymitis using ofloxacin. II. Andrological findings]. Urologe A 1990;29:277–280. [PubMed] [Google Scholar]
- Weidner W, Krause W. Orchitis In: Knobil E, Neill J (eds).. Encyclopedia of Reproduction.. San Diego: Academic Press, 1998;524–527. [Google Scholar]
- Weidner W, Krause W, Ludwig M. Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum Reprod Update 1999;5:421–432. [DOI] [PubMed] [Google Scholar]
- Weidner W, Wagenlehner FM, Marconi M, Pilatz A, Pantke KH, Diemer T. Acute bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: andrological implications. Andrologia 2008;40:105–112. [DOI] [PubMed] [Google Scholar]
- Weidner W, Pilatz A, Diemer T, Schuppe HC, Rusz A, Wagenlehner F. Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World J Urol 2013;31:717–723. [DOI] [PubMed] [Google Scholar]
- Werner CA. Mumps orchitis and testicular atrophy; a factor in male sterility. Ann Intern Med 1950;32:1075–1086. [DOI] [PubMed] [Google Scholar]
- Wesselhoeft C. Orchitis in Mumps. Boston Med Surg J 1920;183:520–524. [Google Scholar]
- Wheeler K, Tardif S, Rival C, Luu B, Bui E, Del Rio R, Teuscher C, Sparwasser T, Hardy D, Tung KS. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc Natl Acad Sci USA 2011;108:7511–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willocks LJ, Guerendiain D, Austin HI, Morrison KE, Cameron RL, Templeton KE, DEL VRF, Ewing R, Donovan W, Pollock KGJ. An outbreak of mumps with genetic strain variation in a highly vaccinated student population in Scotland. Epidemiol Infect 2017;145:3219–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnall WR, Muir JA, Hedger MP. Differential responses of epithelial Sertoli cells of the rat testis to Toll-like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun 2011. a;17:123–136. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J Leukoc Biol 2011. b;90:133–143. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Lloyd SB, De Rose R, Alcantara S, Amarasena TH, Hedger MP, Girling JE, Kent SJ. Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis. J Leukoc Biol 2015;97:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff H. The biologic significance of white blood cells in semen. Fertil Steril 1995;63:1143–1157. [DOI] [PubMed] [Google Scholar]
- Wolin LH. On the etiology of epididymitis. J Urol 1971;105:531–533. [DOI] [PubMed] [Google Scholar]
- Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab 1985;60:444–450. [DOI] [PubMed] [Google Scholar]
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- Wu H, Shi L, Wang Q, Cheng L, Zhao X, Chen Q, Jiang Q, Feng M, Li Q, Han D. Mumps virus-induced innate immune responses in mouse Sertoli and Leydig cells. Sci Rep 2016;6:19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol 2009;327:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 2006;74:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakirevich E, Yanai O, Sova Y, Sabo E, Stein A, Hiss J, Resnick MB. Cytotoxic phenotype of intra-epithelial lymphocytes in normal and cryptorchid human testicular excurrent ducts. Hum Reprod 2002;17:275–283. [DOI] [PubMed] [Google Scholar]
- Yeniyol CO, Sorguc S, Minareci S, Ayder AR. Role of interferon-alpha-2B in prevention of testicular atrophy with unilateral mumps orchitis. Urology 2000;55:931–933. [DOI] [PubMed] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol 1988;141:1161–1167. [PubMed] [Google Scholar]
- Zhou ZZ, Zheng Y, Steenstra R, Hickey WF, Teuscher C. Actively-induced experimental allergic orchitis (EAO) in Lewis/NCR rats: sequential histo- and immunopathologic analysis. Autoimmunity 1989;3:125–134. [DOI] [PubMed] [Google Scholar]
- Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 2014;34:433–454. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.