Abstract
Objective
The aim of this study is to determine the severity of female sexual dysfunction (FSD), quality of life, and depression status in female patients with Cushing’s syndrome (CS).
Material and methods
This study included 29 sexually active women with CS and 30 healthy age and body mass index matched women. The Female Sexual Function Index (FSFI) questionnaire, Beck Depression Inventory (BDI) and Short Form Health Survey (SF-36) were filled by each participant. Plasma levels of FSH, LH, PRL, cortisol, DHEA-S, 17-hydroxyprogesterone, androstenedione, free testosterone, total testosterone and estradiol were measured.
Results
Female sexual dysfunction was present in 88.9% of the women with CS and 24.1% of the control group. The CS group showed a lower total FSFI score [16.6 (IQR: 5–23)] compared to the healthy women [26.8 (IQR: 25.5–30.4) (p<0.001)]. The FSFI scores in the arousal, lubrication, orgasm, pain and satisfaction domains were all lower in the women with CS (p<0.001). Both summary scores of the SF-36 were reduced in women with Cushing’s syndrome compared to the control group (p=0.001). The BDI scores of patients were significantly higher than those of the control subjects (p=0.007). In patients with CS, levels of LH, estradiol, and DHEA-S04 were significantly lower while cortisol (p<0.05), and 17 hydroxyprogestrone levels were higher than control subjects (p<0.05).
Conclusion
This study showed that majority of the women with CS had FSD. This may be related to the inhibitory effect of cortisol on sex hormones.
Keywords: Cortisol, Cushing’s syndrome, female sexual dysfunction
Introduction
Female sexual dysfunction (FSD) is a disorder of sexual desire, arousal, orgasm and sexual pain, with a detrimental effect on a woman’s quality of life. Age, education, chronic diseases, medication, psychological and physical conditions have been shown to affect sexual function.[1] Similarly, many neurotransmitters and neuropeptides such as serotonin, dopamine, epinephrine, norepinephrine, histamine, opioids, and gamma-aminobutyric acid (GABA) have been related to the FSD.[2] Disease-states associated with low estrogen levels such as menopause, dysfunction of the hypothalamic-pituitary axis, hyperprolactinemia and premature ovarian failure and androgen insufficiency are the main endocrine disorders known to cause FSD.[3,4]
Cushing’s syndrome (CS) refers to the clinical manifestations induced by chronic exposure to excessive concentrations of glucocorticoids. Glucocorticoid excess causes several metabolic effects and psychiatric symptoms ranging from anxiety to frank psychosis.[5,6] Exposure to supraphysiologic levels of cortisol over a long period of time reveals abnormal limbic system drive on the hypothalamic-pituitary-adrenal axis and causes depression.[7] Moreover, studies investigating health-related quality of life (HRQoL) in patients with active CS and healthy subjects and patients with other pituitary neoplasms defined worse HRQoL scores in patients with CS.[8,9]
Corticosteroids have a profound effect on many regulatory systems of the body, including the reproductive system. Excess adrenal androgens and cortisol both suppress gonadotropic function, which results in an array of gonadal dysfunctions. Indeed, most female patients have oligomenorrhea and amenorrhea, and frequently show infertility problems. Also hirsutism, due to the excess of adrenocortical androgens, is extremely frequent in women with Cushing’s syndrome.[10] However, scant data exist on sexual function in patients with CS.
The primary aim of our study was to investigate the incidence of sexual dysfunction in women with Cushing’s syndrome and compare it with an age-matched healthy control women using the Female Sexual Function Index (FSFI) questionnaire. The quality of life and depression status were also evaluated in these patients. The secondary aim of the study was to assess the correlation between sexual dysfunction and hormone levels in women with Cushing syndrome.
Material and methods
A total of 29 sexually-active women with CS, followed up and treated at a tertiary referral center between 2012 and 2014, were included and compared with 30 healthy age-and body mass index matched female subjects.
Exclusion criteria were usage of any drug that could possibly affect sexual or psychiatric status like oral contraceptives, heavy smoking, presence of inflammatory genital disease or vaginal discharge; musculoskeletal, neurologic, inflammatory, or clinically significant chronic diseases, pregnancy; menopause or emergennce of these conditions during the study.
All participants had a stable heterosexual relationship and were sexually active with a normal sexually-active male partner. The frequency of sexual activity per week and personal sexual history were obtained from the subjects. Demographic features and a detailed medical history, which included the presence of any systemic disease, use of medication(s), menstruation status and cigarette use were obtained from all subjects. Height and weight values were recorded to calculate the body mass index (BMI). A detailed physical and gynecologic examination was performed. The previous therapies and remission status of CS were examined for the patient group.
For the assessment of sexual satisfaction, the Female Sexual Function Index (FSFI), which was first described by Rosen et al.[11] was applied for all subjects. The FSFI survey was validated for Turkish women. The same cut-off values were defined for Turkish population.[12] The FSFI includes a total of six domains including sexual desire, arousal, lubrication, orgasm, satisfaction and pain during sexual intercourse. The total score ranged between 2–36 points, and a total score of <26.55 indicated sexual dysfunction as described by Wiegel et al. and in various studies.[11,13,14] The questionnaire survey was conducted during the early follicular (days 2–5) phase of the participants’ menstrual cycle.
All the subjects completed the Beck Depression Inventory (BDI), a 21-question multiple-choice self-report inventory, which measures the severity of depression. An analysis of the validity and reliability of its use within the Turkish population has already been conducted and a cut-off score of 17 points was determined.[15]
Short-Form Health Survey instrument (SF-36) was used to evaluate quality of life of the subjects.[16,17] Two summary scores, the mental component summary (MCS) and the physical component summary (PCS), can be derived from the eight domains of SF-36. The MCS and PCS are designed to have a population mean score of 50 with a standard deviation of 10. Validation for SF-36 in the Turkish population was undertaken by Kocyigit et al.[18].
The diagnosis of CS was established using appropriate tests such as 24-h urinary cortisol (UFC), late-night plasma or salivary cortisol, overnight 1 mg or the classic 48 h low-dose dexamethasone suppression test (DST).[10] Remission was defined as finding of overnight 1 mg DST <1.8 mcg/dL and 24 h UFC levels in the normal reference range of the laboratory.
Fasting venous blood samples were drawn into plain tubes from patients with CS and controls in their early follicular phases before nine am after overnight fasting. The blood samples were centrifuged for 10 minutes at 4000 rpm at 4°C. For the determination of hormones freeze serum aliquots were immediately froze and stored at −20°C until further analysis. Prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), dehydroepiandrosterone-SO4 (DHEA-SO4), free testosterone, 17α hydroxyprogestrone (17-OH progesterone), androstenedione, estradiol (E2) and cortisol levels were measured twice using a commercial enzyme-linked immunosorbent assay (ELISA) kit.
The study protocol was approved by the Ethics Committee of Cerrahpasa Medical Faculty, Istanbul University (date:02.18.2013 and no. 3954). All the subjects read and signed the informed consent forms before being enrolled in the study.
Statistical analysis
The data were statistically analyzed with Statistical Package for the Social Sciences (SPSS Inc.; Chicago, IL, USA) 15.0 package. Birth and residency settlement type, education and income levels and associated complications of the patients were expressed as numbers and percentages (%). Chi-square test and Fisher’s exact test were used for categorical variables. The variables with significance were evaluated using the Mann-Whitney U and t test to investigate differences between the groups. The results were presented as median values and interquartile ranges [IQR]. The degree of correlation between variables was evaluated using Pearson and Spearman’s correlation tests. P<0.05 was considered statistically significant. Linear regression analysis was used to determine the most important predictors of FSFI. For the variables which differ significantly between groups and could possibly affect FSFI, a hierarchical multiple regression analysis was performed. The variables included in the model were education status (categorical; over and below 8 years), income levels (categorical; high and low), physical-and mental health domains of SF-36 (continuous), and BDI scores (continuous). The unique contribution of having CS on FSFI was then calculated after having adjusted for these variables.
Results
Table 1 shows the demographic characteristics of patient and control groups. In the CS group, 26 women were diagnosed with Cushing’s disease. The remaining three patients had different disease etiologies as adrenal dysfunction (n=1), ectopic Cushing’s syndrome (n=1) and exogenous CS (n=1). The patients with CS had diabetes mellitus (n=2), hypertension (n=4), hypothyroidism (n=1), and anemia (n=1). All of these women were receiving appropriate medical therapy and their comorbid diseases were under control. There was no significant difference between the patient and control group in terms of age, BMI and birth settlement type. The level of education was significantly higher in the control group than the patient group (p=0.001). Also, the income level was significantly higher in the control group (p=0.01).
Table 1.
Demographics data of female patients with Cushing’s Syndrome and healthy controls
| Variable | Syndrome Controls n=29 |
Healthy Cushing’s n=30 |
p |
|---|---|---|---|
| Age, year | 38 (34–44.5) | 39 (33.7–42) | 0.98 |
| BMI (kg/m2) | 30.5 (27.1–33.6) | 30.0 (28.6–32.1) | 0.41 |
| Birth settlement type (n, %) | 0.08 | ||
| Rural area | 10 (33.3) | 4 (12.9) | |
| Urban area | 19 (66.7) | 26 (87.1) | |
| Education status (n, %) | 0.001 | ||
| Literate | 3(10) | 0 | |
| Primary school | 7 (23) | 4 (12.1) | |
| Middle school | 6 (20) | 0 | |
| High school | 7 (26.7) | 13 (48.5) | |
| University | 6 (20) | 13 (39.4) | |
| Income level ($/month) (n, %) | 0.001 | ||
| <500 | 9 (30) | 0 | |
| 500–<1000 | 12 (43.3) | 13 (40.4) | |
| 1000–2000 | 7 (23.3) | 10 (38.4) | |
| >2000 | 1 (3.3) | 7 (21.2) | |
| Associated complications (n, %) | 0.47 | ||
| Present | 10 (32.1) | 9 (28.1) | |
| Absent | 19 (67.9) | 21 (71.9) |
Data were expressed as median (interquartile range). Bold letters represent p<0.05 and were considered statistically significant
According to FSFI questionnaire, 24 (88.9%) out of 29 women with CS and 7 out of 30 women in the control group (24.1%) had female sexual dysfunction. Women with CS had significantly lower FSFI scores, while the median of total FSFI scores for patients with CS and the control group were 16.6 [IQR: 5–23] and 26.8 [IQR: 25.5–30.4], respectively (p<0.001). The arousal, lubrication, the ability to achieve orgasm, pain and satisfaction subdomains of FSFI were significantly lower in patients with CS (Table 2). While mean FSFI desire domain scores were also lower in patients with CS, but it did not reach a statistically significant level (p=0.23).
Table 2.
FSFI, BDI, Questionnaire Scores female patients with Cushing’s Syndrome and healthy controls
| Cushing’s Syndrome n=29 |
Healthy controls n=30 |
p | |
|---|---|---|---|
| Beck | 16 (10–23) | 10 (6.5–14.5) | 0.007 |
| FSFI Total | 16.6 (5–23) | 26.8 (25.5–30.4) | <0.001 |
| FSFI subscores | |||
| Sexual desire | 3.6 (2.4–3.6) | 3.6 (2.7–4.8) | 0.23 |
| Arousal | 2.1 (0–3.9) | 4.2 (3.4–5.4) | <0.001 |
| Lubrication | 3.6 (0–4.5) | 4.8 (3.9–5.7) | <0.001 |
| Orgasm | 1.6 (0–4) | 4.4 (4–5.2) | <0.001 |
| Satisfaction | 2.8 (0.8–4.4) | 4.8 (4–5.2) | <0.001 |
| Pain | 3.6 (0–4.8) | 4.8 (3.8–6) | 0.006 |
Data were expressed as median (interquartile range). Bold letters represent p<0.05 and were considered statistically significant.
FSFI: Female Sexual Function Index; BDI: Beck Depression Inventory
Mean levels of E2, LH and DHEA-SO4 in patients with CS were significantly lower than the control group (p<0.05), whereas cortisol and 17-OH Progesterone levels were higher in the CS group (Table 3, p=0.006).
Table 3.
Hormone levels in female patients with Cushing’s Syndrome and healthy female controls
| Variable | Cushing’s Syndrome, n=29 | Healthy controls, n=30 | p |
|---|---|---|---|
| Estradiol, pg/mL | 20.2 (13.5–29.2) | 26.0 (20–44.4) | 0.03 |
| FSH, mIU/mL | 7.66 (4.8–12.8) | 8.27 (7.1–12.9) | 0.39 |
| LH, mIU/mL | 6.32 (4.4–8.9) | 9.24 (6–11.9) | 0.03 |
| PRL, ng/mL | 10.6 (6.6–15.2) | 9.37 (4.9–11.8) | 0.27 |
| DHEA-SO4, μg/dL | 0.65 (0.2–2.4) | 1.33 (0.8–2.3) | 0.05 |
| Androstenedion, ng/mL | 1.93 (0.9–3.9) | 1.62 (1–1.9) | 0.33 |
| 17_OH progesteron, ng/mL | 0.42 (0.2–1.1) | 0.30 (0.2–0.4) | 0.05 |
| FF. testesteron, pg/mL | 0.87 (0.1–2.9) | 1.15 (0.4–1.8) | 0.45 |
| Cortisol, ug/dL | 202.4 (106–287) | 112.5 (77–177) | 0.006 |
| Progesteron, ng/mL | 0.13 (0.06–0.24) | 0.13 (0.05–0.21) | 0.66 |
PRL: prolactin; LH: luteinizing hormone; FSH: follicle-stimulating hormone; DHEA-SO4: dehydroepiandrosterone-SO4; F Testesterone; free testosterone, 17-OH P;17α hydroxyprogestrone
Data was expressed as median (interquartile range). Bold letters represent p<0.05 and were considered statistically significant
FSFI: Female Sexual Function Index
The SF-36 scores of the patients with CS and controls are shown in Table 4. The scores were lower in women with CS compared with the HCs in seven of the eight domains of the SF-36. While both physical and the mental summary scores of the SF-36 decreased in CS patients, the physical component summary score (PCS) was more profoundly affected than the mental component summary score (MCS) (p=0.001 and 0.012, respectively). Furthermore, BDI scores were higher in the CS group (Table 2, p=0.007).
Table 4.
Short-Form Health Survey Instrument (Sf36) Questionnaire Scores female patients with Cushing’s Syndrome and healthy controls
| Cushing’s Syndrome, n=29 | Healthy controls, n=30 | p | |
|---|---|---|---|
| PF | 40.4 (31.4–45.6) | 49.5 (42.5–55) | 0.002 |
| RP | 35.0 (35–49.2) | 49.2 (43.8–56.2) | 0.004 |
| BP | 37.5 (31.5–46.5) | 46.5 (37.5–51.6) | 0.025 |
| GH | 34.5 (38.9–41.5) | 50.9 (40.6–55) | 0.000 |
| VT | 39.6 (33.7–46.7) | 46.7 (42–56.2) | 0.007 |
| SF | 40.9 (31.1–43.6) | 49.0 (42.2–57.1) | 0.001 |
| RE | 34.3 (29–34.9) | 44.8 (36.7–44.8) | 0.001 |
| MH | 39.1 (26.1–47) | 49.3 (32.8–52.7) | 0.067 |
| PCS | 39.3 (32.2–43.2) | 49.6 (41.5–55.3) | 0.001 |
| MCS | 40.5 (32.7–42.9) | 45.0 (35.6–54.3) | 0.012 |
Data were expressed as median (interquartile range). Bold letters represent p<0.05 and were considered statistically significant.
PF: Physical Functioning; RP: Role Physical; BP: Bodily Pain; GH: General Health; VT: Vitality; SF: Social Functioning; RE: Role Emotional; MH: Mental Health; PCS: Physical Health; MCS: Mental Health
In the correlation analysis of patients with CS, no correlation was found between basal cortisol levels and FSFI scores. However there was a positive correlation between FSFI scores and LH levels (r=0.28, p=0.03). There was also a positive correlation between income levels and FSFI scores (r=0.3, p=0.03) (Figure 1). Additionally, high cortisol levels were positively correlated with BDI scores (r=0.5, p=0.003).
Figure 1.
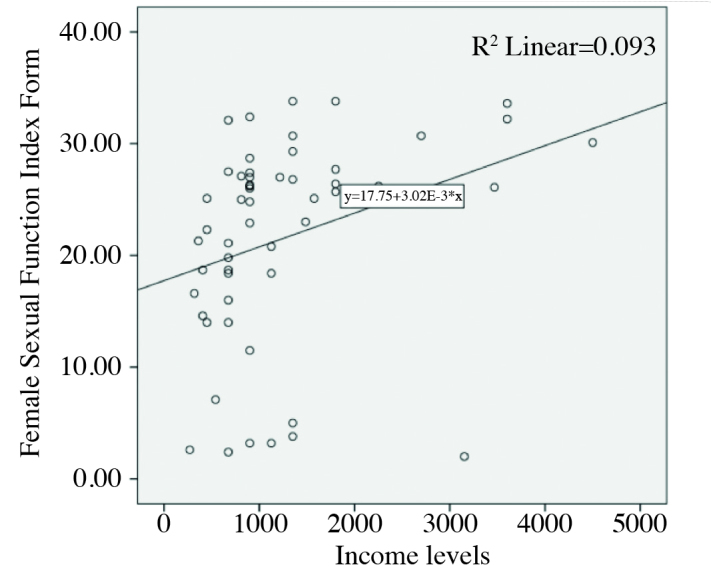
A positive correlation was shown between FSFI total score and income levels
FSFI: Female Sexual Function Index
Discussion
Sexuality is an important part of a woman’s life and wellbeing and it is modulated by a number of factors such as life events, reproduction-related events, relationships, socio-cultural variables, BMI and hormonal status.[19] In our study, we tried to match the patients’ and control group’s age, BMI, racial and relationship status as much as possible, in order to eliminate the possible confounding effects of these variables on sexuality. We demonstrated that patients with CS exhibited higher rate of sexual dysfunction, had poorer quality of life and higher rate of depression as compared to the controls. A total of 88.9% of women with CS had FSD. The total FSFI score and arousal, lubrication, pain, orgasm and satisfaction subdomains of FSFI were significantly lower in female patients with CS. The only domain that did not differ between patients and healthy controls (HCs)was that of sexual desire. Similarly, patients with CS had lower quality of life scores and were additionally more depressed, in comparison to the HCs. The majority of women with CS and gonadotropin deficiency had relatively low LH and estradiol levels during early follicular phase.
Studies investigating the prevalence of FSD in different endocrine diseases have shown decreased FSFI scores in patients with acromegaly and type 2 diabetes mellitus in comparison to the healthy subjects.[4,20] Similarly, both being overweight and obese have been identified as risk factors for sexual dysfunction in men, but the relationship between sexual dysfunction and BMI in females is yet to be determined.[21,22] In a previous study by our group, we showed that obesity did not affect sexual function in women.[23] Likewise, Yaylali et al.[21] investigated FSD in women who were obese and overweight and found no significant difference between obese patients and the nonobese control group in terms of total FSFI scores. All these studies used the same FSFI survey with ours which has been validated for Turkish population by Oksuz et al.[12]. In our study, patients with CS and HCs were matched in terms of BMI. Likewise, we found no difference in terms of FSFI scores between subjects with a BMI over or under 25 suggesting that female sexual function is not related to BMI.
Based on the National Health and Social Life Survey (NHSLS) of 1992, which evaluated a sample of 1749 women aged between 18 and 59 years, a total of 43% of the women reported sexual dysfunction, and low sexual desire being the most common complaint.[24] Similar to this result, the scores of sexual desire domain in our study decreased both in patients with CS and HCs. This supports the NHSLS data. In contrast, in our study, the lowest scores of FSFI were in arousal and orgasm domains in both groups, which differed from NHSLS results. However, scores of these two domains were significantly lower in women with CS relative to healthy women. Additionally women with CS had lower scores in lubrication, satisfaction and pain domains of FSFI when compared to the healthy women. An important point in our study was that HC group had higher socio-cultural status and income level than women with CS. This may have had an effect on high scores of FSFI in the HCs.
There is paucity of data in the literature to establish an association between FSD and chronic hypercortisolism. On the other hand, some data have suggested that, in patients with CS, FSD may be related to the low hormone levels caused by the inhibitory effect of hypercortisolism on gonadotropin release.[25] Hypercortisolism has been shown to block hypothalamic GnRH secretion and hence cause hypogonadotropic hypogonadism.[26] In our study, patients with CS had lower LH levels than HCs. There was also a positive correlation between FSFI scores and LH levels. This might in part explain the higher rate of sexual dysfunction in CS group.
In our study, patients with CS had lower estradiol levels than HCs. As estrogen has a crucial role on maintaining the integrity of vaginal tissues, estradiol levels might also affect sexuality besides the inhibitory effects of high cortisol levels on gonadotropic hormones. Estrogen deficiency results in sexual dysfunction by causing vaginal atrophy and dyspareunia. Furthermore arousal, sexual interest and response also decrease secondarily to estrogen deficiency.[3,27] In line with the latter, arousal, lubrication, pain, orgasm and satisfaction subdomains of FSFI were significantly decreased in patients with CS.
Sexual hormones interact with neurotransmitters in the central nervous system, where the equilibrium between excitatory and inhibitory factors can control sexual functioning.[28] DHEA-SO4 is the major secretory product of the adrenal cortex, an inactive precursor that is metabolized by active androgens and/or estrogens in specific peripheral tissues. Level of DHEA-SO4 decreases with age, irrespective of menopausal status. This may be associated with late-onset hypogonadism and decreased libido in both men and women. Reduction in DHEA-SO4 levels leads to impaired quality of life, low libido and lack of well-being.[29] In our study, the patients with CS had decreased plasma level of DHEA-SO4 when compared to the healthy women. This might be one of the possible factors contributing to the sexual dysfunction in women with CS.
Hypercortisolism does not only affect sexual function but also leads to several comorbidities which themselves might deteriorate sexual well-being. Diabetes, hypertension, cardiovascular diseases, coagulopathy and hypothyroidism are more frequently seen in CS.[30] These systemic disorders may be associated with pelvic atherosclerosis and cause sexual dysfunction. It is worth to remember that, in our study, nearly 50% of the patients with CS had at least one complication associated with hypercortisolism which could lead to sexual dysfunction.
Chronic exposure to hypercortisolism has a significant impact on patient’s health and health-related quality of life (HRQoL). We used the SF-36, a generic health questionnaire, to determine HRQoL among our subjects. Seven of eight domains from the SF-36 had significantly lower scores in the women with CS. We had chosen SF-36 for evaluating quality of life in our subjects because SF-36 is a health questionnaire which can easily predict well-beings of both the patients and the healthy control group.[31]
Women with CS were found to be more depressed than the HCs. The relation between depression and chronic hypercortisolism is very well known. Dysregulation of the biomarker cortisol suggests the presence of an abnormal limbic system drive on the hypothalamic-pituitary-adrenal axis in primary depression.[7] Starkman et al.[7] investigated neuropsychiatric findings in patients with CS and revealed that depressed mood (77%) manifested with a range of symptoms. Most patients described short spells of sadness, whereas others experienced constant hopelessness. Exposure to supraphysiologic stress-level concentrations of cortisol for a long time may be the cause of depression. The positive correlation between cortisol levels and BDI scores in our study confirm this hypothesis. In addition, there was a negative correlation between the SF-36 and BDI scale scores, which could confirm that wellbeing may be directly affected by physiologic mood. In addition, higher SF-36, and lower BDI and, FSFI scores were found in women with CS. Although women with CS have a worse quality of life and more severely depressive mood, we have demonstrated that CS per se affected FSFI independently.
There are some limitations that must be considered when interpreting these results. First, the small number of the patients may limit the statistical significance of the obtained results. Secondly, the patient and control groups were not totally homogeneous, differences such as income level and education status could have affected our results. However, this is the first study which evaluated sexual dysfunction in women with CS.
In conclusion, majority of women with CS in our study had sexual dysfunction. Sexual dysfunction is a multifactorial condition and our findings supported this notion. Additionally, we found that income levels, physiologic mood, wellbeing and sex hormone status are important factors in FSD. Further large-scale multi-center studies are needed to clarify the impact of these factors on the pathogenesis of FSD in CS.
Acknowledgements
The study was supported by the Research Fund of the Istanbul University, Istanbul, Turkey, project number 25885. This research did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sector.
Footnotes
This study was presented in poster presentation form at ESAU Bodrum Meeting, 11.04.2017 as the second best oral presentation in the meeting.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Istanbul University Cerrahpaşa School of Medicine (Date: 18.2.2013/No: 3954).
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – P.K.; Design – P.K., F.E.K., Supervision – P.K., F.E.K., A.K.; Resources – F.E.K., H.M.O., Materials – F.E.K.; Data Collection and/or Processing – F.E.K., H.M.O.; Analysis and/or Interpretation – F.E.K., H.M.O., M.O., E.S., P.K., A.K.; Literature Search – F.E.K., H.M.O., P.K.; Writing Manuscript – F.E.K.; Critical Review – P.K., A.K.; Other – M.O., E.S.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Aslan E, Fynes M. Female sexual dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:293–305. doi: 10.1007/s00192-007-0436-3. https://doi.org/10.1007/s00192-007-0436-3. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AL, Truss MC. Mediators of the female sexual response: pharmacotherapeutic implications. World J Urol. 2002;20:101–5. doi: 10.1007/s00345-002-0271-6. https://doi.org/10.1007/s00345-002-0271-6. [DOI] [PubMed] [Google Scholar]
- 3.Amato P. Categories of female sexual dysfunction. Obstet Gynecol Clin North Am. 2006;33:527–34. doi: 10.1016/j.ogc.2006.10.003. https://doi.org/10.1016/j.ogc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Celik O, Hatipoglu E, Akhan SE, Uludag S, Kadioglu P. Acromegaly is associated with higher frequency of female sexual dysfunction: experience of a single center. Endocr J. 2013;60:753–61. doi: 10.1507/endocrj.ej12-0424. https://doi.org/10.1507/endocrj.EJ12-0424. [DOI] [PubMed] [Google Scholar]
- 5.Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P, et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383–92. doi: 10.1530/EJE-11-0272. https://doi.org/10.1530/EJE-11-0272. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary. 2003;6:81–7. doi: 10.1023/b:pitu.0000004798.27230.ed. https://doi.org/10.1023/B:PITU.0000004798.27230.ed. [DOI] [PubMed] [Google Scholar]
- 7.Starkman MN. Neuropsychiatric findings in Cushing syndrome and exogenous glucocorticoid administration. Endocrinol Metab Clin North Am. 2013;42:477–88. doi: 10.1016/j.ecl.2013.05.010. https://doi.org/10.1016/j.ecl.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Carluccio A, Sundaram NK, Chablani S, Amrock LG, Lambert JK, Post KD, et al. Predictors of quality of life in 102 patients with treated Cushing’s disease. Clin Endocrinol (Oxf) 2015;82:404–11. doi: 10.1111/cen.12521. https://doi.org/10.1111/cen.12521. [DOI] [PubMed] [Google Scholar]
- 9.Webb SM, Badia X, Barahona MJ, Colao A, Strasburger CJ, Tabarin A, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol. 2008;158:623–30. doi: 10.1530/EJE-07-0762. https://doi.org/10.1530/EJE-07-0762. [DOI] [PubMed] [Google Scholar]
- 10.Bertagna X, Guignat L, Groussin L, Bertherat J. Cushing’s disease. Best Pract Res Clin Endocrinol Metab. 2009;23:607–23. doi: 10.1016/j.beem.2009.06.001. https://doi.org/10.1016/j.beem.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. https://doi.org/10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 12.Oksuz E, Malhan S. Reliability and validity of the Female Sexual Function Index in Turkish population. Sendrom. 2005;17:54–60. [Google Scholar]
- 13.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. https://doi.org/10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 14.Witting K, Santtila P, Jern P, Varjonen M, Wager I, Hoglund M, et al. Evaluation of the female sexual function index in a population based sample from Finland. Arch Sex Behav. 2008;37:912–24. doi: 10.1007/s10508-007-9287-8. https://doi.org/10.1007/s10508-007-9287-8. [DOI] [PubMed] [Google Scholar]
- 15.Hisli N. A study on the validity of Beck Depression Inventory. Turkish Journal of Psychology. 1988;6:118–23. [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. https://doi.org/10.1097/00005650-199206000-00002. [PubMed] [Google Scholar]
- 17.Wilson S, Sharp CA, Davie MW. Health-related quality of life in women referred for bone density assessment: relationships with bone mineral density, fracture and co-morbidity. Qual Life Res. 2015;24:1235–43. doi: 10.1007/s11136-014-0851-0. https://doi.org/10.1007/s11136-014-0851-0. [DOI] [PubMed] [Google Scholar]
- 18.Kocyigit H, Aydemir O, Olmez N, Memis A. Reliability and validity of the Turkish version of Short-Form-36 (SF-36) Turkish J Drugs Therap. 1999;12:102–6. [Google Scholar]
- 19.Mimoun S, Wylie K. Female sexual dysfunctions: definitions and classification. Maturitas. 2009;63:116–8. doi: 10.1016/j.maturitas.2009.04.003. https://doi.org/10.1016/j.maturitas.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Erol B, Tefekli A, Ozbey I, Salman F, Dincag N, Kadioglu A, et al. Sexual dysfunction in type II diabetic females: a comparative study. J Sex Marital Ther. 2002;28(Suppl 1):55–62. doi: 10.1080/00926230252851195. https://doi.org/10.1080/00926230252851195. [DOI] [PubMed] [Google Scholar]
- 21.Yaylali GF, Tekekoglu S, Akin F. Sexual dysfunction in obese and overweight women. Int J Impot Res. 2010;22:220–6. doi: 10.1038/ijir.2010.7. https://doi.org/10.1038/ijir.2010.7. [DOI] [PubMed] [Google Scholar]
- 22.Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res. 2005;17:391–8. doi: 10.1038/sj.ijir.3901333. https://doi.org/10.1038/sj.ijir.3901333. [DOI] [PubMed] [Google Scholar]
- 23.Kadioglu P, Yetkin DO, Sanli O, Yalin AS, Onem K, Kadioglu A. Obesity might not be a risk factor for female sexual dysfunction. BJU Int. 2010;106:1357–61. doi: 10.1111/j.1464-410X.2010.09348.x. https://doi.org/10.1111/j.1464-410X.2010.09348.x. [DOI] [PubMed] [Google Scholar]
- 24.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. https://doi.org/10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 25.Lado-Abeal J, Rodriguez-Arnao J, Newell-Price JD, Perry LA, Grossman AB, Besser GM, et al. Menstrual abnormalities in women with Cushing’s disease are correlated with hypercortisolemia rather than raised circulating androgen levels. J Clin Endocrinol Metab. 1998;83:3083–8. doi: 10.1210/jcem.83.9.5084. https://doi.org/10.1210/jc.83.9.3083. [DOI] [PubMed] [Google Scholar]
- 26.Dorn LD, Burgess ES, Dubbert B, Simpson SE, Friedman T, Kling M, et al. Psychopathology in Patients with Endogenous Cushings-Syndrome-Atypical or Melancholic Features. Clin Endocrinol (Oxf) 1995;43:433–42. doi: 10.1111/j.1365-2265.1995.tb02614.x. https://doi.org/10.1111/j.1365-2265.1995.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 27.Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril. 2001;76:456–60. doi: 10.1016/s0015-0282(01)01978-1. https://doi.org/10.1016/S0015-0282(01)01978-1. [DOI] [PubMed] [Google Scholar]
- 28.Palacios S. Hypoactive Sexual Desire Disorder and current pharmacotherapeutic options in women. Womens Health (Lond Engl) 2011;7:95–107. doi: 10.2217/whe.10.81. https://doi.org/10.2217/WHE.10.81. [DOI] [PubMed] [Google Scholar]
- 29.Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–96. doi: 10.1677/joe.1.06264. https://doi.org/10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 30.Arnaldi G, Mancini T, Tirabassi G, Trementino L, Boscaro M. Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J Endocrinol Invest. 2012;35:434–48. doi: 10.1007/BF03345431. https://doi.org/10.1007/BF03345431. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. https://doi.org/10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]


