Abstract
Scleritis is an inflammatory process involving the outer coating of the globe which is characterized by focal or diffuse hyperemia, moderate to severe pain, and frequent impairment of vision. Most cases of scleritis are autoimmune in nature and are managed with topical and/or systemic corticosteroids. Infectious scleritis is a less common entity, occurring in 5%–10% of cases, and requiring directed antimicrobial therapy. We present a case of Nocardia farcinica anterior nodular scleritis diagnosed via positive culture of an excisional biopsy of a scleral nodule. The patient improved after combined surgical and medical therapy with amoxicillin-clavulanate and moxifloxacin for 12 months. Based on a literature review, a summary of reported cases of infectious scleritis is provided, and guidelines pertaining to diagnosis and management are offered.
Keywords: infectious scleritis, infection, scleritis, nodular scleritis, anterior scleritis
CASE REPORT
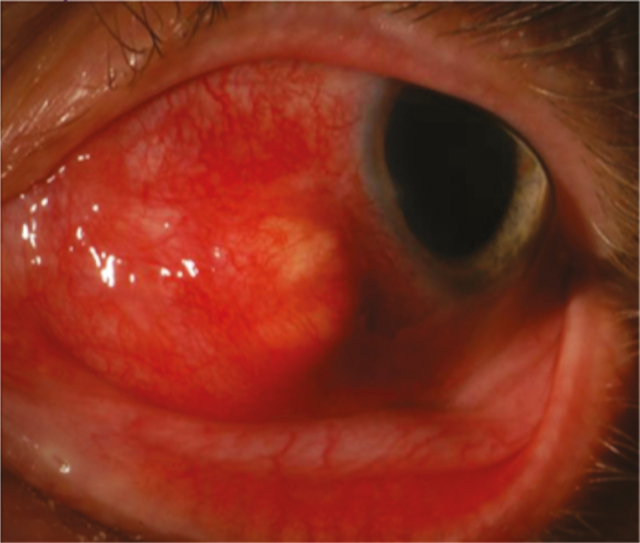
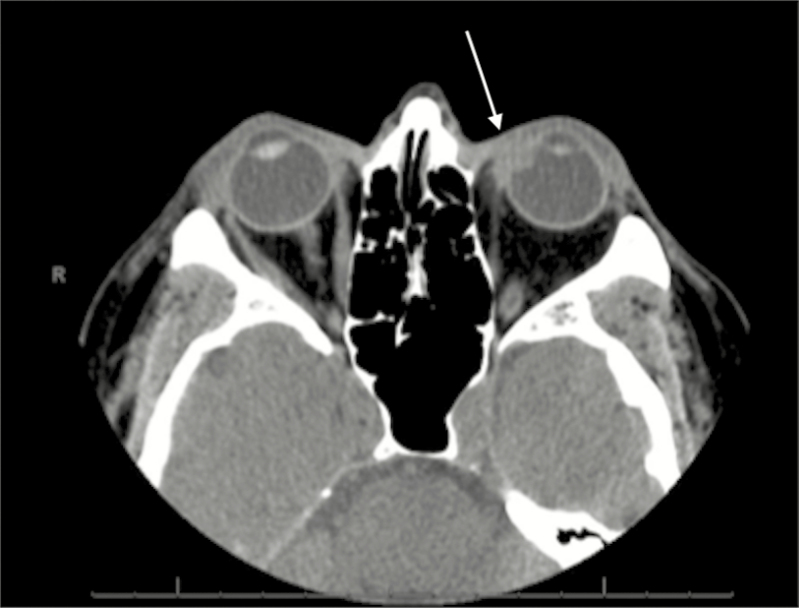
A previously healthy 59-year-old male presented to his ophthalmologist in October 2015 with acute onset left eye pressure and pain, followed by the development of erythema along the medial aspect of the eye. He denied any preceding injury or trauma to the eye, foreign body sensation, or prior ocular surgery. There were no associated systemic symptoms such as fevers, weight loss, rash, ulcers, or musculoskeletal complaints. He did relate that he was involved in a home renovation project for the previous several months that entailed tearing down walls. He also reported that he had been using a leaf blower in his yard quite frequently over the past several weeks, but he did not recall getting any debris in his eye. During those activities, he did not wear any eye protection. Ophthalmologic examination of the left eye revealed nasal injection from 6 o’clock to 11 o’clock with chemosis and a small nodule on the medial aspect of the globe. He was diagnosed with episcleritis of the left medial eye, and therapy with topical prednisone drops was initiated. Seven days later, he returned to his ophthalmologist with minimal resolution of his symptoms and new onset blurry vision. At that time, topical prednisone was discontinued, and ibuprofen 800 mg 3 times a day was initiated. He returned 2 days later for follow-up, at which time oral prednisone 40 mg daily for 1 week was initiated due to poor response to the ibuprofen. The prednisone was subsequently increased to 80 mg daily due to lack of improvement, and he was referred to Wake Forest Baptist Medical Center for specialty consultation in ophthalmology. The preliminary diagnostic impression was episcleritis with possible evolving anterior nodular scleritis. His ocular examination revealed an erythematous and elevated nodule with associated scleral injection of the left eye (Figure 1). There was no associated keratitis or anterior uveitis. Serologies were obtained to rule out infectious and inflammatory etiologies and included rapid plasma reagin, interferon gamma release assay, antinuclear antibody, and rheumatoid factor, all of which were negative or nonreactive. Screening for HIV was not done due to lack of risk factors. Computed tomography of the left orbit revealed focal thickening of the left anteromedial sclera with extension of inflammation into the contiguous vitreous that measured 6 × 9 mm (Figure 2). An aspiration biopsy of the nodule for cultures was performed, and the patient was admitted to the hospital for empiric antimicrobial therapy. Infectious diseases was consulted and recommended empiric treatment with intravenous vancomycin, cefepime, and voriconazole. The aspirate from the nodule subsequently grew only diphtheroids on routine culture, the significance of which was unclear. After 5 days of inpatient parenteral antimicrobial therapy with marginal improvement in local inflammatory signs, the patient was discharged on ongoing empiric oral therapy with linezolid, ciprofloxacin, and voriconazole. He then returned to the ophthalmology clinic 2 days later, at which time he underwent excisional biopsy of the scleral nodule and injection of intralesional moxifloxacin. Per observations at surgery, the nodule was characterized as an abscess that contained a small amount of purulent fluid. Gram stain, fungal smear, and acid-fast smear of the fluid were negative. Cytopathology revealed only acute and chronic inflammation with reactive changes; no organisms were seen. Therapy with oral linezolid, ciprofloxacin, and voriconazole was continued while final culture data were pending. Subsequently, 2 of 3 acid-fast cultures from the biopsy grew Nocardia farcinica. The specimen was then sent to the University of Texas Health Sciences Center microbiology lab for susceptibility testing. Monotherapy with linezolid was initially maintained while awaiting minimum inhibitory concentration data. Treatment was then changed to oral amoxicillin–clavulanate and moxifloxacin for a total of 12 months based on the susceptibility. Trimethoprim–sulfamethoxazole was not utilized as the patient previously had a major allergy to that drug. With ongoing antimicrobial therapy, the patient’s ocular inflammation slowly improved. At his 12-month follow-up visit, all of the patient’s symptoms had resolved, and his ocular exam findings had normalized, aside from some persistent scleral thinning at the infection site.
Figure 1.
Left eye nodule with surrounding chemosis.
Figure 2.
Computed tomography of the orbit showing invasion of infection into the vitreous (white arrow).
DISCUSSION
Infectious scleritis (IS) is an entity not well described outside of the ophthalmologic literature, where it is noted to occur in about 5%–18% of scleritis cases [1–3]. Physicians trained in infectious diseases are generally well versed in the manifestations and causes of infection at most sites in the human host; the eye and associated ocular infections are frequently an area of less familiarity. As such, input from and collaboration with colleagues in ophthalmology are critical if management of eye infections is to be successful.
Scleral inflammatory disorders of the eye can be classified as episcleritis, in which inflammation is superficial, and scleritis, characterized by location as anterior or posterior, which is associated with deeper inflammation that is more destructive [4, 5]. In patients with scleritis, the nature of the scleral inflammatory process is often described as diffuse or nodular and may be necrotizing or non-necrotizing, with each of those descriptions providing some degree of etiologic insight [2]. Scleritis is most often associated with autoimmune or connective tissue disorders but may also be idiopathic or infectious in etiology. Clinically, autoimmune scleritis may be indistinguishable from IS, which creates challenges in the selection of appropriate therapy [2, 6–8]. If initially treated with immunosuppressive or anti-inflammatory therapies, IS can result in a number of vision-threatening complications such as cataract, glaucoma, endophthalmitis, vision loss, and loss of the globe [6, 9].
Clinical presentation of IS includes redness, pain, and tearing of the affected eye [2, 7]. Discomfort is often mild to moderate in the setting of nodular scleritis, whereas pain is frequently severe with necrotizing scleritis [2]. If pain is out of proportion to exam findings, one should consider an infectious etiology [2]. Time to presentation of infection varies from days to years and has been reported to occur up to 30 years after an injury [10, 11]. In patients with nodular IS, nodules can be solitary or multifocal and often appear to be yellow colored under the conjunctiva [2, 7]. The presence of multiple nodules has not been associated with a specific pathogen, but rather may be more related to factors such as the burden of infection [12]. Vision may be normal, but patients frequently present with diminished visual acuity [7].
Bacteria, fungi, parasites, and viruses have all been reported as etiologies of IS [2, 7, 8, 10], but bacterial infection is by far most common, with a reported range of 53%–100% [2, 7, 10]. In case series of patients with IS (Table 1), P. aeruginosa is etiologic in about 25% of patients, whereas all pseudomonas species account for about 40% of cases of IS (Table 1). Although fungal infection is rare, it appears to be most common in climates that are hot or humid, theorized to be due to the heavier spore load in the environment [7]. Bacterial infection tends to present more acutely, whereas infections due to fungi, Nocardia, and mycobacteria are more indolent in onset and presentation [2, 10]. Time to presentation varies and can be months to decades after an inciting event [2, 9, 13].
Table 1.
Summary of Reported Cases of Infectious Scleritis Including Demographics, Risk Factors, Treatment, Outcomes, and Microbiology [4, 5, 7, 8, 9, 10, 12, 13]
| No. (%) | |
|---|---|
| Total, No. eyes | 224 |
| Male | 127 (56.6) |
| Female | 96 (42.8) |
| Age range, y | 12–89 |
| Risk factors | |
| Trauma | 31 (13.8) |
| Surgery | 170 (75.8) |
| Treatment | |
| Topical antibiotics | 221 (98.6) |
| Systemic antibiotics | 154 (68.7) |
| Intraocular antibiotics | 68 (30.4) |
| Surgery | 188 (83.9) |
| Outcomes | |
| Resolved (VAa > 20/200) | 93 (41.5) |
| Recurred | 6 (0.03) |
| Microbiology | 217 (96.8) |
| Pseudomonas aeruginosa | 57 (25.4) |
| Pseudomonas spp. | 28 (12.5) |
| Other gram-negative bacillib | 16 (7.1) |
| Staphylococcus aureus | 16 (7.1) |
| Staphylococcus spp. | 4 (0.9) |
| Streptococcus pneumoniae | 7 (3.1) |
| Streptococcus spp. | 8 (3.50) |
| Enterococcus spp. | 4 (0.9) |
| Gram-positive rodsc | 6 (2.7) |
| Aspergillus spp. | 9 (4.0) |
| Fusarium spp. | 5 (2.2) |
| Scedosporium/Pseudellcheria | 3 (1.3) |
| Other fungid | 18 (8.0) |
| Nocardia spp. | 18 (8.0) |
| Nontuberculous mycobacteria | 14 (6.25) |
| M. tuberculosis | 1 (0.4) |
Abbreviation: VA, visual acuity.
aVisual acuity < 20/200 confers legal blindness [15].
b Klebsiella, Stenotrophomonas, Eikenella, Enterobacter, Chryseobacterium, H. influenzae, Citrobacter, Alcaligenes, Serratia.
c Corynebacterium, Propionibacterium, Brevobacterium.
d Paecilomyces, Candida, Colletotrichum, Cladosporium, Acremonium, Curvularia, Rhodotorula.
Diagnosis of IS can be difficult to achieve as the infection presents very similarly to autoimmune scleritis, which is the most common form of scleritis [6, 8, 10]. History is often a key component in gaining insight as to the cause of infectious scleritis, with the majority of cases having an identifiable inciting factor before symptom onset [10]. The most common predisposing factor is prior ocular surgery including pterygium excision, cataract extraction, and scleral buckle, in which necrosis around the incision site is often seen [1, 6, 7, 10, 12]. It has also been noted that adjunctive use of radiation or antimetabolites increases the risk of developing scleral thinning and avascular necrosis, hence providing a more optimal environment for microbial growth [6, 11]. Trauma to the eye with organic material such as soil or plant matter is also highly associated with IS due to fungi and Nocardia [2, 7, 10]. It is generally recommended that scleral scrapings or lesion debridement be obtained for appropriate staining, as well as bacterial, fungal, and acid-fast cultures, to properly identify the causative organism [2, 7, 8]. Once a microorganism has been isolated in culture, directed antimicrobial therapy can be initiated.
Treatment recommendations for infectious scleritis have not been well established. Initial management with intensive broad-spectrum antibiotics to include coverage for P. aeruginosa and gram-positive organisms with combined topical and systemic modalities has been recommended [1, 6]. In cases of trauma with organic material, empiric antifungal therapy with agents such as voriconazole has been suggested [11]. Early surgical debridement has also been recommended as debridement and debulking of necrotic tissue may facilitate antibiotic access [1, 6, 7, 11, 13]. Sahu et al., and others, reported that the area involved at the time of surgery tended to be much larger than previously anticipated, with a possible “tunnel lesion” requiring further debridement to avoid residual infection [2, 7, 13]. Given the nature of infecting pathogens, time to diagnosis, and poor penetration of antimicrobial therapies into the avascular sclera, multiple surgical interventions may be required [7, 13]. The duration of antimicrobial therapy is poorly defined, though reference texts suggest that it should be “prolonged” [14].
Worse visual outcomes are associated with poor visual acuity at the time of presentation, medical therapy alone, and fungal infections, but they are not significantly associated with any particular organism or the presence of multifocal abscesses [1, 10, 12]. Bacterial biofilm formation is theorized to be responsible for cases of IS refractory to medical management alone [9]. Surgical intervention has been associated with better outcomes in regards to higher rates of globe preservation and improved prognosis, however, not better visual outcomes [9, 10, 12]. Hodson et al. report that only 18% of patients treated with medical therapy alone for a prolonged duration had adequate treatment, with the majority requiring surgery [2, 10]. For the best outcomes, surgical intervention in conjunction with medical therapy has been recommended [7, 9, 11, 13].
CONCLUSION
IS should be considered in patients presenting with a painful unilateral red eye preceded by a history of ocular surgery or trauma. Often nodules or ulcers are present in the affected eye. Infectious etiologies include typical bacteria such as P. aeruginosa and S. aureus, Nocardia, tuberculous and nontuberculous mycobacteria, viruses such as varicella zoster virus, and fungal pathogens such as Aspergillus, Fusarium, Pseudallescheria, and Scedosporium [2, 7]. Debridement of the infected nodule or ulcer is recommended for tissue culture to establish a definitive diagnosis and may result in improved outcomes (Table 1). It would be reasonable to initiate empiric therapy with antimicrobials such as ciprofloxacin and linezolid that would cover P. aeruginosa, gram-positive organisms, and Nocardia until tailored therapy based on culture results is feasible. Those patients with trauma, particularly involving organic material, could potentially be started on empiric antifungal therapy with an agent such as voriconazole. Duration of therapy has not been established for infectious scleritis, and further studies are needed to define the appropriate duration of medical therapy depending on the etiology of the infection and the presence or absence of surgical intervention.
Acknowledgments
Financial suppport. None.
Potential conflicts of interest. Authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ho YF, Yeh LK, Tan HY, et al. Infectious scleritis in Taiwan—a 10-year review in a tertiary-care hospital. Cornea 2014; 33:838–43. [DOI] [PubMed] [Google Scholar]
- 2. Ramenaden ER, Raiji VR. Clinical characteristics and visual outcomes in infectious scleritis: a review. Clin Ophthalmol 2013; 7:2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddy JC, Murthy SI, Reddy AK, Garg P. Risk factors and clinical outcomes of bacterial and fungal scleritis at a tertiary eye care hospital. Middle East Afr J Ophthalmol 2015; 22:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol 2000; 130:469–76. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein DA, Patel SS, Tessler HH. Episcleritis and scleritis. In: Yanoff M, Duker J, ed. Ophthalmology. 4th ed Philadelphia, PA: Elsevier; 2014:209–16. [Google Scholar]
- 6. Paula JS, Simão ML, Rocha EM, et al. Atypical pneumococcal scleritis after pterygium excision: case report and literature review. Cornea 2006; 25:115–7. [DOI] [PubMed] [Google Scholar]
- 7. Kumar Sahu S, Das S, Sharma S, Sahu K. Clinico-microbiological profile and treatment outcome of infectious scleritis: experience from a tertiary eye care center of India. Int J Inflam 2012; 2012:753560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de la Maza M, Hemady RK, Foster CS. Infectious scleritis: report of four cases. Doc Ophthalmol 1993; 83:33–41. [DOI] [PubMed] [Google Scholar]
- 9. Tittler EH, Nguyen P, Rue KS, et al. Early surgical debridement in the management of infectious scleritis after pterygium excision. J Ophthalmic Inflamm Infect 2012; 2:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodson KL, Galor A, Karp CL, et al. Epidemiology and visual outcomes in patients with infectious scleritis. Cornea 2013; 32:466–72. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham MA, Alexander JK, Matoba AY, et al. Management and outcome of microbial anterior scleritis. Cornea 2011; 30:1020–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pradhan ZS, Jacob P. Infectious scleritis: clinical spectrum and management outcomes in India. Indian J Ophthalmol 2013; 61:590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin CP, Shih MH, Tsai MC. Clinical experiences of infectious scleral ulceration: a complication of pterygium operation. Br J Ophthalmol 1997; 81:980–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cunningham ET, Augsburger JJ, Correa ZM, Pavesio C. Uveal tract and sclera. In: Riordan-Eva P, Augsburger JJ, eds. Vaughan and Asbury’s General Opthalmology. 19th ed New York: McGraw-Hill;2018:151–69. [Google Scholar]
- 15. Levenson JH, Kozarsky A. Clinical methods. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed Boston: Butterworths;1990:563–4. [PubMed] [Google Scholar]