Abstract
Chronic pain is a major global healthcare problem that is currently inadequately treated. In addition, the current use of opioids for treatment has reached far beyond the paucity of evidence for long‐term benefits relative to risks. Benefit–risk models for opioid and non‐opioid treatments would benefit from a rational, mechanism‐based understanding of neuroplastic and neurochemical contributions to chronic pain. Here, we evaluate the findings and limitations of representative research investigating brain neuroplasticity and neurochemistry in chronic pain. In sum, the mechanisms of pain‐related neuroplasticity in the brain remain poorly understood because neuroimaging studies have been largely descriptive. We argue that definition is needed of optimal (pain‐resilient) and suboptimal (pain‐vulnerable) functioning of the endogenous opioid system in order to identify neurochemical contributions to aberrant neuroplasticity in chronic pain. We outline the potential benefits of computational approaches that utilize evolutionary and statistical optimality principles, illustrating this approach with mechanistic hypotheses on opioid function. In particular, we discuss the role of predictive mechanisms in perceptual and associative plasticity and evidence for their modulation by endogenous opioids. Future research should attempt to utilize formal computational models to provide evidence for the clinical validity of this approach, thereby providing a rational basis for future treatment and, ideally, prevention.
Linked Articles
This article is part of a themed section on Emerging Areas of Opioid Pharmacology. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.14/issuetoc
Abbreviations
- PAG
periaqueductal grey
- PC
predictive coding
- RL
reinforcement learning
Introduction: The challenge of chronic pain
Chronic pain is a major global health problem that is difficult to manage, because of a number of factors: its high prevalence in the population, comorbidity (e.g. with mental ill‐health), a high degree of heterogeneity (in symptoms and causal mechanisms), and treatments that are largely poorly targeted to underlying mechanisms.
Prevalence and comorbidity
The prevalence of chronic pain varies from 19 to 50% depending on the survey methods used and definitions of severity (Croft et al., 2010), with arthritis as the most common diagnosis. Increased prevalence and disproportionate disability has been reported in the elderly (Gunzelmann et al., 2002; Eriksen et al., 2003; Mottram et al., 2008), with 62% prevalence in those over 75 in the UK (Fayaz et al., 2016). One of the challenges we face is comorbidity with mental health problems. Chronic pain is consistently linked with an increased risk of depression (Breivik et al., 2013). The comorbidity of pain and depression is associated with a greater burden to the individual and society than either condition alone (Mossey and Gallagher, 2004). There has long been debate as to whether depression is a cause or consequence of chronic pain, but it is widely accepted that the presence of one will at least exacerbate the other (Bair et al., 2003). This raises the question as to whether the overlap between chronic pain and depression reflects a true or artificial comorbidity, that is, whether there are shared biological vulnerability factors.
Heterogeneity
This poses a serious challenge for research, because it introduces uncontrolled sources of variance, making it difficult to understand causal processes, and limits generalization of results. How we select patients for research (e.g. according to symptoms criteria) has a bearing on generalisability. We highlight two related types of heterogeneity (Figure 1) that do not have a straightforward relationship. Due to symptom heterogeneity, many patients with chronic pain, particularly those diagnosed with arthritis, have a combination of recurrent acute pain and chronic ongoing pain. Indeed, in osteoarthritis, there is a generally poor relationship between the severity of joint damage and chronic pain (Jordan et al., 1997; Wood et al., 2008). Even in rheumatoid arthritis, widespread pain unrelated to joint pathology is common (Lee and Weinblatt, 2001; Andersson et al., 2013). It can be argued that this is fundamentally due to mechanistic heterogeneity. Chronic pain is increasingly regarded as reflecting a spectrum of mechanisms including nociceptive, neuropathic and central. For example, chronic ongoing pain in arthritis suggests neuropathic elements (Thakur et al., 2014). Chronic pain conditions in which there is very little evidence of nociceptive or neuropathic elements, such as atypical facial pain and chronic widespread pain (or fibromyalgia) have recently been termed ‘nociplastic’ pain (from ‘nociceptive plasticity’) to reflect change in function of nociceptive pathways, probably in the CNS (Kosek et al., 2016).
Figure 1.
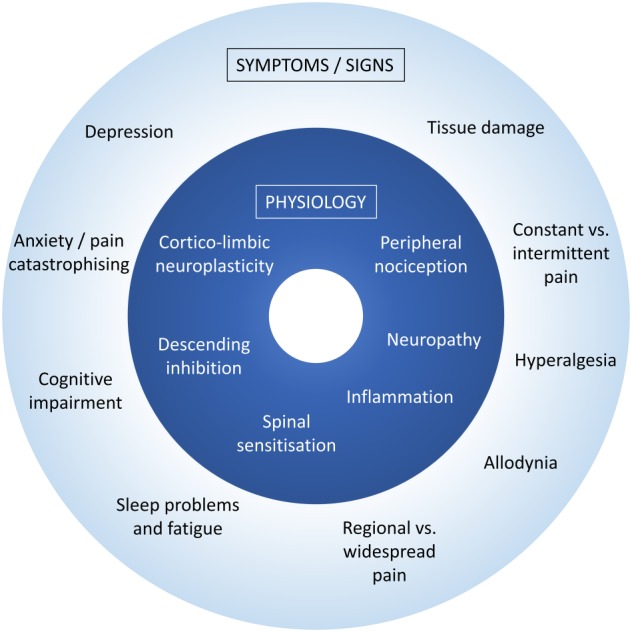
Two types of clinical heterogeneity. Due to symptom heterogeneity, many patients with chronic pain have a combination of recurrent acute pain and chronic ongoing pain, variable evidence of tissue damage and differences in cognitive and mood symptoms. The underlying mechanistic heterogeneity reflects variability in nociceptive, inflammatory, neuropathic and central mechanisms.
Inadequate management
In a European survey, 40% of chronic pain patients reported that their treatment was inadequate (Pain Proposal, 2010). For elderly people in particular, there is a more limited range of pain therapies available because of immobility issues, a lack of efficacy of pain killers, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239 (Roberts et al., 2016) and/or increased side‐effects from pain killers including non‐steroidal analgesics and opiates (Shaheed et al., 2016). In the United States, rates of misuse, abuse and addiction to opioids (three use patterns contributing to the so‐called ‘opioid epidemic’) are concerning, with misuse estimated to range from 21 to 29% and addiction from 8 to 12% (Vowles et al., 2015). This is despite only clinically unimportant short‐term effects on pain and function for guideline‐recommended doses in chronic low back pain (Shaheed et al., 2016) and a complete lack of evidence for long‐term benefits with concomitant dose‐escalating serious risks (Chou et al., 2015). Decision‐making for long‐term use is currently complex and requires individual benefit–risk assessment versus the relative benefits of non‐opioid therapies, a judgement that is currently not evidence‐based (Chou et al., 2015). Generally, patients report a substantial preference for non‐drug therapies (Breivik et al., 2006). Surgical therapies for specific pathologies (e.g. brain stimulation (Cruccu et al., 2007)) are limited in usefulness by eligibility, while other orthopaedic procedures are no better than placebo or exercise (Moseley et al., 2002; Kise et al., 2016). Therapies focused on training the brain to deal with pain better, such as cognitive‐behavioural therapy (Williams et al., 2012; Kong et al., 2016), hypnosis and neuro‐feedback, provide low to moderate effects sizes but potentially long‐term benefits, so further research is needed (Jensen et al., 2014).
There is an urgent need to broaden the choice of safe therapies based on an understanding of the brain's own powerful mechanisms of pain relief and resilience. There is also a need for effective predictive models of benefits and risks of different (e.g. opioid) therapies. In this review, we focus on the potential role of the http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=50 system in neuroplasticity underlying pain vulnerability and resilience, and outline how future computational models have the potential to identify sub‐optimal opioid function and improve patient stratification for treatment.
Neuroplasticity and opioid function in chronic pain
Brain organisation in chronic pain
Neuroplasticity (a change in the neuronal response after repeated experience and use) is a fundamental property of the whole brain (Feldman, 2009) observed in all sensory systems (Seitz and Dinse, 2007). Chronic pain is associated with profound changes in brain structure and function reflecting neuroplasticity in midbrain, thalamus and widespread regions of the cerebral cortex (see Table 1 for example studies). A number of different types of experience‐dependent neuroplasticity occurring in chronic pain have been observed; for example, changes in somatosensory cortices in Complex Regional Pain Syndrome and back pain (Table 1, and reviewed in detail elsewhere; Di Pietro et al., 2013; Kuner and Flor, 2016; Kuttikat et al., 2016), changes in connectivity within the ‘default‐mode network’ (DMN) and connectivity of the DMN with descending modulatory regions (Table 1, especially Baliki et al., 2014; Kucyi et al., 2014) and changes in the midbrain theorized to relate to the balance of inhibition and facilitation in nociceptive processing in fibromyalgia (Lee et al., 2011; Fallon et al., 2013). However, understanding of cellular and molecular mechanisms contributing to central sensitization in musculoskeletal pain conditions is largely limited to the spine (seeThakur et al., 2014). Despite a wealth of observational evidence, we have a very limited understanding of whether and how brain neuroplasticity might contribute to chronic pain symptoms and what vulnerability factors underlie this.
Table 1.
Research methodologies and example results in the neuroimaging of pain
| Pain induction method | Within‐subject comparisons | Between‐subject/group comparisons | Mixed comparisons |
|---|---|---|---|
| 1. Experimental pain induction |
Modulation of pain processing by: Cognitive distraction (e.g. Bantick et al., 2002) Expectation (e.g. Lorenz et al., 2005; Brown et al., 2008a; Atlas et al., 2010) Hypnotic analgesia (e.g. Rainville et al., 1999; Huber et al., 2013) Placebo treatments (e.g. Wager et al., 2004; Watson et al., 2009) Mindfulness meditation (e.g. Brown and Jones, 2010; Zeidan et al., 2011) Endogenous opioid function (Zubieta et al., 2001; Sprenger et al., 2006) |
Cerebral pain processing affected by individual differences in pain catastrophising (e.g. Gracely et al., 2004; Michael and Burns, 2004; Brown et al., 2014; Loggia et al., 2015), fear and anxiety (e.g. Ochsner et al., 2006) and altered sleep (Petrov et al., 2015). | Studies are needed to investigate the moderator effects of between‐subjects characteristics (e.g. pain catastrophising) on within‐subject contextual modulation of pain (e.g. attention, expectation and placebo effects). |
| 2. Chronic pain presence/absence or induction/suppression |
Reductions opioid receptor binding within the medial pain system in patients with post‐stroke pain (Jones et al.,
1999) and arthritic pain (Jones et al.,
1994). Fluctuations in chronic low back pain correlating with functional connectivity of medial prefrontal cortex with other brain regions (Baliki et al., 2011). |
Reduced opioid receptor binding in fibromyalgia versus controls (Harris et al.,
2007), post‐stroke pain versus controls (Willoch et al.,
2004) and central versus peripheral neuropathic pain (Maarrawi et al.,
2007). Changes in resting‐state functional networks between chronic pain conditions and controls (Baliki et al., 2014; Kucyi et al., 2014; Fallon et al., 2016) |
Future research could investigate (i) interactions between psychological factors (e.g. pain catastrophising) and changes in opioid receptor binding in response to chronic pain and (ii) how patients with different patterns of network connectivity in the brain differ in endogenous opioid system functioning. |
| 3. Chronic pain versus acute pain | Greater processing in the medial pain system for chronic arthritic than for acute pain (Kulkarni et al., 2007; Parks et al., 2011). | N/A – requires within‐subject comparisons | Future studies could investigate whether differential medial pain system activity in chronic versus acute pain is related to levels of psychological distress. |
| 4. Chronic pain natural history or treatment response |
Structural brain changes from of arthroplasty in osteoarthritis (Gwilym et al.,
2010). Differences in brain function and structure bertween subacute and chronic pain stages of low back pain (e.g. Baliki et al., 2012). Changes in somatosensory cortical reorganization with recovery in patients with Complex Regional Pain Syndrome (Maihöfner et al., 2004). Changes in prefrontal cortex structure or function with cognitive‐behavioural therapy (e.g. Seminowicz et al., 2013; Čeko et al., 2015) or mindfulness meditation (e.g. Brown and Jones, 2013). |
N/A – requires within‐subject comparisons |
Identifying patient subgroups with different prospective outcomes from baseline brain structure/function (Baliki et al.,
2012). Studies are required to identify predictors of response to difference treatments, such as cognitive‐behavioural therapy, analgesics, physical therapies. |
Studies can be broadly categorised into (i) those that use a standardized acute or tonic pain stimulus to understand different aspects of pain processing, (ii) studies where patients are scanned in different clinical pain states, (iii) where responses are compared between standardized experimental pain and clinical pain, (iv) longitudinal observations of changes in pain processing according to naturalistic changes or treatment‐related changes. Furthermore, in each case, studies can make within‐subject, between subject or mixed comparisons. Examples are provided of studies within each category where possible. Studies further down and/or to the right side of the table generally require greater sophistication and resources, and are therefore less common, but provide greater mechanistic insights into chronic pain vulnerability.
While most types of pain, whether acute or chronic, tend to activate the same network of brain regions (Apkarian et al., 2005), there is evidence that acute experimental pain and clinical pain produce subtle differences in extent and amount of activation. For example, in patients with arthritis, clinical pain results in greater activity within prefrontal, cingulate and insula cortices, amygdala and putamen compared to intensity‐matched experimental acute pain (Kulkarni et al., 2007). However, interpretation of these findings is complicated by the fact that many differences in pain processing are likely to be due to differences in psychological comorbidities. In patients with fibromyalgia, depression is associated with increased experimental pain responses in the amygdala and insula cortex compared to those without depression (Giesecke et al., 2005). More extensive differences, including greater prefrontal and parietal responses (Gracely et al., 2004) have been associated with high levels of pain catastrophising in chronic pain patients. More promisingly, functional MRI studies of natural fluctuations in back pain have demonstrated activations of medial prefrontal cortices that appear to be quite specific to coding chronic pain intensity (Baliki et al., 2006, 2011). Still, the mechanisms underlying these observations are unknown.
Brain reorganisation affecting opioidergic function
Neurochemical differences in chronic pain have been well reviewed (Tracey and Mantyh, 2007; Morton and Jones, 2016) and include changes in http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=20, opioid and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067 receptor systems. Evidence is consistent with the endogenous opioid system being activated mainly within the medial pain system during chronic pain including arthritic and neuropathic pain (Table 1). In chronic pain, observations of neuroplasticity within the network of brain regions mediating opioid‐dependent analgesia point to the possibility of altered output properties of descending controls conferring states of pain vulnerability (Table 1). These might either reflect increased occupation by endogenous opioids or a fall‐out of opioid receptors. In relation to post‐stroke pain, we favour the latter interpretation as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1638 failed to alter the pain in our study (Jones et al., 2004) and this would also explain the requirement for higher doses of synthetic opiates in this type of pain (Rowbotham et al., 2003).
Given the anatomical overlap between brain regions binding exogenous opioids and those undergoing neuroplasticity in chronic pain, an important consideration is whether opioid treatment for chronic pain is one potential driver of observed neuroplasticity. Evidence supporting this thesis is limited to studies of long‐term opiate users showing deficits in certain cognition functions, namely fear‐learning (Basden et al., 2016), prospective memory (Terrett et al., 2014) and episodic foresight (imagining the future) (Terrett et al., 2017), known to involve medial prefrontal interactions with basal ganglia (Peira et al., 2016) that undergo neuroplasticity in chronic pain (Baliki et al., 2014). While cause and effect (between opioid use and cognitive deficits) cannot be established from these studies, observations of changes in opioidergic and dopaminergic circuits and regions predicting chronic pain in the striatum (Baliki et al., 2012), point to the possibility that reinforcement‐related changes in both brain plasticity and behaviour in relation to opioid use and misuse may overlap with some of the observations of chronic pain neuroplasticity. In relation to this it has been hypothesized that changes in motivational learning may be a vulnerability factor for chronic pain (Mansour et al., 2014).
Pain vulnerability resulting from neuroplasticity in the opioid system could be countered by physiologically enhancing pain resilience. One possibility is to inhibit the breakdown of endogenous opioids with inhibitors of natural http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1611 in the brain (Le et al., 2003). Another possibility is increasing OR density through yet‐to‐be‐discovered mechanisms. Indeed, our recent study of patients with arthritis (Brown et al., 2015) suggest that variability in opioid receptor density is a natural aspect of endogenous pain regulation. In particular, patients with greater recent clinical pain had greater opioid receptor binding (consistent with greater opioid receptor density) in the striatum, thalamus, insula and periaqueductal grey (PAG). Evidence for this being a possible mechanism of pain resilience is that binding in the striatum (caudate nucleus) was also correlated positively with acute pain threshold in both patients with arthritis and normal pain‐free volunteers. The mechanism by which this occurs is not clear but one possibility is that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=317 agonism (as a result of endogenous opioid release in response to pain) up‐regulates http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319&familyId=50&familyType=GPCR in humans, as it has been shown to in animals (Wang et al., 1999; Cahill et al., 2001; Morinville et al., 2003).
Cognitive or lifestyle interventions may be able to enhance endogenous opioid mechanisms; for example, exercise in normal volunteers enhances activity of the endogenous opioid system in prefrontal, cingulate and insula cortices (Boecker et al., 2008), but with unknown effects on opioid receptor density. However, the rational use of such interventions for the purposes of enhancing opioid‐dependent pain resilience is limited by a current lack of mechanistic understanding of the function of the endogenous opioid system in the brain.
Investigating pain and opioid mechanisms with neuroimaging
Understanding the role of neuroplasticity and opioid function in chronic pain vulnerability requires a mechanistic understanding of pain processing in the brain. Until the mid‐20th century, pain perception was thought to be a direct reflection of the afferent processes of nociception – pain processing in the brain was considered unidirectional and passively received by a single brain centre (Melzack and Wall, 1965). However, after the introduction of the ‘pain matrix’ theory (Melzack and Wall, 1965), the concept of pain evolved towards active, multidirectional and multicentre cognitive information processing. More recently, the concept of pain‐specific cognitive and emotional networks has been replaced with a view of shared brain functions involved with processing pain as well as other motivationally salient stimuli (Legrain et al., 2011), although it seems likely that pain is likely to be a construct of a specific pattern of activity within this network (Wager et al., 2013). Furthermore, cognitive and emotional processing takes an active role in the endogenous modulation of pain (Table 1). Observations such as these contribute towards our understanding of mechanistic heterogeneity in chronic pain, particularly with regard to understanding individual differences in the efficacy of endogenous pain control, a common factor thought to affect many types of chronic pain (Staud, 2012).
Box 1 – Challenges in understanding the mechanisms of pain vulnerability and resilience
Research question: Investigating a biological mechanism requires asking the right research question. Neuroimaging studies have been largely descriptive, that is, seeking to answer the question of what the brain (including the opioid system) does in response to (or in prediction of) acute and chronic pain, and cognitive and affective modulation of pain. Such descriptions provide useful data for classification, prediction, and for the generation of hypotheses and models, but are unable to test hypotheses to answer why and how; for example, why and how does neuroplasticity in opioidergic brain regions contribute to chronic pain? In other words, we currently lack a comprehensive process theory for the functional role of the opioid system in pain perception and chronic pain vulnerability. Research questions seeking to identify such a theory would help to integrate descriptive research findings and provide greater utility in informing us about what treatments may be appropriate under certain contexts.
Sample selection: Research normally seeks to answer questions in specific populations, involving the selection of recruitment criteria to sample the right patients. Most commonly, selection relies on diagnostic categories and symptom profiles. However, such categories lack predictive clinical validity (e.g. in predicting the outcome of certain treatments), because diagnostic heterogeneity does not currently map on to mechanistic heterogeneity. This means that we cannot distinguish whether descriptive observations of brain abnormalities in a patient group defined by diagnostic category (e.g. osteoarthritis) are due to a unique biological pathology, a psychological comorbidity (that nonetheless has more complex biological substrates), or a difference in cognitive strategy or emotional response to the experiment, for example patient anxiety at being in an MRI scanner.
Design and methodology: While appropriate research design largely follows from the research question and sample, some general considerations are outlined here. Methodological challenges arise from a number of factors including arranging for the subject to be in an appropriate pain state, practical or technological limitations in measuring the target physiological processes, limitations in the number of variables/factors that can be investigated within a single study, and a lack of standardization of how neuroimaging data are acquired, making it difficult (but not impossible) to compare and combine data across sites and studies. More longitudinal studies are also required to establish which brain responses are associative and which causal in relation to pain vulnerability. Future work will benefit from efforts to standardize and share neuroimaging data, enabling larger‐scale and more highly powered research, necessary for longitudinal research and some of the more complex designs (Table 1).
However, the majority of neuroimaging studies of cognitive and emotional influences on pain have been conducted using experimental pain stimuli in healthy people (Table 1). Furthermore, studies are generally descriptive rather than providing evidence of mechanisms underlying cognitive and emotional influences on pain. Hence, while functional brain imaging has made a major contribution to our understanding of pain physiology and pathophysiology over the past few decades, we are still barely scratching the surface of understanding the fundamental mechanisms underlying chronic pain vulnerability.
We identify three main challenges (Box 1): identifying the correct research question, selecting the appropriate participant samples for study, and designing/implementing the appropriate experimental methodology (e.g. Table 1). Here, we focus on the first challenge: identifying the right research question. Descriptive approaches (asking ‘what’ questions) frequently do not provide insight into the causal mechanistic processes that explain observations. By contrast, normative approaches are explicit about the hypothesized function of a system, for example, considering what problem is being solved or goal of the system and how this might be optimally achieved (Niv, 2009), thereby providing a basis for understanding and identifying suboptimal function. Normative approaches commonly refer to the evolutionary principle that the purpose of organisms is to provide optimal or near‐optimal adaption to the (physical and social) environment (Kacelnik, 1997). In this regard, the function of the brain can be thought of as optimizing perception and behaviour through learning processes. This provides the ability to generate and direct test computationally explicit hypotheses about the function of brain systems (Niv, 2009), including neurotransmitters such as opioids, in terms of how they optimize perception and behaviour through learning.
Evolutionary pressures have hard‐wired brain systems towards adaptation through processes that maximize reward (e.g. food, mating opportunities, money and knowledge) and minimize punishment (e.g. pain, hunger and social rejection). These processes can sometimes be related, such that failure to acquire reward is punishing (Tom et al., 2007), and avoiding a punishment or relieving pain is rewarding (Navratilova and Porreca, 2014). In this case, there is a cooperative opposition in the pain and reward systems (Leknes and Tracey, 2008), which has been linked to activity zin the striatum consistent with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940 signalling during associative learning tasks (Seymour et al., 2005, also see Box 2). Likewise, maximizing longer‐term rewards may require suppressing short‐term pain, for example, when reaching limits of physical endurance during exercise in which the long‐term goal is greater physical fitness. In this case, opioidergic activity in the striatum may be important suggested by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1643 release during exercise (Boecker et al., 2008). These reward‐pain motivational interactions have been conceptualised by Fields (2006) according to a motivation‐decision model, in which neural decision‐making is based on the predicted homeostatic value to the organism of each option. Current reinforcement learning (RL) models (Box 2) require theoretical advances to account for how the value of certain actions can be approximated when rewards depend on the long‐term consequences of those actions (Gershman and Daw, 2017). These more advanced RL models are likely to enable a more complete understanding of the cooperation between dopaminergic and opioidergic signalling in the basal ganglia.
In parallel, the specific function of endogenous opioids in the brain requires further elaboration. Evidence summarized in Figure 2 challenges the assumption that opioids only mediate antinociceptive responses, suggesting rather that opioids modulate nociception bi‐directionally via the basal ganglia and insula, converging on a common midbrain descending pathway. An alternative hypothesis of opioid function is called for to account for these considerations. A promising candidate that we will explore in the following sections (see also Figure 2) is that the opioid system within the basal ganglia and midbrain functions to optimize predictions about pain, consistent with striatal functions in learning predictions of reward value and that of the amygdala and anterior insula in learning predictions of aversive value. Testing this hypothesis will require models of the explicit neural computations involved.
Figure 2.
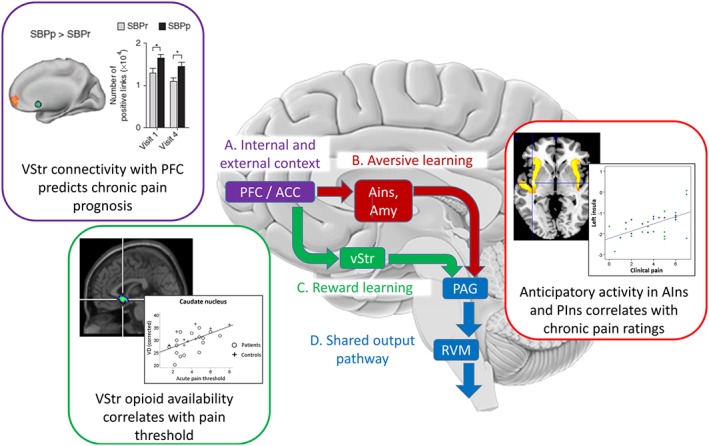
Opioidergic modulation of motivational learning processes play a role in the contextual modulation of pain. For illustrative purposes, the scheme simplifies the main opioid‐mediated, top‐down pathways involved in the modulation of nociception by internal (e.g. beliefs, mood and distress) and external (e.g. placebo treatment) context. Further bottom‐up and recurrent pathways exist but are not illustrated for clarity and were recently reviewed elsewhere (Büchel et al., 2014; Navratilova and Porreca, 2014). (A) Many cortical systems potentially mediate phenomena such as placebo hypoalgesia (Büchel et al., 2014), but all are likely to originate within the prefrontal cortex and its interactions with the perigenual Anterior Cingulate Cortex (ACC) as shown by their activation during anticipation of pain and prediction of placebo analgesia (Wager et al., 2004, 2011). These regions, through interactions with limbic areas, provide contextual cognitive information critical to the learning of reward and aversive value information (see Navratilova and Porreca, 2014). This is consistent with observations of abnormal Ventral Striatum (vStr) activity being a potential vulnerability factor in chronic pain (Purple inset, Baliki et al., 2012). SBPp: Subacute back pain, persistent. SBPr: Subacute back pain recovered. (B) Anterior Insula (AIns) and Amygdala (Amyg) form a circuit required for fear learning (Critchley et al., 2002) that shows opioid release during placebo analgesia (Zubieta et al., 2005). While anterior insula is deactivated during placebo analgesia (Price et al., 2008), it mediates the effects of negative expectations on increased pain (Brown et al., 2008b; Atlas et al., 2010) and shows abnormal anticipatory processing in patients with chronic pain (Red inset, Brown et al., 2014). (C) A role for opioidergic activity in vStr in setting nociceptive sensitivity/salience is evidenced by bidirectional modulation of vStr attenuating or enhancing nociception (Gear and Levine, 2011), which depends on upstream ACC opioid circuits (Navratilova et al., 2015). Consistent with this, pain thresholds in chronic pain patients correlate with opioid receptor availability in vStr (Green inset, Brown et al., 2015) presenting a potential vulnerability/resilience factor. (D) The cortical and subcortical projections all converge onto the PAG‐RVM‐spinal cord system which either inhibits or facilitates nociception (Fields, 2004).
Box 2 – Computational models of learning and perception
Computational neuroscience uses normative principles to understand explicit mechanisms of brain function. A common approach, inspired from David Marr's influential work (Marr, 1982), is to (i) define the computational optimization problem (i.e. identify the problem the brain is trying to solve and its underlying principles), (ii) identify a range of potential process models/algorithms (i.e. what representations and operations are required in the brain to solve the problem), and (iii) constrain the repertoire of process models with reference to biological observations (i.e. how do neurons carry out the algorithm to achieve the computational goal?). A successful example is that of RL. Originally popularized by Rescorla and Wagner (1972, RL models formalize how associative learning occurs. The optimization problem, maximizing future reward, is solved by learning the value of environmental cues according to an algorithm in which organisms are ‘surprised’ (generate a prediction error) when events violate expectations. Prediction errors have been equated to the motivational salience of environmental cues that provide information about rewards (Berridge, 2007). Critically, empirical evidence supports RL models in that both reward and aversive prediction errors are closely related to phasic activation of the ventral striatum consistent with dopaminergic signalling (O'Doherty et al., 2003; Seymour et al., 2004).
While RL provides a compelling model of reward and aversive learning, it does not provide a model for pain‐related neuroplasticity in sensory pain networks. An important consideration is that in order to optimally learn and adapt to the environment, the brain needs to make perceptual decisions (e.g. ‘Is this painful or not?’; ‘Is this situation dangerous?’) as well as behavioural decisions (e.g. avoidance or approach). This leads to the question of how the brain deals with the uncertainty, a major topic of research in the neurosciences (see Bach and Dolan, 2012). Sources of uncertainty in sensory perception include neuronal noise and incomplete/ambiguous information, meaning that there are many possible states of the world that could give rise to any one sensory input (Friston, 2003). This leads naturally to the idea that perception is a process of unconscious, statistical inference (the ‘Bayesian brain’ hypothesis), which to be optimal requires that the brain represents sensory information in the form of probability distributions (with variance indicating uncertainty) (Friston, 2003). However, there is debate as to whether the brain can actually perform such computations due to the problem of bounded rationality that recognizes cognitive processing capacity limitations available to the brain (Gershman et al., 2015). Recent computational theories of perceptual inference borrow theoretical principles from statistical physics and information theory to solve this problem by assuming that the optimality principle the brain uses for sensory perception is to minimize free‐energy, or the upper bound on the surprise (entropy) of the sensory states it experiences (Friston et al., 2006). This principle converts intractable Bayesian inference into a neuronally computable optimization problem.
Bayesian models of pain neuroplasticity
Computational neuroscience has provided normative models for how the brain might optimize perception and behaviour (Box 2), with many approaches converging on the view that the brain applies statistical inference to sensory inputs in order to resolve uncertainty. How this is implemented in the brain is an active topic of research. A leading framework is that of predictive coding (PC), which provides a biologically plausible scheme based on simple rules of synaptic plasticity (Hebbian learning) (Bogacz, 2017). PC models assume that learned knowledge (implicit or explicit) about the world is represented hierarchically as a set of ‘priors’, which capture the statistical regularities of brain activity (reflecting environmental regularities) at lower levels to resolve sensory uncertainty.
PC provides an attractive framework to account for the modulation of pain by learning processes such as those underlying placebo and nocebo effects (Büchel et al., 2014) commonly attributed to the endogenous opioid system. We and others (Edwards et al., 2012; Kuttikat et al., 2016) have also suggested that PC is an appealing approach to understand functional and potentially subsequent structural plasticity occurring in chronic pain. According to the Bayesian optimality principle, pain experience would depend on the extent of the mismatch between the learnt predictions and current sensory inputs, but also on their relative ‘precision’ weights, reflecting the uncertainty of the representation. Precision weights are thought to be learnt over a lifetime (Bogacz, 2017), thus providing stable or slowly changing traits. This may be particularly important in chronic pain conditions associated with psychological trauma in childhood and early adulthood (Gupta et al., 2007). However, to date, there has been no investigation of whether the PC framework provides an explanation for neuroplastic changes in chronic pain.
Our experimental observations in healthy individuals and chronic pain patients have, however, provided some general support for a Bayesian account of pain vulnerability. If the brain uses a Bayesian updating scheme for pain perception, at least three phenomena would need to occur: firstly, that the brain predicts nociceptive inputs; secondly, that the brain models the uncertainty (inverse precision) of those predictions; thirdly, that greater precision (certainty) in predictions more greatly biases pain perception in the direction of those predictions. Our and others research measuring anticipatory brain responses has indeed discovered that neural processes prior to the experience of pain predict the subsequent nociceptive input and serve as the basis for the modulation of pain perception by expectations (Brown et al., 2008a, 2008b; Atlas et al., 2010). Furthermore, we have shown that subjective confidence (as a metacognitive representation of Bayesian precision; Meyniel et al., 2015) in predictions is related to the extent to which expectations influence pain perception and is related to activity in the anterior insula cortex (Brown et al., 2008b), a region through which expectancy effects are mediated (Atlas et al., 2010). While this research does not directly test a Bayesian model or a PC scheme for pain perception, it does point to the importance of predictive processes in acute pain and is consistent with Bayesian optimality principles. Furthermore, predictive processing appear to have relevance to chronic pain: In patients with fibromyalgia, the anterior and posterior insula are over‐active when anticipating pain, while anticipatory posterior insula activity predicts pain symptoms in patients with fibromyalgia as well as those with osteoarthritis (Brown et al., 2014).
However, despite recent interest in the Bayesian and PC framework for acute pain, knowledge is currently limited on how these principles pertain to the situation of chronic pain. Very little work has been done in computational modelling of chronic pain symptoms and behaviour. Such models may provide the ability to test the recent hypothesis (derived from descriptive longitudinal studies of chronic pain neuroplasticity; Mansour et al., 2014) that the value and saliency of both nociceptive and rewarding stimuli are altered in those vulnerable to chronic pain. An important first step in this direction will be to test for the construct validity of PC models in chronic pain, that is, do they effectively account for individual variability, and variability over time, in pain‐related neuroplasticity in the brain? In addition, the validity of generative computational models (such as PC) would rest on their ability to account for how symptoms can arise from underlying hidden mechanisms. A second step required is clinical validation, for example, do such models have predictive validity for future pain symptoms and/or treatment outcomes? If so, once other hurdles (such as test–retest reliability of different models) have been overcome, the clinical potential is substantial. By inferring mechanisms rather than relying on symptom profiles, the approach may finally achieve the lofty ambition of mechanism‐based stratification for treatment.
Opioid function in pain vulnerability and resilience
We argue the need to better understand the precise function in the brain of the opioid system in a normative sense. This will provide a definition of optimal and suboptimal functioning to facilitate diagnosis and treatment. A view of the brain's predictive mechanisms provide insights into the role of the endogenous opioid system in chronic pain vulnerability and resilience.
Endogenous opioids have been intensively studied for their role in the modulation of nociception. Many nociceptive forebrain areas are rich in opioid receptors (μ, δ and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=318) and modulated by endogenous opioids, including the frontal cortices, anterior cingulate and midcingulate cortices, the thalamus, striatum, insula, hypothalamus and central nucleus of the amygdala. Opiate drugs and endogenous opioids act on opioid receptors in these regions to produce analgesia (Jones et al., 1991; Petrovic et al., 2002; Zubieta et al., 2005; Eippert et al., 2009). However, evidence suggests that the actions of endogenous opioids is partly to mediate or reinforce neural predictions. For example, placebo analgesia, thought to be mediated by conscious or unconscious (e.g. conditioned) expectations (Watson et al., 2012), is opioid‐mediated (Benedetti et al., 2005). Furthermore, behavioural and neuroimaging studies show that the analgesic effect of synthetic opiates during experimental pain is blocked by negative suggestion (Bingel et al., 2011). This highlights a potentially important interaction between opioids and predictive mechanisms in the brain.
Further evidence from Pavlovian fear conditioning experiments suggests that endogenous opioids may specifically optimize predictions about pain to promote associative learning. In rodents, when a neutral conditioned stimulus was repeatedly paired with an unconditioned stimulus (a painful shock) and over time came to predict that shock, endogenous opioids were released resulting in analgesia (Fanselow and Baackes, 1982). The effect was blocked with opioid antagonism (e.g. naloxone), which in addition facilitated learning of the conditioned stimulus‐unconditioned stimulus pairing, in rats (Bolles and Fanselow, 1982; McNally et al., 2004) and humans (Eippert et al., 2008). However, a series of experiments (McNally et al., 2004) involving blocking designs discovered that opioid enhancement of fear learning is not due to a greater unconditioned stimulus (i.e. increased pain) from opioid antagonism, but rather results from a specific increase in the prediction error learning signal (the discrepancy between the unconditioned and the conditioned stimulus). Further observations were that over the course of fear conditioning, the conditioned stimulus increasingly resulted in endogenous opioid signalling in the ventrolateral PAG, which acted to limit further fear learning (McNally and Cole, 2006). In addition, endogenous opioid signals act to not only limit fear learning but also fear extinction (McNally, 2009). These observations are consistent with opioid receptor activity encoding the prediction (i.e. the conditioned stimulus) rather than the painful outcome (i.e. the unconditioned stimulus), as the latter would result in the opposite to the observed extinction learning behaviour (McNally, 2009). In other words, endogenous opioids appear to be important for learning the prediction, that is, the transfer of information from the unconditioned stimulus to the prior conditioned stimulus over time. Buchel et al., in discussing predictive mechanisms of placebo (Büchel et al., 2014), speculate that opioidergic signalling specifically represents the precision of predictions, consistent with Bayesian updating scheme for pain perception. However, whether there is a pain vulnerability mechanism involving altered opioid signalling during the encoding or expression of predictions of pain is a hypothesis that remains to be tested.
Conclusions
The brain is best considered as an active participant in shaping the sensory‐perceptual and cognitive/emotional aspects of pain. In chronic pain, observations of neuroplasticity within the network of brain regions mediating opioid‐dependent analgesia may confer states of pain vulnerability, but the meanings of such observations have proved challenging to interpret in light of the potential for a range of influences on these regions such as from nociceptive inputs, cognitive and behavioural strategies, and long‐term use of opiate analgesics. Computational models that use normative optimality principles may help to make sense of pain‐related pathophysiology in individuals with chronic pain. Evidence currently supports (but does not explicitly test) the hypothesis that endogenous opioids function to optimize predictions about pain to serve adaptive perception and behaviour. Further research is required to test whether opioids directly modulate prediction per se or act on the precision of predictions according to a Bayesian updating scheme. Identification of these mechanisms, requiring psychopharmacology experiments in combination with computational modelling, will serve to provide a definition of sub‐optimal opioid system functioning and a rational basis for chronic pain prevention and treatment.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a, 2015b).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Thanks to Nayab Begum, Timothy Rainey and Kate Lees for help with the manuscript. This review was not supported by any funding.
Jones A. K. P., and Brown C. A. (2018) Predictive mechanisms linking brain opioids to chronic pain vulnerability and resilience, British Journal of Pharmacology, 175: 2778–2790, https://doi.org/10.1111/bph.13840.
References
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MLE, Svensson B, Bergman S (2013). Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 40: 1977–1985. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R‐DD, Zubieta J‐KK (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010). Brain mediators of predictive cue effects on perceived pain. J Neurosci 30: 12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Dolan RJ (2012). Knowing how much you don't know: a neural organization of uncertainty estimates. Nat Rev Neurosci 13: 572. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K (2003). Depression and pain comorbidity: a literature review. Arch Intern Med 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV (2011). The cortical rhythms of chronic back pain. J Neurosci 31: 13981–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB et al. (2006). Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour AR, Baria AT, Apkarian AV (2014). Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9: e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ et al. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I (2002). Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310–319. [DOI] [PubMed] [Google Scholar]
- Basden SL, Orr SP, Otto MW (2016). Impaired de novo fear conditioning in opiate‐dependent outpatients. Cognit Ther Res 40: 824–830. [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J‐K (2005). Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl). 191: 391–431. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M et al. (2011). The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 3: 70ra14‐70ra14. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ et al. (2008). The runner's high: opioidergic mechanisms in the human brain. Cereb Cortex 18: 2523–2531. [DOI] [PubMed] [Google Scholar]
- Bogacz R (2017). A tutorial on the free‐energy framework for modelling perception and learning. J Math Psychol 76: 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS (1982). Endorphins and behavior. Annu Rev Psychol 33: 87–101. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D (2006). Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 10: 287–333. [DOI] [PubMed] [Google Scholar]
- Breivik H, Eisenberg E, O'Brien T, OPENMinds (2013). The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 13: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, El‐Deredy W, Jones AK (2014). When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci 39: 663–672. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AKP (2010). Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain 150: 428–433. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AKP (2013). Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness‐based pain management program. Clin J Pain 29: 233–244. [DOI] [PubMed] [Google Scholar]
- Brown CA, Matthews J, Fairclough M, McMahon A, Barnett E, Al‐Kaysi A et al. (2015). Striatal opioid receptor availability is related to acute and chronic pain perception in arthritis: does opioid adaptation increase resilience to chronic pain? Pain 156: 2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El‐Deredy W, Jones AKP (2008a). Modulation of pain perception by expectation and uncertainty: behavioral characteristics and anticipatory neural correlates. Pain 135: 240–250. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, El‐Deredy W, Jones AKP (2008b). Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain 139: 324–332. [DOI] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F (2014). Placebo analgesia: a predictive coding perspective. Neuron 81: 1223–1239. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D et al. (2001). Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen‐specific cellular compartmentalization. J Comp Neurol 440: 65–84. [DOI] [PubMed] [Google Scholar]
- Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA (2015). Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 36: 2075–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I et al. (2015). The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 162: 276. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663. [DOI] [PubMed] [Google Scholar]
- Croft P, Blyth F, van der Windt D (2010). The global occurence of chronic pain: an introduction In: Croft P, Blyth F, van der Windt D. (eds). Chronic Pain Epidemiology: From Aetiology to Public Health. Oxford University Press: Oxford, United Kingdom: pp. 9–18. [Google Scholar]
- Cruccu G, Aziz TZ, Garcia‐Larrea L, Hansson P, Jensen TS, Lefaucheur JP et al. (2007). EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol 14: 952–970. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ (2012). A Bayesian account of ‘hysteria’. Brain 135: 3495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Buchel C (2008). Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. J Neurosci 28: 5465–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J et al. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63: 533–543. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Jensen MK, Sjøgren P, Ekholm O, Rasmussen NK (2003). Epidemiology of chronic non‐malignant pain in Denmark. Pain 106: 221–228. [DOI] [PubMed] [Google Scholar]
- Fallon N, Alghamdi J, Chiu Y, Sluming V, Nurmikko T, Stancak A (2013). Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. NeuroImage Clin 3: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon N, Chiu Y, Nurmikko T, Stancak A (2016). Functional connectivity with the default mode network is altered in fibromyalgia patients. PLoS One 11: e0159198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Baackes MP (1982). Conditioned fear‐induced opiate analgesia on the Formalin test: Evidence for two aversive motivational systems. Learn Motiv 13: 200–221. [Google Scholar]
- Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT (2016). Prevalence of chronic pain in the UK: a systematic review and meta‐analysis of population studies. BMJ Open 75: 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE (2009). Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci 32: 33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H (2004). State‐dependent opioid control of pain. Nat Rev Neurosci 5: 565–575. [DOI] [PubMed] [Google Scholar]
- Fields HL (2006). A motivation‐decision model of pain: the role of opioids In: Flor H, Kalso E, Dostrovsky JO. (eds). Proceedings of the 11th World Congress on Pain. IASP press: Seattle, pp 449–459. [Google Scholar]
- Friston K (2003). Learning and inference in the brain. Neural Netw 16: 1325–1352. [DOI] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L (2006). A free energy principle for the brain. J Physiol Paris 100: 70–87. [DOI] [PubMed] [Google Scholar]
- Gear RW, Levine JD (2011). Nucleus accumbens facilitates nociception. Exp Neurol 229: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Horvitz EJ, Tenenbaum JB (2015). Computational rationality: A converging paradigm for intelligence in brains, minds, and machines. Science 349: 273–278. [DOI] [PubMed] [Google Scholar]
- Gershman SJ, Daw ND (2017). Reinforcement learning and episodic memory in humans and animals: an integrative framework. Annu Rev Psychol 68: 101–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ (2005). The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum 52: 1577–1584. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA et al. (2004). Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127: 835–843. [DOI] [PubMed] [Google Scholar]
- Gunzelmann T, Schumacher J, Brähler E (2002). The prevalence of pain in the elderly German population: results of population‐based studies with the Giessen Subjective Complaints List (Giessener Beschwerdebogen GBB). Schmerz 16: 249–254. [DOI] [PubMed] [Google Scholar]
- Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ et al. (2007). The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population‐based study. Rheumatology 46: 666–671. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I (2010). Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel‐based morphometric study. Arthritis Rheum 62: 2930–2940. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK (2007). Decreased central mu‐opioid receptor availability in fibromyalgia. J Neurosci 27: 10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Lui F, Porro CA (2013). Hypnotic susceptibility modulates brain activity related to experimental placebo analgesia. Pain 154: 1509–1518. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Day MA, Miro J (2014). Neuromodulatory treatments for chronic pain: efficacy and mechanisms. Nat Rev Neurol 10: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Cunningham VJ, Ha‐Kawa S, Fujiwara T, Luthra SK, Silva S et al. (1994). Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol 33: 909–916. [DOI] [PubMed] [Google Scholar]
- Jones AK, Kitchen ND, Watabe H, Cunningham VJ, Jones T, Luthra SK et al. (1999). Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C] diprenorphine and positron emission tomography. J Cereb Blood Flow Metab 19: 803–808. [DOI] [PubMed] [Google Scholar]
- Jones AK, Watabe H, Cunningham VJ, Jones T (2004). Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur J Pain 8: 479–485. [DOI] [PubMed] [Google Scholar]
- Jones AKP, Friston KJ, Qi LY, Harris M, Cunningham VJ, Jones T et al. (1991). Sites of action of morphine in the brain. Lancet 338: 825. [DOI] [PubMed] [Google Scholar]
- Jordan J, Luta G, Renner J, Dragomir A, Hochberg M, Fryer J (1997). Knee pain and knee osteoarthritis severity in self‐reported task specific disability: the Johnston County Osteoarthritis Project. J Rheumatol 24: 1344–1349. [PubMed] [Google Scholar]
- Kacelnik A (1997). Normative and descriptive models of decision making: time discounting and risk sensitivity. In Characterizing Human Psychological Adaptations, pp. 51–66. [DOI] [PubMed]
- Kise NJ, Risberg MA, Stensrud S, Ranstam J, Engebretsen L, Roos EM (2016). Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow‐up. BMJ 354: i3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LJ, Lauche R, Klose P, Bu JH, Yang XC, Guo CQ et al. (2016). Tai Chi for chronic pain conditions: a systematic review and meta‐analysis of randomized controlled trials. Sci Rep 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek E, Cohen M, Baron R, Gebhart GF, Mico J‐A, Rice ASC et al. (2016). Do we need a third mechanistic descriptor for chronic pain states? Pain 157: 1382–1386. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman‐Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC et al. (2014). Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A et al. (2007). Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum 56: 1345–1354. [DOI] [PubMed] [Google Scholar]
- Kuner R, Flor H (2016). Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18: 20–30. [DOI] [PubMed] [Google Scholar]
- Kuttikat A, Noreika V, Shenker N, Chennu S, Bekinschtein T, Brown CA (2016). Neurocognitive and neuroplastic mechanisms of novel clinical signs in CRPS. Front Hum Neurosci 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le GS, Mas NM, Canestrelli C, Chen H, Fournie‐Zaluski MC, Cupo A et al. (2003). Pain management by a new series of dual inhibitors of enkephalin degrading enzymes: long lasting antinociceptive properties and potentiation by CCK2 antagonist or methadone. Pain 104: 139–148. [DOI] [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME (2001). Rheumatoid arthritis. Lancet 358: 903–911. [DOI] [PubMed] [Google Scholar]
- Lee Y, Nassikas N, Clauw D (2011). The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011). The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93: 111–124. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I (2008). A common neurobiology for pain and pleasure. Nat Rev Neurosci 9: 314–320. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Berna C, Kim J, Cahalan CM, Martel M‐O, Gollub RL et al. (2015). The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. J Pain 16: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Hauck M, Paur RC, Nakamura Y, Zimmermann R, Bromm B et al. (2005). Cortical correlates of false expectations during pain intensity judgments‐‐a possible manifestation of placebo/nocebo cognitions. Brain Behav Immun 19: 283–295. [DOI] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M et al. (2007). Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain 127: 183–194. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F (2004). Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63: 693–701. [DOI] [PubMed] [Google Scholar]
- Mansour AR, Farmer MA, Baliki MN, Apkarian AV (2014). Chronic pain: The role of learning and brain plasticity. Restor Neurol Neurosci 32: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1982). Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. W.H. Freeman and Company: San Francisco. [Google Scholar]
- McNally GP (2009). The roles of endogenous opioids in fear learning. Int J Comp Psychol 22: 153–169. [Google Scholar]
- McNally GP, Cole S (2006). Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during Pavlovian fear conditioning. Behav Neurosci 120: 313–323. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G (2004). Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of pavlovian association formation. Behav Neurosci 118: 111–120. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD (1965). Pain mechanisms: a new theory. Science (80‐. ) 150: 971–979. [DOI] [PubMed] [Google Scholar]
- Meyniel F, Sigman M, Mainen ZF (2015). Confidence as Bayesian probability: from neural origins to behavior. Neuron 88: 78–92. [DOI] [PubMed] [Google Scholar]
- Michael ES, Burns JW (2004). Catastrophizing and pain sensitivity among chronic pain patients: moderating effects of sensory and affect focus. Ann Behav Med 27: 185–194. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL et al. (2003). Regulation of delta‐opioid receptor trafficking via mu‐opioid receptor stimulation: evidence from mu‐opioid receptor knock‐out mice. J Neurosci 23: 4888–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Jones AKP (2016). Brain imaging of pain: state of the art. J Pain Res 9: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH et al. (2002). A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 347: 81–88. [DOI] [PubMed] [Google Scholar]
- Mossey JM, Gallagher RM (2004). The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med 5: 335–348. [DOI] [PubMed] [Google Scholar]
- Mottram S, Peat G, Thomas E, Wilkie R, Croft P (2008). Patterns of pain and mobility limitation in older people: cross‐sectional findings from a population survey of 18,497 adults aged 50 years and over. Qual Life Res 17: 529–539. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Porreca F (2014). Reward and motivation in pain and pain relief. Nat Neurosci 17: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A et al. (2015). Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 35: 7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y (2009). Reinforcement learning in the brain. J Math Psychol 53: 139–154. [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003). Temporal difference models and reward‐related learning in the human brain. Neuron 38: 329–337. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC et al. (2006). Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain Proposal Steering Committee (2010). Pain Proposal.
- Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV (2011). Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain 15: 843.e1–e843.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peira N, Ziaei M, Persson J (2016). Age differences in brain systems supporting transient and sustained processes involved in prospective memory and working memory. Neuroimage 125: 745–755. [DOI] [PubMed] [Google Scholar]
- Petrov ME, Goodin BR, Cruz‐Almeida Y, King C, Glover TL, Bulls HW et al. (2015). Disrupted sleep is associated with altered pain processing by sex and ethnicity in knee osteoarthritis. J Pain 16: 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M (2002). Placebo and opioid analgesia‐‐ imaging a shared neuronal network. Science (80‐.) 295: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Pietro FD, McAuley JH, Parkitny L, Lotze M, Wand BM, Moseley GL et al. (2013). Primary somatosensory cortex function in complex regional pain syndrome: a systematic review and meta‐analysis. J Pain 14: 1001–1018. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F (2008). A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol 59: 565–590. [DOI] [PubMed] [Google Scholar]
- Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH (1999). Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82: 159–171. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In Black AH, Prokasy WF. (eds). Classical Conditioning II. Appleton‐Century‐Crofts: New York, pp 64–99. [Google Scholar]
- Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P et al. (2016). Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann. Rheum. Dis 75: 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D (2003). Oral opioid therapy for chronic peripheral and central neuropathic pain. New Engl J Med 348: 1223–1232. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR (2007). A common framework for perceptual learning. Curr Opin Neurobiol 17: 148–153. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA et al. (2013). Cognitive‐behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Doherty JPO, Dayan P, Koltzenburg M, Jones AK, Dolan RJ et al. (2004). Temporal difference models describe higher‐order learning in humans. Nature 429: 664–667. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K et al. (2005). Opponent appetitive‐aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8: 1234–1240. [DOI] [PubMed] [Google Scholar]
- Shaheed CA, Maher CG, Williams KA, Day R, Mclachlan AJ (2016). Efficacy, tolerability, and dose‐dependent effects of opioid analgesics for low back pain: a systematic review and meta‐analysis. JAMA Intern Med 176: 958–968. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F et al. (2006). Opioidergic activation in the medial pain system after heat pain. Pain 122: 63–67. [DOI] [PubMed] [Google Scholar]
- Staud R (2012). Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 12: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrett G, Lyons A, Henry JD, Ryrie C, Suddendorf T, Rendell PG (2017). Acting with the future in mind is impaired in long‐term opiate users. Psychopharmacology (Berl) 234: 99–108. [DOI] [PubMed] [Google Scholar]
- Terrett G, McLennan SN, Henry JD, Biernacki K, Mercuri K, Curran HV et al. (2014). Prospective memory impairment in long‐term opiate users. Psychopharmacology (Berl) 231: 2623–2632. [DOI] [PubMed] [Google Scholar]
- Thakur M, Dickenson AH, Baron R (2014). Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol 10: 374–380. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA (2007). The neural basis of loss aversion in decision‐making under risk. Science 315: 515–518. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007). The cerebral signature for pain perception and its modulation. Neuron 55: 377–391. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015). Rates of opioid misuse, abuse, and addiction in chronic pain. Pain 156: 569–576. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011). Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C‐W, Kross E (2013). An fMRI‐based neurologic signature of physical pain. N Engl J Med 368: 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ et al. (2004). Placebo‐induced changes in fMRI in the anticipation and experience of pain. Science (80‐. ) 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- Wang X‐M, Zhou Y, Spangler R, Ho A, Han J‐S, Kreek MJ (1999). Acute intermittent morphine increases preprodynorphin and kappa opioid receptor mRNA levels in the rat brain. Mol Brain Res 66: 184–187. [DOI] [PubMed] [Google Scholar]
- Watson A, El‐Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA et al. (2009). Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Power A, Brown C, El‐Deredy W, Jones A (2012). Placebo analgesia: cognitive influences on therapeutic outcome. Arthritis Res Ther 14: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC d C, Eccleston C, Morley S (2012). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 11 CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M et al. (2004). Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain 108: 213–220. [DOI] [PubMed] [Google Scholar]
- Wood L, Peat G, Thomas E, Hay E, Sim J (2008). Associations between physical examination and self‐reported physical function in older community‐dwelling adults with knee pain. Phys Ther 88: 33–42. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 31: 5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J‐KK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA et al. (2005). Placebo effects mediated by endogenous opioid activity on mu‐opioid receptors. J Neurosci 25: 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM et al. (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science (80‐. ) 293: 311–315. [DOI] [PubMed] [Google Scholar]


