Abstract
G-protein–mediated signal transduction pathways play an essential role in the developmental program of the simple eukaryotic organism Dictyostelium discoideum. Database searches have yielded 11 Gα-subunits, a single Gβ-subunit, but no Gγ-subunits. We report here the purification, cDNA isolation, and functional analysis of a Gγ-subunit. Like Gβ, the Gγ appears to be unique and hybridization studies show that Gγ and Gβ are expressed in parallel during development. Species-wide sequence comparisons of Gγ-subunits and γ-like domains of RGS proteins reveal short stretches of highly conserved residues as well as the common CXXL motif at the COOH-terminal of Gγs that target Gβγs to plasma membrane. Overexpression of a CSVL-deleted Gγ (GγΔ) in wild-type cells shifts Gβγ to the cytosol and selectively impairs certain G-protein–mediated signal transduction pathways. These cells are able to respond to increments in the stimulus, but are unable to sense chemoattractant gradients. They neither move directionally nor recruit PH-domains to their leading edge. Thus, a full complement of membrane-tethered Gβγ is required for sensing shallow gradients, but is not essential for responses to increments in extracellular stimuli.
INTRODUCTION
The G-protein–linked signaling strategy has evolved to sense chemoattractants in cells ranging from Dictyostelium discoideum to mammalian leukocytes (Devreotes and Zigmond, 1988). In these cells, chemoattractants elicit sharp rises in the proportion of polymerized actin as well as the phosphorylation of myosins, the production of cyclic nucleotides, and the activation of mitogen-activated protein kinases and STATs (Berlot et al., 1985; Caterina and Devreotes, 1991; Thompson et al., 1994; Lai et al., 1996; Maeda et al., 1996; Parent and Devreotes, 1996; Krump et al., 1997; Araki et al., 1998). Evidence suggests that it is the βγ-complexes, released by receptor-catalyzed exchange of GTP for GDP on α-subunits, which directly regulate effectors (Wu et al., 1995; Neptune et al., 1997; Jin et al., 1998). The specific G-proteins involved in chemotaxis consist of αi in mammals and α2 or α4 in D. discoideum (Kumagai et al., 1989; Hadwiger et al., 1994). In mammals, it is not certain which of the βγ-complexes is key, whereas in D. discoideum, there is a single β-subunit. Genetic evidence shows that α2 and β are essential for many responses to chemoattractants; it is expected that the Gγ is also essential (Kumagai et al., 1989; Wu et al., 1995). However, the anticipated Gγ has not been identified in spite of considerable effort by degenerate PCR analysis and database searching.
Gγ-subunits play multiple roles in G-protein signal transduction. Not only are they required for proper Gβ folding and function, the sequence diversity of Gγs also contributes to the specificity of G-protein signaling (Iniguez-Lluhi et al., 1992). Specific Gβγ subtypes appear to interact with different effectors (Yan et al., 1996). Perhaps most significantly, γ-subunits determine the subcellular distribution of the Gβγ complex because isoprenylation of the COOH-terminal of Gγs targets Gβγ to the membrane (Muntz et al. 1992). Interestingly, a Gγ-like-domain (GGL) has been found in a subgroup of mammalian RGSs, indicating that β-subunits can also have additional partners in the cytosol (Cabrera et al., 1998).
The structures and functions of heterotrimeric G-proteins are highly conserved from yeast to human, but the sequences of γ-subunits are quite divergent among different species (Gautam et al., 1998). Gβs have seven WD repeats at their COOH-terminal that fold into β-propeller structures, containing the binding sites for Gαs and effectors (Ford et al., 1998; Jin et al., 1998; Li et al., 1998). The NH2-terminal regions of Gβs are variable and form tight coiled-coil structures with the diverse Gγs. Because Gγs share little homology among diverse species, our strategy was to purify Gγ, isolate a DNA probe based on its amino acid sequence, and then obtain its cDNA by library screening. A gβ − cell line was rescued with a His-tagged Gβ, and the resulting cell line was used to purify the functional His-Gβγ heterodimer. The isolated Gγ allowed us to characterize its function and explore its role in chemoattractant and G-protein–mediated responses.
G-protein–mediated responses can be induced by stimuli presented either as increments or as spatial gradients. Increments of chemoattractant elicit uniform responses around the cell periphery, whereas gradients elicit responses on the side of the cell that faces the higher chemoattractant concentration (Parent et al., 1998). When there is a polarized distribution of Gβγs, uniform increments of stimuli result in a similar polarized recruitment of downstream signaling molecules (Jin et al., 2000). Endogenous G proteins have been found both on the membrane and in the cytosol. Membrane localization may be critical for localized activation of cells in gradients. Previous studies have shown that nonisoprenylated Gγ1 mutant can interact with Gβ1 and is confined in cytosol (Simonds et al., 1991; Iniguez-Lluhi et al., 1992). To test the functional role of membrane localization, a CSVL-deleted Gγ (GγΔ) construct was designed to compete with endogenous Gγ and to shift the membrane Gβγ subunits to the cytosol. The consequences of GγΔ on responses to increments and gradients were examined.
MATERIALS AND METHODS
Materials
Restriction enzymes were purchased from New England Biolabs (Beverly, MA), Boehringer Mannheim (Indianapolis, IN), or Promega Inc. (Madison, WI); Taq polymerase from Perkin Elmer-Cetus (Norwalk, CT); TA cloning kit from Invitrogen (San Diego, CA); Chelating-Sepharose and DEAE-Sepharose from Pharmacia Biotech (Piscataway, NJ); BCA protein assay kit from Pierce (Rockford, IL); cyclic-AMP 3H assay system and cyclic-GMP 3H assay system from Amersham (Arlington Heights, IL). Polyclonal anti-Gβ and anti-Gα2 antibodies were made in our laboratory. All other reagents were reagent grade and were obtained from standard suppliers.
Cell Culture and Development
Cells were grown in shaken suspension (Watts and Ashworth, 1970) or on SM agar plates with Klebsilla aerogenes lawn (Kay, 1987). Cells were developed in shaken suspension at 2 × 107/ml in development buffer (DB; 10 mM Na/K phosphate, 0.2 mM CaCl2, 2 mM MgSO4, pH 6.5) for 1 h and then pulsed with 50 nM cAMP at a 6-min intervals for 5 h.
Protein Assays, SDS-PAGE, and Western Blotting
Protein assays were carried out according to the protocol provided by Pierce. High percentage SDS-PAGE that separated low-molecular-weight proteins was carried out as described by Hoefer Scientific Instruments' protocol (San Francisco, CA). Western blotting was carried out as described in Current Protocol in Molecular Biology with minor modification. PVDF membrane was substituted for nitrocellulose; the transfer was performed at 300 mA at 4°C for 3 h; the secondary antibody was a horseradish peroxidase (HRP), anti-rabbit IgG conjugate (Sigma Chemical Co., St. Louis, MO). Band detection was achieved with the use of enhanced chemiluminescence (ECL).
Purification of Gγ-subunit
Gγ-subunits were copurified with HisGβ-subunits. The Gβ cDNA, with six histidines fused to its NH2-terminal, was cloned into the expression vector p88d1 (Hughes et al., 1994). The construct was transformed into gβ− cells to create a HisGβ/gβ− cell line. HisGβ/gβ− cells were used to purify Gγ-subunit. During the purification process, the total amount of protein was assayed by BCA protein assay (Pierce). The amount of Gβγ-subunits was estimated by Western analysis against Gβ-subunit. Protease inhibitor cocktails, PIC I (1 mg/ml antipain, 2 mg/ml leupeptin, and 2 mg/ml aprotinin) and PIC II (1 mg/ml chymostatin and 1 mg/ml pepstatin in DMSO), were added to buffers to protect the G-protein subunits from degradation.
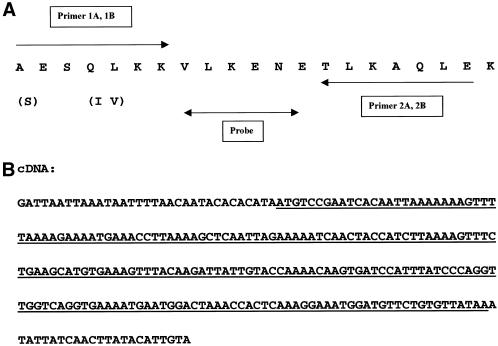
HisGβ/gβ− cells were cultured in HL5 medium with 20 μg/ml G418, shaking at 200 rpm room temperature. When the cell density reached 5 × 106 per ml, 24 liters of HisGβ/gβ− were harvested. The harvested cells were collected by flow-through centrifugation, resuspended in DB to 2 × 107 per ml, and shaken at room temperature for 3 h to reduce protease activities. The following purification steps were carried out at 4°C. Cells were washed with 16 liters of DB twice and resuspended into 1 liter of lysis buffer (LB; 2 mM EDTA, 200 mM sucrose, and 10 mM Tris, pH 8), and cells were lyzed by Parr Bomb. Lysates were centrifuged at 17,000 × g for 40 min. After discarding the supernatant, the pellet was washed with 1 liter LB. The cell pellet was then extracted in 3 liters of running buffer I (RBI; 250 mM NaCl, 1% NP-40, 0.1% of PIC I, 0.2% of PIC II, and 20 mM Tris, pH 7.5), centrifuged at 17,000 × g for 40 min, and the supernatant was saved for the following purification. The supernatant was mixed with 50 ml Ni2+–chelating resin. The mixture was stirred for 0.5 h to achieve the optimal binding and then loaded on a Bio-Rad column. The column was then washed with 250 ml RBI followed by 250 ml RBI plus 20 mM imidazole. The G-protein was eluted with 150 ml elution buffer I (EBI: RBI plus 250 mM imidazole). The eluate was diluted 10-fold with running buffer II (RBII; 10 mM NaCl, 1 mM EDTA, 1% NP-40, and 20 mM Tris, pH 7.5) and loaded on to a 30 ml DEAE-Sepharose column. The column was washed with 300 ml running buffer III (RBIII; 50 mM NaCl, 1 mM EDTA, 0.1% NP-40, and 20 mM Tris, pH 7.5). The G-protein–containing fraction was then eluted with RBII with 250 mM NaCl. The DEAE column eluates were dialyzed against running buffer IV (RBIV; RBI with 0.1% NP-40) and loaded on 1.5 ml freshly made Ni2+-chelating column. After washing the column with 15 ml of RBIV, the G-protein was eluted with 5 ml elution buffer III (EBIII; RBIV in which 0.1% NP-40 was replaced with 0.1% SDS). One hundred fifty microliters of eluates was loaded on a high percentage SDS-PAGE. There were three closely spaced Commassie Blue–stained bands in the 7- to 10-kDa region of the gel. The two upper bands were cut and sent for sequencing (Figure 1A).
Figure 1.
Isolation of Gγ cDNA (A) A NH2-terminal 21 amino acid sequence of Gγ was obtained and used to design PCR primers. A DNA probe between primers was isolated for the screening of a cDNA library. (B) The Gγ cDNA was isolated and sequenced.
PCR and Screen cDNA Library
The PCR was performed in a 50 μl reaction mixture as described in PCR protocols with minor modifications. The sequences of primers were as follows:
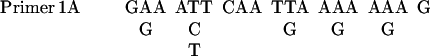 |
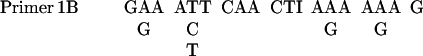 |
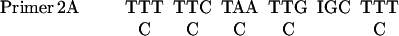 |
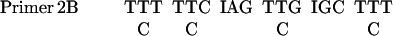 |
The mixture contained 5 μl 10× PCR buffer (Life Technologies), 200 μM each dNTPs, 1.0 mM MgCl2, 0.5 μM primers, template, and 3 U of Taq polymerase. The samples were placed in a DNA thermal cycler from Perkin Elmer-Cetus. The program comprised 5 cycles of low-temperature (40°C) annealing and 30 cycles of high-temperature (46°C) annealing. Screening cDNA was carried out as described in Current Protocols in Molecular Biology (Ausubel et al., 1987).
Isolation of Gγ cDNA
Based on the NH2-terminal amino acid sequence of Gγ, four sets of degenerate PCR primers were designed. With the use of D. discoideum genomic DNA as templates, the PCR was carried out with four possible combinations of primers: 1A with 2A, 1A with 2B, 1B with 2A, and 1B with 2B. A 20-nucleotide sequence was isolated and used as a probe to screen cDNA library. The sequences of the probe were as follows:
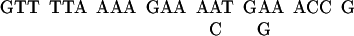 |
Approximately 1.5 × 105 phages from λgt11 cDNA library, prepared from mRNA isolated at 2–4 h of development, were screened (Lilly et al., 1993). Fifty-two positive clones were detected and 4 independent clones were obtained after secondary and tertiary screens. They were picked for subcloning and DNA sequencing (Figure 1B).
Developmental Northern Blotting
Nine 35-mm tissue culture plates were filled with 2 ml DB agar each. Each plate was then covered with 1 × 107 vegetative growing AX3 cells. Cell samples were collected at time 0, 2, 4, 6, 9, 12, 16, 20, and 30 h. Total RNA from each sample was isolated with the use of the Trizol reagents (BRL-Life Technologies, Rockville, MD). Twenty micrograms of total RNA from each time point was loaded onto a 1% formaldehyde gel and blotted onto nylon membrane. The full-length cDNA was random prime labeled, and the blot was probed according to the method described in Current Protocols in Molecular Biology. The quantitative measurement of bands was carried out by a FUJIFILM BAS1500 PhosphorImager (Fuji, Stamford, CT).
Plasmid Construction and Transformation
The hexyl-histidine was fused to the NH2-terminal of Gβ by PCR, and the resulting DNA fragment was cloned into D. discoideum extrachromosomal expression plasmid, p88d1. Four COOH-terminal amino acid residues of Gγ were removed by PCR, and the resulting DNA fragment was cloned into p88d1 to make GγΔ plasmid. Gγ cDNA was cloned into p88d1 to make Gγ plasmid. PHcrac-GFP was the same as that in Parent et al. 1998. The transformation was carried out as described previously (Insall et al., 1994). The plasmid was electroporated into cells with the use of a Bio-Rad gene pulser, and the stable transformants were selected in 20 μg/ml G418.
Functional Assays
Amoebae aggregation was assayed on 35-mm nonnutrient agar as described previously (Devreotes et al., 1987). Chemotaxis assays and cAMP-binding assays were carried out according to the protocols by Xiao et al. (1997). In vitro adenylyl cyclase assays were performed as described by Theibert et al. (1986). In vivo adenylyl cyclase assays were performed as described by Parent et al. (1995). The production of cGMP was assayed by Amersham kits. Fluorescent microscopy analysis on live PHcrac-GFP:GγΔ/AX2 cells was performed as described (Parent et al., 1998).
RESULTS
Purification of the Gγ-subunit and Isolation of Its cDNA
We reasoned that a hexylhistidine-tagged Gβ-subunit, HisGβ, would form a tight association with endogenous Gγ and could be used to isolate the complex. A plasmid expressing HisGβ was transformed into gβ− cells, and its functionality and level of expression was assessed. Although gβ− cells were completely defective in aggregation and most signaling events (Wu et al., 1995), HisGβ/gβ− cells developed appropriately into spores and stalks (our unpublished results). The HisGβ protein was not largely overexpressed, consistent with previous data suggesting tight control of Gβ expression (Lilly et al., 1993). Through purification of HisGβγ, Gγ was isolated from the membrane fractions of 1011 cells (Table 1). The purification used sequential Ni2+-chelating, DEAE-Sepharose, and Ni2+-chelating columns, yielding an overall purification of 2000-fold. A high percentage SDS-PAGE provided an additional 10-fold of purification. There were three Commassie blue–stained bands in the 7- to 10-kDa region of the gel. These bands were absent in parallel samples from wide-type cells that lack HisGβ, indicating that they were released from the HisGβ by SDS (our unpublished results). The purified Gγ-subunits were sequenced and found to contain identical NH2-terminal sequences (Figure 1A).
Table 1.
Gγ isolated from membrane fractions
| Step | Volume (ml) | Protein (mg) | Recovery (%)a | Purification Fold |
|---|---|---|---|---|
| Cell lysate | 1,000 | 12,000 | 100 | 1 |
| Membrane extract | 3,000 | 1,900 | 65 | 4.1 |
| Ni-chelating I | 150 | 98 | 50 | 61.6 |
| DEAE | 100 | 23 | 42 | 218 |
| Ni-chelating II | 5 | 0.74 | 15 | 2,420 |
| SDS-PAGE | N/Ac | 0.08 | N/A | ∼20,000b |
Recovery is based on the estimated intensity of Gβ Western bands.
The purification fold in this step is estimated by the intensity of SDS-PAGE silver strain.
N/A, not applicable.
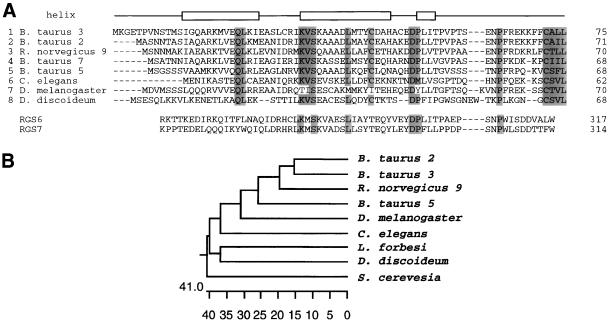
The amino acid sequence data were used to isolate the Gγ cDNA. Based on the NH2-terminal amino acid sequence, four sets of degenerate PCR primers were designed. A 20-bp region of novel sequence isolated from PCR was used as a probe to screen the cDNA library. Four positive clones with identical sequences were isolated. The completed cDNA sequence is shown in Figure 1B. The cDNA sequence encodes a protein of 68 amino acids. Its COOH-terminal contains the sequence CSVL, which is the characteristic signal for isoprenylation. When compared with Gγ-subunits from other species, the D. discoideum Gγ shared 10–18% identity with Gγs from Bos taurus, Rattus norvegicus, Caenorhabditis elegans, and Drosophila melanogaster (Figure 2A). The Gγ-subunits from Saccharomyces cerevisiae showed less homology; only three residues could be aligned. Interestingly, a number of identical amino acid residues were conserved with the GGL domain of mammalian RGSs. A phylogenetic tree is shown in Figure 2B. The secondary structure program, PHD, predicted 2.5 alpha-helixes, which presumably would form coiled-coil domains with the NH2-terminal of Gβ.
Figure 2.
Homology of the Dictyostelium Gγ-subunit to that of other species (A) D. discoideum, B. taurus, R. norvegicus, C. elegans, D. melanogaster Gγ-subunits, and GGL domain of RGSs are aligned. (B) A phylogenetic analysis was carried out on Gγ-subunits.
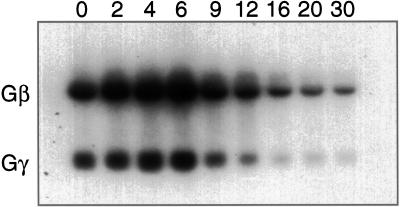
To investigate the expression of Gγ throughout development, Northern analyses were carried out (Figure 3). Based on quantitative phosphorimage analyses, Gβ and Gγ showed identical patterns of expression. Gγ and Gβ mRNAs were expressed in vegetative cells, increased twofold within 6 h after the initiation of development, and gradually decreased after passing the mound stage at 10 h, reaching 20% of the growth level at the final stage. When exogenous cAMP was added at 6-min intervals to assist development, the increase of Gβγ expression was accelerated while the maximum expression levels remained unchanged.
Figure 3.
Northern blotting of Gβ and Gγ in development. Total RNA was isolated from cells at 0, 2, 4, 6, 9, 12, 16, 20, and 30 h of development. Twenty micrograms of RNA was loaded on each lane, and the blot was probed with the mixture of 32P-labeled Gβ and Gγ cDNA.
Overexpression of CSVL-deleted Gγ Impairs Cell Aggregation
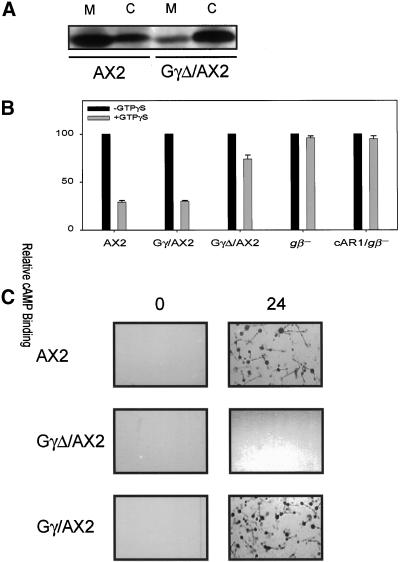
The terminal CSVL of Gγ was deleted, and GγΔ was used as a tool to study Gβγ function. The GγΔ construct was transformed into wild-type AX2 cells. Sixteen clones were screen by Western blotting analysis, and the one with highest level of GγΔ was designated GγΔ/AX2 cell line (our unpublished results). We initially chose AX2 rather than AX3 cells because the partial duplication of chromosome 2 in AX3 might have complicated the analyses of GγΔ dominant negative effects. Later, we found that overexpression of the GγΔ construct caused similar defects in AX3 cells. Wild-type Gγ was transformed into AX2 to create Gγ/AX2. In all of the following experiments, both AX2 and Gγ/AX2 cells were used as controls. Expression of GγΔ caused the endogenous membrane-associated Gβ to shift significantly to the cytosol. In wild-type cells, 70% of Gβγ was on the membrane, whereas in GγΔ/AX2 cells, only 20% of Gβγ remained on membrane (Figure 4A). When GγΔ/AX2 cells were plated on the surface of nonnutrient agar, they failed to aggregate and remained as a smooth monolayer (Figure 4C). Repeated cAMP stimuli at 6-min intervals for 6 h did not alter this phenotype. These observations suggested that GγΔ/AX2 cells were defective in certain G-protein–mediated responses required for aggregation.
Figure 4.
Expression of GγΔ impairs membrane G-protein function. (A) Expression of GγΔ shifts Gβ-subunit to cytosol. The distribution of Gβ protein between membrane (M) and cytosol (C) in AX2 and GγΔ/AX2 cells was examined with Western blotting, with the use of Gβ antibody as a probe. (B) The coupling of cAR1 and membrane G-protein in AX2, Gγ/AX2, GγΔ/AX2, gβ−, and cAR1/gβ− cells was examined by the GTPγS-induced inhibition of 3H-cAMP binding assays. The average results of three representative experiments were presented. (C) The aggregation assays were carried out on nonnutrient plates. The photo was taken at 0 and 24 h of development. Each assay was repeated at least three times, and their average is presented.
The shift of a portion of Gβγ to the cytosol significantly reduced receptor/G-protein coupling. We examined the ability of membrane associated G-proteins to regulate the binding of cAMP to cAR1. Typically, membranes contain a mixture of high- and low-affinity cAMP binding sites (Van Haastert et al., 1992). In the presence of GTPγS, the high-affinity binding sites are lost because of the dissociation of cAR1 from the G-proteins. In membranes from gβ− or cAR1/gβ− cells, cAMP binding sites are insensitive to GTPγS because in the absence of functional G-proteins, all the cAMP binding sites are low affinity (Figure 4B; Wu et al., 1995). As shown in Figure 4B, with wild-type and Gγ/AX2 cells, ∼70% of the apparent cAMP binding sites detected with 2 nM 3H-cAMP were sensitive to GTPγS. With GγΔ/AX2 cells, only 30% of cAMP binding sites were sensitive to GTPγS, suggesting that the functional membrane-associated G-protein level was significantly reduced.
GγΔ/AX2 Cells Fail to Carry Out Chemotaxis but Can Respond to Stimulus Increments
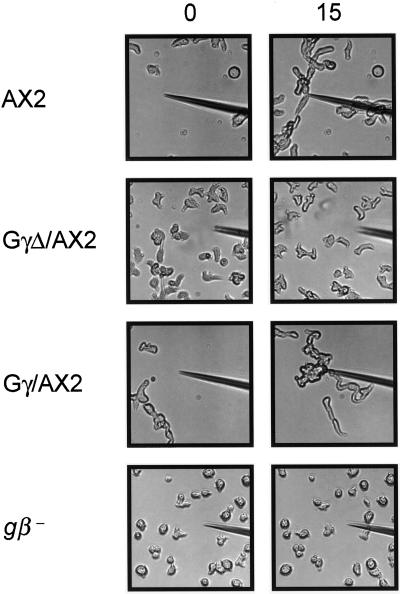
At the 5-h stage of development, wild-type cells acquire a series of characteristics associated with the differentiated state. A set of genes required for chemotaxis and aggregation are fully expressed (Firtel, 1995). The cell shape changes from rounded to elongated, and the cells show increased sensitivity toward external gradients. These cells carry out vigorous chemotaxis toward cAMP at concentrations from 10−10 to 10−6 M. As shown in Figure 5, when exposed to a cAMP gradient from a micropipette containing 1 μM cAMP, AX2 and Gγ/AX2 cells moved toward the source and began to form an aggregate at the micropipette tip within 15 min. The gβ− cells did not acquire an elongated shape and failed to carry out chemotaxis. In contrast, GγΔ/AX2 cells did acquire the characteristic elongated shape and motility of wild-type cells. In addition, cAR1 was expressed when they were stimulated repeatedly with cAMP (AX2: 1.0 ± 0.2 × 105 cAMP-binding sites/cell; GγΔ/AX2: 1.3 ± 0.2 × 105 cAMP-binding sites/cell). However, the GγΔ/AX2 cells were severely impaired in chemotaxis. They maintained random movements in the presence of a cAMP gradient and did not accumulate at the pipette tip (Figure 5). The same chemotaxis defects were observed in GγΔ/AX2 cells by repeating the assays with 0.1 or 10 μM cAMP in the micropipette (our unpublished results).
Figure 5.
GγΔ/AX2 cells fail to carry out chemotaxis. Cells at the 6-h stage of development were plated on a cover slide and a micropipette containing 1 μM cAMP was touched to its surface. The cell-movement images were taken by a CCD camera with Zeiss microscope. The left panels were taken when the micropipette first contacted the cover slide. The right panels were taken in 10 min later. Each chemotaxis assay was repeated at least three times.
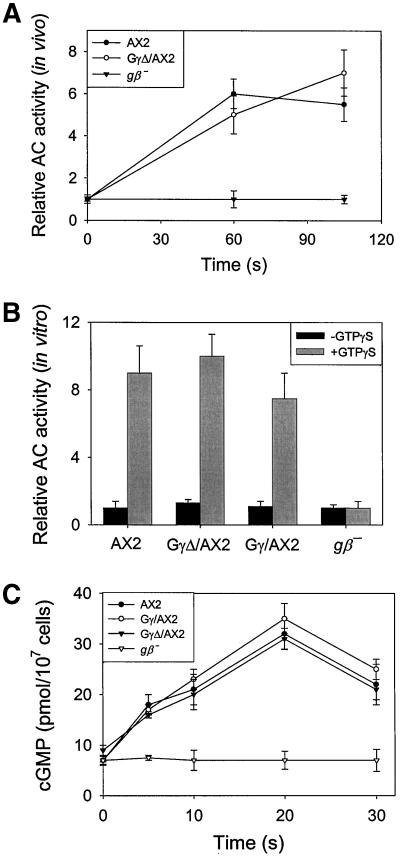
We also monitored several biochemical responses of the cells to stimulus increments. Receptor-induced actin polymerization and the production of cAMP and cGMP are mediated by G-proteins. These responses are absent in gβ− cells (Wu et al., 1995, Jin et al., 1998). In the GγΔ/AX2 cells, the cAMP-induced F-actin formation response was ∼30% as large as that of AX2 (our unpublished results). Surprisingly, chemoattractant-induced adenylyl cyclase activation in GγΔ/AX2 cells reached nearly the same levels as that in wild-type cells (Figure 6A). The similar results were obtained by assaying adenylyl cyclase activation by GTPγS in vitro (Figure 6B). Furthermore, in response to 100 nM cAMP stimuli, GγΔ/AX2 cells produced the same amounts of cGMP as AX2 cells (Figure 6C). Apparently, in GγΔ/AX2 cells, the adenylyl and guanylyl cyclase activation pathways are functional even when the majority of the G-protein is in cytosol.
Figure 6.
Adenylyl cyclase A and guanylyl cyclase assays. AX2, Gγ/AX2, and GγΔ/AX2 cells were allowed to differentiate for 6 h with cAMP. (A) The adenylyl cyclase activity was recorded after stimulation with 100 nM cAMP stimuli at time 0 s. (B) The activation of adenylyl cyclase by GTPγS was measured. (C) The accumulation of cGMP was recorded after stimulation with 100 nM cAMP. Each assay was repeated at least three times, and their average is presented.
YFP-Gβ:GγΔ/AX2 Cells Fail to Carry Out Chemotaxis
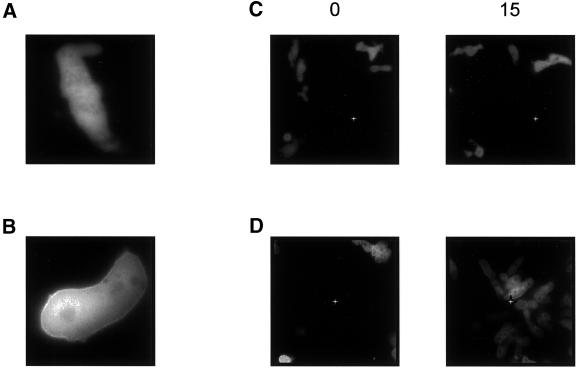
To visualize the redistribution of Gβ from membranes to cytosol, we expressed YFP-Gβ in the presence and absence of GγΔ in gβ− cells. When expressed in wild-type cells, the distribution of YFP-Gβ alone is similar in all cells and reflects the 70% membrane/30% cytosol observed for endogenous Gβ distribution (Jin et al., 2000; also see Figure 4A). Inclusion of GγΔ caused a variation among clones in the partitioning of YFP-Gβ between the membrane and cytosol. Higher levels of GγΔ caused more Gβ to shift to the cytosol. Among 16 independent YFP-Gβ:GγΔ/AX2 clones examined, clone 12 had the lowest expression of GγΔ and clone 5 had eightfold higher expression (our unpublished results). In clone 12 cells, a large portion of YFP-Gβ remained on the membrane, whereas in clone 5, most of YFP-Gβ was in the cytosol (Figure 7, A and B). As shown in Figure 7, C and D, clone 12 cells were able to carry out normal chemotaxis, whereas clone 5 cells were severely impaired in chemotaxis. Furthermore, we examined the activation of adenylyl cyclase by GTPγS. Even although the majority of YFP-Gβ was in cytosol, the adenylyl cyclase of clone 5 was activated to the same level as that of clone 12 (our unpublished results). The YFP-Gβ:GγΔ/AX2 data further confirmed that the expression of GγΔ could selectively impair chemotaxis, as opposed to the activation of adenylyl cyclase.
Figure 7.
YFP-Gβ: GγΔ/AX2 cells fail to carry out chemotaxis. Two representative clones of YFP-Gβ: GγΔ/AX2 were allowed to differentiate for 7 h. (A) YFP-Gβ was predominantly distributed in the cytosol of clone 5 cells. (B) Clone 12 showed wild-type-like distribution of YFP-Gβ. (C) Chemotaxis assay of clone 5 cells was carried out under a Zeiss inverted fluorescent microscope. At time 0 min, a micropipette containing 1 μM cAMP was positioned in a field of cells as indicated by the white star. The photographs were taken at times 0 and 15 min. (D) Chemotaxis assay of clone 12 cells was recorded. At time 0 min, the micropipette was positioned at the white star. These assays were repeated at least three times, and a representative assay is presented.
Incremental versus Gradient Stimulation
Cells are able to respond to chemoattractants presented as either increments or as stable gradients. As shown by Parent et al., GFP-tagged PH domains are useful tools for directly comparing the response to these two different types of stimulation (Parent et al. 1998). When an increment of cAMP is added, PH domains such as that of CRAC and protein kinase B (PKB) transiently associate with plasma membrane at the cell perimeter (Meili et al., 1999). In contrast, in an external cAMP gradient, the PH domains are persistently localized at the side of the cell facing the higher concentration of chemoattractant. These redistributions require functional G-proteins (Parent et al., 1998).
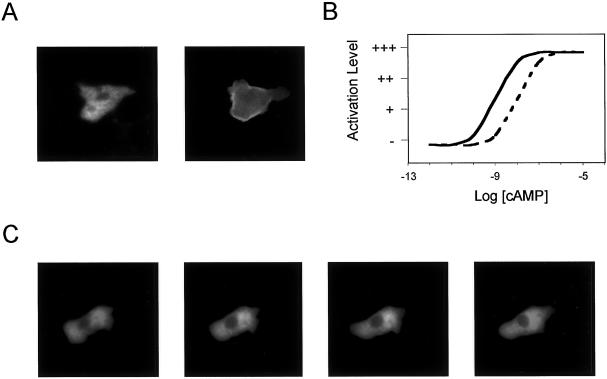
To examine the response of GFP-tagged PH domains in the GγΔ/AX2 cells, we coexpressed PHcrac-GFP and GγΔ in AX2 cells. The clone with highest expression of GγΔ was selected. When a 100 nM cAMP increment was applied uniformly to these cells, the cytosolic PHcrac-GFP transiently associated with the membrane (Figure 8A). Such translocation to the membrane was consistent with the fact that adenylyl cyclase can be activated in the GγΔ/AX2 cells. The dose dependence of this response was examined with the use of cAMP increments ranging from 10−12 to 10−5 M. The translocation of PHcrac-GFP in mutant cells could be induced by as low as 10−8 M cAMP, about one magnitude higher than that in wild-type cells (Figure 8B). In contrast, when exposed to a cAMP gradient from a micropipette containing 1 μM cAMP, the cells failed to carry out chemotaxis and the PHcrac-GFP remained in cytosol and did not associate with the membrane (Figure 8C). Changing the chemoattractant gradient with the use of 0.1 or 10 μM cAMP did not alter these assay results (our unpublished results). These data suggest that, although less sensitive, GγΔ/AX2 cells can sense stimulus increments. However, they are severely impaired in detecting shallow gradients of chemoattractant.
Figure 8.
Incremental versus gradient activation. PHcrac: GγΔ/AX2 cells were allowed to differentiate for 7 h with cAMP stimuli as described above. (A) At time 0 s, cells in 200 μl DB were stimulated with 20 μl of 1 μM cAMP and photographs were taken at 0 and 5 s. (B) The dose dependence of PHcrac-GFP translocation were assayed in PHcrac-GFP: GγΔ/AX2 (dashed line) and PHcrac-GFP/AX2 cells (solid line). The results were rated subjectively, with “−” as no response and “+++” as maximum response. (C) A cell was moving in a cAMP gradient created by a micropipette containing 1 μM cAMP located 20 μm to the left of the cell. The photographs were taken at 5-s intervals. These assays were repeated more than six times on different cells, and no exception was observed.
DISCUSSION
We have isolated a putative Gγ cDNA from D. discoideum based on its association with Gβ. Its cDNA predicts a protein of 68 amino acids in length, similar to most Gγ-subunits. The purification approach was the most expedient method to identify the Gγ gene. When compared with other Gγs sequences, the D. discoideum Gγ contains few identical amino acids, the longest stretch of identity being three residues. In retrospect, the unsuccessful database searches and PCR approaches are understandable. The predicted secondary structure of Gγ contains 2.5 α-helixes as does transducin Gγ (Sondek et al., 1996). In addition, a CSVL motif is found in its COOH-terminal, which is conserved in all Gγ-subunits. Expression of a Gγ containing a deletion of this motif causes the endogenous Gβ of wild-type cells to relocate to the cytosol, providing convincing evidence of a functional interaction of Gγ and Gβ (Simonds et al., 1991). Furthermore, fluorescent resonant energy transfer (FRET) studies demonstrate the direct interaction of Gβ and Gγ in a living cell (Zhang et al., manuscript in preparation).
Our data suggest that there is one Gβγ-dimer in D. discoideum. The genome sequencing project has provided >90% of the genomic sequence, including a single Gβ sequence. Previous studies have shown that Gγs from the same species usually share certain level of homology (Gautam et al., 1998). When we used our Gγ cDNA to search the databases, we found multiple cDNA and genomic clones, all with the same sequence. Of cause, blast-search results cannot rule out the possibility of another Gγ with a very different sequence. However, when we purified Gβγ complex, we found there was only one Gγ associated with Gβ. Moreover, our northern analyses showed that the mRNA levels of Gβ and Gγ at different development stages rose and fell in parallel.
Functional studies of GγΔ/AX2 cells indicate that responses to stimulus increments and gradients, although closely related, display different sensitivities. During development, both responses are manifested and utilize the same G-proteins. When Gβ is disrupted, both responses are eliminated. The GγΔ/AX2 cells enabled us to separate the responses. Redistribution of Gβγ to the cytosol impairs the capacity to sense the gradients, as indicated by the inability to carry out chemotaxis or relocate PHcrac-GFP to the cell's leading edge. However, these cells were able to respond to incremental stimuli. A possible explanation is that both membrane-associated and cytosolic βγ-subunits can participate in incremental sensing, but a full complement of membrane-tethered Gβγ-subunits is required for gradient sensing. Recent FRET studies showed cAMP-induced G-protein dissociation both on the membrane and in the cytosol, suggesting that cytosolic G protein might be activated by an incremental stimulus (our unpublished results).
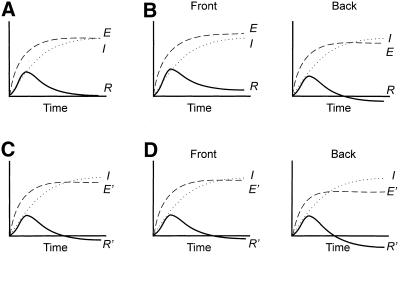
The selective inhibition of gradient sensing in cells lacking a full complement of membrane-associated Gβγ can also be explained by hybrid spatial-temporal models for directional sensing (Fischer, 1990; Parent et al., 1999). In these schemes, receptor occupancy regulates two counteracting biochemical processes: local excitation and global inhibition. When an incremental stimulus is encountered, occupied receptors trigger a rapid excitation followed by a slower rise of inhibition (Figure 9A). Both processes eventually reach the same steady-state level of activity determined by receptor occupancy, and the response subsides. In a gradient, after an initial transient response, a steady state is reached where excitation slightly exceeds inhibition at the front of the cell, whereas inhibition exceeds excitation at the back (Figure 9B). In GγΔ/AX2 cells, the redistribution of Gβγ to cytosol selectively lowers the level of excitation but does not impair inhibition. On addition of an incremental stimulus, the decreased excitation briefly exceeds inhibition, and the decreased membrane Gβγ is sufficient to mediate transient responses such as activation of ACA and translocation of PHcrac-GFP (Figure 9C). However, in a gradient at steady state, the magnitude of excitation above inhibition depends on the gradient across one single cell length of ∼10–20 μm, which is usually very shallow. In the GγΔ/AX2 cells, this difference in excitation levels is further decreased (Figure 9D). Thus, after one initial transient response, the Gβγ left on the membrane of GγΔ/AX2 cells is not enough to sense gradients and to mediate chemotaxis.
Figure 9.
Schematic representation of model for gradient sensing. (A) In wild-type cells, upon an incremental activation, a transient response (R) results from a balance between rapid excitation (E) and slower inhibition processes (I). (B) For a wild-type cell in a chemoattractant gradient, excitation is higher than the inhibition at the front and lower than the inhibition at the back at the steady state. (C) In GγΔ/AX2 cells, the receptor-specified levels of excitation (E′) are lower because less G-protein is on the membrane. On an incremental activation, the response (R′) is slightly impaired. (D) In a chemoattractant gradient, after the initial transient response, the inhibition exceeds the decreased excitation (E′) at the front of the GγΔ/AX2 cells, and gradient is not sensed.
Our isolation of γ-subunit provided a long missing member of G-protein family in D. discoideum. Further analysis of this gene should help us to understand the roles of G-proteins in chemotaxis and to identify other components located downstreams of βγ-subunits. Furthermore, we propose that dominant properties of the CXXL-deletions make these constructs useful tools for studying the functions of Gβγ-subunits in mammalian systems. In mammals, there are 20 α-subunits, 11 γ-subunits, and 5 β-subunits. This large number of subunits contributes to an even larger number of possible combinations and complicates functional analysis. Although it would be very challenging to generate clones containing disruptions of multiple Gγ genes, it might be feasible to select clones expressing specific combinations of COOH-terminal truncated Gγ genes. According to our data, these GγΔs are likely to compete with specific endogenous Gγs, shift the corresponding Gβ-subunits to cytosol, and selectively impair functions. Such cell lines or transgenic animals can be used to carry out the studies on the specific functions of each subtype of Gβγ.
ACKNOWLEDGMENTS
The authors thank Dr. Tian Jin and Dr. Christopher Janetopoulos for critical review of the manuscript, Dr. Carole Parent for providing plasmid PHcrac-GFP, and Dr. Tian Jin for providing plasmid Gβ.
Footnotes
EMBL Accession No(s): AJ312388, DDI312388
REFERENCES
- Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- Araki T, Gamper M, Early A, Fukuzawa M, Abe T, Kawata T, Kim E, Firtel RA, Williams JG. Developmentally and spatially regulated activation of a Dictyostelium STAT protein by a serpentine receptor. EMBO J. 1998;17:14. doi: 10.1093/emboj/17.14.4018. , 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlot CH, Spudich JA, Devreotes PN. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium. Cell. 1985;43:1. doi: 10.1016/0092-8674(85)90036-4. , 307–314. [DOI] [PubMed] [Google Scholar]
- Cabrera JL, de Freitas F, Satpaev DK, Slepak VZ. Identification of the Gbeta5-RGS7 protein complex in the retina. Biochem Biophys Res Commun. 1998;249:898–902. doi: 10.1006/bbrc.1998.9218. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Devreotes PN. Molecular insights into eukaryotic chemotaxis. FASEB J. 1991;5:15. , 3078–3085. [PubMed] [Google Scholar]
- Devreotes P, Fontana D, Klein P, Sherring J, Theibert A. Transmembrane signaling in Dictyostelium. Methods Cell Biol. 1987;28:299–331. doi: 10.1016/s0091-679x(08)61653-2. [DOI] [PubMed] [Google Scholar]
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Firtel RA. Integration of signaling information in controlling cell-fate decisions in Dictyostelium. Genes Dev. 1995;9:12. doi: 10.1101/gad.9.12.1427. , 1427–1444. [DOI] [PubMed] [Google Scholar]
- Fisher PR. Pseudopodium activation and inhibition signals in chemotaxis by Dictyostelium discoideum amoebae. Semin Cell Biol. 1990;1:87–97. [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- Gautam N, Downes GB, Yan K, Kisselev O. The G-protein betagamma complex. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Lee S, Firtel RA. The G alpha subunit G alpha 4 couples to pterin receptors and identifies a signaling pathway that is essential for multicellular development in Dictyostelium. Proc Natl Acad Sci USA. 1994;91:10566–10570. doi: 10.1073/pnas.91.22.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JE, Kiyosawa H, Welker DL. Plasmid maintenance functions encode on Dictyostelium discoideum nuclear plasmid Ddp1. Mol Cell Biol. 1994;14:6117–6124. doi: 10.1128/mcb.14.9.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall R, Kuspa A, Lilly PJ, Shaulsky G, Levin LR, Loomis WF, Devreotes P. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1994;126:1537–1545. doi: 10.1083/jcb.126.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG. G protein beta gamma subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of gamma. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- Jin T, Amzel M, Devreotes PN, Wu L. Selection of gbeta subunits with point mutations that fail to activate specific signaling pathways in vivo: dissecting cellular responses mediated by a heterotrimeric G protein in Dictyostelium discoideum. Mol Biol Cell. 1998;9:2949–2961. doi: 10.1091/mbc.9.10.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Soede RD, Liu J, Kimmel AR, Devreotes PN, Schaap P. Temperature-sensitive Gbeta mutants discriminate between G protein-dependent and -independent signaling mediated by serpentine receptors. EMBO J. 1998;17:5076–5084. doi: 10.1093/emboj/17.17.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- Katz A, Wu D, Simon MI. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kay RR. Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol. 1987;28:433–448. doi: 10.1016/s0091-679x(08)61661-1. [DOI] [PubMed] [Google Scholar]
- Krump E, Sanghera JS, Pelech SL, Furuya W, Grinstein S. Chemotactic peptide N-formyl-met-leu-phe activation of p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils. J Biol Chem. 1997;272:937–944. doi: 10.1074/jbc.272.2.937. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Pupillo M, Gundersen R, Miake-Lye R, Devreotes PN, Firtel RA. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989;57:265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS, Carson WE, Kordula T, Caligiuri MA, Hawley RG, Fey GH, Baumann H. Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. J Biol Chem. 1996;271:13968–13975. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. Sites for Galpha binding on the G protein beta subunit overlap with sites for regulation of phospholipase Cbeta, and adenylyl cyclase. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- Lilly P, Wu L, Welker DL, Devreotes PN. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 1993;7:986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:8. doi: 10.1093/emboj/18.8.2092. , 2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz KH, Sternweis PC, Gilman AG, Mumby SM. Influence of gamma subunit prenylation on association of guanine nucleotide-binding regulatory proteins with membranes. Mol Biol Cell. 1992;3:49–61. doi: 10.1091/mbc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Aubry L, Insall R, Gaskins C, Devreotes PN, Firtel RA. Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium. Role of heterotrimeric G proteins. J Biol Chem. 1996;271:3351–3354. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci USA. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. Isolation of inactive, and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J Biol Chem. 1995;270:22693–22696. doi: 10.1074/jbc.270.39.22693. [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem. 1996;65:411–440. doi: 10.1146/annurev.bi.65.070196.002211. [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. A cell's sense of direction. Cell. 1999;284:701–864. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- Simonds WF, Butrynski JE, Gautam N, Unson CG, Spiegel AM. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J Biol Chem. 1991;266:9. , 5363–5366. [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1Å resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- Theibert A, Devreotes PN. Surface receptor-mediated activation of adenylate cyclase in Dictyostelium. Regulation by guanine nucleotides in wild-type cells and aggregation deficient mutants. J Biol Chem. 1986;261:15121–15125. [PubMed] [Google Scholar]
- Thompson HL, Marshall CJ, Saklatvala J. Characterization of two different forms of mitogen-activated protein kinase kinase induced in polymorphonuclear leukocytes following stimulation by N-formylmethionyl-leucyl-phenylalanine or granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1994;269:9486–9492. [PubMed] [Google Scholar]
- Van Haastert PJ, Wang M, Bominaar AA, Devreotes PN, Schaap P. cAMP-induced desensitization of surface cAMP receptors in Dictyostelium. Different second messengers mediate receptor phosphorylation, loss of ligand binding, degradation of receptor, and reduction of receptor mRNA levels. Mol Biol Cell. 1992;3:603–612. doi: 10.1091/mbc.3.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Ashworth JM. Growth of myxameobae of the cellular slime mold Dictyostelium discoideum in axenic culture. Biochem J. 1970;119:171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Valkema R, Van Haastert PJ, Devreotes PN. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–1675. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Kalyanaraman V, Gautam N. Differential ability to form the G protein betagamma complex among members of the beta and gamma subunit families. J Biol Chem. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]