Abstract
Venous thromboembolism (VTE) in acute medically ill patients is a leading cause of in-hospital morbidity and mortality. A majority of these VTE events occur post-discharge, and patients remain at increased VTE risk for up to 3 months post-discharge. Recent clinical trials of extended-duration thromboprophylaxis with enoxaparin, rivaroxaban, and apixaban in acute medically ill patients did not demonstrate a net clinical benefit compared with in-hospital thromboprophylaxis, and were shown to be associated with higher risks of major bleeding. Betrixaban is a new direct oral anticoagulant (DOAC) with a different pharmacokinetic profile than other DOACs. Betrixaban has the longest half-life among the DOAC class, with a terminal half-life of 35–45 h and an effective half-life of 19–27 h. Betrixaban has a low peak-to-trough ratio compared with other anticoagulants and a predictable duration of drug exposure, leading to overall consistent anticoagulant effect over 24 h. Betrixaban is mainly cleared via the hepatobiliary system and therefore not contraindicated in patients with severe renal insufficiency. Betrixaban was recently approved for the indication of extended thromboprophylaxis in the United States based on the APEX trial of betrixaban 80 mg once daily for 35–42 days compared with low molecular weight heparin enoxaparin for 10 ± 4 days in hospitalized acute medically ill patients. This study demonstrated that extended-duration betrixaban reduced VTE compared with standard-duration enoxaparin in acute medically ill patients, without increased risk of major bleeding. This patient population at risk of VTE may benefit from extended prophylaxis, ensuring continuum of care from in-hospital to post-discharge.
Keywords: Venous thromboembolism, Medically ill, Betrixaban, Direct oral anticoagulant, Thromboprophylaxis, Pharmacokinetics
Introduction
Acute medically ill patients are at risk of venous thromboembolism (VTE), a leading potentially preventable cause of in-hospital morbidity and mortality in Europe and the United States (US).1–3 Patients remain at risk for up to 3 months after hospital discharge, with the highest risk period within the first 6 weeks after discharge.4 Preventative measures to reduce the risk of VTE events while hospitalized or after surgery include physical activity, intermittent pneumatic compression, and prophylactic doses of anticoagulants.2 Until 2017, parenteral anticoagulants including low molecular weight heparins (LMWH), unfractionated heparins, or fondaparinux for 10 ± 4 days were the only recommended drugs for thromboprophylaxis for acute medically ill patients.5 Notably, it has been demonstrated in observational studies that in-hospital prophylaxis alone does not protect patients against post-discharge VTE events.6
Recent clinical trials have investigated whether acute medically ill patients may benefit from extended-duration thromboprophylaxis, i.e. 4 to 6 weeks after discharge, with the LMWH enoxaparin as well as rivaroxaban and apixaban.7–9 In those trials, extended anticoagulant therapy was not associated with a net clinical benefit compared with standard-duration (10 ± 4 days) thromboprophylaxis administered while hospitalized, and extended thromboprophylaxis with enoxaparin, rivaroxaban, or apixaban was associated with a higher risk of major bleeding.7–9
Betrixaban is a direct oral anticoagulant (DOAC) that directly inhibits factor Xa, with a different pharmacokinetic (PK) profile than other DOACs. The APEX study compared betrixaban at a dose of 80 mg once daily for 35 to 42 days with the standard-duration LMWH enoxaparin (at a dose of 40 mg once daily) for 10 ± 4 days in hospitalized acute medically ill patients.10 This study demonstrated that extended prophylaxis with betrixaban led to a reduction in VTE compared with standard-duration enoxaparin, without an increase in major bleeding. Clinical results for betrixaban, including reduced rehospitalization rates,11 are reviewed in further detail in article 3 of this supplement.12
Pharmacodynamics
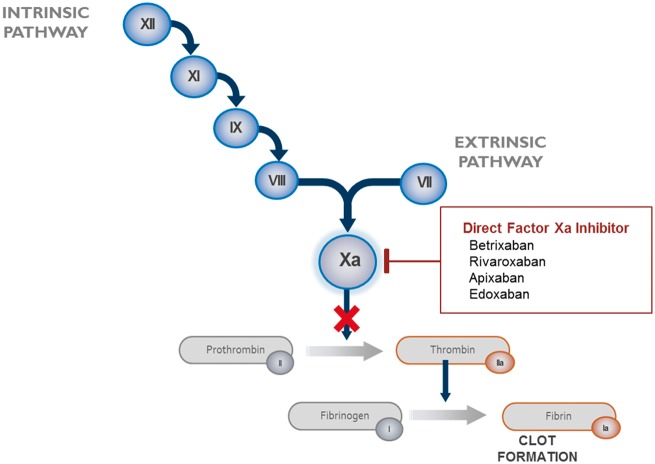
Betrixaban competitively and reversibly inhibits free and prothrombinase-bound factor Xa in a concentration-dependent manner.9,13–15 It has a profound selectivity for factor Xa above other serine proteases, including thrombin, and does not require a cofactor (such as anti-thrombin III) for activity.13,16 In intrinsic and extrinsic coagulation pathways, factor Xa plays a central role in the cascade of blood coagulation for activation of prothrombin to thrombin (Figure 1).13 Direct-acting inhibitors of factor Xa decrease thrombin generation, and thereby prevent clot formation.13
Figure 1.
Coagulation cascade.17 XII, factor XII; XI, factor XI; IX, factor IX; VIII, factor VIII; VII, factor VII; Xa, factor Xa.
Pharmacokinetics
The pharmacology of betrixaban is distinct from that of other DOACs, including the lowest renal clearance and no metabolism by CYP enzymes (Table 1). At a dose of 80 mg, betrixaban is rapidly absorbed, and its plasma concentration peaks after 3–4 h. Bioavailability is 34% and is lowered if taken with fatty food. Betrixaban is less protein-bound (60%) than the other factor Xa inhibitors. Among DOACs, betrixaban has the longest half-life (Table 1), with a terminal half-life of 35 to 45 h and an effective half-life of 19 to 27 h.18 Betrixaban has a low peak-to-trough ratio and a predictable duration of drug exposure, leading to an overall consistent anticoagulant effect over 24 h.18 For the 80-mg betrixaban doses, the anticipated Cmax is 36 ng/mL, and the volume of distribution is ∼32 L/kg. Betrixaban has slightly non-linear kinetics, which show that increasing doses are associated with greater than proportional increases in plasma concentrations.
Table 1.
| Betrixaban | Apixaban | Edoxaban | Rivaroxaban | Dabigatran | |
|---|---|---|---|---|---|
| Target | Factor Xa | Factor Xa | Factor Xa | Factor Xa | Thrombin |
| Half-life (h) | 19–27 | 12 | 10–14 | 5–9 | 12–17 |
| Dosing | o.d. | b.i.d. | o.d. | o.d. (b.i.d.) | b.i.d. |
| Tmax (h) | 3–4 | 1–3 | 1–2 | 2–4 | 2 |
| Bioavailability (%) | 34 | 50 | 62 | 66 | 7 |
| Renal excretion (%) | 17.8a | 25 | 35 | 66 | >80 |
| Faecal excretion (%) | 85b | 46.7–56 | 62.2 | 26.4 | 82–88 |
| CYP450 metabolism | No | Yes | No | Yes | No |
b.i.d., twice daily; o.d., once daily; Tmax: time to reach peak concentration in plasma after oral dose.
Unchanged betrixaban in urine following an intravenous betrixaban dose.
Following oral administration of radio-labelled betrixaban.
Unlike apixaban and rivaroxaban, betrixaban is not metabolized by CYP enzymes (<1%) and does not induce or inhibit cytochrome P450 (CYP450) activity, lowering the risk of adverse drug events or interactions during concomitant administration.18 Drugs that alter or compete for CYP enzyme activity can change the PK of concurrent medications that are metabolized by CYP450 enzymes, including antibiotics (e.g. ciprofloxacin, metronidazole, trimethoprim/sulfamethoxazole, clarithromycin, erythromycin), antifungals (e.g. ketoconazole, nefazodone, itraconazole, fluconazole), anticonvulsants (i.e. carbamazepine, phenytoin, phenobarbital), and antihypertensives (e.g. verapamil, diltiazem), drugs that are often administered to acute medically ill patients.20 In contrast, betrixaban is a substrate of P-glycoprotein.18 Dedicated Phase 1 studies found elevated betrixaban exposure when coadministered with the strong P-glycoprotein inhibitors, such as ketoconazole.19 A PK analysis of the population from the APEX study also confirmed that P-glycoprotein inhibitors increase betrixaban concentrations when coadministered, and a dose reduction to 40 mg is appropriate.21
A proportion of patients at risk for VTE have renal or hepatic insufficiency.22 Betrixaban is primarily excreted in the gut (85%) through bile via the hepatobiliary system, as well as via the P-glycoprotein efflux pump, mostly unmetabolized.18,19 Patients with hepatic impairment [defined as cirrhosis, bilirubin >2× upper limit of normal (ULN), or liver enzymes >3× ULN] should not receive betrixaban, and obstructive jaundice could also cause drug accumulation.18 The low renal clearance of betrixaban (11% following oral administration of radio-labelled betrixaban; 17.8% of the absorbed dose was observed as unchanged betrixaban in urine following intravenous administration); however, creates the possibility of safe administration in patients with severe renal impairment, defined as a reduced glomerular filtration with creatinine clearance ≥15 to <30 mL/min.19 Patients with severe renal insufficiency had higher plasma concentrations of betrixaban after full (80 mg) and half (40 mg) doses compared with patients with normal kidney function in the pivotal APEX trial.23 However, increases in plasma concentration in this patient population were not associated with an increase in major bleeding at the 80 mg or 40 mg dose of betrixaban.19,23 Therefore, the betrixaban label in the US supports use of betrixaban in patients with severe renal impairment (in contrast with other DOACs), but because these patients are still at increased risk of bleeding events, betrixaban dosage should be reduced to 40 mg.19 Even so, decisions on initiation and type of thromboprophylaxis in acute medically ill patients with severe renal insufficiency need to be individually tailored.
Conclusion
Betrixaban, approved in the US for extended thromboprophylaxis in hospitalized acute medically ill patients at risk of VTE, is a new direct factor Xa inhibitor with pharmacological properties that make it particularly appropriate for the acute medically ill population: a long half-life, a low peak-to-trough concentration ratio, low renal clearance, and no metabolism by CYP enzymes. These properties have several important clinical implications for the population where adverse drug events due to renal impairment, comorbidities, and polypharmacy are considerable. Betrixaban has a consistent anticoagulant effect over 24 h, no contraindication in patients with severe renal insufficiency (dose reduction indicated), and a low propensity for drug–drug interactions (dose reduction indicated for patients on P-glycoprotein inhibitors).
Funding: Portola Pharmaceuticals, Inc., South San Francisco, CA supported development of this supplement. The authors wrote all drafts of the article and take responsibility for its content.
Conflict of interest: F.A.K. reports research grants from Bayer, research grants from Bristol-Myers Squibb, research grants from Boehringer-Ingelheim, and non-financial research support from Daiichi-Sankyo. M.V.H. reports independent research grants and honoraria for consulting and giving presentations from Boehringer-Ingelheim, Pfizer BMS, Aspen, Bayer Healthcare, Daiichi-Sankyo, and Merck Sharp and Dohme (MSD).
References
- 1. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M; VTE Impact Assessment Group in Europe (VITAE). Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756–764. [DOI] [PubMed] [Google Scholar]
- 2. Rathbun S. Cardiology patient pages. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation 2009;119:e480–e482. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P;. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 UPDATE: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin AN, Varker H, Princic N, Lin J, Thompson S, Johnston S.. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med 2012;7:231–238. [DOI] [PubMed] [Google Scholar]
- 5. Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad MH.. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahan CE, Fisher MD, Mills RM, Fields LE, Stephenson JJ, Fu AC, Spyropoulos AC.. Thromboprophylaxis patterns, risk factors, and outcomes of care in the medically ill patient population. Thromb Res 2013;132:520–526. [DOI] [PubMed] [Google Scholar]
- 7. Hull RD, Schellong SM, Tapson VF, Monreal M, Samama MM, Nicol P, Vicaut E, Turpie AG, Yusen RD;. EXCLAIM study. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med 2010;153:8–18. [DOI] [PubMed] [Google Scholar]
- 8. Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, Weitz JI;. ADOPT Trial Investigators. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167–2177. [DOI] [PubMed] [Google Scholar]
- 9. Cohen AT, Spiro TE, Buller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos AC, Tapson V; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–523. [DOI] [PubMed] [Google Scholar]
- 10. Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Hernandez AF, Gibson CM; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016;375:534–544. [DOI] [PubMed] [Google Scholar]
- 11. Chi G, Yee MK, Amin AN, Goldhaber SZ, Hernandez AF, Hull RD, Cohen AT, Harrington RA, Gibson CM.. Extended-duration betrixaban reduces the risk of rehospitalization associated with venous thromboembolism among acutely ill hospitalized medical patients: findings from the APEX trial (Acute Medically Ill Venous Thromboembolism Prevention With Extended Duration Betrixaban Trial). Circulation 2018;137:91–94. [DOI] [PubMed] [Google Scholar]
- 12.Beyer-Westendorf J, Verhamme P, Bauersachs P. Betrixaban for prevention of venous thromboembolism in acute medically ill patients. Eur Heart J Supp2018; doi:10.1093/eurheartjsupp/suy017. [DOI] [PMC free article] [PubMed]
- 13. Gomez-Outes A, Suarez-Gea ML, Lecumberri R, Terleira-Fernandez AI, Vargas-Castrillon E, Rocha E.. Potential role of new anticoagulants for prevention and treatment of venous thromboembolism in cancer patients. Vasc Health Risk Manag 2013;9:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morganroth J, Gretler DD, Hollenbach SJ, Lambing JL, Sinha U.. Absence of QTc prolongation with betrixaban: a randomized, double-blind, placebo- and positive-controlled thorough ECG study. Expert Opin Pharmacother 2013;14:5–13. [DOI] [PubMed] [Google Scholar]
- 15. Chan NC, Hirsh J, Ginsberg JS, Eikelboom JW.. Betrixaban (PRT054021): pharmacology, dose selection and clinical studies. Future Cardiol 2014;10:43–52. [DOI] [PubMed] [Google Scholar]
- 16. Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY.. Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N, N-dimethylcarbamimidoyl)benzamido)-5-methoxybenz amide, a highly potent, selective, and orally efficacious factor Xa inhibitor. Bioorg Med Chem Lett 2009;19:2179–2185. [DOI] [PubMed] [Google Scholar]
- 17. Ansell J. Factor Xa or thrombin: is factor Xa a better target? J Thromb Haemost 2007;5:60–64. [DOI] [PubMed] [Google Scholar]
- 18. Chan NC, Bhagirath V, Eikelboom JW.. Profile of betrixaban and its potential in the prevention and treatment of venous thromboembolism. Vasc Health Risk Manag 2015;11:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bevyxxa [package insert]. South San Francisco, CA: Portola Pharmaceuticals Inc.; 2017.
- 20. Lynch T, Price A.. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician 2007;76:391–396. [PubMed] [Google Scholar]
- 21. Leeds JM, Wada DR, Bandman O, Gold A, Cohen AT, Curnutte JT, Conley PB, Analysis of patient characteristics as covariates potentially affecting pharmacokinetics, efficacy, or safety of betrixaban in the APEX study In: European Society of Cardiology. Barcelona, Spain, 2017. [Google Scholar]
- 22. Cohen AT, Gitt AK, Bauersachs R, Fronk EM, Laeis P, Mismetti P, Monreal M, Willich SN, Bramlage P, Agnelli G;. PREFER in VTE Scientific Steering Committee and the PREFER in VTE Investigators (OBOT). The management of acute venous thromboembolism in clinical practice. Results from the European PREFER in VTE Registry. Thromb Haemost 2017;117:1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gibson CM, Halaby R, Korjian S, Daaboul Y, Arbetter DF, Yee MK, Goldhaber SZ, Hull R, Hernandez AF, Lu SP, Bandman O, Leeds JM, Gold A, Harrington RA, Cohen AT; APEX Investigators. The safety and efficacy of full- versus reduced-dose betrixaban in the Acute Medically Ill VTE (Venous Thromboembolism) Prevention With Extended-Duration Betrixaban (APEX) trial. Am Heart J 2017;185:93–100. [DOI] [PubMed] [Google Scholar]