Abstract
Aluminum (Al) is neurotoxic to adults and also to infants. In this study, we investigated the developmental exposure effect of AlCl3 on postnatal hippocampal neurogenesis. Pregnant mice were administered 0-, 900-, or 1800-ppm AlCl3 via drinking water from gestational day 6 to postnatal day (PND) 21, with their offspring examined on PND 21 and PND 77. On PND 21, GFAP-immunoreactive (+) neural stem cells (NSCs) and p21Cip1/Waf1+ cells were decreased in number in the subgranular zone at 900 and ≥900 ppm, respectively. Pcna transcript level examined at 1800 ppm was decreased in the dentate gyrus. These results suggest induction of compromised cell quiescence that caused impaired self-renewal capacity of NSCs accompanying slowing down of cell cycling, which ultimately resulted in exhaustion of the NSC pool. At 1800 ppm, Reelin+ hilar GABAergic interneurons were also decreased, suggesting a contribution to the NSC reduction. At this dose, TBR2+ or DCX+ progenitor and immature granule cells and PVALB+ interneurons were increased. Moreover, COX-2+ granule cells were increased at ≥900 ppm. These results suggest facilitation of transient progenitor cell proliferation and differentiation during exposure. Moreover, TUNEL+ or Morin-stained granule cells were increased, together with Casp12 transcript upregulation, suggesting induction of Al accumulation-related endoplasmic reticulum stress-mediated granule cell apoptosis. Transcript expression changes on cholinergic and glutamatergic signals and synaptic plasticity suggested contribution to disruptive neurogenesis. The NSC-targeting effects sustained through the adult stage despite no sustained Al-accumulation. These results suggest that developmental AlCl3-exposure irreversibly affects postnatal hippocampal neurogenesis involving multiple functions in mice.
Keywords: aluminum chloride, hippocampal neurogenesis, mouse, developmental neurotoxicity, synaptic plasticity
Aluminum (Al) is the third-most abundant element found in the earth’s crust and occurs naturally in the environment, foodstuffs, and drinking water (Priest, 1993). Although the concentration of Al in drinking water and foods has been low, the levels of Al in drinking water are increased by acid rain (Smith, 1996). Conversely, Al products have many modern applications. Adding aluminum sulfate and lime to water causes aluminum hydroxide formation, which leads to settling of pollutants. Al-containing agents are also commonly found as food and medication additives. Infant formulae are especially rich in Al (Burrell and Exley, 2010). Human exposure to Al usually occurs primarily by way of the gastrointestinal tract through oral intake. In a 60-kg adult, estimated daily alimentary Al exposure is 1.6–13 mg (0.2–1.5 mg/kg body weight [BW]/week); however, relative exposure in children is higher at up to 2.3 mg/kg BW/week (EFSA, 2008).
Absorbed Al can cross the blood-brain barrier and accumulate in the brain, especially in hippocampal tissue (Struys-Ponsar et al., 1997). The neurotoxic effect of Al is well known and excessive Al exposure causes multiple abnormalities of the neuronal system, especially in uremic patients with dialytic treatment, called dementia-like encephalopathy, with features similar to Alzheimer’s disease (DeVoto and Yokel, 1994). In addition, mothers and their newborn infants showed nearly identical blood Al concentration in the analysis of human biological samples (Takács et al., 1992), and prolonged intravenous feeding with solutions containing Al has been shown to be associated with impaired neurologic development in preterm infants (Bishop et al., 1997). Experimentally, maternal Al exposure results in a high concentration of Al in the hippocampus in neonatal rats (Yuan et al., 2012), and oral Al exposure during lactation has been shown to cause neurotoxicity, such as short- and long-term memory deficits in rats (Moazedi et al., 2008).
The dentate gyrus in the hippocampal formation of the mammalian brain is crucial for higher brain functions, such as learning, memory, and motivation (Montaron et al., 2004). During postnatal life, neurogenesis continues in the subgranular zone (SGZ) of the dentate gyrus (Zhao et al., 2008). In the SGZ, type-1 neural stem cells (NSCs) differentiate into type-2a, type-2b, and type-3 progenitor cells. The type-3 cells undergo final mitosis to differentiate into immature granule cells, and then to mature granule cells (Hodge et al., 2008). In the hilus of the dentate gyrus, subpopulations of γ-aminobutyric acid (GABA)-ergic interneurons innervate granule cell lineage populations to control neurogenesis in the SGZ (Masiulis et al., 2011). One subpopulation of GABAergic interneurons produces Reelin, an extracellular glycoprotein that is essential for neuronal migration and correct positioning, including that for the granule cell lineage (Gong et al., 2007). In addition to GABAergic neuronal inputs, various types of neurons outside the SGZ also create a synaptic connection with neurons in the dentate gyrus, such as glutamatergic neurons in the entorhinal cortex providing axonal projections to the dentate gyrus (Fonnum et al., 1979) and cholinergic neurons originating from the septal nucleus and nucleus of the diagonal band of Broca innervating neurons in the hilus of the dentate gyrus (Amaral and Kurz, 1985). Glutamatergic inputs to the SGZ are important for maintaining proper proliferation and differentiation of the granule cell lineage (Freund and Buzsáki, 1996).
Recently, we reported that developmental exposure of several neurotoxicants and developmental neurotoxicants, such as manganese, acrylamide, and glycidol, targets granule cell lineages to alter a number of subpopulations during hippocampal neurogenesis (Shibutani, 2015). In addition to the changes in granule cell lineage subpopulations, we have also detected aberrations in the number of hilar GABAergic interneuron subpopulations (Shibutani, 2015). With regard to neuronal signals from outside the hippocampus to the neurons in the dentate gyrus, we also reported decreased glutamatergic or cholinergic inputs in association with disrupted neurogenesis (Itahashi et al., 2015; Tanaka et al., 2016). Therefore, evaluating the effects of toxicants on neurogenesis by analyzing populations of granule cell lineages in combination with interneuron subpopulations, as well as fluctuations in signal inputs, could reveal target mechanisms involved in neurogenesis.
In the present study, to determine whether exogenously administered Al targets developing nervous systems, we examined the maternal exposure effect of aluminum chloride (AlCl3) on developmental neurotoxicity, targeting hippocampal neurogenesis in mouse offspring. We analyzed distribution changes of granule cell lineage subpopulations in the SGZ/GCL, as well as their cell proliferation, apoptosis and synaptic plasticity. We also analyzed distribution changes of GABAergic interneuron subpopulations in the hilus of the dentate gyrus, as well as transcript expression changes of other regulatory systems.
MATERIALS AND METHODS
Chemicals and animals
Aluminum chloride hexahydrate (AlCl3·6H2O; purity: >98.0%; CAS No. 7784-13-6) was purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Thirty-six mated female Slc: ICR mice were purchased from Japan SLC, Inc (Hamamatsu, Japan) at gestational day (GD) 1 (appearance of vaginal plug was designated as GD 0). Maternal animals were individually housed in polycarbonate cages with paper bedding until postnatal day (PND) 21 (where PND 0 is the day of delivery). Animals were maintained in an air-conditioned animal room (temperature: 23 ± 2°C, relative humidity: 55 ± 15%) with a 12-h light/dark cycle. Maternal animals were allowed access to a pelleted basal diet (CRF-1; Oriental Yeast Co. Ltd, Tokyo, Japan) throughout the experimental period and distilled water (DW) ad libitum until the start of exposure to AlCl3. From PND 21 onwards, offspring were reared with 2 or 3 animals per cage and provided with the pellet CRF-1 basal diet and DW ad libitum.
Experimental design
Mated female mice were randomly assigned to 3 groups of 12 animals per group by stratified randomization according to BW and treated with 0, 900, or 1800 ppm AlCl3 (0, 182, or 364 ppm as Al) in DW from GD 6 to PND 21 (Supplementary Figure 1). Based on the appearance of behavioral abnormalities of mouse offspring by postnatal exposure to AlCl3 during PND 1–15 via maternal drinking water at the dose level of ≥300 mg/kg body weight/day (Abu-Taweel et al., 2011), a preliminary maternal exposure study, which was consisted of the dosing groups similar to or exceeding the level to induce behavioral abnormalities, ie, 0, 600, and 1200 ppm in the drinking water, was conducted (N = 5 dams). At 1200 ppm, male offspring exposed to AlCl3 showed transient decrease in the body weight on PND 5 and female offspring decreased absolute brain weight on PND 21. There were no apparent changes in the number of live offspring and body weight of dams at both 600 and 1200 ppm. Thus, we decided to select 1800 ppm as the highest dose level that is expected to show slight maternal toxicity, according to the Organisation for Economic Co-operation and Development (OECD) guideline for the testing of chemicals (Test No. 426: Developmental Neurotoxicity Study; OECD, 2007). The lower dose was subsequently set to 900 ppm.
Body weights and food and water consumption of dams were measured twice a week throughout the experimental period. On PND 4, the litters were randomly culled to preserve 5–7 male pups and 3–5 female pups per litter. The offspring were weighed every twice a week until PND 21.
In the present study, male offspring were selected for immunohistochemical and gene expression analyses, because neurogenesis is influenced by circulating levels of steroid hormones during the estrous cycle (Pawluski et al., 2009). On PND 21, 10–12 male offspring per group (1 male offspring per dam) were subjected to perfusion fixation for histopathological assessment and immunohistochemistry through the left cardiac ventricle with ice-cold 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) at a flow rate of 10 ml/min after deep anesthetization with CO2/O2. For gene expression analysis, 20–24 male offspring per group (2 male pups per dam) were euthanized by exsanguination from the abdominal aorta under CO2/O2 anesthesia and subjected to necropsy, and brains were fixed in methacarn solution at 4°C for 5 h after measuring the weight. All dams were euthanized by exsanguination from the abdominal aorta under CO2/O2 anesthesia and their brains were removed to fix with Bouin’s solution and neutral buffered formalin (pH 7.4), respectively at 4°C overnight. Twenty-24 female offspring per group (2 female pups per dam) were also similarly euthanized for collection of brain samples to store at –20°C for other experimental purposes after measuring the weight. The remaining offspring were maintained without administration of AlCl3 until PND 77, and body weight was measured once a week.
On PND 77, 10–12 male offspring per group (1 male offspring per dam) were subjected to perfusion fixation with 4% PFA buffer solution for histopathological assessment and immunohistochemistry at a flow rate of 10 ml/min. For gene expression analysis, 13–22 male offspring per group (1 or 2 male pups per dam) were euthanized by exsanguination from the abdominal aorta under CO2/O2 anesthesia and subjected to necropsy, and brains were fixed in methacarn solution at 4°C for 5 h after measuring the weight. Six male offspring of 1800 ppm group and 16–28 female offspring per group (1–3 female pups per dam) were also similarly euthanized for collection of brain samples to store at –20°C for other experimental purposes after measuring the weight. All animal experiments were conducted in accordance with the “Guidelines for Proper Conduct of Animal Experiments” (Science Council of Japan, June 1, 2006), and the protocols were approved by the Animal Care and Use Committee of the Tokyo University of Agriculture and Technology. All efforts were made to minimize animal suffering.
Histopathology of the brain
After fixation with 4% PFA buffer solution at PND 21 and PND 77, brains from male offspring (N = 10–12/group; 1 male pups per dam) were additionally immersion fixed with the same solution overnight at 4°C. Three-mm-thick coronal slices were prepared at −2.2 mm from the bregma. Brain slices were further immersion fixed with 4% PFA buffer solution overnight at 4°C and were routinely processed for paraffin embedding and sectioned into 3-μm-thick sections. Brain sections were stained with hematoxylin and eosin and subjected to histopathological examination.
Immunohistochemistry and apoptotic cell detection in the brain
Brain sections of male offspring perfused at necropsies on PND 21 and PND 77 were also subjected to immunohistochemistry using primary antibodies against proliferating cell nuclear antigen (PCNA), and the neuronal stage-defining markers, glial fibrillary acidic protein (GFAP), expressed in type-1 NSCs (radial glial cells) in the SGZ (Kempermann et al., 2004), sex determining region Y-box 2 (SOX2), expressed in type-1 NSCs, type-2a and type-2b progenitor cells (Lugert et al., 2012), T-box brain protein 2 (TBR2), expressed in type-2b progenitor cells in the SGZ (Lugert et al., 2012), and doublecortin (DCX), expressed in type-2b and type-3 progenitor cells and immature granule cells in the SGZ and GCL (Kempermann et al., 2004; Lugert et al., 2012) and neuron-specific nuclear protein (NeuN), expressed in postmitotic neurons of both immature and mature granule cells in the SGZ and GCL (Kempermann et al., 2004; Lugert et al., 2012). Immunohistochemistry of GABAergic interneuron subpopulations was also performed using primary antibodies against Reelin, parvalbumin (PVALB), calbindin-D-28K (CALB1) and somatostatin (SST) (Freund and Buzsáki, 1996; Gong et al., 2007). Immunohistochemistry of glutamatergic excitatory mossy cells was performed using primary antibody against calbindin-D-29K (CALB2; Fujise et al., 1998). Immunohistochemistry of molecules related to synaptic plasticity was also performed using primary antibodies against FBL osteosarcoma oncogene (FOS), activity-regulated cytoskeleton-associated protein (ARC), and cyclooxygenase-2 (COX-2), which are the members of immediate-early genes (IEGs) involved in neuronal plasticity (Fleischmann et al., 2003; Guzowski, 2002; Nahm and Noebels, 1998). Immunohistochemistry of molecules related to cell cycle was also performed using primary antibodies against p21Cip1/Waf1, a cyclin-dependent kinase inhibitor acting in G1/S phase (Roque et al., 2012). Immunohistochemistry of molecules related to astrocyte, microglia, and oligodendrocyte was also performed using primary antibodies against GFAP, an intermediate filament expressed in astrocytes (Eng et al., 2000); ionized calcium-binding adaptor molecule 1 (Iba1), a specific marker of microglia (Ito et al., 1998); and oligodendrocyte transcription factor 2 (OLIG2), a specific marker of oligodendrocyte (Fang et al., 2013). Incubation with the respective antibodies was performed overnight at 4°C. Primary antibodies are listed in the Supplementary Table 1. Deparaffinized sections were incubated in 0.3% hydrogen peroxide solution in absolute methanol for 30 min to quench endogenous peroxidase. Conditions of antigen retrieval applied for some antibodies were shown in Supplementary Table 1. Immunodetection was performed using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) with DAB/H2O2 as the chromogen. Immunostained sections were then counterstained with hematoxylin and coverslipped for microscopic examination. One section per animal was subjected to each immunohistochemistry.
For evaluation of apoptosis in the SGZ or GCL of the dentate gyrus, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was carried out using the ApopTag Peroxidase In situ Apoptosis Detection kit (EMD Millipore, Billerica, MA) according to the manufacturer’s instructions, with DAB/H2O2 as the chromogen. One section per animal was subjected to TUNEL assay.
Morin (3,5,7,2′,4′-pentahydroxyflavone) staining
The Al-Morin fluorescence assay was performed for visualization and detection of Al in the SGZ/GCL, according to the protocol by Shaw and Petrik (2009). Brain sections from 5 male pups per group were stained with Morin, a fluorochrome that forms a fluorescent complex with Al fluorescing green with an excitation wavelength of 420 nm (Crapper et al., 1973; De Boni et al., 1974). Deparaffinized sections were first washed with phosphate buffered saline (pH 7.4) twice for 5 min, and then pretreated for 10 min in a 1% aqueous solution of hydrochloric acid, rinsed in DW twice for 5 min, and immersed in 0.2% solution of Morin (Sigma-Aldrich, St. Luis, MO) in 85% ethyl alcohol containing 0.5% acetic acid for 10 min. The sections were then washed in DW twice for 5 min, dehydrated in 70%, 90%, and 100% ethyl alcohol, cleared with 100% xylene, and mounted with Bioleit (Oken-shoji, Tokyo, Japan). One section per animal was subjected to Al-Morin fluorescence assay.
Morphometry of immunolocalized cells, apoptotic cells, and Morin-stained cells
All immunoreactive cells, ie, GFAP+, SOX2+, TBR2+, DCX+, NeuN+, ARC+, COX-2+, FOS+, PCNA+, or p21Cip1/Waf1+ cells, and all TUNEL+ apoptotic cells in the SGZ and/or GCL of whole dentate gyrus were bilaterally counted and normalized for the entire length of the SGZ (Supplementary Figure 2). GFAP+ radial glia cells were morphologically identified as those having cell bodies that reside in the SGZ and vertical processes that extend into the molecular layer of the dentate gyrus and distinguished from GFAP+ astrocytes in the hilus of the hippocampal dentate gyrus. All immunoreactive cells distributed within the hilus, ie, Reelin+, PVALB+, CALB1+, CALB2+, SST+, NeuN+, GFAP+, Iba1+, or OLIG2+ cells were bilaterally counted and normalized per unit area of the whole hilar area (Supplementary Figure 2). The immunoreactive cells were analyzed by blind trial for the treatment conditions. Cornu ammonis region 3 pyramidal neurons distributed in the hilar area were easily distinguished from hilar interneurons and excluded from counting. For quantitative measurements of each immunoreactive cellular component, digital photomicrographs at 400-fold magnification were taken using a BX53 microscope (Olympus Corp., Tokyo, Japan) attached to a DP72 Digital Camera System (Olympus Corp.), and quantitative measurements were performed using the WinROOF image analysis software package (version 5.7, Mitani Corp., Fukui, Japan).
Detection of the fluorescent complex of Morin with Al was performed as reported previously (Khan et al., 2013) with some modification. To acquire Morin-fluorescence images, BX-53 fluorescence microscope (Olympus Corp.) equipped with a color charge-coupled device camera (DP72; Olympus Corp.) and cellSens imaging software (Olympus Corp.) were used. The excitation and emission filter was 400 to 440 and 460 nm (long pass), respectively. The green channel images corresponding to Morin-fluorescence were used to count the Morin+ cells, following the subtraction of background of fluorescence to normalize sample-to-sample variation. All Morin+ cells in the GCL of whole dentate gyrus were bilaterally counted and normalized for the entire length of the SGZ (Supplementary Figure 2).
Real-time reverse-transcription polymerase chain reaction
Real-time reverse-transcription polymerase chain reaction (RT-PCR) analysis of transcript expression in the hippocampal dentate gyrus was examined in PND 21 and PND 77 offspring. Brain tissues were dissected according to the whole-brain fixation method using methacarn solution (Akane et al., 2013). In brief, 2-mm-thick coronal cerebral slices were prepared at −2.2 mm from the bregma after methacarn-fixation. Hippocampal dentate gyrus tissue was collected from the slice using a punch biopsy device with a 1-mm pore size (Kai Industries Co. Ltd, Gifu, Japan). Total RNA was extracted from tissue samples of the 0-ppm controls and 1800-ppm AlCl3-exposed groups (N = 6 pups from different dams per group) using QIAzol (Qiagen, Hilden, Germany) and RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. First-strand cDNA was synthesized using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA) from 2 μg total RNA. Analysis of the transcript levels for gene targets shown in Supplementary Table 2 was performed with the PCR primers designed with Primer Express software (Version 3.0; Thermo Fisher Scientific). Real-time PCR with Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) was conducted using individual samples (N = 6) and a StepOnePlus Real-time PCR System (Thermo Fisher Scientific). At first, comparison of the expression level was made between 0-ppm controls and 1800-ppm AlCl3 group, and the relative differences in gene expression between the 2 groups were calculated with threshold cycle (CT) values that were first normalized to those of the glyceraldehyde 3-phosphate dehydrogenase (Gapdh) or hypoxanthine guanine phosphoribosyl transferase (Hprt) gene as the endogenous control in the same sample. The relative differences in gene expression compared with control CT values were calculated using the 2−ΔΔCT method as previously described (Livak and Schmittgen, 2001).
Statistical analysis
Numerical data are presented as mean ± SD. Maternal body weights, food and water consumption, and organ weights were analyzed using the individual animal as the experimental unit. Offspring body and organ weights, immunoreactive cell counts for each antigen, and numbers of apoptotic cells were analyzed using the litter as the experimental unit. Data were analyzed using the Levene’s test for homogeneity of variance. Significant differences between the 0-ppm controls and each AlCl3-exposed group were evaluated as follows. If the variance was homogenous, numerical data were assessed using the Dunnett’s test. For heterogeneous data, the Aspin-Welch’s t-test with Bonferroni correction was applied. Numerical data consisting of 2 sample groups were analyzed using the Student’s t-test when the variance was homogenous between the groups, and the Aspin-Welch’s t-test was performed when data were heterogeneous. All analyses were performed using the IBM SPSS Statistics ver. 25 (IBM Japan, Ltd, Tokyo, Japan).
RESULTS
Maternal Parameters
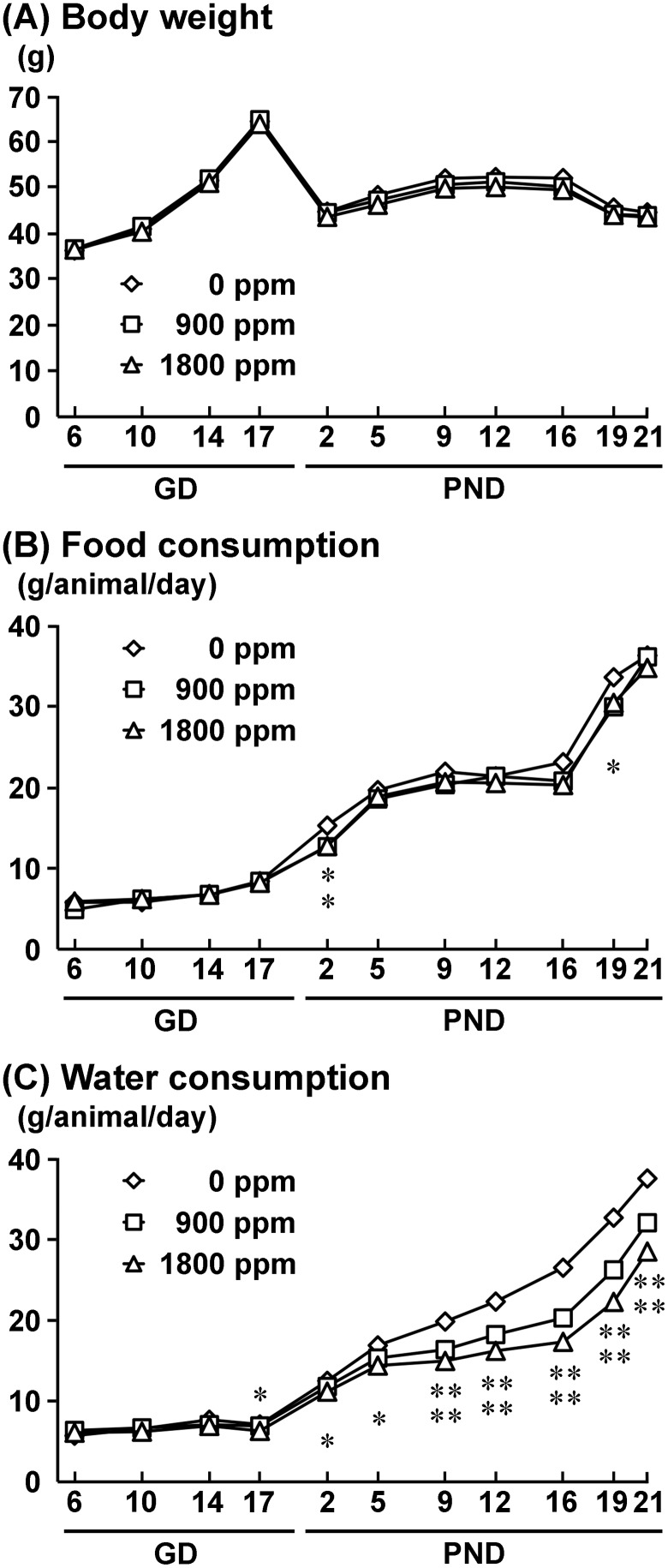
One nonpregnant animal in the 900-ppm group and 2 animals which had abortions, one each in the 900-ppm and 1800-ppm group, as confirmed by examination of implantation sites and the number of live offspring at birth, were excluded from the experiment. Therefore, effective number of dams were 12, 10, and 11 for 0-, 900-, and 1800-ppm group, respectively. The number of implantation sites and live offspring and male ratio were not different between the 0-ppm controls and the AlCl3-exposed groups (Supplementary Table 3). Dams showed no significant changes in body weight during gestation and lactation periods between the 0-ppm and any of the exposed groups (Figure 1). Food consumption was significantly decreased on PND 2 in the 900- and 1800-ppm groups and on PND 19 in the 1800-ppm group compared with 0-ppm controls (Figure 1). Water consumption was significantly decreased from PND 9 to PND 21 in the 900-ppm group, and from GD 17 to PND 21 in the 1800-ppm group, compared with 0-ppm controls (Figure 1). There were no abnormalities in the gait and behaviors of dams in any group. At necropsy on PND 21, there were no change in absolute and relative brain weights in any group (Supplementary Table 3). Based on mean values of water consumption, dams in the 900- and 1800-ppm groups received 107.9 and 207.1 mg/kg body weight/day Al, respectively, during the gestation period. Dams took 319.8 and 574.5 mg/kg body weight/day Al during the lactation period, respectively.
Figure 1.
Body weight (A), food consumption (B), and water consumption (C) of dams during the exposure period. *p < .05, **p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Clinical Observation and Necropsy Data of Offspring
No abnormalities in the gait and behaviors were observed in offspring of any group before necropsy on PND 21 and after weaning. Body weights of male offspring of the 1800-ppm group significantly decreased from PND 4 to PND 77 compared with 0-ppm controls. Body weights of female offspring of the 900-ppm group from PND 4 to PND 12 and from PND 19 to PND 35 and of the 1800-ppm group from PND 4 to PND 56 significantly decreased compared with 0-ppm controls (Supplementary Table 4). At necropsy on PND 21, male offspring of the 1800-ppm group and female offspring of the 900- and 1800-ppm groups showed significantly decreased body weight compared with 0-ppm controls (Table 1). At necropsy on PND 77, male offspring of the 1800-ppm group showed significantly decreased body weight compared with 0-ppm controls. With regard to brain weight in male offspring on PND 21, relative weight was significantly higher in the 1800-ppm group compared with 0-ppm controls (Table 1). In female offspring, absolute weight was significantly lower in the 1800-ppm group and relative weight was significantly higher in the 900- and 1800-ppm groups, compared with 0-ppm controls. With regard to brain weight in male offspring on PND 77, absolute and relative weights showed no change in any group. In female offspring, absolute brain weight was significantly lower in the 1800-ppm group compared with 0-ppm controls.
Table 1.
Body and Brain Weights of Offspring on PND 21 and PND 77
| AlCl3 in Drinking Water (ppm) |
|||
|---|---|---|---|
| 0 (Control) | 900 | 1800 | |
| PND 21 | |||
| Males | |||
| No. of offspring examined | 36 | 30 | 33 |
| Body weight (g) | 17.9 ± 0.9 | 16.9 ± 1.3 | 15.6 ± 0.8** |
| No. of offspring examined | 24 | 20 | 22 |
| Brain weight (g) | 0.45 ± 0.02 | 0.44 ± 0.01 | 0.43 ± 0.01 |
| (g/100g body weight) | 2.50 ± 0.15 | 2.65 ± 0.26 | 2.79 ± 0.18** |
| Females | |||
| No. of offspring examined | 24 | 20 | 22 |
| Body weight (g) | 17.4 ± 0.7 | 15.7 ± 1.5** | 15.1 ± 0.7** |
| Brain weight (g) | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.43 ± 0.02* |
| (g/100g body weight) | 2.58 ± 0.19 | 2.82 ± 0.24* | 2.90 ± 0.13** |
| PND 77 | |||
| Males | |||
| No. of offspring examined | 32 | 23 | 39 |
| Body weight (g) | 53.0 ± 2.8 | 53.0 ± 4.7 | 48.3 ± 2.1** |
| No. of offspring examined | 20 | 13 | 28 |
| Brain weight (g) | 0.50 ± 0.01 | 0.50 ± 0.02 | 0.50 ± 0.02 |
| (g/100g body weight) | 0.98 ± 0.08 | 0.94 ± 0.10 | 1.04 ± 0.08 |
| Females | |||
| No. of offspring examined | 28 | 27 | 16 |
| Body weight (g) | 42.5 ± 4.4 | 43.7 ± 2.7 | 40.5 ± 3.4 |
| Brain weight (g) | 0.51 ± 0.02 | 0.49 ± 0.02 | 0.48 ± 0.03* |
| (g/100g body weight) | 1.85 ± 0.08 | 1.89 ± 0.09 | 1.95 ± 0.17 |
Abbreviations: PND, postnatal day; AlCl3, aluminum chloride.
Data are expressed as the mean ± SD.
p < .05,
p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Brain Histopathology of Male Offspring
There were no histopathological changes in the brain of male offspring in any group at both PND 21 and PND 77.
Distribution of Granule Cell Lineage Subpopulations in the SGZ and GCL of Male Offspring
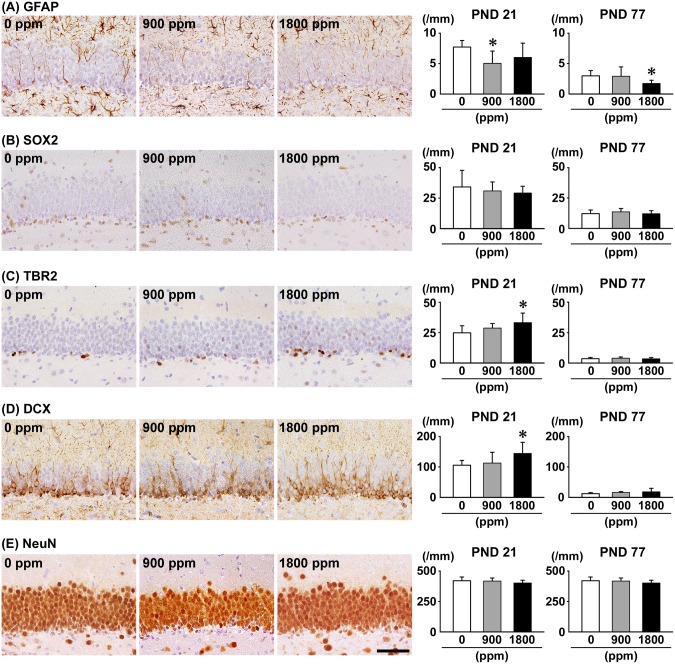
On PND 21, the number of GFAP+ cells in the SGZ was significantly less at 900 ppm, and the number of TBR2+ cells in the SGZ and DCX+ cells in the SGZ/GCL was significantly greater at 1800 ppm compared with 0-ppm controls (Figure 2). With regard to SOX2+ cells in the SGZ and NeuN+ cells in the GCL, there were no significant differences in any exposure group compared with 0-ppm controls. On PND 77, the number of GFAP+ cells in the SGZ was significantly less at 1800 ppm (Figure 2 and Supplementary Figure 3). However, no significant differences were observed in the number of SOX2+ cells and TBR2+ cells in the SGZ, and DCX+ cells in the SGZ/GCL, and NeuN+ cells in the GCL of any exposure group compared with 0-ppm controls.
Figure 2.
Distribution of immunoreactive cells for (A) glial fibrillary acidic protein (GFAP), (B) SRY (sex determining region Y)-box2 (SOX2), and (C) T box brain protein 2 (TBR2) in the subgranular zone (SGZ), (D) doublecortin (DCX) in the SGZ and granule cell layer (GCL), and (E) neuron-specific nuclear protein (NeuN) in the GCL of male offspring at postnatal day (PND) 21 and PND 77 after maternal exposure to aluminum chloride (AlCL3) from gestational day (GD) 6 to PND 21. Representative images from the 0-ppm controls (left), and the 900-ppm (middle), and 1800-ppm (right) AlCL3 groups at PND 21. Magnification ×400; bar 50 μm. Graphs show the numbers of immunoreactive cells in the SGZ and/or GCL. N = 9–12/group (PND 21: 0-ppm controls, 10; 900-ppm AlCL3, 10; 1800-ppm AlCL3, 10; PND 77: 0-ppm controls, 12; 900-ppm AlCL3, 9; 1800-ppm AlCL3, 11). *p < .05, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Distribution of Neuronal Subpopulations in the Hilus of the Dentate Gyrus of Male Offspring
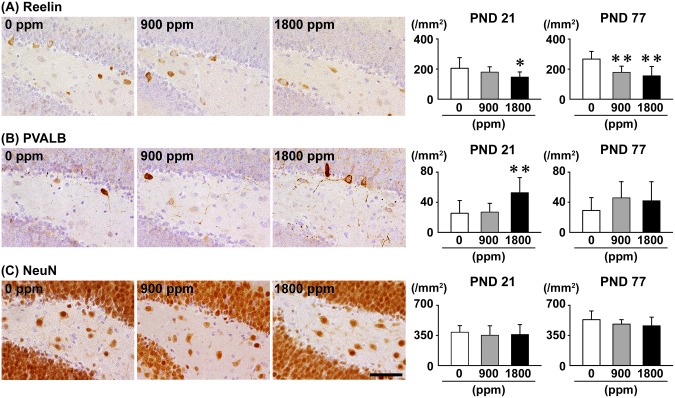
On PND 21, the number of Reelin+ cells in the hilus was significantly less at 1800 ppm compared with 0-ppm controls, and the number of PVALB+ cells was significantly greater at 1800 ppm compared with 0-ppm controls (Figure 3). There were no significant differences in the number of CALB1+ cells, CALB2+ cells and SST+ cells and NeuN+ postmitotic neurons of any exposure group compared with 0-ppm controls (Figure 3 and Supplementary Figures 4 and 5). On PND 77, the number of Reelin+ cells was significantly less at ≥900 ppm compared with 0-ppm controls (Figure 3 and Supplementary Figure 6). No significant differences were observed in the numbers of PVALB+ cells, CALB1+ cells, CALB2+ cells, SST+ cells and NeuN+ mature neurons of any exposure group compared with 0-ppm controls (Figure 3 and Supplementary Figures 4–6).
Figure 3.
Distribution of interneurons immunoreactive for (A) Reelin or (B) parvalbumin (PVALB), and (C) NeuN-immunoreactive postmitotic neurons in the hilus of the hippocampal dentate gyrus of male offspring at PND 21 and PND 77 after maternal exposure to aluminum chloride (AlCL3) from GD 6 to PND 21. Representative images from the 0-ppm controls (left) and the 900-ppm (middle) and 1800-ppm (right) AlCL3 groups at PND 21. Magnification ×400; bar 50 μm. Graphs show the numbers of immunoreactive interneurons in the hilus. N = 9–12/group (PND 21: 0-ppm controls, 10; 900-ppm AlCL3, 10; 1800-ppm AlCL3, 10; PND 77: 0-ppm controls, 12; 900-ppm AlCL3, 9; 1800-ppm AlCL3, 11). *p < .05, **p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Proliferating, Apoptotic and Cycle-Arrested Cells in the SGZ and GCL of Male Offspring
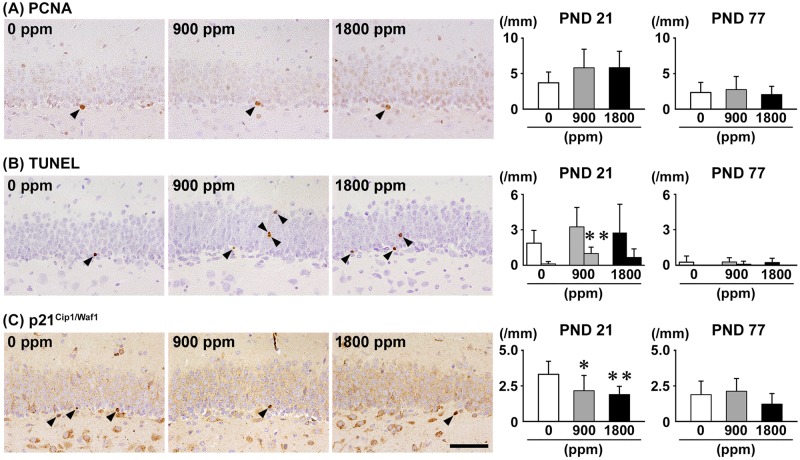
On PND 21, there was no significant difference in the number of PCNA+ proliferating cells and TUNEL+ apoptotic cells in the SGZ in any exposure group compared with 0-ppm controls (Figure 4). On the other hand, the number of TUNEL+ apoptotic cells in the GCL was significantly greater at 900 ppm compared with 0-ppm controls. The number of p21Cip1/Waf1+ cells in the SGZ was significantly less at ≥900 ppm compared with 0-ppm controls. On PND 77, no significant differences were observed in the number of PCNA+ cells in the SGZ, TUNEL+ cells in the SGZ or GCL and p21Cip1/Waf1+ cells in the SGZ in any exposure group compared with 0-ppm controls (Figure 4 and Supplementary Figure 7).
Figure 4.
Distribution of (A) proliferating cell nuclear antigen (PCNA)+ proliferating cells in the SGZ, (B) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ apoptotic cells in the SGZ or the GCL, and (C) p21Cip1/Waf1+ cells in the SGZ of male offspring at PND 21 and PND 77 after maternal exposure to aluminum chloride (AlCL3) from GD 6 to PND 21. Representative images from the 0-ppm controls (left) and the 900-ppm (middle) and 1800-ppm (right) AlCL3 groups at PND 21. Arrowheads indicate immunoreactive or TUNEL+ apoptotic cells. Magnification ×400; bar 50 μm. Graphs show the numbers of immunoreactive cells in the SGZ or GCL. In the graphs of (B), left and right columns at each dose indicate the numbers of TUNEL+ cells in the SGZ and in the GCL, respectively. N = 9–12/group (PND 21: 0-ppm controls, 10; 900-ppm AlCL3, 10; 1800-ppm AlCL3, 10; PND 77: 0-ppm controls, 12; 900-ppm AlCL3, 9; 1800-ppm AlCL3, 11). *p < .05, **p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Distribution of Synaptic Plasticity-Related Molecules in the GCL of Male Offspring
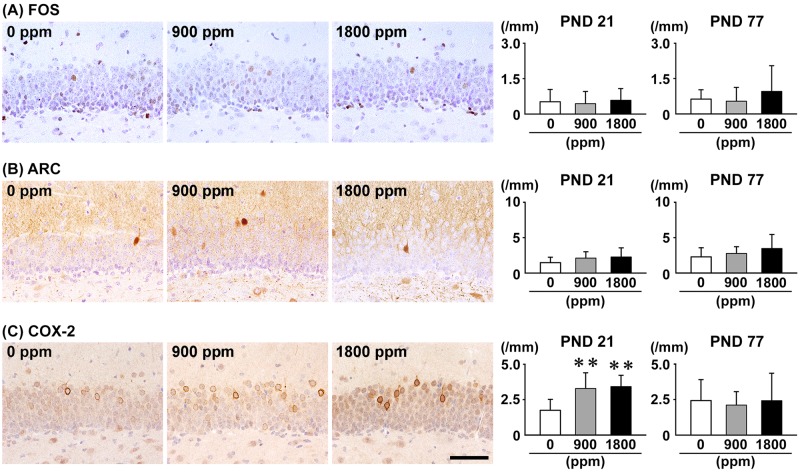
On PND 21, the number of COX-2+ cells in the GCL was significantly greater at ≥900 ppm compared with 0-ppm controls, whereas there were no significant differences in the numbers of ARC+ cells and FOS+ cells in any exposure group compared with 0-ppm controls (Figure 5). On PND 77, no significant differences were observed in the numbers of FOS+ cells, ARC+ cells and COX-2+ cells in any exposure group compared with 0-ppm controls (Figure 5 and Supplementary Figure 8).
Figure 5.
Distribution of immunoreactive cells for (A) FBJ osteosarcoma oncogene (FOS), (B) activity-regulated cytoskeleton-associated protein (ARC), and (C) cyclooxygenase-2 (COX-2) in the GCL of the hippocampal dentate gyrus of male offspring at PND 21 and PND 77 after maternal exposure to aluminum chloride (AlCL3) from GD 6 to PND 21. Representative images from the 0-ppm controls (left) and the 900-ppm (middle) and 1800-ppm (right) AlCL3 groups at PND 21. Magnification ×400; bar 50 μm. Graphs show the numbers of immunoreactive cells in the GCL. N = 9–12/group (PND 21: 0-ppm controls, 10; 900-ppm AlCL3, 10; 1800-ppm AlCL3, 10; PND 77: 0-ppm controls, 12; 900-ppm AlCL3, 9; 1800-ppm AlCL3, 11). **p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Morin+ Cells in the SGZ/GCL of Male Offspring
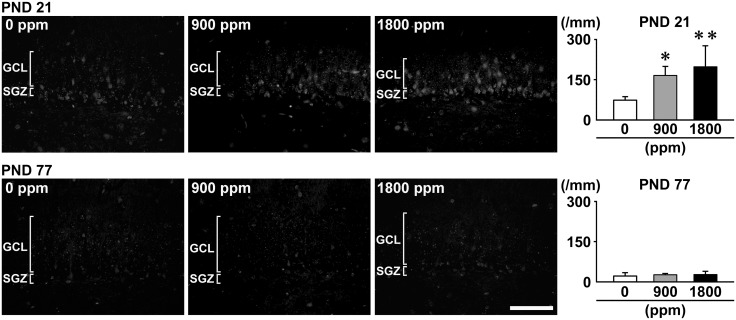
On PND 21, Morin+ cells were observed in the GCL in addition to the SGZ in AlCl3-exposed offspring, whereas Morin+ cells were observed mainly in the SGZ in 0-ppm controls (Figure 6). The number of Morin+ cells was significantly higher at ≥900 ppm compared with 0-ppm controls. On PND 77, no significant differences in the number of Morin+ cells were observed in any exposure group compared with 0-ppm controls (Figure 6).
Figure 6.
Distribution of Morin+ cells in the SGZ/GCL of the hippocampal dentate gyrus of male offspring at PND 21 and PND 77 after maternal exposure to aluminum chloride (AlCL3) from GD 6 to PND 21. Representative images from the 0-ppm controls (left) and the 900-ppm (middle) and 1800-ppm (right) AlCL3 groups at PND 21. Magnification ×400; bar 50 μm. Graphs show the numbers of Morin+ cells in the GCL. N = 5/group. *p < .05, **p < .01, compared with the 0-ppm controls by Dunnett’s test or Aspin-Welch’s t-test with Bonferroni correction.
Distribution of Astrocytes, Microglia and Oligodendrocytes in the Hilus of the Dentate Gyrus of Male Offspring
On PND 21, there were no significant differences in the numbers of GFAP+ cells, Iba1+ cells and OLIG2+ cells in the hilus of any exposure group compared with 0-ppm controls (Supplementary Figures 9 and 10). On PND 77, the number of Iba1+ cells was significantly greater at 900 ppm compared with 0-ppm controls (Supplementary Figures 9 and 10). No significant differences were observed in the number of GFAP+ cells and OLIG2+ cells of any exposure group compared with 0-ppm controls.
Transcript Expression Changes in the Dentate Gyrus of Male Offspring
Transcript expression level was compared between 0-ppm controls and 1800-ppm group on PND 21 and PND 77 (Table 2). With regard to synaptic plasticity-related genes, expression level of Arc significantly increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 21 and decreased after normalization with Gapdh and Hprt at 1800 ppm compared with 0-ppm controls on PND 77. With regard to cell proliferation-related Pcna, expression level significantly decreased after normalization with Gapdh and Hprt at 1800 ppm compared with 0-ppm controls on PND 21 and with Gapdh at 1800 ppm compared with 0-ppm controls on PND 77. With regard to cholinergic receptor genes, transcript expression levels of Chrna4 significantly decreased after normalization with Gapdh at 1800 ppm compared with 0-ppm controls on PND 21. With regard to genes of glutamate transporters and receptors, expression level of Slc17a6 significantly decreased after normalization with Gapdh and Hprt at 1800 ppm compared with 0-ppm controls on PND 21 and increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 77. Expression levels of Gria3 and Grin2a significantly increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 21. In contrast, Grin2d expression levels significantly decreased after normalization with Gapdh at 1800 ppm compared with 0-ppm controls on PND 21 and increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 77. With regard to apoptosis-related genes, expression level of Bax significantly decreased after normalization with Gapdh at 1800 ppm compared with 0-ppm controls on PND 21 and PND 77. Expression level of Casp12 significantly increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 21. Expression level of Casp2 significantly increased after normalization with Hprt at 1800 ppm compared with 0-ppm controls on PND 77. Other genes listed in Table 2 did not show significant fluctuations in transcript expression levels between 0-ppm controls and the 1800-ppm group on both PND 21 and PND 77.
Table 2.
Real-time RT-PCR Analysis Data in the Hippocampal Dentate Gyrus on PND 21 and PND 77
| 0 ppm AlCl3 (Control) |
1800 ppm AlCl3 |
|||
|---|---|---|---|---|
| Relative Transcript Level Normalized to |
Relative Transcript Level Normalized to |
|||
| Gapdh | Hprt | Gapdh | Hprt | |
| No. of animals examineda | 6 | 6 | 6 | 6 |
| PND 21 | ||||
| Synaptic plasticity | ||||
| Arc | 1.02 ± 0.23 | 1.04 ± 0.32 | 1.63 ± 0.87 | 1.83 ± 0.80* |
| Ptgs2 | 1.02 ± 0.24 | 1.01 ± 0.13 | 0.78 ± 0.15 | 0.89 ± 0.10 |
| Fos | 1.02 ± 0.21 | 1.02 ± 0.24 | 0.98 ± 0.31 | 1.14 ± 0.38 |
| Stem cell regulation | ||||
| Kitl | 1.02 ± 0.25 | 1.04 ± 0.32 | 0.78 ± 0.19 | 1.02 ± 0.37 |
| Notch1 | 1.03 ± 0.28 | 1.07 ± 0.42 | 0.89 ± 0.29 | 1.14 ± 0.43 |
| Cell proliferation | ||||
| Pcna | 1.01 ± 0.15 | 1.00 ± 0.06 | 0.71 ± 0.15** | 0.81 ± 0.12** |
| Neurotrophic action | ||||
| Ntrk2 | 1.03 ± 0.29 | 1.09 ± 0.52 | 0.98 ± 0.19 | 1.29 ± 0.45 |
| Bdnf | 1.07 ± 0.42 | 1.08 ± 0.48 | 1.21 ± 0.28 | 1.60 ± 0.60 |
| Cholinergic receptors | ||||
| Chrna7 | 1.03 ± 0.30 | 1.04 ± 0.34 | 1.13 ± 0.39 | 1.29 ± 0.35 |
| Chrna4 | 1.01 ± 0.17 | 1.07 ± 0.43 | 0.66 ± 0.25* | 0.82 ± 0.28 |
| Chrnb2 | 1.02 ± 0.20 | 1.02 ± 0.20 | 0.82 ± 0.11 | 0.95 ± 0.13 |
| Glutamate transporters/receptors | ||||
| Slc17a6 | 1.05 ± 0.41 | 1.05 ± 0.37 | 0.47 ± 0.22* | 0.58 ± 0.21* |
| Slc17a7 | 1.12 ± 0.63 | 1.15 ± 0.76 | 1.94 ± 0.97 | 2.19 ± 0.89 |
| Gria1 | 1.09 ± 0.53 | 1.10 ± 0.48 | 1.12 ± 0.28 | 1.44 ± 0.47 |
| Gria2 | 1.10 ± 0.50 | 1.09 ± 0.53 | 1.52 ± 0.59 | 2.00 ± 0.93 |
| Gria3 | 1.03 ± 0.25 | 1.02 ± 0.22 | 1.37 ± 0.44 | 1.56 ± 0.39* |
| Grin2a | 1.07 ± 0.41 | 1.07 ± 0.45 | 1.77 ± 0.69 | 2.36 ± 1.13* |
| Grin2d | 1.02 ± 0.24 | 1.11 ± 0.52 | 0.55 ± 0.14** | 0.70 ± 0.15 |
| Apoptosis | ||||
| Bax | 1.01 ± 0.18 | 1.01 ± 0.11 | 0.78 ± 0.13* | 0.89 ± 0.09 |
| Bcl2 | 1.02 ± 0.20 | 1.00 ± 0.08 | 1.06 ± 0.32 | 1.22 ± 0.30 |
| Casp2 | 1.01 ± 0.17 | 1.02 ± 0.22 | 1.02 ± 0.23 | 1.16 ± 0.17 |
| Casp3 | 1.04 ± 0.28 | 1.04 ± 0.31 | 0.97 ± 0.12 | 1.27 ± 0.36 |
| Casp9 | 1.02 ± 0.18 | 1.01 ± 0.16 | 0.95 ± 0.16 | 1.09 ± 0.14 |
| Casp12 | 1.02 ± 0.20 | 1.02 ± 0.21 | 1.21 ± 0.28 | 1.38 ± 0.22* |
| PND 77 | ||||
| Synaptic plasticity | ||||
| Arc | 1.02 ± 0.21 | 1.01 ± 0.18 | 0.36 ± 0.14** | 0.45 ± 0.15** |
| Ptgs2 | 1.06 ± 0.44 | 1.01 ± 0.18 | 0.79 ± 0.38 | 0.96 ± 0.25 |
| Fos | 1.01 ± 0.12 | 1.02 ± 0.18 | 0.79 ± 0.31 | 0.98 ± 0.18 |
| Stem cell regulation | ||||
| Kitl | 1.14 ± 0.54 | 1.33 ± 0.55 | 1.49 ± 0.92 | 1.74 ± 0.80 |
| Notch1 | 1.02 ± 0.24 | 1.01 ± 0.35 | 0.91 ± 0.35 | 1.14 ± 0.22 |
| Cell proliferation | ||||
| Pcna | 1.02 ± 0.20 | 1.01 ± 0.16 | 0.72 ± 0.22* | 0.92 ± 0.15 |
| Neurotrophic action | ||||
| Ntrk2 | 1.02 ± 0.26 | 1.00 ± 0.09 | 0.86 ± 0.34 | 1.07 ± 0.17 |
| Bdnf | 1.05 ± 0.39 | 1.04 ± 0.29 | 0.92 ± 0.26 | 1.17 ± 0.10 |
| Cholinergic transporters/receptors | ||||
| Chrna7 | 1.07 ± 0.48 | 1.04 ± 0.32 | 0.89 ± 0.27 | 1.13 ± 0.17 |
| Chrna4 | 1.13 ± 0.60 | 1.13 ± 0.58 | 1.45 ± 0.71 | 1.75 ± 0.45 |
| Chrnb2 | 1.04 ± 0.30 | 1.03 ± 0.30 | 1.10 ± 0.49 | 1.34 ± 0.28 |
| Glutamate receptors | ||||
| Slc17a6 | 1.26 ± 0.82 | 1.26 ± 0.87 | 2.37 ± 1.35 | 2.82 ± 0.98* |
| Slc17a7 | 1.11 ± 0.62 | 1.06 ± 0.39 | 0.79 ± 0.16 | 1.03 ± 0.10 |
| Gria1 | 1.07 ± 0.47 | 1.04 ± 0.30 | 1.02 ± 0.28 | 1.31 ± 0.15 |
| Gria2 | 1.04 ± 0.33 | 1.03 ± 0.26 | 0.81 ± 0.19 | 1.05 ± 0.14 |
| Gria3 | 1.06 ± 0.44 | 1.01 ± 0.18 | 0.86 ± 0.31 | 1.11 ± 0.14 |
| Grin2a | 1.08 ± 0.52 | 1.05 ± 0.34 | 1.04 ± 0.29 | 1.33 ± 0.21 |
| Grin2d | 1.10 ± 0.52 | 1.07 ± 0.44 | 1.60 ± 0.97 | 1.91 ± 0.71* |
| Apoptosis | ||||
| Bax | 1.02 ± 0.25 | 1.00 ± 0.08 | 0.72 ± 0.15* | 0.93 ± 0.08 |
| Bcl2 | 1.05 ± 0.31 | 1.04 ± 0.29 | 0.82 ± 0.45 | 0.99 ± 0.35 |
| Casp2 | 1.02 ± 0.25 | 1.01 ± 0.19 | 1.12 ± 0.48 | 1.41 ± 0.35* |
| Casp3 | 1.02 ± 0.24 | 1.01 ± 0.17 | 0.89 ± 0.36 | 1.11 ± 0.22 |
| Casp9 | 1.04 ± 0.37 | 1.01 ± 0.16 | 1.02 ± 0.45 | 1.27 ± 0.27 |
| Casp12 | 1.09 ± 0.52 | 1.03 ± 0.27 | 0.94 ± 0.36 | 1.22 ± 0.38 |
Abbreviations: Arc, activity-regulated cytoskeletal-associated protein; Bax, BCL2-associated X protein; Bcl2, B-cell leukemia/lymphoma 2; Bdnf, brain derived neurotrophic factor; Casp2, caspase 2; Casp3, caspase 3; Casp9, caspase 9; Casp12, caspase 12; Chrna4, cholinergic receptor nicotinic alpha polypeptide 4; Chrna7, cholinergic receptor nicotinic alpha polypeptide 7; Chrnb2, cholinergic receptor nicotinic beta polypeptide 2; Fos, FBJ osteosarcoma oncogene; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Gria1, glutamate receptor, ionotropic, AMPA1 (alpha 1); Gria2, glutamate receptor, ionotropic, AMPA2 (alpha 2); Gria3, glutamate receptor, ionotropic, AMPA3 (alpha 3); Grin2a, glutamate receptor, ionotropic, NMDA2A (epsilon 1); Grin2d, glutamate receptor, ionotropic, NMDA2D (epsilon 4); Hprt, hypoxanthine guanine phosphoribosyl transferase; Kitl, kit ligand; Notch1, notch 1; Ntrk2, neurotrophic tyrosine kinase, receptor, type 2; Pcna, proliferating cell nuclear antigen; Ptgs2, prostaglandin-endoperoxide synthase 2; Slc17a6, solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 6; Slc17a7, solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 7.
Data are expressed as the mean ± SD.
p < .05,
p < .01, compared with the 0-ppm controls by Student’s t-test or Aspin-Welch’s t-test.
DISCUSSION
In the present study, we observed decreases of GFAP+ type-1 NSCs at 900 ppm and p21Cip1/Waf1+ SGZ cells at ≥900 ppm and hilar Reelin+ cells at 1800 ppm on PND 21. We also observed statistically non-significant decrease of GFAP+ type-1 NSCs at 1800 ppm. The p21Cip1/Waf1 induces cell cycle arrest at the G1/S checkpoint to maintain relative quiescence (Roque et al., 2012). The loss of p21Cip1/Waf1 expression increases NSC proliferation by compromising cell quiescence, especially under stress conditions (Kippin et al., 2005; Qiu et al., 2004). Then, the loss of p21Cip1/Waf1 expression leads to self-renewal exhaustion and progressive decline in NSC proliferation rate by slowing down of cell cycling (Kippin et al., 2005). In addition, Reelin-deficiency in reeler mice causes decreased proliferation of NSCs in the hippocampus, suggesting that Reelin functions to maintain NSC proliferation (Sibbe et al., 2015). Therefore, it may be possible to consider that AlCl3 causes p21Cip1/Waf1 downregulation to trigger a reduction of the NSC pool, and then downregulation of Reelin in interneurons to weaken NSC proliferation at the developmental stage. We also found Pcna transcript downregulation at 1800 ppm on PND 21, suggesting that AlCl3 exposure leads to NSCs slowing down cell cycling. Importantly, decreases of GFAP+ NSCs, Pcna transcript level, and Reelin+ cells were sustained through the adult stage on PND 77. We also observed a tendency to decrease p21Cip1/Waf1+ cells on PND 77. These results suggest an irreversible effect on hippocampal neurogenesis by developmental AlCl3 exposure.
In the present study, we observed increases of TBR2+ and DCX+ cells, reflecting increase of type-2 and type-3 progenitor cells and immature granule cells, in the SGZ/GCL, and also increase of hilar PVALB+ cells at 1800 ppm on PND 21. PVALB+ GABAergic interneurons have been shown to promote differentiation of type-2 progenitor cells (Freund and Buzsáki, 1996; Tozuka et al., 2005), suggesting an increase of PVALB+ interneuron signals to cause an increase of TBR2+ and DCX+ cells. We also found transcript upregulation of Grin2a, which encodes one of the N-methyl-d-aspartate receptor (NMDAR) subunits, ie, NR2A, at 1800 ppm on PND 21. NR2A is essential to maintaining PVALB expression in GABAergic interneurons (Kinney et al., 2006), suggesting that Grin2a transcript upregulation is evidence for increase of PVALB+ interneurons. We further observed an increase of GCL COX-2+ cells at ≥900 ppm on PND 21. Reportedly, COX-2 in mature granule cells activates cell proliferation by producing prostaglandin E2/EP2 signals in SGZ progenitor cells (Ma et al., 2017). Moreover, Ptgs2 knockout mice have decreased DCX+ cells (Nam et al., 2015). Therefore, the increase of GCL COX-2+ cells observed here may cause an increase of progenitor cells during AlCl3 exposure. However, transcript levels of Pcna were decreased at the end of exposure, suggestive of the suppression of progenitor cell proliferation to adjust excessive progenitor cell proliferation at the developmental stage.
In the present study, we did not observe any change in the number of NeuN+ mature granule cells by developmental AlCl3 exposure, despite the increases of type-2b, type-3 and immature cells in the SGZ/GCL at 1800 ppm on PND 21. However, TUNEL+ apoptotic granule cells were increased at ≥900 ppm on PND 21, whereas the increase was statistically insignificant at 1800 ppm. Therefore, a balance between the increases of the progenitor and immature granule cell population and loss of granule cells by apoptosis may result in an unchanged number of GCL NeuN+ cells on PND 21 by AlCl3-exposure. Conversely, we observed downregulation of Bax, a Bcl-2 family gene that is able to initiate the intrinsic apoptosis pathway (Gross et al., 1998), examined at 1800 ppm on PND 21. In contrast, we observed transcript upregulation of Casp12 at 1800 ppm. Considering the crucial role of caspase 12 in the progression of endoplasmic reticulum (ER) stress-mediated apoptosis (Yoneda et al., 2001), facilitation of ER stress-mediated apoptosis and suppression of the intrinsic pathway of apoptosis may result in a nonsignificant increase of granule cell apoptosis at 1800 ppm. Interestingly, Al-accumulated granule cells as evident by Morin-staining were dose-dependently increased at ≥900 ppm on PND 21. Neuronal cell apoptosis has been suggested in relation with their Al accumulation (Abdel Moneim, 2012; Shaw and Petrik, 2009). Because Al can induce ER stress-mediated apoptosis (Mustafa Rizvi et al., 2014), an increase of granule cell apoptosis at ≥900 ppm may be related to the cytotoxic effect of accumulated Al in granule cells in the present study. At 1800 ppm, a protective mechanism, such as suppression of the BAX-mediated intrinsic pathway of apoptosis, may be predominated. On PND 77, AlCl3-exposed animals did not see an increase in Al-accumulated cells and apoptosis in the SGZ/GCL. Considering the functional role of Casp2 as a potent activator of the downstream intrinsic pathway of apoptosis, downregulation of Bax, irrespective of the upregulation of Casp2, suggested suppression of the intrinsic pathway of apoptosis at the adult stage. Detection of Morin+ cells in the SGZ of all groups including 0-ppm controls in the present study may be the reflection of basal Al accumulation in the brain of normal animals (Baydar et al., 2003).
In the present study, we observed transcript downregulation of Grin2d and Chrna4 on PND 21 at 1800 ppm. GRIN2D is a subunit protein of NMDA receptors and CHRNA4 is a subunit of the neuronal nicotinic acetylcholine receptor, both being expressed in GABAergic interneurons (Son and Winzer-Serhan, 2008; Yamasaki et al., 2014), suggesting an involvement of both glutamatergic and cholinergic signals in GABAergic interneurons to indirectly affect neurogenesis. In contrast, Grin2d transcript level was increased on PND 77, probably reflecting a compensatory response against downregulation on PND 21. Although the functional involvement of CHRNA4 in the hippocampal neurogenesis is not reported, CHRNB2 has been shown to play an important role in regulating SGZ cell proliferation (Harrist et al., 2004). Therefore, the decreased CHRNA4 subunit may affect tetramerization involving CHRNB2 to cause downregulation of the nicotinic acetylcholine receptor in GABAergic interneurons at 1800 ppm on PND 21, resulting in insufficient inputs from hilar GABAergic interneurons to the SGZ.
Synaptic plasticity in the hippocampus as a cellular mechanism of learning and memory requires appropriate integration of glutamatergic excitatory and GABAergic inhibitory synaptic inputs (Bliss and Collingridge, 1993). In the present study, we observed transcript upregulation of Arc at 1800 ppm on PND 21. ARC is a member of the IEGs that play a role in the initial steps of neuronal plasticity including axonal and synaptic plasticities (Guzowski, 2002). However, Arc transcript level turned to be suppressed on PND 77, suggestive of suppression of ARC-related synaptic plasticity. Interestingly, NMDA receptor activation upregulates ARC expression (Alaghband et al., 2014). As aforementioned, AlCl3 exposure upregulated transcript of the glutamatergic receptor subunit of NR2A at 1800 ppm on PND 21, suggesting a relation to the transcript upregulation of Arc for increase of GCL synaptic plasticity.
In the present study, we found transcript upregulation of Gria3 at 1800 ppm on PND 21. GRIA3, a subunit of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), is reportedly found at excitatory synapses throughout the brain (Ozawa et al., 1998). The cellular models of synaptic plasticity, especially of long-term potentiation, can be expressed by the synaptic insertion of AMPARs (Kessels and Malinow, 2009). Conversely, ARC interacts with endocytic machinery to regulate AMPAR trafficking and contributes to late-phase synaptic plasticity and memory consolidation (Chowdhury et al., 2006). These results suggest that transcript upregulation of Arc may facilitate membrane trafficking of increased GRIA3 on PND 21 by developmental AlCl3 exposure, whereas the ARC+ granule cells did not fluctuate in number. COX-2 is another molecule regulating synaptic plasticity of GCL (Chen et al., 2002), and the number of COX-2+ granule cells was increased at ≥900 ppm on PND 21 in the present study, suggestive of the increase of synaptic plasticity of GCL. In contrast, we also found transcript downregulation of Slc17a6 on PND 21 at 1800 ppm. On PND 77, Slc17a6 was upregulated in this group. The highest density of boutons expressing VGLUT2, a gene product of Slc17a6, of mossy cells makes an axonal projection to granule cells in the dentate gyrus (Halasy et al., 2004). Additionally, the highest levels of VGLUT2 are expressed in the hippocampus during the early postnatal stage to mediate glutamate release to support synaptogenesis that is necessary for hippocampal development (Boulland et al., 2004). These results suggest unbalanced synaptic plasticity changes within the dentate gyrus through the adult stage.
In conclusion, developmental exposure to AlCl3 via maternal drinking water decreased the NSCs in offspring, suggestive of a reflection of NSC pool exhaustion by loss of p21Cip1/Waf1-mediated compromised cell quiescence to cause an impaired self-renewal capacity of NSCs accompanying slowing down of cell cycling. Decrease in Reelin+ hilar GABAergic interneurons may also contribute to the NSC reduction. In contrast, increase of progenitor and immature granule cells suggested facilitation of transient progenitor cell proliferation and differentiation by enhancing the PVALB+ interneuron signal and COX-2-mediated signals during exposure. In addition, increase of apoptosis or Morin-stained cells in the GCL, together with Casp12 transcript upregulation, suggested an induction of Al accumulation-related ER stress-mediated granule cell apoptosis. Transcript expression changes on cholinergic and glutamatergic signals and synaptic plasticity suggested contribution to disruptive neurogenesis. The NSC-targeting effects were sustained through the adult stage despite no sustained Al-accumulation. These results suggest that developmental AlCl3-exposure irreversibly affects postnatal hippocampal neurogenesis involving multiple functions in mice.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Shigeko Suzuki for her technical assistance in preparing the histological specimens. All authors declare that there are no conflicts of interest that influenced the outcome of the present study.
FUNDING
Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS; grant no. 25292170); Research Fund from Institute of Global Innovation Research, Tokyo University of Agriculture and Technology.
REFERENCES
- Abdel Moneim A. E. (2012). Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol. Trace Elem. Res. 150, 328–336. [DOI] [PubMed] [Google Scholar]
- Abu-Taweel G. M., Jamaan S. A., Hossam E. (2011). Aluminum-induced testosterone decrease results in physiological and behavioral changes in male mice. Afr. J. Biotech. 10, 201–208. [Google Scholar]
- Akane H., Shiraki A., Imatanaka N., Akahori Y., Itahashi M., Ohishi T., Mitsumori K., Shibutani M. (2013). Glycidol induces axonopathy by adult-stage exposure and aberration of hippocampal neurogenesis affecting late-stage differentiation by developmental exposure in rats. Toxicol. Sci. 134, 140–154. [DOI] [PubMed] [Google Scholar]
- Alaghband Y., O’Dell S. J., Azarnia S., Khalaj A. J., Guzowski J. F., Marshall J. F. (2014). Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol. Learn. Mem. 116, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. G., Kurz J. (1985). An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J. Comp. Neurol. 240, 37–59. [DOI] [PubMed] [Google Scholar]
- Baydar T., Papp A., Aydin A., Nagymajtenyi L., Schulz H., Isimer A., Sahin G. (2003). Accumulation of aluminum in rat brain: Does it lead to behavioral and electrophysiological changes? Biol. Trace Elem. Res. 92, 231–244. [DOI] [PubMed] [Google Scholar]
- Bishop N. J., Morley R., Day J. P., Lucas A. (1997). Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. N. Engl. J. Med. 336, 1557–1561. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Boulland J. L., Qureshi T., Seal R. P., Rafiki A., Gundersen V., Bergersen L. H., Fremeau R. T. Jr., Edwards R. H., Storm-Mathisen J., Chaudhry F. A. (2004). Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J. Comp. Neurol. 480, 264–280. [DOI] [PubMed] [Google Scholar]
- Burrell S. A., Exley C. (2010). There is (still) too much aluminium in infant formulas. BMC Pediatr. 10, 63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Magee J. C., Bazan N. G. (2002). Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 87, 2851–2857. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Shepherd J. D., Okuno H., Lyford G., Petralia R. S., Plath N., Kuhl D., Huganir R. L., Worley P. F. (2006). Arc interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapper D. R., Krishnan S. S., Dalton A. J. (1973). Brain aluminum distribution in Alzheimer's disease and experimental neurofibrillary degeneration. Science 180, 511–513. [DOI] [PubMed] [Google Scholar]
- De Boni U., Scott J. W., Crapper D. R. (1974). Intracellular aluminum binding; a histochemical study. Histochemistry 40, 31–37. [DOI] [PubMed] [Google Scholar]
- DeVoto E., Yokel R. A. (1994). The biological speciation and toxicokinetics of aluminum. Environ. Health Perspect. 102, 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [EFSA] European Food Safety Authority. (2008). Safety of aluminium from dietary intake – scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J. 754, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Ghirnikar R. S., Lee Y. L. (2000). Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem. Res. 25, 1439–1451. [DOI] [PubMed] [Google Scholar]
- Fang F., Zhang H., Zhang Y., Xu H., Huang Q., Adilijiang A., Wang J., Zhang Z., Zhang D., Tan Q. et al. , . (2013). Antipsychotics promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 2. Life Sci. 93, 429–434. [DOI] [PubMed] [Google Scholar]
- Fleischmann A., Hvalby O., Jensen V., Strekalova T., Zacher C., Layer L. E., Kvello A., Reschke M., Spanagel R., Sprengel R. et al. , . (2003). Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J. Neurosci. 8, 9116–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F., Karlsen R. L., Malthe-Sørenssen D., Skrede K. K., Walaas I. (1979). Localization of neurotransmitters, particularly glutamate, in hippocampus, septum, nucleus accumbens and superior colliculus. Prog. Brain Res. 51, 167–191. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Buzsáki G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. [DOI] [PubMed] [Google Scholar]
- Fujise N., Liu Y., Hori N., Kosaka T. (1998). Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience 82, 181–200. [DOI] [PubMed] [Google Scholar]
- Gong C., Wang T. W., Huang H. S., Parent J. M. (2007). Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J. Neurosci. 27, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Jockel J., Wei M. C., Korsmeyer S. J. (1998). Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17, 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J. F. (2002). Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus 12, 86–104. [DOI] [PubMed] [Google Scholar]
- Halasy K., Hajszan T., Kovács E. G., Lam T. T., Leranth C. (2004). Distribution and origin of vesicular glutamate transporter 2-immunoreactive fibers in the rat hippocampus. Hippocampus 14, 908–918. [DOI] [PubMed] [Google Scholar]
- Harrist A., Beech R. D., King S. L., Zanardi A., Cleary M. A., Caldarone B. J., Eisch A., Zoli M., Picciotto M. R. (2004). Alteration of hippocampal cell proliferation in mice lacking the β2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse 54, 200–206. [DOI] [PubMed] [Google Scholar]
- Hodge R. D., Kowalczyk T. D., Wolf S. A., Encinas J. M., Rippey C., Enikolopov G., Kempermann G., Hevner R. F. (2008). Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 28, 3707–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi M., Abe H., Tanaka T., Mizukami S., Kimura M., Yoshida T., Shibutani M. (2015). Maternal exposure to hexachlorophene targets intermediate-stage progenitor cells of the hippocampal neurogenesis in rat offspring via dysfunction of cholinergic inputs by myelin vacuolation. Toxicology 328, 123–134. [DOI] [PubMed] [Google Scholar]
- Ito D., Imai Y., Ohsawa K., Nakajima K., Fukuuchi Y., Kohsaka S. (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. (2004). Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452. [DOI] [PubMed] [Google Scholar]
- Kessels H. W., Malinow R. (2009). Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Combadière C., Authier F. J., Itier V., Lux F., Exley C., Mahrouf-Yorgov M., Decrouy X., Moretto P., Tillement O. et al. , . (2013). Slow CCL2-dependent translocation of biopersistent particles from muscle to brain. BMC Med. 11, 99.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J. W., Davis C. N., Tabarean I., Conti B., Bartfai T., Behrens M. M. (2006). A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J. Neurosci. 26, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin T. E., Martens D. J., van der Kooy D. (2005). p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 19, 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lugert S., Vogt M., Tchorz J. S., Müller M., Giachino C., Taylor V. (2012). Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1high intermediate progenitors. Nat. Commun. 14, 670. [DOI] [PubMed] [Google Scholar]
- Ma Y., Matsuwaki T., Yamanouchi K., Nishihara M. (2017). Glucocorticoids suppress the protective effect of cyclooxygenase-2-related signaling on hippocampal neurogenesis under acute immune stress. Mol. Neurobiol. 54, 1953–1966. [DOI] [PubMed] [Google Scholar]
- Masiulis I., Yun S., Eisch A. J. (2011). The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol. Neurobiol. 44, 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazedi A. A., Ehsani V. S., Chinipardaz R. (2008). Effect of oral aluminum chloride administration during lactation on short and long-term memory of their offspring. J. Biol. Sci. 8, 767–772. [Google Scholar]
- Montaron M. F., Koehl M., Lemaire V., Drapeau E., Abrous D. N., Le Moal M. (2004). Environmentally induced long-term structural changes: Cues for functional orientation and vulnerabilities. Neurotox. Res. 6, 571–580. [DOI] [PubMed] [Google Scholar]
- Mustafa Rizvi S. H., Parveen A., Verma A. K., Ahmad I., Arshad M., Mahdi A. A. (2014). Aluminium induced endoplasmic reticulum stress mediated cell death in SH-SY5Y neuroblastoma cell line is independent of p53. PLoS One 9, e98409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm W. K., Noebels J. L. (1998). Nonobligate role of early or sustained expression of immediate-early gene proteins c-Fos, c-Jun, and Zif/268 in hippocampal mossy fiber sprouting. J. Neurosci. 18, 9245–9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S. M., Kim J. W., Yoo D. Y., Choi J. H., Kim W., Jung H. Y., Won M. H., Hwang I. K., Seong J. K., Yoon Y. S. (2015). Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: Effects on adult neurogenesis in the hippocampal dentate gyrus. J. Vet. Sci. 16, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Test Guideline 426. (2007). OECD Guideline for Testing of Chemicals. Developmental Neurotoxicity Study. Organisation for Economic Co-operation and Development, Paris, France; Available at https://www.oecd-ilibrary.org/environment/test-no-426-developmental-neurotoxicity-study_9789264067394-en. Accessed 6 April 2018. [Google Scholar]
- Ozawa S., Kamiya H., Tsuzuki K. (1998). Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 54, 581–618. [DOI] [PubMed] [Google Scholar]
- Pawluski J. L., Brummelte S., Barha C. K., Crozier T. M., Galea L. A. (2009). Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front. Neuroendocrinol. 30, 343–357. [DOI] [PubMed] [Google Scholar]
- Priest N. D. (1993). The bioavailability and metabolism of aluminium compounds in man. Proc. Nutr. Soc. 52, 231–240. [DOI] [PubMed] [Google Scholar]
- Qiu J., Takagi Y., Harada J., Rodrigues N., Moskowitz M. A., Scadden D. T., Cheng T. (2004). Regenerative response in ischemic brain restricted by p21cip1/waf1. J. Exp. Med. 199, 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque T., Haton C., Etienne O., Chicheportiche A., Rousseau L., Martin L., Mouthon M. A., Boussin F. D. (2012). Lack of a p21waf1/cip-dependent G1/S checkpoint in neural stem and progenitor cells after DNA damage in vivo. Stem Cells 30, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. A., Petrik M. S. (2009). Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J. Inorg. Biochem. 103, 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M. (2015). Hippocampal neurogenesis as a critical target of neurotoxicants contained in foods. Food Safety 3, 1–15. [Google Scholar]
- Sibbe M., Kuner E., Althof D., Frotscher M. (2015). Stem- and progenitor cell proliferation in the dentate gyrus of the reeler mouse. PLoS One 10, e0119643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W. (1996). Kinetic aspects of aqueous aluminum chemistry: Environmental implications. Coord. Chem. Rev. 149, 81–93. [Google Scholar]
- Son J. H., Winzer-Serhan U. H. (2008). Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J. Comp. Neurol. 511, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struys-Ponsar C., Kerkhofs A., Gauthier A., Soffié M., van den Bosch de Aguilar P. (1997). Effects of aluminum exposure on behavioral parameters in the rat. Pharmacol. Biochem. Behav. 56, 643–648. [DOI] [PubMed] [Google Scholar]
- Takács S., Tatár A., Barkai L. (1992). Trace elements in the human blood, cerebrospinal and amniotic fluid. Zentralbl. Hyg. Umweltmed. 193, 329–341. [PubMed] [Google Scholar]
- Tanaka T., Abe H., Kimura M., Onda N., Mizukami S., Yoshida T., Shibutani M. (2016). Developmental exposure to T-2 toxin reversibly affects postnatal hippocampal neurogenesis and reduces neural stem cells and progenitor cells in mice. Arch. Toxicol. 90, 2009–2024. [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. (2005). GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815. [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Okada R., Takasaki C., Toki S., Fukaya M., Natsume R., Sakimura K., Mishina M., Shirakawa T., Watanabe M. (2014). Opposing role of NMDA receptor GluN2B and GluN2D in somatosensory development and maturation. J. Neurosci. 34, 11534–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T., Imaizumi K., Oono K., Yui D., Gomi F., Katayama T., Tohyama M. (2001). Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940. [DOI] [PubMed] [Google Scholar]
- Yuan C. Y., Lee Y. J., Hsu G. S. (2012). Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 19, 51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.