 Metal sulfide ion-exchangers (MSIEs) have emerged as a new class of promising sorbents for the removal of toxic and radioactive metals from wastewater.
Metal sulfide ion-exchangers (MSIEs) have emerged as a new class of promising sorbents for the removal of toxic and radioactive metals from wastewater.
Abstract
Metal sulfide ion-exchangers (MSIEs) represent a new addition to the field of ion exchange materials. This is a growing class of materials that display exceptional selectivity and rapid sorption kinetics for soft or relatively soft metal ions as a result of their soft basic frameworks. Without requiring functionalization, they outperform the most efficient sulfur-functionalized materials. This is the first review focusing on this class of materials; it covers the most important MSIEs, focusing on their synthesis, structural features and ion-exchange chemistry. Furthermore, recent developments in the engineered and composite forms of MSIEs are described. Future research opportunities are also discussed in the hope of inspiring additional scientists to engage in this new area of research on sulfidic ion-exchange materials.
1. Introduction
The treatment of various types of aqueous wastes, such as industrial and nuclear waste effluents, is of major concern for countries all over the world. Radionuclides (137Cs, 89Sr, 235U, 59Fe, 57Co, 65Zn, etc.) and toxic heavy metal ions (Hg2+, Pb2+, Cd2+, and Tl+) are major pollutants in these types of waste and pose a serious threat to humans and other species.1 Commonly used and inexpensive methods such as precipitation of the ions from solutions are often not sufficiently effective to lower the concentration of these ions below the acceptable legal limits. For example, precipitation of Hg2+ ions with Na2S cannot reduce the concentration of mercuric ions below 10 to 50 ppb.2 Such levels are 20 to 100-times higher than the legally accepted limits defined by the European Union (1 ppb) and USA-EPA (2 ppb).3 The issue of the treatment of nuclear effluents is even more complex because of the harsh conditions of nuclear waste deriving from nuclear waste manufacture, such as inhomogeneous samples, extreme pH and very high salt concentrations.4 Efficient removal of radioactive elements and minimization of long-term storage space is crucial to enable safer and low cost implementation of nuclear energy.4
Ion exchange is well recognized as a relatively inexpensive and highly effective method for the elimination of various types of ions from aqueous waste solutions.5 Clays6 and zeolites7 are common and abundant cation exchangers; however, they suffer from low selectivity and capacity for toxic heavy metal ions in the presence of high salt concentrations or under acidic conditions. In addition, these materials are unstable in extreme alkaline or acidic conditions (due to immediate dissolution of aluminum/silicon ions) of nuclear wastes.8 Other oxidic sorbents, such as titanates, silicates and manganese oxides, can survive under the conditions of nuclear waste; however, they show decreased selectivity for radioactive ions in the presence of high salt concentrations (e.g. Cs+ absorption by manganese oxides),9 or they are selective for radioactive ions only within a narrow pH range (e.g. Sr2+ absorption by sodium titanate).10
Organic resins, with functional groups suitable for absorption of specific ions, are extensively used for water purification.5 These purely organic materials, however, are of limited chemical, radiolytic and thermal stability.8 In addition, resins display an amorphous porous structure; therefore, they cannot exhibit the molecular sieve separation properties of ordered porous inorganic materials such as zeolites.5
Functionalized silica-based materials show remarkable selectivity and binding affinity for a variety of heavy ions. For example, thiol-functionalized mesoporous materials are famous for their exceptional capability to rapidly absorb Hg2+ from water solutions.11 Silica-based materials, however, cannot be used for remediation of extreme alkaline or acidic waste water (e.g. nuclear waste) due to their instability under such conditions.8
Metal organic frameworks (MOFs) incorporating functional groups with high affinity for toxic or radioactive ions appear to be promising sorbents for various remediation processes.12 The development of these sorbents is still in its infancy.
From the above, it is clear that “perfect” sorbents that can withstand the harsh conditions of various types of wastes, are highly selective for toxic or radioactive ions, and are affordable are still elusive. The search for new sorbent materials is therefore important.
Recently, metal sulfide ion exchangers (MSIEs) with labile extra-framework cations have emerged as a new class of promising sorbents.13 These materials exhibit a variety of structures, ranging from layered and three-dimensional crystalline frameworks13 to porous amorphous materials14 and aerogels.15 They are proving to be particularly effective for the decontamination of water solutions from various heavy metal ions (e.g. Hg2+, Pb2+, Cd2+, Ni2+, and Co2+) as well as ions relevant to nuclear waste (e.g. UO22+, Cs+, and Sr2+).13 The unique properties of MSIEs arise from their soft S2– ligands, which endow these materials with innate selectivity for soft or relatively soft metal ions. MSIEs with a soft basic framework thus do not require the introduction of any functional groups. They exhibit exceptional absorption properties for soft metal ions, superior to those of the best sulfur-functionalized materials.11 Furthermore, hard ions such as H+, Na+, and Ca2+ only weakly interact with the soft S2– ligands of MSIEs, thus affecting their ion exchange properties to a much lesser degree than those of traditional oxidic materials.6,7,9,10 Therefore, MSIEs may be effective for metal ion absorption over a broad pH range and in the presence of high salt concentration. In this review, we describe the most important MSIEs in terms of their synthesis, structural characteristics and metal ion absorption properties. Furthermore, we discuss the recent development of composite and engineered forms of MSIEs, which promise to open up paths for the practical applications of these materials. To our knowledge, this is the first review of these systems. Finally, we provide some directions and perspectives for future research on this new family of ion-exchangers.
2. Layered crystalline MSIEs
Metal sulfides with layered anionic structures and labile interlayer cations constitute the most well studied class of MSIEs. These materials show excellent and selective ion exchange properties due to (a) the facile diffusion of the inserted ions and their easy access to the internal surface of metal sulfide layers and (b) the formation of strong bonds between the incorporated metal ions and S2– ligands. In the following, we present the main layered MSIEs, highlighting their synthesis, structural features and ion exchange chemistry.
2.1. Alkali-ion intercalated metal disulfides
A common route to the preparation of layered MSIEs involves partial reduction of the metal ions of a metal disulfide, such as SnS2 or MoS2, by treating it with an alkali ion (A+)-containing reducing agent (e.g. n-butyl lithium, alkali ion dithionite).16 An anionic layer is thus formed, and its negative charge is compensated by alkali cations provided by the reducing agent. The cations inserted in the interlayer space of the materials can be rapidly and topotactically exchanged by a variety of inorganic and organic cationic species.16 The synthesis of such layered materials is represented by the following equation:
| MS2 + xA+ + xe– → Ax[MS2]x– | 1 |
Actually, the earliest known MSIEs, which were reported on 1979 by R. Schöllhorn et al., were alkali-ion intercalated metal disulfides, specifically the hydrated layered Sn sulfide phases Ax(H2O)y[SnS2]x– (A+ = K+, Rb+, Cs+).17 These materials show facile ion-exchange properties for a series of inorganic cations, such as Li+, Na+, Mg2+, Ca2+, and Ni2+. The insertion of the various ions in the interlayer space of the materials was probed by powder X-ray diffraction (PXRD) studies. It was observed that the higher the hydration energy of the inserted cation, the larger the interlayer spacings, indicating the incorporation of the ions as hydrated complexes in the space between the layers. These materials, however, are susceptible to oxidation, which results in SnS2.
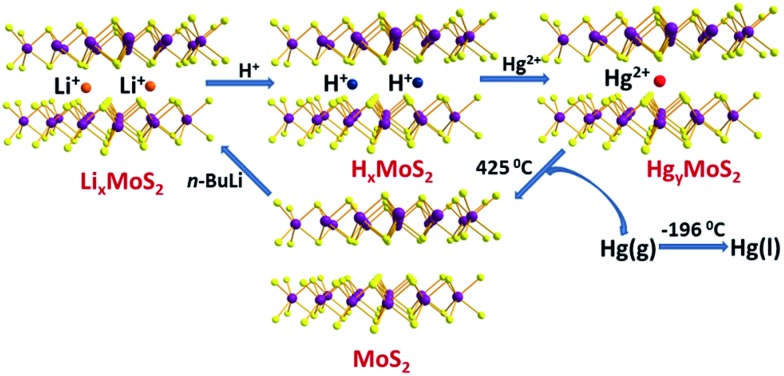
About twenty years after Schöllhorn, the ion-exchange properties of LixMoS2 (0.25 ≤ x ≤ 1.9) were investigated by A. E. Gash et al.18 This material is particularly effective in binding to Hg2+ in acidic conditions. The authors suggested a mechanism for the Hg2+ absorption, which is shown in Fig. 1 and represented by the following equations:
| Li1.3MoS2(s) + 1.3H3O+ → 0.26H2(g) + 1.3Li+(aq) + (H3O)0.78MoS2(s) + 0.52H2O(l) | 2 |
| (H3O)0.78MoS2(s) + 0.20Hg2+(aq) → (H3O)0.38Hg0.20MoS2(s) + 0.40H3O+(aq) | 3 |
Fig. 1. Suggested mechanism of Hg2+ absorption/desorption for the LixMoS2 material. Mo, purple; S, yellow.
Thus, the first step of the ion-exchange reaction involved the transformation of the Li+-intercalated material to a hydronium-containing material, which in turn reacts with Hg2+ to yield the final product. The driving force for the second reaction was the higher affinity of the soft basic MoS2x– layers for the soft acid Hg2+ compared to that for the hard hydronium ions. Inductively coupled plasma-atomic emission (ICP-AES) analytical data indicated that ∼200 ppm of Hg2+ (pH ∼ 1) can be reduced to 6.5 ppb after treatment of the Hg2+ solution with LixMoS2 (the molar ratio of LixMoS2 to Hg was 5). The Hg content of various exchanged products was found to be 0.24 to 0.32 mole per formula unit of the exchanged material, depending on the initial LixMoS2/Hg molar ratio.
Interestingly, Hg can be recovered by heating the Hg-exchanged product at 425 °C, which leads to the vaporization of Hg and the formation of MoS2 (Fig. 1) according to the following equation:
| HgxMoS2(s) → xHg(g) + MoS2(s) | 4 |
The Hg vapor is then collected in a cold trap at 77 K. The MoS2 can be retransformed to LixMoS2via reduction-Li intercalation with n-BuLi. As observed for the alkali-intercalated tin disulfide materials, LixMoS2 sorbents are also air and moisture-sensitive and thus, they should be stored under anaerobic conditions to retain their Hg2+ sorption capacity.
LixMoS2 was also tested for Zn2+, Pb2+ and Cd2+ sorption.18 The results revealed only moderate Pb2+ removal capacity (40–75% removal of the initial Pb content), relatively low Cd2+ (4–40% removal capacity) and negligible Zn2+ sorption (1–4% removal capacity). In contrast, the removal capacities for Hg2+ were 74–100%. The selectivity of the LixMoS2 follows the order Hg2+ > Pb2+ > Cd2+ > Zn2+, which indicates the preference of the material for softer metal ions due to their stronger interactions with the soft MoS2x– layer.
2.2. KMS materials
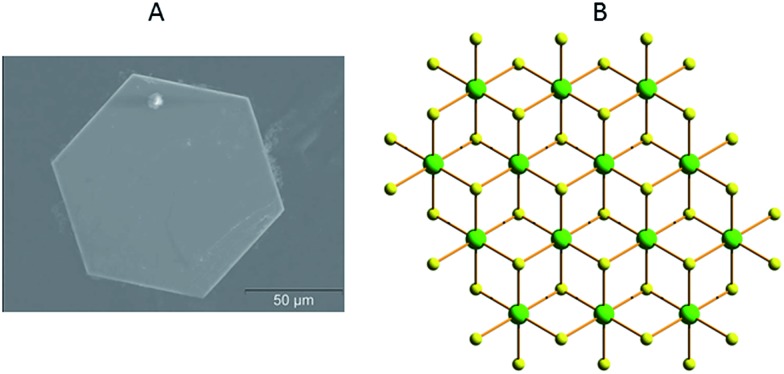
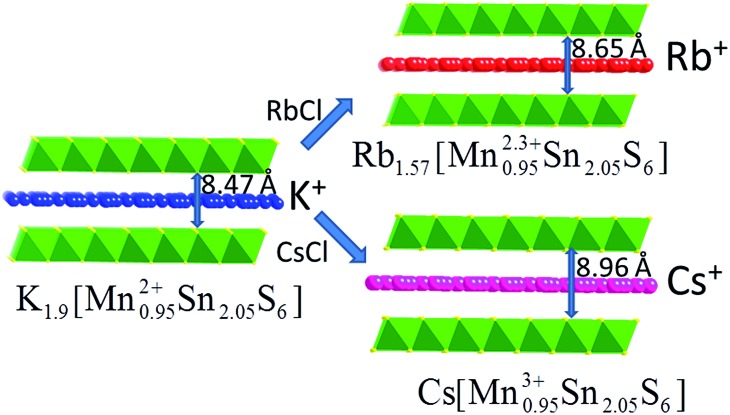
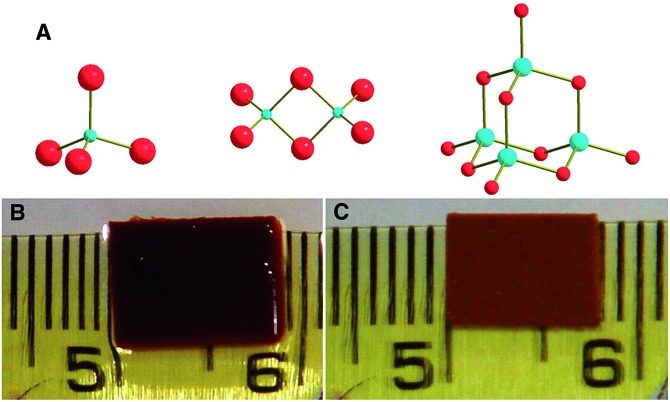
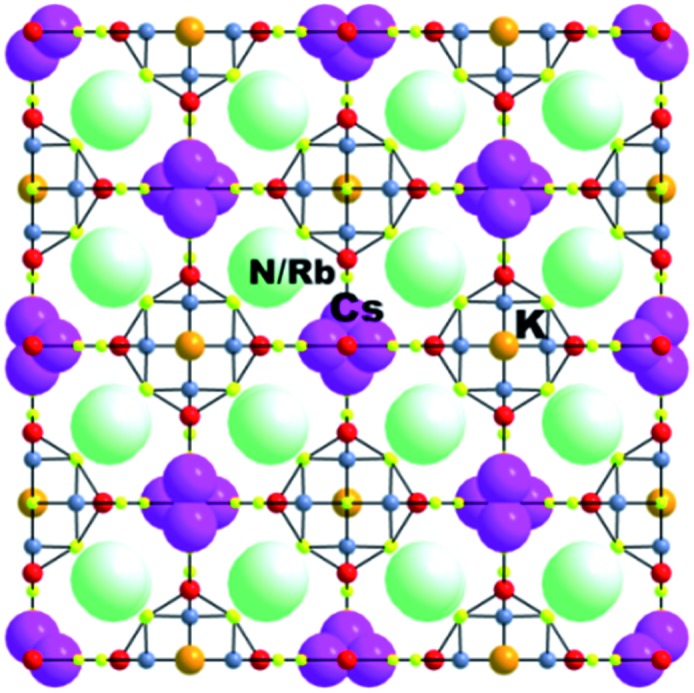
These materials have the general formula K2xMxSn3–xS6 (M = Mn2+, KMS-1; M = Mg2+, KMS-2; x = 0.5 to 1).13c–h They can be prepared on a multigram scale with high purity using solid state and hydrothermal synthetic methods. They are exceptionally stable in air and in highly acidic and basic aqueous solutions. Single crystal X-ray measurements, performed on hexagonal-shaped crystals of KMS-1 and 2 (Fig. 2A), revealed that their structure is based on edge sharing “Sn/M”S6 octahedra (CdI2 structure type) with Sn4+ and M2+ occupying the same crystallographic position and three-coordinated S2– ligands (Fig. 2B). The interlayer space is filled by K+ ions (Fig. 3), which compensate for the negative charge of the metal sulfide layers. There is much more room in the interlayer space than that required for all K+ cations. As a result, these ions are highly disordered and mobile and are thus easily exchanged by a variety of other cationic species (see below). KMS compounds are actually derivatives of SnS2 with partial substitution of Sn4+ by M2+ (Mn2+ or Mg2+) ions. KMS-1 and 2 feature essentially the same structural characteristics; their only differences are related to the stacking of the layers.
Fig. 2. (A) Scanning electron microscopy (SEM) image of a typical crystal of KMS-1. (B) Representation of part of the layered structure of KMS-1 (S, yellow; Sn/Mn, green).
Fig. 3. Representations of the crystal structures of KMS-1 and its Rb+ and Cs+-exchanged analogues and the interlayer spacing.
KMS-1 and 2 materials were investigated in detail for their ion-exchange properties with cations related to nuclear waste, such as Cs+, Sr2+, Ni2+ and UO22+, and heavy metal ions (such as Hg2+, Pb2+, Cd2+) which are common contaminants in industrial wastewater. In the following, we describe the most important ion-exchange results for KMS materials.
2.2.1. Cs+ and Rb+ ion exchange properties
The Cs+ ion-exchange process is highly relevant to nuclear waste remediation, since the 137Cs+ radionuclide represents one of the major contaminants in the fission products of nuclear waste.4 It is thus of particular importance to discover selective Cs+ sorbents and expand the gamut of materials that can be used to capture this ion. For this reason, the Cs+ ion-exchange properties of KMS-1 and 2 were investigated in detail. For comparison, Rb+ absorption was also studied.
K+ ions can be completely and topotactically replaced by Cs+ or Rb+ by treating KMS-1 with an aqueous solution of the corresponding alkali-ion chloride salt. Interestingly, Cs+ and Rb+ sorption can be achieved not only with polycrystalline samples of KMS-1, but also in a single crystal-to-single crystal (SCSC) fashion using large specimens.13d When single crystals of K2xMnxSn3xS6 (x = 0.95, KMS-1) are immersed in a solution of CsCl or RbCl, single crystals of Cs+ or Rb+-exchanged material can be isolated and, thus, their structures can be accurately determined by X-ray crystallography (Fig. 3). X-ray refinement indicated the formulas Rb1.57Mn0.95Sn2.05S6 and CsMn0.95Sn2.05S6 for the Rb+ and Cs+-exchanged materials, respectively. Based on these formulas and the charge balance requirements, Mn should be in the 2.3+ and 3+ oxidation states in the Rb+ and Cs+-containing compounds, respectively. The Mn oxidation states in these exchanged materials were also confirmed using X-ray photoelectron spectroscopic (XPS) data. The stability of the Mn3+ oxidation state in the Cs+-exchanged analogue was explained on the basis of the increased ionic character of the Cs+···S2– interactions. Thus, in the presence of Cs+, the [MnxSn3–xS6]2x– layer becomes more electron-rich and, as a consequence, becomes more prone to lose an electron.13d
Detailed batch Cs+ ion exchange studies were performed for KMS-1, and the data can be described well with the Langmuir model:
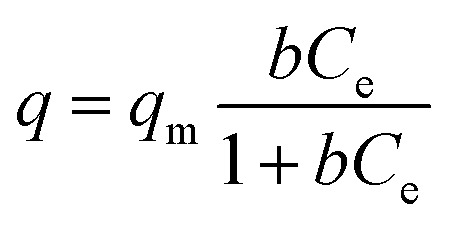 |
5 |
where q (mg g–1) is the amount of the cation absorbed at the equilibrium concentration Ce (ppm), qm is the maximum sorption (exchange) capacity of the sorbent, and b (L mg–1) is the Langmuir constant related to the free energy of the sorption.13d
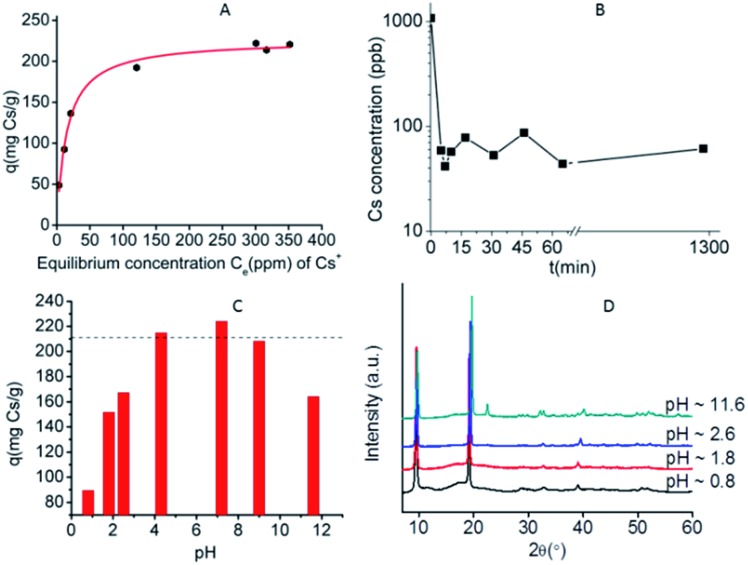
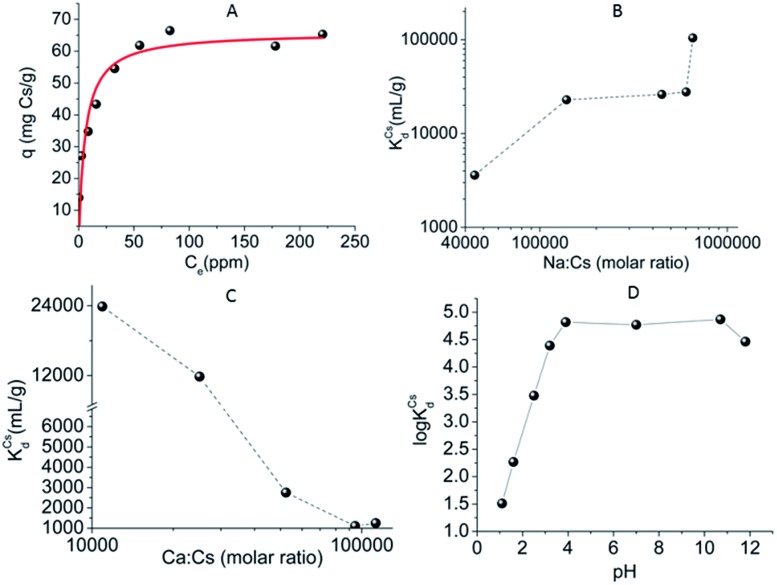
The fitting of the data indicated a maximum sorption capacity of ∼1.7 mmol g–1 (226 ± 2 mg g–1), close to the maximum theoretical capacity (1.6 mmol g–1) calculated for the exchange of two K+ ions by one Cs+ (Fig. 4A). The material can be regenerated by treating the Cs+-loaded compound with a large excess of KCl aqueous solution. The regenerated compound showed almost identical Cs+ exchange capacity to that of the pristine KMS-1 material.
Fig. 4. (A) Cs+ isotherm sorption data for KMS-1 (at pH ∼ 7). The solid line represents the fitting of the data with the Langmuir model. (B) Kinetic data for the sorption of Cs+ (initial concentration ∼ 1 ppm) by KMS-1. (C) Maximum Cs+ sorption capacity of KMS-1 found for different pH values. The dashed line represents the maximum theoretical exchange capacity for KMS-1. (D) PXRD patterns for Cs+-exchanged products isolated from highly acidic and alkaline solutions.
Kinetic studies with trace Cs+ concentrations (∼1 ppm) revealed a very fast sorption process at room temperature. Specifically, within less than 5 min of contact with the KMS-1/solution, the Cs+ ion exchange reached its equilibrium with ∼90% removal of the initial Cs+ content (Fig. 4B). The material was also capable of efficient Cs+ ion-exchange in highly acidic (pH ∼ 1) and alkaline conditions (pH ∼ 12), as shown in Fig. 4C. PXRD studies indicate that the Cs+-exchanged products isolated from either acidic or alkaline solutions were crystalline and retained the layered structure of the parent KMS-1 compound (Fig. 4D).
Finally, ion-exchange studies have been performed with solutions simulating alkaline groundwater contaminated by radioactive elements. These solutions with pH ∼ 11 contained low Cs+ levels (∼1 ppm) and relatively high concentrations (7 to 125 ppm) of Na+, Mg2+ and Ca2+. Despite the presence of various competitive ions, KMS-1 showed high removal capacities (74–99%) for Cs+ from these complex solutions.
KMS-2 also exhibits Cs+ ion-exchange properties.13h The maximum sorption capacity of KMS-2 is 531 ± 28 mg g–1. This is 2.35 ± 0.12 times the capacity of KMS-1 and one of the highest capacities ever reported for Cs+ ion-exchangers.10 KMS-2 is expected to exhibit twice the Cs+ exchange capacity of KMS-1 because in the latter, the sorption capacity is reduced by the oxidation of Mn2+ to Mn3+ (see above). This oxidation cannot occur in KMS-2 containing Mg2+; thus, two equivalents of Cs+ can be absorbed by one mole of KMS-2. Furthermore, KMS-2 shows high Cs+ sorption capacity not only under neutral pH conditions, but also in acidic (pH = 3) and alkaline (pH = 10) environments.
2.2.2. Sr2+ ion exchange properties
Sr2+ ion-exchange and capture is also an important process, since radioactive 90Sr+ represents one of the major heat producers and biohazards in nuclear waste.4 Thus, detailed Sr2+ ion-exchange property studies were performed with KMS-1 and 2.13c,h The isotherm sorption data for these materials can be fitted with the Langmuir model and revealed maximum Sr2+ sorption capacities of 77 ± 2 and 87 ± 2 mg g–1 for KMS-1 and 2, respectively. Both KMS-1 and KMS-2 perform very efficiently in a very broad pH range. The affinity of the sorbents for Sr2+ was expressed in terms of the distribution coefficient Kd, which is calculated by the equation:
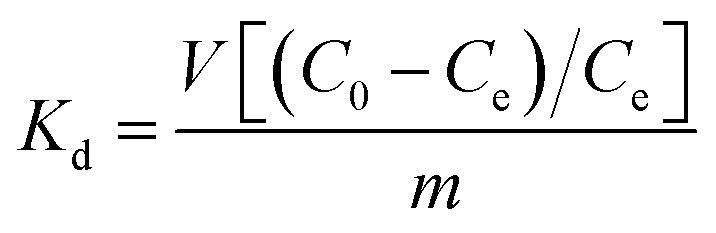 |
6 |
where C0 and Ce are the initial and equilibrium concentrations of Sr2+ (ppm), respectively, V is the volume (mL) of the testing solution and m is the amount of the ion exchanger (g) used in the experiment.13 Values of Kd equal to or above 104 mL g–1 are considered excellent. KMS-1 showed very high Kd values in the range of 104 to 105 mL g–1 from pH 3 to 14.
KMS-1 performs very efficiently for Sr2+ sorption. Its removal capacity is ∼92% (Kd ∼1.2 × 104 mL g–1) at very high salt concentrations (∼5 M Na+) and pH ∼ 14, which are the conditions typically found in alkaline nuclear waste. KMS-1 outperforms oxidic sorbents for Sr2+ ion exchange under acidic conditions. The KMS-1 material contains soft basic S2– ligands with very small affinity for protons. This is not the case for the conventional oxidic exchangers, where the hard H+ ions show great affinity for O2– ligands, which interferes with the process.19–21
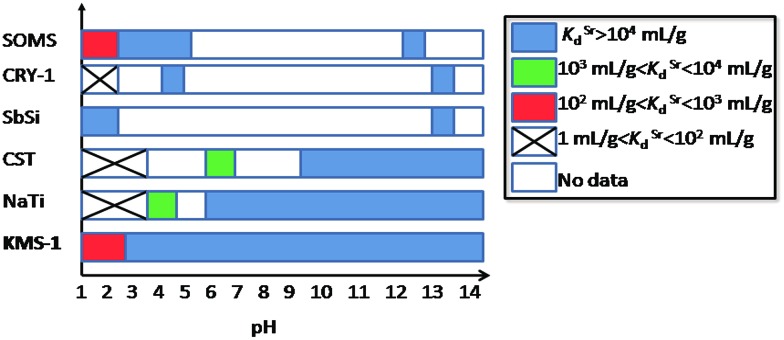
The comparison of KMS-1 with various Sr2+ sorbents, in terms of their Kd values vs. the pH of the solution, is provided in Fig. 5.
Fig. 5. Comparison diagram indicating the dependence of Kd on pH for the Sr2+ ion-exchange of various materials. NaTi: sodium titanate,19 CST: sodium silicotitanate,19b,d CRY-1: cryptomelane-type manganese oxide,20 SOMS: Sandia octahedral molecular sieves21 and SbSi: Sb silicate.19b.
KMS-2 is also efficient in removing Sr2+ under both alkaline and acidic conditions. Thus, Kd values of 6.3 × 104 and 1.5 × 105 mL g–1 were observed for the Sr2+ exchange of KMS-2 at pH ∼ 3 and 10, respectively.
2.2.3. Ni2+ ion-exchange properties
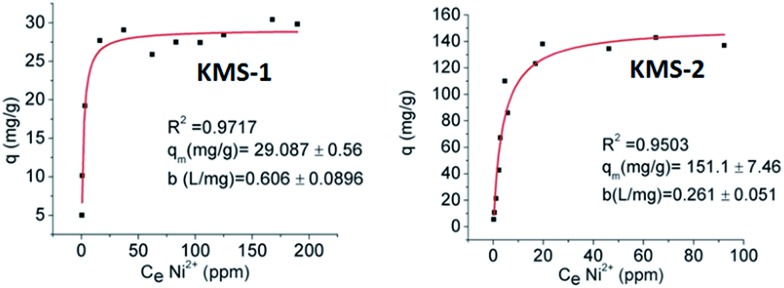
During the nuclear fission process, radioactive byproducts are generated due to the corrosion of the containers by the heat and acidity produced. Among these byproducts, 63Ni is regulated as a low energy beta contaminant.4 Thus, the removal of Ni2+ from aqueous solutions is relevant to the decontamination of nuclear waste from the corrosion products of the fission process. For this reason, the Ni2+ ion-exchange properties of KMS-1 and 2 were investigated (Fig. 6).13h
Fig. 6. Ni2+ isotherm sorption data for KMS-1 and 2. The solid lines represent the fitting of the data with the Langmuir model.
The isotherm Ni2+ sorption data for KMS-1 and 2 follow the Langmuir model and indicate maximum sorption capacities of ∼29 and 151 mg g–1, respectively. The relatively low Ni2+ sorption capacity of KMS-1 is likely due to the oxidation of Mn2+ to Mn3+. In the case of KMS-2, the observed Ni2+ sorption capacity is ∼1.4 larger than the theoretically expected value. This is attributed to the exchange not only of the interlayer K+ ions but also the intralayer Mg2+ ions. KMS materials are highly selective for Ni2+ in the presence of a tremendous excess of Na+ ions. Thus, a reasonably high Kd value of 1.8 × 105 mL g–1 was obtained for Ni2+ exchange of KMS-2 (initial Ni2+ concentration of ∼6 ppm) in the presence of 5 M Na+. The hard Na+ ions exhibit very weak interactions with the soft metal sulfide layer, in contrast to the relatively soft Ni2+, which may interact strongly with the soft S2– ligands. As a result, high Na+ concentrations have a negligible effect on the Ni2+ exchange of KMS materials. This impressive binding to Ni2+ ions also indicates that KMS materials may be effective agents in Ni recovery from industrial effluents arising from massive nickel plating operations worldwide.22
2.2.4. Hg2+, Pb2+ and Cd2+ ion-exchange and capture
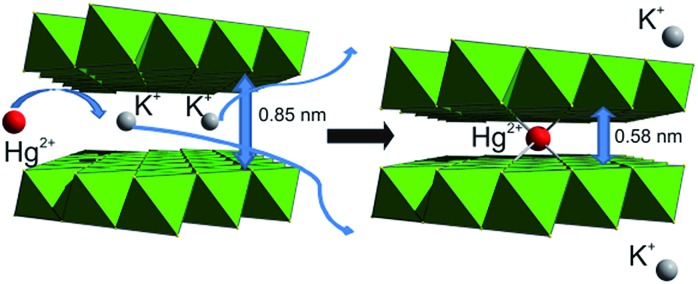
Because Hg2+, Pb2+ and Cd2+ ions present a major health hazard for drinking and industrial wastewater,1 it is important to develop highly selective sorbents for these ions with very high loading capacities.11 The layered structure of KMS-1 sorbent and the presence of soft basic sites (S2– ligands) allow for very rapid kinetics for the exchange of interlayer K+ ions by soft Lewis acids, such as Hg2+, Pb2+ and Cd2+ ions (Fig. 7).13e
Fig. 7. Capture of Hg2+ by KMS-1 through exchange of its interlayer potassium cations.
PXRD studies indicated that significant contraction of the interlayer space occurs upon exchange of K+ ions by Hg2+ (Fig. 7), which is due to the smaller size of the Hg2+ ion compared to K+ and the formation of strong Hg2+–S2– covalent interactions.
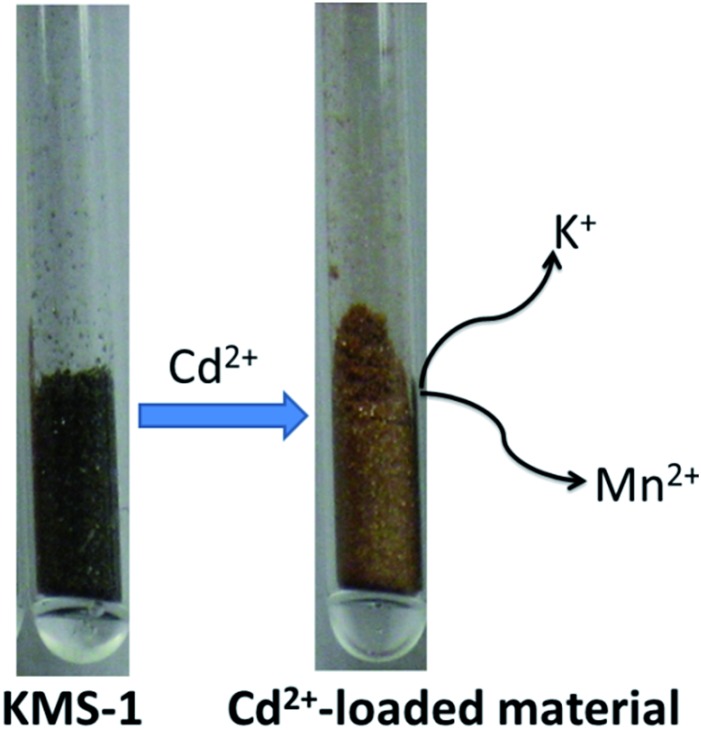
The PXRD pattern for the Pb2+ exchanged product showed the presence of two interlayer spacings because of the different hydrations of the Pb2+ ions ([Pb(H2O)x]2+). The Cd2+ ion-exchange process is particularly interesting. The analytical data for the Cd2+-exchanged product showed the absence not only of K+ ions but also of Mn2+. Specifically, the average formula of the Cd2+-loaded compound was determined as Cd1.8Sn2.1S6, and the ion-exchange process is described by the following equation:
| K1.9Mn0.95Sn2.05S6 + 1.9Cd2+ → [Cd(H2O)1.5]0.95[Cd0.95Sn2.05S6] + 0.95Mn2+ + 1.9K+ | 7 |
The Cd2+-exchanged KMS-1 has a layered structure, as indicated by the PXRD data, and the Cd2+ is inserted as a hydrated ion in the interlayer space and also replaces all intra-layer Mn2+ ions. Due to the complete exchange of Mn2+ by Cd2+, a dramatic color change of the material from dark brown to orange-brown was observed upon Cd2+ exchange (Fig. 8).
Fig. 8. Color change of KMS-1 upon the Cd2+ exchange process.
Isotherm batch sorption data revealed maximum sorption capacities of 320 to 377 mg g–1 for the Hg2+, Pb2+ and Cd2+ exchange processes. The capture of these ions by KMS-1 is not affected by the acidity or basicity of aqueous solutions. Thus, very high Kd values (>104 mL g–1) for these ions in a broad pH range (2.5 to 10) can be achieved. In addition, the presence of a very large excess of Na+ and Ca2+ ions, which are commonly found in high concentrations in drinking water and industrial wastewater, has no effect on the exchange of Hg2+ and Pb2+ and only slightly interferes with the Cd2+ exchange process.
When Hg2+, Pb2+ and Cd2+ are present simultaneously in solution, KMS-1 can capture all three ions with no apparent selectivity. The concentrations of all three ions are reduced well below the safety limits within only 2 min of KMS-1/solution contact (Fig. 9). KMS-2 also exhibits exceptional Hg2+, Pb2+ and Cd2+ exchange properties.13e This result is in marked contrast to thiol-functionalized materials that show selectivity for Hg2+ and exhibit low to moderate sorption capacity for Pb2+ and Cd2+.11
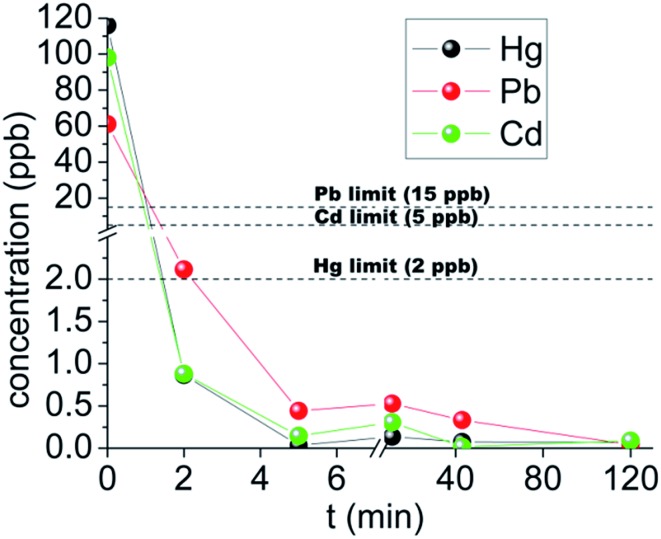
Fig. 9. The kinetics for the decontamination of potable water sample intentionally contaminated by mercury (116 ppb), lead (61 ppb) and cadmium (98 ppb) with KMS-1.
Regeneration of KMS-1/2 from the Hg2+, Pb2+ or Cd2+-loaded materials was not feasible because of the very strong binding of these ions. Because of the extremely high metal ion sorption capacity (up to 50% by weight) and relatively low cost of KMS materials, it may not be necessary to regenerate them; thus, the ion-loaded materials could be considered as a permanent waste form, suitable for safe disposal. Initial investigations indicate negligible leaching of Hg2+, Pb2+ or Cd2+ from the corresponding exchange products after their hydrothermal treatment at 70 °C for 24 h.
Finally, a methylammonium analogue of KMS-1, with the formula [CH3NH3]2xMnxSn3–xS6·0.5H2O (x = 1.0 to 1.1) (CMS),23 was recently described, and its Cd2+ and Pb2+ ion-exchange properties were studied. The results of these investigations revealed rapid sorption kinetics, high sorption capacity and exceptional selectivity of CMS for Cd2+ and Pb2+ (see also the section below for a comparison between MS exchangers, and Table 1).
Table 1. Important ion-exchange characteristics of representative MSIEs.
| Material | q m a (mg g–1) | K d (mL g–1) individual (pH ∼ 7) | K d (mL g–1) competitive (Na+) | K d (mL g–1) competitive (Ca2+) | Active pH range | Kinetic studies-equilibrium time (min) at RT | Reference |
| Cs + | |||||||
| KMS-1 | 226 ± 2 | ≥104 | ≥103 e | ≥102 f | 0.8–12 | 5 | 13d |
| KMS-2 | 531 ± 28 | ≥103 | ≥10 g | NA b | 3–10 | NA | 13h |
| KTS-1 | 205 ± 6 | ≥105 | NA | NA | NA | NA | 33 |
| KTS-3 | 280 ± 11 | ≥104 | ≥103 h | NA | 2–12 | 5 | 13i |
| FJSM-SnS | 409 ± 29 (65 °C) | ≥103 | NA | NA | 0.7–11 | 5 (65 °C), 30 (RT) | 34 |
| GeSbS-2 | 231 ± 15 (65 °C) | ≥103 | NA | NA | 2.8–11 | 2 (65 °C) | 39 |
| K6MS | 66 ± 4 | ≥104 | ≥104 i | ≥103 j | 2.5–12 | NA | 42 |
| Sr 2+ | |||||||
| KMS-1 | 77 ± 2 | ≥105 | ≥105 k | NA | 1–14 | NA | 13c |
| KMS-2 | 87 ± 2 | ≥104 | ≥10 l | NA | 3–10 | NA | 13h |
| KTS-3 | 102 ± 5 | ≥105 | ≥102 m | NA | 2–12 | 5 | 13i |
| FJSM-SnS | 65 ± 5 | ≥104 | NA | NA | 0.7–11 | 5 (65 °C), 60 (RT) | 34 |
| Hg 2+ | |||||||
| KMS-1 | 377 ± 15 c | ≥105 | ≥105 n | ≥9 × 104 n | 2.5–9.5 | 5 | 13e |
| LHMS-1 | 87 ± 6 c | ≥106 | ≥105 n | ≥105 n | 0–9 | 10 | 13f |
| K6MS | NA | ≥106 | ≥106 n | ≥106 n | 3–8 | 60 | 43 |
| Pb 2+ | |||||||
| KMS-1 | 319 ± 4 c | ≥106 | ≥8 × 104 n | ≥104 n | 2.5–9.5 | 5 | 13e |
| CMS | 1053 d | ≥106 | ≥106 n | ≥106 n | 1–7 | 30 | 23 |
| Cd 2+ | |||||||
| KMS-1 | 329 d | ≥107 | ≥6 × 103 n | ≥7 × 103 n | 0–9 | 5 | 13e |
| CMS | 515 d | ≥105 | ≥105 n | ≥105 n | 1–7 | 90 | 23 |
| UO 2 2+ | |||||||
| KMS-1 | 380 ± 20 | ≥105 | ≥105 o | ≥104 p | 2.5–10 | 120 q | 13g |
| KTS-3 | 287 ± 15 | ≥104 | ≥5 × 103 o | NA | 2–12 | 50 r | 13i |
| Ni 2+ | |||||||
| KMS-1 | 29 ± 1 | ≥105 | ≥105 s | NA | 3–10 | NA | 13h |
| KMS-2 | 151 ± 8 | ≥105 | ≥105 s | NA | 3–10 | NA | 13h |
| Cu 2+ | |||||||
| KMS-1 | 155 | ≥105 | ≥104 t | ≥104 t | 2–6 | 20 | 29 |
| Tl + | |||||||
| K6MS | 508 ± 31 c | ≥105 | NA | NA | NA | NA | 43 |
aEstimated from the fitting of the data with the Langmuir model.
bNot available.
cEstimated from the fitting of the data with the Langmuir–Freundlich model.
dEstimated by averaging the metal uptake values corresponding to the saturation of the exchange sites of the material.
eNa : Cs molar ratio ∼655, pH ∼ 7.
fCa : Cs molar ratio ∼21.
gNa : Cs molar ratio ∼1.1 × 105, pH ∼ 7.
hNa : Cs molar ratio ∼1.8 × 103.
iNa : Cs molar ratio ∼1.3 to 6.5 × 105.
jCa : Cs molar ratio ∼105.
kNa : Sr molar ratio ∼1887.
lNa : Sr molar ratio ∼1.9 × 105.
mNa : Sr molar ratio ∼329.
nCompetitive ion: Hg2+ (or Pb2+ or Cd2+) molar ratio ≥ 1000.
oNa : U molar ratio ≥ 104.
pCa : U molar ratio ≥ 104.
q97% of U removal is achieved within 2 min.
r80% removal is achieved within 5 min.
sNa : Ni molar ratio ∼4.8 × 104.
tNa or Ca/Cu molar ratio ∼8.
2.2.5. Extraction of Ag+ and Hg2+ ions from their cyanide complexes
Cyanide ions are widely used for the dissolution of precious metals, such as gold and silver, from their minerals. In addition to the precious metal ions, the minerals also contain several heavy metal ions that must be removed and separated from the precious metals.24 One example is the mineral Ag2Hg2S2. The cyanidation of this mineral results in the formation of [Hg(CN)4]2– and [Ag(CN)2]– complexes.25 Remarkably, KMS materials can extract Hg2+ and Ag+ from their cyanide complexes. KMS-2 was studied for these ion-exchange processes.26 A complete exchange of the K+ ions of KMS-2 by Hg2+ or Ag+ was observed after treatment of KMS-2 with aqueous solutions of [Hg(CN)4]2– or [Ag(CN)2]–. KMS-2 was also capable of simultaneously and quantitatively capturing Hg2+ and Ag+ from water solutions containing both [Hg(CN)4]2– and [Ag(CN)2]– complexes (removal capacities >97%).
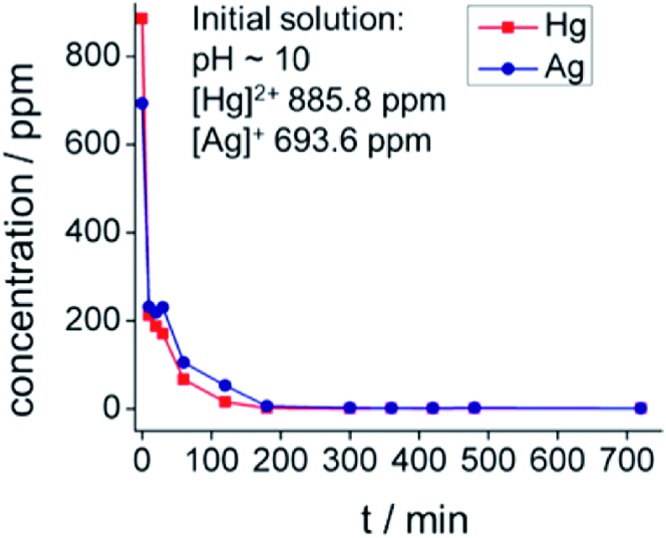
The kinetics of the sorption of Hg2+ and Ag+ from mixed [Hg(CN)4]2––[Ag(CN)2]– solution (pH ∼ 10) show that within 3 h, the Hg2+ and Ag+ exchange reached equilibrium, with ≥99.9% removal capacities observed (Fig. 10). The fast extraction of Hg2+ and Ag+ from their cyanide complexes is attributed to the high mobility of the K+ ions of KMS-2 and the high affinity of the soft Lewis acids Hg2+ and Ag+ for the soft basic sulfide framework of KMS-2. It is suggested that KMS-2 absorbs Hg2+ and Ag+ once the ions are released to the solution and reach equilibrium, according to Le Chatelier's principle (reactions 7–9):
| [Ag(CN)2]– ↔ Ag+ + 2CN– | 8 |
| [Hg(CN)4]2– ↔ Hg2+ + 4CN– | 9 |
| K2MgSn2S6 + xHg2+ + yAg+ → K2–2x–yHgxAgyMgSn2S6 + (2x + y)K+ | 10 |
Fig. 10. Kinetic data for the sorption of Hg2+ and Ag+ from a mixture of Hg(CN)4]2––[Ag(CN)2]– complexes in solution (pH ∼ 10). Reproduced with permission from ref. 26. © 2015, American Chemical Society.
The elemental Hg and Ag can be recovered from the Hg2+/Ag+-loaded KMS-2 via a two step process. First, the exchanged product is heated at 425 °C; HgS sublimes and is collected at the end of a tube which is placed outside the furnace. Then, the Hg-free compound is dissolved in nitric acid, and elemental Ag is isolated by reducing the dissolved Ag+ with hydrazine.
Overall, the results from these studies suggest that MSIEs are very effective for the recovery of precious metal ions and decontamination of mine wastewater.
2.2.6. UO22+ exchange properties
Uranium, which exists primarily as the UO22+ cation, represents the major source of nuclear energy and its waste; it can be released into the environment through various industrial and mining processes.4 Furthermore, a legacy of uranium-contaminated sites resulted from the closure of various nuclear facilities worlwide.4 Selective UO22+ ion exchangers to effectively capture this species are thus particularly attractive. In addition to their environmental remediation applications, UO22+ sorbents can find use in the extraction of U from seawater, which contains 1000 times more U than terrestrial ores.
Traditionally, suitable sorbents for UO22+ are materials with hard basic functional groups,27ae.g. carboxylic, phosphonate, or amidoxime groups, taking into account that U, with a 6+ oxidation state, is expected to behave as a typical hard Lewis acid. However, this may be an oversimplified assumption, since there have been reports of uranyl compounds forming reasonably strong covalent bonds with S2–.27b KMS-1 shows selective UO22+ exchange properties. Complete exchange of the K+ ions of KMS-1 by UO22+ can be accomplished very fast13g according to the following equation:
| K1.9Mn0.95Sn2.05S6 + 0.95UO22+ → [UO2(H2O)1.5]0.95[Mn0.95Sn2.05S6] + 1.9K+ | 11 |
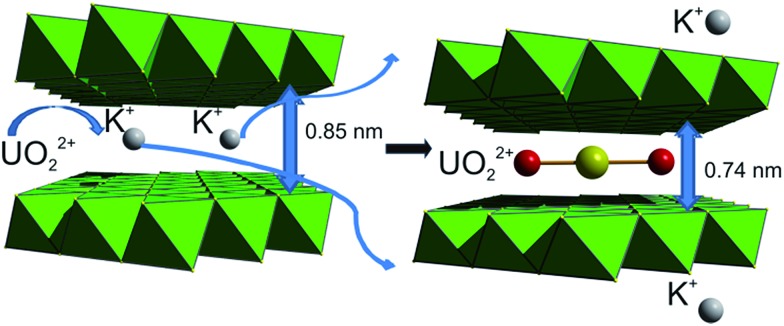
PXRD studies show that the main UO22+-exchanged phase contains the linear [O U O]2+ group, ordered parallel to the layer plane (Fig. 11).
Fig. 11. Schematics for the mechanism of UO22+ capture by KMS-1.
Solid-state reflectance NIR/UV-Vis data indicated a significantly lower band gap for the UO22+ exchanged compound (∼0.95 eV) compared to that (1.3 eV) of pristine KMS-1. This is indicative of relatively strong UO22+···S2– interactions.
Isotherm batch sorption data fitted to the Langmuir model reveal a maximum capacity of 380 ± 20 mg g–1. The material can be regenerated by treating the UO22+-loaded product with Na2CO3 and can be reused several times for UO22+ ion exchange. KMS-1 performs well for UO22+ sorption in a broad pH range (2 to 10). Interestingly, very high UO22+ removal capacities (94–98%) for KMS-1 were observed even in the presence of tremendous excesses (up to 104 times higher concentration) of competitive ions such as Na+ and Ca2+.
Furthermore, KMS-1 was tested for UO22+ exchange under realistic conditions. It was found to be particularly effective for the decontamination of potable and lake water samples intentionally contaminated with traces of U (400 to 1000 ppb). Thus, the treatment of the samples with KMS-1 for only 2 min results in a final U concentration of ∼1 ppb, well below the acceptable limit (30 ppb, defined by USA-EPA).
KMS-1 has been tested for recovery of U from seawater samples which contain U in concentrations of 3 to 4 ppb and very high amounts of Na+, Ca2+, Mg2+ and K+ (200 to 10 000 ppm). KMS-1 efficiently absorbs (removal capacity up to 84%) even this extremely low U content in the presence of reasonably high concentrations of competitive ions. Overall, the results from the UO22+ sorption of KMS-1 revealed that this ion is much softer than previously thought, and MSIEs may provide new strategies for selective UO22+ sorbents. Additional work is required to further evaluate the potential of this material for uranium harvesting from the sea; especially, testing should be performed under more realistic conditions relevant to a scalable practical process.
2.2.7. Cu2+ ion-exchange properties
High concentrations of Cu2+ in drinking water may result in several health problems, such as liver or kidney damage or gastrointestinal distress.1,28 KMS-1 was recently tested as a Cu2+ exchanger.29 The results indicated that Cu2+ is inserted as a hydrated cation in the interlayer space, exchanging all K+; at the same time, it partially replaces Mn2+ ions from the layer. The ion-exchange process is described as follows:
| K1.88Mn0.94Sn2.06S6 + 1.34Cu2+ → [Cu(H2O)5.6]0.94[Cu0.4Mn0.54Sn2.06S6] + 0.4Mn2+ + 1.88K+ | 12 |
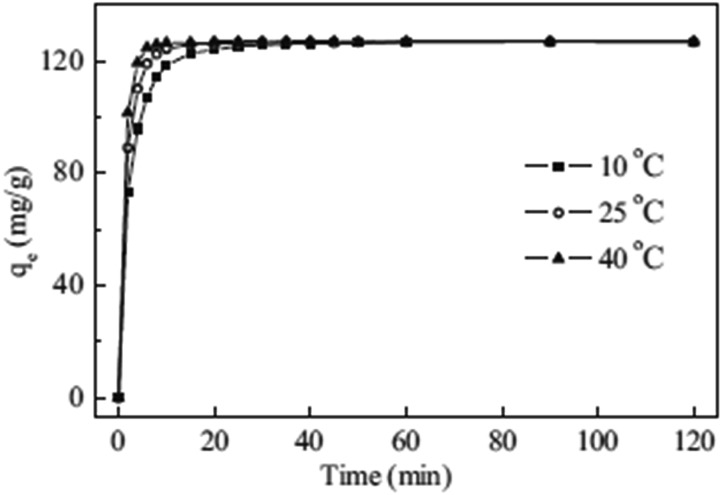
A detailed kinetic study has been performed for Cu2+ exchange by KMS-1. The data revealed that the absorption of Cu2+ is very fast, with ion-exchange equilibrium reached in 40, 20 and 10 min at 10, 25 and 40 °C, respectively (Fig. 12). In addition, fitting of the kinetic data can be readily achieved with the pseudo-second order model, which indicates that the rate limiting step is a chemical adsorption process. Further analysis of the kinetic data revealed that the rate controlling steps were external film and intraparticle diffusion processes.
Fig. 12. Kinetic data for the sorption of Cu2+ by KMS-1, obtained at three different temperatures. Reproduced with permission from ref. 29; © 2014, Elsevier.
2.2.8. Sorption of radionuclides
KMS-1 was also tested for the sorption of a series of radionuclides, including 233U, 239Pu, and 241Am.30 The results revealed that the material was particularly effective for rapid absorption of these radionuclides over a wide pH range (2 to 9), with ≥99% removal capacities observed even in the presence of high Na+ concentrations. This study indicated for the first time that MSIEs can be highly efficient for the decontamination of radioactive waste.
2.3. Protonated KMS:LHMS material
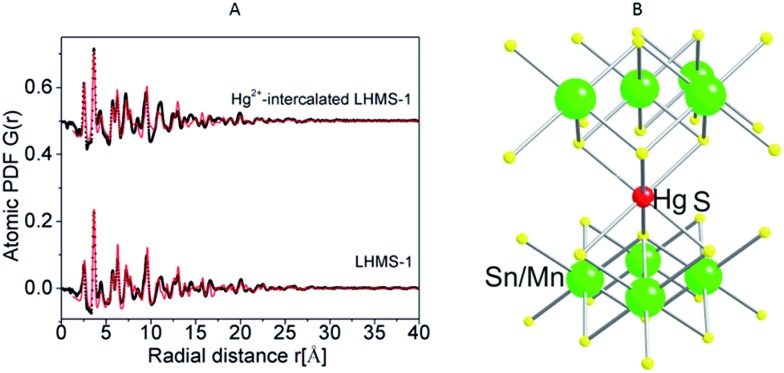
A rare example of a solid acid metal sulfide material is H2xMnxSn3–xS6 (x = 0.11–0.25), or LHMS-1 (layered hydrogen metal sulfide-1) compound.13f This compound is formed from treatment of KMS-1 with highly acidic solution to exchange K+ with H+, a process which also attests to the high stability of the material to strong acids. LHMS-1 can absorb Hg2+ from a very acidic (pH = 0) to strongly alkaline environment (pH = 9). It is remarkable that LHMS-1 achieves almost 100% Hg2+ removal under extremely acidic conditions, e.g. even in the presence of 6 M HNO3. Thus, this compound could be useful for removing Hg2+ from acidic wastewater, e.g. particular types of nuclear waste.4c,31 The local structure of Hg2+ in the interlayer space of the Hg2+-loaded LHMS material was determined by atomic pair distribution function (PDF) studies, performed for the pristine LHMS-1 material and the Hg2+-exchanged product. The results are consistent with an octahedral coordination of Hg2+ with Hg–S distances of ∼2.57 Å (Fig. 13).
Fig. 13. (A) Experimental atomic PDFs (black) for LHMS-1 and Hg2+-exchanged material. The red lines represent the computed atomic PDFs based on the SnS2 structure type. (B) The octahedral coordination of Hg2+ in the exchanged material, which is suggested based on the PDF model.
2.4. Layered materials based on the Sn3S72– net
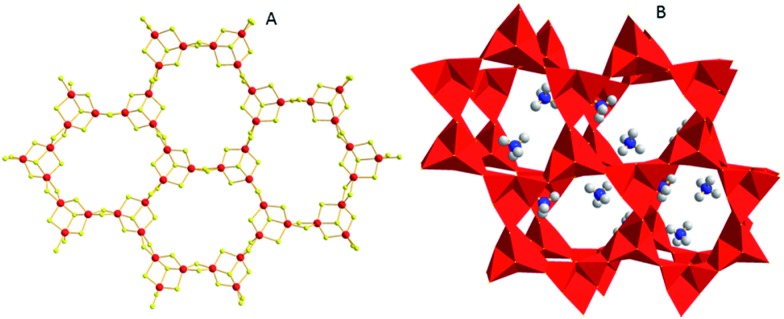
Another family of interesting MSIEs is based on the Sn3S72– layered framework. The first member of this series, (TMA)2(Sn3S7)·H2O (TMA+ = tetramethylammonium ion), was reported by Prof. J. B. Parise and coworkers.32 The compound was isolated by the reaction of SnS2, TMAOH and S under hydrothermal conditions. It features a microporous layered framework consisting of edge-sharing [Sn3S4] semi-cubes (Fig. 14A). Six [Sn3S4] units are connected via S2– bridges to form a ring surrounded by twelve SnS5 trigonal bipyramids. The layers are separated by TMA+ cations and guest water molecules (Fig. 14B). Preliminary ion-exchange experiments indicated that the TMA+ ions are easily exchanged by alkali and alkaline earth ions and some transition metal ions; however, ion exchange with soft metal ions such as Ag+ ions resulted in the breakdown of the layered framework structure.
Fig. 14. (A) The layered structure of (TMA)2(Sn3S7)·H2O (Sn, red; S, yellow). (B) The arrangement of two adjacent layers (with a polyhedral representation) and the TMA+ cations (C, grey; N, blue) located in the interlayer space (guest water molecules were omitted for clarity).
Additional examples of materials with the Sn3S72– layered structure contain a variety of interlayer organic cations, such as DABCOH+ (protonated 1,8-diazabicyclooctane), QUIN (quinuclidinium), TBA+ (tert-butylammonium) and Et4N+ (tetraethylammonium).33
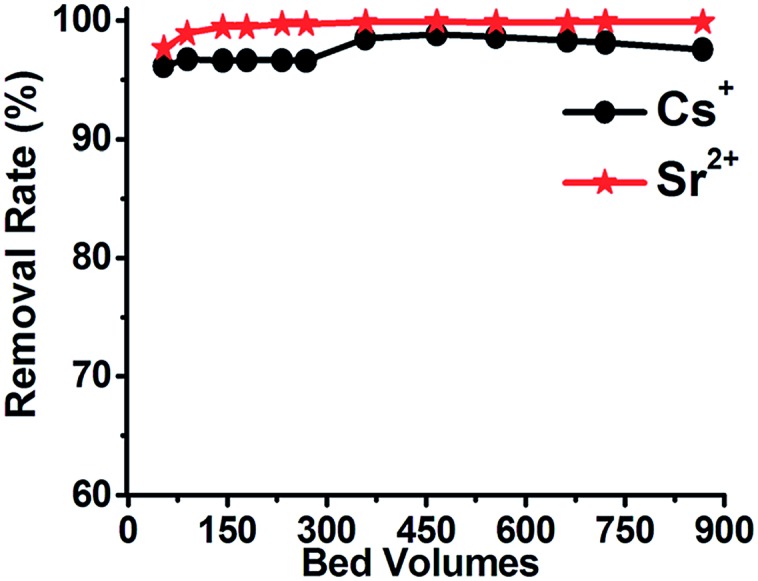
More recently, a new member of the Sn3S72– family templated by mixed MeNH2+ and Me3NH+ cations was described. The formula of this material, denoted as FJSM-SnS, was (Me2NH2)4/3(Me3NH)2/3Sn3S7·1.25H2O.34 The Cs+ and Sr2+ exchange properties of this material at 65 °C indicated that the maximum sorption is achieved within only 5 min, whereas at room temperature, the ion exchange equilibrium is reached within 30 to 60 min. The maximum Cs+ and Sr2+ sorption capacities were 409 ± 29 and 65 ± 5 mg g–1, respectively. These values are among the highest reported for MSIEs. In addition, pH-dependent experiments revealed good Cs+ and Sr2+ removal capacities in a broad pH range (0.7 to 10). Interestingly, FJSM-SnS can be used as the stationary phase in an ion-exchange column, which was found to be particularly effective in absorbing Cs+ and Sr2+ from aqueous solutions containing both ions (initial concentrations: Cs+ = 12 to 15 ppm; Sr2+ = 6 ppm). Thus, removal capacities of 96–100% Cs+ and Sr2+ were observed after passing 900 bed volumes (total volume passed = 2.42 L, one bed volume = 2.79 mL) through the ion exchange column (Fig. 15). This represents one of the first reports of the use of MSIEs in columns. However, for practical ion-exchange column applications, engineered forms of the sorbents may be required (see below).
Fig. 15. % removal of Sr2+ and Cs+vs. the bed volume for a column of the FJSM-SnS material.
2.5. KTS materials
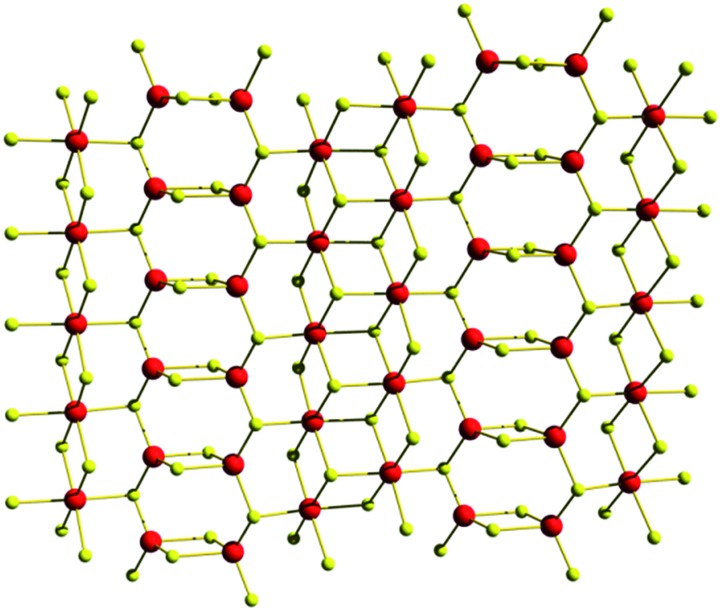
An additional example of a tin sulfide layered material with promising ion-exchange properties is K2Sn4S9 (KTS-1). This compound can be isolated via solid state synthesis. The crystal structure of KTS-1 is related to the structures of Rb2Sn4S9 and Cs2Sn4S9.35 The basic unit of the structure of these materials is the Sn4S92– duster, consisting of two tetrahedrally coordinated Sn4+ ions and two Sn4+ ions adopting trigonal bipyramidal geometry. The clusters are connected through S2– bridges to form a layered structure perforated with relatively large holes (Fig. 16A). The layers are corrugated, and the interlayer space is filled with highly disordered cations (Fig. 16B).
Fig. 16. (A) Part of the Sn4S92– layer (Sn, red; S, yellow). (B) The arrangement of two adjacent layers with the cations (Cs+, pink) filling the interlayer space.
Preliminary investigations of the Cs+ exchange properties of KTS-1 revealed a maximum sorption capacity of 205 ± 6 mg g–1 and very high Kd values in the range of 103 to 105 mL g–1. Furthermore, the K+ ions of KTS-1 can be fully exchanged by soft metal ions, such as Hg2+, Pb2+ and Cd2+.36 Thus, KTS-1 appears to be a promising ion-exchanger, although further studies are required.
KTS-2, another tin sulfide ion-exchanger, is a 3-D material and is discussed below (see Section 2.3).36
Very recently, a new compound, K2xSn4–xS8–x (x = 0.65 to 1, KTS-3) was reported.13i This compound was prepared with a hydrothermal reaction similar to that used for KMS materials, but without adding Mn or Mg to the reaction mixture. The crystal structure of KTS-3 is shown in Fig. 17. SnS6 octahedra form ribbons running along the c-axis and are interconnected through SnS4 units in the form of Sn2S6 bridges. The interlayer space is filled by disordered K+ ions.
Fig. 17. Structural segment of the KTS-1 layer viewed along the b-axis. Sn, red; S, yellow.
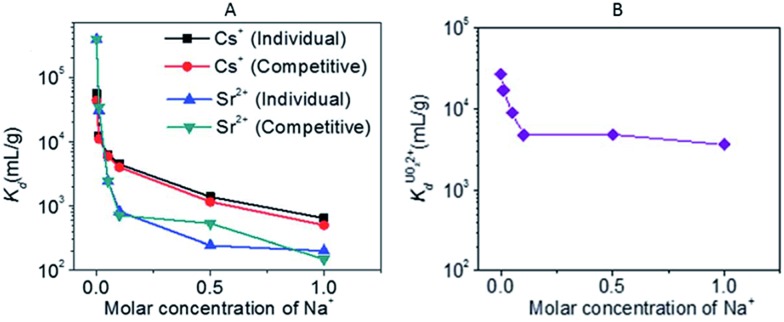
KTS-3 is an excellent ion-exchanger for Cs+, Sr2+ and UO22+. The Sr2+ capacity of this sorbent was found to be 102 ± 5 mg g–1 (by fitting of the data with the Langmuir model), the highest reported among MSIEs. It also shows high Cs+ and UO22+ exchange capacities (Langmuir fitting: 280 ± 11 mg Cs/g, 287 ± 15 mg U/g). Interestingly, it exhibits very good selectivity for Cs+ (Kd value of 4.4 × 103 mL g–1) in the presence of Na+ in a molar concentration (0.1 M) 2000 times higher than the initial Cs+ content (Fig. 18A). Even with a 10 000-fold excess of Na+, the Kd value for Cs+ was >103 mL g–1. The effect of Na+ seems to be more important in the case of ion-exchange of Sr2+; the Kd values dropped sharply when the Na+ concentration was increased (Fig. 18B). Furthermore, competitive Cs+/Sr2+ ion exchange experiments revealed generally higher Kd values for Cs+ exchange than Sr2+ exchange (Fig. 18A). Finally, Na+ even in huge excess (concentration up to 5 M) has a negligible effect on UO22+ exchange because the Kd values show only slight variation when the Na+ concentration is increased (Fig. 18B). This result further indicates that UO22+ behaves more like a typical soft ion (borderline soft) interacting strongly with the soft basic metal sulfide layer, rather than a hard ion, as UO22+ is generally classified. KTS-3 is also an effective sorbent for heavy metals such as Hg2+, Pb2+, Cd2+, Ag+, and Tl+.
Fig. 18. (A) Kd of individual and competitive Cs+ and Sr2+ ion exchange vs. molar concentration of Na+ (initial concentrations were 7.4 and 6.9 ppm for Cs+ and Sr2+ respectively, V m–1 ratio was 1000 mL g–1, and pH ∼ 7). (B) Kd for UO22+ ion exchange vs. molar concentration of Na+ (initial concentration of UO22+ was 1 ppm, V m–1 ratio was 1000 mL g–1, and pH ∼ 7).
2.6. Layered sulfides with trivalent metal ions in their framework
The majority of layered MSIEs are based on tetravalent or combined tetravalent/bivalent metal ions. Generally, these metal ions mainly adopt tetrahedral or octahedral coordination; in only a few cases, trigonal bipyramidal coordination has been also observed. An alternative approach to create sulfide materials involves the combination of trivalent ions such as In3+ or Ga3+, which prefer tetrahedral coordination, and Sb3+, which tends to adopt a trigonal pyramidal coordination geometry.
2.6.1. [(CH3CH2CH2)2NH2]5In5Sb6S19·1.45H2O (InSbS)
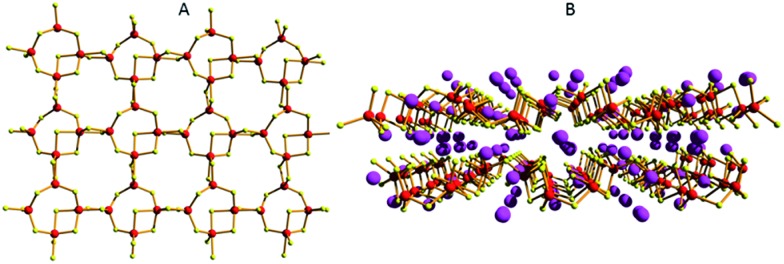
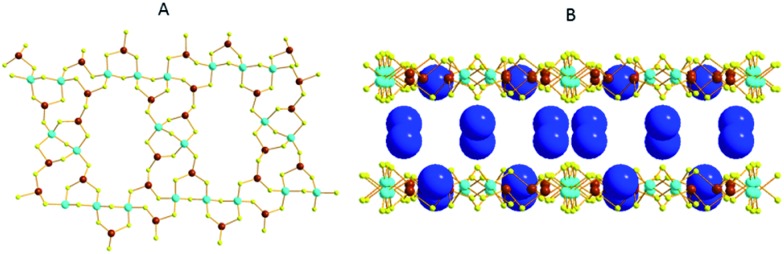
A layered material isolated with this synthetic strategy is [(CH3CH2CH2)2NH2]5In5Sb6S19·1.45H2O (InSbS), prepared by a hydrothermal reaction of In2S3, Sb2S3, S and dipropylamine.37 The [In5Sb6S19]5– layer is composed of corner-sharing InS4 tetrahedra bridged by SbS3 trigonal pyramidal units and Sb2S6 dimers (Fig. 19A). The layer contains large holes with dimensions 13.3 × 3.8 Å2, which are large enough to host some of the organic counter ions, with the remaining organic cations located in the interlayer space (Fig. 19B).
Fig. 19. (A) Part of the [In5Sb6S19]5– layer viewed down the b-axis. (B) Packing of the layers with the N atoms of dipropylammonium cations showing as large balls (C and H atoms were omitted for clarity). In, cyan; Sb, brown; N, blue; S, yellow.
Due to the disordered state of the organic guest ions and the relatively large windows of the layer, this compound displays facile ion-exchange properties with various cationic species. Interestingly, Cs+ can completely exchange the organic cations, whereas Rb+ exchanges only 37% of the cations. In addition, 12% of ions are replaced by Li+, and a negligible amount of organic guests can be exchanged by K+ and Na+.
Competitive experiments with a mixture of equimolar amounts of Na+, K+, Rb+ and Cs+ revealed that the Cs+ uptake of InSbS is 10 times higher than that for the other ions. This significant selectivity of the compound for Cs+ probably results from the size-match of this cation with the aperture of the windows in the [In5Sb6S19]5– layer. Thus, the perforated layers of the compound seem to favor its facile ion-exchange properties. This hypothesis is also supported by the lack of any ion-exchange capacity for the lamellar material [CH2NH2]2In2Sb2S7, whose layers are relatively dense and have no holes.
2.6.2. [CH2NH2]2Ga2Sb2S7·H2O (GaSbS-1)
Another example of a layered MSIE material containing trivalent metal ions is [CH2NH2]2Ga2Sb2S7·H2O (GaSbS-1).38 The building block of the structure is made of two corner-sharing GaS4 tetrahedra and two SbS3 trigonal pyramidal units further bridging the GaS4 moieties (Fig. 20A). There are relatively open windows in the layers that are defined by 16-member rings composed of four building units. Dimethylammonium cations are found in the interlayer space and interact with the layers via N–H···S hydrogen bonding.
Fig. 20. (A) Part of the layer of GaSbS-1. The dashed lines represent hydrogen bonding interactions between the dimethylammonium ions and the layer. (B) Part of the layer of GaSbS-2 and some of the interlayer Cs+ ions. Ga, purple; Sb, brown; S, yellow; Cs, pink.
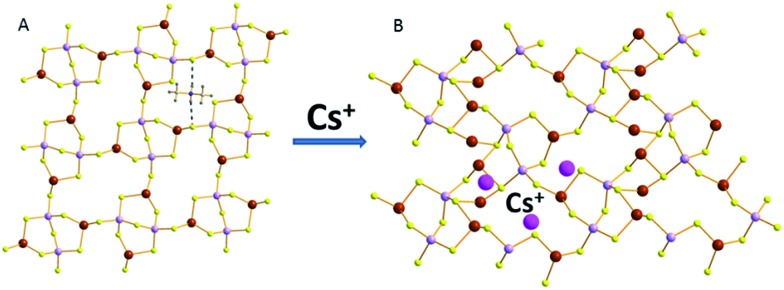
GaSbS-1 shows facile ion-exchange properties for alkali and alkaline earth metal ions due to its layered structure and the relatively large windows in the layers, which allow the diffusion of species in the ion-exchange reactions. Of particular interest is Cs+ sorption by the compound GaSbS-1. This material is particularly selective for Cs+ in the presence of a very large (100-fold) excess of Na+. Interestingly, the Cs+ exchange can be achieved even via a SCSC transformation process. The crystal structure of the Cs+-exchanged product (GaSbS-2) could be thus determined.
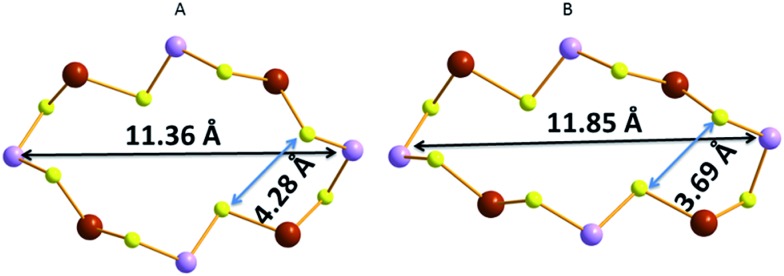
The connectivity of the atoms in GaSbS-2 (Fig. 20B) is the same as in GaSbS-1. There is, however, a significant contraction of the layered framework. Thus, the size of the windows changed from 11.36 × 4.28 Å2 in GaSbS-1 to 11.85 × 3.69 Å2 (including atomic radii) in GaSbS-2 (Fig. 21). Therefore, it appears that there is a framework response when Cs+ ions enter the structure, resulting in shrinkage of the window size in the layers. Two of the three crystallographically independent Cs+ ions are located close to the layer, whereas the third one is found between two layers. These ions are tightly bound in the compound, as no Cs+ can be leached out by treating the material with large excess of Na+ ions. The opening/closing of windows observed for the layered structure of compound GaSbS-1 upon Cs+ exchange is reminiscent of the mechanism of insect capture by the Venus flytrap plant.
Fig. 21. (A) The 16-member ring in GaSbS-1 with indication of its dimension. (B) The 16-member ring in GaSbS-2 with indication of its dimension. Ga, purple; Sb, brown; S, yellow..
2.6.3. [CH3NH3]20Ge10Sb28S72·7H2O (GeSbS-1)
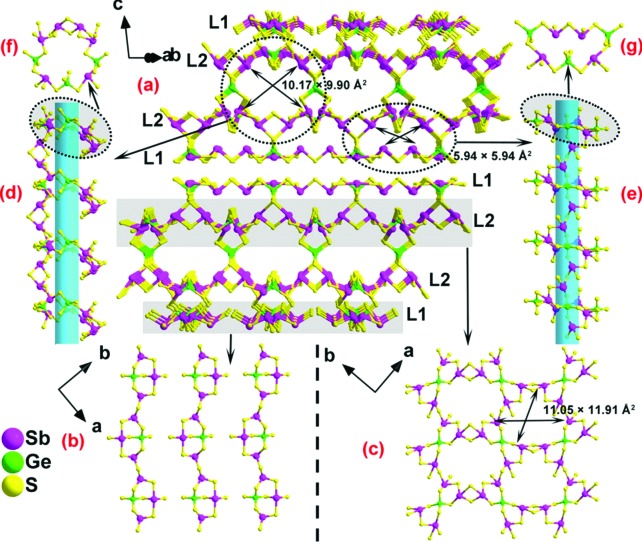
This compound was prepared solvothermally by reacting GeO2, Sb, S and methylammonium salt.39 It features an unusual double-layered structure formed by two symmetry-related thick layers joined by Ge4+ ions (Fig. 22). Each single layer is composed of two parts, L1 and L2. L1 is a 1-D chain of interconnecting [GeSb3S8]3– units, whereas the L2 moiety is a layer based on [GeSb4S11]6– units. Two types of channels are observed in the structure, with dimensions of 10.17 × 9.90 Å2 and 5.94 × 5.94 Å2. The methylammonium cations and guest water molecules are found within these channels as well as in the interlayer space.
Fig. 22. (a) The double-layered network of GeSbS-1. (b) The L1 unit. (c) The L2 unit. (d and e) The two types of channels in the network. (f and g) The 18- and 14-membered rings that form the channels in the structure. Reproduced with permission from ref. 39 © 2015, American Chemical Society.
GeSbS-1 shows impressive Cs+ exchange properties. Specifically, it exhibits a Cs+ exchange capacity of 231 ± 15 mg g–1. The kinetics of the Cs+ sorption is also remarkably fast, with the ion exchange reaching equilibrium within 2 min. In addition, the selectivity of the material for Cs+ is significant, as revealed by the relatively high Kd values (2 to 4 × 103 mL g–1) for Cs+ exchange in the presence of a large excess (20-fold) of Na+ or Ca2+.
2.7. Polysulfide and MoS42–-intercalated layered double hydroxides
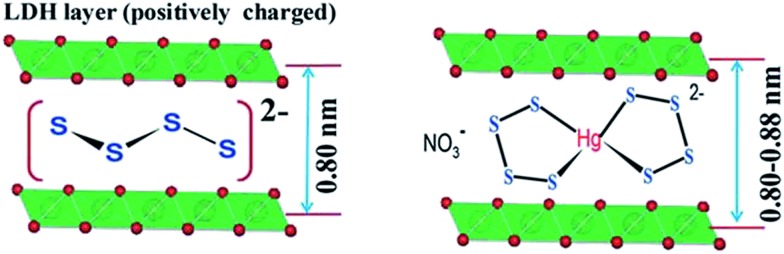
Recently, a new type of metal sulfide-like sorbent, with selective sorption properties for heavy metal ions, has been developed.40 These materials consist of layered double hydroxides (LDHs) intercalated by polysulfide [Sx]2– groups (Fig. 23, left).
Fig. 23. Left: Arrangement of [S4]2– groups in the interlayer space of the LDH material. Right: Proposed binding of metal ions with the polysulfide unit. Metal, green; O, red.
The mechanism of the metal ion capture by LDH(Sx) sorbents depends on the metal ion : LDH(Sx) molar ratio.
In the case of low metal ion concentration and a large excess of sorbent, the following reaction takes place:
| LDH(Sx) + M(NO3)2 → LDH(NO3–)m([M2+(Sx2–)2]2–)n | 13 |
Thus, the [Sx]2– groups act as a second host for the incoming ions (Fig. 23, right).
When, however, the concentration of the metal ion is relatively large, the sorption reactions are stoichiometric; thus, two products, LDH(NO3) and MSx, are formed:
| LDH(Sx) + M(NO3)2 → LDH(NO3) + MSx | 14 |
Sorption experiments with aqueous solutions containing a series of metal ions, such as Ni2+, Co2+, Cu2+, Cd2+, Hg2+, Pb2+, Zn2+ and Ag+, indicate higher selectivity of LDH(Sx) for the softer ions. Extremely high Kd values (up to 107 mL g–1) are found for the sorption of Ag+ and Hg2+, revealing the high potential of LDH(Sx) sorbents for the capture of heavy metal ions.40a
LDH(Sx) materials were also tested for UO22+ sorption.40b A series of UO22+ sorption experiments reveal the exceptional selectivity of the LDH(Sx) sorbents for UO22+ in the presence of very large excesses of Na+ and Ca2+. In addition, LDH(Sx) materials can efficiently absorb U from seawater samples. These results further confirm the potential of sulfide sorbents for selective U capture.
More recently, MoS42– intercalated LDHs have been reported.40c They show extremely high sorption capacities for Hg2+ (∼500 mg g–1) and Ag+ (∼450 mg g–1). They are also efficient to capture additional metal ions such as Cu2+, Pb2+, and Co2+. It is suggested that in low and medium metal (M2+) ion concentrations, the sorption proceeds through the formation of [M(MoS4)2]2– species trapped in the interlayer space of the LDH materials. In high initial metal ion concentrations, neutral amorphous [M(MoS4)] salts are produced.
3. Three-dimensional crystalline MSIEs
Although a large number of three-dimensional crystalline metal sulfide materials have been reported, relatively few examples have been thoroughly studied for their ion-exchange properties. Below, we describe some characteristic 3-D MSIEs and their sorption properties for various cations.
3.1. K6Sn[Sn4Zn4S17] (K6MS)
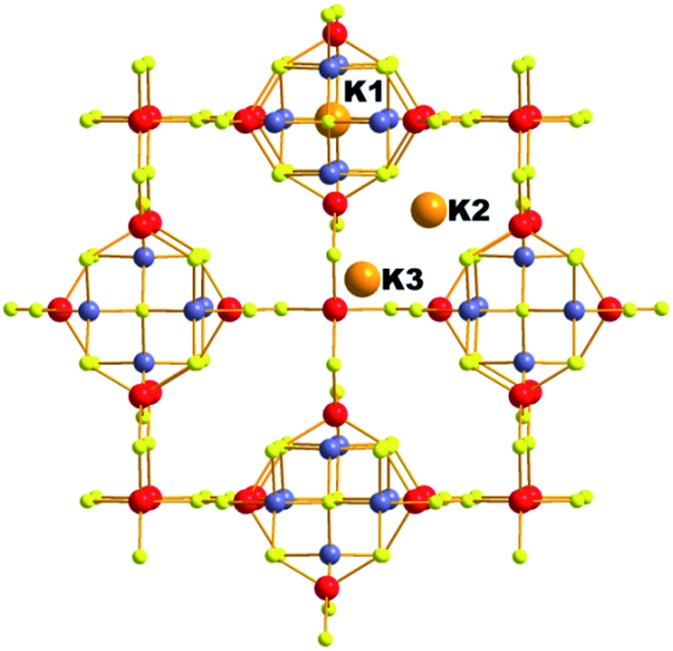
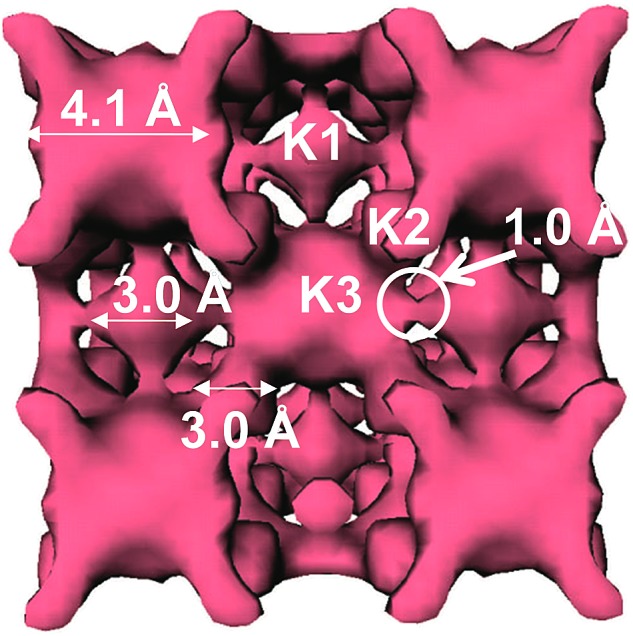
This compound was isolated via solid state flux synthesis.41 Its structure is based on the so-called penta-supertetrahedral (P1) [Zn4Sn4S17]10– cluster, constructed by a central {Zn4S}6+ core (anti-T1 unit) capped by four SnS4 tetrahedral (T1) units. The clusters are interconnected via four-coordinated Sn4+ ions to form a diamond-like framework (Fig. 24).13a There are 3 cavities in the structure which host K+ ions. The K1 cavity accommodates a tightly bound K+ ion (K1), whereas K2 and K3 cavities contain four (K2) and one (K3) K+ ions, respectively, which are relatively labile. The K3 cavity has a relatively large diameter (∼4.1 Å) and communicates through narrow passages (with sizes of ∼1.0 to 1.5 Å) with the smaller K1 and K2 pores (with a diameter of ∼3.0 Å) (Fig. 25).
Fig. 24. The three-dimensional framework of K6MS with labeling of the K+ ions. Sn, red; Zn, blue-grey; S, yellow.
Fig. 25. Plot of the void space of K6MS with labeling of the three different types of pores and indication of the sizes of the cavities.
3.1.1. Cs+ ion-exchange properties
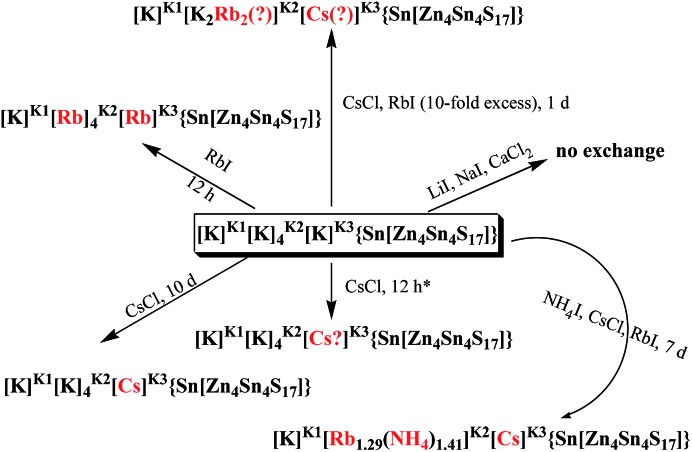
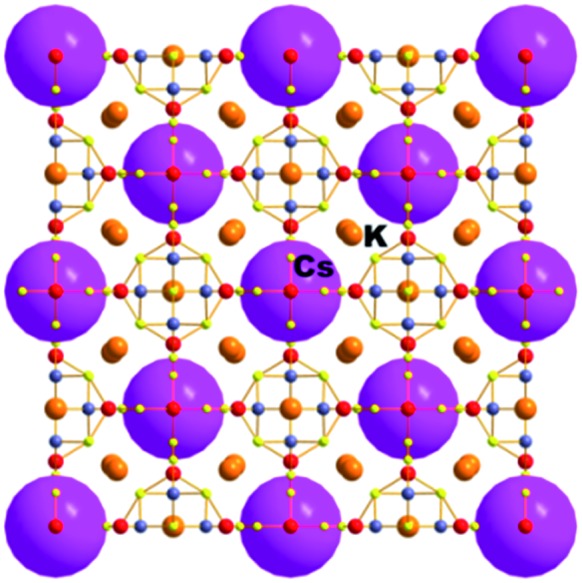
K6MS shows interesting Cs+ ion-exchange properties, which may be observed with either polycrystalline samples or single crystals of the material (SCSC transformation).13b Reaction of K6MS with CsCl for ∼12 h gives K5CsSn5Zn4S17. The SCSC Cs+ ion-exchange reaction resulted in the isolation of single crystals of the Cs+-exchanged product. Determination of its crystal structure revealed the presence of one Cs+ ion in the place of K3+ of K6MS (Fig. 26). For comparison, the corresponding SCSC Rb+ ion exchange led to the isolation of a product with 5 Rb+ replacing K2+ and K3+ ions (only K1+ remained intact). Competitive Rb+/Cs+ ion-exchange reactions using a large excess of Rb+ (Rb : Cs molar ratio = 10 : 1) yielded a material with three K+, two Rb+ and one Cs+ according to analytical data (the crystal structure was not determined). Despite the high concentration of Rb+ related to that of Cs+, the material still absorbs one Cs+ per formula unit, thus indicating its strong selectivity for this ion.
Fig. 26. Representation of the structure of K5CsSn[Zn4Sn4S17]. Sn, red; Zn, blue-gray; S, yellow.
An SCSC exchange experiment was also performed in the simultaneous presence of Cs+, Rb+ and NH4+ in equimolar concentrations. The results revealed that the exchange product contained only K+ in its K1 cavity, the K2 cavity hosted a mixture of Rb+/NH4+ and the K3 cavity was filled exclusively with Cs+ (Fig. 27). Therefore, the K3 cavity seems to be suitably sized for Cs+, which explains the selectivity of K6MS for this ion. Interestingly, K6MS shows absolutely no exchange capacity for Li+, Na+ and Ca2+ because the large hydration sphere of these ions prevents them from entering the framework. The ion-exchange reactions for K6MS are summarized in Scheme 1.
Fig. 27. Representation of the structure of KCsRb1.29(NH4)2.71Sn[Zn4Sn4S17]. Sn, red; Zn, blue-gray; S, yellow.
Scheme 1. Representation of the ion-exchange reactions for K6MS.
Encouraged by the above excellent Cs+ exchange results, more detailed batch ion-exchange studies have been performed.42 Ion-exchange isotherm data, which fit the Langmuir model (Fig. 28A), reveal a maximum Cs+ ion-exchange capacity of 66 ± 4 mg g–1, corresponding to 0.81 ± 0.06 mole of Cs+ per formula unit, i.e. close to the expected maximum Cs+ capacity (1 mole per formula unit).
Fig. 28. (A) Isotherm Cs+ sorption data for K6MS. The solid line represents the fitting of the data with the Langmuir model (qm = 66 ± 4 mg g–1; b = 0.17 ± 0.05 L mg–1, R2 = 0.89). (B) The distribution coefficient Kd for Cs+ ion exchange versus the Na+ : Cs+ molar ratio. (C) The distribution coefficient Kd for Cs+ ion exchange versus the Na+ : Cs+ molar ratio. (D) The logarithm values of the distribution coefficient Kd for Cs+ ion exchange versus the pH.
The sorption of low concentration (∼1 ppm) Cs+ in the presence of extremely high concentrations of Na+ (0.4 to 5 M) is exceptional. Specifically, >95% Cs+ sorption and large distribution coefficients Kd in the range of 104 to 105 mL g–1 were estimated, even with 1.3 to 6.5 × 105-fold excesses of Na+ (Fig. 28B). Surprisingly, an enhancement of the Cs+ sorption by K6MS was observed upon increasing the Na+ concentration.
Very high Ca2+ concentrations (0.1 to 1 M) partially reduce the Cs+ sorption capacity of K6MS. High Cs+ removal capacity (∼68%) and distribution coefficients above 1000 mL g–1 were observed even in the presence of a 105-fold excess of Ca2+ (Fig. 28C).
K6MS also exhibits excellent affinity and selectivity for Cs+, showing Kd values ≥104 mL g–1 within a very wide pH range (3 to 12), Fig. 28D. These data indicate that K6MS may be effective for Cs+ decontamination for both alkaline and acidic waste solutions. The above results confirm the exceptional selectivity of K6MS for Cs+, which results from the perfect fit of the K3 cavity for Cs+ (see above).
3.1.2. NH4+ ion-exchange properties
This material also exhibited very interesting NH4+ exchange properties.13b Treating single crystals of compound K6MS with NH4I for 1 week resulted in the isolation of an exchange product with 5 NH4+ and only one K+ per formula unit. Surprisingly, NH4+ replaced all K+ except the highly disordered K3+ ion. It is remarkable that NH4+ can diffuse even through the very narrow passages connecting the K1 and K3 cavities. This highly unusual NH4+ exchange capability of K6MS is attributed to the flexibility of the framework of this material. The M–S–M′ angles in K6MS are relatively small (∼110°), which facilitates breathing phenomena and, thus, the movement of ions through the tunnel network. This behavior of K6MS is in marked contrast to that of zeolites, which are characterized by wide Al–O–Si angles (160 to 180°) and do not favor swelling processes to the same degree.
3.1.3. Heavy metal ion sorption properties
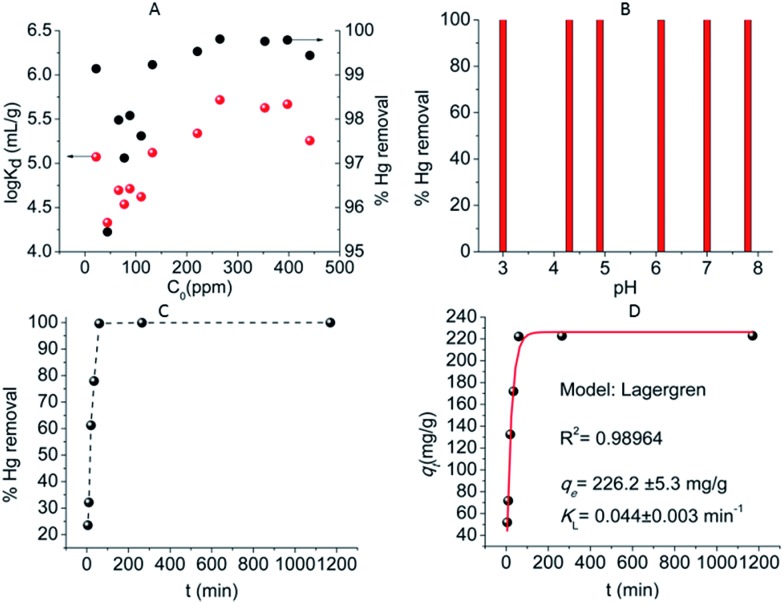
K6MS selectively absorbs soft metal ions.43 Hg2+ exchange experiments with various initial Hg2+ concentrations indicated very high removal capacities (95–100%) and Kd values of up to 2 × 106 mL g–1 (Fig. 29A). In addition, experiments at various pH values (3 to 8) revealed no effect of the pH on the Hg2+ sorption, which reaches almost 100% removal capacity over the whole pH range tested (Fig. 29B). The investigation of kinetics for the Hg2+ ion exchange (initial Hg2+ concentration ∼441 ppm, pH ∼ 5) revealed that equilibrium is reached within only one hour (Fig. 29C). Fitting of the kinetic data was performed with the Lagergren's equation describing first-order kinetics:
| qt = qe[1 – exp(–KLt)], | 15 |
where qe = the amount (mg g–1) of metal ion absorbed in equilibrium, KL = the Lagergren or first-order rate constant (min–1).12b The fitting (Fig. 29D) indicated a maximum Hg2+ sorption capacity of 226 ± 5 mg g–1 (for an initial Hg2+ concentration of 441 ppm) and a rate constant of 0.044 ± 0.003 min–1.
Fig. 29. (A) Representation of the % Hg removal and log Kd values versus the initial Hg2+ concentration for K6MS. (B) % Hg removal vs. pH (initial Hg2+ concentration = 441 ppm, V m–1 = 500 mL g–1). (C) % Hg removal vs. time (min) (initial Hg2+ concentration = 441 ppm, V m–1 = 500 mL g–1). (D) Fitting of the kinetic data with Lagergren's equation (red solid line).
Extremely high Na+ and Ca2+ concentrations (1 to 5 M) had no influence on the Hg2+ sorption by K6MS, and the Kd values obtained under these conditions were ≥106 mL g–1.
K6MS can efficiently remove a variety of other heavy metal ions, such as Pb2+, Cd2+, Ag+ and Tl+. Specifically, K6MS absorbed 98.7–99.7% each of Hg2+ (166 ppm), Pb2+ (67 ppm) and Cd2+ (40 ppm) from a solution containing all three ions. Furthermore, K6MS exhibited very high Kd values (up to 107 mL g–1) for Ag+ exchange.
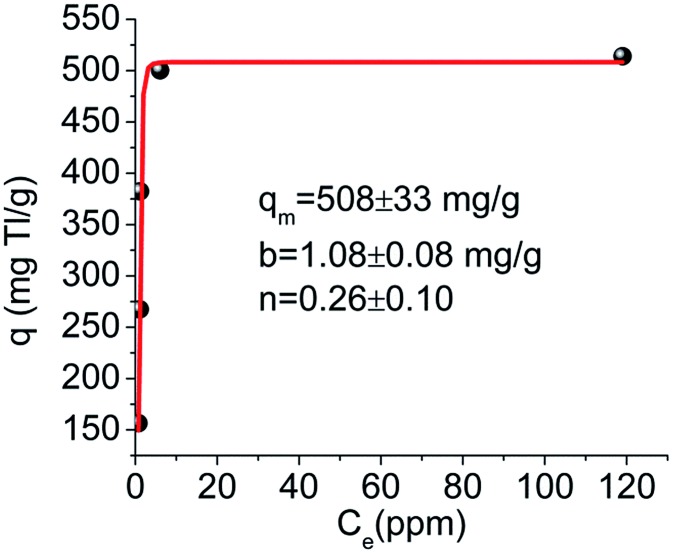
K6MS was also highly efficient for the removal of Tl+. This ion presents very high toxicity, and its removal from contaminated water resources is of high priority.44 Isotherm Tl+ exchange data (Fig. 30) for K6MS can be fitted with the Langmuir–Freundlich model:
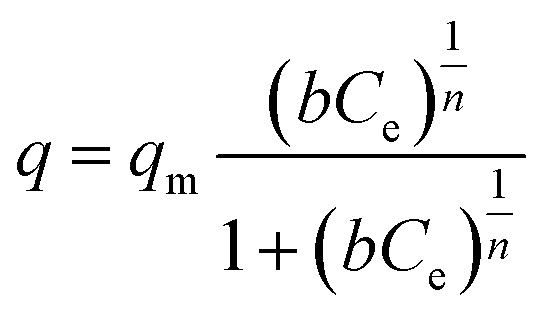 |
16 |
where n and b (L mg–1) are constants and qm (mg g–1) is the maximum sorption capacity at the equilibrium concentration Ce (ppm).13
Fig. 30. Isotherm Tl+ sorption data for K6MS (pH ∼ 7). The solid line represents the fitting of the data with the Langmuir Freundlich model.
The fitting indicated a maximum sorption capacity of 508 ± 33 mg Tl g–1, which is consistent with the absorption of ∼4 moles of Tl+ per formula unit of K6MS. Kd values for Tl+ exchange were found high (up to 2.2 × 105 mL g–1) revealing the high affinity of K6MS for Tl+.
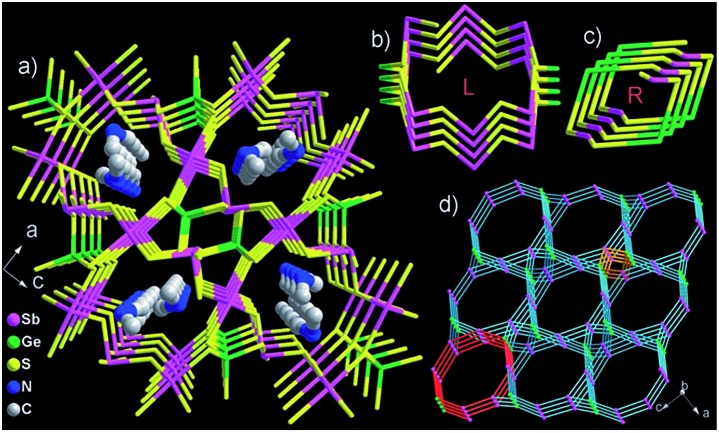
3.2. [(Me)2NH2]2[Sb2GeS6] (GeSbS-2)
This compound was isolated via a solvothermal reaction of GeO2, Sb and S in DMF.45 The structure is chiral and based on the interconnection of helical chains (Fig. 31A). Specifically, there are two types of chains: one chain is left-handed, formed by [Sb3S10] units and GeS4 tetrahedra (Fig. 31B). The second chain is right-handed constructed by GeS4 units linked with the middle trigonal bipyramidal SbS4 moieties (Fig. 31C). The Me2NH2+ ions are located in the pores forming N–H···S hydrogen bonds with the framework (Fig. 31A). From the topological point of view, the structure can be described as a 4-connected net [(32 × 104) net topology], with both GeS4 and SbS4 units acting as 4-connected nodes (Fig. 31D).
Fig. 31. (a) Structure of [(Me)2NH2]2[Sb2GeS6] along the b-axis. (b) The left-handed helical chain parallel to the b-axis. (c) The right-handed helical chain parallel to the b-axis. (d) The (32 × 104) net topology of the structure. The left-handed and right-handed helical chains are highlighted with red and orange, respectively. Reproduced with permission from ref. 45; © 2008, Wiley-VCH.
The compound shows ion-exchange properties for alkali metal ions. The Cs+ ion-exchange study indicated that ∼93% of the organic cations can be replaced with Cs+. The crystal structure of the Cs+-exchanged compound was also determined indicating topotactic ion-exchange. The material shows high selectivity for Cs+, as revealed by competitive Cs+–Na+ exchange studies. Thus, the ion-exchange reaction with a Na+ : Cs+ molar ratio of 10 afforded a product containing only Cs+.
In addition, ion-exchange with a mixture of Na+, K+, Rb+ and Cs+ in a ratio of 10 : 10 : 10 : 1 yielded an exchanged compound with no Na+ and K+, Rb+, Cs+ with a ratio of 1 : 4.6 : 6.3. Thus, despite the large excess of competitive ions, the compound shows high preference for Cs+. The ion-exchange selectivity of this compound is probably due to its microporous framework, which excludes ions with large hydration spheres such as Na+ and allows the entrance of ions with limited hydration shells, such as Cs+. MSIEs containing relatively rare elements such as Ge, In, and Ga are not cost-effective; however, their study contributes to our understanding of Cs+ and other ion capture processes.
3.3. Other crystalline three-dimensional metal sulfides
There are a number of additional MSIEs with 3-D structures for which some ion-exchange properties were reported. Characteristic examples are open framework compounds based on T2 (e.g. Ge4S104–) or T3 (e.g.In10S2010–) supertetrahedral units, which can exchange their extra-framework organic cations with alkali ions.46 Another example is K2Sn2S5 (KTS-2), which shows exchange capacity for Cs+, Sr2+, Pb2+, Cd2+ and Hg2+.36 However, no detailed ion-exchange studies have been performed for these materials.
In this review we focused on metal sulfides rather than selenides and tellurides, because the relatively high toxicity of selenium and tellurium makes them impractical for environmental applications. Nevertheless, it should be mentioned that metal selenides also exhibit interesting ion-exchange properties.47 Characteristic examples are (NH4)4In12Se2047a showing selective heavy metal ion sorption properties and (Cs6Cl)2Cs5[Ga15Ge9Se48]47b displaying both cation and anion exchange capacity.
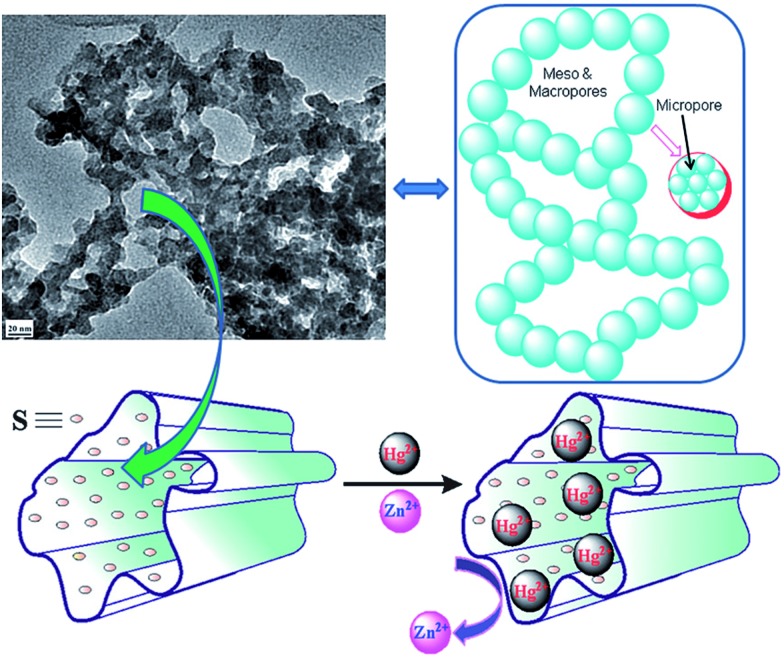
4. Chalcogels with sorption properties for heavy metal ions
Chalcogels are gels based on all chalcogenide frameworks (Fig. 32).48 They are usually prepared via a metathesis reaction involving anionic metal chalcogenide units (Fig. 32) and linking metal ions.
Fig. 32. (A) Different building blocks for the formation of chalcogels. (B) Monolithic gel composed of [Ge4S10]2– building blocks linked by Pt2+ ions before supercritical drying. (C) The chalcogel after supercritical drying. Reproduced with permission from ref. 48a © 2007, American Association for the Advancement of Science.
These are highly porous materials combining various interesting properties, such as catalytic activity, photoluminescence, selective gas adsorption, and sorption of organic and inorganic pollutants.48
Chalcogels have proven to be excellent sorbents for Hg2+.48a Specifically, chalcogen-1 which is composed of [Ge4S10]2– units linked by Pt2+ shows nearly 100% removal capacity for Hg2+ solutions. Kd values for Hg2+ sorption were found to be enormous (up to 107 mL g–1), revealing the potential of chalcogels as heavy metal ion sorbents. The chalcogels show preference for the softer ions, since competitive experiments with the simultaneous presence of Hg2+ and Zn2+ indicated only Hg2+ absorption.48dFig. 33 provides a schematic for the capture of heavy metal ions by chalcogels.
Fig. 33. Schematic for the selective capture of soft heavy metal ions by chalcogels.
Recently, metal polysulfide chalcogels with anionic frameworks and easily exchangeable cations have been reported. Examples of such materials are KCo6S21, K–Pt–Sx and (NH4)0.2MoS4.15 They show ion-exchange capacity, as demonstrated by the complete replacement of their extra-framework cations by Cs+. These materials, with a combination of significant porosity, soft polysulfide ligands and highly mobile cations, appear to be particularly promising as ion-exchange materials. Thus, further studies of their ion-exchange properties would be interesting.
5. Engineered forms-composites of MSIEs
As described above, almost all MSIEs have been tested for their ion exchange properties using the so-called batch (stirring) method. Many industrial and wastewater treatment processes, however, rely on the use of continuous bed flow ion-exchange columns. A material suitable for use as a stationary phase in ion-exchange columns should combine the following characteristics: (i) high sorption capacity and rapid ion-exchange kinetics for the targeted ion, (ii) proper particle size distribution to allow continuous flow through the column and achieve the smallest possible pressure drop of the water coming through the column and (iii) sufficient mechanical strength to tolerate high water pressures. Note that for column applications, the sorbent material should generally display a particle size close to 1 to 2 mm, a size resulting from a practical compromise between limiting the pressure drop and providing adequate surface area of the sorbent for sorption of the ions.49MSIEs are usually isolated as small size crystals (≤300 μm).13 Thus, these materials tend to form fine suspensions in water that may either pass through the column or result in column clogging. To overcome these limitations of MSIEs, new approaches are essential to produce engineered forms of these materials to satisfy the requirements of the column testing. Below, we present some results on this new research direction for MSIEs.
5.1. KMS-2-alginate composite
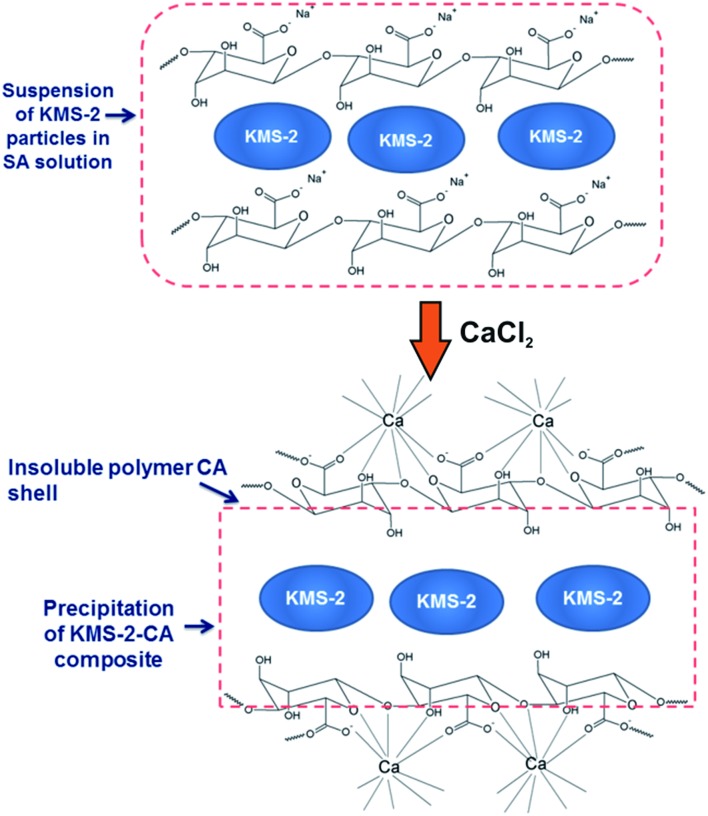
As described above, KMS-2 shows excellent batch ion-exchange properties; however, the small particle sizes (≤50 μm) preclude its use in ion-exchange columns. The alginate encapsulation method is a common way to produce materials with particles of specific shape and of suitable size for column applications.12b This method involves (a) addition of the sorbent to a water solution of sodium alginate (SA) so that the sorbent particles are enclosed by one or more monolayers of alginate-saturated water and (b) addition of CaCl2 to the SA-sorbent suspension, which results in the transformation of the alginate monolayer to a water-insoluble calcium alginate (CA) polymer encapsulating the sorbent particulates (Fig. 34).12b Thus, via the above method, paper-like KMS-2-CA composite can be prepared (Fig. 35).50 Note that only a small alginate content (∼4% wt) is needed to form the composite; thus, KMS-2-CA largely retains the ion-exchange properties of the pristine MSIE material, albeit with somewhat slower kinetics. In addition, the relatively large (mm-size) pieces of the KMS-2-CA composite are suitable for use in columns. To obtain, however, a more stable flow in the column and immobilize the KMS-2-CA particles, the composite was mixed with an inert material such as sand.
Fig. 34. Schematic process for the isolation of the KMS-2-CA composite material.
Fig. 35. (A) Pristine layered metal sulfide (KMS-2) with particle size ≤50 μm. (B) KMS-2-CA composite (particle size ≥ 1 mm). (C) Column made with a mixture of KMS-2/CA composite (particle size 1 to 2 mm) and sand (50 to 70 mesh).
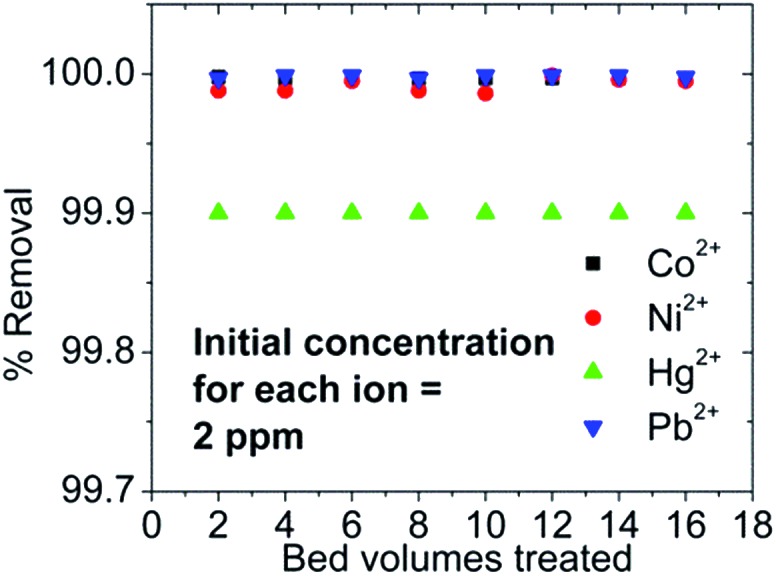
KMS-2-CA/sand (mass ratio 1 : 1) columns were tested for Ag+ ion-exchange. Nearly 100% Ag+ sorption (initial Ag concentration was 100 ppm) was observed for at least 80 bed volumes. Interestingly, the column is capable of simultaneous and almost 100% sorption of Co2+, Ni2+, Hg2+ and Pb2+ from a mixture of these ions (initial concentration ∼ 2 ppm for each of the ions) (Fig. 36). The final concentrations of all ions in the effluents were found to be well below the EPA acceptable limits.
Fig. 36. % removal of Co2+, Ni2+, Hg2+ and Pb2+vs. bed volume for the column ion exchange with a KMS-2-CA/sand column.
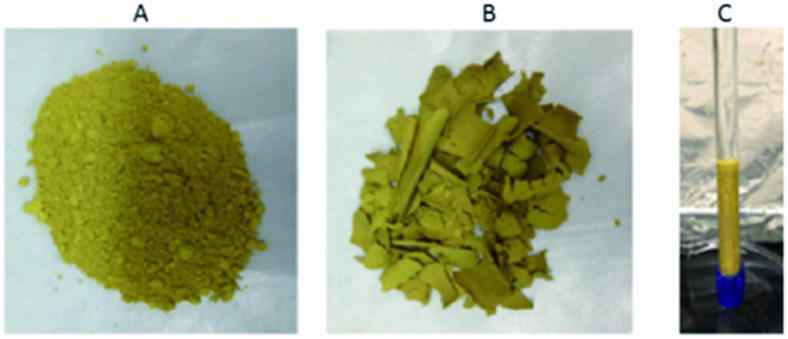
5.2. KMS-1-PAN composite
Polyacrylonitrile (PAN) is a binding polymer suitable for inorganic ion-exchangers. KMS-1-PAN beads (Fig. 37) were prepared by forming a suspension of KMS-1 and PAN in DMSO and adding this suspension to water, resulting in precipitation of the KMS-1-PAN composite.51 KMS-1-PAN composite samples with different KMS contents were prepared and tested for Cs+ ion-exchange with the batch method.
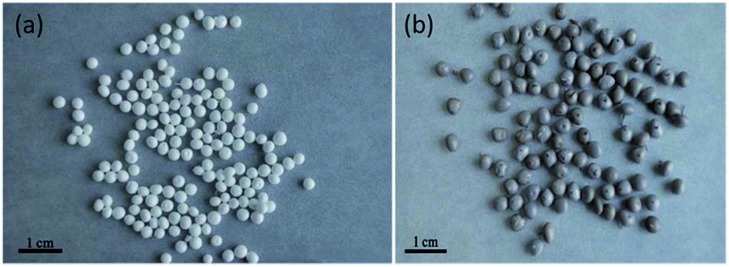
Fig. 37. Images of (a) PAN beads, (b) KMS-1-PAN composite beads.
A sample with ∼71% wt KMS-1 showed the optimum Cs+ absorption. The results revealed that the composite retains the properties of the pristine KMS-1 material in a significant degree, although the Cs+ ion exchange is much slower with the composite. Nevertheless, the separation of the KMS-1-PAN beads from the water solution is easily accomplished.
5.3. Porous amorphous MSIEs
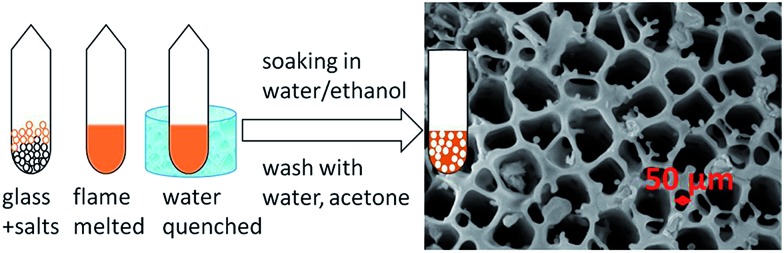
A different approach to produce an engineered form of MSIE consists of the synthesis of porous glassy materials that are melt-processable and, thus, can be made in any user defined shape and size. Porous amorphous sulfides were prepared by mixing inorganic salts with a presynthesized compound of the general formula A2Sn2SbS6 (A = K+ or Cs+), flame-melting the mixture, rapid quenching in water and liquid extraction of the salt (Fig. 38).52
Fig. 38. Procedure for the isolation of porous amorphous MS ion exchangers. Reproduced with permission from ref. 52 © 2015, American Chemical Society.
The materials with the composition Na2–xKxSn2SbS6 and Cs2–xKxSn2SbS6 were tested for Hg2+, Pb2+ and Cd2+ ion-exchange. Both materials perform excellently as ion-exchangers for these soft metal ions. 99.99% Hg2+ removal capacities and enormous Kd values of 6.2 × 107 to 7.2 × 108 mL g–1 were obtained with these ion exchangers. In addition, significant removal capacities (70–90%) were observed for Pb2+ and Cd2+. Considering that these materials are isolated as melts that can be cut in particles of specific size and shape, they may be appropriate for column testing. Column ion-exchange studies therefore would be interesting.
6. Comparison between MSIEs
Some ion-exchange characteristics of representative MSIEs are summarized in Table 1. It can be seen that all materials show highly efficient Cs+ ion exchange properties, with significant sorption capacities in a relatively wide pH range, rapid sorption kinetics and moderate to excellent selectivity for Cs+vs. Na+.
The highest capacity for Cs+ is shown by KMS-2;13i however, the most selective Cs+MSIE is K6MS, which exhibits molecular sieve properties excluding ions with large hydration spheres such as Na+ and Ca2+.13b At the same time, one of its cavities is an exact fit for Cs+; thus, the Kd values for Cs+ exchange are high (103 to 104 mL g–1) even with a ≥105-fold excess of Na+ or Ca2+.
The MSIE with the highest Sr2+ exchange capacity is KTS-3;13i however, the most selective sorbent is KMS-1, showing Kd ≥ 105 mL g–1 in the presence of a ∼1900-fold excess of Na+.13c KMS-1 also showed the highest Hg2+ sorption capacity;13e however, LHMS-1 is an exceptional sorbent for Hg2+, even under extremely acidic conditions (pH ≤ 0).13f K6MS was also studied for its Hg2+ ion exchange properties, and the results also indicated the exceptional capacity and selectivity of this material to absorb Hg2+.43 KMS-113e and its methylammonium analogue CMS23 show excellent selectivity for Pb2+ and Cd2+ even in the presence of a tremendous excess of Na+ or Ca2+. CMS exhibits higher Pb2+ and Cd2+ sorption capacities but somewhat slower sorption kinetics than KMS-1. KMS-1 also exhibits high affinity for Cu2+ ion in the presence of Na+ and Ca2+.
KMS-113g and KTS-313i show similar UO22+ exchange properties. Both materials exhibit very high UO22+ removal capacities which are only slightly affected by hard ions such as H+, Na+ or Ca2+.
Furthermore, KMS-2 has significantly higher Ni2+ sorption capacity13h than KMS-1; however, both materials display similar and exceptional selectivity for Ni2+ (Kd ≥ 105 mL g–1) in the presence of a ≥104-fold excess of Na+.
Finally, K6MS is the only MS exchanger investigated for its Tl+ sorption properties.43 The first results of these investigations revealed the high Tl+ sorption capacity of the material and very high Kd values for Tl+ exchange.
7. Comparison of MSIE with other sorbents
At this point, it will be useful to compare the metal ion sorption properties of MSIEs with those of other sorbents. The unique characteristic of MSIE materials is their soft Lewis basic frameworks, which favor stronger interactions with soft, borderline Lewis acids and the heavier alkali and alkaline earth metal ions, in contrast to the interactions favored by oxidic materials. In addition, typical hard ions, such as H+, Na+, and Ca2+, which strongly interact with oxides, have relatively low or negligible effect on the ion-exchange properties of MSIEs. Thus, MSIE materials are effective for ion-exchange in the pH range of 1 to 12, whereas typical oxidic sorbents are inactive for pH ≤ 3–4.9,10 Furthermore, as discussed above, MSIEs achieve very high metal ion removal capacities in the presence of high excesses of Na+ or Ca2+ (and, presumably, other ions such as Mg2+ and Al3+), which in relatively high concentration cause a drastic decrease of the sorption capacity of oxides.6,7
Comparing the MSIEs with sulfur-functionalized materials, it can be seen that both types of materials are highly efficient and selective for the sorption of heavy metal ions. However, MSIEs, e.g. the KMS layered materials, are effective for simultaneous removal of various toxic metal ions, such as Hg2+, Pb2+, Cd2+,Ni2+, Co2+, and UO22+, whereas thiol-containing mesoporous silica tends to absorb mainly Hg2+.11 Thus, MSIEs may find broader applications in the field of heavy metal ion remediation. The inexpensive hydrothermal synthesis of MSIEs is much more attractive than the multistep preparation of sulfur-functionalized materials requiring high cost organic reagents and solvents.11
Several studies also indicated that MSIEs are extremely stable in air and in both acidic (up to pH ∼ 1) and alkaline (up to pH ∼ 13 to 14) water. Their stability in water seems superior to that of silicates and aluminosilicates, which are soluble in both acidic (pH < 3) and alkaline (pH > 9) environments.8
A drawback of MSIEs is the lack of regeneration capability and reusability after being saturated with heavy metal ions. The very high capacities (up to 50% heavy metal by weight) can compensate for this non-regenerability. An exception to this rule is UO22+-loaded and Cs+ loaded MSIEs, which can be easily converted to pristine phases; the regenerated materials can be reused for Cs+ and UO22+ sorption.13d,g In some cases, MSIEs can often be regenerated after the Cs+ exchange process and reused with no loss of their exchange capacity.13d It is not always necessary, however, to regenerate a sorbent because this causes large amount of secondary liquid waste that needs to be stored. Solid waste occupies much less space, and if it is very stable, it can be properly buried without concern. The heavy metal ion-containing MSIEs can be probably considered as ultimate solid waste safe for disposal, since preliminary investigations indicated minimum leaching of heavy metals from these materials.13e,f,28
8. Conclusions and prospects
MSIEs represent a recently developed and growing class of ion-exchangers, which seems highly promising for environmental remediation applications. They combine various attractive features, such as potential for low cost synthesis, rapid sorption kinetics, high capacity and exceptional selectivity for toxic cations. At the same time, they do not require functionalization, since selectivity for soft ions is innate to these materials. Concerning the sorption of soft heavy metal ions, MSIEs outperform any other known material class. The sorption of heavy metal ions by MSIEs represents a textbook case of the Pearson's hard-soft-acid–base theory53 in action: Soft Lewis acids, such as Hg2+, Cd2+, and Pb2+, are preferentially absorbed by the soft basic (S2–-containing) framework of MSIEs. Waste minimization is one of the most pressing environmental issues currently facing society, and MSIEs by virtue of their high loading capacities could play a significant role in meeting this challenge.
Despite progress in the research on MSIEs, this class of ion-exchange materials remains largely unexplored. There are many known phases that may be excellent candidates as ion exchangers; however, their ion-exchange properties were overlooked in the past and were never studied in detail. These include some microporous46 and layered54 metal sulfide materials templated by organic cations, as well as various all-inorganic compounds with labile extra-framework alkali ions.41 Of course, exploratory synthesis may be also a fruitful source of MSIEs. The recent development of engineered forms and composites of MSIEs is an important milestone, as it forecasts good potential for many real-world applications in the field of wastewater treatment.
Finally, it would be challenging to develop metal sulfide materials that can capture toxic anionic species, such as dichromate, arsenate, and cyanate anions. A possible approach towards this challenge would be the intercalation of species into layered metal sulfide cation exchangers that will have high affinity for specific anions. Of course, the development of MSIEs with anion-exchange properties will open an entirely new research direction in the area of ion exchange materials. This task may require the development of cationic metal sulfides. The synthesis of such materials is feasible, as revealed by the recent reports of layered cationic metal chalcogenides.55 Moving forward, a wealth of research opportunities thus exist for MSIEs, and further progress in the understanding of these materials is anticipated in the next few years.
Acknowledgments
We thank the National Science Foundation for funding this work via Grant DMR-1410169. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Biographies

Manolis J. Manos
Dr Manolis J. Manos is an Assistant Professor of Inorganic Chemistry at the University of Ioannina, Greece. He completed his PhD (2003) at the University of Ioannina, under the supervision of Prof. T. Kabanos. He performed post-doctoral research in the group of Prof. M. G. Kanatzidis at Michigan State and Northwestern University (2004 to the end of 2008) and also in the group of Associate Prof. A. Tasiopoulos at the University of Cyprus (2009 to 2012). He was appointed as a lecturer on September 2012. His research interests are directed toward materials and composites with ion exchange and luminescence sensing properties.

Mercouri G. Kanatzidis
Mercouri Kanatzidis is a Professor of Chemistry and of Materials Science and Engineering at Northwestern University in Evanston, Illinois. He also has a joint appointment at Argonne National Laboratory. His interests include the design and synthesis of new materials, with emphasis on systems with highly unusual structural/physical characteristics or those capable of energy conversion, energy detection, environmental remediation, and catalysis. After obtaining a BSc from Aristotle University in Greece, he received his PhD in chemistry from the University of Iowa and was a post-doctoral research fellow at the University of Michigan and Northwestern University. He holds a Charles E. and Emma H. Morrison Professor Chair at Northwestern University.
References
- (a) Sarkar B., Heavy metals in the environment, Marcel Dekker Incorporated, 2002. [Google Scholar]; (b) Naja G. M. and Volesky B., Toxicity and Sources of Pb, Cd, Hg, Cr, As, and Radionuclides in the Environment, Heavy metals in the environment, CRC Press, Taylor & Francis Group, 2009. [Google Scholar]
- Tonini D. R., Gauvin D. A., Soffel R. W., Freeman W. P. Environ. Prog. 2003;22:167–173. [Google Scholar]
- (a) USA-EPA, http://www.epa.gov/mercury.; (b) European Union (Drinking Water) Regulations, 2014, http://www.irishstatutebook.ie/eli/2014/si/pdf/2014/en.si.2014.0122.pdf.
- (a) Hyman M. and Hladek K., Management of wastes from nuclear facilities, Handbook of complex environmental remediation problems, McGraw-Hill, 2001. [Google Scholar]; (b) Nash K. L. and Lumetta G. J., Advanced separation techniques for nuclear fuel reprocessing and radioactive waste treatment, Woodhead Publishing, 2011. [Google Scholar]; (c) Noyes R., Nuclear waste cleanup technology and opportunities, Elsevier, 1995. [Google Scholar]
- Zagorodni A. A., Ion Exchange Materials, Properties and Applications, Elsevier, 2007. [Google Scholar]
- Fan Q. H., Li Z., Zhao H. G., Jia Z. H., Xu J. Z., Wu W. S. Appl. Clay Sci. 2009;45:111–116. [Google Scholar]
- Benhammou A., Yaacoubi A., Nibou L., Tanouti B. J. Colloid Interface Sci. 2005;282:320–326. doi: 10.1016/j.jcis.2004.08.168. [DOI] [PubMed] [Google Scholar]
- Braun A., et al., Application of the Ion Exchange Process for the Treatment of Radioactive Waste and Management of Spent Ion Exchangers, International Atomic Energy Agency, Vienna, Tech Rep Ser No 408, 2002. [Google Scholar]
- Dyer A., Pillinger M., Harjula R., Amin S. J. Mater. Chem. 2000;10:1867–1861. [Google Scholar]
- Bortun A. I., Bortun L. N., Clearfield A. Solvent Extr. Ion Exch. 1997;15:909–929. [Google Scholar]
- (a) Mercier L., Pinnavaia T. J. Environ. Sci. Technol. 1998;32:2749–2754. [Google Scholar]; (b) Feng X., Fryxell G. E., Wang L. Q., Kim A. Y., Liu J., Kemner K. M. Science. 1997;276:923–926. [Google Scholar]
- (a) Howarth A. J., Katz M. J., Wang T. C., Platero-Prats A. E., Chapman K. W., Hupp J. T., Farha O. K. J. Am. Chem. Soc. 2015;137:7488–7494. doi: 10.1021/jacs.5b03904. [DOI] [PubMed] [Google Scholar]; (b) Rapti S., Pournara A., Sarma D., Papadas I. T., Armatas G. S., Tsipis A. C., Lazarides T., Kanatzidis M. G., Manos M. J. Chem. Sci. 2016;7:2427–2436. doi: 10.1039/c5sc03732h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Carboni M., Abney C. W., Liu S. B., Lin W. B. Chem. Sci. 2013;4:2396–2402. [Google Scholar]; (d) Yee K. K., Reimer N., Liu J., Cheng S. Y., Yiu S. M., Weber J., Stock N., Xu Z. T. J. Am. Chem. Soc. 2013;135:7795–7798. doi: 10.1021/ja400212k. [DOI] [PubMed] [Google Scholar]
- (a) Manos M. J., Iyer R. G., Quarez E., Liao J. H., Kanatzidis M. G. Angew. Chem., Int. Ed. 2005;44:3552–3555. doi: 10.1002/anie.200500214. [DOI] [PubMed] [Google Scholar]; (b) Manos M. J., Chrissafis K., Kanatzidis M. G. J. Am. Chem. Soc. 2006;128:8875–8883. doi: 10.1021/ja061342t. [DOI] [PubMed] [Google Scholar]; (c) Manos M. J., Ding N., Kanatzidis M. G. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3696–3699. doi: 10.1073/pnas.0711528105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Manos M. J., Kanatzidis M. G. J. Am. Chem. Soc. 2009;131:6599–6607. doi: 10.1021/ja900977p. [DOI] [PubMed] [Google Scholar]; (e) Manos M. J., Kanatzidis M. G. Chem.–Eur. J. 2009;15:4779–4784. doi: 10.1002/chem.200900353. [DOI] [PubMed] [Google Scholar]; (f) Manos M. J., Petkov V. G., Kanatzidis M. G. Adv. Funct. Mater. 2009;19:1087–1092. [Google Scholar]; (g) Manos M. J., Kanatzidis M. G. J. Am. Chem. Soc. 2012;134:16441–16446. doi: 10.1021/ja308028n. [DOI] [PubMed] [Google Scholar]; (h) Mertz J. L., Fard Z. H., Malliakas C. D., Manos M. J., Kanatzidis M. G. Chem. Mater. 2013;25:2116–2127. [Google Scholar]; (i) Sarma D., Malliakas C. D., Subrahmanyam K. S., Islam S. M., Kanatzidis M. G. Chem. Sci. 2016;7:1121–1132. doi: 10.1039/c5sc03040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard Z. H., Islam S. M., Kanatzidis M. G. Chem. Mater. 2015;27:6189–6192. [Google Scholar]
- (a) Oh Y., Morris C. D., Kanatzidis M. G. J. Am. Chem. Soc. 2012;134:14604–14608. doi: 10.1021/ja3061535. [DOI] [PubMed] [Google Scholar]; (b) Shafaei-Fallah M., He J., Rothenberger A., Kanatzidis M. G. J. Am. Chem. Soc. 2011;133:1200–1202. doi: 10.1021/ja1089028. [DOI] [PubMed] [Google Scholar]; (c) Subrahmanyam K. S., Malliakas C. D., Sarma D., Armatas G. S., Wu J. S., Kanatzidis M. G. J. Am. Chem. Soc. 2015;137:13943–13948. doi: 10.1021/jacs.5b09110. [DOI] [PubMed] [Google Scholar]
- Oriakhi C. O. and Lerner M. M., Layered Metal Chalcogenides, Handbook of Layered Materials, Marcel Dekker Incorporated, 2004, pp. 509–541. [Google Scholar]
- Schöllhorn R., Roer W., Wagner K. Monatsh. Chem. 1979;110:1147–1152. [Google Scholar]
- Gash A. E., Spain A. L., Dysleski L. M., Flaschenriem C. J., Kalaveshi A., Dorhout P. K., Strauss S. H. Environ. Sci. Technol. 1998;32:1007–1012. [Google Scholar]
- (a) Behrens E. A., Sylvester P., Clearfield A. Environ. Sci. Technol. 1998;32:101–107. [Google Scholar]; (b) Moller T., Harjula R., Pillinger M., Dyer A., Newton J., Tusa E., Amin S., Webb M., Araya A. J. Mater. Chem. 2001;11:1526–1532. [Google Scholar]; (c) Lehto J., Clearfield A. J. Radioanal. Nucl. Chem. 1987;118:1–13. [Google Scholar]; (d) Sylvester P., Behrens E. A., Graziano G. M., Clearfield A. Sep. Sci. Technol. 1999;34:1981–1992. [Google Scholar]; (e) DeFilippi I., Yates S., Sedath R., Straszewski M., Andren M., Gaita R. Sep. Sci. Technol. 1997;32:93–113. [Google Scholar]
- Dyer A., Pillinger M., Newton J., Harjula R., Moller T., Amin S. Chem. Mater. 2000;12:3798–3804. [Google Scholar]
- Nyman M., Tripathi A., Parise J. B., Maxwell R. S., Nenoff T. M. J. Am. Chem. Soc. 2002;124:1704–1713. doi: 10.1021/ja017081z. [DOI] [PubMed] [Google Scholar]
- (a) Du X., Zhang H., Hao X., Guan G., Abudula A. ACS Appl. Mater. Interfaces. 2014;6:9543–9549. doi: 10.1021/am501926u. [DOI] [PubMed] [Google Scholar]; (b) Keränen A., Leiviskä T., Salakka A., Tanskanen J. Desalin. Water Treat. 2015;53:2645–2654. [Google Scholar]; (c) Wołowicz A., Hubicki Z. Chem. Eng. J. 2012;197:493–508. [Google Scholar]
- Lia J. R., Wang X., Yuan B. L., Fu M. L., Cui H. J. Appl. Surf. Sci. 2014;320:112–119. [Google Scholar]
- Torma A. E. and Gundiler I. H., Precious and Rare Metal Technologies, Elsevier, Amsterdam, 1989, pp. 3–15. [Google Scholar]
- (a) Luna R. M., Lapidus G. T. Hydrometallurgy. 2000;56:171–188. [Google Scholar]; (b) Zhang Y., Fang Z., Muhammed M. Hydrometallurgy. 1997;46:251–269. [Google Scholar]
- Fard Z. H., Malliakas C. D., Mertz J. L., Kanatzidis M. G. Chem. Mater. 2015;27:1925–1928. [Google Scholar]
- (a) Chiarizia R., Horwitz E. P., Alexandratos S. D., Gula M. J. Sep. Sci. Technol. 1997;32:1–35. [Google Scholar]; (b) Sutorik A. C., Kanatzidis M. G. Polyhedron. 1997;16:3921–3927. [Google Scholar]
- USA-EPA, Table of Regulated Drinking Water Contaminants, http://www.epa.gov/your-drinking-water/table-regulated-drinking-water-contaminants.
- Li J. R., Wang X., Yuan B., Fu M. L. J. Mol. Liq. 2014;200:205–212. [Google Scholar]
- Sengupta P., Dudwadkar N. L., Vishwanadh B., Pulhani V., Rao R., Tripathi S. C., Dey G. K. J. Hazard. Mater. 2014;266:94–101. doi: 10.1016/j.jhazmat.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Nam A. M., Tavlarides L. L. Solvent Extr. Ion Exch. 2003;21:899. [Google Scholar]
- Parise J. B., Ko Y., Rijssenbeek J., Nellis D. M., Tan K., Koch S. J. Chem. Soc., Chem. Commun. 1994:527. [Google Scholar]
- Jiang T., Lough A., Ozin G. A., Bedard R. L., Broach R. J. Mater. Chem. 1998;8:721–732. [Google Scholar]
- Qi X. H., Du K. Z., Feng M. L., Li J. R., Du C. F., Zhang B., Huang X. Y. J. Mater. Chem. A. 2015;3:5665–5673. [Google Scholar]
- Marking G. A., Evain M., Petricek V., Kanatzidis M. G. J. Solid State Chem. 1998;141:17–28. [Google Scholar]
- Kanatzidis M. G., Mertz J. and Manos M. J., Chalcogenide compounds for the remediation of nuclear and heavy metal wastes, US Pat., US2011290735–A1, 2011.
- Ding N., Kanatzidis M. G. Chem. Mater. 2007;19:3867–3869. [Google Scholar]
- Ding N., Kanatzidis M. G. Nat. Chem. 2010;2:187–191. doi: 10.1038/nchem.519. [DOI] [PubMed] [Google Scholar]
- Zhang B., Feng M. L., Cui H. H., Du C. F., Qi X. H., Shen N. N., Huang X. Y. Inorg. Chem. 2015;54:8474–8481. doi: 10.1021/acs.inorgchem.5b01181. [DOI] [PubMed] [Google Scholar]
- (a) Ma S., Chen Q., Li H., Wang P., Islam S. M., Gu Q., Yang X., Kanatzidis M. G. J. Mater. Chem. A. 2014;2:10280–10289. [Google Scholar]; (b) Ma S., Huang L., Ma L., Shim Y., Islam S. M., Wang P., Zhao L. D., Wang S., Sun G., Yang X., Kanatzidis M. G. J. Am. Chem. Soc. 2015;137:3670–3677. doi: 10.1021/jacs.5b00762. [DOI] [PubMed] [Google Scholar]; (c) Ma L., Wang Q., Islam S. M., Liu Y., Ma S., Kanatzidis M. G. J. Am. Chem. Soc. 2016;138:2858–2866. doi: 10.1021/jacs.6b00110. [DOI] [PubMed] [Google Scholar]
- Kanatzidis M. G., Sutorik A. C. Prog. Inorg. Chem. 1995;43:151–265. [Google Scholar]
- Manos M. J. and Kanatzidis M. G., unpublished results.
- Kanatzidis M. G. and Manos M. J., Process for the removal of heavy metals using an open framework chalcogenide, US Pat., US20080145305–A1, 2008.
- (a) Blain R. and Kazantzis G., Thallium, Handbook on the toxicology of metals, Elsevier, 2014, pp. 1129–1240. [Google Scholar]; (b) Sager M. Toxicol. Environ. Chem. 1994;45:11–32. [Google Scholar]
- Feng M. L., Kong D. N., Xie Z. L., Huang X. Y. Angew. Chem., Int. Ed. 2008;47:8623–8626. doi: 10.1002/anie.200803406. [DOI] [PubMed] [Google Scholar]
- (a) Li H. L., Laine A., O'Keeffe M., Yaghi O. M. Science. 1999;283:1145–1147. doi: 10.1126/science.283.5405.1145. [DOI] [PubMed] [Google Scholar]; (b) Zheng N., Bu X., Wang B., Feng P. Science. 2002;298:2366–2369. doi: 10.1126/science.1078663. [DOI] [PubMed] [Google Scholar]; (c) Lin Q. P., Bu X. H., Mao C. Y., Zhao X., Sasan K., Feng P. Y. J. Am. Chem. Soc. 2015;137:6184–6187. doi: 10.1021/jacs.5b03550. [DOI] [PubMed] [Google Scholar]; (d) Sheldrick W. S., Wachhold M. Angew. Chem., Int. Ed. Engl. 1997;36:206–224. [Google Scholar]
- (a) Manos M. J., Malliakas C. D., Kanatzidis M. G. Chem.–Eur. J. 2007;13:51–58. doi: 10.1002/chem.200600892. [DOI] [PubMed] [Google Scholar]; (b) Huang-Fu S. X., Shen J. N., Lin H., Chen L., Wu L. M. Chem.–Eur. J. 2015;21:9809–9815. doi: 10.1002/chem.201405719. [DOI] [PubMed] [Google Scholar]; (c) Santner S., Heine J., Dehnen S., Angew. Chem., Int. Ed., 2016, 55 , 876 –893 , and references therein . [DOI] [PubMed] [Google Scholar]; (d) Trikalitis P. N., Rangan K. K., Bakas T., Kanatzidis M. G. J. Am. Chem. Soc. 2002;124:12255–12260. doi: 10.1021/ja026367g. [DOI] [PubMed] [Google Scholar]
- (a) Bag S., Trikalitis P. N., Chupas P. J., Armatas G. S., Kanatzidis M. G. Science. 2007;317:490–493. doi: 10.1126/science.1142535. [DOI] [PubMed] [Google Scholar]; (b) Yuhas B. D., Smeigh A. L., Samuel A. P. S., Shim Y., Bag S., Douvalis A. P., Wasielewski M. R., Kanatzidis M. G. J. Am. Chem. Soc. 2011;133:7252–7255. doi: 10.1021/ja111275t. [DOI] [PubMed] [Google Scholar]; (c) Bag S., Gaudette A. F., Bussell M. E., Kanatzidis M. G. Nat. Chem. 2009;1:217–224. doi: 10.1038/nchem.208. [DOI] [PubMed] [Google Scholar]; (d) Bag S., Arachchige I. U., Kanatzidis M. G. J. Mater. Chem. 2008;18:3628–3632. [Google Scholar]; (e) Mohanan J. L., Arachchige I. U., Brock S. L. Science. 2005;307:397–400. doi: 10.1126/science.1106525. [DOI] [PubMed] [Google Scholar]
- Inglezakis V. J. and Poulopoulos S. G., Adsorption, Ion Exchange and Catalysis. Design of Operations and Environmental Applications, Elsevier, 2006. [Google Scholar]
- Kanatzidis M. G., Sarma D. and Manos M. J., Column material for the capture of heavy metal and precious metal ions, US Pat., US20150144568–A1, 2015.
- Wang Y. X., Li J. R., Yang J. C. E., Yuan B., Fu M. L. RSC Adv. 2015;5:91431–91435. [Google Scholar]
- Fard Z. H., Islam S. M., Kanatzidis M. G. Chem. Mater. 2015;27:6189–6192. [Google Scholar]
- Pearson R. G. Science. 1966;151:172–177. doi: 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- (a) Xiong W. W., Miao J. W., Li P. Z., Zhao Y. L., Liu B., Zhang Q. C. CrystEngComm. 2014;16:5989–5992. [Google Scholar]; (b) Xiong W. W., Miao J. W., Ye K. Q., Wang Y., Liu B., Zhang Q. C. Angew. Chem., Int. Ed. 2015;54:546–550. doi: 10.1002/anie.201409653. [DOI] [PubMed] [Google Scholar]; (c) Zhang G. D., Li P. Z., Ding J. F., Liu Y., Xiong W. W., Nie L. N., Wu T., Zhao Y. L., Tok A. I. Y., Zhang Q. C. Inorg. Chem. 2014;53:10248–10256. doi: 10.1021/ic501282d. [DOI] [PubMed] [Google Scholar]; (d) Zhang Q. C., Bu X. H., Han L., Feng P. Y. Inorg. Chem. 2006;45:6684–6687. doi: 10.1021/ic060367q. [DOI] [PubMed] [Google Scholar]
- (a) Biswas K., Zhang Q. C., Chung I., Song J. H., Androulakis J., Freeman A. J., Kanatzidis M. G. J. Am. Chem. Soc. 2010;132:14760–14762. doi: 10.1021/ja107483g. [DOI] [PubMed] [Google Scholar]; (b) Biswas K., Chung I., Song J. H., Malliakas C. D., Freeman A. J., Kanatzidis M. G. Inorg. Chem. 2013;52:5657–5659. doi: 10.1021/ic400782c. [DOI] [PubMed] [Google Scholar]