Abstract
Reactive oxygen species play a key role in vascular disease, pulmonary hypertension, and hypoxic pulmonary vasoconstriction. We investigated contractile responses, intracellular Ca2+ ([Ca2+]i), Rho-kinase translocation, and phosphorylation of the regulatory subunit of myosin phosphatase (MYPT-1) and of myosin light chain (MLC20) in response to LY83583, a generator of superoxide anion, in small intrapulmonary arteries (IPA) of rat. LY83583 caused concentration-dependent constrictions in IPA and greatly enhanced submaximal PGF2α-mediated preconstriction. In small femoral or mesenteric arteries of rat, LY83583 alone was without effect, but it relaxed a PGF2α-mediated preconstriction. Constrictions in IPA were inhibited by superoxide dismutase and tempol, but not catalase, and were endothelium and guanylate cyclase independent. Constrictions were also inhibited by the Rho-kinase inhibitor Y27632 and the Src-family kinase inhibitor SU6656. LY83583 did not raise [Ca2+]i, but caused a Y27632-sensitive constriction in α-toxin-permeabilized IPA. LY83583 triggered translocation of Rho-kinase from the nucleus to the cytosol in pulmonary artery smooth muscle cells and enhanced phosphorylation of MYPT-1 at Thr-855 and of MLC20 at Ser-19 in IPA. This enhancement was inhibited by superoxide dismutase and abolished by Y27632. Hydrogen peroxide did not activate Rho-kinase. We conclude that in rat small pulmonary artery, superoxide triggers Rho-kinase-mediated Ca2+ sensitization and vasoconstriction independent of hydrogen peroxide.
Keywords: Rho-kinase, Hypoxia, Superoxide, Hydrogen peroxide, Pulmonary, Free radicals
Reactive oxygen species (ROS), which include the superoxide anion and hydrogen peroxide (H2O2), are important regulators of cell function in the vasculature and, when produced in excess (oxidative stress), contribute to endothelial dysfunction and proliferative diseases such as atherosclerosis, pulmonary hypertension, and right ventricular hypertrophy [1–4]. Superoxide is normally generated within the cell in response to agonists by oxidoreductase enzymes such as NADPH oxidase and xanthine oxidase [5–8] and in response to hypoxia by the mitochondrial respiratory chain [9,10]. Excessive superoxide can result from a deficiency in the expression or activity of the various antioxidant systems such as superoxide dismutase (SOD) and glutathione peroxidase and catalase [1]. This excess may cause uncoupling of endothelial nitric oxide synthase, inhibition of nitric oxide production, and production of yet more superoxide, along with other defects [1,3,11]. Both superoxide and H2O2 are modulators of vascular tone. Superoxide is usually procontractile in the pulmonary and other vascular beds [12–17], although it is not always clear whether it is acting directly or via its conversion to H2O2 [15]. H2O2 on the other hand has both proconstrictor and prorelaxant actions, depending on the type of vascular bed and experimental conditions used [16–21], but in our hands it constricts rat intrapulmonary artery [22].
The mechanisms underlying hypoxic pulmonary vasoconstriction (HPV), an adaptive process that optimizes pulmonary ventilation–perfusion matching in response to localized alveolar hypoxia, are not fully understood, but they involve changes in intracellular Ca2+ ([Ca2+]i) [10,23–25]; activation of protein kinases [26], particularly Rho-kinase [27,28]; and subsequent Ca2+ sensitization [29–31] and require an intact endothelium for a maximum and sustained response [32–34]. Several models have been proposed to explain how the pulmonary artery senses a reduction in oxygen tension. One of these suggests that hypoxia enhances production of superoxide from complex III of the mitochondrial electron transport chain [9,10]. It is generally assumed that superoxide is rapidly converted to H2O2 by mitochondrial Mn-SOD and cytosolic CuZn-SOD and that H2O2, and not superoxide, acts as the signaling moiety that initiates the resulting vasoconstriction [10]. Indeed, strong evidence has been presented that H2O2 is responsible for the elevation of [Ca2+]i in pulmonary artery smooth muscle cells during hypoxia [10,35]. Nevertheless, HPV has also been reported to be inhibited by SOD and enhanced by SOD antagonists [36,37], whereas, if H2O2 were the sole signaling moiety, one might expect SOD to be stimulatory and SOD antagonists inhibitory. However, the potential involvement of ROS in Rho-kinase-mediated Ca2+ sensitization in pulmonary artery remains to be investigated. It has been shown that exogenous superoxide generated by a xanthine/xanthine oxidase system activates the RhoA/Rho-kinase pathway in aorta [14], and cold-induced vasoconstriction of skin arteries is initiated by an increased production of mitochondrial ROS and subsequent activation of Rho-kinase [12].
In this study, we used LY83583, which generates intracellular superoxide by acting as a substrate for endogenous oxidoreductase enzymes [38], to investigate the mechanisms through which superoxide constricts small intrapulmonary arteries of rat. Our results have implications for the role of ROS in HPV and the wider field of vascular disease, particularly pulmonary hypertension, which is heavily influenced by Rho-kinase [39–41].
Materials and methods
Reagents and chemicals
Fura PE-3/AM was from Sigma (Poole, UK), dihydroethidium (DHE) and MitoSOX red were from Invitrogen (Paisley, UK), and the luminol derivative L-012 (8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H)dione) was from Wako Chemicals USA (USA). Hydrogen peroxide, antioxidants, LY83583, and other pharmacological agents were from Amersham (Buckinghamshire, UK), Biomol (Exeter, UK), Calbiochem (Merck Biosciences, Nottingham, UK), Fisher (Loughborough, UK), Invitrogen, Pierce (Cramlington, UK), or Sigma (Poole, UK). Antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Upstate (UK), or Cell Signalling (UK), as described previously [42].
Tissue isolation and cell culture
This study conformed with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996). Intrapulmonary (IPA; first- to third-order branches) and mesenteric or femoral arteries (second- or third-order) were obtained from male Wistar rats (200–250 g). Pulmonary artery smooth muscle cells (PASMC) were dispersed enzymatically and grown in DMEM with 10% FCS to passage 4 or 5. Cells were growth-arrested in serum-free medium for 24 h before experiments, as described previously [42].
Measurement of superoxide
PASMC were grown on coverslips or in 96-well plates, for measurement of superoxide production. Because it has been suggested that no single ROS indicator is completely reliable [43], we performed these experiments with three different superoxide indicators: DHE, MitoSOX red, and L-012. For DHE and MitoSOX red fluorescence measurement, PASMC were preloaded with the dyes in DMEM for 20 min (10 or 5 μmol/L for DHE or MitoSOX red, respectively) and then washed in medium before treatment with LY83583. Reactions were terminated by adding paraformaldehyde fixative. Intensity of staining within cells was taken as a measure of superoxide production. For L-012 luminosity measurement, PASMC were bathed in Hepes-buffered physiological salt solution and incubated with L-012 (10 μM) in the absence or presence of LY83583 (0–30 μM). Luminescence was measured with a luminometer (Chameleon; Hidex, Turku, Finland) after 30 min incubation. Measurements of L-012 luminescence for each LY83583 concentration in the absence of cells were subtracted as background.
Measurement of force, intracellular Ca2+, and α-toxin permeabilization
Isometric tension was measured using a wire myograph with IPA bathed in physiological salt solution (PSS), at 37°C, pH 7.4, as described previously [32,34,42]. When required, endothelium was removed by rubbing the artery lumen with a human hair. [Ca2+]i was measured in Fura PE-3-loaded, myograph-mounted IPA. Tension was recorded simultaneously with light emitted by the whole artery at >510 nm at excitation wavelengths of 340 and 380 nm. The ratio of the emission intensities (R340/380) was taken as a measure of [Ca2+]i as described previously [24,44]. For Ca2+-sensitization experiments, myograph-mounted IPA were permeabilized with α-toxin and Ca2+ clamped with 10 mmol/L EGTA, as described previously [42]. For the benefit of statistical analysis, the effects of inhibitors were evaluated by applying LY83583 twice to each artery: a 10-min control constriction followed by a second 10-min constriction after preincubation with inhibitor.
Rho-kinase translocation
PASMC were treated for 10 min in DMEM at 37°C. Fixed cells were permeabilized with Triton X-100 and stained overnight with anti-ROCK-2 primary antibody (1:100; Santa Cruz Biotechnology) at 4°C followed by incubation with Alexa Fluor 488-labeled secondary antibody (1:300; Santa Cruz) for 2 h at room temperature. ROCK-2 translocation was expressed as the ratio of cytosol/nucleus intensities, as described previously [42]. Note that ROCK-2 is the isoform of Rho-kinase most associated with phosphorylation of MYPT-1 and Ca2+ sensitization in smooth muscle [45].
Western blot
IPA were prepared, treated, and homogenized and protein was extracted for Western blot as described previously [42]. Membranes were blocked for 1 h with 5% skimmed milk and probed with primary antibody overnight at 4°C (1:1000–1:5000 in 1% milk), followed by horseradish peroxidase-conjugated anti-IgG secondary antibody for 1 h at room temperature (1:5000 in 1% milk). Membranes were first probed with anti-phospho-antibodies, then stripped for 1 h (Pierce stripping buffer), reblocked, and reprobed with corresponding anti-total antibodies.
Statistical analysis
Data were analyzed with SigmaStat (Systat Software, San Jose, CA, USA). Comparisons of the effects of treatments against control and of inhibitors against treatments were performed by one-way ANOVA with appropriate post hoc tests. Pair-wise comparisons were done by Student t test. A probability of p<0.05 was deemed significant.
Results
LY83583 generates superoxide in PASMC
LY83583 is known to generate intracellular superoxide by acting as a substrate for oxidoreductase enzymes within the cell, but may also generate superoxide outside the cell after intracellular modification [38]. In PASMC preloaded with the superoxide selective dye DHE (Figs. 1A–1D) or MitoSOX red (not shown), 10 μmol/L LY83583 caused enhancement of fluorescence, which was abolished by SOD, whereas SOD alone had no effect. LY83583-dependent superoxide generation in PASMC was also demonstrated with the extracellularly applied luminescent indicator L-012. Luminescence increased with increasing concentrations of LY83583, reaching a maximum at ~ 10 μmol/L with an apparent EC50 of 1.4 ± 0.2 μmol/L (Fig. 1E). These results confirm that LY83583 generates superoxide in PASMC and that this effect can be prevented by SOD. Consistent with the selectivity of DHE and MitoSOX red for superoxide, 30 μM H2O2 had no effect on fluorescence (not shown).
Fig. 1. Measurement of superoxide generation by LY83583 in pulmonary artery smooth muscle cells.
(A–C) Representative images of DHE-loaded PASMC, showing fluorescence under (A) control conditions, (B) 10 min exposure to 10 μmol/L LY83583, and (C) LY83583 after 30 min preincubation with 200 U/ml SOD. (D) Average effect of treatments in three or four cell lines. **p<0.001 vs control, ††p<0.001 vs LY83583 alone. SOD alone was without effect. Similar results were obtained in PASMC loaded with MitoSOX red (n = 4–6, not shown). (E) Superoxide measurement in PASMC with L-012 (10 μmol/L), showing concentration-dependent LY83583-mediated increases in luminescence (n = 5).
LY83583 causes constriction in IPA
Constrictor responses to LY83583 were examined in IPA of rat. Ten minutes of exposures to 10 μmol/L LY83583 induced sustained, reversible, and reproducible contractions (Fig. 2A). Reproducibility was confirmed in 20 IPA, in which there was no significant change in contraction amplitude between the first and the second 10-min exposures to LY83583 (first, 17 ± 3% of maximal constriction with 80 mM K+-substituted PSS (KPSS) vs second, 15 ± 2% KPSS). LY83583-induced constrictions were concentration-dependent, comparable to superoxide generation, reaching a maximum at ~ 10 μmol/L with an EC50 of 1.7 ± 0.2 μmol/L (Fig. 2B).
Fig. 2. Contractile responses to LY83583 in IPA.
(A) Example trace of contraction in IPA in response to repeated 10-min exposures to 10 μmol/L LY83583. (B) Concentration dose response to LY83583 in IPA (n = 6). (C and D) Cumulative effects of 0.1, 1.0, and 10 μmol/L LY83583 on IPA preconstricted with 5 μmol/L PGF2α (*p<0.05, **p<0.001 vs PGF2α, n = 3–15).
In the presence of a small degree of preconstriction with 5 μmol/L PGF2α (10–15% of KPSS), LY83583, at concentrations of 0.1 and 1 μmol/L, which alone caused only very small contractions in IPA (~ 1 and 5% of KPSS, respectively), induced much larger responses (at 1 μmol/L, these had mean amplitudes >50% of those evoked by KPSS, an apparent EC50 of between 0.1 and 1 μmol/L). These responses were slightly increased by raising the concentration of LY83583 to 10 μmol/L (Figs. 2C and 2D). Similar enhancements were observed when IPA were preconstricted with PSS containing 30 mmol/L K+ (30 mmol/L K+, 7 ± 2% of KPSS vs 30 mmol/L K+ + 1 μM LY83583, 39 ± 10% of KPSS, n = 3).
LY83583-mediated constriction in IPA is superoxide-dependent
Constrictions induced by 10 μmol/L LY83583 were almost abolished by the superoxide scavenger SOD (which converts superoxide into H2O2) and the membrane-permeant superoxide scavenger tempol (which removes superoxide by recombination), but were unaffected by catalase (which destroys H2O2) (Figs. 3A and 3B). SOD also nearly abolished the 1 μmol/L LY83583-mediated enhancement of the constriction to 5 μmol/L PGF2α, whereas catalase was without significant effect (Figs. 3C and 3D). SOD also inhibited the LY83583-mediated enhancement of a 30 mmol/L K+-induced preconstriction (LY83583 + PGF2α: 79 ± 5% block, p<0.01, n = 6; LY83583 + 30 mmol/L K+: 61 ± 8% block, p<0.05, n = 3). LY83583-induced contraction is therefore mediated by superoxide and not via dismutation of superoxide into H2O2 by endogenous SOD.
Fig. 3. Effects of antioxidants on LY83583-induced contraction in IPA.
(A) Example trace of the effect of 200 U/ml SOD on contraction induced by 10 μmol/L LY83583 and (B) average effects of SOD, tempol (3 mmol/L), and catalase (200 U/ml) on this contraction (**p<0.001 vs control, n = 5–7). There was no significant difference between the effects of SOD and tempol. (C) Example trace of the effect of SOD on constriction induced by 1 μmol/L LY83583 in the presence of 5 μmol/L PGF2α. (D) Average effects of SOD and catalase on this constriction (*p<0.05, **p<0.001 vs control, n = 6).
Contractile responses were also obtained in IPA when superoxide was instead generated with menadione (maximum response at ~ 10 μmol/L) or with the combination of hypoxanthine (10–40 μmol/L) and xanthine oxidase (100–400 mU/ml), and the xanthine/xanthine oxidase-mediated constriction was also abolished by tempol (Supplementary Figs. S1 and S2).
LY83583-mediated constriction in IPA is not via inhibition of guanylate cyclase
As well as being a generator of superoxide, LY83583 is well known as an inhibitor of soluble guanylate cyclase [46]. We therefore tested whether the constrictor actions of LY83583 in IPA were via this pathway. A high concentration of the structurally unrelated guanylate cyclase inhibitor ODQ (10 μM; EC50 ~ 20 nM [47]) did not itself cause any constriction of IPA and did not significantly alter the response to 10 μM LY83583 (Figs. 4A and 4B). On the other hand, like LY83583, ODQ did enhance PGF2α-mediated constriction (0.5 μM PGF2α), but in the presence of ODQ 1 μM LY83583 still caused further enhancement of this constriction (Figs. 4C and 4D). LY83583-mediated inhibition of guanylate cyclase does not therefore contribute significantly to the constriction caused by this drug in IPA.
Fig. 4. Effects of guanylate cyclase inhibitor ODQ on LY83583 and PGF2α-induced constriction in IPA.
(A) Example trace of the effect of ODQ (10 μmol/L) on constriction induced by 10 μmol/L LY83583. (B) ODQ did not significantly affect the size of the LY83583-induced constriction under these conditions (n = 6). (C) Example trace of the effect of ODQ on constriction induced by 1 μmol/L LY83583 in the presence of 0.5 μmol/L PGF2α. (D) Although it enhanced the PGF2α-induced constriction, ODQ did not significantly affect the size of the constriction induced by 1 μmol/L LY83583 under these conditions (n = 6).
We also eliminated the possibility that LY83583 was causing constriction via inhibition of endothelial NO production [3,13] or inhibition of glutathione reductase [48] or by an endogenous constrictor prostanoid produced as a result of cyclooxygenase stimulation [16]. As shown in Supplementary Fig. S3, neither endothelial removal, inhibition of e-NOS with L-NAME, nor inhibition of prostanoid TP receptors with SQ-29548 had any significant effect on LY83583-mediated constriction. The glutathione reductase inhibitor BCNU neither mimicked nor inhibited the effects of LY83583, but did enhance the LY83583-mediated constriction (Supplementary Fig. S3).
LY83583-mediated constriction in IPA is dependent on Rho-kinase and Src-family kinase and independent of changes in [Ca2+]i
Constrictions induced by 10 μmol/L LY83583 were abolished by the Rho-kinase inhibitor Y27632 (3 μmol/L) and partially blocked by SU6656 (30 μmol/L), an inhibitor of Src-family kinases (Figs. 5A and 5B). In confirmation of the role of superoxide in the above responses, vasoconstriction induced by hypoxanthine/xanthine oxidase (10 μmol/L and 100 mU/ml) was also abolished by Y27632 (Supplementary Fig. S2).
Fig. 5. LY83583-mediated constriction in IPA is Rho-kinase- and Src-family-kinase-dependent and intracellular Ca2+-independent.
(A) Example trace of the effect of 3 μmol/L Y27632 on contraction induced by 10 μmol/L LY83583. (B) Average effects of Y27632 and the Src-family kinase inhibitor SU6656 (30 μmol/L) on this constriction (**p<0.001 vs control, *p<0.01 vs control, n = 4–7). Y27632 was also significantly more effective than SU6656 (†p<0.01). (C) Example traces showing lack of change in [Ca2+]i induced by 10 μmol/L LY83583 in Fura PE-3-loaded IPA (typical of 10 others, as measured by the ratio of emission intensity at >510 nm from excitation wavelengths of 340 and 380 nm; upper trace) and simultaneous tension measurement (lower trace) Response to KPSS (maximum is off scale) is included to verify successful loading of tissue with Fura PE-3. (D) LY83583-induced constriction (10 μmol/L) with [Ca2+]i clamped at pCa 6.9 after permeabilization with α-toxin and subsequent reversal with 3 μmol/L Y27632. Average responses in six arteries (*p<0.01 vs pCa 6.9; ††p<0.001 vs LY83583 alone).
The effectiveness of Y27632 against LY83583-induced constriction led us to explore the possibility that superoxide was acting upon a Rho-kinase-mediated Ca2+-sensitization pathway. Fig. 5C shows that in Fura PE-3-loaded IPA, 10 μmol/L LY83583 did not cause any measurable increase in [Ca2+]i. Similar results were obtained in 10 other experiments. To corroborate this observation, we performed additional constriction experiments in α-toxin-permeabilized IPA, wherein [Ca2+]i was clamped at pCa 6.9, and in the presence of cyclopiazonic acid to eliminate the involvement of Ca2+ release from intracellular stores. Under these conditions, LY83583 caused constriction and this was strongly inhibited by Y27632 (Fig. 5D).
LY83583 activates Rho-kinase and induces superoxide-dependent and Rho-kinase-mediated phosphorylation of MYPT-1 and MLC20 in IPA
To investigate further the role of Rho-kinase in the LY83583-mediated constriction we examined the effects of LY83583 on the translocation of ROCK-2 (Rho-kinase), an indicator of Rho-kinase activation, and on the phosphorylation of MYPT-1 and MLC20, the principle pathway through which Rho-kinase mediates Ca2+ sensitization [29–31]. LY83583 (10 μmol/L) caused the translocation of ROCK-2 from nuclei to cytoplasm in PASMC. H2O2 on the other hand did not induce ROCK-2 translocation (Fig. 6). The effect of LY83583 was comparable in magnitude to that observed with a high concentration of PGF2α (not shown, but which we have described previously [42]).
Fig. 6. Effect of LY83583 on ROCK-2 translocation.
(A–C) Representative images of PASMC stained for ROCK-2 under control conditions (A) and after 10 min treatment with 10 μmol/L LY83583 (B) or 30 μmol/L H2O2 (C). (D) Average degree of translocation expressed as a ratio of nuclear/cytosolic staining intensities in cell lines derived from three to eight rats (**p<0.01 vs control).
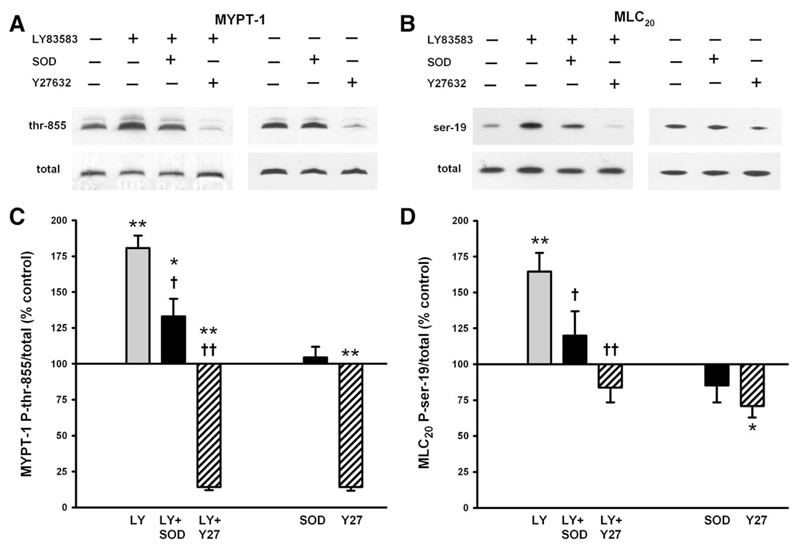
In IPA, the phosphorylation of MYPT-1 at Thr-855 (equivalent to Thr-850 in humans), a target of Rho-kinase [31], and of MLC20 at Ser-19, the principle regulator of cross-bridge cycling, was enhanced by 10 μmol/L LY83583. This enhancement was significantly blocked by SOD and abolished by Y27632. Basal phosphorylation of MYPT-1 was almost abolished by Y27632, as shown previously [42], but unaffected by SOD (Figs. 7A and 7C). Basal phosphorylation of MLC20, however, was only partially reduced by Y27632 and not significantly affected by SOD (Figs. 7B and 7D).
Fig. 7. Effects of LY83583 on phosphorylation of MYPT-1 and MLC20.
(A and B) Representative Western blots of the effects of 10 μmol/L LY83583 in the presence or not of 200 U/ml SOD or 3 μmol/L Y27632 and of SOD and Y27632 alone on (A) MYPT-1 phosphorylation at Thr-855 or (B) MLC20 phosphorylation at Ser-19. (C and D) Average effects of LY83583 alone (n = 13), LY83583 plus SOD (n = 11), LY83583 plus Y27632 (n = 7), SOD alone (n = 15), and Y27632 alone (n = 9), expressed as a percentage of control (untreated) phosphorylation of (C) MYPT-1 and (D) MLC20. *p<0.05 vs control, **p<0.01 vs control, †p<0.05 vs LY83583 alone, ††p<0.001 vs LY83583 alone.
LY83583 also significantly enhanced MYPT-1 and MLC20 phosphorylation at 1 μmol/L, albeit to a lesser extent than 10 μmol/L, and when combined with 5 μmol/L PGF2α, produced an enhancement of MYPT-1 phosphorylation that was similar to the sum of the two separate stimuli (LY83583 alone, 26 ± 14% increase, n = 6; PGF2α alone, 29 ± 7% increase, n = 13; LY83583 + PGF2α, 66 ± 16% increase, n = 7). On the other hand, the effect of the combined actions of 1 μmol/L LY82583 and 5 μmol/L PGF2α on MLC20 phosphorylation was equivalent to almost twice that of the combined actions of 1 μmol/L LY83583 and 5 μmol/L PGF2α alone (LY83583 alone, 30 ± 13% increase, n = 6; PGF2α alone, 25 ± 8% increase, n = 13; LY83583 + PGF2α, 104 ± 18% increase, n = 7). This observation corroborates the apparent synergistic effect of 1 μmol/L LY83583 and 5 μmol/L PGF2α on contraction (as shown in Figs. 2C and 2D).
LY83583-mediated constriction and relaxation in systemic arteries
In contrast to IPA, mesenteric arteries were completely unresponsive to 10 μmol/L LY83583, whereas femoral arteries produced constrictions approximately one-tenth the relative size of those in IPA (Figs. 8A and 8B). In the presence of a small 5 μmol/L PGF2α-induced preconstriction, 1 μmol/L LY83583 did cause significant constrictions in both femoral and mesenteric arteries but these were still relatively small compared to IPA and generally transient. In addition, and in marked contrast to IPA, raising the LY83583 concentration to 10 μmol/L relaxed both types of artery, such that the tension fell to a level significantly below that of the underlying PGF2α constriction (Figs. 8C and 8D). To determine whether these differences between IPA and systemic arteries were due to differential metabolism of superoxide and possible relaxing effects of H2O2 generated by endogenous SOD [49], we repeated these experiments in the presence of catalase. Catalase slightly enhanced constriction to 10 μmol/L LY83583 in femoral artery and allowed a very small but significant constriction in mesenteric artery (Fig. 8B) and also significantly enhanced the response to 1 μmol/L LY83583 in the presence of PGF2α in both artery types (Fig. 8D). However, catalase did not alter the relaxation caused by raising the concentration of LY83583 to 10 μmol/L (Fig. 8D).
Fig. 8. Constrictor and relaxant effects of LY83583 in mesenteric and femoral arteries.
(A) Example trace showing the lack of effect of 10 μmol/L LY83583 in mesenteric artery. (B) Average constrictor effect of 10 μmol/L LY83583 in mesenteric and femoral arteries in the absence and presence of catalase (200 U/ml; *p<0.05, n = 7 or 8). (C) Example trace of cumulative effects of 1 and 10 μmol/L LY83583 on mesenteric artery preconstricted with 5 μmol/L PGF2α. (D) Average constrictor effects of LY83583 in femoral and mesenteric arteries in the absence and presence of catalase (†p<0.05 for 10 μmol/L vs 1 μmol/L LY83583; *p<0.05 for presence vs absence of catalase, n = 9 or 10). Note: The axis in (D) is scaled the same as in Fig. 2D, for comparison, but the axis in (B) is scaled down for the sake of clarity.
Discussion
The results of this study clearly show that the superoxide generating molecule LY83583 (at > 1 μmol/L), causes a sustained, reproducible, SOD- and Y27632-inhibitable constriction in small pulmonary arteries that does not require a change in [Ca2+]i and is independent of the guanylate cyclase inhibitory action of this drug [46]. Our results show that LY83583-derived superoxide activates Rho-kinase, which phosphorylates MYPT-1, the regulatory subunit of myosin phosphatase, resulting in enhanced phosphorylation of the 20-kDa subunit of myosin light chain. Thus, superoxide anion causes Ca2+ sensitization [29–31]. The fact that Y27632 almost abolished all MYPT-1 phosphorylation at Thr-855, both basal and stimulated, confirms the observation that Rho-kinase is solely responsible for phosphorylating this site [42,50] and is therefore being activated by superoxide. Basal phosphorylation of MLC20 was also partially blocked by Y27632, implying that Rho-kinase may contribute to resting tone in IPA, as we have previously suggested [42]. The fact that SOD affected only the LY83583-mediated increase in MYPT-1 phosphorylation and not basal phosphorylation suggests that basal endogenous superoxide levels are not sufficient to cause Ca2+ sensitization in this preparation.
It is of note that extracellularly applied (non-cell permeative) SOD was effective against LY83583-mediated superoxide production, constriction, and MYPT-1 phosphorylation. Because we are potentially measuring superoxide both intracellularly and extracellularly (using DHE or MitoSOX red and L-012, respectively), the immediate site of action of superoxide in this model is unclear. Furthermore, whether SOD is entering cells or it is dismuting superoxide solely at the cell surface is not known. However, regardless of the mechanism, our results clearly indicate that SOD is lowering the superoxide concentration in PASMC and inhibiting the actions of LY83583 in IPA. In addition, the cell-permeative SOD mimetic tempol also inhibited LY83583-mediated constrictor responses.
LY83583-mediated constriction in IPA was independent of inhibition of guanylate cyclase or glutathione reductase by LY83583 [46,48], either directly or via superoxide, because constriction to LY83583 persisted in the presence of the structurally unrelated inhibitors ODQ and BCNU, respectively, which did not themselves cause constriction. The LY83583-mediated constriction in IPA also did not depend on an intact endothelium and was therefore independent of NO production or endogenous prostanoid release, despite evidence suggesting that the harmful vasoconstrictor and proliferative effects of superoxide in the vasculature are due to NO scavenging and subsequent uncoupling of e-NOS by peroxynitrite [1,3,11]. An independence of the vasoconstrictor actions of superoxide from endothelial function has been observed in a model of pulmonary hypertension, using xanthine/xanthine oxidase as a source of exogenous superoxide [4].
Although we deliberately used a high concentration of LY83583 to characterize the pure superoxide-mediated constriction, there were also effects of lower concentrations of LY83583 when applied in combination with other stimuli. These responses were also blocked by SOD and independent of guanylate cyclase inhibition. When measuring constriction and MLC20 phosphorylation, we found an apparent synergy between 0.1 or 1 μmol/L LY83583 (which alone did not greatly raise tone) and either PGF2α (which both elevates [Ca2+]i and activates Rho-kinase [42,51]) or submaximal KCl (which principally elevates [Ca2+]i). The amount of superoxide generated within the cell required to trigger measurable constriction therefore depends on the level of submaximal activation of other pathways, particularly Ca2+ influx, which synergizes with Rho-kinase at the level of MLC20 phosphorylation. Low-level superoxide generation may also contribute to agonist-mediated constriction, probably via activation of oxidoreductase enzymes such as NADPH oxidase [5–8]. Indeed, in this study, PGF2α-mediated constriction was partially sensitive to SOD. Synergy between superoxide generation and other contractile pathways may also be of relevance to HPV, because this is also dependent on Rho-kinase [27,28] and is strongly potentiated by a degree of preconstriction with an agonist such as PGF2α [32].
Our observation that the LY83583-mediated constriction was also partially blocked by inhibitors of Src-family kinases (SU6656) is also of interest. We have recently shown that PGF2α-mediated constriction of rat IPA is dependent on activation of both Src-family kinases and Rho-kinase and that Src-family kinases are likely to be acting upstream of Rho-kinase activation [42]. We have also found that Src-family kinases are activated by hypoxia [52]. Src-family kinases may act as intermediaries between ROS and Rho-kinase, perhaps by phosphorylating guanine-exchange factors or guanine-dissociation inhibitors, thus affecting RhoA translocation and therefore Rho-kinase activation [53,54]. This may tie in with the recent paper by Jernigan et al. [55], who demonstrated enhanced activity of RhoA as well as enhanced MYPT-1 phosphorylation in IPA of chronically hypoxic rats, which was apparently driven by superoxide [55].
There are several models for how ROS are affected by hypoxia and how they may contribute to HPV and responses to chronic hypoxia. The “redox hypothesis” proposes that ROS production is depressed by hypoxia and that the reduced cytosolic redox state inhibits voltage-gated K+ channels, thus causing depolarization and Ca2+ influx [56]. In direct contrast, the “ROS hypothesis” maintains that mitochondrial ROS production is enhanced by hypoxia, leading to release of Ca2+ from intracellular stores and subsequent capacitative Ca2+ entry [9,10,57]. In the latter model, originally proposed by Waypa and Schumacker and colleagues [10], it is generally assumed that the constrictor response to hypoxia in pulmonary artery is entirely due to H2O2, partly because of the short half-life of superoxide and the high concentration of mitochondrial SOD, but also because several studies have shown that overexpression of catalase or glutathione peroxidase suppresses the hypoxic-mediated elevation of [Ca2+]i in PASMC [10,35]. Our findings that both superoxide and H2O2 [22] cause sustained vasoconstriction of IPA are consistent with the concept that an elevation of ROS initiates HPV, but not with the redox hypothesis. However, the facts that LY83583-mediated constriction and MYPT-1 phosphorylation were blocked by SOD and tempol but not by catalase show that the observed effects were direct actions of superoxide and not a result of H2O2 produced by endogenous SOD. In support of this, we also showed that exogenous H2O2 did not activate Rho-kinase. This is also consistent with our recent observations that H2O2 caused Y27632-insensitive constriction and did not stimulate MYPT-1 phosphorylation [22]. Indeed it has been shown that HPV is enhanced by a SOD inhibitor and blocked by SOD itself [36,37]. On the other hand, H2O2 does indeed elevate [Ca2+]i in IPA [22], whereas LY83583 apparently does not. We can speculate that mitochondrial superoxide drives Rho-kinase-mediated Ca2+ sensitization, whereas its product H2O2 drives the elevation in [Ca2+]i. Interestingly, H2O2 also does not enhance MLC20 phosphorylation [22], suggesting a non-myosin-based mechanism of contraction, perhaps via actin polymerization or non-myosin-based regulation of cross-bridge cycling [58].
The pulmonary circulation is the only vascular bed in the adult mammal that constricts in response to hypoxia. In the systemic circulations, including mesenteric and femoral, hypoxia causes relaxation of preconstricted arteries [32]. It is therefore of note that in femoral and mesenteric arteries, LY83583, at a concentration that caused constriction in IPA, either in the absence or in the presence of PGF2α, elicited no response in the absence of preconstriction and a contraction at low dose followed by relaxation at high dose when applied in the presence of PGF2α. Bae et al. have shown that the superoxide generator menadione inhibits relaxation at low dose and inhibits contraction at high dose in rat aorta [49]. They proposed that at low dosage, superoxide causes constriction, but at high dose, there is H2O2-dependent endothelium-independent relaxation. This is consistent with the idea of H2O2 being an endothelium-derived hyperpolarizing factor [18–20]. Our results with LY83583 and catalase in mesenteric and femoral arteries may be partly explained by this model. Catalase did indeed enhance LY83583-mediated constriction, particularly at 1 μmol/L LY83583 in the presence of PGF2α, but had no effect on these responses in IPA. However, catalase was ineffective against the relaxation induced by the higher concentration of LY83583. Whether this is due to poor penetration of catalase or an alternative superoxide-dependent relaxation pathway in systemic arteries remains to be determined.
In conclusion, we have shown that superoxide activates Rho-kinase, resulting in Ca2+ sensitization and constriction of rat IPA. Our results have implications for the roles of superoxide and H2O2 in HPV and for the wider field of vascular disease, particularly the vasoconstriction and vascular remodeling in pulmonary hypertension, in which both superoxide and Rho-kinase are involved [2,41].
Supplementary Material
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.freeradbiomed.2008.11.015.
Acknowledgments
This work was funded by the Wellcome Trust (078075) and British Heart Foundation (FS/06/003, PG/06/151/21995).
Abbreviations
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- HPV
hypoxic pulmonary vasoconstriction
- PASMC
pulmonary artery smooth muscle cells
- IPA
intrapulmonary artery
- PSS
physiological salt solution
- KPSS
K+-substituted physiological salt solution
- DHE
dihydroethidium
References
- [1].Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- [2].Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- [3].Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signaling. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- [4].Sun R, Wang A, Yan Y, Zhang H. The role of activated neutrophils and free radical in the pathogenesis of pulmonary hypertension. Chin Med Sci J. 1993;8:15–19. [PubMed] [Google Scholar]
- [5].Cogolludo A, Frazziano G, Cobeno L, Moreno L, Lodi F, Villamor E, Tamargo J, Perez-Vizcaino F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann N Y Acad Sci. 2006;1091:41–51. doi: 10.1196/annals.1378.053. [DOI] [PubMed] [Google Scholar]
- [6].Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens. 2005;18:81–87. doi: 10.1016/j.amjhyper.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [7].Kanie N, Kamata K. Contractile responses in spontaneously diabetic mice. I. Involvement of superoxide anion in enhanced contractile response of aorta to norepinephrine in C57BL/KsJ(db/db) mice. Gen Pharmacol. 2000;35:311–318. doi: 10.1016/s0306-3623(02)00115-5. [DOI] [PubMed] [Google Scholar]
- [8].Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- [9].Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- [10].Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- [11].Belik J, Jankov RP, Pan J, Tanswell AK. Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med. 2004;37:1384–1392. doi: 10.1016/j.freeradbiomed.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [12].Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2005;289:H243–H250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- [13].Jimenez-Altayo F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther. 2006;316:42–52. doi: 10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- [14].Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- [15].Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- [16].Ullrich V, Brune B, Hecker G, Schmidt KU, Mulsch A, Busse R. Physiological targets of superoxide anion and hydrogen peroxide in reperfusion injury. Free Radic Res Commun. 1989;7:265–274. doi: 10.3109/10715768909087951. [DOI] [PubMed] [Google Scholar]
- [17].Wiklund L, McGregor CG, Miller VM. Effects of prolonged exposure to oxygen-derived free radicals in canine pulmonary arteries. Am J Physiol. 1996;270:H2184–H2190. doi: 10.1152/ajpheart.1996.270.6.H2184. [DOI] [PubMed] [Google Scholar]
- [18].Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2003;285:H2345–H2354. doi: 10.1152/ajpheart.00458.2003. [DOI] [PubMed] [Google Scholar]
- [20].Shimokawa H, Matoba T. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pharmacol Res. 2004;49:543–549. doi: 10.1016/j.phrs.2003.10.016. [DOI] [PubMed] [Google Scholar]
- [21].Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- [22].Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- [23].Robertson TP, Aaronson PI, Ward JP. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- [24].Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525(Pt 3):669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- [26].Ward JP, Knock GA, Snetkov VA, Aaronson PI. Protein kinases in vascular smooth muscle tone—role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207–231. doi: 10.1016/j.pharmthera.2004.08.009. [DOI] [PubMed] [Google Scholar]
- [27].Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- [29].Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- [30].Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- [31].Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- [32].Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am J Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- [33].Liu Q, Sham JS, Shimoda LA, Sylvester JT. Hypoxic constriction of porcine distal pulmonary arteries: endothelium and endothelin dependence. Am J Physiol Lung Cell Mol Physiol. 2001;280:L856–L865. doi: 10.1152/ajplung.2001.280.5.L856. [DOI] [PubMed] [Google Scholar]
- [34].Robertson TP, Aaronson PI, Ward JP. Ca2+ sensitization during sustained hypoxic pulmonary vasoconstriction is endothelium dependent. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1121–L1126. doi: 10.1152/ajplung.00422.2002. [DOI] [PubMed] [Google Scholar]
- [35].Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Abdalla S, Will JA. Potentiation of the hypoxic contraction of guinea-pig isolated pulmonary arteries by two inhibitors of superoxide dismutase. Gen Pharmacol. 1995;26:785–792. doi: 10.1016/0306-3623(94)00245-i. [DOI] [PubMed] [Google Scholar]
- [37].Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- [38].Hasegawa T, Bando A, Tsuchiya K, Abe S, Okamoto M, Kirima K, Ueno S, Yoshizumi M, Houchi H, Tamaki T. Enzymatic and nonenzymatic formation of reactive oxygen species from 6-anilino-5,8-quinolinequinone. Biochim Biophys Acta. 2004;1670:19–27. doi: 10.1016/j.bbagen.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [39].Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- [40].McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van VT, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2008;294:L205–L213. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- [41].Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155:444–454. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Knock GA, Shaifta Y, Snetkov VA, Vowles B, Drndarski S, Ward JP, Aaronson PI. Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery. Cardiovasc Res. 2008;77:570–579. doi: 10.1093/cvr/cvm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- [44].Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kontos HA, Wei EP. Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583. Stroke. 1993;24:427–434. doi: 10.1161/01.str.24.3.427. [DOI] [PubMed] [Google Scholar]
- [47].Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- [48].Luond RM, McKie JH, Douglas KT, Dascombe MJ, Vale J. Inhibitors of glutathione reductase as potential antimalarial drugs: kinetic cooperativity and effect of dimethyl sulphoxide on inhibition kinetics. J Enzyme Inhib. 1998;13:327–345. doi: 10.3109/14756369809021479. [DOI] [PubMed] [Google Scholar]
- [49].Bae ON, Lim KM, Han JY, Jung BI, Lee JY, Noh JY, Chung SM, Lee MY, Lee JY, Chung JH. U-shaped dose response in vasomotor tone: a mixed result of heterogenic response of multiple cells to xenobiotics. Toxicol Sci. 2008;103:181–190. doi: 10.1093/toxsci/kfn023. [DOI] [PubMed] [Google Scholar]
- [50].Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Snetkov VA, Knock GA, Baxter L, Thomas GD, Ward JP, Aaronson PI. Mechanisms of the prostaglandin F2α-induced rise in [Ca2+]i in rat intrapulmonary arteries. J Physiol. 2006;571:147–163. doi: 10.1113/jphysiol.2005.101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Knock GA, Snetkov VA, Shaifta Y, Drndarski S, Ward JP, Aaronson PI. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc Res. 2008;80:453–462. doi: 10.1093/cvr/cvn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- [54].DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- [57].Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hai CM, Gu Z. Caldesmon phosphorylation in actin cytoskeletal remodeling. Eur J Cell Biol. 2006;85:305–309. doi: 10.1016/j.ejcb.2005.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.