Abstract
The transplantation of cranial neural crest (CNC) expressing GFP in Xenopus laevis has allowed researchers not only to assess CNC migration in vivo but also to address many other experimental questions. Coupled with loss- or gain-of-function experiments, this technique can be used to characterize the function of specific genes during CNC migration and differentiation. Although targeted injection can also be used to assess gene function during CNC migration, CNC transplantation allows one to answer specific questions, such as whether a gene’s function is tissue autonomous, cell autonomous, or exerted in the tissues surrounding the CNC. Here we describe a protocol for performing simple CNC grafts.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPES: Please see the end of this protocol for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Ethanol (70%)
H2O (reverse osmosis [RODI] or distilled [dH2O])
Lineage tracer of choice (e.g., 200 pg of GFP mRNA)
Modified Barth’s Saline (MBS) (1×) <R>
For grafting medium, use 1× MBS containing 50 μg/mL of gentamycin. For recovery medium, use 0.1× MBS containing 50 μg/mL of gentamycin. For imaging medium, use 0.1× MBS containing 50 μg/mL of gentamycin and 30 μg/mL of benzocaine.
Xenopus laevis embryos (at the one- or two-cell stage)
Embryos are staged according to Nieuwkoop and Faber (1967).
Equipment
Bridges
Cut coverslips into rectangles (3-mm ×1-cm) using a diamond pen.
Dissecting microscope, equipped with a gooseneck lighting system.
Embryo incubators at the desired temperature (for Xenopus laevis, between 15°C and 19°C)
Eyelash knife
Select a human eyelash with the desired thickness and curvature and thread it through a 23-gauge needle fitted on a 1-cc syringe. For safety purposes, the tip of the needle can be cut off with scissors before the threading. Fix the eyelash with a drop of nail polish or cyanoacrylate glue.
Forceps (blunt, to manipulate bridges)
Forceps (fine, to remove vitelline envelope)
Glass bead tool
Thin out the end of a Pasteur pipette under a benzene burner and melt the end into a ball roughly the size of gastrula-stage embryo (~2 mm).
Glass plate (see Step 19.iv)
Hair loop
Cut a human hair into 3-inch sections. Thread both ends of a section into a 23-gauge needle fitted on a 1-cc syringe. Push the loop into the needle until the desired stiffness is reached. Fix the hair with a drop of nail polish or cyanoacrylate glue.
Petri dishes (60-mm, plastic), coated with a 4-mm layer of 1% agarose.
Petri dishes (60-mm, plastic), coated with plasticine
Roll 2 tsp of plasticine (nondrying, toxin free, appropriate for young children) into a ball. Flatten it out into a plastic Petri dish by hand.
For time-lapse imaging (Step 19), choose plasticine with a low level of autofluorescence in the desired channel. White plasticine usually works best in most channels.
Transfer pipette (disposable plastic or glass) with an opening of ≥2 mm, for transferring embryos.
METHOD
Tissue graft and lineage tracing, including transplantation of tissue containing neural crest, have been routinely performed in urodele such as axolotl (Horstadius 1950; Hall and Horstadius 1988). In 1987, the first interspecies cranial neural crest grafts between Xenopus laevis and Xenopus borealis were reported (Sadaghiani and Thiebaud 1987). The first grafts of cranial neural crest (CNC) expressing GFP in Xenopus laevis were reported a decade later (Carl et al. 1999; Borchers et al. 2000).
The groups who originally grafted CNC used agarose-coated dishes during the extirpation and grafting steps, which allowed the embryos to move freely (Carl et al. 1999; Borchers et al. 2000). The protocol detailed below was modified to use plasticine-coated dishes instead (Alfandari et al. 2001). This offers the option of immobilizing the embryo during the transplantation procedure, which can be an advantage for many experimenters.
CNC grafting can be performed as soon as stage 14 and as late as stage 18; see Step 4.
The grafting procedure should be conducted between 15°C and 19°C.
- One or two days before grafting, inject embryos at the one- or two-cell stage with the lineage tracer of your choice (e.g., 200 pg of GFP mRNA).Embryos kept at 18°C can be grafted in the afternoon of the day after injection. Embryos raised at 14°C can be grafted 48 h after injection.
On the day of grafting, when the embryos reach stage 14, sterilize a plasticine-coated dish with 70% ethanol for 10 min. Rinse briefly with RODI H2O and then fill with grafting medium.
- Using a glass bead tool, dig two trenches corresponding to the length of the embryos. (The depth of the trench should correspond to about half of the embryo width.)The purpose of these trenches is to immobilize the embryos in a position that will allow the experimenter to dissect out and graft in the CNC.
- Select donor and host embryos between stages 14 and 15 and transfer the embryos to the plasticine-coated dish. Remove the vitelline envelope from each embryo using fine forceps. Ensure that the embryos do not breach the liquid surface once the vitelline envelope is removed.If one wishes to graft and observe the migration of all the CNC, stage 14 or 15 embryos are ideal as the segments (mandibular hyoid and branchial) have not yet formed and the entirety of the tissue can be grafted without losing any of the segments in the process. However, stage 14 CNC may be difficult to distinguish from the surrounding neuroepithelium and placodal ectoderm. In that case, CNC grafts can be performed at a later stage. However, by stage 17, the segments are already formed and the loss of a segment will likely occur during the grafting procedure.
- Move the donor and host embryos into the trenches. Orient the embryos according to the experimenter’s preference.The goal is to orient the embryos so that the CNC directly faces the experimenter. In Figure 1A, the embryo is positioned in a ¾ anterior view with the anterior neural plate slanted slightly to the left.Being able to recognize the location of the CNC before immobilizing the embryos is critical but sometimes difficult for the novice. The experimenter should be familiar with the Xenopus developmental table. The CNC makes up the bulk of the anterior neural plate borders. They appear as two bulges on each side of the anterior neural plate and can therefore be easily identified if the gooseneck guide lights are oriented in a manner that highlights the three-dimensional conformation of the embryo. In Figure 1A, the incident light highlights the CNC.
- Once the embryos are oriented, tighten the plasticine around them by pushing gently on the plasticine with the glass bead.This step is optional for experimenters that prefer performing microdissection on free-moving embryos.
- On the donor embryo, insert the tip of the eyelash knife under the ectoderm at the ventral and posterior end of the anterior neural fold (Movie 1). Thread the knife under the ectoderm, move it slightly anteriorly, and cut the ectoderm by lifting the knife swiftly. Repeat the motion anteriorly along the ventral edge of the anterior neural fold until the ventral edge of the CNC is completely uncovered (Movie 1).The goal of this step is to cut the ectoderm ventrally to the anterior neural fold in order to peel it dorsally (i.e., toward the neural plate) in Step 8. However, the experimenter may find it more convenient to cut off the ectoderm dorsally and peel it off ventrally.Cutting a section of ectoderm larger than the size of the CNC is recommended: The experimenter can better see the outline of the CNC and neural plate once the ectoderm is peeled, and the peeled ectoderm is easier to manipulate with the hair loop.
Peel off the ectoderm dorsally, to expose the underlying neural fold, and drape it over the other side of the embryo using the hair loop (Fig. 1B, Movie 1).
- Identify the location of the CNC (Sadaghiani and Thiebaud 1987). Using the knife tip, cut the CNC outline. Cut superficially to avoid damaging or contaminating the explant with the underlying mesoderm and endoderm (Movie 1).In some cases, the CNC inherits some of the pigment deposited in the egg during oogenesis, which makes it easy to distinguish from the neural plate and mesoderm. However, CNC are unpigmented the majority of the time. The experimenter should be familiar with landscape of the embryos and learn to recognize the bulge formed by the neural folds. Adequate lighting (described in Step 5) is critical.
- Lift the explant off the embryo with the knife and hair loop and inspect it for any contaminating mesoderm and endoderm (Fig. 1C). Scrape off contaminating cells with the hair loop and knife.CNC explants are made of small cells which typically give the tissue a blue hue. The large size and high yolk content of mesoderm and endoderm cells give them a white to yellow coloration.
- Place the donor explant adjacent to the host embryo. To move the explant, spear it on the eyelash knife, move it close to the host embryo and push it gently off the knife with the hair loop (Movie 1).This technique helps the experimenter to keep track of the antero–posterior and dorso–ventral orientation of the CNC.
Remove the host CNC by repeating Steps 7–10 on the host embryo. Replace the host CNC with the donor CNC (Fig. 1D, Movie 1). Ensure that orientation of the explant matches that of the host embryo (Movie 1).
Using the hair loop, pull the ectoderm back over the explant as far as possible (Fig. 1E, Movie 1).
Using blunt forceps, mount a fulcrum of plasticine next to the newly grafted embryo (Fig. 1F).
Using blunt forceps, install a cover glass bridge across the fulcrum (Fig. 1G).
Using blunt forceps, push gently down on the part of bridge that contacts the fulcrum. Stop pushing once the bridge touches the graft and slightly flattens it (Fig. 1H, Movie 1).
Allow the epidermal ectoderm to heal for a minimum of 10–20 min.
Remove the bridge with the blunt forceps and transfer the grafted embryo into an agarose-coated dish filled with recovery medium. Grow the grafted embryo between 15°C and 18°C.
-
Perform a lime-lapse movie of CNC migration.
- Transfer the embryo into a new plasticine-coated dish containing imaging medium. Ensure that the plasticine chosen has a low level of autofluorescence in the desired channel.The benzocaine in the imaging medium prevents somitic contraction once the embryos reach tailbud stage. It can be replaced with other anesthetics, such as tricain.
Using the glass bead tool, dig a trench in the plasticine as deep as the embryo is wide and as long as a stage 16 embryo. While making the depression, ensure that one end of the trench is slightly deeper than the other end.
- Move the embryo into the trench with its anterior side in the deepest part of the trench.Because gravity will compensate for the movement caused by the ectodermal cilia, the incline ensures that the embryo will move minimally during the movie.
To prevent optical distortions caused by evaporation, fill the dish with imaging medium until the liquid bulges over. Slide a glass cover over the dish (i.e., for a 60-mm dish, use an 8- × 10-cm glass plate used to cast SDS-PAGE gels). Ensure that no air is trapped below the plate.
Record a time-lapse movie between 18°C and 20°C, at the rate of one image every 3 min for 8–12 h (Movie 2).
FIGURE 1.
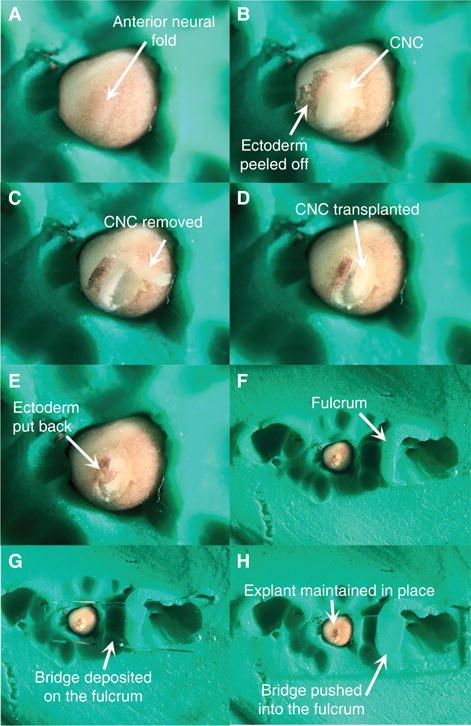
Key steps of the CNC transplantation procedure.
DISCUSSION
Respecting the polarity of the CNC in relation to the host yields the best results in terms of quality and timing of the migration. While CNC grafted with any of their polarity reversed will migrate, they may be delayed and the migration of individual segments may be perturbed (H Cousin, unpubl.).
The CNC grafting experiment is invaluable to assessing whether a gene’s function is cell autonomous, CNC autonomous, or exerted in the tissues surrounding the CNC (ectoderm, placode, or mesoderm). Grafting modified crest (i.e., in which the gene function has been perturbed) into a wild-type host and grafting wild-type CNC into a modified host allows the experimenter to assess whether the function of the studied gene is necessary in the CNC, the surrounding tissues, or both (Alfandari et al. 2001; Cousin et al. 2011, 2012). To assess whether the function of a particular gene is cell autonomous or tissue autonomous, one can co-graft unmodified and modified CNC that express different fluorescent markers (Cousin et al. 2012).
RECIPE
Modified Barth’s Saline (MBS) (1×)
| CaCl2 | 0.41 mM |
| CaNO3 | 0.3 mM |
| HEPES-NaOH | 15 mM |
| KCl | 1 mM |
| MgSO4 | 0.82 mM |
| NaCl | 88 mM |
| NaHCO3 | 2.4 mM |
| Adjust pH to 7.6. Store at room temperature for up to one month |
Supplementary Material
Acknowledgments
H.C. is supported by NIH/NIDCR DE025691.
References
- Alfandari D, Cousin H, Gaultier A, Smith K, White JM, Darribere T, DeSimone DW. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest- cell migration. Curr Biol. 2001;11:918–930. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Borchers A, Epperlein HH, Wedlich D. An assay system to study migratory behavior of cranial neural crest cells in Xenopus. Dev Genes Evol. 2000;210:217–222. doi: 10.1007/s004270050307. [DOI] [PubMed] [Google Scholar]
- Carl TF, Dufton C, Hanken J, Klymkowsky MW. Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev Biol. 1999;213:101–115. doi: 10.1006/dbio.1999.9320. [DOI] [PubMed] [Google Scholar]
- Cousin H, Abbruzzese G, Kerdavid E, Gaultier A, Alfandari D. Trans-location of the cytoplasmic domain of ADAM13 to the nucleus is essential for Calpain8-a expression and cranial neural crest cell migration. Dev Cell. 2011;20:256–263. doi: 10.1016/j.devcel.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin H, Abbruzzese G, McCusker C, Alfandari D. ADAM13 function is required in the 3 dimensional context of the embryo during cranial neural crest cell migration in Xenopus laevis. Dev Biol. 2012;368:335–344. doi: 10.1016/j.ydbio.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, Horstadius S. The neural crest. Oxford University Press; 1988. [Google Scholar]
- Horstadius S. The neural crest: Its properties and derivatives in the light of experimental research. Oxford University Press; 1950. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus Laevis (Daudin) 2nd. North-Holland; Amsterdam: 1967. [Google Scholar]
- Sadaghiani B, Thiebaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


