Significance
The arrival of humans and human-introduced species to Pacific islands resulted in significant, long-lasting transformations to local ecosystems. However, direct measurements of deep-time human effects can be difficult to quantify from archaeological datasets. Isotopically reconstructed diet of the Pacific rat (Rattus exulans), a commensal species introduced by humans during one of the most dramatic archaeological examples of past human migration and ecosystem alteration, can provide insight into resource availability and utilization within the human-commensal niche. Our results highlight significant long-term restructuring of nutrient flows through ecosystems resulting from human arrival and subsequent land use on three Polynesian islands. We also demonstrate that stable isotope analysis of often-ignored commensal taxa represents a tool for tracking human activities and ecosystem effects more broadly.
Keywords: stable isotopes, Pacific islands, commensalism, nutrient flows, Rattus exulans
Abstract
The role of humans in shaping local ecosystems is an increasing focus of archaeological research, yet researchers often lack an appropriate means of measuring past anthropogenic effects on local food webs and nutrient cycling. Stable isotope analysis of commensal animals provides an effective proxy for local human environments because these species are closely associated with human activities without being under direct human management. Such species are thus central to nutrient flows across a range of socionatural environments and can provide insight into how they intersected and transformed over time. Here we measure and compare stable carbon and nitrogen isotope data from Pacific rat (Rattus exulans) skeletal remains across three Polynesian island systems [Mangareva, Ua Huka (Marquesas), and the Polynesian Outlier of Tikopia] during one of the most significant cases of human migration and commensal introduction in prehistory. The results demonstrate widespread δ15N declines across these islands that are associated with human land use, intensification, and faunal community restructuring. Local comparison of rat stable isotope data also tracks human activities and resource availability at the level of the settlement. Our results highlight the large-scale restructuring of nutrient flows in island ecosystems that resulted from human colonization and ecosystem engineering activities on Pacific islands. They also demonstrate that stable isotope analysis of often-ignored commensal taxa can provide a tool for tracking human land use and environmental effects.
The long-term effects of human alteration of Earth systems have become a focal point of research, as humans are increasingly recognized as a force for global geological change (1). Archaeological and paleoecological datasets are now being used to highlight past human–environment interactions with widespread and lasting consequences for global ecosystems, including landscape modification (2), deforestation (3), species translocations (4), and human-influenced extinctions (5, 6). The identification of such ecosystem engineering processes in the archaeological record indicates a human capacity for inducing long-term ecological consequences as early as the Late Pleistocene (4, 7). However, although archaeologists and paleoecologists can discern broad-scale effects, the local-scale study of trophic ecology, food web disruptions, and thresholds of change witnessed in contemporary ecosystems are often elusive for historical and deep-time datasets (although see refs. 8 and 9).
Commensal species are closely associated with humans, entangled in human food webs, and deeply embedded in anthropogenic ecosystem engineering processes. In contrast to species that have been deliberately managed by humans, like domesticated pigs and chickens, commensal animals are unintentionally supported and transformed by the ever-expanding human niche. In particular, small rodents such as mice and rats have become widely distributed as a result of human activities. The close association between these species and people has allowed them to be used in studies of transoceanic human migrations (10), patterns of human mobility and trade (11, 12), and vector-borne diseases (13). However, the potential for commensal faunal remains from archaeological sites to provide data on resource flows within anthropogenically altered food webs has yet to be fully appreciated.
In the Pacific, processes of human dispersal and island colonization resulted in the translocation of a range of plant and animal species, including cultivars such as taro, breadfruit, and yams and domestic pig, dog, and chicken, across extraordinary geographic distances. Intensive agricultural and animal husbandry regimes centered around these translocated species, which included the use of fire in forest clearance and slash-and-burn agriculture, resulting in significant transformations to Pacific biomes (14). A combination of human predation, the introduction of new faunal predators and competitors, and habitat alteration led to the extirpation or extinction of a large component of the native Pacific island biota, including endemic forest, land birds and seabirds, and terrestrial gastropods and arthropods (15, 16). The nearly ubiquitous transport of the commensal Pacific rat (Rattus exulans) into virtually every island ecosystem in the Pacific likewise contributed to extinctions of local avifauna and reduction in native plant diversity (16, 17).
Although lasting changes to resource flows are a characteristic outcome of such ecosystem engineering processes (18, 19), direct measurements of these effects can be difficult to trace in archaeological contexts. Stable carbon (δ13C) and nitrogen (δ15N) isotope measurements from archaeologically recovered commensal species, such as the Pacific rat, offer an opportunity to track changes in diet and environment that are related to anthropogenic modulation of nutrient flows through food webs. Proportions of C3 (e.g., taro, yams, and breadfruit) versus C4 (e.g., sugarcane) plants can be discerned as a result of differential fractionation of 13C during photosynthesis (20). This leads to distinct δ13C values in primary producers which are then passed reliably up the consumer chain (21). Nitrogen isotopes provide insight into the trophic level of an organism, as a stepwise 15N-enrichment between trophic levels causes consumers to have δ15N values approximately 3–5‰ higher than their food (22–24). Marine food webs tend to be larger and more complex, leading to generally higher δ15N values than their terrestrial counterparts, although some shellfish and reef fish can overlap with δ15N values of terrestrial animals. The sources of carbon in marine systems lead to values resembling those of C4 plants (25). δ13C and δ15N values are also influenced by environmental factors such as precipitation and soil nutrient dynamics (26–28). Thus, collagen δ13C and δ15N, particularly from small omnivorous species such as the Pacific rat, can reveal food sources available within the commensal niche as well as broader environmental changes.
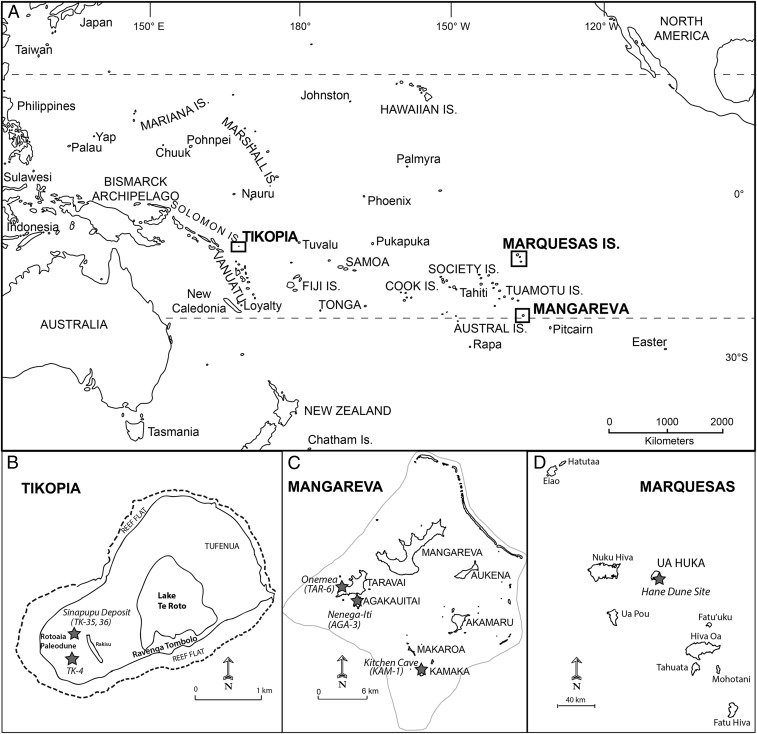
Here we apply stable isotope analyses of rat bone collagen to track changing nutrient flows and ecosystem alterations on Pacific islands before European contact. We examine δ13C and δ15N values of Pacific rat bone collagen from seven archaeological sites across the islands of Mangareva, Ua Huka (Marquesas Islands), and Tikopia (Fig. 1 and SI Appendix, Table S1). Polynesian islands have proven particularly effective as model systems for investigating Late Holocene human–ecosystem dynamics, due to their small size, isolation, and colonization by people with a shared ancestral Polynesian origin (14, 29). The sites selected for analysis provide long-term, stratified deposits across three contrastive island socioecosystems. The Polynesian Outlier of Tikopia uniquely saw ∼2,200 y of occupation by people of Austronesian origins before the arrival of Polynesians (approximately AD 1200) and thus provides a longer chronology of human activities. This interisland comparison allows both local and regional trends in human alteration of nutrient flows to be identified within relatively short, well-controlled archaeological chronologies.
Fig. 1.
(A) Map of Oceania and maps of (B) Tikopia, (C) the Gambier Islands (Mangareva), and (D) the Marquesas Islands, with study sites labeled.
Results
Full stable isotope results, bone collagen evaluation criteria, and contextual information for Tikopia, Mangareva, and Ua Huka archaeological materials are presented in SI Appendix, Tables S2 and S3, with statistical analyses of rat δ13C and δ15N from all sites reported in SI Appendix, Tables S4–S9.
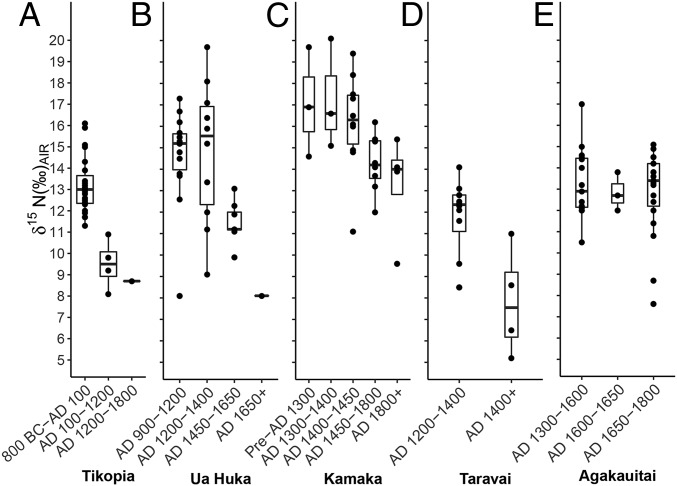
Statistically significant temporal declines in rat δ15N values across archaeological strata are seen across all islands studied except for Agakauitai Island, Mangareva (Fig. 2). On Kamaka Island (Mangareva), a Kruskal–Wallis test indicates a significant decline in δ15N (P < 0.05), although the pairwise Wilcoxon rank-sum test did not produce clear differences between layers. However, when samples were grouped into culturally meaningful time periods (SI Appendix, Table S8), differences in rat δ15N at Kamaka were significant (from 16.6 ± 2.3‰ to 13.9 ± 1.8‰, P < 0.05). On Taravai Island (Mangareva) this shift to lower δ15N values occurs from layer II to I in the TAR-6 site (from 11.7 ± 1.8‰ to 7.8 ± 2.5‰, P < 0.05). On Ua Huka Island (Marquesas), it occurs from phase I to III in the Hane site (14.6 ± 2.2‰ to 11.5 ± 1.0‰, P < 0.05), cooccurring with a transition in site use from intensive occupation to a more ceremonial function (SI Appendix, Table S1). Similarly, declines in rat δ15N on Tikopia Island occur at the transition between two archaeologically defined cultural periods on the island: the early Kiki and subsequent Sinapupu Phases (13.2 ± 1.3‰ to 9.5 ± 1.2‰, P < 0.05). Taken together, a trend of declining rat δ15N after human colonization across Polynesian islands is strongly evidenced (Fig. 2). The single exception to this pattern is the Agakauitai Island rat sequence, which displays no significant change in δ15N values through time. The largest shifts in δ15N values occur on Taravai, Ua Huka, and Tikopia Islands, with mean δ15N values declining by ∼4‰.
Fig. 2.
Boxplots illustrating temporal declines of δ15N values across (A) Tikopia, (B) Ua Huka (Marquesas), and (C–E) three islands in the Mangarevan archipelago (Kamaka, Taravai, and Agakauitai Islands; refs. 61 and 62).
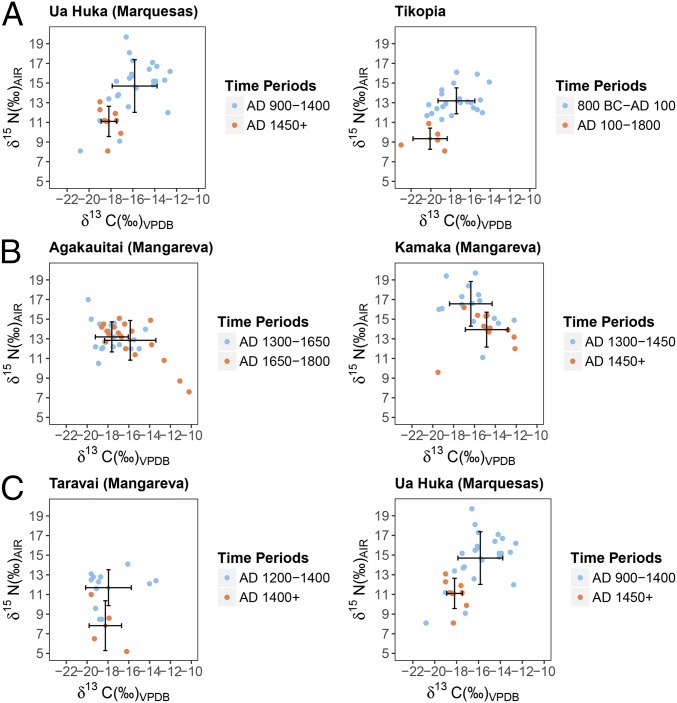
Smaller shifts occur in rat collagen δ13C on Agakauitai, Ua Huka, and Tikopia Islands (Fig. 3 and SI Appendix, Tables S4–S9). Changes in δ13C reflect broad transformations to terrestrial landscapes, as well as changes in human subsistence practices. On Agakauitai Island, rat δ13C becomes significantly higher from layer IV to layer II, suggesting greater inputs of C4 or marine resources (from −17.7 ± 1.7‰ to −15.9 ± 2.5‰, P < 0.05). Because most introduced Polynesian crops (except sugarcane) are C3 and thus display lower δ13C values, it is likely that these higher δ13C values reflect increased dietary inputs of marine resources. This is further supported by the elevated δ15N values also displayed in the same samples. Because Pacific rats are strictly terrestrial, they are most likely to have obtained marine resources as anthropogenic subsidies in the form of human meal scraps. In contrast, on both Ua Huka and Tikopia Islands, rat δ13C becomes significantly lower over time, indicating larger inputs of terrestrial C3 resources. On Ua Huka Island, this shift occurs alongside declines in δ15N, from phase I to phase III (from −15.5 ± 2.2‰ to −18.2 ± 0.7‰, P < 0.05). Similarly, on Tikopia, lower δ13C occurs simultaneously with declining δ15N values at the transition from the Kiki to Sinapupu Phase. Differences in rat δ13C between phases on Tikopia were statistically significant (Kruskal–Wallis, P < 0.05), with post hoc analysis not producing statistically significant differences between layers. However, when the Sinapupu and Tuakamali Phase samples are grouped together, differences in δ13C become significant (from −17.4 ± 1.9‰ to −20.1 ± 1.7‰, P < 0.05). Lower δ13C values coupled with declining δ15N suggests an increasing dietary focus on introduced, terrestrial C3 resources such as breadfruit, taro, and yams.
Fig. 3.
Significant shifts in mean δ13C and δ15N values occurring at key transitions in site use history. (A) Significant shifts to lower δ15N and lower δ13C on Ua Huka and Tikopia mark transition from reliance on native fauna to intensive terrestrial agricultural production. (B) Continued access to marine resources is apparent on Agakauitai and Kamaka Islands, where in both cases, δ15N values remain consistently high, and δ13C values become lower through time. (C) Intensity of human site activity influences rat δ13C and δ15N values, where rat δ15N becomes significantly lower (Taravai and Ua Huka) and δ13C becomes significantly higher (Ua Huka) after humans are no longer present to subsidize rat diet with marine and other high trophic level resources (61, 62).
Discussion
The temporal changes in rat collagen δ13C and δ15N presented here from three island systems demonstrate wide-scale reorganizations of island nutrient flows across Pacific islands over the course of pre-European human occupation. The biogeographic characteristics of each island system, coupled with unique human cultural developments (including agricultural regimes and subsistence strategies), influenced the spatiotemporal patterning of rat collagen δ13C and δ15N across the study islands. A significant trend of declining rat δ15N values through time is evident in all cases with a single exception (Agakauitai Island, Mangareva; Fig. 2). Because the observed declines in δ15N occurred at different times on each island, and oceanic δ15N baselines appear to have been relatively stable through this period until approximately AD 1850 (30, 31), it is not likely that these declines are related to climate-induced shifts in island baselines. Rather, these declines in rat δ15N can largely be attributed to a suite of human land use activities and ecological effects, the most important of which include faunal extinctions (particularly seabirds), resource depression, and the intensification of agricultural systems.
Human land use practices are known to alter soil and plant baseline δ15N values in different ecosystems around the world (e.g., refs. 32 and 33). Natural and anthropogenic fires have been shown to enrich foliar δ15N up to +8.6‰ (27). These effects can last for decades and produce plant δ15N values similar to those observed with manuring. Fire was likely important on the Polynesian Outlier Tikopia, where excavations in the Rakisu agricultural zone document an early period of intensive fire use for forest clearance and swidden agriculture [Kiki Phase, approximately 800 BC to AD 100 (34)], followed in the subsequent Sinapupu Phase (approximately AD 100–1200) by a decline in the use of fire and the development of a multistory arboricultural system (“agroforestry”) that mimicked natural forest cover. By the Tuakamali Phase (approximately AD 1200), fire was no longer in use on the island (35). These changing fire management practices were influential in driving rat δ15N declines through time on Tikopia, especially as the development of intensive agroforestry renewed available avifaunal habitat across the island (14). Although fire use may also have affected δ15N values on other islands, there is no similar archaeological evidence for a transition from intensive fire use to its complete elimination in the other cases presented here.
Declines through time in rat δ15N values in Pacific island ecosystems are also likely linked to human effects on native faunal populations, especially birds. Land birds and ground-nesting seabirds were particularly vulnerable to extinction from habitat destruction and predation by humans and introduced species, including the Pacific rat (16, 36). Major reductions in the populations of both seabirds and land birds, leading in many cases to extirpation and extinction, are well documented for Tikopia, the Marquesas, and Mangareva (37–40). Rat δ15N likely decreased through time as contributions of avifauna—particularly seabirds—to rat diet declined. These effects were compounded by the gradual removal of 15N-enriched seabird guano from terrestrial systems. Seabird guano can be a critical source of soil nutrient (P and N) inputs, particularly on small islands (41). Experimental studies have shown that animal fertilizers can significantly increase plant δ15N values, with seabirds having the largest potential effect of up to 44.7‰ (42). It has previously been argued that seabird population declines on Mangareva accelerated soil nutrient depletion, lowering island baseline δ15N values and terrestrial productivity (39, 43).
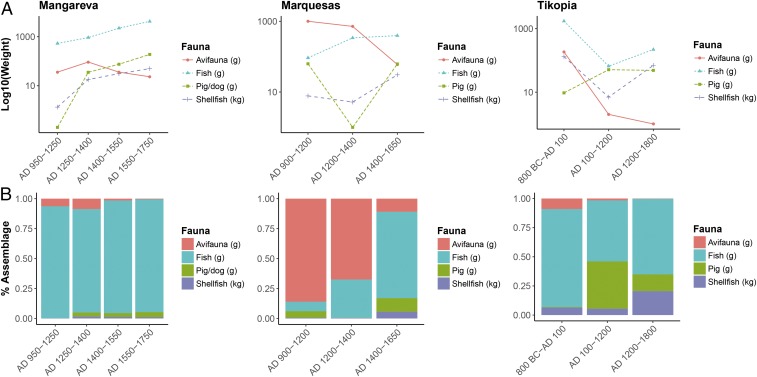
On both Ua Huka (Marquesas) and Tikopia, rat δ13C values also decreased alongside declining δ15N values (Fig. 3A and SI Appendix, Table S8). Although fire ecology and avifaunal extinctions largely influence δ15N values, lower δ13C values indicate a rat dietary change toward greater inputs of terrestrial, C3 resources through time. The larger size of Ua Huka and the geologically young and nutrient-rich soil substrates of Tikopia provided ample environments for the development of intensive agricultural systems. By European contact, both of these islands featured intensive agricultural and arboricultural systems focused on C3 crops (35, 44, 45). There is also evidence for marine resource depression and changing fishing practices on both islands. In the case of Tikopia, natural shoreline progradation significantly increased areas of cultivable land on the island, while simultaneously decreasing the area of exploitable reef habitats over time (46). Faunal remains from the site support declines in shellfish procurement and increased pig populations from the Kiki to Sinapupu Phases (Fig. 4). On Ua Huka, analyses of fishhook assemblages and fish remains indicate a shift from offshore pelagic fishing to exploitation of lower trophic level resources in the nearshore environment (47). Increasing quantities of pig and shellfish remains at the Hane Dune site after AD 1400 further suggest a later focus on terrestrial and nearshore resources (Fig. 4). The trend toward lower δ15N and δ13C in Pacific rat remains marks this transition from early exploitation of native fauna to the generation of new, anthropogenic subsistence landscapes dominated by introduced terrestrial C3 cultivars and domesticates.
Fig. 4.
Faunal change across time periods on Tikopia, Ua Huka (Hane Dune site), and Mangareva, by (A) total weight and (B) assemblage composition (SI Appendix, SI Text and refs. 58 and 59).
The relatively consistent δ15N values displayed by rats on the island of Agakautai within Mangareva are the exception that proves the general rule of direct human influence on rat diet, particularly with regard to marine resource availability to rat populations. The agricultural potential of the Mangarevan Islands was limited by steep slopes with old, nutrient-poor soils. These islands were subject to severe deforestation with limited opportunity for regeneration (48), further exacerbating seabird extirpations through habitat destruction. In contrast, Mangareva’s extensive and rich barrier reef/lagoon system appears to have experienced little to no resource depression following human colonization. Ethnohistoric evidence indicates that the Mangarevan people depended more heavily than most Polynesians on fishing for their subsistence base (49), and the dominance of fish remains across archaeofaunal assemblages from Mangareva indicates that this pattern extended into the deeper past (Fig. 4). The long-term access by rats to abundant marine resources in the form of human meal scraps is reflected in the elevated δ15N values at the continuously occupied Nenega-iti Rockshelter site on Agakauitai. Rat δ15N values are observed to decline on the other two study islands within Mangareva, Kamaka and Taravai. However, the sites on these two islands were not continuously occupied by humans throughout their archaeological sequences. Kamaka Island was intermittently inhabited, whereas the Onemea site on Taravai Island was abandoned after phase II. During periods of low site activity, rats would have received fewer marine dietary inputs through anthropogenic subsidies. In addition, these rats would have been reliant on foraging within a terrestrial environment that may have simultaneously undergone a decline in baseline δ15N from the removal of seabird guano inputs. Rat collagen δ13C at the Mangarevan sites changes little, or becomes higher over time, further suggesting frequent access to marine resources and a terrestrial landscape less dominated by introduced C3 agricultural plants in comparison with Tikopia or Ua Huka (Fig. 3B). This interpretation conforms to previous isotopic analyses of human remains from the Fiji islands, which suggest that people inhabiting smaller islands with limited agricultural potential retain marine-focused diets into late prehistory (50). Pacific rat δ13C and δ15N values are also influenced by the intensity and nature of human activities on the scale of the individual site. This is particularly apparent at the sites on Taravai and Ua Huka Islands (Fig. 3C), which both contain rats from a late precontact phase that postdates intensive site occupation. The Onemea site on Taravai Island underwent intensive use throughout phase II and was subsequently abandoned in phase I (40). Similarly, activities at the Hane dune site on Ua Huka Island in the Marquesas appear to transition from an intensive habitation site to ceremonial complex at approximately AD 1400 (51). Rat diet during intensive human activity phases in both instances appears to include inputs from high trophic level marine resources which rats likely procured from human meal scraps. The departure of humans from these sites resulted in δ15N declines of around 4.0‰ [approximately one trophic level (22, 24)], reflecting the removal of anthropogenic marine subsidies from rat diet, and shifting to a diet more reminiscent of wild foraging (43, 52).
Pacific rat stable isotope analysis demonstrates the significant influence of human land use on restructuring local landscapes and nutrient pathways within the context of one of the most dramatic archaeological examples of past human migration and ecosystem alteration. Temporal shifts in rat δ13C and δ15N reflect cultural and environmental transformations on islands that had not previously been subject to human occupation, including species extinctions and introductions, terrestrial and marine resource depression, landform change, and intensification. Our data show that these processes affected resource abundances and nutrient flows throughout entire island systems, which can then be traced through the diets of archaeologically recovered commensal remains. Stable isotope data, as a proxy for the diets of commensal rats sharing the human niche, can demonstrably distinguish differences in human activity on a spatial scale between sites, as well as a temporal scale between contexts within a given site.
Conclusion
The arrival of humans and human-translocated plant and animal species to previously uninhabited Pacific islands had profound effects on nutrient cycling throughout entire island systems. Our results reveal a consistent pattern of lasting resource flow restructuring across islands and in particular a trend of declining δ15N values that can be linked to patterns of island resource depression and agricultural intensification. Although the role of human activity in shaping Pacific island environments has previously been identified (14, 53, 54), our study provides a direct, quantitative measurement of the deep-time effects of human land use on restructuring nutrient pathways through anthropogenic island food webs.
We have shown that stable isotope analysis of the commensal Pacific rat is an effective method for investigating the effects of human ecosystem engineering activities on nutrient cycling at both region-wide and local scales. This is significant as interest grows in identifying the scale and nature of human ecosystem alterations in the past. Measuring the extent of anthropogenic influences on ecological processes through quantitative changes in commensal diets also allows archaeological research to begin to make contributions to discussions of contemporary human–ecosystem crises. With adequate spatial and chronological control, such analyses can be applied toward investigating the timing, intensity, and nature of anthropogenic effects in both island and continental regions, as well as enabling past instances of human landscape transformation to be compared with modern datasets.
Materials and Methods
Sample Selection.
Tikopia samples were selected from assemblages excavated by Patrick Kirch and Douglas Yen in 1977–1978 and housed in the Bernice P. Bishop Museum, Honolulu, Hawaii. Samples were analyzed with permission from the museum through a destructive analysis loan. All samples used in analysis, including those previously published, were preliminarily identified as Pacific rat by site excavators. Identifications were reconfirmed using comparative skeletons of brown rat (Rattus norvegicus), black rat (Rattus rattus), and Pacific rat (Rattus exulans) on loan from the Museum of Vertebrate Zoology at the University of California, Berkeley. Species determinations were based primarily on size categories (55). The Pacific rat is the only rat species present on Mangareva and the Marquesas until European contact. The large spiny rat (Rattus praetor) was also present prehistorically on Tikopia; however, the femoral elements of these two species can be distinguished with a high degree of confidence based on size differences (56).
Minimum number of individuals was calculated within each site, unit, and layer to maximize sample sizes while minimizing the chance of sampling from the same individual. Femora were selected for analysis whenever possible because these elements preserve well and often possess species-diagnostic metric traits. Between levels, femora were compared for potential matching pairs to further eliminate double sampling. From the Tikopia site, a total of 87 Pacific rat femora were selected from sites representing the Kiki (TK-4 and TK-36), Sinapupu (TK-35), and Tuakamali (TK-35) cultural phases (35).
Stable Isotope Analysis.
The stable isotope data reported here from rat specimens recovered from Tikopia, as well as the previously published results from Mangareva and the Marquesas, were all derived using the same collagen extraction methods (after ref. 57, detailed in SI Appendix, Bone Collagen Extraction). Analyses were conducted at the Center for Stable Isotope Biogeochemistry at the University of California, Berkeley. Dry samples were weighed into tin caps and analyzed simultaneously for C and N contents (percent dry weight) and C and N stable isotope ratios using a CHNOS (carbon, hydrogen, nitrogen, oxygen, sulfur) Elemental Analyzer (vario ISOTOPE cube; Elementar) and Isoprime 100 mass spectrometer (Isoprime Ltd.). Samples were normalized to international scales [Vienna Pee Dee Belemnite (VPBD) and atmospheric nitrogen; Ambient Inhalable Reservoir (AIR)] using Standard Reference Materials (SRMs) certified by the National Institute of Standards and Technology (NIST): NIST SRM 1547 (peach leaves) and in-house standards of fish meal (−17.7 ± 0.1‰, 16.3 ± 0.2‰) and spirulina (−32.1 ± 0.1‰, 11.0 ± 0.2‰). Long-term external precisions based on reference material NIST SRM 1577b (bovine liver) are 0.1‰ and 0.2‰ for δ13C and δ15N, respectively.
Collagen was evaluated for preservation and contamination using the ratio of carbon to nitrogen (percent by weight). Samples displaying C:N ratios outside the range of 2.7–3.6 were eliminated from analysis (57–59). A total of 46 samples were eliminated from the Tikopia analysis for not meeting this criterion, with the unfortunate effect of reducing representation of the Tuakamali phase to a single sample. The percent by weight carbon, percent by weight nitrogen, and percent collagen are reported in SI Appendix, Table S3. Modern bone collagen contains roughly 35% carbon and 11–16% nitrogen, and well-preserved, uncontaminated archaeological samples should approximate these values. A minimum of 0.5% preserved collagen is recommended for archaeological bone samples recovered from tropical environments (60), and all samples included in the analysis met this criterion.
Statistically significant differences in rat δ13C and δ15N values between archaeological layers were evaluated using a Kruskal–Wallis test followed by a post hoc Pairwise Mann–Whitney–Wilcoxon test when P < 0.05 (SI Appendix, Tables S5–S7). To compensate for low sample sizes, archaeological strata were further grouped into early and late periods based on culturally meaningful distinctions for each island (SI Appendix, Table S8). A Kruskal–Wallis test was not run for samples from Taravai Island, because only two samples were compared. Statistical analyses were performed in R 3.4.2 (61).
Supplementary Material
Acknowledgments
Melanie Miller, Stefania Mambelli, Todd Dawson, and Paul Brooks of Berkeley’s Center for Stable Isotope Biogeochemistry provided assistance in stable isotope preparation and analysis. Guillaume Molle and Eric Conte provided the Marquesan rat samples from their excavations of the Hane Dune site. Stable isotope analysis was funded by a National Science Foundation Grant (Doctoral Dissertation Research Improvement Award BCS-1452364 awarded to P.V.K. and J.A.S.). Additional support was provided by the National Science Foundation Graduate Research Fellowship Program (Grant DGE 1106400). We thank the Bernice P. Bishop Museum for a destructive analysis loan of the Tikopia faunal materials and in particular Mara Mulrooney and Charmaine Wong for facilitating access to the collections. J.A.S., P.R., and N.B. are funded through the Max Planck Institute for the Science of Human History.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805787115/-/DCSupplemental.
References
- 1.Steffen W, Grinevald J, Crutzen P, McNeill J. The Anthropocene: Conceptual and historical perspectives. Philos Trans A Math Phys Eng Sci. 2011;369:842–867. doi: 10.1098/rsta.2010.0327. [DOI] [PubMed] [Google Scholar]
- 2.Bayon G, et al. Intensifying weathering and land use in Iron Age Central Africa. Science. 2012;335:1219–1222. doi: 10.1126/science.1215400. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson QD, Coomber T, Passmore S, Greenhill SJ, Kushnick G. Cultural and environmental predictors of pre-European deforestation on Pacific islands. PLoS One. 2016;11:e0156340. doi: 10.1371/journal.pone.0156340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin NL, et al. Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc Natl Acad Sci USA. 2016;113:6388–6396. doi: 10.1073/pnas.1525200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnosky AD, et al. Prelude to the Anthropocene: Two new North American Land Mammal Ages (NALMAs) Anthropocene Rev. 2014;1:225–242. [Google Scholar]
- 6.Kirch PV. Archaeology and global change: The Holocene record. Annu Rev Environ Resour. 2005;30:409–440. [Google Scholar]
- 7.Roberts P, Hunt C, Arroyo-Kalin M, Evans D, Boivin N. The deep human prehistory of global tropical forests and its relevance for modern conservation. Nat Plants. 2017;3:17093. doi: 10.1038/nplants.2017.93. [DOI] [PubMed] [Google Scholar]
- 8.Szpak P, Orchard TJ, McKechnie I, Gröcke DR. Historical ecology of late Holocene sea otters (Enhydra lutris) from northern British Columbia: Isotopic and zooarchaeological perspectives. J Archaeol Sci. 2012;39:1553–1571. [Google Scholar]
- 9.Szpak P, Orchard TJ, Salomon AK, Gröcke DR. Regional ecological variability and impact of the maritime fur trade on nearshore ecosystems in southern Haida Gwaii (British Columbia, Canada): Evidence from stable isotope analysis of rockfish (Sebastes spp.) bone collagen. Archaeol Anthropol Sci. 2013;5:159–182. [Google Scholar]
- 10.Matisoo-Smith E. The commensal model for human settlement of the Pacific 10 years on—What can we say and where to now? J Island Coastal Archaeol. 2009;4:151–163. [Google Scholar]
- 11.Jones EP, Eager HM, Gabriel SI, Jóhannesdóttir F, Searle JB. Genetic tracking of mice and other bioproxies to infer human history. Trends Genet. 2013;29:298–308. doi: 10.1016/j.tig.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Weissbrod L, et al. Origins of house mice in ecological niches created by settled hunter-gatherers in the Levant 15,000 y ago. Proc Natl Acad Sci USA. 2017;114:4099–4104. doi: 10.1073/pnas.1619137114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young HS, Parker IM, Gilbert GS, Sofia Guerra A, Nunn CL. Introduced species, disease ecology, and biodiversity-disease relationships. Trends Ecol Evol. 2017;32:41–54. doi: 10.1016/j.tree.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Kirch PV. Three islands and an archipelago: Reciprocal interactions between humans and island ecosystems in Polynesia. Earth Environ Sci Trans R Soc Edinburgh. 2007;98:85–99. [Google Scholar]
- 15.Christensen CC, Kahn JG, Kirch PV. Nonmarine mollusks from archaeological sites on Mo’orea, Society Islands, French Polynesia, with descriptions of four new species of recently extinct land snails (Gastropoda: Pulmonata: Endodontidae) Pac Sci. 2018;72:95–123. [Google Scholar]
- 16.Steadman DW. Extinction and Biogeography of Tropical Pacific Birds. Univ Chicago Press; Chicago: 2006. [Google Scholar]
- 17.Meyer J-Y, Butaud J-F. The impacts of rats on the endangered native flora of French Polynesia (Pacific Islands): Drivers of plant extinction or coup de grâce species? Biol Invasions. 2009;11:1569–1585. [Google Scholar]
- 18.Jones CG, Lawton JH, Shachak M. Ecosystem Management. Springer; New York: 1994. Organisms as ecosystem engineers; pp. 130–147. [Google Scholar]
- 19.Laland KN, O’Brien MJ. Niche construction theory and archaeology. J Archaeol Method Theory. 2010;17:303–322. [Google Scholar]
- 20.Bender MM. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry. 1971;10:1239–1244. [Google Scholar]
- 21.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 22.Bocherens H, Drucker D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol. 2003;13:46–53. [Google Scholar]
- 23.Deniro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta. 1981;45:341–351. [Google Scholar]
- 24.Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta. 1984;48:1135–1140. [Google Scholar]
- 25.Schoeninger MJ, DeNiro MJ. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta. 1984;48:625–639. [Google Scholar]
- 26.Austin AT, Vitousek PM. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia. 1998;113:519–529. doi: 10.1007/s004420050405. [DOI] [PubMed] [Google Scholar]
- 27.Szpak P. Complexities of nitrogen isotope biogeochemistry in plant-soil systems: Implications for the study of ancient agricultural and animal management practices. Front Plant Sci. 2014;5:288. doi: 10.3389/fpls.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caut S, et al. Seabird modulations of isotopic nitrogen on islands. PLoS One. 2012;7:e39125. doi: 10.1371/journal.pone.0039125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitousek PM. Oceanic islands as model systems for ecological studies. J Biogeogr. 2002;29:573–582. [Google Scholar]
- 30.Sherwood OA, Guilderson TP, Batista FC, Schiff JT, McCarthy MD. Increasing subtropical North Pacific Ocean nitrogen fixation since the Little Ice Age. Nature. 2014;505:78–81. doi: 10.1038/nature12784. [DOI] [PubMed] [Google Scholar]
- 31.Wiley AE, et al. Millennial-scale isotope records from a wide-ranging predator show evidence of recent human impact to oceanic food webs. Proc Natl Acad Sci USA. 2013;110:8972–8977. doi: 10.1073/pnas.1300213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aranibar JN, et al. Nitrogen isotope composition of soils, C3 and C4 plants along land use gradients in southern Africa. J Arid Environ. 2008;72:326–337. [Google Scholar]
- 33.Bogaard A, et al. Crop manuring and intensive land management by Europe’s first farmers. Proc Natl Acad Sci USA. 2013;110:12589–12594. doi: 10.1073/pnas.1305918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirch PV, Swift JA. New AMS radiocarbon dates and a re-evaluation of the cultural sequence of Tikopia Island, Southeast Solomon Islands. J Polyn Soc. 2017;126:313–336. [Google Scholar]
- 35.Kirch PV, Yen DE. 1982. Tikopia. The Prehistory and Ecology of a Polynesian Outlier, Bernice P Bishop Museum Bulletin (Bishop Museum Press, Honolulu), Vol 238.
- 36.Steadman DW. Extinctions of Polynesian Birds: Reciprocal Impacts of Birds and People. Yale Univ Press; New Haven, CT: 1997. [Google Scholar]
- 37.Steadman D, Rolett B. A chronostratigraphic analysis of landbird extinction on Tahuata, Marquesas Islands. J Archaeol Sci. 1996;23:81–94. [Google Scholar]
- 38.Steadman DW, Pahlavan DS, Kirch PV. Extinction, biogeography, and human exploitation of birds on Tikopia and Anuta, Polynesian outliers in the Solomon Islands. Bishop Mus Occas Pap. 1990;30:118–153. [Google Scholar]
- 39.Kirch PV, et al. Human ecodynamics in the Mangareva Islands: A stratified sequence from Nenega-Iti Rock Shelter (site AGA-3, Agakauitai Island) Archaeol Oceania. 2015;50:23–42. [Google Scholar]
- 40.Kirch PV, Conte E, Sharp W. The Onemea site (Taravai Island, Mangareva) and the human colonization of southeastern Polynesia. Archaeol Oceania. 2010;45:66–79. [Google Scholar]
- 41.Anderson WB, Polis GA. Nutrient fluxes from water to land: Seabirds affect plant nutrient status on Gulf of California islands. Oecologia. 1999;118:324–332. doi: 10.1007/s004420050733. [DOI] [PubMed] [Google Scholar]
- 42.Szpak P, Longstaffe FJ, Millaire J-F, White CD. Stable isotope biogeochemistry of seabird guano fertilization: Results from growth chamber studies with maize (Zea mays) PLoS One. 2012;7:e33741. doi: 10.1371/journal.pone.0033741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swift JA, Miller MJ, Kirch PV. Stable isotope analysis of Pacific rat (Rattus exulans) from archaeological sites in Mangareva (French Polynesia): The use of commensal species for understanding human activity and ecosystem change. Environ Archaeol. 2017;22:283–297. [Google Scholar]
- 44.Firth R. We the Tikopia: A Sociological Study of Kinship in Primitive Polynesia. Allen & Unwin; London: 1936. [Google Scholar]
- 45.Handy E. 1923. Native Culture in the Marquesas, Bernice P. Bishop Museum Bulletin (Bishop Museum Press, Honolulu), Vol 9.
- 46.Kirch PV. Man’s role in modifying tropical and subtropical Polynesian ecosystems. Archaeol Oceania. 1983;18:26–31. [Google Scholar]
- 47.Dye T. Occasional Papers in Prehistory. Vol 18. Australian National University, Canberra; Australia: 1990. The causes and consequences of a decline in the prehistoric Marquesan fishing industry; pp. 70–84. [Google Scholar]
- 48.Rolett B, Diamond J. Environmental predictors of pre-European deforestation on Pacific islands. Nature. 2004;431:443–446. doi: 10.1038/nature02801. [DOI] [PubMed] [Google Scholar]
- 49.Buck PH. 1938. Ethnology of Mangareva, Bernice P. Bishop Museum Bulletin (Bishop Museum Press, Honolulu), Vol 157.
- 50.Field JS, Cochrane EE, Greenlee DM. Dietary change in Fijian prehistory: Isotopic analyses of human and animal skeletal material. J Archaeol Sci. 2009;36:1547–1556. [Google Scholar]
- 51.Conte E, Molle G. Reinvestigating a key site for Polynesian prehistory: New results from the Hane dune site, Ua Huka (Marquesas) Archaeol Oceania. 2014;49:121–136. [Google Scholar]
- 52.Swift JA, Molle G, Conte E. Coastal subsistence and settlement at the Hane dune site, Ua Huka (Marquesas Islands): New insights from Pacific rat (Rattus exulans) stable isotope analysis. J Archaeol Sci. 2017;15:161–168. [Google Scholar]
- 53.Braje TJ, Leppard TP, Fitzpatrick SM, Erlandson JM. Archaeology, historical ecology and anthropogenic island ecosystems. Environ Conserv. 2017;44:286–297. [Google Scholar]
- 54.Kirch PV, Hunt TL. Historical Ecology in the Pacific Islands: Prehistoric Environmental and Landscape Change. Yale Univ Press; New Haven, CT: 1997. [Google Scholar]
- 55.Matisoo-Smith E, Allen JS. Name that rat: Molecular and morphological identification of Pacific rodent remains. Int J Osteoarchaeol. 2001;11:34–42. [Google Scholar]
- 56.White JP, Clark G, Bedford S. Distribution, present and past, of Rattus praetor in the Pacific and its implications. Pac Sci. 2000;54:105–117. [Google Scholar]
- 57.Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci. 1990;17:431–451. [Google Scholar]
- 58.DeNiro MJ, Weiner S. Chemical, enzymatic and spectroscopic characterization of “collagen” and other organic fractions from prehistoric bones. Geochim Cosmochim Acta. 1988;52:2197–2206. [Google Scholar]
- 59.van Klinken GJ. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J Archaeol Sci. 1999;26:687–695. [Google Scholar]
- 60.Pestle WJ, Colvard M. Bone collagen preservation in the tropics: A case study from ancient Puerto Rico. J Archaeol Sci. 2012;39:2079–2090. [Google Scholar]
- 61.R Development Core Team 2017. R: A Language and Environment for Statistical Computing (R foundation for Statistical Computing, Vienna), Version 3.4.2.
- 62.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.