Significance
Determining the structure of water on metal oxide surfaces is a key step toward a molecular-level understanding of dissolution, corrosion, geochemistry, and catalysis, but hydrogen bonding and large, complex unit cells present a major challenge to modern theory. Here, we utilize state-of-the-art experimental techniques to guide a density functional theory (DFT)-based search for the minimum-energy configurations of water on Fe3O4(001). A subsurface reconstruction dominates adsorption at all coverages. An ordered array of partially dissociated water agglomerates form at low coverage, and these serve to anchor a hydrogen-bonded network. We argue that similar behavior will occur whenever a surface presents a well-spaced array of active sites for dissociation. Given the propensity of metal oxides to undergo surface reconstructions, this is likely often.
Keywords: water, magnetite, Fe3O4, cooperativity, H-bond network
Abstract
Determining the structure of water adsorbed on solid surfaces is a notoriously difficult task and pushes the limits of experimental and theoretical techniques. Here, we follow the evolution of water agglomerates on Fe3O4(001); a complex mineral surface relevant in both modern technology and the natural environment. Strong OH–H2O bonds drive the formation of partially dissociated water dimers at low coverage, but a surface reconstruction restricts the density of such species to one per unit cell. The dimers act as an anchor for further water molecules as the coverage increases, leading first to partially dissociated water trimers, and then to a ring-like, hydrogen-bonded network that covers the entire surface. Unraveling this complexity requires the concerted application of several state-of-the-art methods. Quantitative temperature-programmed desorption (TPD) reveals the coverage of stable structures, monochromatic X-ray photoelectron spectroscopy (XPS) shows the extent of partial dissociation, and noncontact atomic force microscopy (AFM) using a CO-functionalized tip provides a direct view of the agglomerate structure. Together, these data provide a stringent test of the minimum-energy configurations determined via a van der Waals density functional theory (DFT)-based genetic search.
The ubiquity of water in the ambient environment ensures that its interaction with solid surfaces is of fundamental importance (1). To understand processes such as dissolution, corrosion, and weathering at the molecular level requires an understanding of how water adsorbs on surfaces, and what governs their reactivity. Atomic-scale investigations on single-crystal samples have revealed that interfacial water almost never forms an ice-like structure (2) and aims to simultaneously maximize its interaction with the surface and intermolecular hydrogen bonding (H bonds). The surface and hydrogen bonds have similar magnitude on metals, and the adlayer is often stabilized if some fraction of the water dissociates (2). This body of work has culminated in the so-called “2D ice rules” (3, 4), which suggest that water likes to form closed-looped structures and prefers to adsorb atop metal atoms, and each molecule donates and accepts a single hydrogen bond in a stable structure.
The situation is somewhat different on metal oxides, where the water–surface bonds are significantly stronger than hydrogen bonds. It has long been established (5) that the lone pair on the oxygen atom forms a strong dative bond with electron-deficient cation sites, and that surface defects are active sites for dissociation (6). On particularly reactive surfaces such as ZnO (7) and Cr2O3(0001) (8, 9), dissociative adsorption occurs, leading to the adsorption of terminal (OwaterH) and surface hydroxyl groups on cations and anions, respectively. The behavior of water on the prototypical TiO2(110) surface is an interesting case and has been discussed at length (10, 11). While it is clear that water adsorbs preferentially at surface oxygen vacancies (Vos), resulting in a pair of OsurfaceH hydroxyl groups (12), the question whether water dissociates on the defect-free surface has remained controversial (13–16). Interestingly, Dohnálek and coworkers (17) recently demonstrated that molecular adsorption is marginally preferred (0.035 eV) on the defect-free surface with a small barrier for dissociation. Thus, it seems measurements of partial dissociation result from the equilibrium concentration of both states at finite measurement temperatures.
In recent years, several groups have reported that partially dissociated water dimers are the most stable configuration for water on some metal oxide surfaces (18–21). There is also mounting evidence that long-range, partially dissociated networks can be energetically favored, as on metals (22–26), but accurately determining the structure of such systems is extremely difficult. It is tempting to employ genetic search algorithms to locate the global minimum from a myriad of local minima (25–27), but this is complicated by the large, complex unit cells involved, and the well-documented problems associated with accounting for dispersion in density functional theory (DFT) calculations (28).
Problems studying water adlayers on metal oxides are not restricted to theory. Another key issue has been the difficulty achieving molecular resolution of water clusters and adlayers in scanning-probe experiments. While significant progress has been made recently using scanning tunneling microscopy (STM) (4), noncontact atomic force microscopy (nc-AFM) has now emerged as a technique capable of superior resolution, particularly when the tip is functionalized by a CO molecule (29). We resolved to apply the latter method to the particularly complex case of water adsorption on the (√2 × √2)R45°-reconstructed Fe3O4(001) surface. In combination with quantitative temperature-programmed desorption (TPD) and high-resolution X-ray photoelectron spectroscopy (XPS), the images provide a stringent test of the minimum-energy structures determined by a computational search. Our approach is similar to a genetic search, but we analyze the trends underlying the stability of different structures at each stage and use this information to design the subsequent generation of trial structures.
Ultimately, we find that an isolated molecule adsorbs intact on Fe3O4(001), but significant energy is gained through the formation of partially dissociated water dimers. The surface reconstruction limits the coverage of such species to one per (√2 × √2)R45° unit cell, however, because only a subset of the surface O atoms can accept an H+ to form an OsurfaceH group. The partially dissociated water dimers act as an anchor for further water as the coverage increases, leading first to partially dissociated water trimers, and then to a ring-like H-bonded network, which covers the entire surface. The nc-AFM images allow us to rule out one of two isoenergetic water trimers predicted by a thorough DFT search, suggesting that van der Waals (vdW) DFT does not accurately handle the cooperative energy balance in this system.
Results
A key feature of the spectroscopic measurements described here is the ability to deposit an accurately determined number of water molecules on the Fe3O4(001) surface using a calibrated molecular-beam source (30). Fig. 1A shows TPD spectra obtained for various initial D2O coverages ranging from 0 to 14 molecules per (√2 × √2)R45° unit cell (D2O/u.c.). D2O was utilized to ensure that the measured signal originates solely from the sample surface, but spectra obtained for H2O are indistinguishable from those presented in Fig. 1A. A complex spectrum with seven distinct desorption features was reproducibly observed from several different single crystal samples, and we label the peaks α, α′ β, γ, δ, ε, and φ in order of ascending temperature. A plot of the integrated peak area versus exposure (Fig. 1B) yields a straight line, consistent with the measured sticking probability of unity at all coverages (SI Appendix, Fig. S1). The onset of multilayer ice desorption (peak α at 155 K) occurs for a coverage close to 8.5 molecules per (√2 × √2)R45° unit cell (8.5 D2O/u.c. = 1.28 × 1015 D2O/cm2), which is close to the density of an ice monolayer on close-packed metal surfaces, and we thus consider everything desorbing at higher temperatures a constituent of the first water monolayer. The saturation of the φ (550 K) and ε (310 K) peaks (Fig. 1A, Inset) occurs for coverages significantly less than 1 D2O/u.c., and we assign these states to surface defects. Peaks β, γ, and δ saturate at coverages close to 8, 6, and 3 D2O/u.c., respectively, which suggests that stable surface phases are completed at these coverages. α′ is a small shoulder between the saturation of the β peak and the onset of multilayer desorption (peak α).
Fig. 1.
Quantification of water adsorbed on Fe3O4(001) by TPD. (A) Experimental TPD spectra obtained for initial D2O coverages ranging from 0 to 14 molecules per Fe3O4(001)–(√2 × √2)R45° unit cell (Inset: higher temperature range showing desorption peaks ε and φ, which originate from surface defects). The colored curves indicate the coverages for which a particular desorption feature (labeled α′, β, γ, and δ) saturates, α marks the multilayer desorption peak. (B) Plot of the integrated TPD peak areas as a function of beam exposure. The colored data points correspond to the colored curves in A. Based on these data, we conclude the β, γ, and δ peaks saturate at coverages of eight, six, and three molecules per (√2 × √2)R45° unit cell, respectively. (C) Inversion analysis of the TPD data for D2O on Fe3O4(001) for the different peaks. The filled area marks the uncertainty range of the coverage-dependent desorption energies for each peak.
To extract information regarding the desorption energetics from the TPD data, we performed an inversion analysis (31). Full details are contained in SI Appendix. Briefly, the analysis assumes that the desorption follows first-order Arrhenius kinetics and yields the coverage-dependent activation energy for desorption, Ed (Fig. 1C), by direct inversion of the well-known Polanyi–Wigner equation. The uncertainty in Ed is related to the uncertainty in the preexponential factor, ν, which is unknown, but optimized during the analysis (31). The resulting Ed is equivalent to the adsorption energy (Ead) if the adsorption is a reversible process with no activation barrier. The results, shown in Fig. 1C, show that Ed decreases with increasing coverage from a maximum of 0.85 ± 0.05 eV in the low-coverage limit to 0.52 ± 0.05 eV for the first molecules desorbing from the first monolayer. Interestingly, the corresponding values of ν (νδ = 1017 ± 1 s−1, νγ = 1016 ± 1 s−1, νβ = 1016 ± 1 s−1, να′ = 1014 ± 2 s−1) are relatively high, which suggests that the adsorbed state is highly constrained (31). For comparison, utilizing ν = 1013 s−1 (appropriate for a 2D gas, but not here) in Fig. 1C would see all desorption energies lowered by ∼0.15 eV. For a full discussion of TPD preexponential factors, see refs. 32 and 33.
To understand the origin of the complex multipeak desorption profile, we studied the adsorbed water structures with STM and nc-AFM. Fig. 2A shows an STM image of the as-prepared Fe3O4(001) surface acquired at 78 K. Rows of protrusions in the [110] direction are due to the octahedrally coordinated surface Feoct atoms of a stoichiometric surface layer (see surface model in Inset). The surface oxygen atoms are not imaged because there are no O-related states in the vicinity of the Fermi level. The undulating appearance of the Feoct rows and associated (√2 × √2)R45° periodicity (white square) are linked to a subsurface rearrangement of the cation sublattice (34). We have previously shown that the O* atoms (i.e., surface oxygen without a tetrahedrally coordinated Fetet neighbor in the second layer) are active sites for adsorption. These atoms differ electronically from the others [DFT predicts a small magnetic moment (34)], and they stabilize metal adatoms to high temperatures (35). Crucially for what follows, the O* atoms are also preferred sites for the formation of OsurfaceH groups (36, 37). There is always a small coverage of O*H following in situ preparation due to the reaction of water from the residual gas with oxygen vacancies, and they cause pairs of surface Feoct protrusions to be imaged slightly brighter in empty-states STM images. An example is highlighted by a white arrowhead in Fig. 2A. Recombination of the O*H species with lattice O to desorb water is responsible for the φ peak (550 K) observed in TPD (38).
Fig. 2.
Water monomers, dimers, and multiple neighboring protrusions on the Fe3O4(001) surface imaged by low-temperature (78 K) STM. (A) The as-prepared Fe3O4(001) surface. The (√2 × √2)R45° periodicity is indicated by the white square, and the white arrow highlights an O*H group. (Inset) Top and side views of the Fe3O4(001)–(√2 × √2)R45° surface structure with the SCV structure (the top view is aligned with the STM image, and the gray vector indicates the viewing direction to locate the side view). Only the Feoct atoms are imaged in STM. (B) STM image acquired after 0.05 L of water was adsorbed and heated to 255 K. The surface is clean, except for protrusions located at surface defects including antiphase domain boundaries in the (√2 × √2)R45° reconstruction (cyan arrow). (C) STM image following adsorption of 0.1 L of water at 120 K. Isolated single protrusions (yellow arrow), double protrusions (red arrow), and multiple neighboring protrusions (green arrow) are due to water molecules adsorbed on the Feoct rows.
To confirm the ε TPD peak at 310 K was defect related, we exposed the as-prepared Fe3O4(001) surface to 0.05 L (1 L = 1.33 × 10−6 mbar⋅s) water, heated to 255 K, and imaged the surface using STM. Fig. 2B shows bright protrusions adsorbed at an antiphase domain boundary in the (√2 × √2)R45° reconstruction (39), and there is also evidence for adsorption at Fe2+-related point defects and step edges (SI Appendix, Fig. S3). Similar behavior was recently observed for methanol on this surface (40). In the current paper, we are primarily interested in water adsorbed at regular lattice sites.
Fig. 2C shows an STM image of the Fe3O4(001) surface after exposure to 0.1 L of H2O at 120 K. At this temperature, far below the desorption threshold, surface mobility is low, and we observe a nonequilibrium state. The image, acquired at 78 K, exhibits isolated, bright protrusions on the Feoct rows due to adsorbed water (yellow arrow). It is not straightforward to determine whether the molecules are intact or dissociated from this image, but several water dimers are observed already at this coverage (red arrow). Interestingly, dimers have two apparent heights, so there may be two types of water dimers under these conditions.
Finally, there are instances of multiple neighboring protrusions (green arrow), but it is difficult to know how much water is involved, and these could simply be two dimers. Nevertheless, the STM data suggest that water molecules can form larger agglomerates if adsorbed close by already adsorbed molecules. STM images of higher water coverages were acquired (SI Appendix), revealing limited additional information. The Feoct rows are increasingly occupied by extended protrusions, but it is not possible to resolve the internal structure (SI Appendix, Fig. S3).
To learn more about water in the submonolayer regime, we imaged the surface using nc-AFM. The best images were obtained in constant-height mode using a CO-functionalized tip (Fig. 3). This experimental setup was recently utilized to image water clusters on different surfaces (41–44). The observed image contrast was attributed to electrostatic interaction between the CO quadrupole field and strongly polar water molecules, which can yield up to submolecular resolution. Due to the long-range nature of these contrast-forming interactions, the imaging is remarkably stable and the tip does not perturb the water clusters during scanning (44).
Fig. 3.
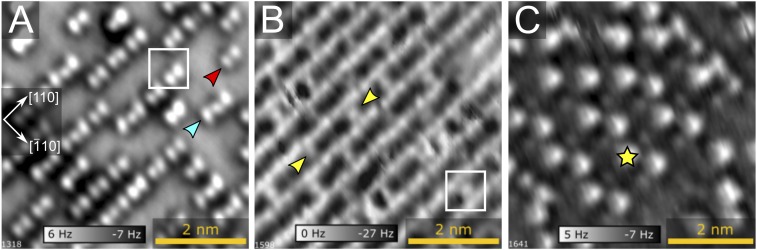
Imaging water agglomerates on Fe3O4(001) with nc-AFM using a CO-functionalized tip. nc-AFM images obtained after exposing the as-prepared Fe3O4(001) surface to (A) 2.5 ± 0.5 H2O/u.c., (B) ∼6 H2O/u.c., and (C) ∼8 H2O/u.c. The Q+ oscillation amplitudes were (A) 45 pm, (B) 110 pm, and (C) 65 pm, and the bias was set to 0 V in all images. In each case, water was dosed at 105 K, and the sample preheated to ∼155 K before imaging at 78 K. The coverages in A–C correspond roughly to the partial populations of the δ, γ, and β peaks in TPD, respectively. Partially dissociated water dimers and trimers on the Feoct rows are indicated by red and cyan arrows in A, respectively, and yellow arrows highlight protrusions bridging the Feoct rows in B. Additional water deposited on the surface appears as bright protrusions (yellow star), suggesting it protrudes significantly above the surface and the previously adsorbed molecules (C). The (√2 × √2)R45° surface unit cell is shown by a white square. Representative STM images of the same structures are shown in SI Appendix, Fig. S4.
Fig. 3A shows a nc-AFM image of the Fe3O4(001) surface after 2.5 ± 0.5 H2O/u.c. was adsorbed at 105 K. The tip was functionalized by picking up a CO molecule from a Pt cluster (details in SI Appendix). Before imaging, the sample was heated to 155 K, which is short of the desorption onset of the δ peak. The image, acquired at 78 K, exhibits double (red arrow) and triple (cyan arrow) protrusions aligned with the [110] direction, which we assign to water dimers and water trimers, respectively. The bright spots originate from repulsive electrostatic interaction between the CO tip and the O atom of the water molecule or OH group. The distance measured between neighboring protrusions within each dimer/trimer is 0.3 nm, consistent with adsorption at the surface Feoct cations on the underlying surface (see structural model in Fig. 2A). Again, it is impossible to know from the AFM images alone whether the species within the dimer/trimer are intact or dissociated.
Fig. 3B was acquired after the water coverage was increased to ∼6 H2O/u.c., and the sample again heated to 155 K before imaging at 78 K. The image exhibits full rows of bright protrusions along [110]; four protrusions are observed per unit cell, consistent with adsorption on all surface Feoct atoms. In addition, protrusions are observed in between the rows (yellow arrows). In most cases, the distance between these bridging protrusions along [110] is 1.19 nm, which corresponds to the periodicity of the (√2 × √2)R45° reconstruction in that direction. Finally, when the coverage is increased (Fig. 3C), the contrast becomes dominated by new features, which protrude further from the surface than the rest of the water layer. This suggests that there are additional stable binding sites available on the 6 H2O/u.c. structure, or that the layer restructures above this coverage.
To ascertain the chemical state of the water within the adlayers, we performed XPS experiments. Fig. 4 shows the O 1s region for the as-prepared Fe3O4(001) surface (black curve), and after 2.6 (blue curve) and 7.7 (magenta curve) D2O/u.c. was adsorbed and the sample was heated to 175 K and 155 K, respectively, with a 1 K/s ramp. The as-prepared surface exhibits a single, slightly asymmetric peak at 530.1 eV due to the lattice oxygen (45). Exposure to water creates a clear peak at 533.4 eV due to D2O, which shifts slightly to lower binding energy with increasing coverage. Fitting the 2.6 D2O/u.c. data with Voigt functions (46), we find that (at least) one additional peak at 531.5 eV is required to accurately model the data. This peak position is close to that observed previously for OsurfaceH groups (531.3 eV) (47). Of course, the XPS binding energy of OwaterH groups could be slightly different, particularly when it is part of an agglomerate, but calculated core level shifts (48) for the OsurfaceH and the OwaterH of the linear water trimer (Fig. 5) found a difference of only 0.1 eV. Since D2O dissociation yields two OD groups, the similar peak areas at 533.4 and 531.5 eV suggest that approximately a third of the D2O is dissociated. At the higher coverage, the area of the D2O peak increases significantly and shifts to lower binding energy. The peak area in the OD region remains roughly constant with respect to the substrate peak, which suggests that the additional water adsorbs molecularly.
Fig. 4.
O 1s XPS data showing that the water agglomerates formed on Fe3O4(001) are partially dissociated. The as-prepared surface exhibits a single peak at 530.1 eV due to the lattice oxygen atoms. The 2.6 D2O/u.c. data should be compared with the surface shown in Fig. 3A and show roughly equal contributions from OD and D2O, consistent with one dissociated molecule per water dimer/trimer. Most of the additional water adsorbed at a coverage of 7.7 H2O/u.c. is molecular. Data were measured at 95 K, with monochromatic Al Kα radiation and at a grazing exit of 80° for the emitted photoelectrons.
Fig. 5.
Top view of the minimum-energy structures determined by DFT for water coverages of 1, 2, 3, 6, and 8 H2O/u.c. Fe atoms are blue, O are red, and H are white. (A) An isolated molecule adsorbs intact, but partially dissociated water dimers and trimers are energetically preferred. Two partially dissociated trimer structures are calculated to be energetically degenerate. The (√2 × √2)R45° unit cell and both O* are highlighted. (B) DFT-based model at 6 H2O/u.c. showing a ring-like structure based on full occupation of the Feoct rows with OH or H2O, and water molecules bridging the O* sites. These bridging molecules are adsorbed partly through H bonds to surface O*H groups. The O*H groups beneath the adsorbed molecules are shown in the topmost white circle. Alternatively, the structure can be viewed as based on a pair of H2O–OH–H2O trimers (labeled 1 and 2). (C) DFT-based model at 8 H2O/u.c. showing a complex structure utilizing dangling bonds in the 6 H2O/u.c. structure to form a second bridge in the region of the yellow star. All adsorption energies are given in electronvolts.
To understand the formation of different water structures, we now turn to our computational results. As explained below, we employed a systematic approach to determine the lowest-energy configurations of water molecules in the coverage regime 0–8 H2O/u.c. It is important to note that this is not an automated genetic algorithm but rather proceeds by identifying factors that make certain trial structures more stable than others at each coverage and using this information to build subsequent generations. A complete account of the theoretical approach and discussion of all computed structures will be published separately. Selected results relevant to the discussion here are shown in Fig. 5. Before continuing, it is important to note that our calculations utilize the GGA+U approach (Ueff = 3.61 eV) (49, 50) with the optPBE-DF exchange-correlation (XC)-functional (51–53), which is modified to include long-range vdW interactions. We also take into account the correct geometry, the so-called subsurface cation vacancy (SCV) model of Fe3O4(001)–(√2 × √2)R45° (34). Thus, our setup differs markedly from the prior work of Mulakaluri et al. (23, 54), who utilized a standard GGA+U functional and a bulk-truncated surface model and calculated only coverages of 1, 2, and 4 H2O/u.c.
Interestingly, we find that an isolated water molecule prefers to adsorb molecularly on the Fe3O4(001) surface (Fig. 5A, Ead = −0.64 eV). The optimum configuration has the O atom close to atop a substrate Feoct cation, with the molecule in the plane of the surface and oriented such that the H atoms interact with nearby surface O atoms via very weak H bonds, 2–2.2 Å. This configuration is 0.05 eV more stable than a dissociated molecule (Ead = −0.59 eV), where the OH group adsorbs upright atop an Feoct cation, with the H+ deposited at the neighboring O* forming a surface hydroxyl (O*H).
The most stable configuration of water on the Fe3O4(001) surface occurs at a coverage of 2 H2O/u.c. with the formation of a partially dissociated water dimer (Ead = −0.92 eV per molecule). This species comprises one terminal OH and one H2O, bound to neighboring surface Feoct atoms along the row, connected by an intermolecular H bond (1.41 Å). The H+ liberated by the dissociation forms an O*H group. Further details of the adsorption geometry are included in Discussion, in which we explain the cooperative origin of this species’ stability.
The DFT-based search at 3 H2O/u.c. yields two partially dissociated water trimers degenerate in energy (Fig. 5A, labeled E3 and E3 ISO). Both species are based on the partially dissociated water dimer described above but differ in the location of a third molecule. In the linear H2O–OH–H2O trimer, the third molecule binds on the surface Feoct row and donates an H bond into the OH. In the alternative nonlinear isomer trimer, the third water molecule binds by H bonds only. It receives an H bond from the surface O*H and donates an H bond to the nearby, unoccupied O* atom. Electrostatic repulsion renders the adsorption of an H+ at both O* sites energetically unfavorable at low coverage, and thus dissociation is limited to one molecule per (√2 × √2)R45° unit cell.
The lowest-energy structure determined by our DFT search at 6 H2O/u.c. exhibits a ring-like appearance, in agreement with the nc-AFM image shown in Fig. 3B. This is the first coverage at which all adsorbed molecules are involved in an H-bonded network that covers the surface. All four Feoct sites in each (√2 × √2)R45° unit cell are occupied by either H2O or OH, and the rows are bridged by two further water molecules attached solely through H bonds. In general, the structure is characterized by H2O–OH–H2O trimers and facilitates more ideal intermolecular bonding angles of 122–124° for intact water molecules. Interestingly, the repulsive behavior of the two O*H species observed at lower coverage is mitigated through the additional H bonding with the bridge molecules. The structure at 8 H2O/u.c. is shown in Fig. 5C. It is rather complex, but essentially water utilizes the remaining dangling H bonds in the 6 H2O/u.c. structure to form a second bridge of molecules near the center of the previously ring-like feature (in the vicinity of the yellow star in Fig. 5B). However, additional reorganization occurs to optimize the H bonding, including a modification of the original bridge structure formed at 6 H2O/u.c. Since the coverage at 8 H2O/u.c. is already close to that of a close-packed ice layer, it is straightforward to understand why further water adsorption results in the formation of multilayer ice. All structures shown in Fig. 5 can be downloaded as part of SI Appendix.
Discussion
Based on the experimental and theoretical evidence presented above, we conclude that partially dissociated water dimers are the most stable species on the Fe3O4(001) surface, closely followed by structurally related, partially dissociated water trimers. Our nc-AFM images clearly show the adsorbed dimers and trimers, and XPS spectra reveal them to be partially dissociated. Moreover, the theoretically determined adsorption energies agree remarkably well with the Ed values obtained from an inversion analysis of the δ-peak, and the highly constrained adsorption geometry predicted by DFT is consistent with the high preexponential factor (ν = 1017 ± 1 s−1). For higher coverages, the inversion analysis reveals the Ed necessary to desorb the most weakly bound molecule(s) and thus should not be compared with the average adsorption energies calculated by DFT.
Clearly, the (√2 × √2)R45° reconstruction plays a crucial role in the adsorption behavior. At low coverages, the partially dissociated water dimers and trimers order with (√2 × √2)R45° symmetry, while at high coverages the structure of the H-bonded network also belies the periodicity of the underlying substrate. Ultimately, this stems from the strong preference to form surface O*H groups, which limits the density of dimers/trimers to one per unit cell. Later, the O*H groups provide a hydrogen bond to bridge the Feoct rows and complete the H-bonded network.
Despite the importance of the O* sites, the primary contribution to the adsorption energy at low coverage arises from the Fe3+–Owater bond. An isolated water molecule binds strongly atop an Feoct row atom (−0.64 eV) and prefers this state to dissociation by 0.05 eV. This result differs from the prior calculations of Mulakaluri et al. (23, 54), and after extensive testing, we have found that the discrepancy originates in the structural model used, and not the functional applied. As suggested by Mulakaluri et al. (54), the Fe2+ cations in the subsurface layers of a bulk-truncated structure interact with the adsorbates and promote dissociation. The SCV reconstruction contains only Fe3+ cations in the outermost four layers, and molecular adsorption is preferred.
Given the lack of dissociation in the monomer case, it is somewhat surprising that partially dissociated water dimers form on the Fe3O4(001) surface. Recently, Freund’s group (19) proposed that partial dissociation requires two molecules to meet on the Fe3O4(111) surface, but later revised their IRAS analysis in favor of the “traditional picture” where dissociation occurs first in isolation on an undercoordinated anion–cation pair (27). Our STM images show that dimerization occurs already at very low coverages on Fe3O4(001), and there is no evidence for monomer dissociation in the form of additional isolated O*H groups. It is, however, difficult to know if the dimers are molecular or partially dissociated from STM alone. This ambiguity does not exist for higher coverages: nc-AFM images of 2 H2O/u.c. (Fig. 3A) show dimerization occurs already at 155 K, and analysis of XPS spectra suggests that roughly one molecule per agglomerate is dissociated.
What, then, drives the partial dissociation of water dimers in the water/Fe3O4(001) system? To answer this question, we first analyze the DFT results for a molecular water dimer (Fig. 6A). Somewhat surprisingly, the energy gain of molecular dimerization is small; an isolated molecule has a binding energy of −0.64 eV, while the average binding energy in the molecular dimer is just −0.66 eV per molecule. This difference is significantly less than the binding energy of an H bond in a gas-phase water dimer (−0.10 eV per molecule). Since the H-bond length in the present system (1.89 Å) is significantly shorter than that of a gas-phase water dimer (2.0 Å), some energy must be lost in the final structure. In this regard, we consider the Feoct–Owater bond lengths. The water that donates an H bond has an Feoct–O bond of 2.20 Å, comparable to the isolated water monomer (2.22 Å), but the acceptor molecule has an Feoct–O bond length of 2.34 Å. This suggests that receiving an H bond weakens the interaction of a water molecule with the substrate, consistent with the idea that forming Feoct–O bonds and receiving H bonds both involve the lone-pair (O 2p) orbitals (55), and that the competition leads to the marginal total-energy gain.
Fig. 6.
The geometry of partially dissociated water dimers and trimers reveals a cooperative binding effect. (A) A molecular water dimer exhibits a relatively long intermolecular H bond, and the H-bond acceptor has a weakened interaction with the surface compared with an isolated molecule. (B) The partially dissociated water dimer exhibits a strong intermolecular H bond, and the H-bond–donating water molecule binds more strongly to the substrate. (C) In the partially dissociated water trimer, the second water molecule donates an H bond to the OH group, further weakening its bond to the substrate. All bond lengths are given in angstroms, and energies are in electronvolts.
The situation is very different for a partially dissociated water dimer (Fig. 6B). Here, the average adsorption energy per molecule is −0.92 eV, so the small energetic cost of dissociating one molecule (0.05 eV for an isolated monomer) is easily compensated. The intermolecular H bond is significantly shorter (1.41 Å) in the partially dissociated water dimer, as expected on electrostatic grounds (the OH species is negatively charged). Moreover, the H-bond–donating water molecule has a significantly shorter Feoct–Owater bond (2.06 eV) than in the molecular water dimer, suggesting a stronger interaction with the substrate. This phenomenon has been observed in gas-phase water clusters (56), and on metal surfaces (55, 57), and is known as cooperativity (58). Essentially, water molecules seek a balance in their H-bonding interactions. If a molecule donates a strong H bond, it accepts stronger H bonds. Since H-bond acceptance utilizes the same O orbital as the Feoct–Owater bond, the balance can be achieved through an enhanced interaction with the substrate. Thus, the formation of the negatively charged OH group induces water to donate a strong H bond, which in turn induces a stronger water–surface interaction. The energy gain is so substantial that the system can accommodate a weakened terminal-OH Feoct–O bond, which is 0.12 Å longer than for an isolated terminal OH. Note that diffusion of the partially dissociated water dimer is likely hampered by the anisotropic nature of the surface, on which neighboring Fe cations are separated by 3 Å. Breaking the OH–H2O bond is highly unfavorable, while a concerted diffusion mechanism would require a transition state in which either the water molecule or the OH is completely detached from the substrate.
Next, we turn our attention to the partially dissociated water trimer (Fig. 6C). The linear trimer is a natural consequence of the arguments outlined above, as there is an undercoordinated Feoct atom available to which a water molecule can bind and simultaneously donate an H bond into the OH group of the partially dissociated dimer. Of course, the Fe–Fe separation along the Feoct rows is considerably larger (3 Å) than the sum of OH and H-bond lengths, so the partially dissociated water trimer forms with the OH group directly atop an Feoct atom, with both water molecules leaning in toward the OH from their favored position atop an Feoct (Fig. 6). The H bonds are slightly longer, as is the OH Feoct–O bond. This results in the slightly lower adsorption energy of −0.88 eV per molecule compared with the OH–H2O water dimer.
To this point in the discussion, the DFT search predicts what is observed in experiment and allows us to understand why the partially dissociated water agglomerates form and are so strongly bound. However, DFT finds a nonlinear partially dissociated water trimer (E3 ISO in Fig. 5B) with a comparable adsorption energy to the partially dissociated linear trimer, which is not observed in the experiments. The fact that we can resolve water bridging the Feoct rows upon the formation of the H-bonded network with nc-AFM in Fig. 3B gives us confidence we could detect the nonlinear trimer if it were present. This indicates that the energy balance involved in adsorbed water agglomerates is not perfectly handled by DFT. To investigate further, we compared several alternative functionals, with and without vdW corrections, and obtained similar trends, albeit with a variation of ±0.1 eV in the absolute energies. We conclude that DFT is insufficiently accurate to predict the relative stabilities of water adlayers, and that calculating reliable structures requires guidance from experiment or the adoption of superior approaches such as hybrid functionals or the random-phase approximation (59, 60).
Once the partially dissociated water trimer (Fig. 6C) forms, it is not possible to H-bond additional water along the Feoct row. The TPD data suggests that the next stable structure occurs at a coverage of 6 H2O/u.c., where the nc-AFM images (Fig. 3B) clearly resolve a ring-like structure with additional protrusions bridging the molecules adsorbed at the Feoct rows. This is in line with the minimum-energy structure determined at 6 H2O/u.c., which is the first configuration to establish an H-bonded network extending over the whole surface. The stability of the proposed structure stems from the presence of H2O–OH–H2O trimers, which utilize H2O molecules stabilized at the O* bridge sites. The structure is consistent with the 2D ice rules (3, 4) in that water forms a closed-loop structure with a preference for atop adsorption sites, although one molecule per unit cell is a “double acceptor,” protruding by more than 3 Å above the surface and where the absent surface bond is compensated by a second H bond, making it appear similar to a water molecule in 3D ice. We note, however, that the experimental data do not allow to unambiguously confirm the fine details of the structure, and that the XPS fitting at 7.7 D2O/u.c. suggests that less water is dissociated. The same is true at a coverage of 8 H2O/u.c., the next coverage where the TPD data indicates that a stable structure exists. The nc-AFM images show that new, ordered protrusions emerge when additional water is added to the ring-like structure (Fig. 3C), and DFT predicts that this water binds via the remaining dangling H bonds present in the 6 H2O/u.c. structure. That these molecules bind solely by H bonds to other water explains why the desorption temperature is so close to that of multilayer ice. The reason for the observed strong AFM contrast is not yet known, and we cannot discount the possibility of additional rearrangement at this coverage. What is clear is that the β and α′ peaks observed in TPD are due to water squeezed into the 6 H2O/u.c. structure, leading to a reoptimization of the available H bonds.
As mentioned above, partially dissociated water dimers have recently been reported to be the most stable species on RuO2(110) (18) and Fe3O4(111) (19, 20), and appear to be common on metal oxide surfaces. Based on the analysis presented here, we expect these species to form whenever there are undercoordinated surface cations sufficiently close together that a H2O–OH bond can be established, provided there are undercoordinated O atoms available to form a stable surface hydroxyl. Here, the SCV reconstruction of Fe3O4(001) limits the availability of the latter sites, which is why the water dimer coverage saturates at one per unit cell. On RuO2(110) (18), for example, where the undercoordinated surface O atoms are homogeneous and plentiful, a complete coverage of H2O–OH dimers is achieved.
Before concluding, it is worth to consider whether the low-temperature–low-pressure phenomena observed here bear any resemblance to the adsorption–desorption of water on Fe3O4(001) under more realistic conditions. At first glance, the adsorption threshold of 10−2 mbar observed by Kendelewicz et al. (47) at 273 K in ambient-pressure XPS studies suggests a significant pressure gap. However, this threshold is entirely consistent with our assertion that isolated molecules are weakly bound and that a strongly bound partially dissociated water dimer species forms when two molecules meet on the surface. Given the binding energy of the water monomer (−0.64 eV) determined here, the 10−2-mbar threshold corresponds to an instantaneous coverage of 0.2 H2O/u.c. at 273 K. This is sufficient to expect that two monomers can meet before desorbing. The as-formed dimer is more strongly bound, so a stable coverage will develop rapidly. Alternatively, the 10−2-mbar threshold corresponds to a chemical potential of −0.70 eV, which agrees very well with the adsorption energy of the partially dissociated water dimer determined by the inversion analysis (−0.85 eV). As such, the surface-science approach utilized here appears directly applicable to understand the adsorption–desorption of water at pressures relevant to catalysis. To our knowledge, the reactivity of partially dissociated water dimers has not been studied directly, and it will be fascinating to see whether these species play an active role in geochemical or corrosion processes, or in catalysis where metal oxides are frequently used as the active component or as the support for metal nanoparticles. In particular, it will be interesting to learn whether partially dissociated species play a role in the water–gas shift reaction, since industry currently utilizes an Fe3O4 based catalyst (61–64) and partially dissociated species have now been directly identified on both major facets.
In summary, the formation of partially dissociated water agglomerates on Fe3O4(001) is driven by the formation of strong intermolecular H bonds and facilitated by the close proximity of undercoordinated cations. The presence of the SCV reconstruction ensures that partially dissociated water dimers and trimers remain isolated because there is only one O site per unit cell that can accommodate an H+. The partially dissociated agglomerates act as an anchor to build a ring-like H-bonded network as the coverage is increased, and the water layer completes by saturating dangling H bonds within this stable structure. A similar evolution can be expected wherever a surface presents well-spaced active sites for dissociation.
Materials and Methods
Experimental Setup.
The TPD and XPS experiments were performed in a vacuum system optimized for the study of the surface chemistry of metal oxide single crystals. The system has been described in detail elsewhere (30). Briefly, the single-crystal Fe3O4 sample (6 × 6 × 1 mm; SurfaceNet) is mounted on a Ta backplate in thermal contact with a liquid-He flow cryostat. The sample can reach a base temperature of ∼30 K and can be heated to 1,200 K by direct current heating of the sample plate. Temperatures are measured by a K-type thermocouple spot-welded to the sample plate and calibrated by the multilayer desorption of simple gases (65). The sample was prepared by cycles of 1 keV Ne+ sputtering and annealing at 900 K for 20 min in a partial pressure of 1 × 10−6 mbar O2. D2O was adsorbed directly onto a 3.5-mm-diameter spot in the center of the sample surface using an effusive molecular beam source. The beam has top-hat profile and a precisely calibrated flux (9.2 ± 0.5 × 1012 D2O molecules/cm2⋅s) at the sample. Coupled with sticking-probability measurements, this provides accurate prediction of the absolute water coverage (30, 66). This is particularly straightforward to achieve here, because the sticking probability for water is unity at all coverages at 100 K (SI Appendix, Fig. S1 and Fig. 1B). For TPD experiments, the sample is exposed to water at 100 K, and then heated with a linear ramp of 1 K/s. XPS utilizes a SPECS Phoibos 150 analyzer with a monochromatized FOCUS 500 Al Kα X-ray source. STM and nc-AFM measurements were performed in a separate vacuum system using an Omicron LT-STM equipped with a QPlus sensor with an improved preamplifier (67). Here, water exposures were performed using a high-precision leak valve. The water coverage is defined in H2O molecules per (√2 × √2)R45° unit cell (H2O/u.c.), where 1 H2O/u.c. is a coverage of 1.42 × 1014 cm−2. The tip was functionalized by a CO molecule picked up from a Pt cluster (details in SI Appendix).
Computational Details.
The Vienna ab initio simulation package (68, 69) was used for all DFT calculations. The projector augmented wave (70, 71) method describes the electron and ion interactions, with the plane wave basis set cutoff energy set to 550 eV. A Γ-centered k-mesh of 5 × 5 × 5 was used for bulk calculations, adjusted to 5 × 5 × 1 for (001) surface calculations. Convergence is achieved when the electronic energy step of 10−6 eV is obtained, and forces acting on ions become smaller than 0.02 eV/Å. The calculations are based on the SCV reconstructed model of the Fe3O4(001) surface (34). Adsorption energies Ead are corrected for the zero-point energy (details in SI Appendix) and are quoted as an average (per molecule, if >1 H2O/u.c.), unless otherwise mentioned.
The optPBE-DF (51–53) functional (details in SI Appendix) accounts for the vdW corrections and ultimately delivers results that correlate well with the experiments. The same functional was recently used to simulate water adsorbed on NaCl(001) and MgO(001) surfaces (72) and water clusters (28).
The optimum configuration for each water coverage was determined via a systematic search inspired by genetic algorithms. For each coverage, the results of at least 10 trial calculations were analyzed to identify factors leading to a low total energy. These insights, together with those found at other coverages, were used to build a next generation of trial structures. This process eventually leads to the lowest-energy configuration the system can reach for the given coverage of water. In the end, over 500 configurations have been investigated.
Supplementary Material
Acknowledgments
The computational results presented have been achieved by using the Vienna Scientific Cluster. We gratefully acknowledge funding through projects from the Austrian Science Fund [START-Prize Y 847-N20 (to M.M., J.H., R.B., and G.S.P.); Special Research Project “Functional Surfaces and Interfaces,” FOXSI F4505-N16 and F4507-N16 (to M. Schmid and U.D.)], the European Research Council [ERC-2011-ADG_20110209 Advanced Grant “OxideSurfaces” (to U.D.)], and the Doctoral College TU-D (Z.J.) and Solids4fun [W1243 (to R.B.)].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801661115/-/DCSupplemental.
References
- 1.Björneholm O, et al. Water at interfaces. Chem Rev. 2016;116:7698–7726. doi: 10.1021/acs.chemrev.6b00045. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson A, Haq S. Water adsorption and the wetting of metal surfaces. Surf Sci Rep. 2009;64:381–451. [Google Scholar]
- 3.Liriano ML, et al. Water-ice analogues of polycyclic aromatic hydrocarbons: Water nanoclusters on Cu(111) J Am Chem Soc. 2017;139:6403–6410. doi: 10.1021/jacs.7b01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier S, Salmeron M. How does water wet a surface? Acc Chem Res. 2015;48:2783–2790. doi: 10.1021/acs.accounts.5b00214. [DOI] [PubMed] [Google Scholar]
- 5.Henderson MA. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf Sci Rep. 2002;46:1–308. [Google Scholar]
- 6.Uhlenbrock S, Scharfschwerdt C, Neumann M, Illing G, Freund H-J. The influence of defects on the Ni 2p and O 1s XPS of NiO. J Phys Condens Matter. 1992;4:7973–7978. [Google Scholar]
- 7.Önsten A, et al. Water adsorption on ZnO(0001): Transition from triangular surface structures to a disordered hydroxyl terminated phase. J Phys Chem C. 2010;114:11157–11161. [Google Scholar]
- 8.Henderson MA, Chambers SA. HREELS, TPD and XPS study of the interaction of water with the α-Cr2O3(001) surface. Surf Sci. 2000;449:135–150. [Google Scholar]
- 9.Costa D, Sharkas K, Islam MM, Marcus P. Ab initio study of the chemical states of water on Cr2O3(0001): From the isolated molecule to saturation coverage. Surf Sci. 2009;603:2484–2493. [Google Scholar]
- 10.Diebold U. Perspective: A controversial benchmark system for water-oxide interfaces: H2O/TiO2(110) J Chem Phys. 2017;147:040901. doi: 10.1063/1.4996116. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z-T, et al. Probing equilibrium of molecular and deprotonated water on TiO2(110) Proc Natl Acad Sci USA. 2017;114:1801–1805. doi: 10.1073/pnas.1613756114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugenschmidt MB, Gamble L, Campbell CT. The interaction of H2O with a TiO2(110) surface. Surf Sci. 1994;302:329–340. [Google Scholar]
- 13.Duncan DA, Allegretti F, Woodruff DP. Water does partially dissociate on the perfect TiO2(110) surface: A quantitative structure determination. Phys Rev B. 2012;86:045411. [Google Scholar]
- 14.Walle LE, Borg A, Uvdal P, Sandell A. Experimental evidence for mixed dissociative and molecular adsorption of water on a rutile TiO2(110) surface without oxygen vacancies. Phys Rev B. 2009;80:235436. [Google Scholar]
- 15.Kumar N, Kent PRC, Wesolowski DJ, Kubicki JD. Modeling water adsorption on rutile (110) using van der Waals density functional and DFT+U methods. J Phys Chem C. 2013;117:23638–23644. [Google Scholar]
- 16.Lindan PJD, Zhang C. Exothermic water dissociation on the rutile TiO2(110) surface. Phys Rev B. 2005;72:075439. [Google Scholar]
- 17.Mu R, Zhao ZJ, Dohnálek Z, Gong J. Structural motifs of water on metal oxide surfaces. Chem Soc Rev. 2017;46:1785–1806. doi: 10.1039/c6cs00864j. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M-T, et al. Dynamics, stability, and adsorption states of water on oxidized RuO2(110) J Phys Chem C. 2017;121:18505–18515. [Google Scholar]
- 19.Dementyev P, et al. Water interaction with iron oxides. Angew Chem Int Ed Engl. 2015;54:13942–13946. doi: 10.1002/anie.201506439. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Paier J. Adsorption of water on the Fe3O4(111) surface: Structures, stabilities, and vibrational properties studied by density functional theory. J Phys Chem C. 2016;120:1056–1065. [Google Scholar]
- 21.Kan HH, Colmyer RJ, Asthagiri A, Weaver JF. Adsorption of water on a PdO(101) thin film: Evidence of an adsorbed HO−H2O complex. J Phys Chem C. 2009;113:1495–1506. [Google Scholar]
- 22.Zhang Z, Bondarchuk O, Kay BD, White JM, Dohnálek Z. Imaging water dissociation on TiO2(110): Evidence for inequivalent geminate OH groups. J Phys Chem B. 2006;110:21840–21845. doi: 10.1021/jp063619h. [DOI] [PubMed] [Google Scholar]
- 23.Mulakaluri N, Pentcheva R, Wieland M, Moritz W, Scheffler M. Partial dissociation of water on Fe3O4(001): Adsorbate induced charge and orbital order. Phys Rev Lett. 2009;103:176102. doi: 10.1103/PhysRevLett.103.176102. [DOI] [PubMed] [Google Scholar]
- 24.Halwidl D, et al. Adsorption of water at the SrO surface of ruthenates. Nat Mater. 2016;15:450–455. doi: 10.1038/nmat4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Włodarczyk R, et al. Structures of the ordered water monolayer on MgO(001) J Phys Chem C. 2011;115:6764–6774. [Google Scholar]
- 26.Kenmoe S, Biedermann PU. Water aggregation and dissociation on the ZnO surface. Phys Chem Chem Phys. 2017;19:1466–1486. doi: 10.1039/c6cp07516a. [DOI] [PubMed] [Google Scholar]
- 27.Mirabella F, et al. Cooperative formation of long-range ordering in water ad-layers on Fe3O4(111) Angew Chem Int Ed. 2018;57:1409–1413. doi: 10.1002/anie.201711890. [DOI] [PubMed] [Google Scholar]
- 28.Gillan MJ, Alfè D, Michaelides A. Perspective: How good is DFT for water? J Chem Phys. 2016;144:130901. doi: 10.1063/1.4944633. [DOI] [PubMed] [Google Scholar]
- 29.Gross L, Mohn F, Moll N, Liljeroth P, Meyer G. The chemical structure of a molecule resolved by atomic force microscopy. Science. 2009;325:1110–1114. doi: 10.1126/science.1176210. [DOI] [PubMed] [Google Scholar]
- 30.Pavelec J, et al. A multi-technique study of CO2 adsorption on Fe3O4 magnetite. J Chem Phys. 2017;146:014701. doi: 10.1063/1.4973241. [DOI] [PubMed] [Google Scholar]
- 31.Tait SL, Dohnálek Z, Campbell CT, Kay BD. n-alkanes on MgO(100). II. Chain length dependence of kinetic desorption parameters for small n-alkanes. J Chem Phys. 2005;122:164708. doi: 10.1063/1.1883630. [DOI] [PubMed] [Google Scholar]
- 32.Campbell CT, Sellers JRV. The entropies of adsorbed molecules. J Am Chem Soc. 2012;134:18109–18115. doi: 10.1021/ja3080117. [DOI] [PubMed] [Google Scholar]
- 33.Weaver JF. Chemistry. Entropies of adsorbed molecules exceed expectations. Science. 2013;339:39–40. doi: 10.1126/science.1231552. [DOI] [PubMed] [Google Scholar]
- 34.Bliem R, et al. Subsurface cation vacancy stabilization of the magnetite (001) surface. Science. 2014;346:1215–1218. doi: 10.1126/science.1260556. [DOI] [PubMed] [Google Scholar]
- 35.Bliem R, et al. Cluster nucleation and growth from a highly supersaturated adatom phase: Silver on magnetite. ACS Nano. 2014;8:7531–7537. doi: 10.1021/nn502895s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkinson GS, et al. Semiconductor-half metal transition at the Fe3O4(001) surface upon hydrogen adsorption. Phys Rev B. 2010;82:125413. [Google Scholar]
- 37.Mulakaluri N, Pentcheva R. Hydrogen adsorption and site-selective reduction of the Fe3O4(001) surface: Insights from first principles. J Phys Chem C. 2012;116:16447–16453. [Google Scholar]
- 38.Parkinson GS, Novotný Z, Jacobson P, Schmid M, Diebold U. Room temperature water splitting at the surface of magnetite. J Am Chem Soc. 2011;133:12650–12655. doi: 10.1021/ja203432e. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson GS, et al. Antiphase domain boundaries at the Fe3O4(001) surface. Phys Rev B. 2012;85:195450. [Google Scholar]
- 40.Gamba O, et al. The role of surface defects in the adsorption of methanol on Fe3O4(001) Top Catal. 2017;60:420–430. doi: 10.1007/s11244-016-0713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiotari A, Sugimoto Y. Ultrahigh-resolution imaging of water networks by atomic force microscopy. Nat Commun. 2017;8:14313. doi: 10.1038/ncomms14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, et al. Real-space imaging of interfacial water with submolecular resolution. Nat Mater. 2014;13:184–189. doi: 10.1038/nmat3848. [DOI] [PubMed] [Google Scholar]
- 43.Meng X, et al. Direct visualization of concerted proton tunnelling in a water nanocluster. Nat Phys. 2015;11:235–239. [Google Scholar]
- 44.Peng J, et al. Weakly perturbative imaging of interfacial water with submolecular resolution by atomic force microscopy. Nat Commun. 2018;9:122. doi: 10.1038/s41467-017-02635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taguchi M, et al. Temperature dependence of magnetically active charge excitations in magnetite across the Verwey transition. Phys Rev Lett. 2015;115:256405. doi: 10.1103/PhysRevLett.115.256405. [DOI] [PubMed] [Google Scholar]
- 46.Hesse R, Streubel P, Szargan R. Product or sum: Comparative tests of Voigt, and product or sum of Gaussian and Lorentzian functions in the fitting of synthetic Voigt‐based X‐ray photoelectron spectra. Surf Interface Anal. 2007;39:381–391. [Google Scholar]
- 47.Kendelewicz T, et al. X-ray photoemission and density functional theory study of the interaction of water vapor with the Fe3O4(001) surface at near-ambient conditions. J Phys Chem C. 2013;117:2719–2733. [Google Scholar]
- 48.Köhler L, Kresse G. Density functional study of CO on Rh(111) Phys Rev B. 2004;70:165405. [Google Scholar]
- 49.Kiejna A, Ossowski T, Pabisiak T. Surface properties of the clean and Au/Pd covered Fe3O4(111): DFT and DFT+U study. Phys Rev B. 2012;85:125414. [Google Scholar]
- 50.Bernal-Villamil I, Gallego S. Charge order at magnetite Fe3O4(001): Surface and Verwey phase transitions. J Phys Condens Matter. 2015;27:12001. doi: 10.1088/0953-8984/27/1/012001. [DOI] [PubMed] [Google Scholar]
- 51.Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI. van der Waals density functional for general geometries. Phys Rev Lett. 2004;92:246401, and erratum (2005) 95:109902. doi: 10.1103/PhysRevLett.92.246401. [DOI] [PubMed] [Google Scholar]
- 52.Klimeš J, Bowler DR, Michaelides A. Chemical accuracy for the van der Waals density functional. J Phys Condens Matter. 2010;22:022201. doi: 10.1088/0953-8984/22/2/022201. [DOI] [PubMed] [Google Scholar]
- 53.Lee K, Murray ÉD, Kong L, Lundqvist BI, Langreth DC. Higher-accuracy van der Waals density functional. Phys Rev B. 2010;82:081101. [Google Scholar]
- 54.Mulakaluri N, Pentcheva R, Scheffler M. Coverage-dependent adsorption mode of water on Fe3O4(001): Insights from first principles calculations. J Phys Chem C. 2010;114:11148–11156. [Google Scholar]
- 55.Schiros T, et al. Cooperativity in surface bonding and hydrogen bonding of water and hydroxyl at metal surfaces. J Phys Chem C. 2010;114:10240–10248. [Google Scholar]
- 56.Xantheas SS. Cooperativity and hydrogen bonding network in water clusters. Chem Phys. 2000;258:225–231. [Google Scholar]
- 57.Schiros T, et al. Structure and bonding of the water−hydroxyl mixed phase on Pt(111) J Phys Chem C. 2007;111:15003–15012. [Google Scholar]
- 58.Frank HS, Wen W-Y. Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: A suggested picture of water structure. Discuss Faraday Soc. 1957;24:133–140. [Google Scholar]
- 59.Stroppa A, Termentzidis K, Paier J, Kresse G, Hafner J. CO adsorption on metal surfaces: A hybrid functional study with plane-wave basis set. Phys Rev B. 2007;76:195440. [Google Scholar]
- 60.Schimka L, et al. Accurate surface and adsorption energies from many-body perturbation theory. Nat Mater. 2010;9:741–744. doi: 10.1038/nmat2806. [DOI] [PubMed] [Google Scholar]
- 61.Martos C, Dufour J, Ruiz A. Synthesis of Fe3O4-based catalysts for the high-temperature water gas shift reaction. Int J Hydrogen Energy. 2009;34:4475–4481. [Google Scholar]
- 62.Estrella M, et al. In situ characterization of CuFe2O4 and Cu/Fe3O4 water−gas shift catalysts. J Phys Chem C. 2009;113:14411–14417. [Google Scholar]
- 63.Newsome DS. The water-gas shift reaction. Catal Rev. 1980;21:275–318. [Google Scholar]
- 64.Rhodes C, Williams BP, King F, Hutchings GJ. Promotion of Fe3O4/Cr2O3 high temperature water gas shift catalyst. Catal Commun. 2002;3:381–384. [Google Scholar]
- 65.Schlichting H, Menzel D. High resolution, wide range, thermal desorption spectrometry of rare gas layers: Sticking, desorption kinetics, layer growth, phase transitions, and exchange processes. Surf Sci. 1992;272:27–33. [Google Scholar]
- 66.Hulva J, et al. Adsorption of CO on the Fe3O4(001) surface. J Phys Chem B. 2018;122:721–729. doi: 10.1021/acs.jpcb.7b06349. [DOI] [PubMed] [Google Scholar]
- 67.Huber F, Giessibl FJ. Low noise current preamplifier for qPlus sensor deflection signal detection in atomic force microscopy at room and low temperatures. Rev Sci Instrum. 2017;88:073702. doi: 10.1063/1.4993737. [DOI] [PubMed] [Google Scholar]
- 68.Kresse G, Hafner J. Ab initio molecular dynamics for open-shell transition metals. Phys Rev B Condens Matter. 1993;48:13115–13118. doi: 10.1103/physrevb.48.13115. [DOI] [PubMed] [Google Scholar]
- 69.Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6:15–50. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 70.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B. 1999;59:1758–1775. [Google Scholar]
- 71.Blöchl PE. Projector augmented-wave method. Phys Rev B Condens Matter. 1994;50:17953–17979. doi: 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- 72.Kebede GG, Spångberg D, Mitev PD, Broqvist P, Hermansson K. Comparing van der Waals DFT methods for water on NaCl(001) and MgO(001) J Chem Phys. 2017;146:064703. doi: 10.1063/1.4971790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.