Significance
Langerhans cells (LCs) are the exclusive antigen-presenting cells of the epidermis, capable of mounting immunity and tolerance. LCs maintain themselves locally by self-renewing throughout life, a process that is regulated by both LCs and keratinocytes. Nevertheless, the mechanisms underlying this process are not clearly understood. Using targeted genetic ablation, we demonstrate that lack of protein S (PROS1) in keratinocytes, but not in LCs, results in reduced numbers of terminally developed LCs. This is due to increased apoptosis of LCs and associated with altered expression of cytokines involved in tissue homeostasis. Furthermore, PROS1 also down-regulates LC differentiation from bone marrow precursors. Together, these identify PROS1 as a regulator of LC development and homeostasis.
Keywords: Langerhans cells, protein S, TAM signaling, epidermis, Pros1
Abstract
AXL, a member of the TYRO3, AXL, and MERTK (TAM) receptor tyrosine kinase family, has been shown to play a role in the differentiation and activation of epidermal Langerhans cells (LCs). Here, we demonstrate that growth arrest-specific 6 (GAS6) protein, the predominant ligand of AXL, has no impact on LC differentiation and homeostasis. We thus examined the role of protein S (PROS1), the other TAM ligand acting primarily via TYRO3 and MERTK, in LC function. Genetic ablation of PROS1 in keratinocytes resulted in a typical postnatal differentiation of LCs; however, a significant reduction in LC frequencies was observed in adult mice due to increased apoptosis. This was attributed to altered expression of cytokines involved in LC development and tissue homeostasis within keratinocytes. PROS1 was then excised in LysM+ cells to target LCs at early embryonic developmental stages, as well as in adult monocytes that also give rise to LCs. Differentiation and homeostasis of LCs derived from embryonic precursors was not affected following Pros1 ablation. However, differentiation of LCs from bone marrow (BM) precursors in vitro was accelerated, as was their capability to reconstitute epidermal LCs in vivo. These reveal an inhibitory role for PROS1 on BM-derived LCs. Collectively, this study highlights a cell-specific regulation of LC differentiation and homeostasis by TAM signaling.
Stratified epithelia, such as the skin epidermis and certain mucosal epithelia, contain a special type of antigen-presenting cells, named Langerhans cells (LCs). LCs represent the first line of defense against invading pathogens but are also capable of maintaining homeostasis with commensal bacteria (1–3). Epidermal LCs originate from embryonic precursors that seed the epithelium until embryonic day 18 (E18). Immediately after birth, these precursors massively proliferate while differentiating into a radioresistant and self-renewing population (4, 5). LCs in mucosal epithelia have a distinct ontogeny as they arise from bone marrow (BM) precursors—pre-DCs (predendritic cells) and monocytes. Unlike their equivalents in the epidermis, mucosal LCs are radiosensitive and continuously replaced by circulating precursors (6). Monocytes can also differentiate into epidermal LCs under certain conditions (5, 7, 8). Regardless of their origin, the differentiation of both epidermal and mucosal LCs is instructed by the epithelium. Nevertheless, intrinsic features unique to each precursor type are also likely to regulate LC development.
AXL, a member of the TYRO3, AXL, and MERTK (TAM) tyrosine kinase receptor family, is expressed on epidermal LCs and has been reported to play a role in their differentiation and activation (9). The TAM receptors and their ligands, protein S (PROS1) and growth arrest-specific 6 (GAS6), are expressed by many hematopoietic and nonhematopoietic cells and are involved in various biological, developmental, and immunological functions (10–12). GAS6 is the predominant ligand of AXL while PROS1 acts mainly via TYRO3 and MERTK (13–15). Despite the proposed role of AXL in LC development, no alteration in the epidermal LC network was found in Axl−/− mice, which could be explained by functional redundancy known to exist among the TAM receptors (9). Nevertheless, since AXL is expressed by both LCs and keratinocytes, it is hard to conclude how the absence of AXL in each cell type affects LCs. As the TAM ligands interact differentially with the various TAM receptors, exploring their function should shed light on the involvement of the TAM signaling pathways in LC ontogeny. Here, we show that, whereas GAS6 is dispensable for LC differentiation, ablation of PROS1 in specific cell types exerts distinct regulatory functions on the development of LCs.
Results
Expression of TAM Receptors and Ligands in the Murine Ear Skin.
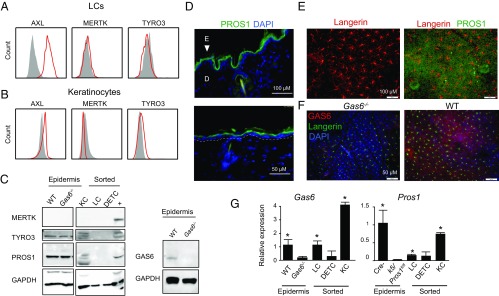
To study the impact of TAM ligands on epidermal LCs, we first characterized the expression of TAM receptors in epidermal cells. LCs (CD45+ MHCII+ CD11c+ EpCAM+ langerin/CD207+) were found to express AXL, but not MERTK or TYRO3 (Fig. 1A). A large fraction of CD45-negative epidermal cells, representing keratinocytes, expressed AXL whereas MERTK expression was not detected (Fig. 1B). Expression of TYRO3 was observed in part of the keratinocytes using both flow cytometry and Western blot analyses (Fig. 1 B and C). We next examined the expression of the TAM ligands. PROS1 and GAS6 were detected via Western blot in epidermal sheet lysate (Fig. 1C). Immunofluorescence staining further visualized both ligands in keratinocytes while PROS1 appeared to be located in the suprabasal epidermal layers (Fig. 1 D–F). Western blot analysis of sorted keratinocytes, LCs, and γδT cells revealed that PROS1 is expressed mainly by keratinocytes (Fig. 1C). PROS1 was undetectable in LCs also using flow cytometry (SI Appendix, Fig. S1). Similar staining for GAS6 using different antibodies did not provide technically reliable results. To overcome this technical hurdle, we fractionated epidermal cell populations and quantified Gas6 and Pros1 mRNA using RT-qPCR (reverse transcription quantitative polymerase chain reaction). As depicted in Fig. 1G, significant Gas6 and Pros1 mRNA levels were detected in keratinocytes and, to a lesser extent, in LCs, but not in cells derived from Gas6−/− and keratin 5 (K5)/Pros1fl/fl, which served as negative controls, respectively. In K5/Pros1fl/fl mice, the Pros1 alleles are homozygously deleted in keratinocytes. Taken together, among the various TAM receptors, AXL is strongly expressed by most keratinocytes and LCs whereas TYRO3 is expressed at lower levels or only by a small fraction of keratinocytes. Such an expression pattern fits well with previous observations demonstrating that expression of TAM receptors and ligands is restricted to certain cellular layer/s within the stratified epithelium (9, 16). Regarding TAM ligands, both PROS1 and GAS6 are expressed by keratinocytes while LCs might express either no protein or only a limited amount of the ligands.
Fig. 1.
Expression of TAM receptors and ligands in the murine skin epidermis. Epidermal cells were prepared from 8-wk-old mice, and representative flow cytometry plots present the expression of AXL, MERTK, and TYRO3 receptors on (A) LCs and (B) keratinocytes. Data of one of three independent experiments are provided, and each experiment included at least five separately analyzed mice. (C) Western blot analysis showing the presence of MERTK, TYRO3, PROS1, GAS6, and GAPDH as a control in lysates of total epidermal cells from adult WT Gas6+/+ and Gas6−/− mice or sorted keratinocytes, LCs, and dendritic epidermal T cells (DETC) from 8-wk-old WT mice. +, positive control. Representative results of one of three independent experiments are shown. (D–F) Ear cross-sections (D, Upper image presents the actual image while the Lower image provides a magnification of a selected area) or whole epidermal sheets (E) were stained for PROS1 (green), langerin (red), and DAPI (blue) for nuclear visualization. The white dotted line demarcates the basal membrane and the arrowhead indicates the epidermis. (F) Whole epidermal sheets were stained with GAS6 (red) and langerin (green). Immunofluorescence microscopy images are shown representing one of three independent experiments. (G) Relative expression levels of Pros1 and Gas6 in whole epidermal tissues and sorted cells isolated from 8-wk-old mice were analyzed by RT-qPCR. Bar graphs present the fold change in mRNA levels among the various groups, which was normalized to Gas6+/+ or Cre− mice, respectively, ±SD (n = 5). D, dermis; E, epidermis. *P < 0.05. Results of one of two independent experiments are presented.
Ablation of GAS6 Minimally Affects the Development of Epidermal LCs.
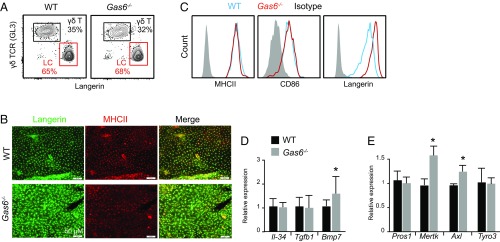
Since AXL has been reported to play a role in LC development and homeostasis (9), we examined whether GAS6, the high affinity ligand of AXL, is also involved in this process. Similar frequencies of LCs, as well as γδT cells, the other major leukocyte population in the epidermis, were found in the ear epidermis of adult Gas6−/− mice and littermate controls (Fig. 2A). A whole-mount immunofluorescence staining of epidermal sheets with MHCII and langerin further demonstrated the presence of a typical LC network in the epidermis of Gas6−/− mice (Fig. 2B). Interestingly, the staining intensity of langerin in Gas6−/− mice appeared to be brighter in comparison with the control, a phenomenon that was further verified using flow cytometry (Fig. 2C). Nevertheless, expression of the maturation markers MHCII and CD86 was not altered in Gas6−/− mice. Concurring with the above findings, expression of Tgfb1, a cytokine controlling LC development and maturation, was similar in the epidermis of both groups (Fig. 2D). We also quantified Il-34 and Bmp7 expression, other cytokines involved in LC development (17, 18), and, whereas Il-34 remained unchanged, expression of Bmp7 was up-regulated in GAS6-depleted epidermis. We then checked the expression of other TAM receptors and ligands in Gas6−/− mice by RT-qPCR. Elevated Axl and Mertk mRNA levels, but not Pros1 or Tyro3, were found in the epidermis of Gas6−/− mice compared with their littermate controls (Fig. 2E). In line with our overall results, postnatal differentiation of LCs was normal in 10-d-old Gas6−/− mice, based on the frequencies of epidermal MHCII+ cells, langerin expression, and expression of AXL on LC precursors and keratinocytes (SI Appendix, Fig. S2). Taken together, although AXL was proposed to regulate epidermal LC development, the lack of its predominant ligand GAS6 hardly affected murine LCs.
Fig. 2.
LC differentiation is not impaired due to the lack of GAS6. (A) Epidermal cells were purified from adult Gas6−/− mice and littermate controls (WT). Representative flow cytometry plots present the frequencies of LCs (langerin+) and γδ T cells (γδ TCR+) among total CD45+ leukocytes. Data are representative of three independent experiments, and each experiment included at least four separately analyzed mice. (B) Whole-mount immunofluorescence staining on epidermal layers prepared from adult Gas6−/− mice and littermate controls, stained for MHCII (red) and langerin (green). Representative confocal microscopy images are presented, representing one of four independent experiments (n = 3 mice in each experiment). (C) Representative flow cytometry plots demonstrating the expression levels of MHCII, CD86, and langerin on epidermal LC cells of adult Gas6−/− mice and littermate controls. Data of one of three independent experiments are provided (n = 5 in each experiment). (D and E) Relative expression of various cytokines or TAM receptors and ligands in epidermal layers of adult Gas6−/− mice and littermate controls were analyzed by RT-qPCR (n = 5 mice). RNA expression data of one of two independent experiments are presented as the mean values ± SD. *P < 0.05.
Excision of PROS1 in Keratinocytes Reduces LC Frequencies.
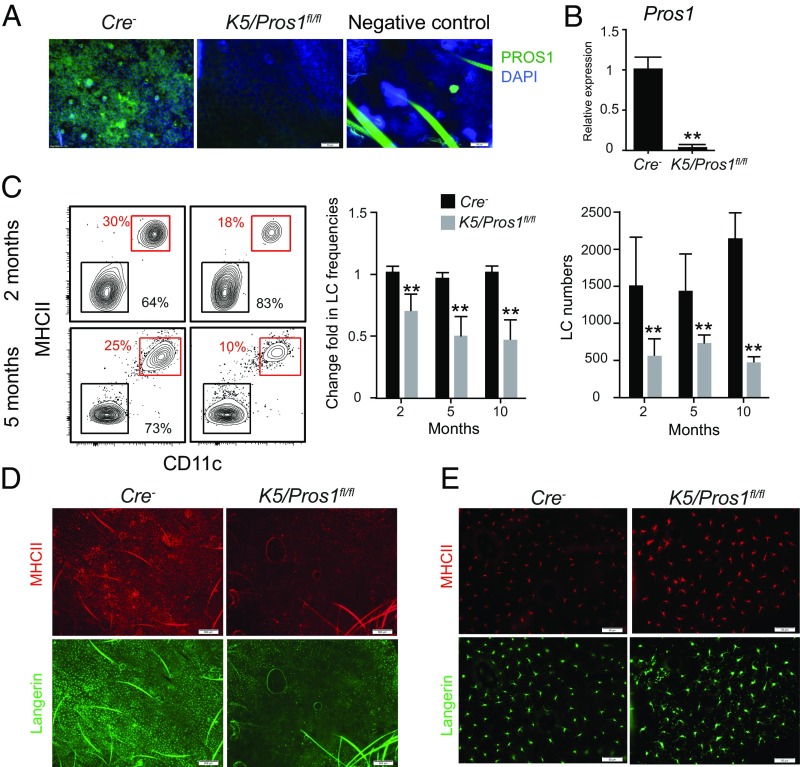
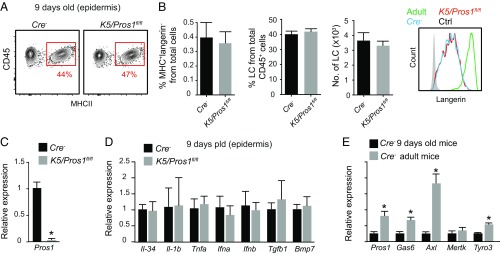
We next investigated the impact of PROS1 on LCs. Since total knockout of PROS1 yields nonviable mice (19), we crossed K5-cre mice with Pros1fl/fl mice to generate K5/Pros1fl/fl mice in which the Pros1 alleles were homozygously deleted in keratinocytes, as demonstrated in SI Appendix, Fig. S3. Analyzing epidermal tissues of K5/Pros1fl/fl adult mice by immunofluorescent staining and RT-qPCR showed a considerable reduction in PROS1 expression, thus confirming the effectiveness of the system (Fig. 3 A and B). The LC network was then examined, and a significant reduction in the frequencies and absolute numbers of LCs was found in K5/Pros1fl/fl mice compared with Cre− littermate controls (Fig. 3C). In fact, a gradual decrease in LC frequencies was observed during the second (∼30% reduction) and fifth (∼50% reduction) months after birth while no further reduction was found 10 mo after birth. A whole-mount immunofluorescence staining of epidermal tissues with MHCII and langerin antibodies further revealed empty patches in the LC network only in K5/Pros1fl/fl mice (Fig. 3D). Moreover, in epidermal areas of K5/Pros1fl/fl mice that contain LCs, expression of langerin and MHCII appeared to be more distributed in the cell body and dendrites compared with littermate controls (Fig. 3E). These data suggest that expression of PROS1 by keratinocytes is important for the development or maintenance of an intact LC network in the epidermis.
Fig. 3.
Ablation of PROS1 in keratinocytes reduces LC frequencies. (A) Whole-mount immunofluorescence staining on epidermal layers prepared from adult K5/Pros1fl/fl and littermate Cre− mice, stained for PROS1 (green) and DAPI (blue). Representative fluorescent images are shown, representing one of two independent experiments (n = 3 mice in each experiment). Negative control, primary antibody was omitted. (B) Quantification of mRNA levels of PROS1 in epidermal layers of adult K5/Pros1fl/fl and Cre− mice using RT-qPCR (n = 5 mice). Data of one of two independent experiments are presented as the mean values ± SD. (C) Frequencies and absolute numbers (per a half ear) of LCs (CD45+MHCII+CD11c+ cells) in epidermal cells prepared from 2-, 5-, and 10-mo-old K5/Pros1fl/fl and Cre− mice. Representative flow cytometry plots, as well as bar graphs, present the fold change in LC frequencies (normalized to Cre− mice) or absolute LC numbers in the epidermis of these mice. Data are representative of four independent experiments, and each experiment included at least five separately analyzed mice. (D and E) Whole-mount immunofluorescence staining on epidermal layers prepared from adult K5/Pros1fl/fl and littermate Cre− mice, stained for langerin (green) and MHCII (red). Representative fluorescent images are presented representing one of three independent experiments (n = 3 mice in each experiment). **P < 0.01. (Scale bars: A and E, 50 μm; D, 500 μm.)
Maturation and Activation of LCs Is Not Altered in K5/Pros1fl/fl Mice.
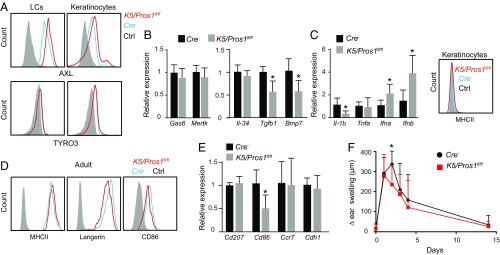
We next sought to check whether the reduction in LCs was due to an accelerated maturation/activation of these cells. First, we examined the impact of PROS1 deletion on the expression of other TAM molecules in epidermal cells of adult mice. Using flow cytometry, we found an up-regulation of AXL on both LCs and keratinocytes of K5/Pros1fl/fl mice whereas TYRO3 expression was not altered (Fig. 4A). Epidermal expression of Gas6 and Mertk in these mice was normal based on RT-qPCR quantification (Fig. 4B). We also quantified the expression of Il-34, Tgfb1, and Bmp7 and found reduced expression of Tgfb1 and Bmp7 but not Il-34 (Fig. 4B). It has been shown that AXL is up-regulated in antigen-presenting cells (APCs) and epithelial cells upon activation, acting as a negative regulator on the positive feedback loop of IFN-I signaling that facilitates inflammatory cytokines production (16, 20, 21). Therefore, we examined, by RT-qPCR, the levels of certain cytokines involved in this regulatory process. An up-regulation in the expression of IFN-α and IFN-β was detected in the epidermis of K5/Pros1fl/fl mice (Fig. 4C). However, expression of the proinflammatory cytokines TNF-α and IL-1β was either unchanged or significantly reduced, respectively. In line with the latter finding, MHCII expression on keratinocytes was not induced due to the intrinsic absence of PROS1 (Fig. 4C). These results suggest that, although the IFN-I/AXL regulatory pathway was modified in the epidermis of K5/Pros1fl/fl mice, based on the inflammatory indicators assayed, the epidermis of these mice is not more inflamed in comparison with littermate controls.
Fig. 4.
LCs are not activated in K5/Pros1fl/fl. (A) Epidermal cells were prepared from adult K5/Pros1fl/fl and littermate Cre− mice. Representative flow cytometry plots present the expression of AXL and TYRO3 on LCs and keratinocytes. Data of one of three independent experiments are provided, and each experiment included at least five separately analyzed mice. (B and C) Relative expression of various genes in epidermal layers of adult K5/Pros1fl/fl and littermate Cre− mice were analyzed by RT-qPCR (n = 5 mice). RNA expression data of one of two independent experiments are presented as the mean values ± SD. Representative FACS plot demonstrates MHCII expression levels on keratinocytes of adult K5/Pros1fl/fl and littermate Cre− mice. (D) Expression levels of MHCII, langerin, and CD86 on epidermal LCs of adult K5/Pros1fl/fl and littermate Cre− mice (n = 5). Representative FACS plots of one of three independent experiments are presented. (E) Relative expression of genes involved in LC maturation and migration to the LNs as measured by RT-qPCR. Data are representative of two independent experiments, and each experiment included five separately analyzed mice. (F) K5/Pros1fl/fl and littermate Cre− mice were sensitized with 1% TNCB on the shaved abdomen, and, 5 d later, the mice were challenged on one ear with 0.5% TNCB while the unchallenged ear was used as control. The graph presents the change in ear swelling at the indicated time points and represents the mean values ± SD (n = 5). Data of one of two independent experiments are provided. Ctrl, control. *P < 0.05.
We then examined LC maturation using flow cytometry and found normal expression of MHCII and CD86 while langerin was slightly reduced in K5/Pros1fl/fl mice (Fig. 4D). Using RT-qPCR, we detected reduced Cd86 expression in K5/Pros1fl/fl mice whereas expression of Ccr7 and Cdh1 (E-cadherin), known to be involved in LC migration to the lymph nodes (LNs) upon maturation, was not altered (Fig. 4E). Concurringly, no increase in migratory DCs was found in the draining LNs of K5/Pros1fl/fl mice; in fact, this population was reduced compared with controls due to the reduction of LCs (SI Appendix, Fig. S4). These results suggest that LC reduction in K5/Pros1fl/fl mice is not due to an accelerated activation of the LCs or keratinocytes. Finally, we examined the development of contact-hypersensitivity (CHS) response in K5/Pros1fl/fl mice since elevated CHS responses were previously reported in TAM KO mice 2 wk after challenge (9). Nevertheless, such a phenomenon was not observed in K5/Pros1fl/fl mice (Fig. 4F), suggesting that regulation of keratinocyte activation is intact in our system.
Postnatal Differentiation of LCs Is Not Impaired in K5/Pros1fl/fl Mice.
We next checked postnatal development of LCs using flow cytometry at postnatal day 9 (P9) in K5/Pros1fl/fl and Cre− mice. The frequencies of MHCII+ LC precursors and langerin expression levels were similar in both groups (Fig. 5 A and B). Of note, a considerable reduction in the mRNA levels of Pros1 was already evident at this time point (Fig. 5C), indicating that Cre is expressed by keratinocytes early after birth, as previously reported (22). Since the reduction of LCs in adult mice was accompanied with an altered expression of various cytokines, we examined whether a similar phenomenon occurs also during postnatal differentiation. In contrast to adult mice, no differences were found in the expression of the tested cytokines at P9 in K5/Pros1fl/fl and Cre− mice (Fig. 5D). Because TAM signaling regulates the expression of these cytokines in adult mice (23), we compared the expression of TAM molecules in the epidermis of adult and P9 Cre− mice. The comparison revealed low mRNA levels of all TAM molecules in P9 mice compared with adult mice (except for Mertk, which is not expressed in the adult epidermis), suggesting that TAM signaling might have less impact on keratinocytes early after birth (Fig. 5E). These results suggest that early differentiation of LCs is not altered in K5/Pros1fl/fl mice presumably because PROS1 ablation minimally affects epidermal function postnatally.
Fig. 5.
Intact postnatal differentiation of LCs in K5/Pros1fl/fl. Epidermal cells were prepared from 9-d-old (P9) K5/Pros1fl/fl mice and littermate Cre− controls. (A) Representative flow cytometry plots and graph present the frequencies of CD45+MHCII+ cells in the epidermis. (B) Frequencies and absolute numbers (per a half ear) of LCs (CD45+MHCII+langerin+) in the epidermis. Representative flow cytometry histogram demonstrates langerin expression in P9 K5/Pros1fl/fl and Cre− mice, as well as adult Cre− mice. Data are representative of two independent experiments, and each experiment included at least three separately analyzed mice. (C–E) Relative expression of Pros1 and various genes in epidermal layers of (C and D) P9 K5/Pros1fl/fl and Cre− mice, or (E) adult vs. P9 Cre− mice were analyzed by RT-qPCR (n = 5 mice). RNA expression data of one of two independent experiments are presented as the mean values ± SD. Ctrl, control. *P < 0.05.
Altered Proliferation and Increased Apoptosis of LCs Following PROS1 Ablation in Keratinocytes.
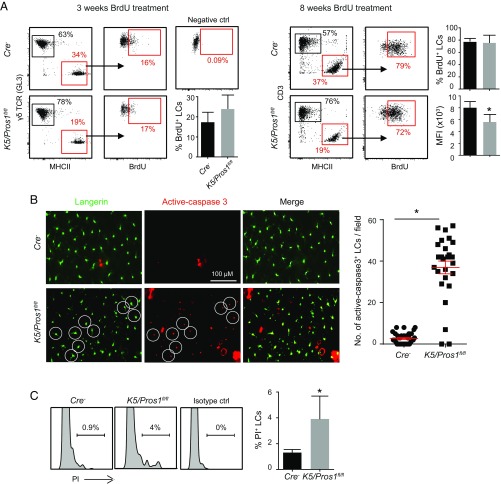
Since postnatal development of LCs is normal in K5/Pros1fl/fl mice, we examined whether the reduced LC levels observed in adult mice are a result of impaired homeostatic proliferation. To address this issue, we treated 21-d-old K5/Pros1fl/fl mice and littermate controls with BrdU for 3 wk or 8 wk and analyzed the incorporation of BrdU into LCs during cell proliferation. Of note, 21-d-old mice were chosen for this assay since we found that, at this age, the frequencies of LCs are normal in K5/Pros1fl/fl mice. This allows tracking alterations in LC proliferation until adulthood, a time in which LCs are significantly reduced. Exposing the mice to BrdU for 3 wk resulted in comparable levels of BrdU-labeled LCs in K5/Pros1fl/fl mice and littermate controls (Fig. 6A). However, following an 8-wk treatment, whereas the percentages of BrdU-labeled LCs were similar in both groups, the mean fluorescence intensity (MFI) of BrdU-labeled LCs was lower in K5/Pros1fl/fl mice compared with littermates (Fig. 6A). This suggests that, in K5/Pros1fl/fl mice, some of the LCs failed to undergo additional rounds of proliferation, which could be explained by increased apoptosis of LCs. To measure apoptosis, we prepared epidermal sheets from 6- to 8-wk-old K5/Pros1fl/fl mice and littermate controls and performed whole-mount immunofluorescence staining against cleaved-caspase 3 and langerin. Indeed, we found, in K5/Pros1fl/fl mice, a four- to fivefold increase in the numbers of cleaved-caspase 3–positive LCs compared with littermate controls (Fig. 6B). We also measured the frequencies of dead LCs in the epidermis by flow cytometry using propidium iodide (PI) and detected elevated percentages of PI+ LCs in K5/Pros1fl/fl mice compared with control (Fig. 6C). Taken together, these results suggest that the reduced numbers of LCs in K5/Pros1fl/fl mice is due to increased apoptosis of these cells.
Fig. 6.
Increased apoptosis rate of LCs in K5/Pros1fl/fl mice. (A) Three-week-old K5/Pros1fl/fl and Cre− mice were treated with BrdU in the drinking water for 3 or 8 wk. Representative flow cytometry plots demonstrate the frequencies of CD45+MHCII+ (LCs) in the epidermis, as well as the frequencies of BrdU-labeled LCs at the end of the treatments. Bar graphs present the frequencies of BrdU-labeled LCs and the mean florescence intensity (MFI) of BrdU staining in LCs (n = 5). Data of one of two independent experiments are provided and present the mean values ± SD. (B) Epidermal sheets of 6- to 8-wk-old mice were stained against langerin and anti-caspase 3 to detect LCs undergoing apoptosis. Representative images of whole-mount immunofluorescence staining and graph demonstrating the numbers of active-caspase 3+ langerin+ cells are presented and present the mean values ± SD. Data are representative of three independent experiments. Each experiment included at least three separately analyzed mice, and 20 fields per mouse were analyzed. White circles exemplify langerin+ cells stained positively to active-caspase 3. (C) Representative flow cytometry plots and graph present the frequencies of dead PI+ LCs in the epidermis of K5/Pros1fl/fl and Cre− mice (n = 5). Results of one of two independent experiments are provided and present the mean values ± SD. ctrl, control. *P < 0.05.
Excision of PROS1 in LysM+ Cells Affects BM-Derived LCs but Not Embryonic LCs.
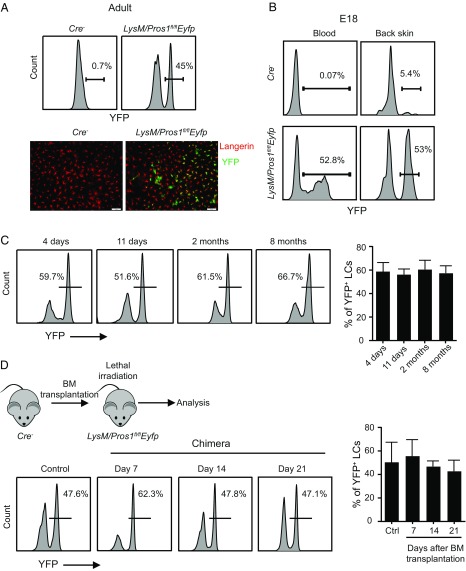
To examine whether intrinsic expression of PROS1 by LCs is required for their development, we employed LysM-cre mice and crossed them with Pros1fl/fl mice. The LysM promoter is active during embryonic development of LCs (24), allowing one to study the role of intrinsic PROS1 expression on LCs during embryonic development and throughout adulthood. Moreover, the LysM promoter is expressed by monocytes (25) which also express PROS1 (SI Appendix, Fig. S5) and give rise to LCs in adult mice under certain conditions (7, 8, 26). To visualize LCs that were also deleted for PROS1, we crossed the LysM-cre mice to both Pros1fl/fl mice and Rosa26-lslEyfp mice to generate LysM/Pros1fl/flEyfp mice. In these mice, YFP+ cells are expected to have an excision of the Pros1 gene as shown in SI Appendix, Fig. S6. In agreement with a previous study (24), analysis of LysM/Pros1fl/flEyfp mice revealed that only 45 to 60% of LCs were YFP+ and, by inference, also Pros1-ablated (Fig. 7A). Examining LC precursors in the skin epidermis and in the blood of E18 embryos demonstrated similar levels of partial YFP labeling, confirming that ablation of the Pros1 gene in LCs occurred during embryogenesis (Fig. 7B). Since the epidermis of LysM/Pros1fl/flEfyp mice contains both Pros1-excised LCs and intact LCs, we used this phenotype as an internal control to examine the role of Pros1 in LC development. First, time-course analysis revealed that the percentages of YFP+ LCs in the epidermis remain constant, suggesting that PROS1 does not affect homeostatic proliferation of LCs (Fig. 7C). Additionally, PROS1 deletion did not alter the radioresistant feature of LCs as the frequencies of YFP+ and YFPneg LCs remained similar after lethal irradiation (Fig. 7D).
Fig. 7.
Normal development and homeostasis of LCs in LysM/Pros1fl/flEyfp mice. (A) Epidermal cells were prepared from adult LysM/Pros1fl/flEyfp and littermate Cre− mice. Representative flow cytometry plots present the percentages of YFP+ LCs among total epidermal LCs. Representative whole-mount immunofluorescence staining on epidermal sheet stained against langerin (red) and YFP (green). Data are representative of four independent experiments, and each experiment included at least three separately analyzed mice. (B) Blood and back skin epidermis of E18 embryos were sampled from LysM/Pros1fl/flEyfp and Cre− mice. Representative flow cytometry plots present the percentages of YFP+ cells among LC precursors in the blood (CD45+MHCII+) and epidermis (CD45+MHCII+F4/80+). Results of one of two independent experiments are presented. (C) YFP+ LCs were analyzed in the epidermis of LysM/Pros1fl/flEyfp mice at the indicated time points. Representative flow cytometry plots and graph present the frequencies of YFP+ LCs among total LCs (n = 3–5). Data of one of three independent experiments are provided and present the mean values ± SD. (D) Adult LysM/Pros1fl/flEyfp mice were lethally irradiated and, 24 h later, were administered i.v. with BM cells purified from littermate Cre− mice. The epidermis of the chimeric mice was analyzed 7, 14, and 21 d after BM transplantation to quantify the frequencies of YFP+ LCs. Representative flow cytometry plots and graph present the frequencies of YFP+ LCs among total LCs (n = 3). Data of one of two independent experiments are provided and present the mean values ± SD. Ctrl, control. (Scale bars:, 50 μm.)
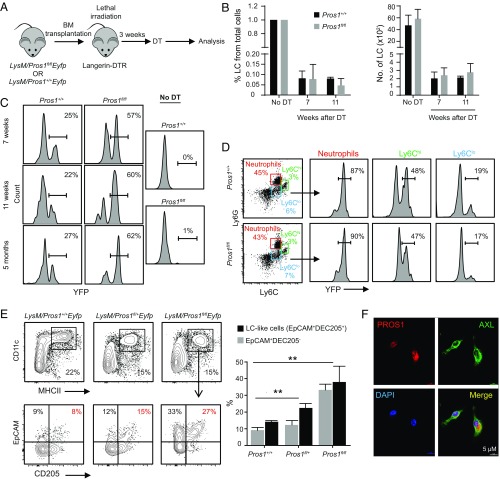
We then asked whether the repopulation of epidermal LCs from BM-derived precursors is altered due to Pros1 excision. For this, we lethally irradiated langerin-DTR (diphtheria toxin receptor) mice and adoptively transferred them with BM cells purified from LysM/Pros1fl/flEyfp or LysM/Pros1+/+Eyfp mice as a control. Eight weeks later, epidermal LCs were depleted by diphtheria toxin (DT), and LC repopulation was tracked in the epidermis (Fig. 8A). Two to 3 mo after LC depletion, a small population of LCs repopulated the epidermis of both groups with comparable levels (Fig. 8B). In this experimental paradigm, circulating BM-derived precursors gave rise to epidermal LCs, and YFP+ cells were derived only from the transplanted donor BM cells. Nevertheless, the percentages of YFP+ LCs in mice receiving BM of LysM/Pros1fl/flEyfp were considerably higher in comparison with the control group (Fig. 8C). This phenomenon cannot be explained by the presence of increased YFP+ circulating precursors in the donor since comparable frequencies of YFP+ Ly6Clo and Ly6Chi monocytes were found in the blood of LysM/Pros1fl/flEyfp and LysM/Pros1+/+Eyfp mice (Fig. 8D). Interestingly, in a separate experiment, we examined the capacity of PROS1-deficient monocytes to repopulate the epidermis under inflammatory conditions induced by tape stripping. In contrast to the above results, we found that PROS1 was crucial for infiltration of monocytes to the epidermis, resulting in a higher differentiation to MHCII+CD11c+ cells (SI Appendix, Fig. S7). These suggest that lack of PROS1 in monocytes facilitates their differentiation into LCs only during steady-state or low inflammatory conditions. We next hypothesized that the excision of Pros1 might facilitate the differentiation of BM precursors in the epidermis, leading to increased numbers of YFP+ LCs in mice receiving BM from LysM/Pros1fl/flEyfp mice. To address this issue, we cultured BM cells purified from both mice with GM-CSF and TGF-β1 to drive their differentiation into LC-like cells. Indeed, BM cells heterozygously or homozygously deleted for Pros1 developed larger percentages of MHCII+CD11c+CD205+EpCAM−/+ cells, representing LC-like cells (27), in a dose-dependent manner (Fig. 8E). In line with these results, LC-like cells generated by these BM cells presented higher percentages of YFP compared with intact BM cells (SI Appendix, Fig. S8). We further demonstrated that AXL-expressing cells in the culture, representing LCs (9), also express PROS1 (Fig. 8F). Taken together, in contrast to PROS1 expressed by keratinocytes, intrinsic expression of PROS1 by embryonic-derived LCs is suggested to be dispensable for their development and maintenance. Expression of PROS1 in BM precursors, however, inhibits their differentiation into epidermal LCs under steady-state conditions.
Fig. 8.
Differentiation of LCs from circulating BM precursors is augmented in LysM/Pros1fl/flEyfp mice. (A) Lethally irradiated langerin-DTR mice were adoptively transferred with BM cells purified from LysM/Pros1fl/flEyfp mice or LysM/Pros1+/+Eyfp mice as a control. Eight weeks later, LCs were ablated by a single injection of DT, and the repopulation of LCs was examined 7 and 11 wk after the depletion. (B) The frequencies and numbers of repopulating LCs in the epidermis of the chimeric mice are presented (n = 3). (C) FACS plots present the percentages of YFP+ cells among the repopulating LCs (n = 3). Results of one of two independent experiments are provided and present the mean values ± SD. (D) Representative flow cytometry plots demonstrate the percentages of YFP+ cells among Ly6Clow, Ly6Chigh, and neutrophils in the blood of LysM/Pros1fl/flEyfp or LysM/Pros1+/+Eyfp mice before the transplantation. Data are representative of two independent experiments, and each experiment included at least three separately analyzed mice. (E) BM cells were purified from LysM/Pros1+/+Eyfp, LysM/Pros1fl/+Eyfp, or LysM/Pros1fl/flEyfp mice and then cultured for 5 d in serum containing media supplemented with GM-CSF and TGF-β1 to drive differentiation of LC-like cells. Representative flow cytometry plots demonstrate the percentages of MHCII+CD11c+ cells and further expression of CD205+EpCAM+, representing LC-like cells. The graph presents the frequencies of LC-like cells, as well as MHCII+CD11c+CD205−EpCAM+ cells, which are also known to differentiate to LC-like cells (n = 3). Data of one of four independent experiments are provided and present the mean values ± SD. (F) LCs were differentiated in vitro from BM cells of WT mice and stained for PROS1 (red), AXL (green), and DAPI (blue) for nuclear visualization at 5 d in culture. Representative immunofluorescence images taken from one of two independent experiments are shown. **P < 0.01.
Discussion
This study examined the role of TAM ligands on the development of epidermal LCs. The negligible impact of GAS6 on murine LC development was unexpected since its predominant receptor AXL was proposed to play a major role in the differentiation and activation of human LCs (9). Whereas it is possible that the reported impact of AXL on LCs is mediated by PROS1 rather than GAS6, previous studies have shown that binding of PROS1 to AXL fails to induce intracellular signaling (13, 15). Alternatively, PROS1 may modify the interaction of AXL with a non-TAM tyrosine kinase receptor, which could affect LCs. However, it is also possible that AXL is dispensable for LC differentiation since, in Axl−/− mice, the LC network is intact (9). In fact, the LC network was reported to be intact also in Tyro3−/− or Mertk−/− mice, and only ablation of all three TAM receptors displayed reduced LC frequencies (9). Whereas the inability of AXL to control LC differentiation in Axl−/− mice was explained by functional redundancy among the various TAM receptors, our study proposes an alternative explanation. We demonstrate here that PROS1 and its predominant receptor TYRO3 are expressed by keratinocytes, and specific ablation of PROS1 in keratinocytes dysregulated cytokine expression in these cells. As keratinocytes play an instructive role in LC differentiation and homeostasis, it is likely that TRYO3/PROS1 signaling in keratinocytes is required to maintain epidermal homeostasis and prevent apoptosis of LCs. The lack of effect of PROS1 on embryonic-derived LCs in LysM/Pros1fl/flEyfp mice further suggests that PROS1 expressed by keratinocytes regulates epidermal LCs.
As for AXL, careful examination of recent data supported a role for AXL in regulating LC activation rather than LC differentiation (9), which is in line with the immunoregulatory roles of AXL in other systems (21). This function of AXL is likely to involve binding to GAS6 since GAS6 is known to be up-regulated upon activation in both APCs and epithelial cells (16, 20, 21). Of note, ablation of PROS1 in keratinocytes resulted in an up-regulation of AXL in both LCs and keratinocytes. AXL was reported to be up-regulated in LCs upon stimulation to limit their activation (9). It is thus likely that, in K5/Pros1fl/fl mice, AXL was up-regulated to compensate for the reduced levels of TGF-β1 known to down-regulate the activation of LCs and keratinocytes (28–30). Indeed, elevated expression of type I interferons known to regulate tissue homeostasis was detected in the epidermis of adult K5/Pros1fl/fl mice (31). Nevertheless, no augmentation in the expression of proinflammatory cytokines, such as TNF-α and IL-1β, was found, indicating that keratinocyte activation is under control in K5/Pros1fl/fl mice and the epidermis is not inflamed. A previous study demonstrated that type I IFN production by APCs is modulated by AXL upon interaction of AXL with IFN α receptor 1 (INFAR1) (21). We reported recently that similar mechanisms take place also in stratified epithelia to regulate epithelial cell activation and to maintain tissue homeostasis (16). It is thus possible that ablation of PROS1 in keratinocytes induces temporary activation of keratinocytes, leading to up-regulation of AXL to prevent further production of type I interferons. Such activity is likely to take place only in adult mice as we demonstrated that TAM signaling has minimal effect on keratinocytes and LCs early after birth. This proposes the establishment of new homeostatic conditions in the epidermis of K5/Pros1fl/fl mice, which involves higher apoptotic rates of LCs. Interestingly, a study by Sparber et al. (32) recently reported a reduced density of LCs in adulthood caused by reduced postnatal proliferation of LCs and increased apoptosis. Collectively, these observations suggest that homeostasis of LC in adulthood is shaped during the early weeks after birth.
Unlike LC differentiation from embryonic precursors, ablation of PROS1 facilitated the differentiation of LCs from adult BM precursors both in vitro and in vivo. Pre-DCs are not labeled in LysM-driven cre recombination (33), and thus monocytes represent the origin of YFP+ LCs upon DT treatment. Interestingly, monocyte-derived LCs were detectable in the epidermis up to 5 mo after LC depletion, demonstrating their capacity to generate long-lived LCs. This result contradicts a recent publication reporting that monocytes give rise to short-lived LCs following exposure to UV (8). Nevertheless, since depletion of LCs is not considered to induce severe inflammation as UV treatment, it is likely that such differences are related to the different inflammatory milieu within the epidermis upon each treatment. Indeed, the necessity of PROS1 for efficient infiltration of monocytes to the epidermis upon tape stripping-induced inflammation further highlights the impact of the inflammatory milieu on this process.
Finally, we demonstrate in this study that PROS1, but not GAS6, plays a role in LC differentiation in a cell-specific manner. Considering the previously reported function of AXL on the activation of these cells, the current work further highlights the multifaceted involvement of TAM signaling on epidermal LCs.
Experimental Procedures
Mice.
CD45.2+ C57BL/6 mice were purchased from Harlan. CD45.1+ C57BL/6, LysM-cre mice (25) and Rosa26-lslEyfp mice (34) were purchased from The Jackson Laboratory. Langerin (CD207)-DTR mice were obtained from Björn E. Clausen, Mainz University, Mainz, Germany (35). Gas6−/− mice and Pros1fl/fl mice were previously described (19, 36). K5-cre mice were kindly provided by Angel Ramirez, Centro de Investigaciones Energéticas, Medio Ambientales y Tecnológicas (CIEMAT), Madrid, Spain (22). K5/Pros1fl/fl mice were generated by crossing K5-cre mice with Pros1fl/fl mice. To generate the LysM/Pros1fl/flEyfp mice, we crossed the LysM-cre mice to both Pros1fl/fl mice and Rosa26-lslEyfp mice. The mice were maintained under specific pathogen-free (SPF) conditions and analyzed between 8 and 12 wk of age unless otherwise described in the text. All animal protocols were approved by the Hebrew University Institutional Animal Care and Use Committee.
Antibodies and Reagents.
Antibodies and reagents are described in SI Appendix.
Chimeric Mice.
LysM/Pros1fl/flEyfp recipient mice were lethally irradiated with 900 rad, and, 24 h later, the mice were injected i.v. with 5 × 106 BM cells obtained from donor Cre− mice. The frequencies of YFP+ among total epidermal LCs were analyzed at various times as explained in SI Appendix.
Ablation of Langerin-Expressing Cells.
Mice were injected once intraperitoneally with 1 μg of DT (Sigma-Aldrich) in 150 μL of PBS per mouse. Control mice received PBS only.
Isolation and Identification of LCs.
Ear skin was excised and separated into two halves, and epithelial sheets were prepared as explained in SI Appendix.
BrdU Incorporation.
Three- to 4-wk-old mice were injected once intraperitoneally with BrdU (2 mg per mouse; Sigma) and subsequently received BrdU (0.8 mg/mL) in the drinking water as explained in SI Appendix.
CHS Assay.
Adult K5/Pros1fl/fl mice and littermate controls were sensitized with 1% TNCB (2,4,6-trinitrochlorobenzene) (Sigma) in acetone/olive oil (4:1) on the shaved abdomen and, 5 d later, was challenged as explained in SI Appendix.
LC-Like Cell Differentiation Cultures.
The femur was isolated, cleaned from soft tissues in RPMI 1640, and soaked in 70% ethanol for 1 min for sterilization as explained in SI Appendix.
RNA Extraction and RT-qPCR.
For RNA isolation, the ear epidermis was homogenized in 300 µL of TRI reagent (Sigma) using an electric homogenizer as explained in SI Appendix.
Western Blot.
Ear epidermis was isolated and homogenized in ice-cold lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 0.1% SDS, 0.5 mM EDTA) supplemented with a protease inhibitor mixture (Sigma) as explained in SI Appendix.
Immunofluorescence Staining.
For whole-mount staining, ear skin was separated into two halves and fixed in ice-cold 95% ethanol for 40 min and subsequently digested as described in SI Appendix.
Statistics.
Data are expressed as means ± SD. Statistical tests were performed using one-way ANOVA and a Student t test (*P < 0.05; **P < 0.01).
Supplementary Material
Acknowledgments
This work was supported by Israel Science Foundation Grants 766/16 (to A.-H.H.) and 984/12 (to T.B.-C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800303115/-/DCSupplemental.
References
- 1.Doebel T, Voisin B, Nagao K. Langerhans cells–The macrophage in dendritic cell clothing. Trends Immunol. 2017;38:817–828. doi: 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol. 2017;18:1068–1075. doi: 10.1038/ni.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chorro L, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capucha T, et al. Distinct murine mucosal Langerhans cell subsets develop from pre-dendritic cells and monocytes. Immunity. 2015;43:369–381. doi: 10.1016/j.immuni.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Nagao K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seré K, et al. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity. 2012;37:905–916. doi: 10.1016/j.immuni.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Bauer T, et al. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. J Exp Med. 2012;209:2033–2047. doi: 10.1084/jem.20120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci. 2010;1209:23–29. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burstyn-Cohen T. TAM receptor signaling in development. Int J Dev Biol. 2017;61:215–224. doi: 10.1387/ijdb.160285tb. [DOI] [PubMed] [Google Scholar]
- 13.Lew ED, et al. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. eLife. 2014;3:e03385. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stitt TN, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsou WI, et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem. 2014;289:25750–25763. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassar M, et al. GAS6 is a key homeostatic immunological regulator of host-commensal interactions in the oral mucosa. Proc Natl Acad Sci USA. 2017;114:E337–E346. doi: 10.1073/pnas.1614926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasmin N, et al. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J Exp Med. 2013;210:2597–2610. doi: 10.1084/jem.20130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest. 2009;119:2942–2953. doi: 10.1172/JCI39325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizraji G, et al. Porphyromonas gingivalis promotes unrestrained type I interferon production by dysregulating TAM signaling via MYD88 degradation. Cell Rep. 2017;18:419–431. doi: 10.1016/j.celrep.2016.12.047. [DOI] [PubMed] [Google Scholar]
- 21.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 23.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubzick C, et al. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 26.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopin M, et al. Langerhans cells are generated by two distinct PU.1-dependent transcriptional networks. J Exp Med. 2013;210:2967–2980. doi: 10.1084/jem.20130930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobr A, et al. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan DH, et al. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. 2010;185:3248–3255. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- 31.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparber F, et al. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of Langerhans cell homeostasis. Blood. 2014;123:217–227. doi: 10.1182/blood-2013-08-518555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angelillo-Scherrer A, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.