The increasing availability of -omics data is driving the development of computational methods for integrating these datasets to connect the underlying molecular mechanisms to phenotypes. Building gene regulatory networks (GRNs) from transcriptomic studies often results in a static view of gene expression, which can make it difficult to disentangle pathway structure. Increasing the temporal resolution of any experimental design provides the opportunity to model the dynamic nature of a regulatory pathway response to a stimulus. Many transient intermediate states can separate the initial prestimulus state from the final poststimulus state; time-series analyses permit the detection and integration of these intermediate steps into the pathway. The difficulty is to incorporate these time-dependent changes to determine causal relationships within the GRN, such as which transcription factor (TF) regulates which target genes. In PNAS, Varala et al. (1) address this challenge by integrating time into their GRN to unravel the temporal cascade of nitrogen signaling. Their time-based analysis offers a potent and general approach to uncover the temporal transcriptional logic for any plant or animal response system.
Nitrogen (N) commonly limits plant production and the widespread application of mineral N fertilizer has greatly increased crop yields (2). Unfortunately, the production of mineral N fertilizer is expensive in terms of fossil energy. Furthermore, plant assimilation of applied N is inefficient; for example, cereals such as maize, rice, and wheat take up less than 40% of the applied N (3). The remaining N is lost to the environment through processes including denitrification and volatilization, releasing greenhouse gases, leaching, contaminating groundwater, and surface runoff, causing eutrophication of fresh and estuarine waters (4). Therefore, the excessive use of N fertilizer both increases the cost of crop production and causes environmental pollution. One might expect that, consequently, plant N use efficiency (NUE) would represent a major target for crop improvement, but NUE has not been a priority because of the ready availability of N fertilizer (5). However, predicted increases in energy costs and increased sensitivity to the environmental consequences make improved NUE an immediate goal for crop improvement (5). Developing transcriptional models that capture the dynamics of N signaling will provide more accurate predictions for targeted breeding in crops.
An important challenge in the era of genomics is to infer causality in networks to predict GRN responses to an untested stimulus. This is not a trivial task; the DREAM (dialogue for reverse engineering assessment and methods) challenge of reverse-engineering networks to predict withheld data found only a few team solutions performed slightly better than the null model (6). One conclusion from this is that context matters and not all datasets can be treated equally; the experimental design and data-collection strategies need to reflect the biological system being modeled. Building dynamic networks that cross scales of either time or space can provide the connectivity needed to assign causal relationships and improve prediction. The selection of method for network modeling is not easy and projects like DREAM reveal that there is not necessarily a single solution; rather, the method should be selected according to the dataset and experimental design (7).
The complexity of biological signaling networks poses major challenges to their understanding and manipulation. At the heart of all GRNs are arrays of regulatory TFs that dictate the dynamics of the system. Plants have amplified large families of TFs; for example, Arabidopsis has an estimated 2,451 TFs compared with the 1,052 in Drosophila melanogaster and 934 in Caenorhabditis elegans (8). This proliferation of TFs exacerbates the challenge to define GRNs at the scale of an individual TF–gene target interaction. Chromatin immunoprecipitation sequencing (ChIP-seq) is widely used to identify TF targets. New techniques to identify regions open to TF binding, such as DNase-seq and ATAC-seq (assay for transposase-accessible chromatin using sequencing), provide insight into genome structure and permit the identification of regions containing regulatory elements accessible to TFs (9, 10). However, identifying specific binding sequences for individual TFs requires more targeted approaches, such as the in vitro elucidation of TF binding sites using SELEX-seq (systematic evolution of ligands by exponential enrichment) (11) and protein binding microarrays that use affinity-tagged TFs to probe DNA oligos (12). The DNA affinity purification sequencing (DAP-seq) method, while still requiring tagged TFs, has the advantage of targeting genomic DNA fragments, thereby maintaining the genomic structure and affording a more accurate assessment of the TF binding site. This method was used to generate the Plant Cistrome Database containing binding locations for 529 TFs and sequence motifs covering 9.3% of the Arabidopsis genome (13). This study revealed the complexity of TF binding and the diversity in motif clusters and distribution that likely impact timing and strength of TF binding (13). Additional factors to consider are the relationship between TF concentration in the nucleus and search time for target regions, as well as residence time on the promoter, both of which will impact mRNA output levels (14). With a collection of known TF gene targets, the question becomes when and where are these interactions occurring in the GRN and can this relationship be predicted?
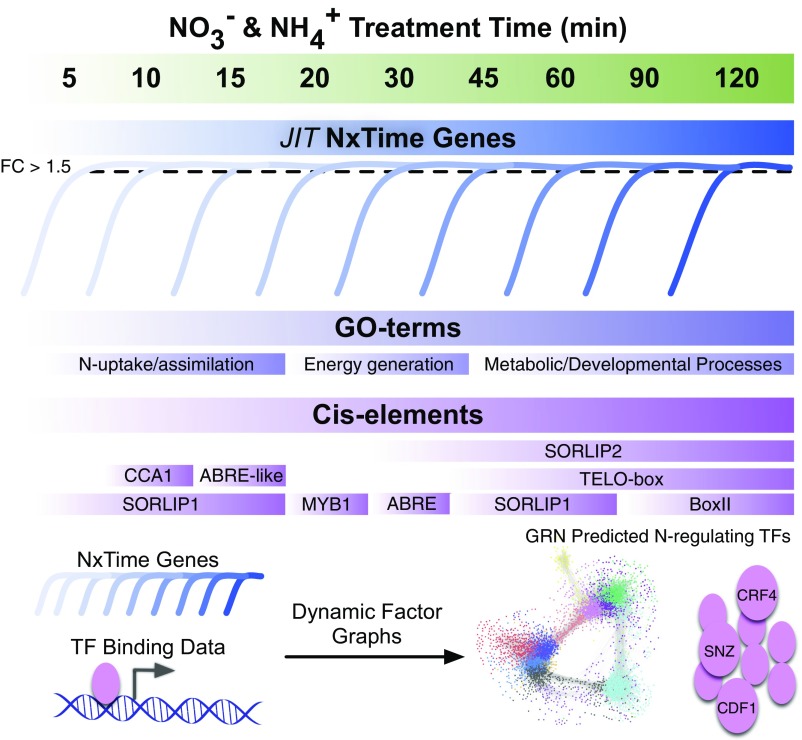
Varala et al. (1) designed a time-scale study and applied time-based machine learning to investigate the regulatory networks underlying N signaling and use in plants. Based on prior transcriptome studies in Arabidopsis following nitrate treatment, the authors selected nine densely spaced time points over a 2-h window to capture the early-to-late inorganic- and organic-N responses. Using a Cubic-Spline Model to analyze their RNAseq time-series data, they classified time-based N responding genes (NxTime genes) in shoots and roots. Applying their just-in-time (JIT) analysis to assign NxTime genes to the time they are first expressed during the N response created a temporal cascade of overrepresented cis-elements and Gene Ontology (GO) terms associated with N-responsive genes (Fig. 1). Although many cis-elements are enriched in very narrow (5 min) time windows, certain motifs are enriched for prolonged periods of time (Fig. 1), demonstrating the importance of time-resolution datasets to tease apart these differential responses. This information can now be used to position past transcriptome studies of N response of lesser temporal resolution or even of single time points into the context of this larger time-sensitive GRN and should facilitate future experimental design for targeting specific TF families.
Fig. 1.
Temporal cascade of N response coupled with TF-binding data reveal a predictive GRN of N signaling. A time-series transcriptome study of N response uncovers the timing of N-responsive genes (NxTime) that are enriched for known cis-elements and GO terms associated with N response. Incorporating the NxTime genes with available TF-binding data into a DFG-predicted GRN identifies novel N-responsive TFs.
To focus in on individual TFs, Varala et al. (1) leverage the available TF binding data in the DAP-seq database (13) to identify the N-responding TFs whose targets are enriched for NxTime genes. The 19 TFs identified include 4 previously characterized N-response TFs and 15 novel TFs, further supporting the improved sensitivity of their time-series design. Instead of being limited by the TFs found in the database, Varala et al. (1) applied a dynamic factor graphs (DFG) machine-learning approach to identify TFs predicted to be associated with their set of NxTime genes. The DFG method uses time-series data to assign dependencies between states in time to infer causal relationships from the most likely sequence of events (15). That is, TFs that increase in abundance at time n are likely to regulate genes whose promoters include binding sites to the TF and which increase (or decrease) in abundance at time n + 1. To improve the precision of their GRN and reduce the number of false positives, seven TF hubs selected from the top 10% of the DFG set were experimentally validated using in planta overexpression lines and the cell-based TARGET (transient assay reporting genome-wide effects of transcription factors) assay for direct target identification (16). Three of the validated targets include CRF4, SNZ, and CDF1, all novel N-regulating TFs. These confirmed targets allowed Varala et al. (1) to further refine their GRN to impose an edge score cut-off that produced higher-confidence predictions. All GRNs suffer from high false positives that can be reduced with additional supporting data. As new technologies for TF binding discovery are developed or refined, the precision of this and other GRNs can be improved. Additionally, this N-response GRN has predicted a set of N-regulating TFs that would be excellent candidates for future DAP-seq studies.
With the incorporation of time into their analysis, Varala et al. (1) define a GRN that includes 155 N-responsive TFs in shoots along with their predicted targets. The use of TF-binding data to refine the GRN identified 15 TFs not previously known to be involved in the N response. Six TFs (CRF4, SNZ, CDF1, HHO5, HHO6, and PHL) target more than half of the genes in the GRN. Overexpression of CRF4, a novel TF identified as an “early” regulator in their GRN, alters N uptake, root development, and plant biomass. Not only has this work enriched our understanding of the N-response GRN, but the findings offer multiple relevant targets for breeding toward improved NUE. Most importantly, though, this study offers a general means to harness the inexorable march of time toward the elucidation of signaling cascades responding to any environmental perturbation. One of many advantages of building GRNs is to continue to improve predictive power by refining the connectivity through additional experimentation. Rather than recreating a GRN with every study, perhaps a more collective effort to improve existing GRNs is a more constructive way forward. With a toolbox of externally validated and refined GRNs, can we start to make predictions of plant responses using static measures of field-grown crops? Time will tell.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6494.
References
- 1.Varala K, et al. Temporal transcriptional logic of dynamic regulatory networks underlying nitrogen signaling and use in plants. Proc Natl Acad Sci USA. 2018;115:6494–6499. doi: 10.1073/pnas.1721487115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson GP, Vitousek PM. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu Rev Environ Resour. 2009;34:97–125. [Google Scholar]
- 3.Kant S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin Cell Dev Biol. 2018;74:89–96. doi: 10.1016/j.semcdb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, et al. Managing nitrogen for sustainable development. Nature. 2015;528:51–59. doi: 10.1038/nature15743. [DOI] [PubMed] [Google Scholar]
- 5.van Bueren ETL, Struik PC. Diverse concepts of breeding for nitrogen use efficiency. Agron Sustain Dev. 2017;37:50. [Google Scholar]
- 6.Prill RJ, et al. Towards a rigorous assessment of systems biology models: The DREAM3 challenges. PLoS One. 2010;5:e9202. doi: 10.1371/journal.pone.0009202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Y, Kelemen A. Dynamic modeling and network approaches for omics time course data: Overview of computational approaches and applications. Brief Bioinform. 2017 doi: 10.1093/bib/bbx036. [DOI] [PubMed] [Google Scholar]
- 8.Ouma WZ, Pogacar K, Grotewold E. Topological and statistical analyses of gene regulatory networks reveal unifying yet quantitatively different emergent properties. PLOS Comput Biol. 2018;14:e1006098. doi: 10.1371/journal.pcbi.1006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cumbie JS, Filichkin SA, Megraw M. Improved DNase-seq protocol facilitates high resolution mapping of DNase I hypersensitive sites in roots in Arabidopsis thaliana. Plant Methods. 2015;11:42. doi: 10.1186/s13007-015-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol. 2015;109:1–9. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smaczniak C, Angenent GC, Kaufmann K. SELEX-Seq: A method to determine DNA binding specificities of plant transcription factors. In: Kaufmann K, Mueller-Roeber B, editors. Plant Gene Regulatory Networks. Methods in Molecular Biology. Vol 1629. Humana Press; New York: 2017. pp. 67–82. [DOI] [PubMed] [Google Scholar]
- 12.Berger MF, Bulyk ML. Universal protein-binding microarrays for the comprehensive characterization of the DNA-binding specificities of transcription factors. Nat Protoc. 2009;4:393–411. doi: 10.1038/nprot.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Malley RC, et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell. 2016;165:1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swift J, Coruzzi GM. A matter of time—How transient transcription factor interactions create dynamic gene regulatory networks. Biochim Biophys Acta. 2017;1860:75–83. doi: 10.1016/j.bbagrm.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirowski P, LeCun Y. Dynamic Factor Graphs for Time Series Modeling. Springer; Berlin: 2009. pp. 128–143. [Google Scholar]
- 16.Bargmann BO, et al. TARGET: A transient transformation system for genome-wide transcription factor target discovery. Mol Plant. 2013;6:978–980. doi: 10.1093/mp/sst010. [DOI] [PMC free article] [PubMed] [Google Scholar]