Significance
Parkinson’s disease (PD) is a chronic and progressive movement disorder; however, the precise mechanisms of its etiology remain largely unknown. Soluble epoxide hydrolase (sEH) plays a key role in the inflammation associated with PD pathogenesis. The sEH inhibitor or deletion of the sEH gene protected against MPTP-induced neurotoxicity in mouse brain. Furthermore, expression of the sEH protein (or mRNA) was higher in the striatum of MPTP-treated mice, patients with dementia of Lewy bodies (DLB), and neurons from iPSCs of a PD patient with PARKIN mutations. Interestingly, treatment with sEH inhibitor protected against apoptosis in human PARK2 iPSC-derived dopaminergic neurons. Our findings indicate that sEH inhibitors or epoxy fatty acids mimics may be promising prophylactic or therapeutic drugs for PD.
Keywords: epoxyeicosatrienoic acid, ER stress, iPSCs
Abstract
Parkinson’s disease (PD) is characterized as a chronic and progressive neurodegenerative disorder, and the deposition of specific protein aggregates of α-synuclein, termed Lewy bodies, is evident in multiple brain regions of PD patients. Although there are several available medications to treat PD symptoms, these medications do not prevent the progression of the disease. Soluble epoxide hydrolase (sEH) plays a key role in inflammation associated with the pathogenesis of PD. Here we found that MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced neurotoxicity in the mouse striatum was attenuated by subsequent repeated administration of TPPU, a potent sEH inhibitor. Furthermore, deletion of the sEH gene protected against MPTP-induced neurotoxicity, while overexpression of sEH in the striatum significantly enhanced MPTP-induced neurotoxicity. Moreover, the expression of the sEH protein in the striatum from MPTP-treated mice or postmortem brain samples from patients with dementia of Lewy bodies (DLB) was significantly higher compared with control groups. Interestingly, there was a positive correlation between sEH expression and phosphorylation of α-synuclein in the striatum. Oxylipin analysis showed decreased levels of 8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid in the striatum of MPTP-treated mice, suggesting increased activity of sEH in this region. Interestingly, the expression of sEH mRNA in human PARK2 iPSC-derived neurons was higher than that of healthy control. Treatment with TPPU protected against apoptosis in human PARK2 iPSC-derived dopaminergic neurons. These findings suggest that increased activity of sEH in the striatum plays a key role in the pathogenesis of neurodegenerative disorders such as PD and DLB. Therefore, sEH may represent a promising therapeutic target for α-synuclein–related neurodegenerative disorders.
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. Although the precise pathogenesis of PD is unknown, the pathological hallmark of PD involves the progressive loss of dopaminergic neurons in the substantia nigra (SN) (1, 2). In addition, the deposition of protein aggregates of α-synuclein, termed Lewy bodies, is evident in multiple brain regions of patients from PD and dementia with Lewy bodies (DLB) (3–7). Although there are many medications available to treat symptoms in PD patients, these do not prevent the progression of the disease, and, to date, no agent with a disease-modifying or neuroprotective indication for PD has been approved (8, 9). Therefore, the development of new drugs possessing disease-modifying and/or neuroprotective properties is critical.
Accumulating evidence suggests that various inflammatory processes play a central role in the pathogenesis of PD (10–13). Studies using postmortem brain samples have demonstrated the involvement of inflammation, mitochondrial dysfunction, and oxidative stress in affected brain regions in PD patients (14–18). Taken together, it is likely that antiinflammatory drugs, as well as neuroprotective drugs, may mitigate several symptoms in PD patients.
Many epoxy fatty acids (EpFAs) are produced from the corresponding olefin by cytochrome P450 enzymes. Epoxyeicosatrienoic acids (EETs) and epoxydocosapentaenoic acids (EDPs) are produced from arachidonic acid and docosahexaenoic acid (DHA), respectively. EETs, EDPs, and some other EpFAs have potent antiinflammatory properties. However, these mediators are broken down into their corresponding diols by soluble epoxide hydrolase (sEH), and inhibition of sEH enhances the beneficial effects of EETs (19–22). These potent antiinflammatory effects of EETs have been reported in multiple animal models (19–21, 23, 24). Recently, we reported that sEH plays a key role in the depressive symptoms (25, 26) observed in PD patients (27–29) and DLB patients (30). Thus, it seems that EETs may play a role in the pathogenesis of PD (31).
The purpose of this study was to examine the role of sEH in the pathogenesis of PD. First, we examined the effects of TPPU [1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea], a potent sEH inhibitor (25, 32, 33), on dopaminergic neurotoxicity, endoplasmic reticulum (ER) stress, and oxidative stress in the mouse striatum after repeated administration of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). Second, using sEH knockout (KO) mice, we examined the role of sEH in dopaminergic neurotoxicity, ER stress, and oxidative stress in the striatum from MPTP-treated mice. Third, we measured the protein expression of sEH in the striatum of MPTP-treated mice. We also performed oxylipin analysis of the striatum from MPTP-treated mice. Fourth, we measured the protein expression of sEH, the dopamine transporter (DAT), tyrosine hydrogenase (TH), and other PD-related proteins (α-synuclein and phosphorylated α-synuclein) in postmortem brain samples from DLB patients, as well as age-matched control subjects. Finally, using induced pluripotent stem cells (iPSCs), we examined whether sEH appeared to play a role in the pathogenesis of one case of familial PARK2 PD with mutations in the PARKIN (34).
Results
MPTP-Induced Neurotoxicity Was Attenuated After Subsequent Repeated Administration of TPPU.
First, we examined the effects of MPTP on dopaminergic neurotoxicity in the mouse striatum and SN. For immunohistochemistry of DAT and TH, mice were perfused 7 d after MPTP injection (SI Appendix, Fig. S1A). All doses of MPTP caused significant reductions of DAT and TH density in the striatum and TH-positive cell number in the SN compared with control mice (SI Appendix, Fig. S1 B–D). Therefore, MPTP (10 mg/kg) was used for subsequent experiments.
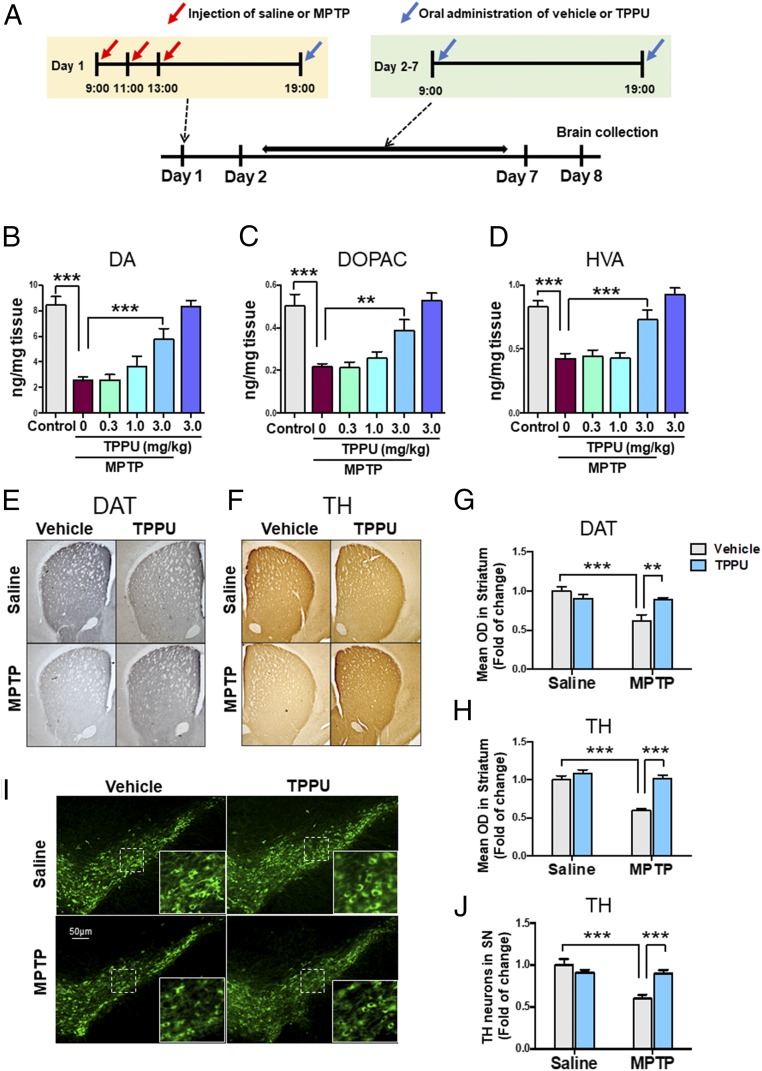
We examined the effects of TPPU on MPTP-induced dopaminergic neurotoxicity in the striatum. After repeated injections of saline or MPTP, vehicle or TPPU (0.3, 1.0, or 3.0 mg/kg) was administered orally to mice. Subsequently, vehicle or TPPU was administered orally to mice from day 2 to day 7 (Fig. 1A). Mice were killed by decapitation on day 8 for measurement of dopamine (DA) and its metabolites [DOPAC (3,4-dihydroxyphenylacetic acid) and HVA (homovanillic acid)]. Repeated administration of TPPU (3 mg/kg, twice daily, PO) significantly attenuated the reduction of DA, DOPAC, and HVA in the striatum after MPTP injection, although TPPU alone did not alter the levels of these markers (Fig. 1 B–D).
Fig. 1.
Effects of TPPU on MPTP-induced neurotoxicity in the mouse brain. (A) Schedule of treatment and brain collection. (B–D) Effects of TPPU (0.3, 1.0, or 3.0 mg/kg, PO) attenuated reductions of DA and its metabolites (DOPAC and HVA) in the striatum after repeated administration of MPTP. Data are shown as mean ± SEM (n = 7 or 8). **P < 0.01, ***P < 0.001 compared with vehicle + MPTP group. (E and F) Typical immunohistochemistry of DAT and TH in the striatum. (G and H) The data of DAT and TH immunoreactivity in the striatum. Data are shown as mean ± SEM (n = 6). **P < 0.01, ***P < 0.001 compared with control group. (I) Typical immunofluorescence of TH-positive cells in the SN. (J) TH immunoreactivity in the SN. Data are shown as mean ± SEM (n = 6). ***P < 0.001 compared with control group. Detailed statistical analysis data are in SI Appendix, Table S1.
Next, we performed immunohistochemistry of DAT and TH in the striatum and SN of mouse brain samples. Repeated administration of MPTP significantly reduced DAT and TH immunoreactivity in the striatum (Fig. 1 E–H) and the number of TH-positive cells in the SN (Fig. 1 I and J). Interestingly, MPTP-induced neurotoxicity in the striatum and SN was significantly attenuated after subsequent repeated administration of TPPU.
Deletion of the sEH Gene Protected Against MPTP-Induced Neurotoxicity.
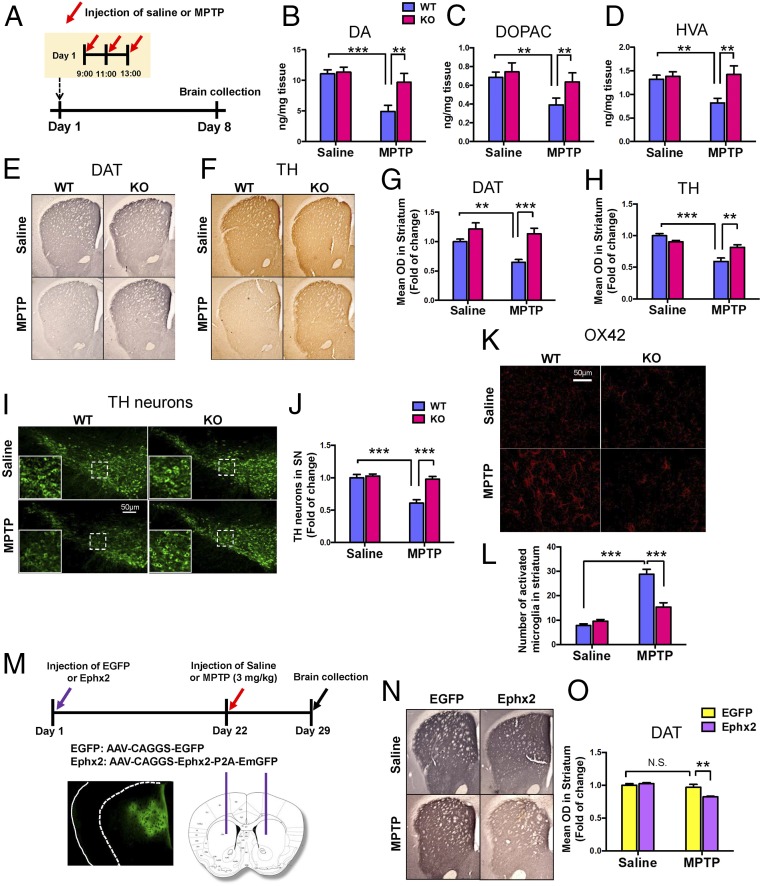
To investigate the role of sEH in MPTP-induced neurotoxicity in the striatum, wild-type and sEH-KO mice received repeated injections of MPTP (Fig. 2A). Seven days after the administration of MPTP, mice were killed for HPLC analysis or immunohistochemistry. Repeated administration of MPTP caused the reduction of DA and its metabolites (DOPAC and HVA) in the striatum of wild-type mice, although MPTP did not alter tissue levels of DA or its metabolites in the striatum of KO mice (Fig. 2 B–D). In addition, MPTP-induced reductions of DAT and TH in the striatum and SN were not detected in sEH KO mice (Fig. 2 E–J). Moreover, MPTP-induced increased expression of OX42 (microglial activation) in the striatum was significantly attenuated in the sEH KO mice (Fig. 2 K and L). These results suggest a key role of sEH in MPTP-induced neurotoxicity.
Fig. 2.
Lack of MPTP-induced neurotoxicity in the sEH KO mice. (A) Schedule of treatment and brain collection. (B–D) Repeated administration of MPTP did not decrease tissue levels of DA, DOPAC, and HVA in the striatum of sEH KO mice. Data are shown as mean ± SEM (n = 8). **P < 0.01, ***P < 0.001 compared with vehicle + MPTP group. (E and F) Typical immunohistochemistry of DAT and TH in the striatum. (G and H) The data of DAT and TH immunoreactivity in the striatum. Data are shown as mean ± SEM (n = 8). **P < 0.01, ***P < 0.001 compared with control group. (I) Typical immunofluorescence of TH-positive cells in the SN. (J) The data of TH immunoreactivity in the SN. Data are shown as mean ± SEM (n = 8). ***P < 0.001 compared with control group. (K) Typical immunofluorescence of OX42-positive cells in the striatum. (L) The data of OX42 immunoreactivity in the striatum. Data are shown as mean ± SEM (n = 4). **P < 0.01, ***P < 0.001 compared with control group. (M) Schematic of AAV-mediated Ephx2 expression in the striatum. The diagram shows the AAV constructs and stereotaxic injection of AAV into the striatum. (N and O) Typical immunohistochemistry of DAT in the mouse striatum. Data are shown as mean ± SEM (n = 6). **P < 0.001 compared with control group. Detailed statistical analysis data are in SI Appendix, Table S1.
Overexpression of sEH Enhanced MPTP-Induced Neurotoxicity.
To further investigate the role of sEH in MPTP-induced neurotoxicity, we applied sEH (or Ephx2)-AAV (adeno-associated virus) to overexpress sEH in the mouse striatum (Fig. 2M). Overexpression of sEH in the striatum by AAV significantly enhanced low-dose MPTP-induced neurotoxicity, although this treatment schedule did not alter DAT immunoreactivity in the striatum of the control group (Fig. 2 N and O). These results suggest that increased levels of sEH play an important role in MPTP-induced dopaminergic neurotoxicity in the brain.
Role of sEH in MPTP-Induced ER Stress and Oxidative Stress in the Striatum.
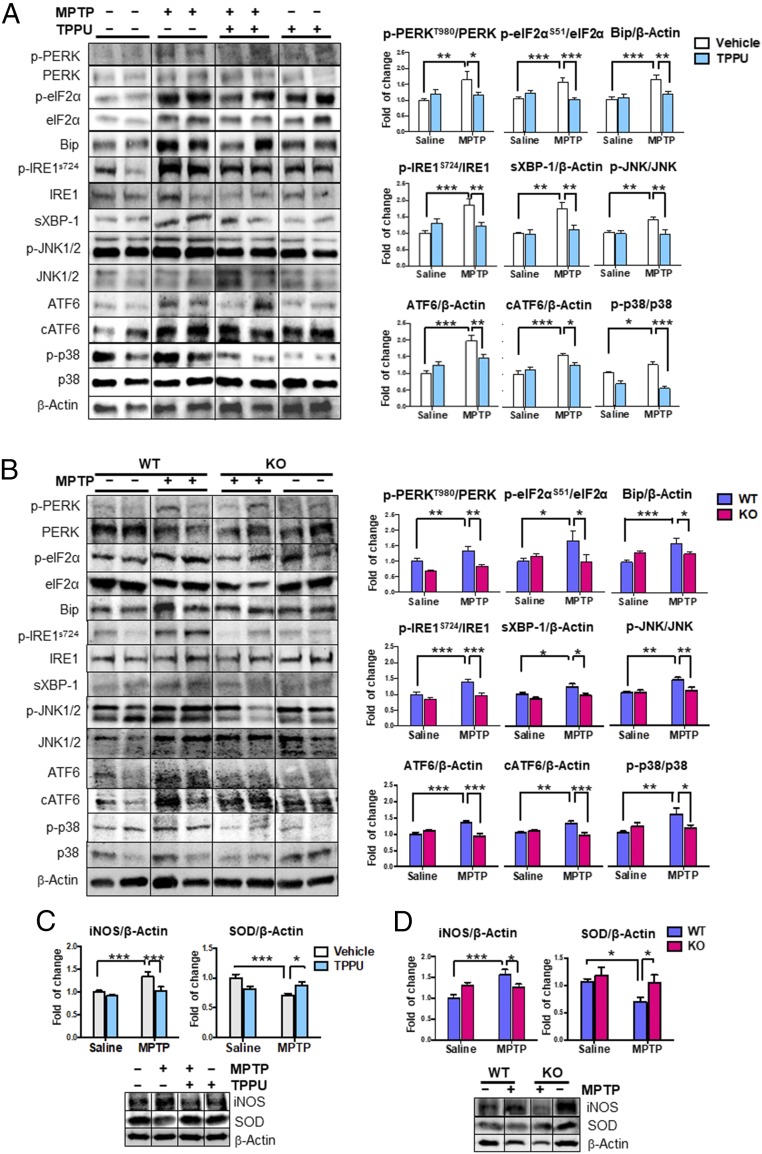
It is well-known that ER stress plays a role in the pathogenesis of PD (35) and that the sEH inhibitor attenuates activation of the ER (36–39). In this study, we examined the effects of a highly selective sEH inhibitor TPPU and sEH deletion in the MPTP-induced activation of ER stress in the striatum. We found increased levels of three key sensors in the ER stress-signaling pathway, including PKR-like ER-resident kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (Fig. 3 A and B). Levels of the associated downstream targets were elevated, suggesting full-scale activation of the ER stress pathway (37). Accordingly, phosphorylation of eukaryotic initiation factor 2 subunit α (eIF2α), mediated by phospho-PERK, was also increased. Phosphorylation of IRE1α led to a significant rise in total protein levels of spliced X-box binding protein 1 (Xbp1), as well as levels of the ER chaperone binding Ig protein. Increased phosphorylation of p38 and c-jun NH2-terminal kinase (JNK) 1/2 was also observed. Pharmacological inhibition by TPPU or sEH deficiency protected against MPTP-induced ER stress in the striatum (Fig. 3 A and B).
Fig. 3.
Effects of TPPU and deletion of sEH on elevated ER stress and oxidative stress in the MPTP-treated mice. (A) Markers of ER stress from saline or MPTP and vehicle or TPPU-treated mice were measured. Representative immunoblots were shown from two mice of the four groups. Data are shown as mean ± SEM (n = 7). *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group. (B) Markers of ER stress from WT or sEH-KO mice treated with saline or MPTP were measured. Representative immunoblots were shown from two mice of the four groups. Data are shown as mean ± SEM (n = 6 or 7). *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group. (C) Markers of oxidative stress from MPTP-treated mice were measured. Representative immunoblots are shown from mice in the four groups. Data are shown as mean ± SEM (n = 6 or 7). *P < 0.05, ***P < 0.001 compared with control group. (D) Markers of oxidative stress from MPTP-treated KO mice were measured. Representative immunoblots are shown from mice in the four groups. Data are shown as mean ± SEM (n = 6 or 7). *P < 0.05, ***P < 0.001 compared with control group. Detailed statistical analysis data are in SI Appendix, Table S1.
It is also known that oxidative stress plays a role in the pathogenesis of PD and in MPTP-induced neurotoxicity (14, 15, 40, 41). MPTP caused alterations in the levels of inducible nitric oxide (iNOS) and superoxide dismutase (SOD) in the striatum. Pharmacological inhibition by TPPU or sEH deficiency protected against MPTP-induced oxidative stress in the striatum (Fig. 3 C and D). These results suggest that the inhibition of sEH protected against MPTP-induced ER stress and oxidative stress in the brain.
Protein Expression and Oxylipin Profile in the Striatum from MPTP-Treated Mice.
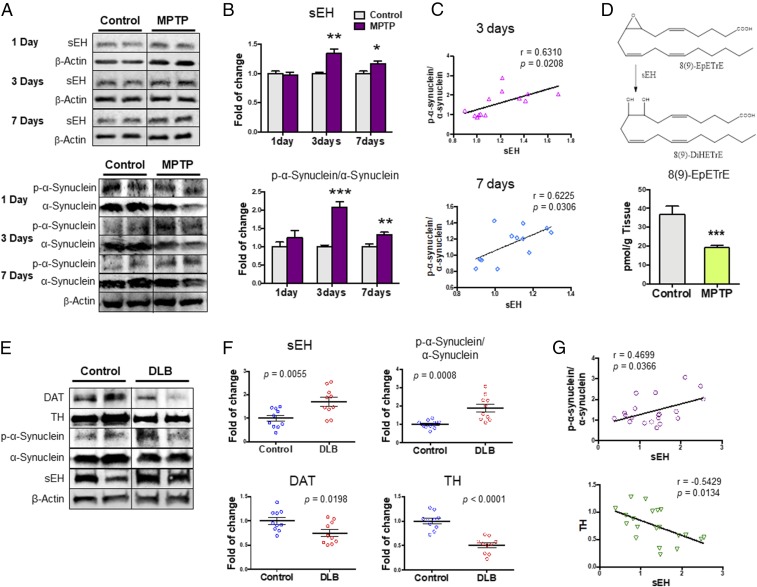
Three or seven days after the injections of MPTP, we found a significant increase in the sEH protein in the striatum of MPTP-treated mice compared with control mice (Fig. 4 A and B). Increasingly, the sEH protein is being viewed as a marker of inflammation. Furthermore, we found a significant increase in the phosphorylated α-synuclein/α-synuclein ratio in the striatum of MPTP-treated mice compared with control mice (Fig. 4 A and B). Interestingly, there was a positive correlation (3 d: r = 0.6310, P = 0.0208; 7 d: r = 6.225, P = 0.0306) between sEH levels and the phosphorylated α-synuclein/α-synuclein ratio in the striatum (Fig. 4C). These results suggest that increased sEH plays a role in the phosphorylation of α-synuclein in the striatum of MPTP-treated mice.
Fig. 4.
Protein levels of sEH and oxylipin analysis in the striatum from MPTP-treated mice and protein levels of sEH, DAT, and TH in the postmortem brain samples. (A) Protein expressions of sEH, α-synuclein, and phosphorylated α-synuclein (p-α-synuclein) in the striatum from MPTP-treated mice were measured. (B) Levels of sEH and p-α-synuclein/α-synuclein ratio in the striatum. The values are the mean ± SEM (n = 6 or 7). *P < 0.05, **P < 0.01 compared with control group. (C) A positive correlation between sEH levels and p-α-synuclein/α-synuclein ratio in the mouse striatum. (D) Tissue levels of 8 (9)-EpETrE in the striatum. The values represent the mean ± SEM (n = 8). ***P < 0.001 compared with control group. (E) Protein expression of sEH, DAT, TH, α-synuclein, and phosphorylated α-synuclein in the striatum from DLB patients (n = 10) and controls (n = 10). Representative immunoblots were shown from two subjects of the two groups. (F) Tissue levels of sEH, DAT, TH, and the ratio of phosphorylated α-synuclein/α-synuclein in the striatum from DLB patients and controls. The values represent the mean ± SEM (n = 10). (G) There was a positive correlation between sEH levels and the ratio of phosphorylated α-synuclein/α-synuclein in the subject (n = 20). Furthermore, there was a negative correlation between sEH levels and TH levels in the subjects.
Next, we measured tissue levels of eicosanoid metabolites (SI Appendix, Fig. S2 and Table S2) in the striatum of MPTP-treated mice and control mice. Tissue levels of antiinflammatory 8 (9)-EET [8 (9)-EpETrE or 8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid], a metabolite of arachidonic acid, in the MPTP-treated mice were significantly lower than those of control mice, supporting the increased activity of sEH in the striatum from MPTP-treated mice (Fig. 4D and SI Appendix, Table S2). Tissue levels of PGF2a (prostaglandin F2α) and 9 (10)-DiHOME [(12Z)-9,10-dihydroxyoctadec-12-enoic acid] in MPTP-treated mice were also lower than those of control mice. In contrast, tissue levels of 10 (11)-EpDPE [10 (11)-epoxy-4Z,7Z,13Z,16Z,19Z-docosapentaenoic acid] in MPTP-treated mice were significantly higher than those of control mice (SI Appendix, Table S2).
Increased Expression of sEH in the Striatum from DLB Patients.
Dementia with Lewy bodies (DLB) is pathologically characterized by α-synuclein and its phosphorylated α-synuclein aggregates in the brain (7, 42). Since deposition of α-synuclein has been shown in multiple brain regions of PD and DLB patients (3–7), postmortem brain samples from patients with DLB were used. We measured the protein expression of sEH, DAT, and TH in the striatum from these patients (n = 10) and age-matched control subjects (n = 10). Protein levels of sEH in the striatum from DLB patients were significantly higher than those of the controls, whereas protein levels of DAT and TH in the striatum from DLB patients were significantly lower than those of controls (Fig. 4 E and F). Furthermore, the ratio of phosphorylated α-synuclein to α-synuclein in the striatum from DLB patients was significantly higher than that of controls (Fig. 4 E and F). Interestingly, there was a positive correlation (r = 0.470, P = 0.036) between sEH levels and the ratio of phosphorylated α-synuclein to α-synuclein in all subjects (n = 20) (Fig. 4G). Furthermore, there was a negative correlation (r = −0.543, P = 0.0013) between sEH levels and TH levels in all subjects (n = 20) (Fig. 4G). Collectively, it is likely that increased sEH and resulting alteration in its substrates and products play a role in the pathogenesis of PD, as well as MPTP-induced neurotoxicity.
TPPU Prevents Apoptosis in PARK2 iPS–Derived Dopaminergic Neurons.
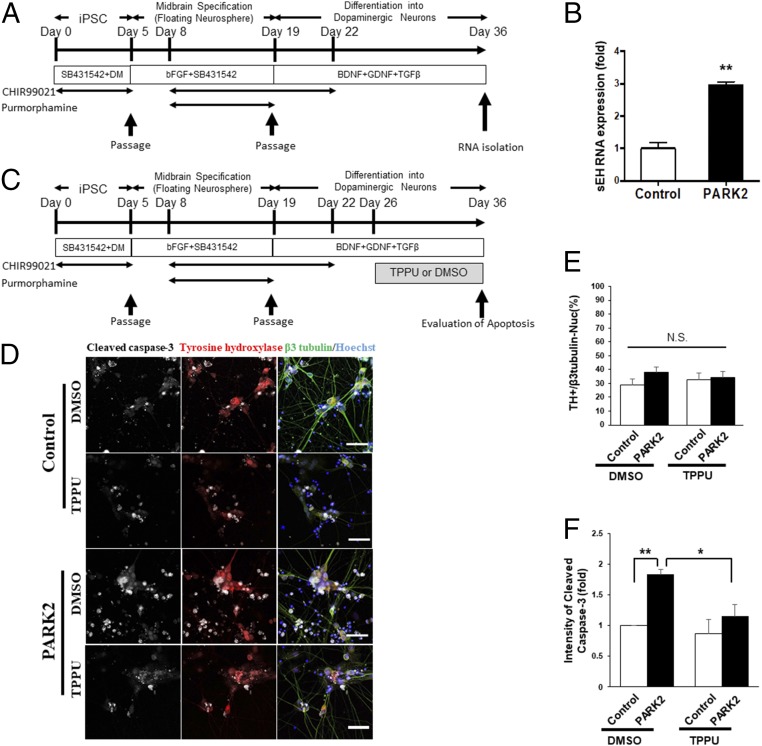
To assess the role of sEH in the pathogenesis of PD, we attempted to suppress sEH in dopaminergic neurons using TPPU. The iPSCs derived from PARK2, one of the familial forms of PDs caused by a mutation in the PARKIN, were differentiated into midbrain dopaminergic neurons (Fig. 5A). We confirmed that the expression of sEH (or EPHX2) mRNA in the PARK2 iPSC-derived neurons was significantly higher than that from control iPSCs (Fig. 5B). Next, we examined whether TPPU treatment could prevent progressive apoptosis in PARK2 iPSC-derived dopaminergic neurons. Dopaminergic neurons were differentiated and evaluated by cleaved caspase-3 expression (Fig. 5C). At first, we confirmed that the numbers of TH-positive DA neurons in the differentiated cells were not changed by the presence of the PARK2 mutation or TPPU treatment (Fig. 5 D and E). The amounts of cleaved caspase-3 were then evaluated in TH-positive dopaminergic neurons using immunocytochemistry and an IN-Cell analyzer 2200 (Fig. 5 D and F). While PARK2 iPSC-derived TH-positive neurons expressed significantly increased amounts of cleaved caspase-3, TPPU treatment decreased cleaved caspase-3 expression in PARK2 neurons to similar levels as control neurons (Fig. 5F). These results suggest that the suppression of sEH by TPPU protected against apoptosis in PARK2 neurons and that increased sEH may be involved in the degeneration of DA neurons in PD patients.
Fig. 5.
Role of sEH in the apoptosis of PARK2 iPSC-derived dopaminergic neurons. (A) Schedule of the culture protocol used for the induction of midbrain dopaminergic (mDA) neurons from hiPSCs. (B) qPCR analysis of sEH gene expression in control and PARK2 iPSC-induced mDA neurons (n = 2 or 3, mean ± SEM). **P < 0.01 compared with control group (Student t test). (C) Schedule of the culture protocol used for the induction of mDA neurons from hiPSCs and TPPU treatment. (D) Representative images of TPPU-treated PARK2 and control DA neurons visualized by IN Cell Analyzer 2200. Immunostaining was performed using cleaved caspase-3 and TH antibodies. (Scale bar, 50 µM.) (E) The numbers of TH-positive mDA neurons in the differentiated cells were calculated. The numbers of TH positive cells were not affected by TPPU treatment in both control and PARK2 iPSC-derived neurons. Data are shown as mean ± SEM (n = 4). (F) Apoptosis in mDA neurons was quantified and evaluated by cleaved caspase-3 staining and IN Cell Analyzer 2200. Apoptotic cells were significantly increased in PARK2 iPSC-derived dopaminergic neurons compared with control cells. Treatment with TPPU significantly decreased apoptotic cells in PARK2 iPSC-derived dopaminergic neurons. Data are shown as mean ± SEM (n = 4). *P < 0.05, **P < 0.01 compared with DMSO-treated PARK2 group. Detailed statistical analysis data are the SI Appendix, Table S1.
Discussion
The present results demonstrate a key role of sEH in the pathogenesis of PD. The major findings of the present study are as follows. First, MPTP-induced dopaminergic neurotoxicity in the striatum and SN was significantly attenuated by subsequent repeated administration of the potent sEH inhibitor, TPPU. Furthermore, MPTP-induced dopaminergic neurotoxicity in the striatum and SN was not detected in sEH KO mice. Second, MPTP-induced ER stress and oxidative stress in the striatum were significantly attenuated by subsequent repeated administration of TPPU or deletion of the sEH gene. Third, protein levels of sEH in the striatum from MPTP-treated mice or postmortem brain (striatum) from DLB patients were higher than those of controls. Interestingly, there was a positive correlation between sEH expression and the ratio of phosphorylated α-synuclein to α-synuclein in the striatum, suggesting a relationship between increased sEH expression and phosphorylation of α-synuclein in the striatum. Levels of 8 (9)-EpETrE in the striatum from MPTP-treated mice were significantly lower than those of control mice, supporting the increased activity of sEH in the striatum from MPTP-treated mice. Finally, we found an increase level of sEH (or EPHX2) mRNA in PARK2 iPSC-derived neurons. Interestingly, treatment with TPPU significantly attenuated apoptosis in PARK2 iPSC-derived dopaminergic neurons. Collectively, these findings suggest that sEH plays a key role in the pathogenesis of PD and that sEH inhibitors may prove to be promising prophylactic or therapeutic drugs for PD.
In this study, we found that MPTP-induced neurotoxicity (e.g., loss of DAT, TH-positive cells, ER stress, and oxidative stress) in the striatum and SN was attenuated after subsequent repeated oral administration of TPPU. In contrast, it has been reported that MPTP-induced loss of TH-positive cells in the SN is attenuated by pretreatment with another sEH inhibitor, AUDA, although posttreatment with AUDA did not attenuate MPTP-induced neurotoxicity (43). The difference may be due to the following reasons. TPPU is a more potent inhibitor than AUDA and more resistant to metabolism to inactive compounds (44, 45). Furthermore, sEH KO mice were resistant to MPTP-induced neurotoxicity in the striatum, consistent with previous reports (43, 46). Thus, the results using the genetic deletion of sEH or the pharmacological inhibition of sEH suggest a crucial role of sEH in MPTP-induced neurotoxicity in the striatum and SN.
Tissue levels of the sEH protein in the striatum of MPTP-treated mice were higher than those of control mice, consistent with a previous report (43). We also found that the phosphorylated α-synuclein/α-synuclein ratio in the striatum from MPTP-treated mice was higher than that of control mice. Interestingly, we found a positive correlation between the sEH protein and the phosphorylated α-synuclein/α-synuclein ratio in the striatum. Furthermore, we found that levels of sEH in the striatum from DLB patients were higher than those of controls. A positive correlation between sEH levels and phosphorylated α-synuclein/α-synucleinin ratio in the striatum with DLB patients is noteworthy since α-synuclein is strongly linked to the pathogenesis of both familial and sporadic PD (3). There was a negative correlation between sEH levels and TH levels in all subjects, suggesting a crucial role of sEH in the dopaminergic neurotoxicity of PD. Collectively, it is likely that increased sEH in the striatum plays a role in the increased phosphorylation of α-synuclein in the striatum from MPTP-treated mice and DLB patients. Given the role of the increased phosphorylation of α-synuclein in the pathogenesis of α-synuclein–related neurodegenerative disorders such as PD and DLB, it is likely that pharmacological inhibition of sEH may attenuate the phosphorylation of α-synuclein, as well as dopaminergic neurotoxicity in the brain, resulting in a delay in the disease progression of α-synuclein–related neurodegenerative disorders.
Among four epoxyeicosatrienoic acids (EETs), tissue levels of 8 (9)-EpETrE were significantly lower in MPTP-treated mice than those of control mice, supporting an increased activity of sEH in the striatum from MPTP-treated mice. The 8 (9)-EpETrE is metabolized to its corresponding diol, dihydroxyeicosatrienoic acid (DiHETrE), by sEH. It is known that EETs such as EpETrE are important components of many intracellular signaling pathways in both cardiac and extracardiac tissues (47) and that EETs and some other EpFAs possess potent antiinflammatory properties (48, 49). Interestingly, Collino et al. (50) reported increased serum concentrations of 8 (9)-EpETrE in centenarians compared with elderly and young subjects, suggesting increased activity of CYP enzymes or decreased activity of sEH in the centenarians. These findings may support the promotion of cellular detoxification mechanisms through specific modulation of the arachidonic acid metabolic cascade in centenarians (50). Although the precise mechanisms underlying the relationship between 8 (9)-EpETrE and sEH in the striatum from MPTP-treated mice are currently unclear, it seems that decreased levels of 8 (9)-EpETrE by increased levels of sEH in the striatum may be involved in MPTP-induced neurotoxicity. By contrast, we found increased levels of 10 (11)-EpDPE in the striatum of MPTP-treated mice, although levels of sEH were lower in this region. Although the reasons underlying this discrepancy are currently unknown, it seems that multiple pathways may contribute to formation and degradation of 10 (11)-EpDPE in the striatum. Further detailed study of the metabolism of EpFAs and their diol metabolites in α-synuclein–related neurodegenerative disorders is needed.
Using patient-specific iPSCs, we found an increased expression of sEH in PARK2 iPSC-derived neurons compared with controls, and TPPU protected against apoptosis in PARK2 iPSC-derived dopaminergic neurons, suggesting that increased activity of sEH may play a role in the pathogenesis of the PD patient with PARK2 mutations. From the present study, we do not fully account for the molecular mechanisms of other familial or sporadic PD since PARKIN is a causative gene of autosomal recessive juvenile PD (51, 52). Therefore, further studies using human iPSCs from other familial or sporadic PD patients are needed. In addition, transplanted human neural stem cells may open a new venue of research for our understanding of the pathology and treatment of PD (52, 53).
Epidemiological and clinical data suggest that ω-3 polyunsaturated fatty acids (PUFAs) may constitute a therapeutic strategy for several brain disorders, including PD and DLB (54–56). Multiple studies have reported that the EDPs derived from DHA are more antiinflammatory and analgesic than EETs from arachidonic acid (23, 57, 58). Therefore, it is likely that an ω-3 enriched and an omega ω-6 depleted diet may have a beneficial effect on PD patients if the sEH can be depleted. Linoleic acid is also metabolized to 9,10- or 12,13-epoxyoctadecenoate, and arachidonic acid is metabolized to EETs. These epoxides are metabolized to their corresponding diols by sEH (59). A recent study demonstrated that the diol 19 (20)-dihydroxydocosapentaenoic acid [19 (20)-DHDP] generated from DHA by sEH had proinflammatory and reduced cellular barrier function in diabetic retinopathy (60), suggesting that inhibition of sEH can prevent progression of the disease.
It is well-known that PD or DLB patients have depressive symptoms (27–30). Previously, we reported the prophylactic and therapeutic effects of TPPU in the inflammation and chronic social defeat stress models of depression (25), suggesting that sEH inhibitors may prevent the onset of the depression-like phenotype by inflammation or repeated stress. Given the comorbidity of depressive symptoms in PD or DLB patients, it is likely that sEH inhibitors may serve as prophylactic drugs to prevent the progression of PD or DLB in patients.
In conclusion, this study shows that genetic deletion or pharmacological inhibition of sEH may protect against MPTP-induced neurotoxicity in the mouse brain. Furthermore, the data using postmortem brain suggest that sEH may play a role in the aggregation of α-synuclein and phosphorylated α-synuclein in the striatum, supporting a crucial role of sEH in the pathogenesis of PD and DLB. Moreover, the sEH inhibitor used in this study protected against apoptosis in PARK2 iPSC-derived dopaminergic neurons. Therefore, sEH inhibitors appear to be prophylactic or therapeutic drugs for α-synuclein–related neurodegenerative disorders such as PD and DLB.
Materials and Methods
The protocol using animals was approved by the Chiba University Institutional Animal Care and Use Committee. This study using postmortem brain samples was approved by Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology and the Research Ethics Committee of the Graduate School of Medicine, Chiba University. The experiment using human iPSCs was approved by the Juntendo University School of Medicine Ethics Committee. Details of the experimental protocols, including animals, materials, MPTP-induced model, HPLC analysis, immunohistochemistry, viral vector injection, Western blot analysis, oxylipin analysis, human iPSCs, and statistical analysis, are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (to Q.R., 16K19751; to K.H., 17H04243), SENSHIN Medical Research Foundation, Japan (to Q.R.), GSK Japan Research Grant 2017 (to Q.R.), the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H., JP17dm0107119), Rare/Intractable Disease Project from AMED (to K.-i.-I., S.S., N.H., and W.A., JP17ek0109244), JSPS KAKENHI (to S.M., 16H06277), the National Institute of Environmental Health Sciences (NIEHS) R01 ES002710 (to B.D.H.), and the NIEHS Superfund Program P42 ES004699 (to B.D.H.). We are also thankful for the Platform of Supporting Cohort Study and Biospecimen Analysis Grant-in-Aid for Scientific Research on Innovative Areas―Platforms for Advanced Technologies and Research Resources, Ministry of Education, Culture, Sports, Science and Technology, Japan (to S.M.). Q.R. was supported by a Research Fellowship for Young Scientists of JSPS (Tokyo, Japan).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6322.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802179115/-/DCSupplemental.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, et al. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olanow CW, Kordower JH. Targeting α-synuclein as a therapy for Parkinson’s disease: The battle begins. Mov Disord. 2017;32:203–207. doi: 10.1002/mds.26935. [DOI] [PubMed] [Google Scholar]
- 6.Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46:S30–S33. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeith I, et al. International Psychogeriatric Association Expert Meeting on DLB Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 8.Dehay B, et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieburtz K, Katz R, Olanow CW. New drugs for Parkinson’s disease: The regulatory and clinical development pathways in the United States. Mov Disord. 2017 doi: 10.1002/mds.27220. [DOI] [PubMed] [Google Scholar]
- 10.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8(Suppl 1):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 13.Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. 2017;96:379–390. doi: 10.1002/jnr.24185. [DOI] [PubMed] [Google Scholar]
- 14.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36, discussion S36–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Sleiman PM, Muqit MMK, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 17.Schapira AHV. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 18.Toulorge D, Schapira AHV, Hajj R. Molecular changes in the postmortem parkinsonian brain. J Neurochem. 2016;139:27–58. doi: 10.1111/jnc.13696. [DOI] [PubMed] [Google Scholar]
- 19.Morisseau C, Hammock BD. Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris TR, Hammock BD. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113-115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Vicario C, et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proc Natl Acad Sci USA. 2015;112:536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Q, et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA. 2016;113:E1944–E1952. doi: 10.1073/pnas.1601532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto K. Soluble epoxide hydrolase: A new therapeutic target for depression. Expert Opin Ther Targets. 2016;20:1149–1151. doi: 10.1080/14728222.2016.1226284. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL. Depression and Parkinson’s disease: A review. Am J Psychiatry. 1992;149:443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 28.Goodarzi Z, et al. Detecting depression in Parkinson disease: A systematic review and meta-analysis. Neurology. 2016;87:426–437. doi: 10.1212/WNL.0000000000002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi S, Mizukami K, Yasuno F, Asada T. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9:56–61. doi: 10.1111/j.1479-8301.2009.00292.x. [DOI] [PubMed] [Google Scholar]
- 31.Lakkappa N, Krishnamurthy PT, Hammock BD, Velmurugan D, Bharath MMS. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med Hypotheses. 2016;93:161–165. doi: 10.1016/j.mehy.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose TE, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostermann AI, et al. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea (TPPU): bioavailability, resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imaizumi Y, et al. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain. 2012;5:35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercado G, Castillo V, Soto P, Sidhu A. ER stress and Parkinson’s disease: Pathological inputs that converge into the secretory pathway. Brain Res. 2016;1648:626–632. doi: 10.1016/j.brainres.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Bettaieb A, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inceoglu B, et al. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci USA. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris TR, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–111. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediat. 2017;133:68–78. doi: 10.1016/j.prostaglandins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul R, et al. Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: Involvement of mitochondrial dysfunctions and oxidative stress. PLoS One. 2017;12:e0171285. doi: 10.1371/journal.pone.0171285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 43.Qin X, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol Neurobiol. 2015;52:187–195. doi: 10.1007/s12035-014-8833-3. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, et al. The antiinflammatory effect of laminar flow: The role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JY, et al. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009;156:284–296. doi: 10.1111/j.1476-5381.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang HJ, Wang YT, Lin HC, Lee YH, Lin AMY. Soluble epoxide hydrolase inhibition attenuates MPTP-induced in the nigrostriatal dopaminergic system: Involvement of α-synuclein aggregation and ER stress. Mol Neurobiol. 2018;55:138–144. doi: 10.1007/s12035-017-0726-9. [DOI] [PubMed] [Google Scholar]
- 47.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 49.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017;180:62–76. doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collino S, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8:e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 52.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet. 1999;353:SI29–SI30. doi: 10.1016/s0140-6736(99)90229-5. [DOI] [PubMed] [Google Scholar]
- 53.Napoli E, Borlongan CV. Cell therapy in Parkinson’s disease: Host brain repair machinery gets a boost from stem cell grafts. Stem Cells. 2017;35:1443–1445. doi: 10.1002/stem.2636. [DOI] [PubMed] [Google Scholar]
- 54.Bousquet M, Calon F, Cicchetti F. Impact of ω-3 fatty acids in Parkinson’s disease. Ageing Res Rev. 2011;10:453–463. doi: 10.1016/j.arr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Lei E, Vacy K, Boon WC. Fatty acids and their therapeutic potential in neurological disorders. Neurochem Int. 2016;95:75–84. doi: 10.1016/j.neuint.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Burckhardt M, et al. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst Rev. 2016;4:CD009002. doi: 10.1002/14651858.CD009002.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulu A, et al. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2013;62:285–297. doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W, et al. ω-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113-115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD. Novel metabolic pathways for linoleic and arachidonic acid metabolism. Biochim Biophys Acta. 1996;1290:327–339. doi: 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 60.Hu J, et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552:248–252. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.