Abstract
The integrin α9 subunit forms a single heterodimer, α9β1. The α9 subunit is most closely related to the α4 subunit, and like α4 integrins, α9β1 plays an important role in leukocyte migration. The α4 cytoplasmic domain preferentially enhances cell migration and inhibits cell spreading, effects that depend on interaction with the adaptor protein, paxillin. To determine whether the α9 cytoplasmic domain has similar effects, a series of chimeric and deleted α9 constructs were expressed in Chinese hamster ovary cells and tested for their effects on migration and spreading on an α9β1-specific ligand. Like α4, the α9 cytoplasmic domain enhanced cell migration and inhibited cell spreading. Paxillin also specifically bound the α9 cytoplasmic domain and to a similar level as α4. In paxillin−/− cells, α9 failed to inhibit cell spreading as expected but surprisingly still enhanced cell migration. Further, mutations that abolished the α9-paxillin interaction prevented α9 from inhibiting cell spreading but had no effect on α9-dependent cell migration. These findings suggest that the mechanisms by which the cytoplasmic domains of integrin α subunits enhance migration and inhibit cell spreading are distinct and that the α9 and α4 cytoplasmic domains, despite sequence and functional similarities, enhance cell migration by different intracellular signaling pathways.
INTRODUCTION
Integrins are a family of transmembrane receptors composed of at least 25 different αβ heterodimers that mediate both cell-substrate and cell-cell adhesion (Hynes, 1992). Among their other functions, integrins play a central role in cell migration. Integrin-dependent migration is important in many biologic processes including embryonic development, wound healing, inflammation, and tumor metastasis.
Cell migration is a complex and poorly understood process that involves both actin reorganization and integrin-dependent focal adhesion remodeling. For cells to migrate on a substrate, they must adhere and de-adhere in a coordinated manner and generate tensile force in the direction of migration. The force required to promote migration is generated by the actin cytoskeleton and integrin-dependent protein complexes that anchor actin to specific sites on the cell membrane (Lauffenburger and Horwitz, 1996). The actin reorganization required to promote cell polarization and directional migration is regulated by specific intracellular signaling pathways (Lauffenburger and Horwitz, 1996; Horwitz and Parsons, 1999). Although most members of the integrin family are capable of mediating cell migration (Lauffenburger and Horwitz, 1996), experiments utilizing chimeric or truncated integrins indicate that the cytoplasmic domain of the α4 subunit preferentially enhances cell migration and inhibits cell spreading compared with other α subunits of the β1 subclass of integrins (Chan et al., 1992; Kassner et al., 1995). In addition, unlike most integrins, α4β1 is relatively excluded from mature focal adhesion complexes (Kassner et al., 1995). These findings support the hypothesis that α4-containing integrins, which are widely expressed on leukocytes, play a specialized role in promoting rapid cell migration. Further, these findings support a model that links inhibition of cell spreading (i.e., cell rounding) to enhanced migration. Recently, the integrin α9β1, an integrin that is structurally most closely related to α4β1, was shown to promote transendothelial neutrophil migration through its interactions with vascular cell adhesion molecule-1 on activated endothelial cells (Taooka et al., 1999). The cytoplasmic domain of the α9 subunit shares 52% homology with the α4 subunit but is divergent from all other integrin-cytoplasmic domains. We therefore questioned whether the α9 cytoplasmic domain would also specifically enhance cell migration and inhibit cell spreading.
Recently, α4β1-dependent cell migration was shown to be dependent on the specific interaction of the α4 cytoplasmic domain with the adapter protein paxillin (Liu et al., 1999). Paxillin participates in several intracellular signaling pathways that influence cell migration (Clark and Brugge, 1995; Turner, 2000). Site-directed mutagenesis of a tyrosine (Y) residue to an alanine (A) residue at position 991 (Y991A) in the α4 cytoplasmic domain that inhibited the α4-paxillin interaction also inhibited α4β1-dependent migration and cell shape changes (Liu et al., 1999; Liu and Ginsberg, 2000). In the current study, we utilized a series of chimeric and truncated versions of the α9 subunit to determine whether the α9 cytoplasmic domain preferentially enhances migration and inhibits cell spreading on an α9β1-specific ligand and what role, if any, paxillin plays in these processes.
MATERIALS AND METHODS
Reagents and Antibodies
The α9β1-specific ligand used in this study was a recombinant form of the third fibronectin type III repeat of chicken tenascin-C (Prieto et al., 1993) containing alanine (A) substitutions for both glycine (G) and aspartate (D) residues within the arginine (R)-G-D site (TNfn3RAA; Yokosaki et al., 1998). The cDNA for TNfn3RAA was obtained from Anita Prieto and Kathryn Crossin (Scripps Research Institute, La Jolla, CA) and was prepared in Escherichia coli as previously described (Prieto et al., 1993). The mouse mAb, Y9A2, increased against human α9β1, was prepared as previously described (Wang et al., 1996). The following monoclonal antibodies were purchased commercially: monoclonal antibodies against paxillin (clone 349, BD-Biosciences-Transduction Laboratories, Lexington, KY) and against hemagglutinin (HA)-tag (12CA5, American Type Culture Collection [ATCC], Rockville, MD).
Generation of α9 Constructs
The previously described pBlueScript (BS)-SKα9 cDNA plasmid was used as the template to generate all α9 constructs (Yokosaki et al., 1994). To generate the α9 chimeras containing the cytoplasmic domains of α2, α5, and α4, a mutation in the α9 sequence was generated at amino acid position 972 in the transmembrane domain near the start of cytoplasmic domain that changed a leucine residue to a valine residue and created an SpeI restriction site. The mutation was created by polymerase chain reaction (PCR) with the use of a 5′ forward primer that was upstream of an EcoRI site in the extracellular domain, 5′-tttcctttcatgaggtca-3′ and a 3′ reverse primer that created the SpeI restriction site for subcloning into the multiple-cloning site of pBS-SKα9, 5′-ttct ta ctag tacggccagcagcaggaagat-3′. The nucleotides in bold type represent the sites of mutagenesis. The PCR product was digested with EcoRI and SpeI, purified, and subcloned into pBS-SKα9. The α2 and α5 cDNA used in the PCR reactions to generate the cytoplasmic domains was from a teratocarinoma-2 cell line (ATCC). To generate the α9α2 chimera, a 5′ forward primer that was specific for the cytoplasmic domain of α2 and contained an SpeI restriction site, 5′-ggcttactagtctggaagctcggcttcttc-3′, and a 3′ reverse primer specific for the cytoplasmic domain of α2 that contained a NotI restriction site for subcloning into the multiple-cloning site of pBS-SKα9, 5′-atcttgcggccgcaagaaatccatgcacgcaaa-3′, were used. The PCR product was digested with SpeI and NotI, purified, and subcloned into pBS-SKα9. To generate the α9α5 chimera, a 5′ forward primer that was specific for the cytoplasmic domain of α5 and contained an SpeI restriction site, 5′-ttcttactagtctggaaacttggattcttcaaacgc-3′, and a 3′ reverse primer specific for the cytoplasmic domain of α5 that contained an XbaI restriction site for subcloning into the multiple-cloning site of pBS-SKα9, 5′-atctttctagagtggggggactggttcttca-3′, were used. To generate the α9α4 chimera, the α4 expression plasmid, pcα4DM8 (generously provided by Dr. David Erle), was used as a template, and a 5′ forward primer that was specific for the cytoplasmic domain of α4 and contained an SpeI site, 5′-gctccactagtctggaaggctggcttcttt-3′, and a 3′ reverse primer specific for the cytoplasmic domain of α4 that contained a NotI site, 5′-ctgctgctgctggcggccgcggtaccttattaatcatcatt-gcttttac-3′, were used. To generate the α9 cytoplasmic deletion mutants (α9DMs), the pBS-SKα9 was used as the template in PCR reactions that used the 5′ forward primer, 5′-tttcctttcatgaggtca-3′, for all of the α9DMs (Figure 1), and the 3′ reverse primers specific for the α9 cytoplasmic domain that contained a NotI restriction site, 5′-ttcttgcggccgcttacatcttccagagcagcacggc-3′, 5′-ttcttgcggccgcttatcggcgaaagaagcccatctt-3′, 5′-ctgctgctgctggcggccgctctagattattattctttgtacc-ttcggcg-3′, 5′-ctgctgctgctggcggccgctctagattattacttctcagcttcgataat-3′, 5′-ctgctgctgctggcggccgctctagattattattcattctctttccggtt-3′, 5′-ctgctgctgctggcggccgctctagattattactggacccagtcccaact-3′, for α9DM1-α9DM6, respectively. All reverse primers were designed to introduce two translation stop codons in tandem at the end of the coding sequence before the restriction sites, and all constructs were confirmed by nucleotide sequencing. The α9 site-directed mutants containing a tryptophan (W) to A substitution at either position 999 (W999A) or 1001 (W1001A) in the α9 subunit were generated from pBS-SKα9 with the use of a QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. The 5′ forward and 3′ reverse primers used to generate α9(W999A) were 5′-ggaaagagaatgaagacagt gcggactgggtccagaaaaacc-3′ and 5′-ggtttttctggac ccagtccgcactgtcttcattctctttcc-3′, and the primers used to generate α9(W1001A) were 5′-ggaaagagaatgaagacagttgggac gcggtccagaaaaacc-3′ and 5′-ggtttttctggaccgcgtc ccaactgtcttcattctctttcc-3′. The nucleotides in bold type represent the sites of mutagenesis. All constructs were confirmed by nucleotide sequencing. All α9 constructs were subcloned into the previously described full-length α9 expression plasmid pcDNAIneoα9 (Yokosaki et al., 1994) after excision of the pBS-SKα9 constructs with HindIII and NotI and subcloning into pcDNAIneoα9. For subcloning into pBABEpuro, the α9 constructs were excised from pBS-SKα9.
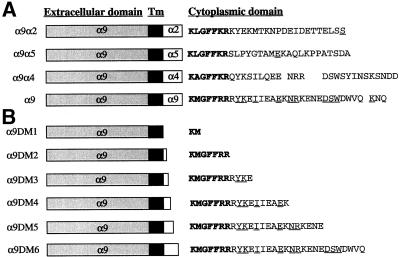
Figure 1.
Schematic diagram of α9, α9 chimeras, and α9DMs. The cytoplasmic amino acid sequences of α9, α9 chimeras (A), and α9DMs (B) are shown. Amino acids indicated in bold type represent areas of homology between all constructs and the underlined amino acids represent areas of homology with the α4 cytoplasmic domain.
Generation of Stable Cell Lines
The CHO cells lines were generated by calcium phosphate precipitation with vectors made in pcDNAIneo (Invitrogen, San Diego, CA.) and were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and the neomycin analogue G418 (1 mg/ml; Life Technologies, Rockville, MD). Transfected cells were analyzed for expression of α9β1 integrins by flow cytometry with the anti-α9β1 antibody, Y9A2. Mouse embryonic fibroblasts (MEF; from ATCC) and MEF paxillin−/− cell lines were infected with the use of α9 constructs in the retroviral vector pBABEpuro (Morgenstern and Land, 1990). Retroviruses were generated by calcium phosphate-mediated transfection into the Phoenix-E replication-incompetent ecotropic virus packaging cell line (Kinoshita et al., 1998; Swift et al., 1999). Specifically, 8 μg of plasmid DNA were added to 70% confluent Phoenix-E cells growing in 60-mm tissue culture dishes at 37°C in 3 ml of 10% FCS DMEM for 16 h. The medium was removed, 3 ml of fresh 10% FCS DMEM were added, and the cells were cultured for 16 h. Virus-containing supernatants were harvested and filtered through a 0.22-μm filter and then added to 50% confluent cultures in the presence of 5 μg/ml polybrene and cultured for 18–20 h. The virus-containing medium was removed and the cells were cultured in 10% FCS DMEM supplemented with 10 μg/ml puromycin (Sigma, St. Louis, MO). MEF and paxillin−/− MEF cells expressing the α9β1 constructs were identified by flow cytometry with the anti-α9β1 antibody, Y9A2. Fluorescence-activated cell sorting was performed to isolate heterogeneous populations of cells expressing high levels of α9β1 integrins on their cell surfaces (Yokosaki et al., 1998). All cell lines continuously expressed high surface levels of α9β1 as determined by flow cytometry with Y9A2.
Flow Cytometry
Cultured cells were harvested by trypsinization and rinsed with phosphate-buffered saline (PBS). Nonspecific binding was blocked with normal goat serum at 4°C for 10 min. Cells were then incubated with primary antibody for 20 min at 4°C, followed by a secondary goat anti-mouse antibody conjugated with phycoerythrin (Chemicon, Temecula, CA). Between incubations cells were washed twice with PBS. The stained cells were resuspended in 100 μl of PBS, and fluorescence was quantified on 5000 cells with a FACScan (Becton Dickinson, Rutherford, NJ) flow cytometer.
Cell Adhesion Assays
The wells of nontissue culture 96-well microtiter plates (Nunc, Naperville, IL) were coated by incubation with 100 μl of TNfn3RAA for 1 h at 37°C. After incubation, wells were washed with PBS and then blocked with 1% bovine serum albumin (BSA) in DMEM at 37°C for 30 min. Control wells were filled with 1% BSA in DMEM. The cells were detached with the use of 2.5 ml of trypsin solution (Sigma), followed by 2.5 ml of trypsin-neutralizing solution (Sigma), washed once in DMEM, and resuspended in DMEM at 5 × 105 cells/ml. The cells were incubated with or without 50 μg/ml Y9A2 for 20 min at 4°C before plating. Plates were centrifuged (top side up) at 10 × g for 5 min before starting the incubation for 1 h at 37°C in humidified 5% CO2. Nonadherent cells were then removed by centrifugation (top side down) at 48 × g for 5 min. Attached cells were fixed and stained in 40 μl of a 1% formaldehyde, 0.5% crystal violet, 20% methanol solution for 30 min, after which the wells were washed three times with PBS. The relative number of cells in each well was evaluated after solubilization in 40 μl of 2% Triton X-100 by measuring the absorbance at 595 nm in a microplate reader (Bio-Rad, San Francisco, CA). All determinations were carried out in triplicate, and the data represent the means ± SEM for a minimum of three experiments.
Cell Migration Assays
For chemotactic migration assays, 24-well Transwell plates (Costar, Cambridge, MA) were used. The lower side of the Transwell filters (6.5-mm diameter, pore size 8.0 μm) were coated with TNfn3RAA dissolved in 250 μl of DMEM for 60 min at 37°C. After incubation with TNfn3RAA, filters were washed by adding 100 μl of PBS to the top well and 500 μl of PBS to the bottom well. After washing twice, filters were blocked with 1% BSA in DMEM for 30 min and again washed once in PBS. Cells were detached as described above and resuspended in DMEM at 5 × 105 cells/ml. Migration and adhesion assays were performed at the same time, and the cells from the same dishes were used for both assays. Cells were incubated for 20 min on ice with or without the anti-α9β1 antibody, Y9A2 (50 μg/ml), and then 100 μl were loaded (50,000 cells/chamber) in each chamber. Each chamber was inserted into a well containing 600 μl of DMEM supplemented with 1% FCS to serve as a chemoattractant and incubated at 37°C in humidified 5% CO2 for 2 h for the CHO cells or 3 h for the MEF cells. Medium was then aspirated and the filters washed once with PBS. Cells on the bottom of the filters were fixed for 20 min in 500 μl of DifQuik fixative (Fisher, Springfield, NJ), and the nonmigrated cells on the top of the filter were gently removed with a Q-tip. Filters were allowed to completely dry, stained by DifQuik, washed in running distilled H20 and allowed to destain in distilled H2O for 1 h. Filters were air-dried (≥3 h), removed from the chamber with a scalpel, and mounted onto glass slides with the use of a Permamount/xylene solution, and the migrated cells were counted. Migrated cells were counted under a 25× objective with the use of a gridded eyepiece (reticule). Ten high-powered fields (HPF) per slide were counted, the average was taken, and the number of migrated cells was expressed as migrated cells per 10 HPF. The data represent means ± SEM from a minimum of three experiments
Cell-spreading Assays
Glass coverslips (12-mm circle, Fisher) sterilized in 100% ethyl alcohol were placed into the wells of 24-well plates, washed twice with 500 μl of PBS, and coated with TNfn3RAA in 400 μl of serum-free DMEM for 60 min at 37°C. After incubation with TNfn3RAA, coverslips were washed twice with 500 μl of PBS and then blocked with 1% BSA in DMEM for 30 min at 37°C. Cells were detached (as described above) and resuspended in DMEM at 5 × 105 cells/ml with 100 μl (50,000 cells), loaded onto coated coverslips, and incubated for either 3 h (MEF cells) or 6 h (CHO cells) at 37°C in humidified 5% CO2. Medium was then removed, and the cells were washed in PBS once and fixed in 2% (freshly made) paraformaldehyde for 20 min at room temperature. Coverslips were mounted and analyzed under a 25× objective with the use of a reticule. Ten HPF per slide were observed, and the number of spread cells and the number of total cells were counted. Time-course experiments were first performed to determine the time required to display the greatest difference in cell spreading for each cell type studied. The difference in the rate of cell spreading for a given time was expressed as the number of spread cells/number of total cells in 10 HPF × 100. Data represent the mean percentages of spread cells ± SEM for a minimum of three experiments.
Coimmunoprecipitation and Western Blot Analysis
CHO cell lines expressing different chimeric α9β1 integrins were surface labeled with sulfo-N-hydroxysuccinimide-biotin (Pierce, Rockford, IL) according to the manufacturer's protocol. Cells were then lysed on ice for 30 min in an immunoprecipitation buffer: 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10 mM EDTA, 10 mM benzamidine HCl, 0.02% sodium azide, 1% Triton X-100, 0.05% Tween 20, 2 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, and 5 μg/ml leupeptin, as previously described (Liu et al., 1999). Briefly, cell lysates were clarified by centrifuging at 16,000 × g for 20 min at 4°C and then incubated with protein G-Sepharose coated with the anti-α9β1 antibody, Y9A2, or an irrelevant mouse immunoglobulin (Ig) G at 4°C overnight. The beads were washed with the same buffer four times, and precipitated polypeptides were extracted with SDS sample buffer. Precipitated cell surface biotin-labeled polypeptides were separated by SDS-PAGE under nonreducing conditions and detected with streptavidin peroxidase followed by ECL (Amersham Pharmacia Biotech, Piscataway, NJ). In parallel, lysates of unmodified cells were precipitated with the anti-α9β1 antibody, Y9A2, and coimmunoprecipitated paxillin was detected with biotin-labeled anti-paxillin antibodies (BD-Biosciences-Transduction Laboratories) as previously described (Liu and Ginsberg, 2000).
Integrin Cytoplasmic Domain Model Proteins and Affinity Chromatography
The design and recombinant production of cytoplasmic domain model proteins was performed as previously described (Liu et al., 1999; Liu and Ginsberg, 2000). Briefly, PCR was used to generate a HindIII-BamHI fragment for each wild-type or mutant integrin cytoplasmic domain, and this fragment was subcloned into the modified pET15b vector as previously described (Liu and Ginsberg, 2000). Recombinant proteins were expressed in BL21 (DE3) pLysS cells (Novagen, Madison, WI), isolated by Ni2+-charged resins, and further purified to >90% homogeneity with the use of a reverse phase C18 high-performance liquid chromatography column (Vydac, Hesperia, CA). Masses of all proteins were assessed by electrospray ionization mass spectrometry on an API-III quadrupole spectrometer (Sciex, Toronto, Canada) and varied by <0.1% from the predicted masses. Recombinant integrin cytoplasmic tails were bound to Ni2+-charged His-Bind resins (Novagen) and used for affinity chromatography as previously described (Liu and Ginsberg, 2000). Briefly, 1 mg of each recombinant integrin tail dissolved in 5 ml of 20 mM 1,4-piperazinediethanesulfonic acid, 50 mM NaCl, pH 6.8 (PN buffer), plus 1 ml of 100 mM sodium acetate (pH 3.5) was bound to 100 μl of Ni2+-charged His-Bind resins (Novagen) at 4°C overnight. Resins were then washed with PN buffer twice and stored in an equal volume of PN buffer plus 0.1% sodium azide. The expression and isolation of recombinant human paxillin was performed as previously described (Liu et al., 1999; Liu and Ginsberg, 2000). Aliquots of recombinant HA-tagged glutathione S-transferase (GST)-paxillin were mixed with 300 μl of buffer A plus 20 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 0.1% Triton X-100, 3 mM MgCl2, and 1 mg/ml BSA, added to integrin tail-loaded resins, and incubated at room temperature with rotation for 2 h (Liu et al., 1999; Liu and Ginsberg, 2000). Resins were washed three times with the same buffer, and bound proteins were extracted with SDS sample buffer, separated on SDS-PAGE, and detected with antibody specific for HA-tag.
RESULTS
The α9 Cytoplasmic Domain Specifically Enhances Cell Migration
To determine whether the cytoplasmic domain of the α9 subunit specifically enhances cell migration, chimeric α subunits composed of the extracellular and transmembrane domain of the α9 subunit fused to the cytoplasmic domains of α4, α2, or α5 were constructed (Figure 1A). A comparison of the cytoplasmic sequences of α9, α4, α2, and α5 reveals that all 4α subunits contain a similar membrane-proximal region but that otherwise only the α9 and α4 sequences are similar. Overall, the α9 cytoplasmic domain is 52% homologous to the α4 cytoplasmic domain.
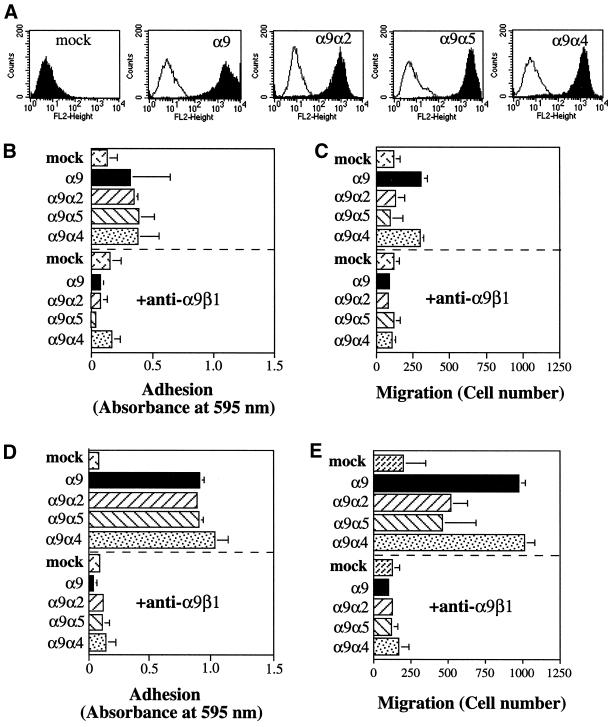
The full-length α9 subunit, the α9α4, α9α2, and α9α5 chimeras, and vector alone were stably expressed in CHO cells that do not express endogenous α9 and were examined for their ability to promote migration on the α9β1-specific ligand, TNfn3RAA. To determine whether the α9 constructs were expressed on the cell surface as α9β1 integrins and to sort cells expressing high levels of the α9β1 integrins, flow cytometry was performed with the anti-α9β1 antibody, Y9A2 (Figure 2A). All stably transfected cells used in subsequent adhesion and migration assays expressed similar high surface levels of α9β1 integrins. Cell adhesion assays performed on TNfn3RAA (Figure 2, B and D) demonstrated that each chimera supports equivalent cell adhesion at both 3 μg/ml (Figure 2B) and 10 μg/ml (Figure 2D), and in each case adhesion was blocked by the anti-α9β1 antibody, Y9A2. To determine whether the α9 cytoplasmic domain preferentially enhances migration compared with the α2 and α5 cytoplasmic domains, as had been previously demonstrated for the α4 cytoplasmic domain, chemotactic migration assays were performed on filters coated with either 3 μg/ml (Figure 2C) or 10 μg/ml (Figure 2E) of TNfn3RAA. As expected, wild-type α9 and each of the chimeras tested supported greater migration on TNfn3RAA than that seen in mock-transfected cells. However, both the α9 and the α4 cytoplasmic domains caused similar enhancement of cell migration compared with the cytoplasmic domains of α2 and α5. Migration of all α9-expressing cells was also inhibited by the anti-α9β1 antibody, Y9A2, demonstrating that the enhanced migration was specific to the α9β1 integrins. These results indicate that the α9 cytoplasmic domain preferentially enhances cell migration and to the same level as the α4 cytoplasmic domain.
Figure 2.
Adhesion and migration of α9-expressing CHO cells. (A) Flow cytometric evaluation of cell surface expression of the α9β1 integrins on α9-, α9 chimera-, and mock-expressing CHO cells. Open peaks represent fluorescence (FL) of unstained CHO cells, and shaded peaks represent fluorescence of CHO cells stained with the anti-α9β1 antibody, Y9A2. (B and D) α9-, α9 chimera-, or mock-expressing CHO cells were added to 96-well plates coated with either 3 μg/ml TNfn3RAA (B) or 10 μg/ml TNfn3RAA (D) after incubation with (below the dashed line) or without (above the dashed line) the anti-α9β1 mAb, Y9A2. Cells were allowed to attach for 60 min, and nonadherent cells were removed by centrifugation. Adherent cells were stained with crystal violet and quantified by measurement of absorbance at 595 nm. (C and E) α9-, α9 chimera-, and mock-expressing CHO cells suspended in serum-free medium were seeded onto membranes coated with 3 μg/ml TNfn3RAA (C) or 10 μg/ml TNfn3RAA (E) in the upper well of 24-well plates after preincubation with (below the dashed line) or without (above the dashed line) the anti-α9β1 mAb, Y9A2. After a 2-h incubation in the presence of 1% FCS in the bottom well, nonmigrated cells on the top side of the membrane were removed, and migrated cells on the bottom side of the membrane were fixed, stained, and counted with the use of a phase-contrast microscope in 10 HPF and expressed as number of migrated cells. Data (B–E) represent the means (±SEM) of triplicate experiments.
The Membrane-proximal 17 Amino Acids of the α9 Cytoplasmic Domain Are Sufficient to Mediate Enhanced Cell Migration
To identify the cytoplasmic sequences critical for mediating α9-dependent enhancement of cell migration, a series of α9DMs (Figure 1B) was stably expressed in CHO cells and examined for their ability to mediate α9β1-dependent adhesion and migration. All of the α9DMs were stably expressed on the cell surface at similarly high levels (Figure 3A). The cells expressing α9DM3-α9DM6 all bound to TNfn3RAA-coated plates at similar levels and to the same level as cells expressing full-length α9 (Figure 3, B and D). The most severe truncations, α9DM1 and α9DM2, impaired α9β1-mediated adhesion, especially to 10 μg/ml TNfn3RAA. As expected from their lack of adhesion, α9DM1 and α9DM2 were unable to mediate α9β1-dependent migration to the same level as the full-length α9 subunit (Figure 3, C and E). In contrast, the α9DM4-α9DM6 all mediated migration comparable to full-length α9. However, α9DM3 was unable to mediate α9β1-dependent migration, although it mediated adhesion to TNfn3RAA to the same level as the α9DM4-α9DM6. These results indicate that α9-mediated enhancement of cell migration requires the 17 amino acids retained in the α9DM4 construct and is particularly sensitive to the loss of amino acids within the sequence IIEAEK that is present in α9DM4 but absent in α9DM3.
Figure 3.
Adhesion and migration of α9- and α9DM-expressing CHO cells. (A) Flow cytometric evaluation of cell surface expression of the α9β1 integrin from α9- and α9DM-expressing CHO cells as described in Figure 2. (B and D) Adhesion of α9- and α9DM-expressing CHO cells on 3 μg/ml TNfn3RAA (B) or 10 μg/ml TNfn3RAA (D) with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. (C and E) Migration of α9- and α9DM-expressing CHO cells on 3 μg/ml TNfn3RAA (C) or 10 μg/ml TNfn3RAA (E) with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. Data (B–E) represent the means (±SEM) of triplicate experiments.
The α9 Cytoplasmic Domain Inhibits Cell Spreading
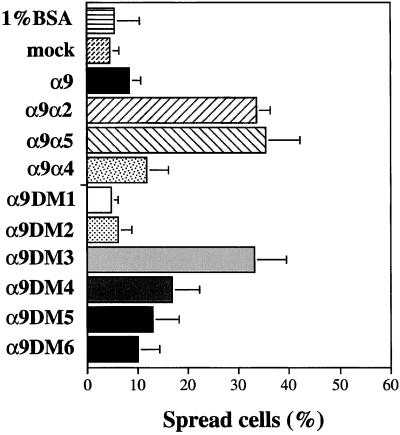
The cytoplasmic domain of α4 has previously been demonstrated to inhibit cell spreading compared with other α subunits of the β1 subclass of integrins (Kassner et al., 1995). To determine whether the α9 cytoplasmic domain could also inhibit cell spreading, spreading assays were performed with cells expressing each of the constructs described above on coverslips coated with 10 μg/ml TNfn3AA (Figure 4). After 6 h, the greatest difference in cell spreading was evident with cells expressing the full-length α9β1 and the α9α4 chimera being less spread (∼10% spread) than cells expressing either the α9α2 or α9α5 chimeras (∼35% spread). Similarly, cells expressing the α9DM3, which mediates adhesion but not migration on TNfn3RAA, were spread to the same level as cells expressing the α9α2 and α9α5 chimeras that do not mediate enhanced migration. The α9DM4-α9DM6 that mediate enhanced migration also inhibited cell spreading to similar levels as that of α9β1- and α9α4β1-expressing cells. These results indicate that the membrane-proximal 17 amino acids of the α9 cytoplasmic domain are sufficient to mediate both enhanced migration and impaired spreading and that the α9 and α4 cytoplasmic domains share both functional properties.
Figure 4.
Spreading of α9-, α9 chimera-, and α9DM-expressing CHO cells. Cells were seeded onto sterile coverslips coated with TNfn3RAA (10 μg/ml) and allowed to spread for 6 h at 37°C. Percentage of spreading was determined by phase-contrast microscopy. Data represent the means (±SEM) of triplicate experiments.
The α9 Cytoplasmic Domain Associates with the Focal Adhesion Adapter Protein, Paxillin
Recently, the α4 cytoplasmic domain was demonstrated to associate with and directly bind to the adapter protein, paxillin, and this interaction was shown to be critical for α4-dependent enhanced migration and impaired cell spreading (Liu et al., 1999). Because the α9 cytoplasmic domain is highly homologous to the α4 cytoplasmic domain (52% homology), we predicted that the α9 cytoplasmic domain would also associate with paxillin. Indeed, bacterially expressed GST-paxillin directly and specifically bound to the recombinant α9 cytoplasmic domain immobilized on a Ni2+ resin-charged column but not the αIIb cytoplasmic domain in vitro (Figure 5A). To determine whether α9 binds paxillin with a similar affinity as α4, recombinant protein-binding assays were again performed. Both the α9 and α4 cytoplasmic domains specifically bound paxillin in a concentration-dependent manner with very similar binding affinities, suggesting that the strength of the α9-paxillin and α4-paxillin interactions is quite similar (Figure 5B). To determine whether paxillin associates with α9 in vivo and to the same level as α4, cell lysates from CHO cells expressing α9β1, α9α4β1, and α9α2β1 were immunoprecipitated with the anti-α9β1 antibody, Y9A2. The precipitates were resolved with the use of SDS-PAGE and immunoblotted with an anti-paxillin antibody. Paxillin was coimmunoprecipitated with full-length α9 and the α9α4 chimera to similar levels but not with the α9α2 chimera (Figure 5C). These combined results demonstrate that the α9 cytoplasmic domain, like the α4 cytoplasmic domain, directly interacts with paxillin both in vitro and in vivo.
Figure 5.
Direct association of paxillin with α9. (A) HA-tagged recombinant GST-paxillin was added to Ni2+-charged resins loaded with the α9 or αIIb cytoplasmic domains. Bound fractions were collected and separated on 4–20% SDS-PAGE under reducing conditions, transferred to a nitrocellulose membrane, and stained with antibody specific for the HA-tag, 12CA5. S.M., starting material. Depicted are results of one of three experiments performed with similar results. (B) HA-tagged recombinant GST-paxillin was added to Ni2+-charged resins loaded with the α9, α4, or the αIIb cytoplasmic domains as described above. Depicted are results of one of three experiments performed with similar results. ●, α9; ▴, α4; ▪, αIIb.(C) CHO cells stably expressing α9β1, α9α2β1, or α9α4β1 integrins were surface labeled with biotin and subjected to immunoprecipitation with the anti-α9β1 antibody, Y9A2, or an irrelevant mouse IgG. The precipitates were separated on 4–20% SDS-PAGE and transferred to a nitrocellulose membrane. Paxillin coimmunoprecipitation was detected with an anti-paxillin antibody (top), and precipitated surface proteins were detected with streptavidin peroxidase followed by ECL (bottom). Depicted are results of one of three experiments performed with similar results.
The Binding of Paxillin to α9DM4 and α9DM5 Correlates with Enhanced Migration and Inhibition of Cell Spreading
To determine whether paxillin binds to the α9DM3-α9DM5, recombinant protein-binding assays were performed as described above. Both α9DM4 and α9DM5 bound paxillin to similar levels and to the same level as the α9 cytoplasmic domain (Figure 6). However, the αIIb cytoplasmic domain, as expected, and α9DM3 did not bind paxillin. The inability of α9DM3 to bind paxillin and promote enhanced migration and inhibit cell spreading suggests that an α9-paxillin interaction may be required for α9β1-dependent migration and inhibition of cell spreading. The fact that both α9DM4 and α9DM5 bind paxillin and support enhanced migration and inhibition of cell spreading further suggests that α9, like α4, may require paxillin to mediate enhanced migration and inhibit cell spreading.
Figure 6.
Association of paxillin with α9 deletion mutants. (A) Binding of recombinant paxillin to α9, α9DM3, α9DM4, α9DM5, or the αIIb cytoplasmic domains as described in Figure 5. Depicted are results of one of two experiments performed with similar results. ♦, α9DM3; ▪, α9DM4; ●, α9DM5; ▴, α9; ▾, αIIb.
The α9 Cytoplasmic Domain Mediates α9β1-dependent Migration in Paxillin Null Cells
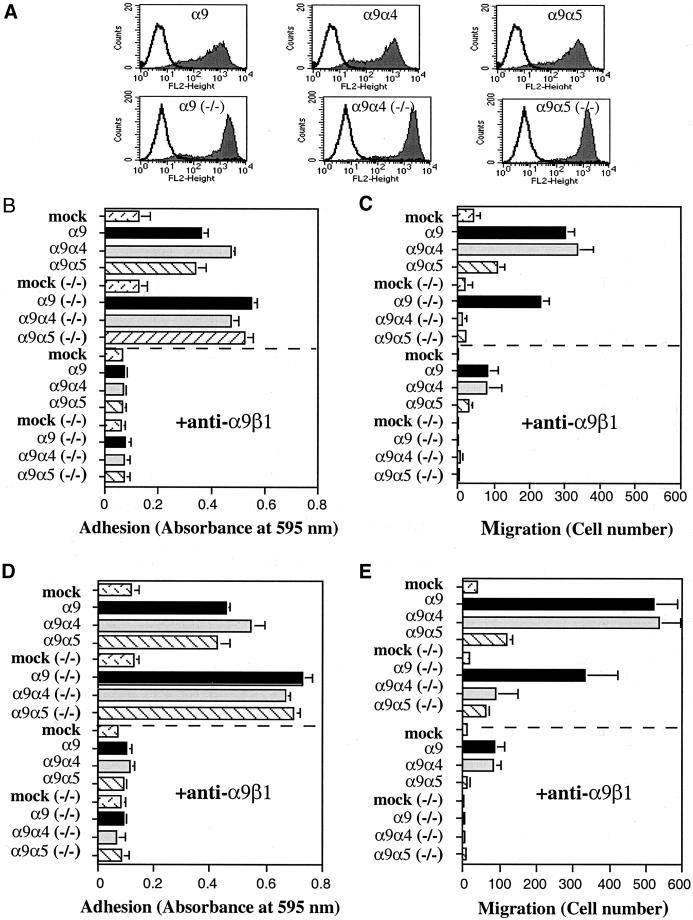
To determine whether paxillin is required for α9-mediated enhanced migration, MEF cells null for paxillin (paxillin−/−) were infected with retroviruses encoding α9, α9α4, α9α5, or vector alone and examined for their ability to promote migration on TNfn3RAA. As a control, wild-type MEF cells that contain endogenous paxillin were similarly infected. Neither the wild-type MEF nor the paxillin−/− MEF cells express endogenous α9. As in CHO cells, all constructs were surface expressed at similar high levels (Figure 7A), and all supported adhesion to TNfn3RAA (Figure 7, B and D). In the wild-type MEF cells, α9 and α9α4 mediated enhanced migration compared with α9α5 on both 3 μg/ml (Figure 7C) and 10 μg/ml (Figure 7E) TNfn3AA, as expected. In the paxillin−/− MEF cells, however, the α9 cytoplasmic domain mediated enhanced migration (Figure 7, C and E). At both 3 μg/ml (Figure 7C) and 10 μg/ml (Figure 7E) TNfn3RAA, the α9β1-expressing paxillin−/− MEF cells demonstrated enhanced migration, whereas the α9α4-expressing paxillin−/− MEF cells did not. Migration mediated by wild-type α9 and each α9 chimeric integrin was inhibited by the anti-α9β1 antibody, Y9A2, in both the wild-type MEF and paxillin−/− MEF cells. Surprisingly, these findings suggest that paxillin is not required for α9-dependent enhancement of cell migration, as is the case for α4.
Figure 7.
Adhesion and migration of α9-expressing MEF and paxillin−/− MEF cells. (A) Flow cytometric evaluation of cell surface expression of the α9β1 integrins from α9- and α9 chimera-expressing MEF cells (top row) and α9- and α9 chimera-expressing paxillin−/− MEF cells (−/−; bottom row) as described in Figure 2. (B and D) Adhesion of α9- and α9 chimera-expressing MEF and paxillin−/− MEF cells (−/−) on 3 μg/ml TNfn3RAA (B) or 10 μg/ml TNfn3RAA (D) with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. Note: twice the amount of the anti-α9β1 antibody, Y9A2, was used to block adhesion of paxillin−/− MEF cells (−/−) on 10 μg/ml TNfn3RAA. (C and E) Migration of α9- and α9 chimera-expressing MEF and paxillin−/− MEF cells (−/−) on 3 μg/ml TNfn3RAA (C) or 10 μg/ml TNfn3RAA (E) for 3 h with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. Data (B–E) represent the means (±SEM) of triplicate experiments.
Paxillin Is Required for α9β1-dependent Inhibition of Cell Spreading
To determine whether paxillin is required for α9-mediated inhibition of cell spreading, MEF and paxillin−/− MEF cells stably infected with α9, α9α4, α9α5, or vector alone were analyzed in cell-spreading assays on 10 μg/ml TNfn3AA (Figure 8). After 3 h, the greatest difference in cell spreading was evident. The wild-type MEF cells expressing α9α5 were approximately twice as spread as cells expressing either α9 or α9α4, as expected. However, the paxillin−/− MEF cells expressing either α9 or α9α4 were at least as well spread as cells expressing α9α5. Thus, paxillin appears to be critical for both α9- and α4-dependent inhibition of cell spreading.
Figure 8.
Spreading of α9- and α9 chimera-expressing MEF and paxillin−/− MEF cells. α9- and α9 chimera-expressing MEF cells and α9- and α9 chimera-expressing paxillin−/− MEF cells (−/−) were seeded onto sterile coverslips coated with TNfn3RAA (10 μg/ml) and allowed to spread for 3 h at 37°C. Percentage of spreading was determined by phase-contrast microscopy. Data represent the means (±SEM) of triplicate experiments.
Point Mutations in the α9 Cytoplasmic Domain That Abolish Paxillin Binding Do Not Inhibit Cell Migration
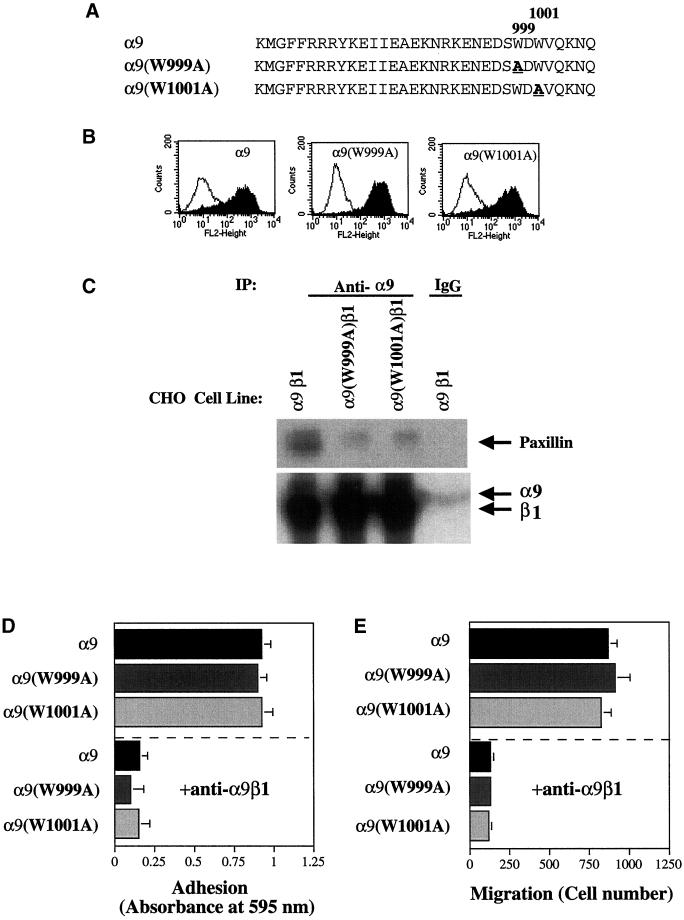
Point mutations in the α4 cytoplasmic domain have previously been shown to both inhibit paxillin binding and abolish α4-mediated enhancement of cell migration and inhibition of cell spreading (Liu et al., 1999; Liu and Ginsberg, 2000). To confirm our results that an α9-paxillin interaction is not required for α9-dependent enhancement of cell migration, two point mutations were made in the α9 cytoplasmic domain, α9(W999A) and α9(W1001A), based on mutations in the α4 cytoplasmic domain shown to inhibit the α4-paxillin interaction (Figure 9A). Although, as shown in Figure 6, the region of the α9 cytoplasmic domain containing these residues is not required for paxillin binding, recombinant protein-binding studies indicated that the α9(W999A) and α9(W1001A) mutations abolished the α9-paxillin interaction in vitro and the interaction of α9 with the paxillin family member, Hic-5 (Young, Taooka, Liu, Askins, Yokosaki, Thomas, and Sheppard, unpublished results). Thus, these mutations were used as additional tools to evaluate the in vivo significance of the α9-paxillin interaction. To evaluate the effects of the α9(W999A) and α9(W1001A) mutations in vivo, α9(W999A) and α9(W1001A) were stably expressed in CHO cells. Both mutants were similarly expressed at the same high surface levels as wild-type α9 (Figure 9B). Immunoprecipitation studies performed with the use of the anti-α9β1 antibody, Y9A2, demonstrate that both the W999A and W1001A point mutations in α9 dramatically inhibit coimmunoprecipitation of paxillin with α9 (Figure 9C). In functional assays, cells expressing either α9(W999A) or α9(W1001A) mediated adhesion (Figure 9D) and migration (Figure 9E) to TNfn3AA (10 μg/ml), as well as cells expressing the wild-type α9β1 integrin. These results confirm our earlier findings and indicate that an α9-paxillin interaction is not required for α9β1-dependent enhancement of cell migration.
Figure 9.
Adhesion and migration of CHO cells expressing α9 cytoplasmic domain mutations that abolish paxillin binding. (A) The cytoplasmic amino acid sequences of α9, α9(W999A), and α9(W1001A) are shown. Amino acids depicted in bold type represent sites of mutagenesis. (B) Flow cytometric evaluation of cell surface expression of the α9β1 integrins from α9-, α9(W999A)-, and α9(W1001A)-expressing CHO as described in Figure 2. (C) CHO cells stably expressing α9β1 or the α9(W999A)β1- or α9(W1001A)β1-chimeric integrins were surface labeled with biotin and subjected to immunoprecipitation (IP) with the anti-α9β1 antibody, Y9A2, or an irrelevant mouse IgG as described in Figure 5. Depicted are results of one of three experiments performed with similar results. (D) Adhesion of α9-, α9(W999A)-, and α9(W1001A)-expressing CHO cells on 10 μg/ml TNfn3RAA with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. (E) Migration of α9-, α9(W999A)-, and α9(W1001A)-expressing CHO cells on 10 μg/ml TNfn3RAA with or without preincubation with the anti-α9β1 antibody, Y9A2, as described in Figure 2. Data (D–E) represent the means (±SEM) of triplicate experiments.
α9β1-dependent Inhibition of Cell Spreading Is Dependent on an α9-Paxillin Interaction
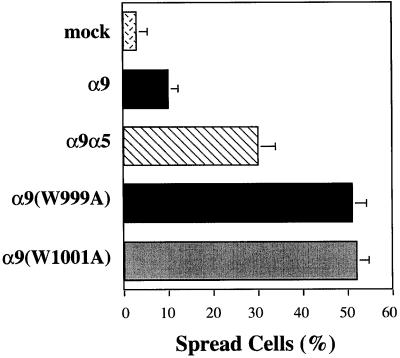
To confirm that an α9-paxillin interaction is required for α9β1-dependent inhibition of cell spreading, the α9 mutants, α9(W999A) and α9(W1001A), that do not bind paxillin described above were analyzed in a cell-spreading assay on 10 μg/ml TNfn3AA (Figure 10). After 6 h, the greatest difference in cell spreading was evident. Cells expressing wild-type α9 were less spread (∼12%) than cells expressing the α9α5 chimera (∼30%), as expected. However, cells expressing the α9 mutants, α9(W999A) and α9(W1001A), were even more spread (∼50%) than cells expressing the α9α5 chimera, indicating that an α9-paxillin interaction is required for α9 to inhibit cell spreading. These results confirm our earlier findings and suggest that an α9-paxillin interaction is required for α9β1-dependent inhibition of cell spreading. In addition, these combined results (Figures 7–10) suggest that α9 may use different intracellular signaling pathways to promote enhanced migration and inhibition of cell spreading.
Figure 10.
Spreading of α9-, α9(W999A)-, and α9(W1001A)-expressing CHO cells. α9-, α9(W999A)-, and α9(W1001A)-expressing CHO cells were seeded onto sterile coverslips coated with TNfn3RAA (10 μg/ml) and allowed to spread for 6 h at 37°C. Percentage of spreading was determined by phase-contrast microscopy. Data represent the means (±SEM) of triplicate experiments.
DISCUSSION
In this study, we demonstrate that the α9 cytoplasmic domain specifically promotes cell migration and inhibits cell spreading. In these experiments, all performed on a ligand specific for the α9β1 extracellular domain, TNfn3RAA, the α9 cytoplasmic domain preferentially enhanced cell migration and inhibited cell spreading compared with the cytoplasmic domains of α2 or α5. These results were nearly identical to those reported previously for the α4 cytoplasmic domain utilizing similarly constructed chimeric integrin subunits containing the extracellular domain and transmembrane domains of either the α2 or α4 subunit (Chan et al., 1992; Kassner et al., 1995). As expected, based on these earlier studies, the α4 cytoplasmic domain also enhanced migration and inhibited spreading in our study. These results, together with recent studies demonstrating that α9β1 and α4 integrins are both critical to transendothelial leukocyte migration (Issekutz et al., 1996; Gao and Issekutz, 1997; Taooka et al., 1999), establish α9 and α4 integrins as members of a functional subfamily of integrins. This classification is further supported by the sequence similarity between α9 (Palmer et al., 1993) and α4 (Takada et al., 1989) and by several recent reports of overlapping ligand-binding specificity for the α9 and α4 integrins (Taooka et al., 1999; Eto et al., 2000; Takahashi et al., 2000)
Critical α9 cytoplasmic sequences required for promotion of α9β1-dependent migration and inhibition of cell spreading were localized to a region encompassing the last six amino acids in the α9DM4 (Figures 3 and 4). The α9DM4, which contains 17 of the 33 amino acids of the α9 cytoplasmic domain (Figure 1B), was able to mediate α9β1-dependent migration to the same extent as the full-length α9 subunit or the α9α4 chimera. In addition, α9 DM4 was able to inhibit cell spreading compared with cells expressing either the α2 or the α5 cytoplasmic domains (Figure 4). The deletion mutant, α9DM3, which was unable to promote enhanced α9β1-dependent migration, did not inhibit cell spreading onTNfn3RAA with cells expressing the α9DM3 being as well spread as cell expressing the α9α2 and α9α5 chimeras (Figure 4). The α4 cytoplasmic domain has previously been shown to directly bind to the adaptor protein, paxillin, and this α4-paxillin interaction has been reported to be critical to α4β1-dependent enhancement of cell migration and inhibition of cell spreading (Liu et al., 1999). Based on the high degree of sequence similarity between the α9 and α4 cytoplasmic domains, we were not surprised that the α9 cytoplasmic domain also associates with paxillin (Figures 5, 6, and 9). In addition, the α9 cytoplasmic domain specifically and directly bound paxillin (Figure 5 and 6) and to the same level as the α4 cytoplasmic domain (Figure 5). However, the site(s) of interaction between each cytoplasmic domain and paxillin appears to differ. Whereas the paxillin binding site in α4 has been localized to a region close to the carboxy terminus (Liu and Ginsberg, 2000), the analogous region can be deleted in α9 without eliminating binding, demonstrating the existence of a more membrane-proximal paxillin-binding site in α9. The elimination of paxillin binding by point mutations in the carboxyl terminal region of α9 (i.e., residues 999 and 1001) suggests that either these mutations change the conformation of the more proximal binding site or the α9 cytoplasmic domain contains more than one such binding site.
As for the α4 cytoplasmic domain, the α9-paxillin interaction appears to be required for α9-mediated inhibition of cell spreading. This conclusion is supported by the fact that the α9 cytoplasmic domain did not inhibit cell spreading in the paxillin−/− MEF cells (Figure 8) and by the fact that two point mutations in the α9 cytoplasmic domain that abolish association with paxillin (W999A and W1001A) also abolished α9-dependent inhibition of cell spreading (Figure 10). Surprisingly, the α9-paxillin interaction does not appear to be required for the α9-dependent enhancement of cell migration. The α9 cytoplasmic domain was able to promote enhanced migration in the paxillin−/− MEF cells to a similar level as that seen in wild-type MEF cells (Figure 7). In addition, the same mutations in α9 (W999A and W1001A) that abolished the α9-paxillin interaction had no effect on α9-dependent enhanced migration (Figure 9). These results indicate that an α9-paxillin interaction is not required for α9β1-dependent enhanced migration. Although it is possible that other known or as yet to be identified paxillin family members could be required for α9-dependent enhanced migration, the α9(W999A) and α9(W1001A) mutants that inhibited the α9-paxillin interaction also inhibited the interaction of α9 with Hic-5, the only other paxillin family member known to be expressed in our cell lines. The fact that α9 mediates enhanced migration in a paxillin-independent manner and inhibits cell spreading in a paxillin-dependent manner suggests that α9 may use different intracellular signaling pathways to regulate cell migration and cell spreading. The intracellular signaling pathway(s) activated by α9 and α4 that promote enhanced migration and inhibit cell spreading remains to be determined. Our finding that the first 17 membrane-proximal amino acids of the α9 cytoplasmic domain are sufficient to mediate these effects and our identification of several mutants that do or do not support enhanced migration and inhibit spreading provide important tools for mapping the intracellular signaling pathway(s) responsible for α9β1-dependent enhancement of cell migration and inhibition of cell spreading.
It is important to note that the α9- and α4-containing integrins are clearly not unique in their ability to support cell migration. In fact, extensive previous studies suggest that all members of the integrin family can support substrate-specific migration to varying degrees (Lauffenburger and Horwitz, 1996). This conclusion is further supported by the findings in previous studies utilizing integrin α subunit chimeras (Chan et al., 1992; Kassner et al., 1995; Liu et al., 1999) and in the current study showing that the chimeras containing the structurally dissimilar α2 or α5 cytoplasmic domains still supported levels of migration greater than that seen in mock transfectants. However, the unique contributions of the α9 and α4 cytoplasmic domain are that they enhance the rate of cell migration. One explanation for this effect would be that these cytoplasmic domains simply alter the confirmation of the extracellular domains and reduce avidity of ligand binding. Such an explanation is unlikely, however, because enhanced migration was seen over a range of ligand-coating concentrations and all of the chimeras and most of the deletion mutants examined mediated comparable degrees of cell adhesion. Further, the two deletion mutants that inhibited cell adhesion both supported decreased, not increased migration.
In summary, the findings in this paper contribute to a body of evidence defining the α9 and α4 integrins as members of a unique integrin subclass that preferentially promotes enhanced cell migration and inhibits cell spreading. Most leukocytes, cells that require rapid migration to exit the vasculature and traffic to extravascular sites of inflammation and immune response, express either the α9 integrin, α4 integrins, or both. The α9 and α4 cytoplasmic domains each contain specific amino acid sequences that enhance cell migration and inhibit cell spreading. Both cytoplasmic domains associate with the adaptor protein, paxillin, and this association is critical for the ability of the α9 and α4 subunits to inhibit cell spreading. However, whereas paxillin binding is critical for α4-mediated enhancement of cell migration, paxillin-independent pathways are sufficient for α9-mediated enhanced migration. These combined findings suggest that, despite their structural and functional similarities, the α9 and α4 cytoplasmic domains can activate different intracellular signaling pathways to regulate enhanced cell migration.
ACKNOWLEDGMENTS
We thank Mark Ginsberg for his many helpful suggestions. This work was supported by National Institutes of Health grants HL 47412, HL53949, and HL56385 (to D.S.) and CA 75621, by a Leukemia and Lymphoma Society Scholar Award (to S.M.T.), by training grant GM 20839 (to B.A.Y.), and by a Scientist Development Grant from the American Heart Association (to S.L.).
Abbreviations used:
- α9DM
α9 cytoplasmic deletion mutant
- ATCC
American Type Culture Collection
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- FCS
fetal calf serum
- GST
HA, hemagglutinin
- HPF
high-powered fields
- Ig
immunoglobulin
- MEF
mouse embryonic fibroblasts
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- TNfn3RAA
an RAA for RGD mutant fragment of the third fibronectin type III repeat of tenascin-C
REFERENCES
- Chan BM, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- Gao JX, Issekutz AC. The beta 1 integrin, very late activation antigen-4 on human neutrophils can contribute to neutrophil migration through connective tissue fibroblast barriers. Immunology. 1997;90:448–454. doi: 10.1111/j.1365-2567.1997.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AR, Parsons JT. Cell migration: movin' on [comment] Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Issekutz TB, Miyasaka M, Issekutz AC. Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J Exp Med. 1996;183:2175–2184. doi: 10.1084/jem.183.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional properties of the integrin alpha 4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–674. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Liu S, Ginsberg MH. Paxillin binding to a conserved sequence motif in the alpha 4 integrin cytoplasmic domain. J Biol Chem. 2000;275:22736–22742. doi: 10.1074/jbc.M000388200. [DOI] [PubMed] [Google Scholar]
- Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci USA. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Prot Immunol. 1999;10(suppl):28. doi: 10.1002/0471142735.im1017cs31. , 31. [DOI] [PubMed] [Google Scholar]
- Takada Y, Elices MJ, Crouse C, Hemler ME. The primary structure of the alpha 4 subunit of VLA-4: homology to other integrins and a possible cell-cell adhesion function. EMBO J. 1989;8:1361–1368. doi: 10.1002/j.1460-2075.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Isobe T, Horibe S, Takagi J, Yokosaki Y, Sheppard D, Saito Y. Tissue transglutaminase, coagulation factor XIII, and the pro-polypeptide of von Willebrand factor are all ligands for the integrins alpha 9beta 1 and alpha 4beta 1. J Biol Chem. 2000;275:23589–23595. doi: 10.1074/jbc.M003526200. [DOI] [PubMed] [Google Scholar]
- Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signaling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, Sheppard D. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]