Significance
The gut microbiota affects several physiological processes, including gut motility. Here we observed that germ-free mice have an immature enteric nervous system (ENS) that is normalized upon colonization with a normal microbiota. We identified the mechanism of communication between the microbiota and enteric neurons as the initiation of serotonin release and subsequent activation of the 5-HT4 receptor. This demonstrates a strong interaction between the microbiota and the ENS and indicates potential mechanisms linking microbial dysbiosis to gastrointestinal disorders. The ability to modulate the microbiota, e.g., by diet, will open new perspectives of research in neurogastroenterology.
Keywords: microbiota, enteric nervous system, serotonin, 5-HT4R
Abstract
The enteric nervous system (ENS) is crucial for essential gastrointestinal physiologic functions such as motility, fluid secretion, and blood flow. The gut is colonized by trillions of bacteria that regulate host production of several signaling molecules including serotonin (5-HT) and other hormones and neurotransmitters. Approximately 90% of 5-HT originates from the intestine, and activation of the 5-HT4 receptor in the ENS has been linked to adult neurogenesis and neuroprotection. Here, we tested the hypothesis that the gut microbiota could induce maturation of the adult ENS through release of 5-HT and activation of 5-HT4 receptors. Colonization of germ-free mice with a microbiota from conventionally raised mice modified the neuroanatomy of the ENS and increased intestinal transit rates, which was associated with neuronal and mucosal 5-HT production and the proliferation of enteric neuronal progenitors in the adult intestine. Pharmacological modulation of the 5-HT4 receptor, as well as depletion of endogenous 5-HT, identified a mechanistic link between the gut microbiota and maturation of the adult ENS through the release of 5-HT and activation of the 5-HT4 receptor. Taken together, these findings show that the microbiota modulates the anatomy of the adult ENS in a 5-HT–dependent fashion with concomitant changes in intestinal transit.
The gastrointestinal tract is unique in the body, as it has an intrinsic nervous system, the enteric nervous system (ENS), composed of the outer myenteric plexus and the inner submucosal plexus. The ENS has several essential physiologic functions, such as regulation of gastrointestinal motility, fluid secretion and absorption, and blood flow (1). In rodents, most enteric neurons are formed during embryogenesis and early postnatal life (2–5). During this process, a small subpopulation of Sox10-expressing neural crest-derived cells colonizes the foregut, subsequently undergoes proliferation, and then colonizes the entire bowel, giving rise to both neurons and glial cells (6–8). Furthermore, enteric neuronal stem cells, which express markers such as Nestin, have been reported in the postnatal murine intestine (9–11). While it was long thought that no enteric neurons were formed after postnatal day 21 in mice, except in cases of inflammation or injury (2–4), a recent study has demonstrated that the ENS undergoes continuous renewal in adult mice, with apoptosis balancing neurogenesis (12). Thus, this report demonstrates that the adult ENS is capable of maturation and plasticity, but the mechanisms driving these processes remain unknown.
The microbiota colonizes the gastrointestinal tract after birth with continuous maturation during the first year of life (13). Concomitantly with colonization of the gastrointestinal tract and maturation of the mucosal immune system, the ENS undergoes extensive development during the early postnatal life (14). Accordingly, germ-free (GF) mice, which lack a gut microbiota, have early postnatal structural and functional abnormalities of the ENS (15). These features can be reversed by colonization with the microbiota from a conventionally raised (CONV-R) donor (16).
About 90% of the body’s serotonin (5-hydroxytryptamine, 5-HT) is produced by enterochromaffin (EC) cells, a type of enteroendocrine cells which are present in the epithelium of the gut (17). Recently, two studies have reported that the gut microbiota is able to induce mucosal 5-HT secretion in the gut (18, 19). The concept that mucosal and neuronal 5-HT are distinct pools is supported by the fact that different forms of the rate-limiting synthetic enzyme tryptophan hydroxylase (TPH) are used by neuronal and nonneuronal cells, with TPH2 used by neurons and TPH1 used by EC cells (20). While mucosal 5-HT is strongly proinflammatory (21, 22), activation of the 5-HT4 receptor (5-HT4R) in the ENS exerts neurogenerative and neuroprotective actions (4, 23).
In this study, we tested the hypothesis that the gut microbiota modulates the function and the anatomy of the ENS through release of 5-HT and activation of the 5-HT4 receptor (5-HT4R). Colonization of GF mice reduced intestinal transit time and was associated with the release of 5-HT. We studied GF and colonized Tph1-deficient mice and demonstrated that mucosal 5-HT is neuroprotective in the early phases of colonization. Next, we studied the respective roles of mucosal and neuronal 5-HT in the observed phenotype through inhibition of TPH1 and TPH2 with parachlorophenylalanine (PCPA) or depletion of neuronal 5-HT with reserpine. Finally, using pharmacological modulation of the 5-HT4 receptor, we established a link between the gut microbiota and neuronal activation of 5-HT4Rs.
Results
The Gut Microbiota Regulates Intestinal Transit Accompanied by Neuroanatomic Changes of the ENS.
Recent work has shown that the gut microbiota is essential for normal brain development and function in mice (24), but much less is known about the implications of the gut microbiota in the development of the ENS. A recent study established that the microbiota is required for the postnatal development of enteric glial cells of the mucosa (25), but so far mechanistic studies on the role of the gut microbiota in the maturation of ENS neurons, and particularly colonic enteric neurons, are lacking.
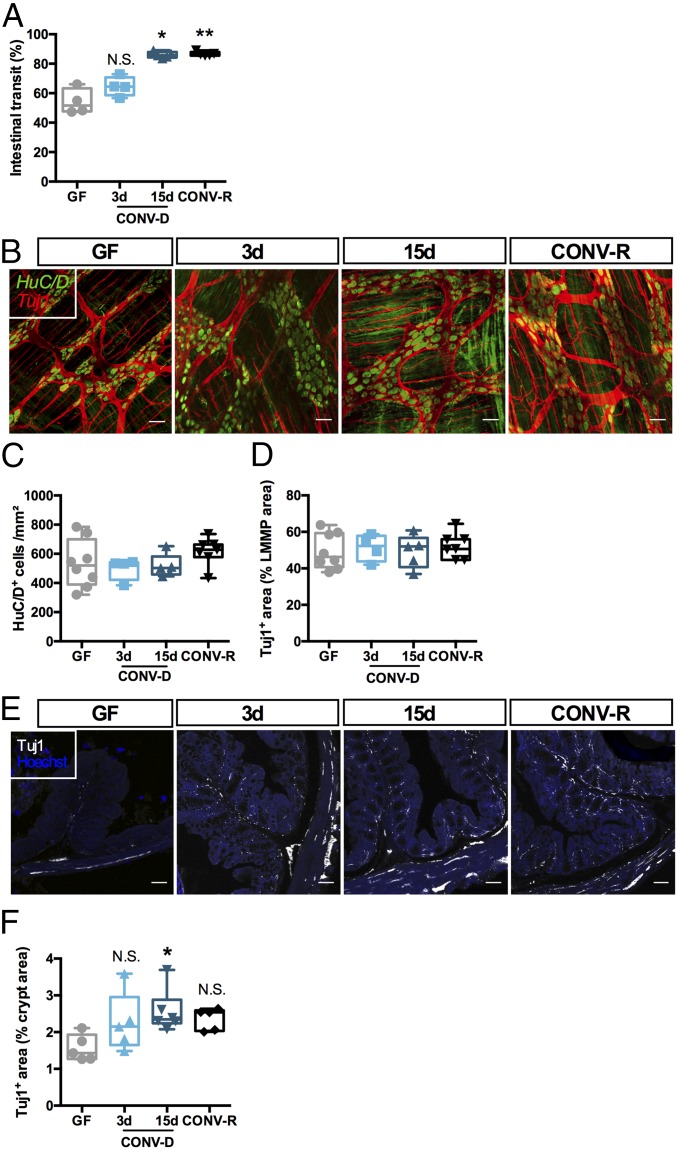
We determined a link between the gut microbiota and ENS function and anatomy by colonizing 12-wk-old GF mice with the microbiota from the cecum of age-matched donor mice, generating CONV-D mice. In agreement with previous findings (26), we found that GF mice exhibited significantly slower intestinal transit than CONV-R controls and that intestinal transit was normalized after 15 d, but not 3 d, of colonization, i.e., when the microbial community is fully established after colonization (Fig. 1A) (27, 28). While we did not observe any difference in the number of myenteric neurons or innervation of the longitudinal muscle/myenteric plexus (LMMP) between GF, CONV-D, and CONV-R mice, as revealed by immunohistochemical quantification of the pan-neuronal marker HuC/D and the neurite marker Tuj1 (Fig. 1 B–D), innervation of the colonic epithelium was reduced in GF animals. These phenotypes were restored 15 d after colonization (Fig. 1 E and F), mirroring the faster transit (Fig. 1A). It is noteworthy that, in accordance with a previous study (25), colonization increased the density of the glial network (as revealed by immunostaining for the glia-specific marker S100β; see SI Appendix, Fig. S1 A and B). However, the number of Sox10+ cells (a marker of neuronal and glial progenitors) was not affected by the microbial status of the mice (SI Appendix, Fig. S1 C and D), suggesting that (i) glia are larger after colonization or (ii) a greater percentage of glia express S100β. Sox10+ cells have been shown to give rise to neurons and glial cells in vitro and in vivo (2, 3, 25). A previous study using reporter mice found that, after colonization, Sox10+ cells undergo proliferation in the myenteric plexus, followed by migration to the mucosa, giving rise to enteric glial cells (25). In line with that finding, we found that about 1% of Sox10+ cells expressed Ki67 in the myenteric plexus 15 d after colonization (SI Appendix, Fig. S2).
Fig. 1.
The gut microbiota regulates ENS anatomy and function. (A) Intestinal transit in GF mice, GF mice colonized (CONV-D) with microbiota from a CONV-R donor for 3 or 15 d, and CONV-R mice. *P < 0.05; **P < 0.01 vs. GF; N.S., not significant; one-way ANOVA followed by Dunnett’s post hoc test. (B) Representative images of the LMMP of the colon showing pan-neuronal marker HuC/D (green) and neuron-specific beta-III tubulin (Tuj1, red). (C and D) Quantification of HuC/D+ cells (C) and the Tuj1+ area (D). (E and F) Representative images (E) and quantification (F) of the innervation of the colonic crypts of the mice using the peripheral neuronal marker Tuj1. *P < 0.05 vs. GF; N.S., not significant; Kruskal–Wallis test followed by Dunn’s post hoc test. (Scale bars: 50 µm.)
Next, we investigated whether depletion of the gut microbiota by 3-wk antibiotic treatment in CONV-R mice reduced innervation of the colon mucosa. Microbial depletion was confirmed by qPCR and was associated with cecum enlargement (SI Appendix, Fig. S3A and Table S1). Unlike GF mice, the depletion resulted in reduced innervation of the colonic mucosa and LMMP, which was associated with a decrease in the glial network (SI Appendix, Fig. S3 B–H). This suggests that the presence of a gut microbiota is not necessary for the development of the nervous myenteric network but is crucial for the maintenance of the network.
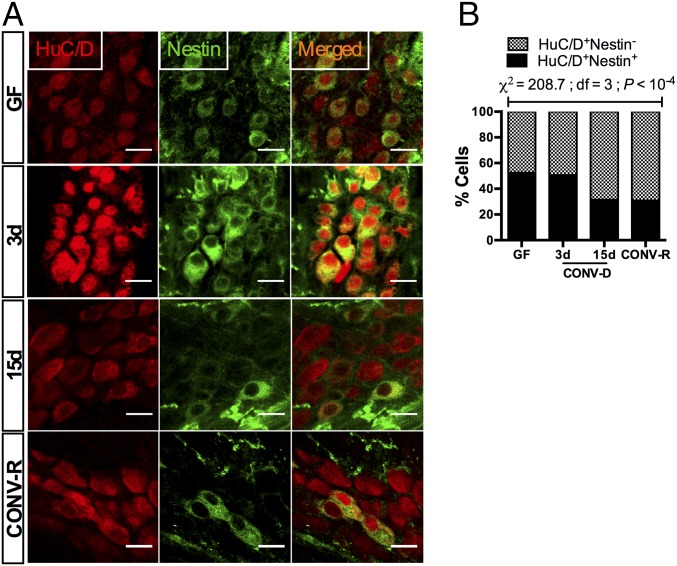
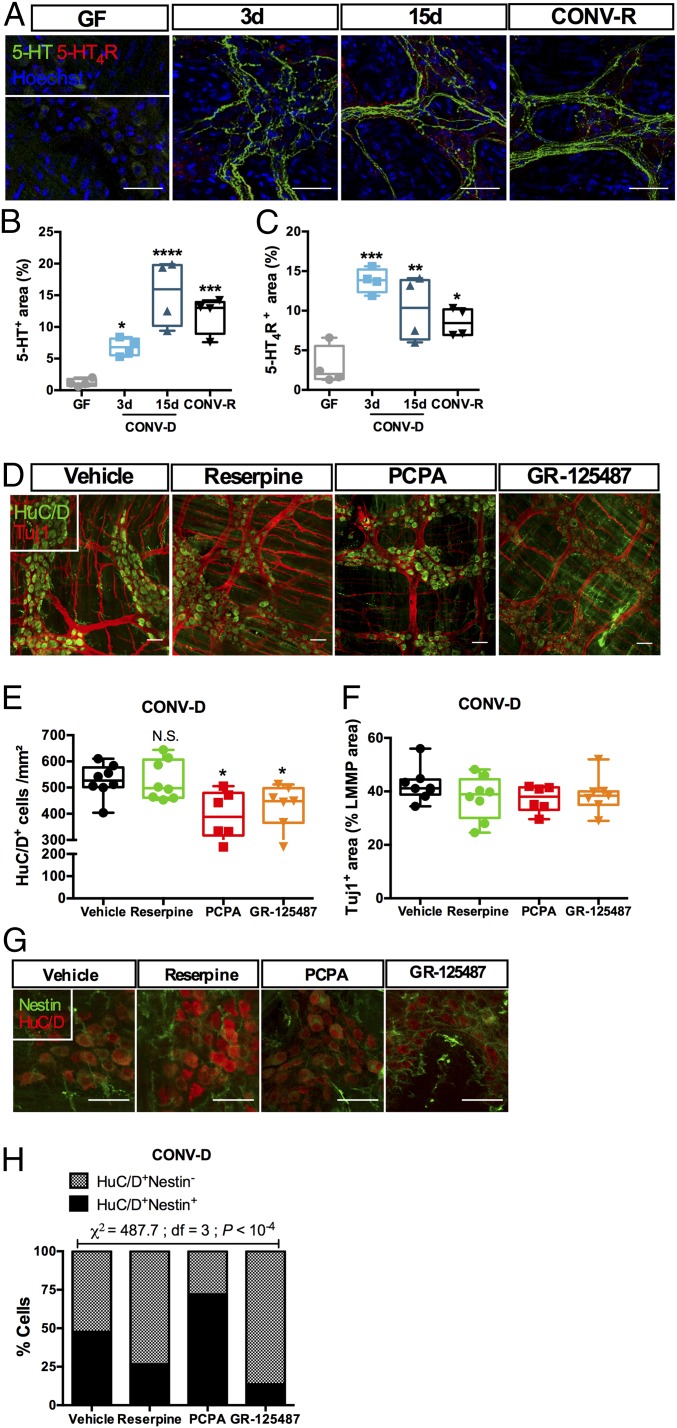
Nestin is a cytoskeletal protein that is expressed by a variety of neural stem cells (10, 12, 29, 30). Nestin+ cells in the myenteric plexus of adult mice give rise to neurons in vivo (12), and studies of Nestin-GFP transgenic mice suggest that coexpression of Nestin and HuC/D is a signature of neuronal plasticity in the adult ENS (10). Thus, we determined the proportion of Nestin+ neurons in GF, CONV-D, and CONV-R mice (Fig. 2A). Colonization of GF mice induced a global decrease in the proportion of Nestin+ neurons (χ2 = 208.7; df = 3; P < 10−4) (Fig. 2B), suggesting that GF mice retain a higher plasticity in the ENS, which is maintained for at least 3 d after colonization but disappears when the microbiota has reached a steady state.
Fig. 2.
Colonization of GF mice with a gut microbiota induces maturation of neuronal precursors in the myenteric plexus of the colon. (A) Immunostaining with HuC/D (red) and the neuronal precursor marker Nestin (green). (B) Colonization reduced the proportion of Nestin+ neurons in the myenteric ganglia (n > 1,000 cells counted per group). (Scale bars: 20 µm.)
Colonization of GF Mice Induces Proliferation of a Preexisting Nestin+ Subpopulation of Neural Precursors.
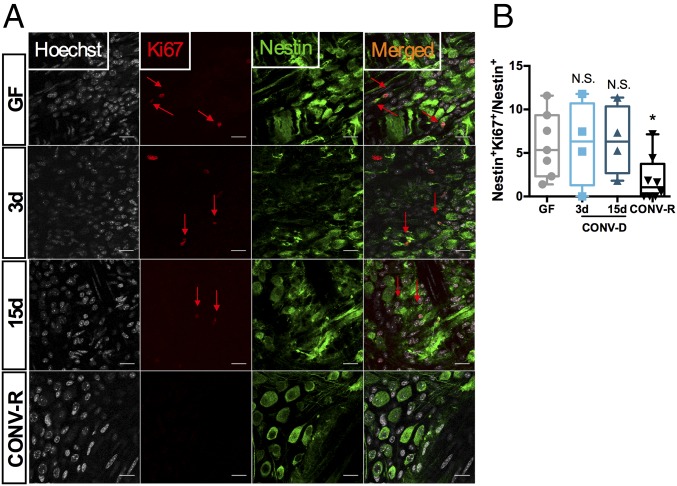
To test the hypothesis that colonization of GF mice with a normal microbiota induces the proliferation of neural precursor cells, we examined the coexpression of Nestin with the cycling marker Ki67 in the myenteric ganglia of the colon. We found that in GF mice as many as 5% of the Nestin+ cells retained the capacity to undergo proliferation and that this state persisted 3 and 15 d after colonization (Fig. 3 A and B). In contrast, less than 1% of Nestin+ cells in CONV-R mice expressed Ki67 (P = 0.03 vs. GF; Kruskal–Wallis test followed by Dunn’s post hoc test). We also observed that some Ki67+ cells had a small rim of Nestin+ cytoplasm (Fig. 3).
Fig. 3.
Colonization of adult GF mice with a gut microbiota results in cycling of neuronal progenitors. (A) Representative images of a colonic myenteric ganglion of a mouse stained with the cycling cell marker Ki67 (red arrows), neuronal precursor marker Nestin (green), and with nuclei counterstained with Hoechst (gray). (B) Quantification of double-positive Nestin/Ki67 cells. *P < 0.05 vs. GF; N.S., not significant; Kruskal–Wallis test followed by Dunn’s post hoc test. (Scale bars: 20 µm.)
Since myenteric Nestin+ cells are responsible for adult neurogenesis in the ENS (12), our results suggest that GF mice retain a potential for neurogenesis and maturation of the ENS which can be activated upon colonization. However, since we observed no difference in the density of neurons in GF mice, proliferation is likely compensated for by cell loss. Taken together, our results suggest that neuronal differentiation from Nestin+ cells occurs after exposure to the gut microbiota.
Interactions Between the Microbiota and Mucosal 5-HT are Neuroprotective.
5-HT has been implicated in neurogenesis as well as in promoting the survival of neurons. We confirmed previous observations that colonization of GF mice partially restored serum levels of 5-HT (SI Appendix, Fig. S4A) (18, 19, 31), likely by inducing de novo 5-HT synthesis by increasing the expression of the rate-limiting enzyme for 5-HT synthesis, Tph1, in the mucosa (SI Appendix, Fig. S4B). Furthermore, depletion of the microbiota with antibiotics reduced circulating 5-HT levels (SI Appendix, Fig. S4C).
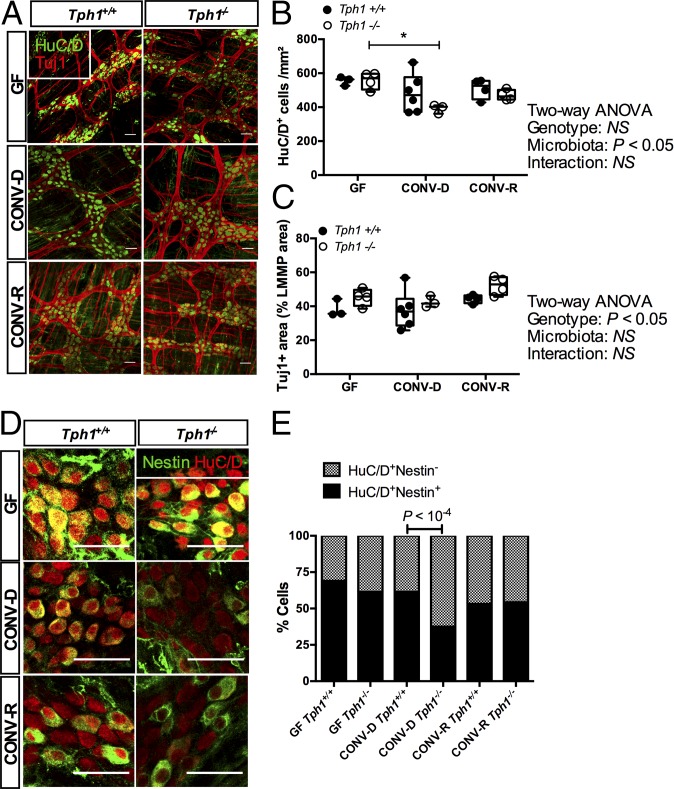
To investigate whether mucosal 5-HT production was required to maintain the neuroanatomy of the ENS, we quantified neuronal density in Tph1-knockout (Tph1−/−) mice. After confirming that Tph1 was not expressed in the colon of our knockout mice (SI Appendix, Fig. S4D), we analyzed the anatomy of the ENS in Tph1−/− mice and wild-type littermates. CONV-R Tph1−/− mice do not show any evident alterations in the neuroanatomy of the ENS (32), but no study so far has focused on these mice under GF conditions. In line with previous studies in the ileum of CONV-R mice (32), we did not observe significant changes in the neuroanatomy of the ENS of CONV-R and GF mice (Fig. 4). However, when GF mice were colonized for 3 d with the microbiota of wild-type C57BL/6 CONV-R mice (yielding CONV-D mice), we found that Tph1−/− mice (Fig. 4 A and B) had a decreased number of myenteric neurons (effect of microbiota: 35% of total variation; two-way ANOVA, P = 0.02), highlighting the importance of mucosal 5-HT in maintaining the integrity of the ENS during the early colonization period. Moreover, the proportion of Nestin+ neurons was significantly reduced in CONV-D Tph1−/− mice (Fig. 4 D and E and SI Appendix, Fig. S4E). Taken together, our data show that the microbiota-induced release of mucosal 5-HT is neuroprotective for the ENS in the early phases of colonization.
Fig. 4.
Mucosal 5-HT is neuroprotective in CONV-D mice. (A) Representative images of the colonic LMMP showing pan-neuronal marker HuC/D (green) and neuron-specific β-III tubulin (Tuj1, red). (B and C) Quantification of HuC/D+ cells (B) and the Tuj1+ area (C). P values are reported after two-way ANOVA followed by Dunnett’s multiple comparisons test. *P < 0.05. (D) Immunostaining with HuC/D (red) and the neuronal precursor marker Nestin (green). (E) Quantification of results in D. Split panels are provided in SI Appendix, Fig. S4E. P values reported are knockout vs. wild type; Fisher’s exact test (n > 750 cells were counted per group). (Scale bars: 50 µm.)
The Gut Microbiota Induces Neuronal 5-HT Production.
It has been shown that the release of 5-HT from enteric neurons influences the development and survival of dopaminergic neurons (32), showing the importance of serotonergic neurons in organizing more mature ENS network components. We performed immunohistochemistry of 5-HT in the LMMP and found that serotonergic neuronal networks were almost absent in GF mice but were gradually restored by colonization with a gut microbiota (Fig. 5 A and B). Importantly, the presence of a gut microbiota was crucial to maintain the serotonergic networks, since depletion with antibiotics abolished 5-HT immunoreactivity (SI Appendix, Fig. S5 B and C). To distinguish the specific roles of serotonin on the observed phenotype, we chronically treated CONV-D mice for 3 d with PCPA, a selective and irreversible inhibitor of TPH1 and TPH2, or with reserpine, an irreversible blocker of vesicular monoamine transporter (thus depleting neuronal 5-HT). We confirmed that PCPA and reserpine diminished 5-HT immunoreactivity in the LMMP (SI Appendix, Fig. S5 E–H).
Fig. 5.
The gut microbiota regulates neuronal 5-HT release and 5-HT4R activation in the myenteric plexus. (A) Representative images of the colonic myenteric plexus showing immunoreactivity to 5-HT (green) and 5-HT4R (red). Localization of 5-HT and 5-HT4R was altered by colonization. (B and C) Quantification of immunoreactivity to 5-HT (B) and 5-HT4R (C). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 10−4 vs. GF; Kruskal–Wallis test followed by Dunn’s post hoc test. (D) Representative images of the colonic LMMP of CONV-D mice treated with a vehicle solution, the 5-HT4R antagonist GR-125487, the TPH inhibitor PCPA, or the vesicular monoamine transporter blocker reserpine, showing the pan-neuronal marker HuC/D (green) and neuron-specific β-III tubulin (Tuj1, red). (E and F) Quantification of HuC/D+ cells (E) and the Tuj1+ area (F). *P < 0.05 vs. GF; N.S., not significant; Kruskal–Wallis test followed by Dunn’s post hoc test. (G) Immunostaining of HuC/D (red) and the neuronal precursor marker Nestin (green). Split panels are shown in SI Appendix, Fig. S6. (H) Quantification of the proportion of Nestin+ neurons (n > 500 cells counted per group). (Scale bars: 50 µm.)
Treatment with the TPH inhibitor PCPA resulted in reduced density of myenteric neurons (Fig. 5 D and E). Surprisingly, the treatment also led to a significant increase in the proportion of Nestin+ neurons (Fig. 5 G and H and SI Appendix, Fig. S6). These data suggest that blocking 5-HT production results in (i) loss of myenteric neurons similar to that observed in Tph1−/− CONV-D mice and (ii) inhibition of neuronal differentiation from Nestin+ progenitors.
While there was no significant difference in the number of neurons or in Tuj1+ neurite density in reserpine-treated CONV-R (SI Appendix, Fig. S5 J and K) or CONV-D (Fig. 5 D–F) mice, the treatment significantly decreased the proportion of Nestin+ neurons (Fig. 5 G and H and SI Appendix, Fig. S6). It is noteworthy that reserpine is an irreversible blocker of the vesicular monoamine transporter, which also transports dopamine and noradrenaline, so we cannot exclude the possibility that these neurotransmitters contribute to the phenotype. Further studies with GF and CONV-D Tph2-deficient mice might confirm the specificity of the action of neuronal 5-HT on maturation of the ENS after colonization.
Activation of the 5-HT4R in GF Mice Induces Maturation of the Adult ENS.
The 5-HT4R is widely expressed in the intestine (33, 34), and mucosal application of 5-HT4R agonists activates mucus discharge by goblet cells and Cl− secretion by enterocytes, whereas 5-HT4R antagonists block these actions (33). Stimulation of 5-HT4R also induces adult neurogenesis and neuroprotection in the gut (4, 23). Here, we confirmed that 5-HT4R was expressed in the ENS, and specifically in myenteric neurons (SI Appendix, Fig. S5A), and found that its expression was dependent upon the presence of a gut microbiota (Fig. 5 A and C and SI Appendix, Fig. S5 B and D). Similar to 5-HT, we observed that 5-HT4R expression was located in the soma of the cells in GF mice but in serotonergic neurites after colonization (Fig. 5A and SI Appendix, Fig. S5A).
We next colonized GF mice and concomitantly treated them for 3 d with chronic injections of the 5-HT4R antagonist GR-125487. Treatment with the antagonist did not affect the density of the neuronal network but resulted in a decrease in the number of myenteric neurons (Fig. 5 D–F). The proportion of Nestin+ neurons (Fig. 5 G and H and SI Appendix, Fig. S6) was significantly reduced by the treatment. This suggests that neuronal 5-HT release regulates the stemness of neuronal progenitors in the ENS, while specific activation of 5-HT4R also regulates proliferation.
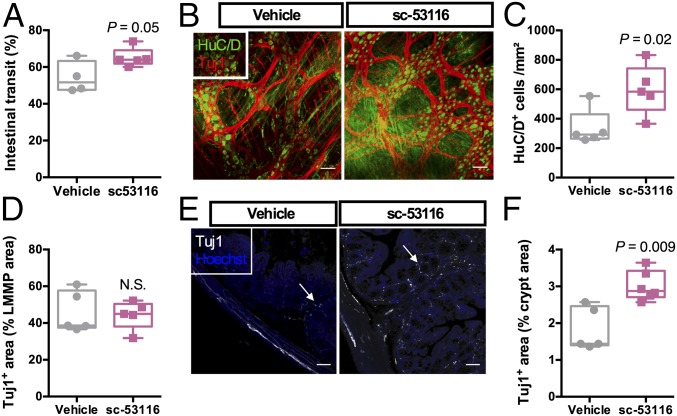
To test directly whether the effects of colonization on ENS anatomy could be phenocopied in GF mice, we treated GF mice with the 5-HT4R agonist sc-53116 for 4 d. We observed that activation of 5-HT4R in GF mice was associated with faster intestinal transit (Fig. 6A), concomitant with an increased number of myenteric neurons (Fig. 6 B–D) and increased innervation of the colonic mucosa (Fig. 6 E and F).
Fig. 6.
5-HT4R regulates ENS anatomy and function in GF mice. (A) Intestinal transit in GF mice that were given the 5-HT4R antagonist sc-53116 or a vehicle solution. P = 0.05; Student’s unpaired t test. (B) Representative images of the colonic LMMP of the aforementioned mice showing the pan-neuronal marker HuC/D (green) and neuron-specific β-III tubulin (Tuj1, red). (C and D) Quantification of HuC/D+ cells (C) and the Tuj1+ area (D). (E) Representative images of the innervation of the colonic crypts of the mice (white arrows) using the peripheral neuronal marker Tuj1. (F) Quantification of the Tuj1+ area. P values were determined by the Mann–Whitney test. N.S., not significant. (Scale bars: 50 µm.)
Taken together, our data suggest that, upon colonization, the gut microbiota stimulates neuronal and mucosal 5-HT release and that 5-HT4R activation in GF mice leads to differentiation and maturation of enteric neurons.
Discussion
The ENS contains cells that give rise to neurons in vitro and in vivo (2, 3, 9). In this study, we showed that colonization of adult GF mice induced maturation of the ENS associated with the proliferation of Nestin+ progenitors precursors present in GF mice. While blocking 5-HT4R or depleting endogenous 5-HT prevented the process, GF mice had restored innervation of the gut when treated with 5-HT4R agonists. Here we show that maturation of the adult ENS requires 5-HT4R–specific signaling and that colonization by gut microbes of GF Tph1−/− mice resulted in fewer myenteric neurons, suggesting that both pools of enteric 5-HT are crucial for maintenance of the adult ENS.
Our findings confirm that the maturation capacity of the ENS (i.e., growth of neurites and neurogenesis) continues in adult mice in a microbiota-naive gut. Interestingly, GF rodents show an abnormal proportion of calbindin and nitrergic myenteric neurons, suggesting that the microbiota influences the maturation of intestinal neural networks (15, 16, 35). Furthermore, functional activity of the ENS can be regulated by the microbiota, either directly through Toll-like receptors (36) or by inducing the secretion of BMP2 by macrophages (37). A recent study showed that adult enteric neurogenesis is an active process that is balanced with apoptosis (12). Our results complement these findings and suggest that Nestin+ cells can undergo proliferation and differentiation into functional neurons. However, the mechanisms by which the gut bacteria impact the organization of the ENS remained unexplored. For example, in cases of dysbiosis, NO production in the ENS is impaired (38). Thus, it is likely that nitrergic and serotonergic pathways interact with the microbiota to establish a functional ENS.
The gastrointestinal tract is the site where most 5-HT is synthesized, and recently two studies have shown that the gut microbiota plays a key role in promoting 5-HT secretion (18, 19). We confirmed these findings, but our results suggest that neuronal and mucosal 5-HT play complementary roles in the ENS maturation (SI Appendix, Fig. S7). Neuronal 5-HT has been identified as a major neuronal growth factor during development (32) while also acting as a promoter of growth and a suppressor of inflammation in the intestinal mucosa (22, 39). We complemented these findings by rederiving Tph1−/− mice as germ-free. Similar to the PCPA-treated mice, colonization of these mice resulted in a decreased number of myenteric neurons, showing that mucosal 5-HT is crucial for the integrity of the ENS in the early stages of colonization. However, unlike Tph1−/− and reserpine-treated CONV-D mice, PCPA induced an increase in the ratio of Nestin+ cells in the myenteric plexus, an observation that requires future investigation.
The altered organization and properties of enteric neurons in GF mice also influence gastrointestinal function. Treatment with the 5-HT4R agonist prucalopride protects enteric neurons against oxidative stress (23), and activation of 5-HT4R in the epithelium reduces inflammation in mice with colitis (40). Changes in the gut microbiota triggered, for example, by infection, stress, or antibiotic usage have been suggested to cause irritable bowel syndrome (41). Thus, improved understanding of the dialogue between the microbiota and the ENS opens the possibility for new treatments for inflammatory diseases of the gut.
Materials and Methods
For detailed procedures, see SI Appendix, SI Methods.
Animals.
Adult C57BL/6J female mice, aged 12 wk at the beginning of the experiments, were housed in a climate-controlled room (22 ± 2 °C) subjected to a 12-h light/dark cycle (lights on, 7:00 AM–7:00 PM), with free access to water and food. Tph1−/− mice were described previously (42). All procedures in mice were approved by the Ethics Committee on Animal Care and Use in Gothenburg, Sweden. Antibiotic treatment is described in SI Appendix, SI Methods.
Statistical Analysis.
Data are presented as box plots showing maximum, minimum, median, and interquartile range. Each dot represents a single mouse. Statistical analysis was performed using GraphPad Prism 7 software.
Supplementary Material
Acknowledgments
We thank Anna Hallén, Carina Arvidsson, Louise Helldén, Sara Nordin-Larsson, Ulrica Enqvist, Caroline Wennberg, and Zakarias Gulic for excellent assistance with the animal studies; Anna Hallén for generating SI Appendix, Fig. S7; Thomas Greiner (University of Gothenburg) for breeding and genotyping Tph1−/− mice; and Gabriel Lepousez (Institut Pasteur, Paris) for fruitful discussions regarding neurogenesis. Confocal microscopy was performed at the Center for Cellular Imaging of the University of Gothenburg. This work was funded by the Swedish Research Council, the Novo Nordisk Foundation, the Torsten Söderberg Foundation, the Swedish Heart Lung Foundation, the Göran Gustafsson Foundation, the IngaBritt och Arne Lundberg Foundation, and the Knut and Alice Wallenberg Foundation. F.D.V. is a recipient of European Molecular Biology Organization Long-Term Fellowship ALTF 1305-2014 (Marie Curie Actions LTFCOFUND2013, GA-2013-609409) and a La Ligue Contre Le Cancer postdoctoral fellowship. E.G. is funded by a postdoctoral fellowship from the Wenner-Gren foundations. G.K. is a recipient of NIH Grant 2P01 AG032959-06A1 and a research grant from the Columbia Aging Center. F.B. is a recipient of European Research Council Consolidator Grant 615362–METABASE and is a Torsten Söderberg Professor in Medicine.
Footnotes
Conflict of interest statement: F.B. is cofounder of and shareholder in Metabogen AB.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720017115/-/DCSupplemental.
References
- 1.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 2.Joseph NM, et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laranjeira C, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu M-T, Kuan Y-H, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: Sequence in relation to phenotype. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Chan AKK, Sham MH, Burns AJ, Chan WY. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141:992–1002.e1–6. doi: 10.1053/j.gastro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Lasrado R, et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science. 2017;356:722–726. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- 8.Memic F, et al. Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology. 2018;154:624–636. doi: 10.1053/j.gastro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger GM, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundmann D, Markwart F, Scheller A, Kirchhoff F, Schäfer K-H. Phenotype and distribution pattern of nestin-GFP-expressing cells in murine myenteric plexus. Cell Tissue Res. 2016;366:573–586. doi: 10.1007/s00441-016-2476-9. [DOI] [PubMed] [Google Scholar]
- 11.Belkind-Gerson J, et al. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil. 2013;25:61–69.e7. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni S, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bäckhed F, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703, and erratum (2015) 17:852. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J Clin Invest. 2015;125:956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 16.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 2015;27:627–636. doi: 10.1111/nmo.12534. [DOI] [PubMed] [Google Scholar]
- 17.Mawe GM, Hoffman JM. Serotonin signalling in the gut–Functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reigstad CS, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling–The gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14:412–420. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghia J-E, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Margolis KG, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianco F, et al. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2016;310:G768–G775. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabouridis PS, et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wichmann A, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 27.El Aidy S, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5:567–579. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 28.Molinaro A, et al. Host-microbiota interaction induces bi-phasic inflammation and glucose intolerance in mice. Mol Metab. 2017;6:1371–1380. doi: 10.1016/j.molmet.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker L, Peterson J, Kulkarni S, Pasricha PJ. Ex vivo neurogenesis within enteric ganglia occurs in a PTEN dependent manner. PLoS One. 2013;8:e59452. doi: 10.1371/journal.pone.0059452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikoff WR, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman JM, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854.e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–G1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 35.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183–e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 36.Brun P, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323–1333. doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313, and erratum (2014) 158:1210. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grasset E, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. 2017;25:1075–1090.e5. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Gross ER, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417.e2, and erratum (2013) 144:249. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spohn SN, et al. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology. 2016;151:933–944.e3. doi: 10.1053/j.gastro.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497–505. doi: 10.1038/nrgastro.2014.40. [DOI] [PubMed] [Google Scholar]
- 42.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.