Significance
BP180 is a cell–cell matrix adhesion molecule and is critical for attachment of epidermis to underlying dermis. We generated a dysfunctional BP180 mouse strain (ΔNC16A mice). The ΔNC16A mice at 8 weeks start developing spontaneous itch and skin inflammation characterized by increased skin thickness, infiltrating immune cells, increased levels of IgE in circulation, proinflammatory mediators in the skin, and impaired skin barrier. Bullous pemphigoid (BP) patients also present increased TSLP in lesional skin and circulation. Our findings suggest that BP180 regulates pruritic skin inflammation. Elucidation of molecular and cellular mechanisms underlying the role of BP180 in skin inflammation should help better understanding of pathophysiology of skin inflammation and identify therapeutic targets for skin inflammatory diseases such as BP and atopic dermatitis.
Keywords: collagen XVII, hemidesmosome, atopic dermatitis, TSLP, keratinocyte
Abstract
BP180, also known as collagen XVII, is a hemidesmosomal component and plays a key role in maintaining skin dermal/epidermal adhesion. Dysfunction of BP180, either through genetic mutations in junctional epidermolysis bullosa (JEB) or autoantibody insult in bullous pemphigoid (BP), leads to subepidermal blistering accompanied by skin inflammation. However, whether BP180 is involved in skin inflammation remains unknown. To address this question, we generated a BP180-dysfunctional mouse strain and found that mice lacking functional BP180 (termed ΔNC16A) developed spontaneous skin inflammatory disease, characterized by severe itch, defective skin barrier, infiltrating immune cells, elevated serum IgE levels, and increased expression of thymic stromal lymphopoietin (TSLP). Severe itch is independent of adaptive immunity and histamine, but dependent on increased expression of TSLP by keratinocytes. In addition, a high TSLP expression is detected in BP patients. Our data provide direct evidence showing that BP180 regulates skin inflammation independently of adaptive immunity, and BP180 dysfunction leads to a TSLP-mediated itch. The newly developed mouse strain could be a model for elucidation of disease mechanisms and development of novel therapeutic strategies for skin inflammation and BP180-related skin conditions.
BP180, also known as collagen XVII, is a 180-kDa transmembrane glycoprotein of the hemidesmosome anchoring basal keratinocytes into the underneath basal membrane of the skin (1). The intracellular region of BP180 is linked to the intermediate filament network, and its extracellular portion is anchored into the basement membrane zone (BMZ) through interacting with extracellular matrix proteins (2, 3). Dysfunction of BP180 by gene mutations can lead to junctional epidermolysis bullosa (JEB) (4, 5), a subepidermal blistering disease in humans. In the skin autoimmune subepidermal blistering disease bullous pemphigoid (BP), anti-BP180 autoantibodies attack and impair function of BP180 autoantigen in basal keratinocytes causing dermal-epidermal separation (6). Pathogenic anti-BP180 autoantibodies mainly target the juxtamembranous noncollagenous NC16A domain (7). These findings in JEB and BP establish BP180 as a key cell–cell matrix adhesion molecule in the skin. However, whether BP180 is involved in other biological processes and pathological conditions is largely unknown.
Both BP and JEB share some features of skin inflammation. BP is characterized by skin infiltration of immune cells, increased IgE, and pruritus (1, 8). JEB is a very rare genetic disease, and some JEB patients developed atopic dermatitis (AD)-like skin inflammation including inflammatory cell infiltration and elevated serum IgE (9). Snauwaert et al. (10) found that itch occurred in 100% of the JEB patients in their study. Previously, another group generated a BP180 dysfunctional mouse strain (termed ΔNC14A mice), which shows an increased itch, eosinophil influx, and serum IgE (11). However, potential molecular and/or cellular mechanisms underlying the BP180 dysfunction-associated phenotypes were not determined.
To uncover functions of BP180 beside its cell-matrix adhesion property, we generated a BP180 dysfunctional mouse model by removal of the NC16A domain (termed ΔNC16A mice). Mice expressing the NC16A-truncated BP180 developed subepidermal blistering with severe skin inflammation and itch. The spontaneous skin inflammation and itch in ΔNC16A mice were neither dependent on histamine nor adaptive immunity, but dependent on TSLP. Moreover, high TSLP expression is also found in BP patients. This report demonstrates that BP180 dysfunction leads to TSLP-mediated skin inflammation.
Results
Generation of ΔNC16A Mice.
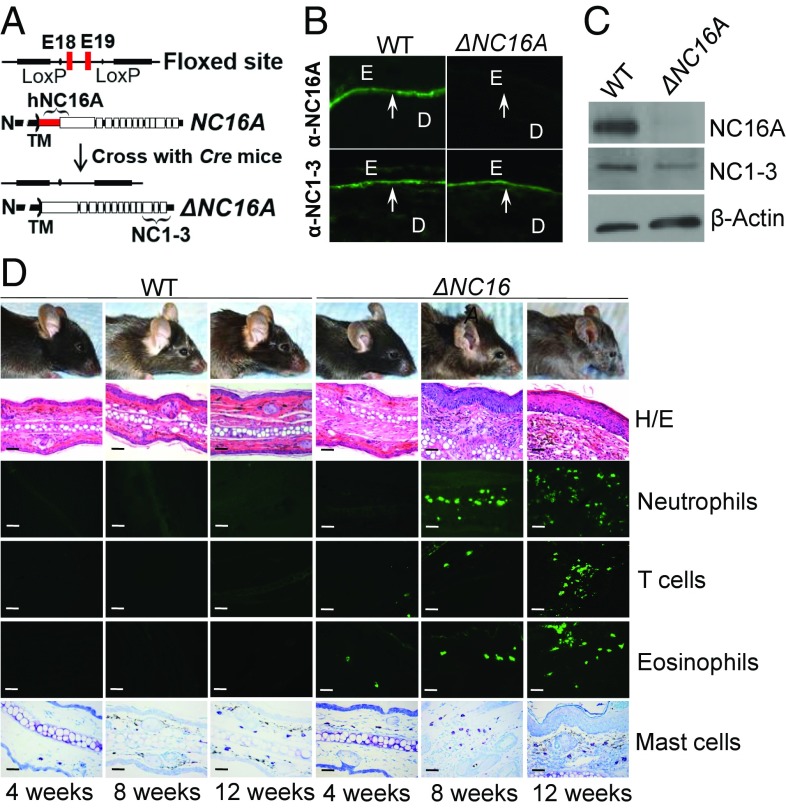
Human and mouse BP180 lack immune cross-reactivity (12). To study the immunopathogenesis of BP using patients’ autoantibodies, we previously generated the humanized NC16A mice by replacing mouse NC14A domain with the human NC16A counterpart (7). The NC16A domain is encoded by exons 18 and 19, which were flanked by lox-P sites (Fig. 1A). In this study, we use NC16A mice as wild-type (WT) control mice. When crossed with the germ-line Cre mice, Cre recombination removes the loxP-flanked exons 18 and 19 and maintains the remaining reading frame, resulting in mice expressing NC16A domain-truncated BP180 (termed ΔNC16A mice). Indirect immunofluorescence exhibited that anti-NC16A antibody stained the basement membrane zone (BMZ) of WT but not ΔNC16A mouse skin, while anti-NC1-3 antibody stained the BMZ of both WT and ΔNC16A mouse skin (Fig. 1B). Immunoblotting showed that anti-NC16A antibody recognized full-length BP180 in WT mice but not in ΔNC16A mice, while anti-NC1-3 antibody recognized both WT and ΔNC16A mice (Fig. 1C).
Fig. 1.
ΔNC16A mice exhibit skin inflammation. (A) NC16A mice (WT) with NC16A-encoding exons 18 and 19 flanked by loxP sites were crossed with germ-line Cre mice, resulting in mice expressing NC16A-truncated BP180 (ΔNC16A). (B) Immunofluorescence (IF) Anti-NC16A antibody stained the BMZ of only WT skin, while anti-NC1-3 antibody stained the BMZ of both WT and ΔNC16A skin. D, dermis; E, epidermis; arrows, BMZ; n = 5 per group. (C) Immunoblotting. Anti-NC16A antibody recognized full-length BP180 in WT skin, while anti-NC1-3 antibody recognized both full-length and NC16A-truncated BP180 in both WT and ΔNC16A skin (n = 5 per group). (D) ΔNC16A showed clinical skin lesion starting around 8 wk old and became more severe at 12 wk old. Ear skin revealed minor skin inflammation with increased infiltrating immune cells at 8 wk old and minor epidermal/dermal separation at 12 wk old in ΔNC16A mice. (Original magnifications: 200×.) (Scale bars: 50 μm.)
ΔNC16A Mice Develop Spontaneous Skin Inflammation with Pruritus.
ΔNC16A mice showed no clinical skin phenotypes after birth but began to develop minor skin lesions at the age of 8 wk and severe skin lesions at 12 wk (Fig. 1D). These ΔNC16A mice had overt skin inflammation, with eczematous lesions occurring mainly on the ears, eyelid, snout, and dorsal skin weeks (Fig. 1D). Ear skin histology showed that ΔNC16A mice have marked acanthosis and prominent orthokeratosis with dermal inflammatory cells infiltration starting at 8 wk after birth (Fig. 1D and SI Appendix, Fig. S1A). A low degree of dermal-epidermal separation in the skin of adult ΔNC16A mice became evident at the age of 12 wk with increased inflammatory cell infiltration (Fig. 1D). The epidermal thickness of ΔNC16A mice were significantly increased compared with WT mice (n = 8) (Fig. 2A). ΔNC16A mice exhibited spontaneous pruritus, starting to show a significantly increased scratching at 8 wk after birth and reaching the plateau at 12–16 wk after birth (n = 8) (Fig. 2B). These results indicate that BP180 dysfunction promotes spontaneous skin inflammation with aberrant itch.
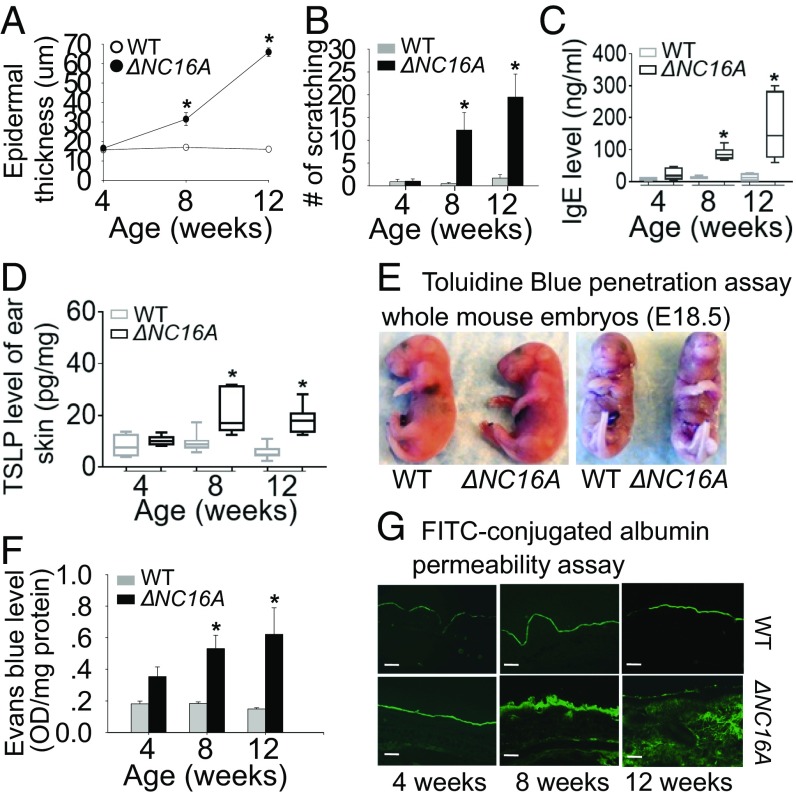
Fig. 2.
ΔNC16A mice exhibit aberrant itch, increased TSLP level, and defective skin barrier. Compared with WT mice, ΔNC16A mice starting 8 wk old exhibited a significantly increased epidermis thickness (A), itch (scratch motion) (B), serum IgE levels (C), and TSLP level in ear skin (D) (n = 8 per group). (E) Toluidine blue penetration assay showed no barrier defect in E18.5 embryos of ΔNC16A and WT mice (n = 8 per group). Adult ΔNC16A mice started to show a significant reduction in barrier function at 8∼12 wk after birth, by Even Blue (F) (n = 6 per group) and FITC-conjugated BSA permeability assays (G). (Scale bars: 50 μm.) *P < 0.05, Student’s t test, graphs A, B, and F show mean ± SE.
In addition, ΔNC16A mice exhibited a significant increase in serum IgE (Fig. 2C) and the infiltrating inflammatory immune cells into the skin, including neutrophils and eosinophils, T cells, and mast cells (Fig. 1D). Whole transcriptome microarray of ΔNC16A ear skin tissue also showed that ΔNC16A mice exhibited a significant increase in the expression of genes associated with skin inflammation (SI Appendix, Table S1), especially TSLP, IL-1β, and IL-13 that are typically increased in patients with skin inflammatory diseases (13) (SI Appendix, Fig. S1). The increased TSLP level of skin in ΔNC16A mice was also confirmed by ELISA (n = 8) (Fig. 2D). However, levels of TNFα, IFN-γ, and IL-4 were not significantly different between WT and ΔNC16A mice (SI Appendix, Fig. S1). Because skin inflammation is often correlated with barrier permeability (13, 14), we tested whether BP180 dysfunction may also reduce skin barrier function. Dye-exclusion assay on embryonic day (E)18.5 embryos showed that like WT mice, the skin barrier of ΔNC16A mice was fully developed and intact before birth (n = 8) (Fig. 2E). However, the skin barrier of adult ΔNC16A mice was significantly impaired as determined by Evans blue dye (Fig. 2F) and fluorescein isothiocyanate (FITC)-conjugated albumin (BSA) permeability assay (n = 6) (Fig. 2G), respectively. Taken together, these results showed that ΔNC16A mice develop spontaneous skin inflammation accompanied with severe pruritus, increased serum IgE, and reduced skin barrier function.
Skin Local BP180 Dysfunction Is Sufficient to Promote Skin Inflammation.
BP180 is expressed in the skin as well as many other tissues (15). To determine whether the skin inflammation in ΔNC16A mice is caused by BP180 dysfunction locally in the skin and/or systemically, we developed tamoxifen-inducible ERCre+NC16Afl/fl mice (termed TamCre-NC16A mice). When treated with tamoxifen topically, TamCre-NC16A mice become local skin-specific ΔNC16A (termed skinΔNC16A). The efficacy of NC16A deletion in skinΔNC16A mice was confirmed by qPCR (SI Appendix, Fig. S2A) and immunoblotting (SI Appendix, Fig. S2B).
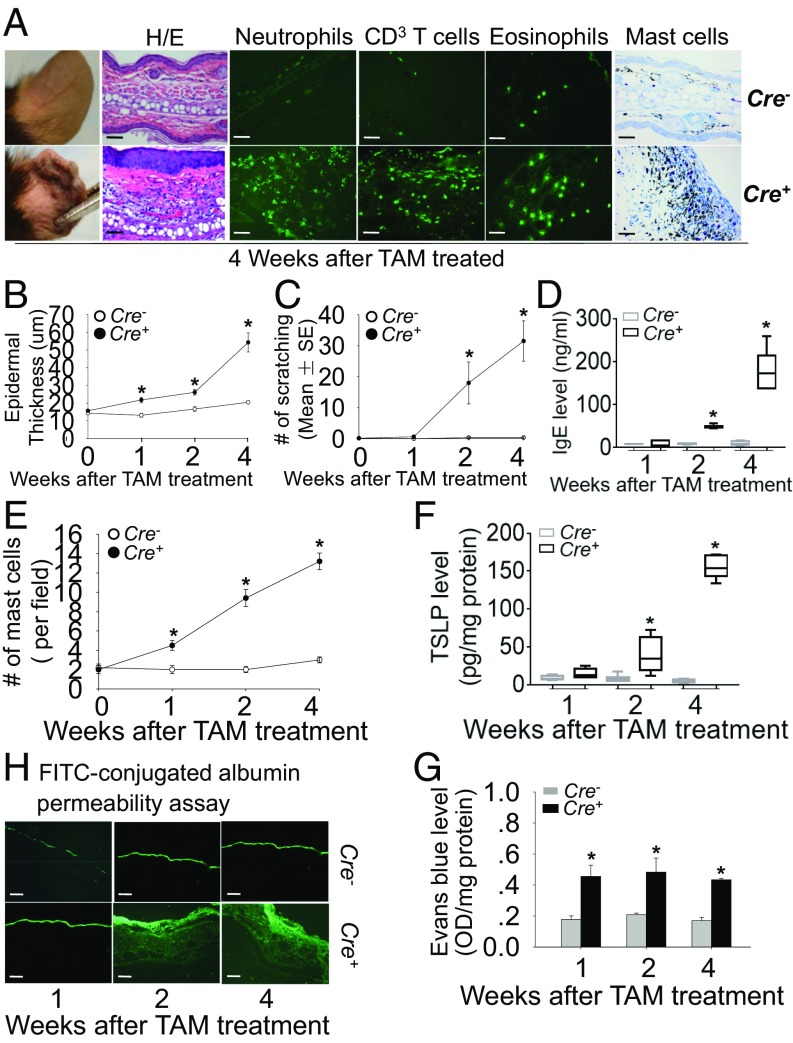
SkinΔNC16A mice started showing minor skin lesions and inflammation around 2 wk after a single tamoxifen treatment (Fig. 3A and SI Appendix, Fig. S2C). No skin lesion or inflammation was seen in the ear of tamoxifen-treated ERCre−NC16Afl/fl mice (Cre− control mice), ruling out the possibility that the skin inflammation was induced by tamoxifen itself (Fig. 3A and SI Appendix, Fig. S2C). Similar to whole body ΔNC16A, the skinΔNC16A mice also showed skin inflammation, including epidermis hyperplasia, severe pruritus, a significantly increased IgE level in serum (Fig. 3 B–D), and a significant increase in infiltrating neutrophils, eosinophils, T cells, and mast cells (n = 8) (Fig. 3 A and E and SI Appendix, Fig. S2 D–G). SkinΔNC16A mice also showed significantly increased skin TSLP level (n = 8) (Fig. 3F) and defective barrier function compared with control mice (n = 6) (Fig. 3 G and H). These results demonstrated that similar to whole body ΔNC16A mice, ΔNC16A in the local skin is sufficient to develop skin inflammation.
Fig. 3.
SkinΔNC16A mice develop skin inflammation with increased immune cell infiltration, epidermal thickness, itch, serum IgE, TSLP, and defective barrier. (A) The ears of skinΔNC16A mice started showing skin lesions clinically and histologically at 4 wk after tamoxifen treatment compared with control mice (Cre−). Immune staining of ear skin also showed that skinΔNC16A mice (Cre+) have significantly increased infiltrating immune cells at 4 wk after tamoxifen treatment. Toluidine blue (TB) staining indicated that there was a significant increase in mast cells in skinΔNC16A mice starting at 1 wk after tamoxifen treatment (A and E). (Original magnifications: 200×.) Compared with Cre− mice, Cre+ mice show increased epidermis thickness (B), spontaneous itch (C), serum IgE level (D), and TSLP (F) in skin starting at 2 wk after tamoxifen treatment (n = 8 per group). (G) Evans Blue dye penetration assay showed barrier defect in skinΔNC16A but not control mice starting 1 wk after tamoxifen treatment. (H) FITC-conjugated BSA permeability assays also revealed barrier defect in skinΔNC16A but not control mice starting 2 wk after tamoxifen treatment (n = 6 per group). (Scale bars: 50 μm.) *P < 0.05, Student’s t test, graphs B, C, E, and G show mean ± SE.
BP180 is expressed in many organs, including skin, ocular conjunctiva, epithelial basement membrane of the cornea, upper esophagus, transitional epithelium of the bladder, and brain (15). To investigate whether BP180 dysfunction triggers skin inflammation through basal keratinocytes, we generated basal keratinocyte-specific ΔNC16A mice (termed K14Cre/ΔNC16A). NC16A deletion was confirmed using Western blot (SI Appendix, Fig. S3A). Similar to whole body ΔNC16A and skinΔNC16A mice, K14Cre/ΔNC16A mice also exhibited spontaneous dermatitis with significantly increased itchiness, immune cells infiltration (including neutrophils, eosinophils, T cells, and mast cells) (SI Appendix, Fig. S3 B–F), increased serum IgE, high TSLP expression, and impaired skin barrier (n = 8) (SI Appendix, Fig. S3 G–I). Therefore, BP180 dysfunction in basal keratinocytes is sufficient to promote skin inflammation.
Skin Inflammation Occurs in ΔNC16A Mice in the Absence of Adaptive Immunity.
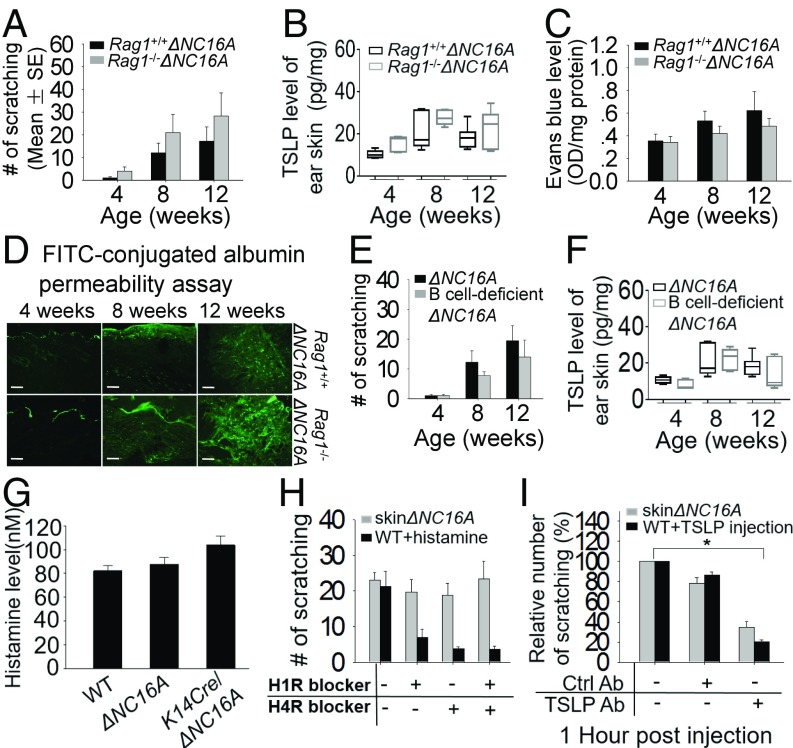
Two hypotheses concerning the mechanism of skin inflammation have been proposed: One hypothesis proposes that the primary defect resides in an immunologic disturbance that causes skin inflammation; the other hypothesis proposes that an intrinsic defect in the epithelial cells (for example, keratinocytes) leads to skin inflammation (16, 17). A previous report suggested that BP180 dysfunction may trigger a spontaneous autoimmune response, which leads to BP-like skin inflammation (11). To assess whether adaptive immunity plays a major role in the spontaneous skin inflammation in our model, we crossed the ΔNC16A mice with T cell- and B cell-deficient Rag1−/− mice to generate adaptive immunity-deficient ΔNC16A (termed Rag1−/−ΔNC16A) (SI Appendix, Fig. S4 A and B). Rag1−/−ΔNC16A and ΔNC16A mice were then compared for development of skin inflammation at 4, 8, and 12 wk old using Rag1−/−NC16A mice as a negative control (SI Appendix, Fig. S4C). Rag1−/−ΔNC16A and ΔNC16A mice developed the same degree of skin inflammation including skin lesion, increased epidermal hyperplasia, increased infiltration of neutrophils, eosinophils, and mast cells into the dermis (SI Appendix, Fig. S4C) and pruritus (n = 6) (Fig. 4A). Similar to ΔNC16A mice, Rag1−/−ΔNC16A mice had significantly increased TSLP levels in the lesional skin (Fig. 4B) and a defective skin barrier (n = 6) (Fig. 4 C and D). These results suggest that adaptive immunity plays a minimal role in skin inflammation in ΔNC16A mice.
Fig. 4.
Itch in ΔNC16A mice is independent on IgE and histamine but dependent on TSLP. (A) Similar to ΔNC16A mice, Rag1−/−ΔNC16A mice exhibited similar degree of itch (A), increased TSLP in skin (B), and barrier defect determined by Evans Blue penetration assay (C) and FITC-conjugated BSA permeability assays (D) (n = 6 per group). (Scale bars: 50 μm.) (E and F) B cell-deficient ΔNC16A mice exhibited a similar degree of itch and TSLP level as ΔNC16A mice. (G) Serum histamine levels in ΔNC16A, K14Cre/ΔNC16A, and WT mice were similar. (H) ΔNC16A mice at 8 wk old were administrated orally with H1R and H4R antagonist, respectively. The blockade of H1R and/or H4R had no effect on itch. (I) The ear of skinΔNC16A mice were injected with TSLP neutralizing antibody or IgG2a control antibody (20 μg per ear). Meanwhile, 8-wk-old WT mice were injected with TSLP after injection with the same amount of TSLP-neutralizing antibody or IgG2a control antibody. The mice were videotaped in the absence of antibody and 1 h after antibody injection for 15 min. TSLP blockade significantly reduced itch in skinΔNC16A mice compared with the control antibody-treated skinΔNC16A mice. *P < 0.05, Student’s t test, n = 6 per group. Graphs A, C, E, and G–I show mean ± SE.
Pruritus in ΔNC16A Mice Is Independent on IgE and Histamine but Dependent on TSLP.
Pruritus in skin inflammation is orchestrated by the complex interplay of numerous mediators (18). A previous report showed that there is increased IgG and IgE in the circulation of BP180 dysfunctional mice, and the authors suggested that the increased IgE may be the cause of spontaneous itch and skin inflammation triggered by BP180 dysfunction (11). ΔNC16A mice exhibited significantly elevated IgE levels, but Rag1−/−ΔNC16A, which lack antibodies, and ΔNC16A mice showed similar degree of pruritus, strongly suggesting that IgE is not involved in the itch phenotype. To further support this conclusion, B cell-deficient ΔNC16A mice (Ighmtm1cgnΔNC16A) were generated and examined for itch (SI Appendix, Fig. S5). ΔNC16A mice lacking B cells, hence lacking IgE, still showed the spontaneous pruritic skin inflammation and increased level of TSLP similar to ΔNC16A and Rag1−/−ΔNC16A mice (Fig. 4 E and F).
Induction of pruritus can be generally divided into two categories: histaminergic and nonhistaminergic (15). We measured serum histamine level of ΔNC16A, K14Cre/ΔNC16A, and WT mice and found no difference among these three groups of mice (Fig. 4G). We then used antagonists of the histamine receptors H1R and H4R, two HRs expressed and identified as potential mediators of pruriproception in the skin (19). To demonstrate that the histamine response can be blocked by these HR antagonists, the 8-wk-old WT mice were injected with histamine after HR antagonist was administered directly. This administration reduced the itch, while neither separate nor concomitant blockade of H1R and H4R resulted in significant reduction of scratching in ΔNC16A mice (n = 6) (Fig. 4H). Therefore, these results suggest that itch in ΔNC16A mice is independent on IgE and histamine.
Numerous studies have reported that TSLP acts as a master switch of skin inflammation (20–22). We found that both concentration of TSLP and the number of scratches were significantly increased in skinΔNC16A (Fig. 3F) and ΔNC16A lacking both T and B cells (Rag1−/−) and B cells (Ighmtm1cgn) (Fig. 4 B and F). To determine whether TSLP is required for itch in ΔNC16A mice, first, WT mice were injected with anti-TSLP neutralizing antibody or control antibody before TSLP injection to confirm the efficiency of neutralizing antibody. Then, TSLP neutralizing antibody and control antibody were injected into the ear of skinΔNC16A mice 2 wk after tamoxifen treatment. Anti-TSLP antibody treatment reduced scratching in ΔNC16A mice compared with control antibody (n = 6) (Fig. 4I). Taken together, these results suggest that TSLP, but not IgE or histamine pathway, is the mechanism underlying severe itch in ΔNC16A mice.
ΔNC16A Promotes the Release of TSLP from Keratinocytes in Vitro and in Vivo.
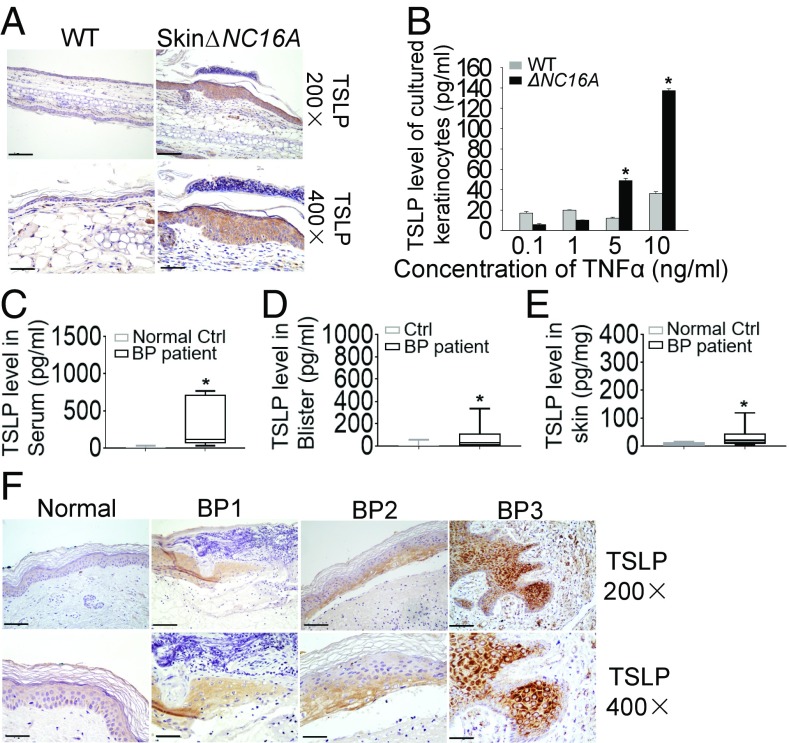
TSLP is expressed predominantly by epithelial cells in the thymus, lung, skin, intestine, and tonsils as well as stromal cells and mast cells, but is not found in most hematopoietic cell types and endothelial cells. Our data showed that dysfunction of BP180 can induce the expression of TSLP even in the absence of T cells and B cells (Fig. 4B). Increased TSLP was seen in both skinΔNC16A and K14Cre/ΔNC16A mice, suggesting that keratinocytes could be the cellular source of TSLP production. Immunohistochemical staining confirmed this is the case: TSLP was mainly expressed in epidermal keratinocytes in skinΔNC16A compared with WT (Fig. 5A). To further confirm this immune localization finding, we generated primary keratinocyte culture from WT and ΔNC16A mice and compared their capacity of producing and releasing TSLP. ΔNC16A keratinocytes stimulated with mouse TNFα showed a significantly increased TSLP level in cell culture medium compared with WT keratinocytes (n = 6) (Fig. 5B). These data supported that BP180 dysfunction promotes the release of TSLP by keratinocytes.
Fig. 5.
Increased TSLP is produced by keratinocytes in ΔNC16A mice and BP patients. (A) Compared with WT, ear skin from skinΔNC16A mice showed a higher expression of TSLP mainly in epidermis. (B) WT and ΔNC16A keratinocytes were stimulated with mouse TNFα for 24 h. ΔNC16A keratinocytes released significantly higher amount of TSLP in culture medium than WT keratinocytes (n = 6 per group). (C–E) The serum, blister fluids, and lesional skin from BP patients exhibited an increased level of TSLP compared with normal control, blister fluids from herpes zoster, and normal control, respectively (n = 12 per group). (F) The increased expression of TSLP in BP patients is mainly seen in epidermis (n = 9 per group). (Original magnifications: A, 200×; B, 400×.) (Scale bars: A, 100 μm; F, 50 μm.) *P < 0.05. Student’s t test, graph B shows mean ± SE.
BP Patients Exhibit High TSLP Expression.
To sustain our conclusions and make our animal model findings more clinically relevant, we investigated whether increased TSLP expression is associated with BP, a skin disease associated with dysfunctional BP180 caused by autoantibody insult. TSLP expression at protein level was determined in serum, blister fluid, and lesional skin of BP patients. A significantly increased expression of TSLP was seen in serum of BP patients compared with healthy control (n = 12) (Fig. 5C). BP blister fluids also had significantly higher level of TSLP than those of herpes zoster, a skin blistering disease without itch (n = 12) (Fig. 5D). In addition, a high level of TSLP was found in BP lesional skin protein extracts compared with control by ELISA (n = 12) (Fig. 5E). Like ΔNC16A mice, TSLP was detected mainly in keratinocytes especially in the lower layers of the epidermis of BP lesional skin by immunohistochemical staining, while TSLP was below detection limit in normal control skin (n = 9) (Fig. 5F). These results suggest that BP180 dysfunction in BP also leads to increased expression of TSLP.
Discussion
BP180 is well documented as a key cell–cell matrix adhesion molecule in the skin; however, other biological functions remain largely unknown (15). BP180 mutations lead to partial or complete BP180 functional loss, which cause most commonly non-Herlitz JEB (23, 24). From a study based on 43 European JEB patients with BP180 mutations, the patient group exhibited a wide clinical variability from mild to severe phenotypes (24). In general, the milder forms of JEB are associated with missense or splice-site mutations and the presence of truncated BP180 protein in skin (24). In this study, we generated a mouse model with the deletion of NC16A domain (termed ΔNC16A mice). Because our ΔNC16A mice still express a truncated form of BP180, we consider ΔNC16A mice as a BP180 dysfunctional mouse model. ΔNC16A mice developed severe spontaneous skin inflammation characterized by immune cell infiltration, increased concentration of IgE and TSLP, pruritus, and defective skin barrier. We further showed that the skin inflammation in ΔNC16A mice is caused by NC16A domain deletion in basal keratinocytes since skin- and basal keratinocyte-specific ΔNC16A mice phenocopy ΔNC16A mice. These findings suggest that BP180 also plays an important role in regulation of skin inflammation.
Dysfunction of BP180 can be caused by BP180 gene mutations in JEB or anti-BP180 autoantibody in BP (4, 6). JEB is a rare autosomal recessive disorder caused by defects in any one of six genes including BPAG2, LAMA3, LAMB3, LAMC2, ITGB4, and ITGA6, which encode the hemidesmosomal proteins BP180, laminin-332, and integrin α6β4, respectively (5, 25, 26). Patients with JEB develop subepidermal blisters within the lamina lucida of the BMZ, and itch is one of the most bothersome symptoms in JEB (10, 27). Case reports indicate that JEB patients with BP180 gene mutations show increased itch and skin inflammation (28, 29). BP is the most common skin autoimmune blistering disease characterized by inflammatory cell infiltration, anti-BP180 IgG and IgE autoantibodies, and pruritus (8). Some rare childhood BP cases shared some clinical similarity with AD (30, 31), and patients can suffer from AD and BP at the same time (30). Taken together, these clinical observations, previous animal model studies, and our BP180 dysfunctional mouse models suggest that dysfunction of BP180 can lead to skin inflammation.
Persistent pruritus is the most important symptom in skin inflammatory diseases. Some JEB patients suffer from severe itch with skin influx of proinflammatory cells (10). Transmission of pruritus can be divided into two categories: histaminergic and nonhistaminergic (15). Both histamine receptors, H1R and H4R, have been identified as potential mediators of itch in the skin (19). We rule out the histamine-dependent pathway as a cause of severe itch in ΔNC16A mice by showing that ΔNC16A mice did not have increased histamine levels (Fig. 4G), and blockade of H1R and H4R did not reduce severity of itch in ΔNC16A mice (Fig. 4H).
Recent work has highlighted TSLP in various inflammatory diseases, including skin inflammation (22). TSLP is highly expressed in acute and chronic lesions in patients with skin inflammation (32). A mutation that increases TSLP expression in the skin has direct consequences on the development of a severe atopic disease in both humans and mice (33). Interestingly, cytokines, which are commonly found at high levels in lesional skin of patients with skin inflammation, including IL-1β, TNFα, IL-4, and IL-13, can synergize to induce TSLP expression by keratinocytes (13), suggesting a positive feedback loop to amplify inflammation and itch. Significantly increased levels of TSLP (Figs. 2D, 3F, and 4 B and F), IL-1β, and IL-13 (SI Appendix, Fig. S1) were present in the skin of ΔNC16A mice, which correlated with severity of itch. More importantly, blockade of TSLP activity using TSLP neutralizing antibody reduced scratching in ΔNC16A mice (Fig. 4I). Thus, TSLP in the skin is required for severe itch in ΔNC16A mice.
TSLP is primarily expressed by epithelial cells in the gut, lungs, ocular tissue, thymus, epidermal keratinocytes in the skin, and also some other types of cells, such as mast cells, cancer cells, basophils, and dendritic cells (DCs) (20). In our study, we rule out T and B cells as a cellular source of TSLP since Rag1−/−ΔNC16A mice show no change in skin TSLP levels and itch (Fig. 4). Both whole body and basal keratinocyte-specific ΔNC16A mice show significantly increased TSLP and itch. ΔNC16A primary keratinocytes produce and release significantly more TSLP compared with normal control keratinocytes when stimulated with TNFα (Fig. 5B). These in vivo and in vitro results suggest that basal keratinocytes are the cellular origin of the increased TSLP, which is caused by BP180 dysfunction and initiates itch. However, it is still unclear how BP180 dysfunction promotes the increased expression and secretion of TSLP by keratinocytes. It is possible that some cytokines (such as TNFα and IL-1β) and/or skin infection may be a trigger to initiate TSLP expression since these proinflammatory mediators are able to induce TSLP production and secretion by keratinocytes (34). Future studies should address this important issue and identify the exact molecular interactions/pathways involved in this process.
A role for TSLP in the development of AD was hypothesized when high levels of TSLP were found in the lesional skin of AD patients (32) and in a variety of AD-like mouse models (35–37). Patients with Netherton syndrome, a severe ichthyosis in which affected individuals have a significant predisposition to AD, have increased levels of TSLP in their skin (33). However, the association of TSLP with itch in BP and JEB has not been investigated; therefore, our findings that BP patients have increased TSLP in the lesional skin and mainly express in epidermal keratinocytes represent a demonstration implicating TSLP in skin inflammation and itch in BP. Future studies should provide direct evidence whether JEB with itch are also associated with an increased TSLP level in the skin. Epithelial cells can directly communicate to cutaneous sensory neurons via TSLP to promote itch (38). However, whether TSLP effects on itch is directly through its binding to TSLPR on neurons or indirectly through other pruritic mediators in ΔNC16A mice remains to be determined.
Previously, another group also generated a BP180 dysfunction mice termed ΔNC14A mice (11). The ΔNC14A mice show phenotypes similar to ΔNC16A mice, including increased itch, eosinophil influx into skin, and increased concentration of serum IgE (11). Based on the increased IgE found in the ΔNC14A mice, the authors proposed that BP180 dysfunction may trigger autoimmunity, which leads to skin inflammation (11). Because adaptive immunity has been considered as a key factor in the development of skin inflammation (16, 17), it is possible that the components of adaptive immunity may play a role in the skin inflammation triggered by BP180 dysfunctional, especially elevated IgE. This possibility was tested by the Rag1−/−ΔNC16A and B cell-deficient ΔNC16A mice. Although both mouse strains lack IgE, they continue to scratch and develop skin inflammation as much as ΔNC16A mice (Fig. 4 and SI Appendix, Fig. S5). Therefore, we ruled out that the increased IgE or any component of adaptive immunity is the cause of severe itch in ΔNC16A mice. These findings suggest that T and B cells are not required for spontaneous skin inflammation in ΔNC16A mice, and local deficiency of BP180 function in basal keratinocytes is necessary and sufficient to drive skin inflammatory pathology.
The onset of spontaneous skin inflammation and itch is different between the whole body ΔNC16A mice (starting at 8 wk old) and skinΔNC16A mice (starting 2 wk after tamoxifen treatment). We speculate that a buildup of skin microbiota/skin infection is necessary for the BP180 dysfunction-caused skin inflammation and itch, and in the SPF environment in an animal facility, it may take 8–10 wk to build up sufficient level of skin microbiome/infection for its effects. The previously described ΔNC14A mice also start the disease at 10–12 wk old (11). This speculation is further supported by clinical practice—antibiotic treatment improves BP, AD, and JEB (39–41). Another possibility is that skin barrier impairment caused by BP180 dysfunction is age-dependent, which works alone or in concert with skin microbiota/infection for the skin inflammation and itch. Future studies should address these scenarios.
In summary, we developed a BP180 dysfunction (ΔNC16A) mouse model to investigate the role of BP180 in vivo. Our results demonstrate that BP180, a cell–cell matrix adhesion molecule of the hemidesmosome, plays an important role in regulating skin inflammation. Dysfunction of BP180 in basal keratinocytes leads to increased TSLP expression, itch, immune cell infiltration, and defective skin barrier. More importantly, BP patients also show high expression of TSLP in keratinocytes, indicating that BP and JEB patients may have a higher risk to develop skin inflammation with itch (28, 29). Our animal model could also provide a research angle for better understanding the mechanisms of BP180 dysfunction-related skin inflammatory diseases including BP and JEB. Future investigations to elucidate the regulatory pathways underlying the molecular link between BP180 and skin inflammation should help identify new therapeutic targets for skin inflammatory diseases associated with altered BP180 expression.
Materials and Methods
Details of mouse generation and human tissue collection, itch analysis, cytokine analysis and blockade, barrier function measurement, qPCR, immunohistology, cell culture, and statistical analysis are described in the SI Appendix. All of the animal experiments were approved by the local ethics committees of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. After obtaining informed consent from patients and normal healthy individuals, human samples were taken and the study was approved by the local ethics committees of the Second Affiliated Hospital, School of Medicine, Xi’an Jiaotong University.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants R01 AI40768 and R01 AR070276 (to Zhi Liu), NS079683 (to M.A.S.), DOD CA140238 (to M.A.S.), 2016LCZX-03 (to S.X.), and China Scholarship Council (to Y.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721805115/-/DCSupplemental.
References
- 1.Hammers CM, Stanley JR. Mechanisms of disease: Pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J Cell Sci. 2003;116:387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- 3.Has C, Kern JS. Collagen XVII. Dermatol Clin. 2010;28:61–66. doi: 10.1016/j.det.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bauer JW, Lanschuetzer C. Type XVII collagen gene mutations in junctional epidermolysis bullosa and prospects for gene therapy. Clin Exp Dermatol. 2003;28:53–60. doi: 10.1046/j.1365-2230.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- 5.Intong LRA, Murrell DF. Inherited epidermolysis bullosa: New diagnostic criteria and classification. Clin Dermatol. 2012;30:70–77. doi: 10.1016/j.clindermatol.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Diaz LA, Giudice GJ. Autoimmune response against the bullous pemphigoid 180 autoantigen. Dermatology. 1994;189(Suppl 1):34–37. doi: 10.1159/000246925. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun. 2008;31:331–338. doi: 10.1016/j.jaut.2008.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: Clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2018;54:26–51. doi: 10.1007/s12016-017-8633-4. [DOI] [PubMed] [Google Scholar]
- 9.Sibaud V, Roul S, Leaute-Labreze C, Memeguzi G, Taïeb A. Atopic dermatitis: Therapeutic challenge in an infant with dystrophic epidermolysis bullosa. Br J Dermatol. 2002;147:350–352. doi: 10.1046/j.1365-2133.2002.04774.x. [DOI] [PubMed] [Google Scholar]
- 10.Snauwaert JJL, et al. Burden of itch in epidermolysis bullosa. Br J Dermatol. 2014;171:73–78. doi: 10.1111/bjd.12885. [DOI] [PubMed] [Google Scholar]
- 11.Hurskainen T, et al. Deletion of the major bullous pemphigoid epitope region of collagen XVII induces blistering, autoimmunization, and itching in mice. J Invest Dermatol. 2015;135:1303–1310. doi: 10.1038/jid.2014.443. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boguniewicz M, Leung DY. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharmage SC, et al. Atopic dermatitis and the atopic march revisited. Allergy. 2014;69:17–27. doi: 10.1111/all.12268. [DOI] [PubMed] [Google Scholar]
- 15.Papoiu ADP, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 17.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 18.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: The molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossbach K, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–852. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He R, et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52:211–223. doi: 10.1007/s12026-012-8264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine J-D. Inherited epidermolysis bullosa. Orphanet J Rare Dis. 2010;5:12. doi: 10.1186/1750-1172-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiritsi D, et al. Molecular mechanisms of phenotypic variability in junctional epidermolysis bullosa. J Med Genet. 2011;48:450–457. doi: 10.1136/jmg.2010.086751. [DOI] [PubMed] [Google Scholar]
- 25.Ng Y-Z, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522–3534. doi: 10.1158/0008-5472.CAN-11-2996. [DOI] [PubMed] [Google Scholar]
- 26.Sawamura D, Nakano H, Matsuzaki Y. Overview of epidermolysis bullosa. J Dermatol. 2010;37:214–219. doi: 10.1111/j.1346-8138.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohn HI, Teng JMC. Advancement in management of epidermolysis bullosa. Curr Opin Pediatr. 2016;28:507–516. doi: 10.1097/MOP.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 28.Mabuchi E, et al. Oral steroid improves bullous pemphigoid-like clinical manifestations in non-Herlitz junctional epidermolysis bullosa with COL17A1 mutation. Br J Dermatol. 2007;157:596–598. doi: 10.1111/j.1365-2133.2007.08046.x. [DOI] [PubMed] [Google Scholar]
- 29.Cifuentes L, et al. A case of junctional epidermolysis bullosa with prurigo-like lesions and reduction of collagen XVII and filaggrin. Br J Dermatol. 2013;169:195–198. doi: 10.1111/bjd.12241. [DOI] [PubMed] [Google Scholar]
- 30.Kamiya K, Aoyama Y, Nishio E, Horio A, Tokura Y. Management of erythematous skin lesions in bullous pemphigoid associated with atopic dermatitis. J Dermatol. 2016;43:1102–1103. doi: 10.1111/1346-8138.13330. [DOI] [PubMed] [Google Scholar]
- 31.Kuenzli S, et al. Childhood bullous pemphigoid: Report of a case with life-threatening course during homeopathy treatment. Pediatr Dermatol. 2004;21:160–163. doi: 10.1111/j.0736-8046.2004.21215.x. [DOI] [PubMed] [Google Scholar]
- 32.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 33.Briot A, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumari V, Babina M, Hazzan T, Worm M. Thymic stromal lymphopoietin induction by skin irritation is independent of tumour necrosis factor-α, but supported by interleukin-1. Br J Dermatol. 2015;172:951–960. doi: 10.1111/bjd.13465. [DOI] [PubMed] [Google Scholar]
- 35.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, et al. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci USA. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson SR, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalmers JR, et al. BLISTER study group A randomized controlled trial to compare the safety and effectiveness of doxycycline (200 mg daily) with oral prednisolone (0.5 mg kg(-1) daily) for initial treatment of bullous pemphigoid: A protocol for the bullous pemphigoid steroids and tetracyclines (BLISTER) trial. Br J Dermatol. 2015;173:227–234. doi: 10.1111/bjd.13729. [DOI] [PubMed] [Google Scholar]
- 40.Lakhani F, Lee K, Lio PA. Case series study of the efficacy of compounded antibacterial, steroid, and moisturizer in atopic dermatitis. Pediatr Dermatol. 2017;34:322–325. doi: 10.1111/pde.13141. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein AM, Davenport T, Sheridan RL. Junctional epidermolysis bullosa: Diagnosis and management of a patient with the Herlitz variant. J Pediatr Surg. 1998;33:756–758. doi: 10.1016/s0022-3468(98)90210-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.