Significance
One of the most fascinating questions in psychology and neuroscience pertains to how young children gain the capacity to remember their past. Early hippocampal processes have been implicated in this ability, but a lack of viable methods has hindered assessments of their contribution in early human development. We employed a functional magnetic resonance imaging paradigm that captures memory-related hippocampal function during natural nocturnal sleep in toddlers. Our results provide direct evidence of a connection between hippocampal function and early memory ability. This experimental approach overcomes previous challenges and promises to pave the way to investigations linking changes in brain function to early development of learning mechanisms, including applications to typical and atypical development.
Keywords: hippocampal development, episodic memory, early childhood development, fMRI
Abstract
Nonhuman research has implicated developmental processes within the hippocampus in the emergence and early development of episodic memory, but methodological challenges have hindered assessments of this possibility in humans. Here, we delivered a previously learned song and a novel song to 2-year-old toddlers during natural nocturnal sleep and, using functional magnetic resonance imaging, found that hippocampal activation was stronger for the learned song compared with the novel song. This was true regardless of whether the song was presented intact or backwards. Toddlers who remembered where and in the presence of which toy character they heard the song exhibited stronger hippocampal activation for the song. The results establish that hippocampal activation in toddlers reflects past experiences, persists despite some alteration of the stimulus, and is associated with behavior. This research sheds light on early hippocampal and memory functioning and offers an approach to interrogate the neural substrates of early memory.
Episodic memory (i.e., the ability to remember past events along with elements of the spatial and temporal context in which they occurred; ref. 1) is critical for the human experience. It is fundamental to recall one’s unique past, identify what is common across events, and make predictions about what the future might bring (1, 2). This capacity emerges in infancy and undergoes substantial improvement in the first 2 or 3 years of life (3, 4). Infants often encounter difficulty at recognizing past events if some of their elements are modified, but they overcome some of these difficulties by the end of the second year of life (5, 6). This improvement affords the adaptive capacity to use memories across a broader range of situations. Nonhuman animal models have implicated postnatal maturation processes in the hippocampus (cf. refs. 7 and 8), such as the full integration of the dentate gyrus and CA3 within the hippocampal circuitry, in the development of increasingly precise and flexible episodic memories (9–11). Initial evidence from human toddlers reveals volumetric changes in the hippocampus in the first few years of life (12), which might coincide with the timing of early development of episodic memory.
However, direct assessment of hippocampal function and its contribution to early episodic memory in human infants has proved challenging. Neuroscientific methods used with infants and young children, such as event-related potentials, are better suited for the examination of cortical activity. Functional magnetic resonance imaging (fMRI) techniques are robust to detecting subcortical activity but present methodological difficulties with populations that cannot endure the demands of completing behavioral tasks within an MRI environment during wakefulness. Here, we developed an fMRI paradigm that overcomes these challenges to examine memory-related hippocampal function in the toddler years.
Our primary goal was to determine whether we could detect hippocampal activation in response to a previous experience in the toddler brain during natural nocturnal sleep. We tagged a laboratory experience with a song (i.e., the target song), a stimulus that can be delivered during sleep. Songs can be learned and carry social meaning even in young infants (13), and comparative studies have demonstrated neuronal replay of song memories during sleep (14, 15). Moreover, presentation of past experiences during sleep reactivates neural correlates of episodic memories (16, 17) and studies of sleeping infants have documented the processing of sensory properties of linguistic stimuli (18). Altogether, this work provides reassurance of the viability of our approach to yield the only available evidence of experimentally induced hippocampal activation in the toddler years.
We assessed the extent to which the hippocampus was successfully activated by the target song, which directly captured toddlers’ previous experience, and a reversed song, which corresponded to the target song played backward and thus maintained most of its features (e.g., voice, rhythm, tempo, and key) but modified its melodic pattern. Behavioral research has indicated that adults flexibly recognize learned songs in the face of some change in melody (19), suggesting that both types of stimuli successfully reinstate previous experiences. However, hippocampal activation in adults discriminates among similar memories; for example, adults show greater hippocampal activation when they remember events accurately with all of the details compared with when some details are incorrectly recalled (20, 21). In contrast, children as old as 8–10 years of age show less—if any—differentiated hippocampal activation between items that are remembered with and without accurate details (22). Thus, we compared hippocampal activation in response to the target and reversed songs to that associated with a completely novel song, and expected to identify significant clusters in the hippocampus, reflecting a general response to a past experience. We reasoned that the examination of both target and reversed songs, compared with the novel song, would provide the best chance of achieving our primary goal to detect hippocampal activation during sleep.
Nonetheless, we also conducted additional analyses to establish whether there was any difference between the target and reversed songs within clusters showing this general memory response. On the one hand, the target song may drive the general memory hippocampal response, given evidence in children of memory facilitations and stronger hippocampal activation under conditions that fully reinstate past experiences (23, 24). However, the hippocampus is also active—and at times more—when adults are presented with altered details of experienced episodes that violate expectations, such as when the end of learned stimulus sequences is altered (25). Activation in the toddler’s hippocampus consistent with this pattern would suggest that hippocampal processes involved in comparing experiences are functional in early childhood.
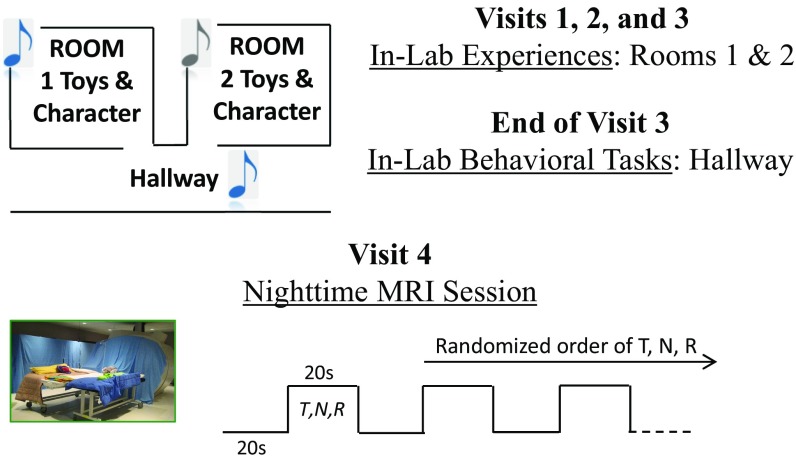
We report data from 22 typically developing toddlers [mean (M), 28.9 mo; SD, 2.06 mo; range, 25–32.5 mo; 11 females]. Toddlers took part in three laboratory sessions and one nighttime MRI session. During visits 1, 2, and 3, occurring over about 1 week (M, 7.32 d; SD, 2.92 d), toddlers participated in two play sessions, each involving distinct games with a unique toy character (e.g., a stuffed dog) in a specific room, while a unique song played in the background. At the end of the third laboratory session, one of the two songs (i.e., the target song) was played in a third environment and toddlers were asked to indicate in which room they had heard the song and with which toy character they played. Within 2 days of visit 3 (M, 1.55 d; SD, 1.10 d), we assessed hippocampal activation in nonsedated sleeping toddlers in response to the target song, reversed song, and a completely novel song, which served the function of the distractor item (see Fig. 1 for a schematic of the experimental design).
Fig. 1.
During three in-laboratory visits (mean days between visits 1 and 3, 7.32), children played with unique toys, including a toy character, in each of two separate rooms while they listened to two distinct songs in the background (the target song in one room and the nontarget song in the other). At the end of visit 3, memory for the association between the target song and the room, as well as the target song and toy character, was assessed in an adjacent hallway. A nighttime MRI session occurred during visit 4 (on average, 1.55 d after visit 3), during which a functional scan delivered nine active, randomized 20-s song blocks of target (T), novel (N), and reversed (R) songs, alternated with blocks of silence. We counterbalanced between subjects whether the first block was a T, N, or R block.
Our primary goal was to determine whether the hippocampus was active when processing aspects associated with a past experience. To do so, we used a general memory contrast [(target + reversed) > novel] intended to capture hippocampal representations of either exact (i.e., target) or altered (i.e., reversed) characteristics of a past episode. Because we were interested in assessing hippocampal function, all analyses were constrained to the hippocampus using left and right hippocampal masks obtained from an age-appropriate template (26). We expected overall activation of the hippocampus for the target and reversed songs, compared with activation for the novel song, thus providing evidence of memory-related activation in the hippocampus. We also examined whether, within the cluster of hippocampal activation, the exact reinstatement or the inclusion of mismatching elements would result in stronger activation.
Our secondary goal was to determine whether hippocampal activation was associated with previously assessed overt recall of a past experience. Thus, we could establish whether toddlers formed memory associations between a unique song and the unique location in which they experienced it. The hippocampus is critical for remembering these types of associations (27), and we sought to use a measure that was distinctly hippocampal in nature. We predicted that the degree of activation in the target > novel contrast would be associated more strongly with previously assessed overt recall of where toddlers heard the song. In addition, toddlers reported with which toy character they played when they listened to the song. We further predicted that toddlers who were able to overtly remember more specific details (i.e., the location and the toy character) would exhibit greater activation in the target > novel contrast, putatively reflecting activation of specific memory representations triggered by the target song.
Results
The Hippocampus Is Engaged by Presentation of Past Experiences During Sleep.
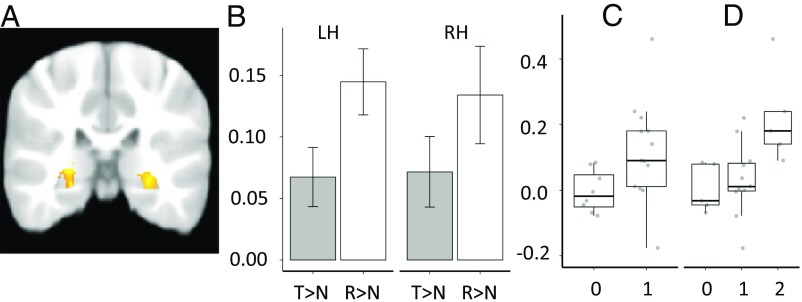
Using left and right hippocampal masks obtained from an age-appropriate template (26), we assessed the most general contrast corresponding to activation for past experiences [(target + reversed) > novel, Z = 2.30, cluster corrected; P < 0.025)], which yielded bilateral hippocampal clusters (Fig. 2A). The opposite contrast, novel > (target + reversed), did not yield any significant clusters. We then extracted mean parameter estimates separately for target > novel and reversed > novel contrasts from these clusters. We chose to examine parameter estimates separately for these contrasts to determine the differential contribution of the target and reversed song to the general memory activation. Thus, while these assessments are nonindependent, they allowed us to obtain a more nuanced view of the conditions triggering memory-related activation.
Fig. 2.
Group analyses were constrained to the left (LH) and right hippocampus (RH) using age-appropriate hippocampal templates and a cluster thresholding process (z = 2.30, cluster corrected, P < 0.025). (A) These analyses isolated active bilateral clusters for the memory (target + reversed) > novel contrast (left, max: x = −26, y = −33, z = 1; 1,839 voxels; right, max: x = 23, y = −27, z = 1; 1,107 voxels). (B) Mean parameter estimates (PEs) (y axis) were extracted from these active clusters separately for target > novel (T > N) and reversed > novel (R > N) contrasts (x axis). Activation for R > N was significantly stronger than T > N [F(1,21) = 6.88, P = 0.016, η2p = 0.25] [LH, t(21) = 3.21, P = 0.004; RH, t(21) = 1.81, P = 0.085]. (C and D) The relation between PE (y axis) for T > N in the RH and the number of correct items in our two behavioral measures (x axis), room choice (C) [r = 0.43, t(19) = 2.10, P = 0.049, d = 0.77] and the composite memory measure (D) (ρ = 0.60, P = 0.004) was significant.
A repeated-measures ANOVA to assess the effect of contrast (target > novel vs. reversed > novel) and laterality (right vs. left hippocampus) on hippocampal activation yielded a main effect of contrast [F(1,21) = 6.88, P = 0.016, η2p = 0.25], but no main effect of laterality or interaction between these two factors [values of F(1,21) < 0.33, values of P > 0.571, η2p < 0.02] (Fig. 2B). Specifically, the reversed > novel contrast yielded stronger hippocampal activation (M, 0.14; SD, 0.16) than the target > novel contrast (M, 0.07; SD, 0.14) across the two hemispheres. Each of the four contrasts entered in the ANOVA was reliably different from zero [left hippocampus: target > novel: t(21) = 2.81, P = 0.011; reversed > novel: t(21) = 5.38, P < 0.001; right hippocampus, target > novel: t(21) = 2.50, P = 0.021; reversed > novel: t(21) = 3.83, P = 0.003]. Thus, reliable activation clusters were observed bilaterally in the hippocampus during processing of past experiences, with stronger activation for reversed songs, providing direct evidence of functional engagement of this structure in response to stimuli that capture elements of an experience.
Overt Memory for an Experience Is Associated with Hippocampal Activation.
We first examined toddlers’ performance in the room choice task because the hippocampus is strongly implicated in memory for location (27, 28). In the room choice task, children were asked to select in which room they experienced the target song. We found that 62% of toddlers selected the correct room in which they had heard the target song. A binomial test revealed that, as a group, children were no different from chance in selecting the correct room (P = 0.38). A point-biserial correlation revealed that activation for target > novel in the right hippocampus was significantly correlated with correct identification of the room in which the target song was experienced [r = 0.43, t(19) = 2.10, P = 0.049, d = 0.77] (Fig. 2C). The correlation between activation in the reversed > novel contrast in the right hippocampus and room choice accuracy was not significant [r = 0.23, t(19) = 1.01, P = 0.325] nor were any correlations in the left hippocampus with the corresponding target > novel [r = 0.18, t(19) = 0.81, P = 0.426] and reversed > novel [r = 0.16, t(19) = 0.69, P = 0.501] contrasts (SI Appendix, Fig. S1). Thus, the correlation with behavior was only significant for the target > novel contrast, although its magnitude was not statistically different from that observed in the other contrasts (values of Fisher’s z < 1.51, values of P ≥ 0.06; one-tailed).
The second overt memory task was the toy character choice task, wherein children were asked to select the toy character associated with the target song from three possible toy characters. We found that 40% of the toddlers identified the correct toy character; a binomial test found that this performance was also not significantly different from chance (P = 0.636). We used this measure to obtain a more comprehensive assessment of memory performance; thus, we computed a composite memory score by summing the location and character measures for a range of scores between 0 and 2 (M, 0.95; SD, 0.72). We took advantage of individual variability because toddlers were at chance as a group. The correlation between this composite score and the target > novel contrast in the right hippocampus was significant (ρ = 0.60, P = 0.004) (Fig. 2D). This correlation was stronger than that observed for reversed > novel in the right hippocampus [ρ = 0.15, P = 0.522 (z = 2.33, P = 0.009, one-tailed)], target > novel in the left hippocampus [ρ = 0.36, P = 0.10 (z = 1.60, P = 0.05, one-tailed)], and reversed > novel in the left hippocampus [ρ = 0.18, P = 0.429 (z = 2.02, P = 0.02, one-tailed)] (SI Appendix, Fig. S2).
These results suggest that the correlation with memory for our comprehensive memory measure may be specific to the target > novel contrast in the right hippocampus. To constrain our interpretation, we tested for the specificity of hippocampal activation across all individual factors assessed in this study. We conducted an omnibus mixed ANOVA including the effect of contrast (target > novel vs. reversed > novel), laterality (right vs. left hippocampus), room choice (correct vs. incorrect), and toy character choice accuracy (correct vs. incorrect) on hippocampal activation; the first two factors were varied within participants and the second two were varied between participants. This analysis yielded a significant interaction between room choice accuracy and laterality [F(1,17) = 4.86, P = 0.042, η2p = 0.22] such that toddlers who selected the correct room recruited the right hippocampus more strongly (M, 0.14; SE, 0.04) than those who did not (M, 0.04; SE, 0.02); the same difference was not evident in the left hippocampus (correct location: M, 0.11, SE, 0.03; incorrect location: M, 0.07, SE, 0.02). This interaction was further qualified by a three-way interaction between room choice accuracy, toy character choice accuracy, and laterality [F(1,17) = 4.47, P = 0.049, η2p = 0.21]. Toddlers who selected both the correct room and toy character recruited the right hippocampus more strongly (M, 0.23; SE, 0.04) than those who only remembered the correct location (M, 0.08; SE, 0.03), only remembered the correct toy character (M, −0.01; SE, 0.01), or remembered none of the details (M, 0.06; SE, 0.03) (values of P < 0.027). Again, these effects were not reliable in the left hippocampus (both, M, 0.16, and SE, 0.03; only correct location, M, 0.08, and SE, 0.02; only correct toy character, M, 0.09, and SE, 0.01; none of the details, M, 0.06, and SE, 0.02) (values of P > 0.050). The main effect of type of contrast was marginally significant in this omnibus analysis [F(1,17) = 4.23, P = 0.055, η2p = 0.20] and did not interact with any of the other factors. Overall, these analyses lend support to the prediction that overt memory performance is associated with hippocampal activation assessed a few days later. Although we leveraged individual differences in memory to examine associations with hippocampal activation, we also conducted additional analyses to exclude the possibility that the results are driven by overall differences between toddlers who could or could not engage in the task (SI Appendix).
Discussion
The present study was designed to assess whether it was possible to induce activation of the hippocampus in response to a previous experience in early childhood during natural nocturnal sleep. To date, neuroimaging research examining the hippocampus with infants and toddlers has been limited to assessments of volume or resting-state connectivity (12, 29, 30), which do not provide insight into task-specific functional properties. In a sample of 2-year-old children, we used an fMRI paradigm and observed hippocampal activation in response to previously learned stimuli.
We predicted that a general contrast including a target and reversed song would reveal hippocampal activation because children exhibit less differentiated hippocampal activation for similar memories (e.g., event memories with some accurate or inaccurate additional detail) compared with adults (22). Our results indicate that the hippocampus is recruited bilaterally upon presentation of the target and reversed songs, suggesting a role of this structure in memory reinstatement at this point in development. These results counter the possibility that early memory for specific events might be supported exclusively by cortical mechanisms in the medial temporal lobes (31).
We also sought to differentiate hippocampal activation for target and reversed songs within the activated general memory clusters. We found that the reversed song resulted in stronger activation compared with the target song. This heightened hippocampal activation to the reversed song may reflect additional processing beyond that associated with reinstatement of the experience when hearing the target song. Previous research showed that the hippocampus plays a significant role in detecting novelty within a familiar environment, and this might be particularly true when violations of predicted associations occur; the hippocampus has been found to respond to these situations through a match–mismatch mechanism implemented in the CA1 subfield (32). This is an early developing component of hippocampal circuitry based on nonhuman evidence (7). The ability to recognize past events despite superficial changes is important to identify commonalities across experiences and support generalization. Furthermore, the ability to identify novel information in the context of something learned may be particularly critical when children are tasked with building a body of knowledge for the first time (24). Thus, the combined reinstatement and novelty processing in the reversed condition in comparison with the reinstatement processing in the target condition may have contributed to the heightened hippocampal activation to this contrast.
Recent research has begun to underscore powerful dynamics during sleep-related memory reactivation. For example, a recent study showed that auditory information delivered after a verbal cue for a learned association disrupts neurophysiological reactivation and stabilization processes triggered by the cue (33); this was true regardless of the content of this information (i.e., consistent or inconsistent with the original memory). While the reversed condition in the present study is markedly different (e.g., it did not solely follow the target song but was presented in alternating blocks with the target and novel song), this work raises the question of whether the inclusion of altered stimuli during sleep disrupts memory stabilization in service of future performance. Our study cannot speak to this since memory testing preceded the neuroimaging session, but future research would benefit from testing this possibility directly.
We found evidence consistent with our prediction that hippocampal activation would be associated with previously assessed overt memory. We found significant correlations between both our overt memory for location and comprehensive memory measure (i.e., the summed score of accurate location and toy character choice) and later activation for the target song in the right hippocampus. The correlation with our comprehensive memory measure was statistically different from the correlations observed with the other contrasts, suggesting that activation during the target song, specifically in the right hippocampus, may reflect reactivation of the original memory representation (cf. ref. 34). However, when we examined the effects of all individual variables assessed in this study concurrently in an omnibus ANOVA, only the interactive effect between behavior and laterality persisted: toddlers who remembered all details—compared with those who remembered either the room, the toy character, or neither— recruited the right hippocampus more strongly overall. Thus, early memory of an experience is associated with hippocampal activation resulting from exposure to elements of the same experience. Previous research showed that hippocampal involvement is coupled with cortical systems to reactivate memory representations during wake (35); the examination of this coupled activation falls beyond the scope of the present study but should be investigated in future research.
We did not predict differences based on laterality. Although there is evidence of strong lateralization during infancy in cortical regions involved in other functions (e.g., left lateralization in speech perception; ref. 17), questions of laterality in hippocampal function are debated (28), including the possibility that the right hippocampus may be functional earlier than the left hippocampus because of the importance of visuospatial information during infancy and early childhood (36). Direct manipulations are required to examine this question further. Along the same vein, future research should also examine whether activation changes along the anterior–posterior axis of the structure as prior work has identified this as an important dimension to understand development (29, 37).
We recognize that we used a limited memory assessment. We chose few memory tasks because our primary goal was to first establish that hippocampal activation could be induced and detected at all during toddlers’ sleep. To this end, we succeeded. Our tasks were challenging in that they required overt recall of the arbitrary association between the song and where and with what toy character it had been learned. We took advantage of individual variability because the sample was overall at chance in these tasks and found meaningful associations with activation across the entire sample. Moreover, when we excluded children who were unable to remember either the room or character detail, the association between memory and right hippocampal activation persisted (SI Appendix). Thus, the relations between memory and activation in the right hippocampus cannot be explained by differences between general ability in engaging in the task and hippocampal activation. Nevertheless, we recognize that the examination of other forms of memory that include match–mismatch detection, recall of additional types of content (e.g., temporal, spatial, verbal), and the use of tasks that yield stronger levels of performance at the group level would provide a more comprehensive understanding of hippocampal function in early childhood.
The songs delivered during the play sessions in the laboratory were played in the background and did not correspond to the content of the central activities in the play session. Children were therefore involved in three play sessions to provide sufficient exposure to the songs and the associated experiences. This is a common approach in early memory research. Repetition may affect hippocampal activation as it does behavioral retention (38). Furthermore, repetition may engage more strongly the CA1 subfield. The CA1 subfield is implicated in slower learning after repeated exposure and its function emerges earlier than that of the CA3 and dentate gyrus regions, which are implicated in more flexible, single-trial learning (7). Thus, associations between the hippocampal activation in the target condition and overt memory could reflect a dominance of CA1-supported processes (39–41). However, we cannot draw conclusions from the present results about hippocampal function after a single exposure or about the contribution of specific subregions to the associations reported.
We did not definitively verify toddlers’ sleep stage during the scan because we did not obtain polysomnographic data. We chose not to acquire EEG data to increase the likelihood that children would stay asleep. Furthermore, we collected data during a time window when children in this age range are likely to be in stage 3 non–rapid-eye movement sleep (42).
In the present study, we focused on hippocampal function given that current accounts of early memory underscore the role of this structure (6), but its importance does not preclude the additional contribution of cortical mechanisms (refs. 11, 29, and 31; SI Appendix, Fig. S3). For example, prior work has suggested that hippocampal replay of past experiences during sleep may be triggered by cortical activity patterns in the auditory cortex (43).
The delivery of songs during sleep as used in this study to activate the hippocampus affords opportunities to assess memory-related brain function, and critically, to study the neural representations that support development in infancy, childhood, and other populations that are not traditionally amenable to fMRI. The methodology introduced in this paper allows researchers to address questions about the neural specificity of early behavior, how changes in neural representations impact developmental trajectories, and how these changes might reflect typical vs. atypical development. The present study, thus, offers an initial glimpse at how the hippocampus supports memory in early life and sets the foundation for further explorations of the neural representations that support early development.
Methods
Participants.
Functional imaging data were collected from 22 typically developing children (M, 28.9 mo; SD, 2.06 mo; range, 25–32.5 mo; 11 females). Behavioral data were collected from an additional 11 children, but they were excluded from this analysis because they were unable to fall (n = 9) or stay (n = 2) asleep during the nighttime MRI portion of the study. Based on parental reports of productive vocabulary using the MacArthur–Bates Communicative Development Inventory (https://mb-cdi.stanford.edu/) (one parent did not complete the form), these children (M, 34.27; SD, 26.77) did not differ diagnostically from our experimental sample (M, 40.52; SD, 28.24) [t(30) = 34.27, P = 0.550]. Data from another two participants were excluded due to computer error during the MRI session. Recruitment was restricted to toddlers whose parents reported no history of neurodevelopmental disorder or prematurity and who were able to speak English fluently. All families were recruited from the greater Sacramento region. Families were given introductory materials detailing their participation in the behavioral and neuroimaging visits. Researchers answered all questions and concerns before parents were provided consent forms. Parents were asked to sign consent forms for both the behavioral and neuroimaging portions of their participation. Toddlers provided verbal assent. All participants received a book at the end of each laboratory visit and $50 after the nighttime MRI visit. Finally, all procedures described below were approved by the University of California, Davis, Institutional Review Board Administration.
Materials.
For each participant, three songs were selected from a library of four African lullabies unknown to the participants. The songs differed from one another in terms of the combination of their rhythm, tempo, and key (SI Appendix, Table S1). Twenty-second clips were created using Audacity’s music-editing software (www.audacityteam.org). Two songs were heard during the three in-laboratory visits (the target and nontarget song), and one song was heard only during the MRI visit (the novel song). Song selection and condition assignment were counterbalanced between subjects. In the laboratory, the song stimuli were delivered using a portable wireless speaker. During the neuroimaging session, the song stimuli were presented using Neurobs’ Presentation stimulus delivery software (www.neurobs.com/), and the sound was delivered using the MR Confon auditory delivery system (www.crsltd.com/tools-for-functional-imaging/audio-for-fmri/mr-confon/).
Procedure.
Participation involved three laboratory visits and one nighttime visit to the MRI facility.
Laboratory visits.
The three in-laboratory visits were, on average, spread out over 1 week (M, 7.32 d; SD, 2.92 d; max, 14 d). At each visit, children were exposed to two songs. Each of the songs was played in one specific laboratory room while toddlers were involved in game activities during which they interacted with a unique toy character and played with at least eight distinct objects (e.g., a toy car, a crayon, and a yo-yo). Children were shown a distinct and simple action for each object by the experimenter (e.g., move the car) and joined the experimenter with completing each action. Each action was brief and simple, and toddlers spent ∼20 s with each object (cf. refs. 44 and 45). This procedure was used to make sure that the play session was consistent across participants and as unencumbered as possible with distractions. We reasoned that this procedure would facilitate children’s formation of memory associations between the song that was playing in the background and the room. Notably, the toy character (e.g., a doll or a bear) was present throughout the whole session and children could engage with the toy character along with each object. During the third and last laboratory session, after experiencing the song and activities in each room, children’s memory for the association between song and room was assessed. The target song (to be tested during the fMRI session) was played while children were in front of the two unmarked closed doors that led to the two rooms in which songs and activities were experienced; toddlers were asked to indicate (by saying or pointing) where they had heard the song. The second song (i.e., the nontarget song) was not tested, but its inclusion in the sessions prevented toddlers from simply forming an association between hearing a song and a room (as opposed to that particular song). The assignment of song to game, game to room, and the order in which children played them were counterbalanced between participants. In addition to responding to this room choice question, toddlers were also asked to indicate which of three toy characters (the one associated with target song, the one associated with the nontarget song, and a toy character never met in either room) they played with while listening to the song. One toddler responded by identifying the correct toy character after the presentation of two characters (i.e., associated with the target and nontarget song), instead of three characters.
In preparation for the neuroimaging session, parents were sent home with a practice kit that consisted of earplugs and headphones, as well as information about the online location of audio files of sample scanner sounds. Parents were instructed to insert the earplugs and have children wear headphones while listening to the scanner sounds for approximately 1 hour at the beginning of each night for 1 week before the scan. This allowed parents and children to become familiar with the MR environment. Experimenters were in frequent contact with parents during this time to assess their preparation and determine a plan of action specific to the child’s needs for the neuroimaging session.
Neuroimaging session.
Nonsedated toddlers underwent their neuroimaging session during nocturnal sleep a few days after the last laboratory visit (M, 1.55 d; SD, 1.10 d; max, 4 d) (cf. refs. 17, 18, and 42). One participant was unable to complete this session within this time due to unexpected family reasons and completed it 11 days following his third laboratory visit. To reinstate memory for the target song and associated experience, this participant was given a fourth laboratory session that was identical to the first three and was followed by the fMRI 2 days later. However, memory for song–location and song–toy character associations were only assessed during the third visit as for all other participants. During the night visit, families arrived between 7 PM and 10 PM depending on their toddler’s sleeping schedule and the session lasted approximately 2 hours. Five toddlers fell asleep on the way to the Imaging Research Center, while others fell asleep after arrival at the center. In the latter case, children were able to fall asleep either in a separate room outfitted for the occasion with mattress, night lamp, etc., or on the scanner bed itself. The scanner bed was outfitted with a memory foam mattress and pillows, as well as several blankets and stuffed animals for the child’s comfort. Parents could bring comfort items for their child, which were properly screened for safety ahead of time. Earplugs and headphones were placed on the child for sound protection and auditory stimulus delivery ∼10–15 min after they had fallen asleep on the scanner bed. We confirmed children were asleep if they had been lying on their back with earplugs and headphones on the scanner bed and had not moved for 10 min. At this point, the parent and experimenter would attempt to move the child’s arm to determine whether they would wake up with the movement. If the child continued to sleep through this disruption, the scan proceeded. Using this procedure, the scan generally began at least 20 min after sleep onset and was completed within the first hour and a half after sleep onset to increase our chances that children were in slow-wave sleep during the functional scans (16, 17). Although the examination of the effects of slow-wave sleep falls beyond the scope of the current research, it was critical to maximize our chances of observing reliable hippocampal activation. A parent and a researcher were present inside the scanner room within arm’s reach of the child for the duration of the scan. Lights in the back of the scanner allowed the parent and experimenter to have full view of the child’s face and body, and monitor for signs of waking up (e.g., any body, face, or eye movements). When the child showed such signs, it was determined that the child was (or close to being) awake and the experimenter stopped the scan (n = 9). We are confident that all children in this sample remained asleep during the scan.
fMRI acquisition and design.
Images were acquired using a 3-T Siemens TIM Trio MRI System at the University of California, Davis, Imaging Research Center using an eight-channel coil. This coil accommodates the auditory stimuli delivery and noise-dampening materials, while maintaining comfort within it. Functional images were acquired using a gradient echo-planar imaging pulse sequence [repetition time (TR), 1,500 ms; echo time (TE), 24 ms; field of view (FOV), 216 mm; number of slices, 46; voxel size, 3 mm isotropic; 248 volumes acquired]. During the functional scan, children heard three 20-s blocks of three different songs: the target song heard during laboratory visits (T), a novel song to the child (N), and the target song played in reverse (R). Twenty-second blocks of silence separated the song blocks. Altogether, there were nine song blocks, three blocks of each song, interspersed by silence, with each 20-s song block modeled as a distinct T, R, or N event block. We counterbalanced between subjects whether the first block was a T, R, or N block and used a randomized sequence to present the order of T, R, and N blocks for the remaining eight blocks for each participant (Fig. 1 provides a depiction of this model). A T1-weighted high-resolution magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan in the sagittal plane was also obtained (TR, 2,500 ms; TE, 3.23 ms; FOV, 226 mm; voxel size, 0.70 mm isotropic). Overall, the MPRAGE and functional scan took 20 min to complete.
Statistical analysis.
Data were preprocessed and analyzed using FEAT in FSL5.0.8 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Preprocessing included slice timing and motion correction, as well as spatial smoothing using a 6-mm FWHM isotropic Gaussian kernel and was coregistered to the MPRAGE. FSL’s motion outliers function (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers) identified motion outliers by measuring relative intensity differences and defining outliers using the upper threshold for creating boxplots. We planned to exclude scans with more than 25% of the volumes with motion outliers as defined by this function. We found a negligible number of outlier volumes (M, 2% of total volumes or 4.82 outlier volumes; range, 0–15 of 248 volumes) and, as such, did not exclude any scans from our analyses based on motion.
However, to ensure that our results were not affected by absolute motion not fully captured by the FSL motion outlier function, we compared motion volume-by-volume to a fixed frame displacement threshold (0.50 mm). The mean absolute frame displacement for a scan across our sample was 0.17 mm (SD, 0.14) with a range of 0.06–0.69 mm across all scans. This resulted in an average of 5% (SD, 9%) of the volumes (SD, 22 volumes; range, 0–29%, or 0–72 of 248 volumes) exceeding a fixed threshold of 0.50 mm. Correlational analyses found that the mean proportion of volumes above this fixed threshold did not correlate with either of our behavioral measures, room choice performance [point-biserial r = −0.04, t(19) = 0.17, P = 0.867] or our composite memory measure (ρ = 0.17, P = 0.463). They also were not related with activation levels in any of the contrasts [−0.27 < values of r < −0.07, values of t(20) < 0.77, values of P > 0.223]. We found the same results when comparing activation levels and behavioral results with the mean absolute frame displacement of each participant’s scan. This measure of motion was not correlated with room choice accuracy [point-biserial r = 0.05, t(19) = 0.22, P = 0.828] nor with our combined memory measure (ρ = 0.19, P = 0.308). It was also not correlated with activation levels in any of the contrasts [−0.24 < values of r < 0.09, values of t(20) < 1.10, values of P > 0.280]. Thus, the motion that was apparent did not influence either behavioral or hippocampal results. Functional and structural data were all registered to a standard toddler template created by the University of North Carolina (26) from a sample of 95 toddlers (M, 33.65 mo) (bric.unc.edu/ideagroup/free-softwares/). Each registration was visually inspected by investigators to ensure the registration procedure was successful. We confirmed that all were successfully standardized to the toddler template.
General linear models were conducted using FEAT in FSL to model target, novel, and reverse song types during the time series and were convolved with a double-gamma hemodynamic response function to yield a general contrast corresponding to activation for past experiences: (target + reversed) > novel. Motion parameters were incorporated into the model to regress out effects of movement. Our hypotheses centered on hippocampal function, and thus we restricted our analyses to left and right hippocampal masks, which were obtained from parcellation maps provided by the University of North Carolina baby template. Significant clusters of activation for the (target + reversed) > novel contrast were identified using a cluster threshold of z > 2.30 and corrected for multiple comparisons using a P threshold of P < 0.025 within the left and right hippocampus mask. A P value of 0.025, instead of P < 0.05, was used for multiple comparisons since analyses were conducted separately for the left and right hippocampus. Parameter estimates were extracted from each hippocampal cluster (right, 1,107 voxels; left, 1,839 voxels) for target > novel and reversed > novel contrasts, respectively. We conducted a repeated-measures ANOVA to determine the effects of contrast (target > novel vs. reversed > novel) and laterality (right vs. left hippocampus) on the strength of hippocampal activation.
We also conducted two sets of correlations to compare previously assessed overt memory performance and parameter estimates from the target > novel and reversed > novel contrasts. The first set of correlations focused on memory for room location. Altogether, four point-biserial correlations were computed to determine relations between an accurate or inaccurate response in the location task and parameter estimates from the two contrasts within each of the two hippocampal masks, left and right. The same analytical approach was repeated with the more comprehensive memory measure. For this measure, we calculated a composite score that summed source and toy character accuracy for each participant. A total of four Spearman’s ρ correlations were computed to examine the relations between the two contrasts within each of the two hippocampal masks, left and right, and the composite memory measure. Out of 22 participants, one participant refused to provide an answer to the location and toy character questions. Therefore, all correlations with behavior reflect a total of 21 participants. Following an anonymous reviewer’s suggestion, we tested for specificity of our associations between behavior and activation. We thus conducted a mixed ANOVA including 2 (room: accurate vs. inaccurate) × 2 (character: accurate vs. inaccurate) × 2 (contrast: target > novel vs. reversed > novel) × 2 (laterality: left vs. right) with the first two factors varied between subjects and the second two factors varied within.
Supplementary Material
Acknowledgments
We thank all families who volunteered their time. We also thank Ethan Fox and all research assistants for their help in completing the study. Finally, we are indebted to Yana Fandakova, Sangeet Khemlani, and three anonymous reviewers for their valuable input. This work was supported by The National Institute of Child Health and Human Development at the National Institutes of Health, Grant 1R21HD088928 (to S.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in Open Science Framework and can be accessed using the following website: https://osf.io/6nu9s/. Data is provided in wide form and has identifying and demographic information (subject ID number, age in months, sex), as well as outcome level data for each subject (mean parameter estimates for target > novel and reversed > novel contrasts in the left and right hippocampus, source memory accuracy, and composite memory score).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805572115/-/DCSupplemental.
References
- 1.Tulving E. Episodic and semantic memory. Org Mem. 1972;1:381–403. [Google Scholar]
- 2.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 3.Bauer PJ. Developments in declarative memory. Psychol Sci. 2005;16:41–47. doi: 10.1111/j.0956-7976.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer PJ, Wenner JA, Dropik PL, Wewerka SS, Howe ML. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monogr Soc Res Child Dev. 2000;65:i–vi, 1–204. [PubMed] [Google Scholar]
- 5.Robinson AJ, Pascalis O. Development of flexible visual recognition memory in human infants. Dev Sci. 2004;7:527–533. doi: 10.1111/j.1467-7687.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 6.Olson I, Newcombe N. The Wiley Handbook on the Development of Children’s Memory. Wiley-Blackwell; West Sussex, UK: 2013. Binding together the elements of episodes: Relational memory and the developmental trajectory of the hippocampus; pp. 285–308. [Google Scholar]
- 7.Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Jabès A, Nelson C. 20 years after “the ontogeny of human memory: A cognitive neuroscience perspective.” Where are we? Int J Behav Dev. 2015;39:315–317. [Google Scholar]
- 9.Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sluzenski J, Newcombe NS, Satlow E. Knowing where things are in the second year of life: Implications for hippocampal development. J Cogn Neurosci. 2004;16:1443–1451. doi: 10.1162/0898929042304804. [DOI] [PubMed] [Google Scholar]
- 11.Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore JH, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2012;22:2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehr SA, Song LA, Spelke ES. For five-month-old infants, melodies are social. Psychol Sci. 2016;27:486–501. doi: 10.1177/0956797615626691. [DOI] [PubMed] [Google Scholar]
- 14.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margoliash D. Sleep, learning, and birdsong. ILAR J. 2010;51:378–386. doi: 10.1093/ilar.51.4.378. [DOI] [PubMed] [Google Scholar]
- 16.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dongen EV, et al. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc Natl Acad Sci USA. 2012;109:10575–10580. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 19.Davies S. Perceiving melodies and perceiving musical colors. Rev Philos Psychol. 2010;1:19–39. [Google Scholar]
- 20.Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- 21.DeMaster D, Pathman T, Ghetti S. Development of memory for spatial context: Hippocampal and cortical contributions. Neuropsychologia. 2013;51:2415–2426. doi: 10.1016/j.neuropsychologia.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Sastre M, 3rd, Wendelken C, Lee JK, Bunge SA, Ghetti S. Age- and performance-related differences in hippocampal contributions to episodic retrieval. Dev Cogn Neurosci. 2016;19:42–50. doi: 10.1016/j.dcn.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson J, Sheffield E. The role of reminders in young children’s memory development. In: Balter L, Tamis-LeMonda C, editors. Child Psychology: A Handbook of Contemporary Issues. Psychology Press; New York: 1999. pp. 193–214. [Google Scholar]
- 24.DeMaster D, Coughlin C, Ghetti S. Retrieval flexibility and reinstatement in the developing hippocampus. Hippocampus. 2016;26:492–501. doi: 10.1002/hipo.22538. [DOI] [PubMed] [Google Scholar]
- 25.Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi F, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2011;6:e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;56:1803–1813. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 29.Riggins T, Geng F, Blankenship SL, Redcay E. Hippocampal functional connectivity and episodic memory in early childhood. Dev Cogn Neurosci. 2016;19:58–69. doi: 10.1016/j.dcn.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JK, et al. Assessing hippocampal development and language in early childhood: Evidence from a new application of the Automatic Segmentation Adapter Tool. Hum Brain Mapp. 2015;36:4483–4496. doi: 10.1002/hbm.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez RL, Edgin JO. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev Cogn Neurosci. 2016;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: Hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18:523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiner T, Lehmann M, Rasch B. Auditory feedback blocks memory benefits of cueing during sleep. Nat Commun. 2015;6:8729. doi: 10.1038/ncomms9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robin J, Moscovitch M. Familiar real-world spatial cues provide memory benefits in older and younger adults. Psychol Aging. 2017;32:210–219. doi: 10.1037/pag0000162. [DOI] [PubMed] [Google Scholar]
- 35.Ritchey M, Yonelinas AP, Ranganath C. Functional connectivity relationships predict similarities in task activation and pattern information during associative memory encoding. J Cogn Neurosci. 2014;26:1085–1099. doi: 10.1162/jocn_a_00533. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu A, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: Developmental differences along the anterior/posterior axis. Cereb Cortex. 2014;24:3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- 38.Hayne H, Barr R, Herbert J. The effect of prior practice on memory reactivation and generalization. Child Dev. 2003;74:1615–1627. doi: 10.1046/j.1467-8624.2003.00627.x. [DOI] [PubMed] [Google Scholar]
- 39.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 41.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 42.Manning JH, Courchesne E, Fox PT. Intrinsic connectivity network mapping in young children during natural sleep. Neuroimage. 2013;83:288–293. doi: 10.1016/j.neuroimage.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothschild G, Eban E, Frank LM. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat Neurosci. 2017;20:251–259. doi: 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer PJ, Hertsgaard LA, Wewerka SS. Effects of experience and reminding on long-term recall in infancy: Remembering not to forget. J Exp Child Psychol. 1995;59:260–298. doi: 10.1006/jecp.1995.1012. [DOI] [PubMed] [Google Scholar]
- 45.Meltzoff AN. Infant imitation after a 1-week delay: Long-term memory for novel acts and multiple stimuli. Dev Psychol. 1988;24:470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.