The scale of life on Earth is shaped by a confluence of biophysical, evolutionary, ecological, and, recently, human forces. Measuring the scale of life offers insights about these forces and raises many more questions. In PNAS, Bar-On et al. (1) offer the most comprehensive quantification to date of the biomass of life on Earth, broken down by major taxonomic groups, ecological strategies, and environments. Despite high uncertainty in some estimates, their findings shed fascinating light on how biomass is distributed. Although many of the detailed findings will likely surprise most readers, the study also builds a foundation for exploring major ecological, evolutionary, and environmental questions. We highlight two such questions as examples of the future impact of this work. One of the findings is the striking contrast between marine and terrestrial biomes. Can we account for the differences on the basis of what we know about how these disparate ecosystems function? The findings also raise important questions about the future. What scale of human activities can be supported by marine and terrestrial environments, looking forward? How will climate change alter the answers?
Land–Ocean Differences
Total primary productivity in the oceans [48.5 Gt C/y net primary production (NPP)] is similar to that on land (56.4 Gt C/y NPP), even though the oceans have more than twice as much surface area (2). However, despite similar total primary productivity, Bar-On et al. (1) estimate that there is roughly 80 times more biomass on land than in the oceans. Terrestrial plants—which comprise ∼80% of the total biomass on Earth—make up most of this difference. In striking contrast to the land’s dominance of producer biomass, Bar-On et al. (1) estimate that more than 70% of global animal biomass is found in the ocean. Earth has a plant-dominated landscape and an animal-dominated seascape. What could explain these fundamental differences?
The lower productivity of the ocean per unit area is due largely to poorer light penetration in water (2). Across marine and terrestrial environments, higher local productivity has been shown to be associated with higher local producer-to-consumer ratios and higher local biomass per unit productivity (3). Thus, it is not surprising that marine environments—which are less locally productive, on average—have less overall biomass and a higher proportion of consumers than terrestrial environments. Given Bar-On et al.’s (1) estimate that consumers make up >80% of marine biomass and producers make up >95% of terrestrial biomass, the sheer magnitude of the difference they estimate between total marine biomass and total terrestrial biomass can be largely explained by the energetic inefficiency of food chains.
Mechanistically, several factors might explain why biomass concentrates in marine consumers but terrestrial producers. One key contributor is the contrasting patterns of energetic efficiencies of marine versus terrestrial food chains. On average, about 10% of energy is transferred from one trophic level to the next in the ocean across all trophic levels (4), whereas on land herbivores assimilate as little as 1% of primary production (5). This order-of-magnitude difference arises mostly because evolution has favored woody and stem structures in plants (6) to help them rise above their competitors for light in the absence of water’s buoyancy. These structures are heavy, relatively inaccessible to consumers, and make up the bulk of terrestrial plant biomass (1). Woody terrestrial plants also have slow turnover, meaning that their standing biomass represents the accumulation of years to decades of primary production, compared with much shorter timescales in ocean producers. Ocean currents make limiting nutrients highly mobile in the oceans (7), which selects for fast turnover—and thus small size—among producers and other sessile organisms.
Marine environments have higher trophic transfer efficiencies and larger predator-to-prey body size ratios, which theory predicts should lead to more top-heavy trophic pyramids (4). The much faster turnover, and more accessible carbon stores, of plankton compared with terrestrial plants makes the transfer of energy between producers and consumers much more efficient in aquatic systems (5). This may explain why Bar-On et al. (1) estimate marine consumer biomass is especially concentrated in basal consumers such as krill, other arthropods, mollusks, and cnidarians. The greater prevalence of ectothermy among marine animals may also increase the efficiency of marine, relative to terrestrial, food chains. Factors explaining the larger predator-to-prey body size ratios in the oceans may include (i) buoyancy, which reduces gravitational pressures on large-sized animals and increases the body sizes that optimize movement speed (8); and (ii) the fact that endothermy limits the size of terrestrial mammals (9) but may select for larger body sizes in marine mammals due to thermoregulation challenges (10).
Although some of the estimates that highlight the striking contrasts between land and sea biomes are highly uncertain (1), the stark qualitative differences between biomass distributions on land and in the sea that are described by Bar-On et al. (1) resonate with these differences in the environmental characteristics that shape the ecology and evolution of food webs in these systems. Looking forward, Bar-On et al.’s (1) land–sea contrasts raise the question of how these fundamental ecosystem differences will interact with different looming threats from future climate change [e.g., changing water distributions on land versus acidification and altered current dynamics in oceans (e.g., see ref. 7)] to reshape the alternative scales of life on land versus in the sea.
The Interacting Scales of Life and Humanity
Bar-On et al. (1) estimate that humans and human livestock combined now make up ∼8% of total animal biomass and roughly a quarter of terrestrial animal biomass (Fig. 1A). This measure underestimates this component of the human biomass footprint, because it ignores the animal biomass in aquaculture. Froehlich et al. (11) estimate that the biomass of animal aquaculture is about 14% as large as that of livestock (thus ∼0.014 Gt C, assuming similar average carbon content) and growing rapidly. Although these numbers represent only one type of human impact on global biomasses, they underscore the unprecedented scale of humanity today and raise the question of what scales of humanity and human activity can be supported sustainably by the planet’s ecosystems. Climate change compounds the urgency of these questions, and, indeed, the differences Bar-On et al.’s (1) analysis highlights between marine and terrestrial environments may be important for understanding the different challenges these environments will face under future climates.
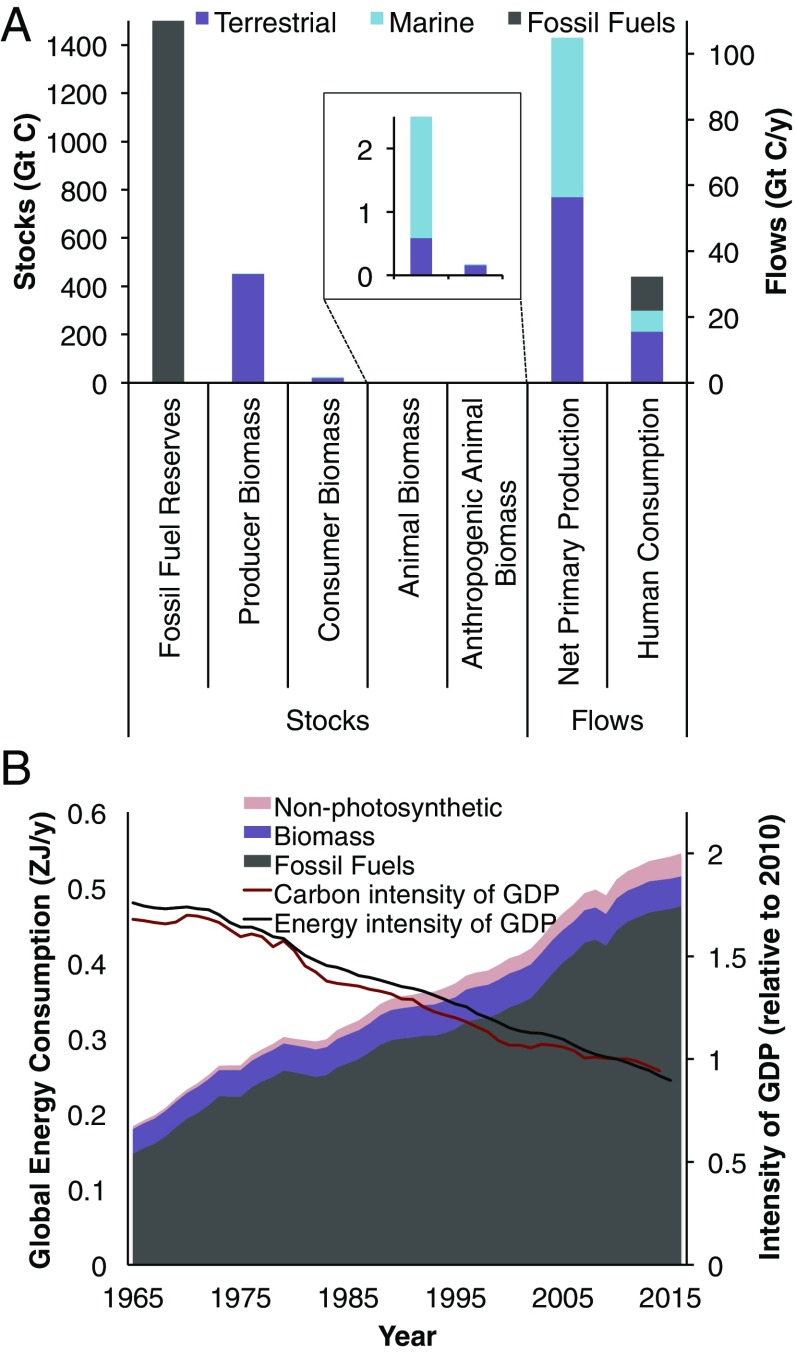
Fig. 1.
A compares scales of human and natural stocks and flows of carbon (data from refs. 1, 2, 11–13, and 18; see text for description). “Animal Biomass” includes both human and nonhuman biomass, from Bar-On et al.’s (1) table 1. “Anthropogenic Animal Biomass” is the subset including human biomass, livestock biomass, and animal aquaculture biomass. For fossil fuel reserves, 1,500 Gt C is shown, which is the midpoint of the IPCC’s approximate range (18). B shows the changes in energy consumption from photosynthetic (biomass and fossil fuels) and nonphotosynthetic (nuclear and renewables) sources over the last ∼50 y (data from ref. 16), as well as the carbon (from energy, cement production, and flaring) and energy intensities of the global economy (gigatons of CO2/2011 international dollars, and zettajoules/2011 international dollars, respectively; both shown as a fraction of their 2010 value) over the same period (data from refs. 16, 17, and 19).
In addition to consuming cultivated biomass from agriculture and aquaculture, humans also consume biomass through logging, fishing, hunting, land clearing, anthropogenic fires, and other forms of consumption. Haberl et al. (12) estimate that this all adds up to an annual terrestrial harvest of about 15.6 Gt C—equivalent to roughly 3% of Bar-On et al.’s (1) estimate of total terrestrial biomass, and roughly 25% of terrestrial NPP. Watson et al. (13) estimate that the demands of global fisheries are equivalent to ∼6.3 Gt C/y, which is ∼13% of marine NPP (Fig. 1A). The primary production demands of fed aquaculture are partly accounted for in these numbers via feeds from wild fisheries and agriculture, but demands from unfed aquaculture (e.g., kelp, shellfish, some carps) would be additional and possibly substantial. In both terrestrial and marine systems, the increasing human appropriation of global primary production has put disproportionate ecological pressure on other energy-intensive forms of life, namely large-bodied animals and top predators. Extinction of megafauna and widespread trophic downgrading have marked human history (14, 15) and are likely to continue as the scale of humanity continues to expand.
Moreover, the scale of humanity is supported not only by current primary production and biomass stores. It is also largely supported by consumption of fossil fuels, which are produced over hundreds of millions of years from degraded biomass (15). Annual global consumption of fossil fuel energy is currently ∼0.48 ZJ (16) (1 ZJ = 1021 J = 2.78 × 105 TWh), which converts—based on CO2 emissions and oxidation rates (17)—to ∼10.3 Gt C. Thus, through biomass consumption and fossil fuel use, the combined scale of human carbon appropriation and consumption might be ∼30% as large as total NPP (Fig. 1A). The magnitude of economically recoverable fossil fuel reserves is uncertain (15), but the Intergovernmental Panel on Climate Change (IPCC) estimated it at ∼1,000–2,000 Gt C in 2011 (table 2.2 in ref. 18). This would make annual fossil fuel consumption ∼0.5–1% of reserves, although reserves are likely to grow as technology improves. Energy use from nonphotosynthetic sources (nuclear, hydro, wind, solar, and other renewables) is increasing but is still a relatively small fraction (<6%) of global primary energy use (16).
Historical economic growth—and the resulting improvements in living standards—has been tightly coupled to energy use, and the global economy’s current energy and carbon footprints are unsustainable (15, 18). However, the energy and carbon intensities of the global economy have actually been decreasing steadily for the past several decades (Fig. 1B; calculated from refs. 16, 17, and 19), likely due to factors such as urbanization, technological improvement, increases in the size of the service sector, and—more recently—increases in renewable energy use. How much economic growth can be sustainably supported in the long term is a topic of active debate.
The food system—the other major source of human appropriation of primary production—also faces stark sustainability challenges. Climate change is likely to pose significant challenges to both land and ocean food-production systems, unique in each system (e.g., refs. 7 and 18). However, recent studies (e.g., refs. 11 and 20) suggest that, through interventions such as shifting diets, reducing waste, and increasing the role of aquaculture in meeting demands, the food system could become substantially less carbon intensive while actually increasing living standards by improving human health outcomes.
Bar-On et al.’s (1) analysis makes many assumptions, comes with considerable uncertainty, and raises more questions than it answers. Nonetheless, its elegance lies in its asking a broad and important question, and answering this question using the most rigorous approaches currently available. Science spends much of its time in the trees, and rightly so. Richness and precision are the lifeblood of science. However, as global sustainability challenges increase in urgency and societies become ever more interconnected, analyses like Bar-On et al.’s (1) remind us of the importance of periodically stepping back to survey the forest.
Acknowledgments
We are grateful to Halley Froehlich and Steve Davis for advice on aquaculture and energy accounting, respectively. This work was supported by the Waitt Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6506.
References
- 1.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 3.Hatton IA, et al. The predator-prey power law: Biomass scaling across terrestrial and aquatic biomes. Science. 2015;349:aac6284. doi: 10.1126/science.aac6284. [DOI] [PubMed] [Google Scholar]
- 4.Trebilco R, Baum JK, Salomon AK, Dulvy NK. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol Evol. 2013;28:423–431. doi: 10.1016/j.tree.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Hairston NG, Jr, Hairston NG., Sr Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am Nat. 1993;142:379–411. [Google Scholar]
- 6.Swenson NG, Enquist BJ. Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. Am J Bot. 2007;94:451–459. doi: 10.3732/ajb.94.3.451. [DOI] [PubMed] [Google Scholar]
- 7.Moore JK, et al. Sustained climate warming drives declining marine biological productivity. Science. 2018;359:1139–1143. doi: 10.1126/science.aao6379. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Nielsen K. Locomotion: Energy cost of swimming, flying, and running. Science. 1972;177:222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 9.Burness GP, Diamond J, Flannery T. Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proc Natl Acad Sci USA. 2001;98:14518–14523. doi: 10.1073/pnas.251548698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gearty W, McClain CR, Payne JL. Energetic tradeoffs control the size distribution of aquatic mammals. Proc Natl Acad Sci USA. 2018;115:4194–4199. doi: 10.1073/pnas.1712629115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froehlich HE, Runge CA, Gentry RR, Gaines SD, Halpern BS. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc Natl Acad Sci USA. April 30, 2018;115:5295–5300. doi: 10.1073/pnas.1801692115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberl H, et al. Quantifying and mapping the human appropriation of net primary production in earth’s terrestrial ecosystems. Proc Natl Acad Sci USA. 2007;104:12942–12947. doi: 10.1073/pnas.0704243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson R, Zeller D, Pauly D. Primary productivity demands of global fishing fleets. Fish Fish. 2014;15:231–241. [Google Scholar]
- 14.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 15.Schramski JR, Gattie DK, Brown JH. Human domination of the biosphere: Rapid discharge of the earth-space battery foretells the future of humankind. Proc Natl Acad Sci USA. 2015;112:9511–9517. doi: 10.1073/pnas.1508353112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie H, Roser M. 2018 Energy Production and Changing Energy Sources. Available at https://ourworldindata.org/energy-production-and-changing-energy-sources. Accessed May 1, 2018.
- 17.Boden TA, Marland G, Andres RJ. Global, Regional, and National Fossil-Fuel CO2 Emissions. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; Oak Ridge, TN: 2017. [Google Scholar]
- 18.Intergovernmental Panel on Climate Change . In: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Pachauri RK, Meyer LA, editors. IPCC; Geneva: 2014. [Google Scholar]
- 19.Roser M. 2018 Economic Growth. Available at https://ourworldindata.org/economic-growth. Accessed May 1, 2018.
- 20.Clark M, Tilman D. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ Res Lett. 2017;12:064016. [Google Scholar]