Abstract
Ca2+ and Zn2+ dynamics have been identified as important drivers of physiological processes. In order for these dynamics to encode function, the cell must have sensors that transduce changes in metal concentration to specific downstream actions. Here we compare and contrast the native metal sensors: calmodulin (CaM), the quintessential Ca2+ sensor and metal-responsive transcription factor 1 (MTF1), a candidate Zn2+ sensor. While CaM recognizes and modulates the activity of hundreds of proteins through allosteric interactions, MTF1 recognizes a single DNA motif that is distributed throughout the genome regulating the transcription of many target genes. We examine how the different inorganic chemistries of these two metal ions may shape these different mechanisms transducing metal ion concentration into changing physiologic activity. In addition to native metal sensors, scientists have engineered sensors to spy on the dynamic changes of metals in cells. The inorganic chemistry of the metals shapes the possibilities in the design strategies of engineered sensors. We examine how different strategies to tune the affinities of engineered sensors mirror the strategies nature developed to sense both Ca2+ and Zn2+ in cells.
Introduction
There are many parallels between calcium and zinc: they are abundant in biological systems where they exist as divalent cations, they are redox inactive, they bind to proteins where they serve as critical cofactors, and for most forms of life they are essential micronutrients. Living organisms concentrate and buffer these ions such that ion acquisition and distribution is tightly regulated. Despite exquisitely coordinated mechanisms to maintain tight homoeostatic regulation of ion concentrations, organisms use dynamic changes in the concentrations of both labile calcium (Ca2+) and zinc (Zn2+) to drive physiological processes [1,2]. Ca2+ transients are important for organismal and cellular processes ranging from fertilization and division to disease and apoptosis [3] and have been well characterized in cells using a variety of Ca2+ indicators [4,5]. While recognition of Zn2+ transients in cells is only beginning to emerge, studies point to Zn2+ fluxes as playing a regulatory or signalling role in cells. Such Zn2+ dynamics include ‘zinc sparks’ upon mammalian egg fertilization and ‘zinc waves’ in immune cells [6,7].
A noted feature of Ca2+ transients and Zn2+dynamics is that they are organized into distinct patterns in space and time [1,2]. In order for these dynamic patterns to encode information, cells must be able to sense the changes in metal concentration and translate that change into a specific downstream action, with different patterns encoding different functions. For Ca2+, many native sensing proteins have been identified, with calmodulin (CaM) as the quintessential Ca2+ effector [3]. The identity of the proteins that transduce mammalian Zn2+ dynamics is less clear, although proteins that multimerize and become active upon Zn2+ binding have been identified as possible Zn2+ sensors [8,9].
This essay will compare and contrast CaM, the textbook calcium-sensing protein, with metal-responsive transcription factor 1 (MTF1), a candidate protein for cellular zinc sensing and signal transduction in mammalian cells [10,11]. As noted above, there are many similarities between these two ions. However, there are also notable differences in the chemistry and biology of these two important ions that hint at orthogonal signalling roles in biological organisms. In this essay, we focus on proteins that sense and transduce changes in Ca2+ or Zn2+, highlighting the fundamental inorganic and protein chemistry features of these sensors that suggest these metal sensors operate by divergent mechanisms.
In addition to native metal sensors that decode natural dynamics in Ca2+ or Zn2+, scientists have sought to engineer protein-based and small molecule metal sensors to spy on these changes [4,12]. Engineered sensors translate changes in metal concentration in live cells to changes in a fluorescence signal that can be detected by microscopy. A challenge in sensor engineering is to tune the affinity of the sensor, while maintaining the specificity, so that the fluorescence changes report only on the metal of interest, ignoring the multitude of confounding and competing factors that could be present in the cellular environment. Ideally, the binding constants of sensors are tuned such that the sensor is ~50% saturated in the resting cell in the subcellular location of interest [13,14]. Distinct approaches have been used to modify the affinities of protein-based sensors for Ca2+ than have been employed for Zn2+ sensors. For Ca2+ sensors, a common approach for tuning the apparent binding constant has been to manipulate the interaction of CaM (or an analogous Ca2+-sensing protein) with a partner binding protein [15–17]. Alternatively, the most widely used approach for tuning the apparent binding constant of Zn2+ sensors is to alter the metal coordination site [14,18]. Although these approaches are distinct, they lead to robust, selective metal sensors to examine the dynamics and distribution of Ca2+ and Zn2+ in cells.
While native and engineered sensors have two very different purposes – to inform the cell of dynamic changes and to let scientist glimpse the inner workings of cell biology – we propose that lessons learned from studying one might inform our study of the other. CaM modulates the cell’s response to Ca2+ through its structural plasticity that allows it to bind and regulate over 300 partner proteins [19]. On the other hand, binding of Zn2+ to six different sites induces MTF1 to recognize a single DNA motif repeated throughout the genome modifying the transcription of target genes. The engineering of sensors has mirrored these native proteins, leveraging both the allosteric flexibility of sequences near Ca2+ sites and the variable coordination preference of Zn2+ to broaden our insight into the biology of these metals.
Native metal sensors
CaM
Ca2+ exists in the cell in two populations: labile Ca2+ that is not tightly bound to proteins and protein-bound Ca2+. Resting cells maintain a gradient of labile Ca2+ from 2 mM in the extracellular space to much lower concentrations in the cell, ranging from 100 nM in the cytosol to hundreds of μM in the endoplasmic reticulum [20]. Upon stimulation of Ca2+ signalling, the cytosolic Ca2+ concentration spikes [2,10,15,20]. Labile Ca2+ concentrations are regulated by Ca2+ channels, buffering proteins that act as sinks for excess Ca2+, and Ca2+ sensors that serve as effectors by binding downstream proteins upon changes in Ca2+ status [10,21]. Sensing proteins coordinate Ca2+ with oxygen ligands, as would be predicted by hard-soft acid-base theory [22], contributed by aspartate or glutamate amino acids. The most common Ca2+-binding motif in proteins is called an EF hand. Based on genetic data, 2540 or approximately 70% of the known Ca2+-binding proteins in animals contain an EF hand motif [23]. This 30-residue helix–loop–helix structure binds Ca2+ through six or seven oxygen atoms from six coordinating amino acids in a pentagonal bipyramidal structure [24]. The affinity of these structures for Ca2+ can be tuned over a 100000-fold range through the identity and conformation of amino acids in the EF hand and side chain packing through the core of the protein [10]. This ability to tune affinity makes EF hands versatile Ca2+ sensors over a wide range of concentrations and may explain why EF hands are found in such diverse proteins including troponin C, a Ca2+ sensor in muscle cells; calcineurin, a phosphatase essential for T-cell activation; and the S100 proteins, regulatory proteins found in a variety of tissues [25].
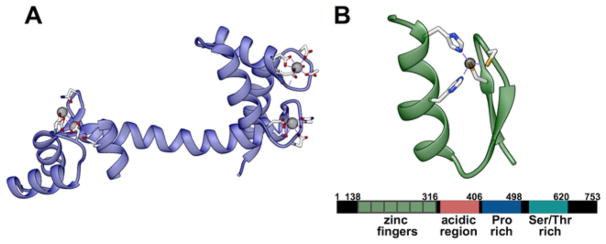
In CaM, EF hand motifs are essential for transducing Ca2+ binding into global protein conformation changes that lead to interactions with partner proteins. CaM senses cytosolic Ca2+ through four EF hands paired in two lobes separated by a flexible linker (Figure 1A) [10,26]. When CaM is in the apo form, the helices of the EF hands are antiparallel and assume a closed geometry. As Ca2+ binds the EF hands, core amino acids throughout CaM rearrange to a more open conformation that exposes hydrophobic interfaces which bind other proteins [27]. It is through this allosteric interaction, and associations with the flexible linker, that CaM modulates the activity of other proteins. Interestingly, this allostery is not unidirectional; the interaction of proteins with the hydrophobic patches on both holo- and apo-CaM can affect the affinity and cooperativity of the EF hands for Ca2+, making CaM responsive over a wide range of Ca2+ concentrations from 10−12 to 10−6 M [26,28,29].
Figure 1. Structures of CaM and MTF1.
(A) Crystal structure of CaM with coordinating ligands highlighted (PDB entry 4BW8). (B) Crystal structure of ZIF-268 as an example of a αββ Zn2+ finger fold. MTF-1 encodes six similar Zn2+ fingers and three transactivation domains as shown in the schematic below the structure (PDB entry 1ZAA).
A remarkable characteristic of CaM is its ability to interact in a variety of conformations with over 300 proteins and to recognize those proteins specifically. The proteins that are binding partners for CaM share little to no homology, and occupy many categories of cellular machinery from G-coupled receptors to ion channels to kinases [21]. Shukla and co-workers employed molecular dynamics (MD) to probe the molecular basis of this ability to recognize diverse partners. A proposed hypothesis for how CaM binds a diverse set of proteins is that the binding partners induce the fit on unstructured CaM. On the contrary, the folding landscapes of both holo-C-CaM and apo-C-CaM include well-populated conformations that provide the intermolecular interfaces described in crystal structures of CaM with binding partners. This evidence implies that it is not the binding partners that prescribe the interaction with CaM, but intramolecular hydrophobic interactions in the CaM sequence that initiate the interaction with binding partners [21].
Upon binding Ca2+, CaM directly modifies the activity of its binding partners. This is accomplished through a number of mechanisms including displacement of autoinhibitory domains, active site remodelling, dimerization and/or increased autophosphorylation [30]. Each of these mechanisms occurs with different kinetics and different thermodynamic stabilities, enabling CaM to orchestrate complex downstream effects in response to Ca2+ dynamics. For example, apo-CaM is constitutively bound to small-conductance Ca2+-activated K+ channels (SK channels), and when Ca2+ binds to the N-lobe of CaM, conformational changes in CaM cause both dimerization and opening of the SK channel subunits to allow K+ flow across the membrane. Ca2+ release upon decreased Ca2+ concentration closes the channel [31]. In contrast, binding of Ca2+-CaM to CaM-dependent kinase II (CaMKII) leads to an increase in autophosphorylation of CamKII’s neighbouring subunits, increasing the activation state of the kinase for its downstream substrates. Because CamKII must be dephosphorylated, its increased activity continues after the initial Ca2+ flux, perhaps creating stability between Ca2+ pulses [32]. Complex allosteric interactions between CaM Ca2+-binding sites and hydrophobic intramolecular interfaces confer plasticity on CaM’s structure, and this plasticity is instrumental in allowing CaM to regulate hundreds of proteins, thereby modulating multiple diverse pathways in cells. One of the only established Zn2+ sensors, MTF1, responds to Zn2+ dynamics in a fundamentally different way.
MTF1
Like Ca2+, Zn2+ levels are regulated by a complex network of transporters, and buffered by proteins and other ligands. Labile Zn2+ in the cytosol of mammalian cells is maintained at a concentration in the hundreds of pM, even though total Zn2+ levels exceed hundreds of μM [33]. There is not strong evidence that organelles store and release excess Zn2+ [14,34,35], although in certain cell types, Zn2+ is packaged and concentrated into vesicles [36,37]. An intriguing feature of biological Zn2+ sensing is the sheer number of zinc-binding proteins present in an organism, and the diversity in binding sites used to coordinate Zn2+. Critical analysis of the Structural Classification of Proteins (SCOP) database identified increasing abundance of zinc-binding structural domains from archea to bacteria to eukaryotes [38]. Analysis of 23 archea, 233 bacteria and 57 eukaryote species revealed that while abundance typically scales with genome size, eukaryotic zinc-binding proteins exceed the predicted power law, and hence eukaryotes devote a greater percentage of their genome to zinc-binding proteins [38]. Further bioinformatics studies estimate that up to 10% of the proteins encoded by the human genome are predicted to be zinc-binding proteins [39]. In biological systems, proteins coordinate Zn2+ through sulfur, nitrogen or oxygen moieties and a variety of coordination spheres from four- to six-coordinate [40]. Protein coordination sites are thought to derive their selectivity for Zn2+ over other cations from the lack of ligand field stabilization cost of desolvating Zn2+ [41]. Although many Zn2+-binding sites coordinate Zn2+ with high affinity (dissociation constants of a few pM or lower), examples of lower affinity or kinetically exchangeable sites have been reported [42].
While the number and variety of Zn2+-binding sites in the proteome is fascinating, the redundancy of Zn2+ proteins has made it difficult to clearly define how cells sense and manage Zn2+. Several outstanding questions remain: how and where do cells load so many diverse binding sites with Zn2+ specifically? How does the cell organize the expression and regulation of the many Zn2+ proteins? As new examples of Zn2+ dynamics are discovered, what proteins sense the dramatic changes in Zn2+ flux? A few characteristics of MTF1 make it an attractive candidate to sense and regulate a response to Zn2+: (i) It binds Zn2+ with a low enough affinity to be partially unsaturated in resting cytosolic concentrations, (ii) once fully Zn2+ bound it translocates from the cytosol to the nucleus and (iii) in the nucleus it binds a specific DNA motif thereby regulating the expression of Zn2+-binding proteins and a Zn2+ export channel. Here we will present MTF1 as an example of a Zn2+ sensor, but hypothesize that other Zn2+ sensors may exist.
Human MTF1 contains six zinc-finger motifs, three transactivation domains and a conserved cysteine-rich cluster (Figure 1B) [11]. Each zinc finger binds one Zn2+ in a pseudo-tetrahedral geometry through two cysteine residues and two histidine residues. Scores of structural and biophysical studies have revealed that two to three of the zinc fingers bind Zn2+ with relatively high affinity and three to four of the fingers bind Zn2+ with low affinity [43–45]. In metal-binding studies of the complete six finger domain the Kd (Zn2+) was estimated to be ~30 pM, and spectroscopic studies of the individual fingers binding to Co2+ indicate individual finger dissociation constants vary 25-fold [45]. This range of in vitro affinities in the picomolar regime supports the hypothesis that lower affinity fingers may be responsible for the Zn2+-sensing capabilities of MTF1 in the cytosol, while the high affinity fingers constitutively bind Zn2+ [11].
The function of MTF1 is to bind to and modulate the transcription of DNA in response to changes in cellular Zn2+. When MTF1 is replete with metal it translocates from the cytosol to the nucleus where it recognizes a DNA motif called the metal-response element (MRE) that is found in the promoter region of MTF1 target genes [11]. Zn2+ association with the four N-terminal fingers is necessary for tight binding of MTF1 to the MRE, while the two C-terminal fingers have been implicated in providing specificity to the protein–DNA interface [44]. Upon DNA binding, the three transactivation domains recruit transcription machinery to the promoter region to regulate transcription of downstream target genes [43].
Until recently the genes identified to be under the control of MTF1 in response to Zn2+ were the genes for metallothioneins, proteins that buffer Zn2+ in the cytosol and Znt1, a Zn2+ exporter [46]. To identify more genes under the control of MTF1, Hardyman and co-workers examined the differential expression of genes in normal and excess Zn2+ in wild-type Caco-2 and MTF1 knockdown Caco-2 cells. They found that, as expected, in the MTF1 knockdown, cells expression of the previously identified MTF1 target genes was no longer sensitive to Zn2+ increases. However, they also discovered that expression of a number of genes was modulated by increasing Zn2+ in the MTF1-depleted cells as compared with wild type. As one example, the expression of genes encoding zinc uptake transporters was decreased upon exposure to increased Zn2+ concentration in the MTF1 knockdown cells. These data led to the hypothesis that MTF1 controls a hierarchy of Zn2+ responsive proteins. When MTF1 is available, it responds to Zn2+ increase by amplifying transcription of Zn2+ buffering and export proteins that lower cellular Zn2+ concentrations. In the absence of this safeguard, increases in Zn2+ were dramatic enough to uncover the expression of other Zn2+-sensitive genes that may be under the control of unknown transcription factors [46].
These data suggest that dynamic changes in Zn2+ impact the proteome of the cell through transcription of a variety of genes. While this mechanism is effective at eliciting a cellular response, it is intriguing to imagine other scenarios for Zn2+ communication based on the unique coordination chemistry of Zn2+. For instance, there is evidence for Zn2+ binding to be kinetically labile, and such labile sites could be exploited to sense fluxes of Zn2+ [42]. Alternatively, Zn2+ can be coordinated at the interface of proteins, modulating their activity [9,47,48]. Could this be an additional mechanism for sensing Zn2+ concentration changes? These scenarios stand in contrast with what is known about Ca2+ sensing and coordination. Coordination sites that are specific to Ca2+ function amid a sea of Mg2+, which is present at much higher concentrations than Ca2+. This pressure requires that coordination sites sensitive to physiologic transients of Ca2+ be carefully tuned to coordinate Ca2+ [49]. Because of this competition, perhaps nature accomplishes sensing and signal transduction through the plasticity of protein conformations of a single protein rather than a library of proteins decode the cell’s response to Ca2+.
Engineered sensors
In order for scientists to visualize and measure dynamic changes in metals in cells, artificial sensors have been engineered to quantify metal concentrations in live cells. While both protein based and small molecule sensors have been developed, protein-based sensors allow useful comparisons with native metal sensors. One class of protein-based sensors is the family of genetically encoded sensors based on FRET. These sensors are fusions of a donor fluorescent protein (FP), a metal-sensing domain and an acceptor FP. When the sensor is metal bound, it shifts conformations leading to a change in FRET between the two FPs. A number of FRET-based sensors have been engineered for both Ca2+ and Zn2+. Scientists often seek to engineer the binding affinity such that the sensor is partially occupied by metal in the environment of interest while maintaining a large change in the fluorescence upon binding to confer a high dynamic range in the cellular milieu. In order to solve this design challenge, FRET sensors for Ca2+ have leveraged the allostery of CaM, while Zn2+ sensors have exploited the dramatic conformational restructuring and flexible coordination of zinc fingers.
A number of powerful Ca2+ indicators have been engineered over the past decades [4,5], but here we will pull one case study to highlight a design strategy that piggybacks off a native sensor characteristic. To create a FRET-based sensor for Ca2+, CaM and a fragment of the CaM-binding partner, smooth muscle myosin light chain kinase (smMLCK), were fused between a donor protein, CFP and an acceptor protein, YFP. In the resulting sensor, called cameleon, Ca2+ binding causes association of CaM with the smMLCK peptide altering the FRET signal between the two FPs. In the original sensors, the affinity for Ca2+ was tuned by mutating the EF hands. Two weaknesses of this design were that the sensor was susceptible to binding by native CaM, and the apparent dissociation constant for Ca2+ was weak (60 μM). To overcome these issues the interface between CaM and the peptide was engineered to include more hydrophobic bumps and holes. This re-engineering led to a series of sensors that were unperturbed by the native CaM with a range of apparent dissociation constants from ~0.1 to 49 μM [15]. This new generation of cameleons was not susceptible to binding to native CaM, making them robust sensors for application in live cells.
As with Ca2+ sensors a common Zn2+ FRET sensor design is to fuse a donor FP to a metal-sensing domain followed by acceptor FP [14,18,50]. A major difference in the design of the sensors as compared with cameleons is the ability to tune Zn2+ affinity altering the identity of the amino acids that coordinate Zn2+ without losing specificity for labile Zn2+ [14,18]. For example, mutating a native cysteine to a histidine in each of the two Zn2+-binding sites of the Zap family of sensors alters the apparent dissociation constant from 2 to 800 pM [14]. This approach is possible because Zn2+ is fairly amenable to different coordination geometries and ligating residues.
By examining native sensors for metals new approaches can be harnessed for engineering more robust sensors for measuring dynamics metals in cells. Here we have compared approaches to tune the affinity of engineered sensors for metals that make the use of native characteristics of both protein and metals. Ca2+-binding proteins are more permissive to alteration at allosteric sites, while Zn2+-binding sites can remain selective with changes to the identity of the coordinating moieties. As scientists continue to examine metal transients, particularly in the developing field of Zn2+ dynamics, it is equally important and challenging to designate the native sensors and targets of those signals. Because there are still many open questions about Zn2+ signalling it is useful to study Zn2+ through the lens of what is known about Ca2+. As we uncover similar patterns and statuses of Ca2+ and Zn2+ cations, it will be essential to remember the fundamental differences in the inorganic chemistry between the two metals that may shape their role in cell biology.
Summary.
Ca2+ and Zn2+ dynamics are important drivers of physiological processes. In order for these dynamics to regulate cell physiology and function, cells must sense the changes.
CaM and MTF1 are compared and contrasted as natural sensors of Ca2+ and Zn2+ respectively.
Engineered protein sensors spy on these dynamic changes in metal concentrations and engineered sensor design borrows features from native sensors.
The inorganic chemistry of metal ions shapes their cell biology and the design of engineered sensors.
Abbreviations
- CaM
calmodulin
- CaMKII
CaM-dependent kinase II
- FP
fluorescent protein
- MRE
metal-response element
- MTF1
metal-responsive transcription factor 1
- SK
small-conductance Ca2+-activated K+ channels
- smMLCK
smooth muscle myosin light chain kinase
Footnotes
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases. J Biol Inorg Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Mank M, Griesbeck O. Genetically encoded calcium indicators. Chem Rev. 2008;108:1550–1564. doi: 10.1021/cr078213v. [DOI] [PubMed] [Google Scholar]
- 5.Rose T, Goltstein PM, Portugues R, Griesbeck O. Putting a finishing touch on GECIs. Front Mol Neurosci. 2014;7:88. doi: 10.3389/fnmol.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird AJ, Zhao H, Luo H, Jensen LT, Srinivasan C, Evans-Galea M, et al. A dual role for zinc fingers in both DNA binding and zinc sensing by the Zap1 transcriptional activator. EMBO J. 2000;19:3704–3713. doi: 10.1093/emboj/19.14.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim PW, Sun ZYJ, Blacklow SC, Wagner G, Eck MJ. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science. 2003;301:1725–1728. doi: 10.1126/science.1085643. [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Günther V, Davis AM, Georgiev O, Schaffner W. A conserved cysteine cluster, essential for transcriptional activity, mediates homodimerization of human metal-responsive transcription factor-1 (MTF-1) Biochim Biophys Acta. 2012;1823:476–483. doi: 10.1016/j.bbamcr.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Carter KP, Young AM, Palmer AE. Fluorescent sensors for measuring metal ions in living systems. Chem Rev. 2014;114:4564–4601. doi: 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JG, Palmer AE. In: Fluorescent Protein-Based Biosensors: Methods and Protocols. Zhang J, Ni Q, Newman HR, editors. Humana Press; Totowa, NJ: 2014. pp. 29–47. [Google Scholar]
- 14.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci USA. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [cited 2016 May 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue M, Takeuchi A, Horigane S, Ohkura M, Gengyo-Ando K, Fujii H, et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat Methods. 2015;12:64–70. doi: 10.1038/nmeth.3185. [DOI] [PubMed] [Google Scholar]
- 18.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gsponer J, Christodoulou J, Cavalli A, Bui JM, Richter B, Dobson CM, et al. A coupled equilibrium shift mechanism in calmodulin-mediated signal transduction. Structure. 2008;16:736–746. doi: 10.1016/j.str.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla D, Peck A, Pande VS. Conformational heterogeneity of the calmodulin binding interface. Nat Commun. 2016;7:10910. doi: 10.1038/ncomms10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertini I, Gray HB, Stiefel EI, Valentine JS. Biological Inorganic Chemistry: Structure and Reactivity. University Science Books; Sausalito, California: 2007. pp. 413–419. [Google Scholar]
- 23.Plattner H, Verkhratsky A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium. 2015;57:123–132. doi: 10.1016/j.ceca.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lewit-Bentley A, Réty S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 25.Nelson MR, Chazin WJ. Structures of EF-hand Ca(2+)-binding proteins: diversity in the organization, packing and response to Ca(2+) binding. BioMetals. 1998;11:297–318. doi: 10.1023/a:1009253808876. [DOI] [PubMed] [Google Scholar]
- 26.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 27.Evenäs J, Forsén S, Malmendal A, Akke M. Backbone dynamics and energetics of a Calmodulin domain mutant exchanging between closed and open conformations. J Mol Biol. 1999;289:603–617. doi: 10.1006/jmbi.1999.2770. [DOI] [PubMed] [Google Scholar]
- 28.Piazza M, Taiakina V, Guillemette SR, Guillemette JG, Dieckmann T. Solution structure of calmodulin bound to the target peptide of endothelial nitric oxide synthase phosphorylated at Thr495. Biochemistry. 2014;53:1241–1249. doi: 10.1021/bi401466s. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Abrams C, Wang L, Gizzi A, He L, Lin R, et al. Structural basis for calmodulin as a dynamic calcium sensor. Structure. 2012;20:911–923. doi: 10.1016/j.str.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 32.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter MC, Lo MN, Palmer AE. Techniques for measuring cellular zinc. Arch Biochem Biophys. 2016;611:20–29. doi: 10.1016/j.abb.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hessels AM, Taylor KM, Merkx M. Monitoring cytosolic and ER Zn2+ in stimulated breast cancer cells using genetically encoded FRET sensors. Metallomics. 2016;8:211–217. doi: 10.1039/c5mt00257e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JG, Qin Y, Galati DF, Palmer AE. New sensors for quantitative measurement of mitochondrial Zn2+ ACS Chem Biol. 2012;7:1636–1640. doi: 10.1021/cb300171p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [cited 2017 Jan 6] [DOI] [PubMed] [Google Scholar]
- 37.Dunn MF. Zinc–ligand interactions modulate assembly and stability of the insulin hexamer – a review. BioMetals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 38.Dupont CL, Yang S, Palenik B, Bourne PE. Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc Natl Acad Sci USA. 2006;103:17822–17827. doi: 10.1073/pnas.0605798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 40.Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 41.Berg JM, Godwin HA. Lessons from zinc-binding peptides. Annu Rev Biophys Biomol Struct. 1997;26:357–371. doi: 10.1146/annurev.biophys.26.1.357. [DOI] [PubMed] [Google Scholar]
- 42.Maret W. New perspectives of zinc coordination environments in proteins. J Inorg Biochem. 2012;111:110–116. doi: 10.1016/j.jinorgbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Agarwal A, Giedroc DP. Structural and functional heterogeneity among the zinc fingers of human MRE-binding transcription factor-1. Biochemistry. 1998;37:11152–11161. doi: 10.1021/bi980843r. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Chu M, Giedroc DP. MRE-Binding transcription factor-1: weak zinc-binding finger domains 5 and 6 modulate the structure, affinity, and specificity of the metal-response element complex. Biochemistry. 1999;38:12915–12925. doi: 10.1021/bi9913000. [DOI] [PubMed] [Google Scholar]
- 45.Guerrerio AL, Berg JM. Metal ion affinities of the zinc finger domains of the metal responsive element-binding transcription factor-1 (MTF1) Biochemistry. 2004;43:5437–5444. doi: 10.1021/bi0358418. [DOI] [PubMed] [Google Scholar]
- 46.Hardyman JEJ, Tyson J, Jackson KA, Aldridge C, Cockell SJ, Wakeling LA, et al. Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics. 2016;8:337–343. doi: 10.1039/c5mt00305a. [DOI] [PubMed] [Google Scholar]
- 47.Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BAL, et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- 48.Callaghan AJ, Redko Y, Murphy LM, Grossmann JG, Yates D, Garman E, et al. “Zn-link”: a metal-sharing interface that organizes the quaternary structure and catalytic site of the endoribonuclease, RNase E. Biochemistry. 2005;44:4667–4675. doi: 10.1021/bi0478244. [DOI] [PubMed] [Google Scholar]
- 49.Haiech J, Klee CB, Demaille JG, Haiech J. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Theoretical approach to study of multiple ligand binding to a macromolecule. Biochemistry. 1981;20:3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- 50.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [cited 2016 Sep 15] [DOI] [PMC free article] [PubMed] [Google Scholar]