Abstract
Background
Despite intensive therapy, children with metastatic and recurrent sarcoma or neuroblastoma have a poor prognosis. Magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) is a non-invasive technique allowing the delivery of targeted ultrasound energy under MR imaging guidance. MR-HIFU may be used to ablate tumors without ionizing radiation or to target chemotherapy using hyperthermia. Here, we evaluated the anatomic locations of tumors to assess the technical feasibility of MR-HIFU therapy for children with solid tumors.
Procedure
Patients with sarcoma or neuroblastoma with available cross-sectional imaging were studied. Tumors were classified based on location and surrounding structures within the ultrasound beam path as: a) not targetable, b) completely or partially targetable with the currently available MR-HIFU system, c) potentially targetable if a respiratory motion compensation technique was used.
Results
Of the 121 patients with sarcoma and 61 patients with neuroblastoma, 64% and 25% of primary tumors were targetable at diagnosis, respectively. Less than 20% of metastases at diagnosis or relapse were targetable for both sarcoma and neuroblastoma. Most targetable lesions were located in extremities or in the pelvis. Respiratory motion compensation may increase the percentage of targetable tumors by 4% for sarcomas and 10% for neuroblastoma.
Conclusions
Many pediatric sarcomas are localized at diagnosis and are targetable by current MR-HIFU technology. Some children with neuroblastoma have bony tumors targetable by MR-HIFU at relapse, but few newly diagnosed children with neuroblastoma have tumors amenable to MR-HIFU therapy. Clinical trials of MR-HIFU should focus on patients with anatomically targetable tumors.
Keywords: MR-HIFU, sarcoma, neuroblastoma, ablation, hyperthermia
Introduction
Sarcoma and neuroblastoma are the most common extracranial solid tumors in children. Together, osteosarcoma (OS), Ewing sarcoma (EWS), rhabdomyosarcoma (RMS), and non-rhabdomyosarcoma soft tissue sarcoma (NRSTS), account for about 13% of all childhood malignancies and neuroblastoma accounts for an additional 7%.[1–3] Advances in therapies have led to significant improvements in the outcome for children with these malignancies. However, children with metastatic or recurrent sarcoma continue to have poor prognosis with a 5 year overall survival of 20–25%.[1] Approximately 50% of children with high risk neuroblastoma have tumor recurrence with a dismal outcome of <10% overall survival at 5 years.[2] Given such poor prognosis, new therapeutic advances are needed.
Magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) is a non-invasive technique that can deliver acoustic energy to an image-defined region. Current clinical applications use this energy for thermal coagulation of tissue.[4] MR-HIFU ablation has been demonstrated to be safe and effective in the treatment of uterine fibroids and painful bony metastases.[5–8] MR-HIFU does not use ionizing radiation, offering an advantage over radiation therapy, and the potential for fewer late effects. Magnetic resonance imaging (MRI) provides visualization of anatomical targets, monitoring of heating within the tumor via proton resonance frequency (PRF) shift MR thermometry,[9] and evaluation of the therapeutic effects.[4] A large, geometrically focused ultrasound transducer integrated into the MRI bed provides the source of energy for non-invasive deep tissue heating. At the focal spot where acoustic energy is concentrated, localized regions are heated rapidly to temperatures sufficient for thermal coagulation (>60°C), resulting in irreversible cell damage and tissue destruction with millimeter accuracy.[10] In addition to ablation, MR-HIFU can generate mild hyperthermia (40–45°C) as an adjuvant to radiotherapy and/or chemotherapy, or for selective tumor delivery of anticancer drugs such as thermosensitive liposomal doxorubicin.[11–14]
Though potentially promising, the feasibility of MR-HIFU in childhood cancer has never been addressed in a large clinical setting. To begin to understand this, we reviewed the anatomic location of all forms of pediatric sarcoma and neuroblastoma at diagnosis and at relapse diagnosed at our institution over a 5-year period. We evaluated the anatomic site, surrounding structures, and size of each tumor to determine the targetability by MR-HIFU. In our study, we aim to establish the number of pediatric sarcomas and neuroblastomas in a targetable anatomic location, to guide the design of initial clinical trials of MR-HIFU therapy in children.
Methods
Study Oversight
The study protocol was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center at Dallas. All patient identifying information was kept confidential and all guidelines set forth by the Health Insurance Portability and Accountability Act (HIPAA) Privacy rule were employed.
Study Patients
Pediatric patients with a pathological diagnosis of sarcoma or neuroblastoma from 1/1/2009 to 4/1/2014 were identified via the pediatric oncology tumor registry at Children’s Health. Of 143 patients diagnosed with sarcoma and of 66 patients diagnosed with neuroblastoma, 22 and 5 were excluded due to lack of available imaging (Computed tomography (CT) or MRI), respectively. The remaining 121 patients with sarcoma and 61 patients with neuroblastoma were analyzed.
Study Procedure
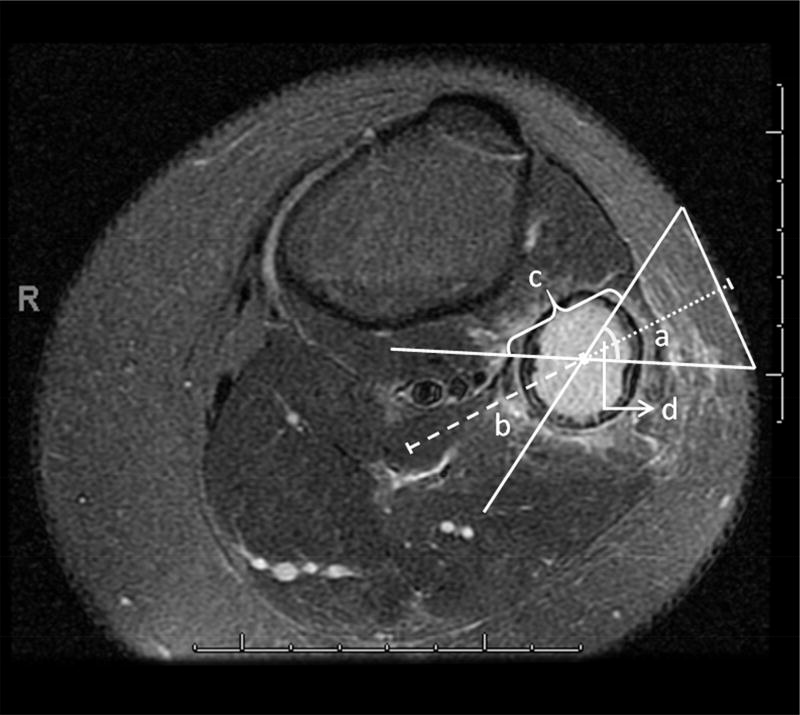
Clinical data was identified using the electronic medical records system, EPIC (Epic Systems, Verona, WI). Data collected included age, gender, number of CT scans, number of MRIs, therapies received, and survival. The hospital picture archival and communications system (PACS iSite Version 3.6.120.0, Philips Healthcare Informatics, Foster City, CA) was used to review CT or MR images. The primary lesion at diagnosis was determined based on the patient’s initial presentation and radiology reports. Up to four additional metastatic tumors at diagnosis and five tumors at relapse were reviewed. The PACS measurement tool was used to record and draw the nominal angle of the focused ultrasound beam cone, distance from the skin to center of target, length of lesion, and distance to the closest safety margin past the center of the target where the ultrasound energy can still cause considerable heating (Figure 1). Initial measurements were performed by a pediatric resident (JS) and each image was reviewed by a pediatric oncologist (TL) and MR physicists with expertise in MR-HIFU (RS and RC) until there was consensus about the targetability and approach to each tumor. Depth to tumor and tumor volumes were recorded, but were not considered a criterion for targetability.
Figure 1.
Dimensions of ultrasound beam and tumor measurements. “a” represents the distance from skin to center of lesion. “b” represents the safety margin of 4 cm past the focus. “c” represents the length of the lesion. Nominal angle of the ultrasound beam is 60 degrees “d.”
Study Parameters
At least two orthogonal views of the CT or MRI images were reviewed for three dimensional spatial considerations of the ultrasound beam path (Figure 2). Lesions were deemed not targetable if they had highly reflecting or absorbing structures such as bowel, trachea, or bone within the beam path between the ultrasound transducer and tumor or within a safety margin of 4 cm in depth beyond the tumor because of the risk for thermal damage to those structures. Lesions were also deemed not targetable if the ultrasound beam path fell within 1 cm of the brain or spinal cord, as this proximity carried risk of injury to the central nervous system. Lesions within 1 cm of skin were considered not targetable due to the risk of skin burn. Tumors within bone were considered targetable unless there was another obstruction in the ultrasound path. These criteria were based on the characteristics of an existing clinical MR-HIFU system (Sonalleve V2, Philips Healthcare, Vantaa, Finland).[15]
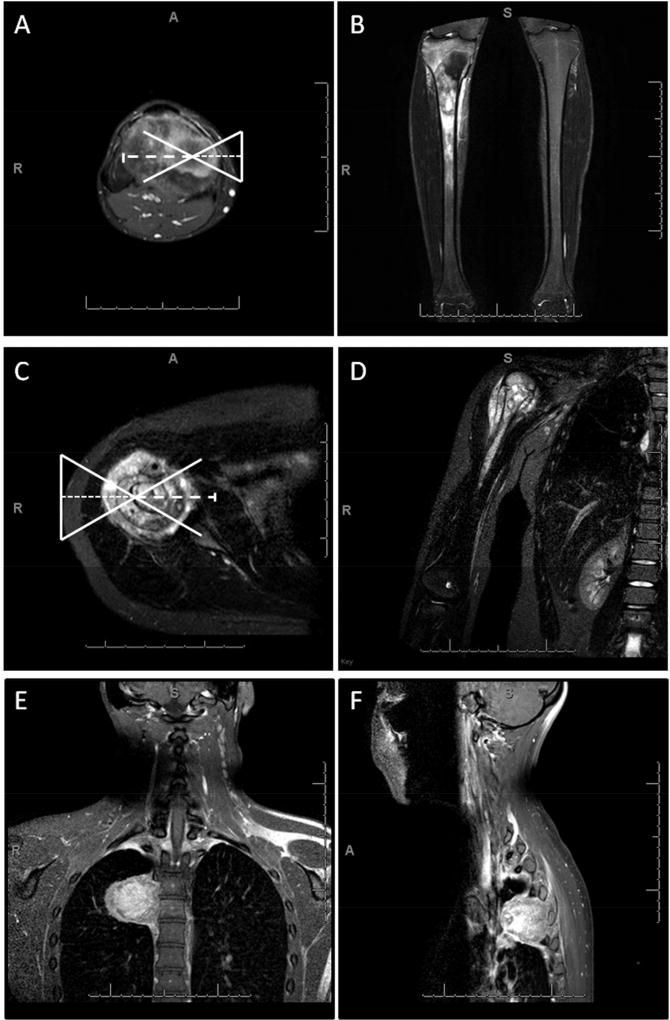
Figure 2.
Radiographic examples of targetable and non-targetable lesions. A, B) axial and coronal captures of right tibia osteosarcoma targetable with the current MR-HIFU system. C, D) axial and coronal captures of right proximal humerus osteosarcoma targetable if breath holding was used to reduce MR temperature mapping artifacts caused by motion of the nearby lungs. E, F) coronal and sagittal captures of thoracic spine Ewing sarcoma not targetable due to presence of ribs in the way and presence of highly reflecting lung within the far field safety margin.
Based on above criteria, each image of tumor was classified into one of the following categories: a) not targetable, b) completely or partially targetable with the currently available MR-HIFU system, c) potentially targetable if respiratory motion compensation (“breath holding”) was used (Figure 2). The last category applied to tumors within or immediately adjacent to organs affected by respiratory motion (lung, heart, liver, spleen, or intestines) but that otherwise met the above criteria. Partially targetable tumors were those in which only a portion (<50%) of the tumor was not targetable by the ultrasound beam due to one of the above limitations.
Statistical analysis
The binomial test was used to determine if the distribution between two groups was significantly different than 50% in each comparison. The Chi-square test for independence (all expected frequencies greater than or equal to 5) or Fisher’s exact test (at least one expected frequency less than 5) was used to analyze whether an association between two categorical variables existed. Means or medians were reported for continuous measures, and Student’s t-test or the Wilcoxon rank-sum test was used to test for differences by groups, respectively. All analyses were completed at the 0.05 significance level using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Demographics
As expected, OS, EWS, and RMS were the three most common sarcomas and 31% of patients had metastatic disease at diagnosis and 42% experienced recurrences.[1] As expected, OS and RMS more commonly occurred in males but this was not significant in our cohort (p=0.0705 and p=0.0771, respectively) (Table I). The majority of OS (76%) and EWS (58%) were diagnosed at ≥10 years while 69% of RMS were diagnosed at <10 years. Of the patients with neuroblastoma, 54% were male and the vast majority (84%) was diagnosed at <5 years.
TABLE I.
Demographics of 121 Patients with Sarcoma and 61 Patients with Neuroblastoma
| Gender | Age at diagnosis (years) | |||||
|---|---|---|---|---|---|---|
| Tumor Histology | Female | Male | 0 – 4 | 5 – 9 | 10 – 14 | 15 – 19 |
| Osteosarcoma | 13 | 24 | 1 | 8 | 14 | 14 |
| Rhabdomyosarcoma | 11 | 21 | 15 | 7 | 5 | 5 |
| Ewing sarcoma | 12 | 14 | 4 | 7 | 6 | 9 |
| Other sarcoma | 6 | 6 | 4 | 1 | 4 | 3 |
| Undifferentiated sarcoma | 4 | 5 | 4 | 2 | 3 | 0 |
| Synovial sarcoma | 2 | 2 | 0 | 1 | 1 | 2 |
| Malignant peripheral nerve sheath tumor | 0 | 1 | 0 | 0 | 0 | 1 |
|
| ||||||
| Neuroblastoma | 28 | 33 | 51 | 9 | 0 | 1 |
Targetability of Tumors at Diagnosis
Of the 121 patients with sarcoma, 64% had targetable primary lesions at diagnosis (Figure 3). Of these, 10 (13%) were only targetable using respiratory motion compensation, while 5 (6%) were only partially targetable with the existing system. The rest (81%) were completely targetable with the existing system. Thirty-seven patients (31%) had metastatic disease at diagnosis, with 123 metastatic tumors; of these, only 14% were targetable. Less than half of patients (45%) had targetable primary tumors at diagnosis and no metastatic disease.
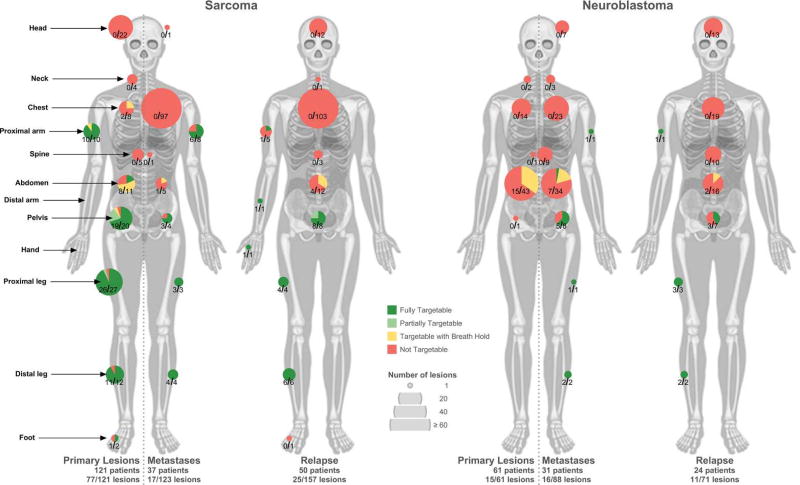
Figure 3.
Number of sarcoma lesions and neuroblastoma lesions and their targetability by anatomical location for primary vs. metastatic lesions (left vs. right sides of the body), at initial diagnosis and relapse. One patient with sarcoma at relapse was excluded due to inadequate imaging.
Of the 61 patients with neuroblastoma, no patients had primary lesions targetable with the current system, but 25% were targetable with respiratory motion compensation. Significantly fewer patients with neuroblastoma had targetable lesions at diagnosis than patients with sarcoma (p<0.0001) (Figure 3). Thirty-one patients (51%) had metastatic disease at diagnosis with a total of 88 tumors; of these, only 18% were targetable. Only 5% of patients had targetable primary tumors at diagnosis and no metastatic disease, significantly fewer than patients with sarcoma (p<0.0001).
Targetability of Tumors at Relapse
Of the patients with sarcoma, 42% had relapsed disease, of which 37 (73%) had multiple tumors (Figure 3). There were 157 sarcoma lesions at relapse and only 16% were targetable, of which 48% were bony lesions. In all, 35% of relapsed patients had at least one targetable tumor, significantly fewer than at diagnosis (p=0.0006).
Of the patients with neuroblastoma, 39% had relapsed disease, of which 19 (79%) had multiple tumors (Figure 3). There were 71 neuroblastoma tumors at relapse with only 15% targetable, of which 64% were bony lesions. In all, 29% of relapsed patients had at least one targetable tumor, not significantly different than at diagnosis (p=0.6645).
Targetability of Primary Tumors based on Histology and Location
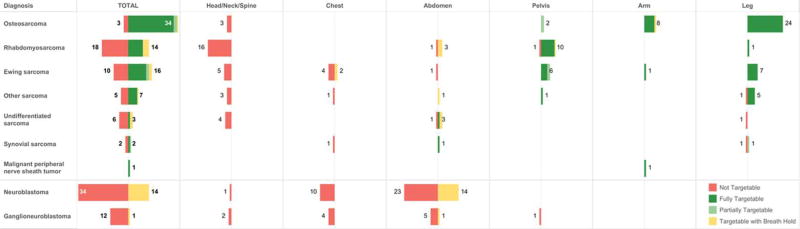
In children with sarcoma, the primary tumor was targetable in >50% of patients with OS, EWS, malignant peripheral nerve sheath tumor, and other sarcoma (Figure 4). The majority of targetable tumors were OS and EWS, which comprised 31% and 21% of the targetable tumors, respectively. Extremity and pelvic tumors were significantly more likely to be targetable than tumors in other sites, with 94% of extremity tumors and 95% of pelvic tumors being targetable (p<0.0001) (Figures 3 and 4).
Figure 4.
Targetability of primary sarcoma and neuroblastoma lesions at diagnosis based on histology and location.
All of the targetable primary neuroblastoma lesions were located in the abdomen (Figures 3 and 4). All targetable neuroblastoma lesions would require the use of respiratory motion compensation techniques. No tumors located in the chest, neck, pelvis, and spine were targetable.
Targetability of Metastatic Lesions and Relapse Lesions based on Location
For sarcoma, 79% of metastatic tumors at diagnosis and 66% of tumors at relapse were located in the chest, the vast majority being pulmonary metastases (Figure 3). None of these lesions were targetable. Extremity and pelvic tumors were significantly more likely to be targetable than those in other sites, comprising 79% and 92% of targetable non-primary lesions, respectively (p<0.0001).
For neuroblastoma, targetable metastatic lesions at diagnosis were mostly located in the abdomen (44%), pelvis (31%), and extremities (25%) (Figure 3). At relapse, targetable lesions were primarily located in the leg (45%), arm (9%), and pelvis (9%). Significantly fewer abdominal tumors were targetable at relapse compared to those in extremities and pelvis (p=0.0027).
Dimensions of Targetable Lesions
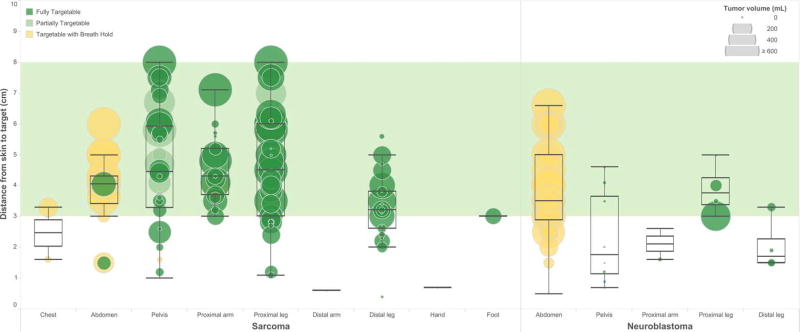
The median volume of targetable sarcoma tumors was 173.6 cm3 while for neuroblastoma was 176.7 cm3 (p=0.7556). However, the volume of targetable sarcoma and neuroblastoma lesions located in the abdomen was significantly larger than those in other locations (p<0.0001 and p=0.0015, respectively) (Figure 5). The mean distance from the skin to targetable sarcoma tumors was 4.1 cm, which is significantly greater than that of neuroblastoma tumors (3.3 cm) (p=0.0062). Both are within the target range for the current MR-HIFU system.
Figure 5.
Distance from skin to center of targetable sarcoma and neuroblastoma tumors (including primary and metastatic tumors at diagnosis and relapse) by location. For each lesion, color of circle indicates targetability, size indicates tumor volume. Green band indicates range of ideal target distance for existing MR-HIFU system.
Of the 94 targetable sarcoma tumors at diagnosis (primary and metastatic tumors), 80% were within the optimal target distance of 3–8 cm, 53% were within the optimal target volume of less than 200 cm3, and 38% were within both (Figure 5). Of these favorable tumors within target distance and volume, 44% were located in the leg or foot, 28% in the arm, 19% in the pelvis, 6% in the abdomen, and 3% in the chest. Of the 25 targetable sarcoma tumors at relapse, 68% were within the optimal target distance and 60% within the optimal target volume. 36% met both criteria. Of these favorable tumors, 67% were located in the pelvis and 33% in the leg. There was no significant difference in the proportion of targetable lesions meeting optimal size and distance criteria at diagnosis or relapse. However, at relapse the proportion of optimal lesions located in the pelvis was significantly greater (p=0.0106) and the proportion in the extremities was significantly less (p=0.0500).
Out of the 31 targetable neuroblastoma tumors at diagnosis, 61% were within the optimal distance and 39% were within the ideal volume range, and 6% met both criteria (Figure 5). Of these favorable tumors, all were located in the pelvis. Of the 11 targetable neuroblastoma tumors at relapse, 64% were within the optimal distance range and 73% were within the optimal volume range, and 45% were within both. Of these 5 favorable lesions, 3 were located in the proximal leg, 1 in the distal leg, and 1 in the pelvis. Significantly fewer neuroblastoma lesions were within optimal target parameters at diagnosis than at relapse (p=0.0085).
Respiratory Motion Compensation
Because MR-HIFU uses image subtraction to evaluate temperature, current clinical applications are limited to non-moving organs. We found that for sarcoma tumors in locations expected to be affected by respiratory motion, respiratory motion compensation may increase the percentage of targetable primary tumors by 9% (Figure 3). Respiratory motion was a greater problem for neuroblastomas, affecting 100% of targetable primary tumors (p<0.0001). Respiratory motion most commonly affected tumors located in the abdomen (p<0.0001). Of all tumors analyzed at diagnosis and at relapse, respiratory motion compensation may increase the percentage of targetable tumors by 4% for sarcomas and 10% for neuroblastoma.
Discussion
In our study, more than half of newly diagnosed children with sarcoma have a tumor that could be targeted by MR-HIFU. Our patient population is representative of children with sarcoma. OS, EWS, and RMS were the most common, and approximately one third of patients had metastatic disease at diagnosis and about one third experienced recurrences.[1] We found that most tumors located in the extremities and pelvis was targetable. As these are the most common location for sarcomas to arise, this suggests that MR-HIFU should be further explored for treatment of children with sarcoma. Neuroblastoma was less commonly targetable at diagnosis due to its frequent intra-abdominal location and proximity to the spine.
Despite the potential for broad application of MR-HIFU in newly diagnosed patients with sarcoma, only a small percentage of metastatic or relapsed tumors were targetable. All metastatic lung lesions were deemed not targetable due to the challenges posed by ultrasound propagation and respiratory motion. At relapse, all sarcoma tumors in the proximal arm were in axillary lymph nodes for which an adequate acoustic window was difficult to define. Also, at relapse, fewer of the targetable sarcoma tumors were located in the extremities, with more tumors in the pelvis. These findings may pose a challenge for initial clinical trials of MR-HIFU therapy as pelvic tumors are more likely to be affected by respiratory motion and peristalsis within the bowel than extremity tumors. Only a minority of neuroblastoma tumors were treatable at diagnosis or relapse. At diagnosis, all treatable neuroblastomas were intra-abdominal and not-targetable without the use of respiratory motion compensation. At relapse, many neuroblastoma patients had intracranial, intrathorassic, or intraabdominal tumors that were not targetable due to adjacent critical anatomic structures.
For the MR-HIFU system considered in this study, the ideal distance from the skin to the focal depth is 3–8 cm. However, in mild hyperthermia, the effective heating zone extends up to 3 cm beyond the target,[16] and thus lesions up to 11 cm deep may be targetable. While we evaluated the ability of the Philips MR-HIFU system to target these lesions, the focal depth limitation is not intrinsic to MR-HIFU therapy, but is dependent on the design of the ultrasound transducer. Thus, transducers could be designed to extend the depth of target. The ideal volume using this MR-HIFU system is 1–200 cm3 for mild hyperthermia,[16] or 0.1–10 cm3 for ablation.[15] Yet, recent tests from our research team show that larger volumes may be targetable, so the ideal volume of maximum 200 cm3 was a conservative target. Only 55% of targetable sarcoma lesions were less than 200 cm3, but since MR-HIFU can be delivered in multiple sessions, lesions within the targetable distance but beyond the ideal volume could still be targeted.
Here we found that using respiratory motion compensation or breath holds could expand the number of targetable primary tumors by 9% in sarcomas, and was particularly critical in neuroblastoma as all targetable primary tumors at diagnosis required the use of breath holds. PRF shift MR thermometry is susceptible to motion because of the image subtraction used to calculate temperature. Therefore, sources of motion such as respiration or peristalsis are obstacles of MR-HIFU therapy, even if an effective ultrasound beam path is available.[17] It is hypothesized that tumors affected by such motion may be targetable if breath holding was used in ventilated patients under general anesthesia,[18–20] or if another periodic motion compensation technique was implemented.[21, 22] Breath holding describes the use of intermittent periods of apnea in ventilated patients under general anesthesia to overcome the problem of respiratory movement for MR temperature mapping,[18–20] and general anesthesia is routinely used for pediatric patients undergoing diagnostic MR imaging. A study of MR thermometry in the breast with voluntary breath holds and background field correction showed reduction of motion-induced temperature mapping errors, but further investigation is needed in the context of pediatric patients.[23]
Our study has implications on the types of pediatric cancer that may be suitable for treatment using MR-HIFU and clinical trial design for MR-HIFU in children. MR-HIFU ablation using the ExAblate device (Insightec, Haifa, Israel) has been Food and Drug Administration (FDA) approved for painful bony metastases in adults who have failed radiation therapy based on the results of a randomized phase III study demonstrating improvement in pain response and quality of life.[5] In pediatric patients with osteoid osteoma, a prospective multicenter evaluation of MR-HIFU ablation of the nidus demonstrated durable pain reduction in 90% of patients,[24] and a comparative study matched to radiofrequency ablation identified no differences in response or complications.[25] We found that at relapse, about half of the targetable sarcoma tumors and two thirds of targetable neuroblastoma tumors are bony lesions in the pelvis or the extremities and would be targetable via MR-HIFU for ablation to provide pain palliation. This indication should be studied in pediatric patients with both sarcoma and neuroblastoma.
In addition to ablation, phase III clinical studies using other hyperthermia techniques have demonstrated improved local control when combined with radiation in recurrent breast, head and neck, and melanoma lesions,[26] or with traditional neo-adjuvant chemotherapy in soft tissue sarcoma.[27] In preclinical studies, MR-HIFU hyperthermia has been demonstrated to induce local release of doxorubicin from thermosensitive liposomes,[11] achieving significant drug uptake in targeted tumors,[13] and reduction in tumor growth.[28] The ability to target chemotherapy with MR-HIFU to a tumor and augment chemotherapy response would be a major clinical advance and could potentially decrease systemic toxicity, increase local control, or both. This strategy should be further studied for the approximately 50% of newly diagnosed children with sarcoma who have MR-HIFU targetable lesions at diagnosis without metastatic disease.
One challenge for initial studies of MR-HIFU drug delivery will be that a significantly fewer number of relapse patients have targetable tumors, and of those, most are located in the pelvis. Pelvic tumors may be technically more challenging to target than extremity tumors which are more common at diagnosis. Without further technological advances, use of MR-HIFU for drug delivery in neuroblastoma would be primarily limited to the relapsed setting as only a minority of tumors are targetable at diagnosis, and of those, most are outside of the optimal depth and/or volume.
The strengths of this study include the large sample size and the review of each radiologic image by the team members who are familiar with the MR-HIFU technology. A limitation of this study is the use of two-dimensional images to measure angles and distances. A more advanced approach using three-dimensional modeling of the MR-HIFU system and the images may improve accuracy in predicting targetable lesions. In light of this, we applied a conservative bias in our radiographic review, which may have led to an under-estimated number of patients with targetable tumors. Furthermore, future improvements in MR-HIFU technology will increase the number of targetable lesions by improving the ability to compensate for respiratory motion and by extending the targetable depth and volume ranges through the use of new ultrasound transducers. As a retrospective study at a large pediatric oncology center, a referral bias toward more advanced disease may be present. However, the percentage of patients with metastatic or relapsed disease is consistent with that expected, suggesting this is not a major limitation.[1]
In conclusion, the majority of pediatric sarcomas are targetable by MR-HIFU at initial diagnosis, but neuroblastoma and relapsed sarcoma pose greater technical challenges. Further technical development of MR-HIFU should focus on the primary obstacles identified here, especially the need for respiratory motion compensation. Prospective clinical trials evaluating MR-HIFU ablation for children with solid tumors and painful bony metastases and MR-HIFU hyperthermia for drug delivery in children with sarcoma are warranted.
Acknowledgments
The authors would like to thank Dr. Stephen Skapek for his support and review of the article. This work was funded by The Hyundai Hope on Wheels Foundation (to TWL), the Cancer Prevention Research Institute of Texas (R1308 to RC), and the National Cancer Institute (R01 CA199937 to TWL and RC).
Abbreviations
- MR-HIFU
Magnetic Resonance-guided High Intensity Focused Ultrasound
- OS
Osteosarcoma
- EWS
Ewing sarcoma
- RMS
Rhabdomyosarcoma
- NRSTS
Non-rhabdomyosarcoma soft tissue sarcoma
- MRI
Magnetic Resonance Imaging
- HIPAA
Health Insurance Portability and Accountability Act
- PRF
Proton Resonance Frequency
- CT
Computed Tomography
- PACS
Picture Archival and Communications System
- FDA
Food and Drug Administration
Footnotes
Conflict of Interest
Robert Staruch is a paid employee of Philips. No other authors report any conflicts of interest.
References
- 1.HaDuong JH, Martin AA, Skapek SX, Mascarenhas L. Sarcomas. Pediatr Clin North Am. 2015;62:179–200. doi: 10.1016/j.pcl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Irwin MSP, Park JR. Neuroblastoma. Pediatr Clin North Am. 2015;62:225–256. doi: 10.1016/j.pcl.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Arndt CAS, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues DB, Stauffer PR, Vrba D, Hurwitz MD. Focused ultrasound for treatment of bone tumours. Int J Hyperthermia. 2015;31:260–27. doi: 10.3109/02656736.2015.1006690. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, Kuten A, Meyer JE, LeBlang SD, Roberts A, Choi J, Lamer JM, Napoli A, Turkevich VG, Inbar Y, Tempany CMC, Pfeffer RM. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106:1–9. doi: 10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huisman M, Lam MK, Bartels LW, Nijenhuis RJ, Moonen CT, Knuttel FM, Verkooijen HM, van Vulpen M, van den Bosch MA. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound. 2014;2:16. doi: 10.1186/2050-5736-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15:1–86. [PMC free article] [PubMed] [Google Scholar]
- 8.Yu W, Tang L, Lin F, Yao Y, Shen Z, Zhou X. High-intensity focused ultrasound: noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg Oncol. 2015;24:9–15. doi: 10.1016/j.suronc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, Suzuki Y. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- 10.Brown MR, Farquhar-Smith P, Williams JE, ter Haar G, deSouza NM. The use of high-intensity focused ultrasound as a novel treatment for painful conditions-a description and narrative review of the literature. Br J Anaesth. 2015;115:520–530. doi: 10.1093/bja/aev302. [DOI] [PubMed] [Google Scholar]
- 11.Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. 2011;27:156–171. doi: 10.3109/02656736.2010.518198. [DOI] [PubMed] [Google Scholar]
- 12.Staruch R, Chopra R, Hynynen K. MRI-controlled ultrasound thermal therapy. IEEE Pulse. 2011;2:39–47. doi: 10.1109/MPUL.2011.942604. [DOI] [PubMed] [Google Scholar]
- 13.Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. 2012;28:776–787. doi: 10.3109/02656736.2012.736670. [DOI] [PubMed] [Google Scholar]
- 14.Hijnen N, Langereis S, Grull H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Deliv Rev. 2014;72:65–81. doi: 10.1016/j.addr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Trillaud H, Rhim H, Lim HK, Mali W, Voogt M, Barkhausen J, Eckey T, Kohler MO, Keserci B, Mougenot C, Sokka SD, Soini J, Nieminen HJ. MR thermometry analysis of sonication accuracy and safety margin of volumetric MR imaging-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Radiology. 2012;265:627–637. doi: 10.1148/radiol.12111194. [DOI] [PubMed] [Google Scholar]
- 16.Tillander M, Hokland S, Koskela J, Dam H, Andersen NP, Pedersen M, Tanderup K, Ylihautala M, Kohler M. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43:1539–1549. doi: 10.1118/1.4942378. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS. Advances in MR image-guided high-intensity focused ultrasound therapy. Int J Hyperthermia. 2015;31:225–232. doi: 10.3109/02656736.2014.976773. [DOI] [PubMed] [Google Scholar]
- 18.Anzidei M, Napoli A, Sandolo F, Marincola BC, Di Martino M, Berloco P, Bosco S, bezzi M, Catalano C. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Intervent Radiol. 2014;37:1611–1617. doi: 10.1007/s00270-014-0861-x. [DOI] [PubMed] [Google Scholar]
- 19.Gedroyc WM. New clinical applications of magnetic resonance-guided focused ultrasound. Top Magn Reson Imaging. 2006;17:189–194. doi: 10.1097/RMR.0b013e318038f782. [DOI] [PubMed] [Google Scholar]
- 20.Kopelman D, Inbar Y, Hanannel A, Freundlich D, Castel D, Perel A, Greenfeld A, Salamon T, Sareli M, Valeanu A, Papa M. Magnetic resonance-guided focused ultrasound surgery (MRgFUS): ablation of liver tissue in a porcine model. Eur J Radiol. 2006;59:157–162. doi: 10.1016/j.ejrad.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Quesson B, Laurent C, Maclair G, de Senneville BD, Mougenot C, Ries M, Carteret T, Rullier A, Moonen CT. Real-time volumetric MRI thermometry of focused ultrasound ablation in vivo: a feasibility study in pig liver and kidney. NMR Biomed. 2011;24:145–153. doi: 10.1002/nbm.1563. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook AB, Ghanouni P, Santos JM, Dumoulin C, Medan Y, Pauly KB. Respiration based steering for high intensity focused ultrasound liver ablation. Magn Reson Med. 2014;71:797–806. doi: 10.1002/mrm.24695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt CR, Soher BJ, MacFall JR. Correction of breathing-induced errors in magnetic resonance thermometry of hyperthermia using multiecho field fitting techniques. Med Phys. 2010;37:6300–6309. doi: 10.1118/1.3515462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger D, Napoli A, Conchiglia A, Gregori LM, Arrigoni F, Bazzocchi A, Busacca M, Moreschini O, Mastantuono M, Albisinni U, Masciocchi C, Catalano C. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: a prospective multicenter evaluation. J Bone Joint Surg Am. 2014;96:743–751. doi: 10.2106/JBJS.M.00903. [DOI] [PubMed] [Google Scholar]
- 25.Masciocchi C, Zugaro L, Arrigoni F, Gravina GL, Mariani S, La Marra A, Zoccali C, Flamini S, Barile A. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol. 2015 doi: 10.1007/s00330-015-4111-7. [DOI] [PubMed] [Google Scholar]
- 26.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 27.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, Vujaskovic Z, Wessalowski R, Jauch KW, Durr HR, Ploner F, Baur-Melnyk A, Mansmann U, Hiddemann W, Blay JY, Hohenberger P, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: therapeutic effect in rabbit Vx2 tumours. Int J Hyperthermia. 2015;31:118–133. doi: 10.3109/02656736.2014.992483. [DOI] [PubMed] [Google Scholar]