Abstract
BACKGROUND
In the Trial to Reduce Alloimmunization to Platelets (TRAP) study, 101 of 530 subjects became clinically refractory (CR) to platelets without lymphocytotoxicity assay (LCA) detectable anti-HLA antibodies. The LCA only detects complement-binding antibodies, and is less sensitive than newer assays. Utilizing a more sensitive bead-based assay that does not distinguish between complement-binding versus non-complement-binding antibodies, we have previously shown that while many LCA-negative (LCA−) patients do have anti-HLA antibodies, these low-to-moderate level antibodies do not predict refractoriness. As complement can contribute to platelet rejection, we assessed if previously undetected complement-binding antibodies account for refractoriness among LCA− patients.
STUDY DESIGN AND METHODS
Samples from 169 LCA− (69 CR, 100 non-CR) and 20 LCA-positive (LCA+) (10 CR, 10 non-CR) subjects were selected from the TRAP study serum repository. Anti-class I HLA IgG and C1q-binding antibodies were measured in serum or plasma with bead-based detection assays. Levels of C1q-binding antibodies were compared between CR and non-CR subjects, and correlated with corrected count increments (CCIs).
RESULTS
While some of the LCA− subjects had detectable C1q-binding anti-class I HLA antibodies, and some LCA+ subjects did not, levels were significantly higher among LCA+ subjects. C1q-binding anti-class I HLA antibody levels did not differ significantly between CR and non-CR among either the LCA− or LCA+ subjects. Furthermore, there was no significant correlation observed between CCIs and either C1q-binding or any anti-HLA IgG antibodies.
CONCLUSIONS
This work confirms that low to mid level anti-class I antibodies do not drive platelet rejection, suggesting a role for antibody-independent mechanisms.
Keywords: Alloimmunization, HLA Antibodies, Complement, Platelet Refractoriness
INTRODUCTION
Blood transfusion exposes recipients to a wide range of alloantigens expressed on the surface of red cells, lymphocytes, and platelets. In response to these exposures, many recipients develop antibodies against some of these antigens, which can complicate subsequent transfusions and solid organ transplants.1–6 Recipients of platelet transfusions most commonly develop antibodies against human leukocyte antigens (HLA) that are expressed on the surface of platelets and white blood cells.1 These antibodies are found in 7–55% of platelet recipients, depending on a number of factors including the number of transfusions, platelet preparation, and patient population.1,7–15 Anti-class I HLA antibodies can lead to platelet refractoriness, requiring HLA matching of subsequent platelet transfusions, which can lead to delays in treatment and roughly doubles the cost per unit. Leukoreduction has been shown to reduce the frequency of alloimmunization, as well as the magnitude and persistence of this antibody response, but has not eliminated this complication.8,10–13,15–17
Anti-class I HLA antibodies can facilitate rejection of allogeneic platelets through several different mechanisms. Once antibodies bind their target class I HLA antigen on the surface of an allogeneic platelet, they can facilitate uptake by macrophages or other scavenger cells via Fc receptor binding.18,19 Alternatively, a subset of these antibodies can bind C1q protein, which can activate the classical complement cascade leading to direct lysis of the platelets as well as enhance phagocytosis by scavenger cells expressing the C1q receptor.20,21
A number of different assays are used to detect anti-HLA antibodies, with varying sensitivities and specificities. The lymphocytotoxicity assay (LCA) is an older assay that detects only complement-binding antibodies by incubating the serum to be screened with cells expressing various HLA antigens, and measuring cell lysis.7,22,23 More recently a range of new assays have been developed utilizing either multianalyte bead-based platforms, enzyme-linked immunosorbant assays (ELISAs), or flow cytometry.24–30 These newer assays are generally more sensitive than the LCA, and some of them have the ability to detect and/or distinguish between different types of antibodies including complement binding and non-complement binding.31
The Trial to Reduce Alloimmunization to Platelets (TRAP) study evaluated the effectiveness of leukoreduction and ultraviolet light (UV) treatment in prevention of alloimmunization and platelet refractoriness among a large cohort of acute myeloid leukemia patients receiving multiple platelet transfusions.7 The study found that these treatments did reduce the rates of new anti-HLA antibody generation, from 45% for non-leukoreduced to 17% or 21% for leukoreduced or UV treated, and that this also reduced platelet refractoriness. Intriguingly, 101 of the 530 subjects became refractory without detectable anti-HLA antibodies. As the study used the LCA to detect antibodies, this suggested that either the antibodies were below the limits of detection of this assay, or that antibody-independent mechanisms were responsible for platelet refractoriness in these subjects. Using a bead-based assay, we have previously shown that while many of the subjects who previously tested negative for anti-HLA antibodies with the LCA did have detectable antibodies using this more sensitive assay, these low-to-moderate level antibodies were not associated with platelet refractoriness.32 The assay used in the previous study did not, however, distinguish between complement binding and non-complement binding antibodies, which have been shown to be more clinically relevant to platelet recovery.31 In addition, the previous study did not assess if these low-to-moderate level antibodies were associated with lower CCIs that did not meet the threshold to be classified as refractory. As a result, though our findings suggested non-immune mediated mechanisms were responsible for these LCA− refractory cases, we were not able to completely discount a role for these lower level antibodies in platelet rejection among these subjects.
As the impact of low-to-moderate level complement-binding antibodies in platelet rejection may have been masked by non-complement-binding antibodies, we have now measured complement-binding antibodies using sensitive bead-based assays in these subjects to determine if previously undetected complement-binding antibodies might be driving platelet refractoriness among the LCA− subjects. In addition, as platelet refractoriness is defined using a categorical cutoff in corrected count increment (CCI), we have now examined the relationship of both complement-binding and all anti-class I HLA antibodies with CCIs to determine if these lower level antibodies are associated with even small reductions in platelet CCI following transfusion.
MATERIALS AND METHODS
Subjects and samples
One hundred sixty-nine LCA− (69 clinically refractory (CR+), 100 clinically non-refractory (CR−)) and 20 LCA+ (10 CR+, 10 CR−) subjects were selected from the TRAP study7 as previously described.15,32 Patients were categorized as CR+ if they had a one-hour post-transfusion corrected count increment (CCI) of less than 5000 after 2 sequential transfusions of ABO-compatible platelets as described in the original TRAP analysis.7 Single samples were selected for screening for each patient from among the available longitudinal samples according to where the peak anti-HLA IgG response had been detected in earlier studies. Samples were collected under IRB approved protocols that included written informed consent.
Antibody detection
Both any anti-class I HLA IgG and specific C1q-binding anti-class I HLA IgG antibodies were screened using three different methods: the LabScreen mixed Luminex assay (LSM), the LabScreen single antigen class I assay (LS1A04), and the LabScreen single antigen class I assay with added EDTA (LS1A04 + EDTA) (One Lambda, Canoga Park, CA). To detect C1q-binding antibody, the C1qScreen assay (One Lambda, Canoga Park, CA) was used to test the HLA coated beads (LSM and LS1A04). This assay uses R-phycoerythrin labeled anti-human C1q antibody for detection of C1q-binding anti-HLA antibodies bound to HLA coated antigen beads. For the LabScreen mixed Luminex assay, normalized background (NBG) ratios for each of the eight multi-antigen beads were determined, with the highest value for each sample reported. For the LabScreen single antigen class I assay with or without added EDTA, the highest trimmed mean for each sample is reported.
Statistical analysis
Comparisons between groups were made with unpaired t-tests using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA). Correlation between variables was evaluated using GraphPad Prism. The decline in CCIs was calculated by subtracting the lowest recorded 1 hour CCI from the 1 hour CCI measured following the first measured platelet transfusion for the 1hr CCI decline, or by subtracting the lowest recorded 24 hour CCI from the 24 hour CCI measured following the first measured platelet transfusion for the 24hr CCI decline.
RESULTS
Assay reproducibility and optimization
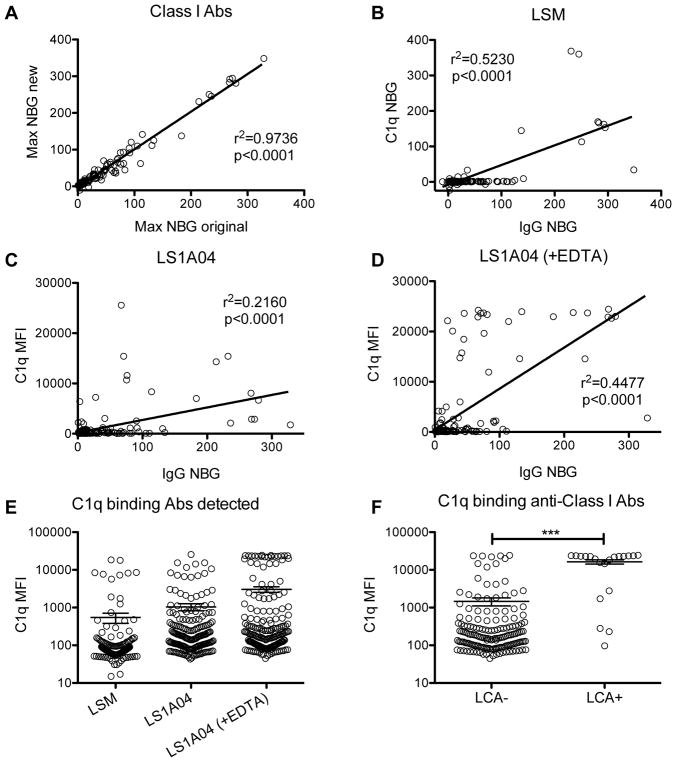
As the assays for anti-class I C1q-binding IgG were run at a different study site than the previous analyses using sample remnants, there was some concern of site and kit variability as well as potential sample degradation. To address this, we first compared the levels of any detected anti-class I HLA IgG antibodies between the current assays and previously published results.32 Max NBG ratios were plotted for each sample and correlations between the old and new measurements were evaluated (Figure 1A). A strong correlation was observed between these two measurements with an r2 = 0.9736 (p<0.0001), demonstrating good reproducibility with the original measurements.
Figure 1. Higher levels of C1q-binding class I HLA antibody among LCA+ subjects.
Serum samples from TRAP study subjects that were previously screened for anti-class I HLA IgG antibodies were retested for both any anti-class I HLA IgG and C1q-binding anti-class I HLA antibodies at a different study site. (A) To assess reproducibility of the original findings the max NBG ratios of any detected anti-class I HLA IgG antibodies were compared between the current assays and the previously published results. Correlation between these two values was evaluated, and the r2 was 0.9736 with p<0.0001. Three different assays were used to measure C1q-binding class I HLA antibodies, (B) the LabScreen mixed Luminex assay (LSM) with NBG ratios reported, (C) the LabScreen single antigen class I assay (LS1A04) with MFI values reported, and (D) the LabScreen single antigen class I assay with added EDTA (LS1A04 (+EDTA)) with MFI values reported and each was correlated with the max NBG ratio for any detectable anti-class I HLA IgG antibodies. (E) As NBG ratios were not available for the LS1A04 assays, the distribution of max MFIs from each assay were plotted for comparison. Bars indicate mean and standard error. (F) Samples of platelet transfusion recipients from the TRAP study were previously screened for anti-HLA antibodies using the lymphocytotoxicity assay (LCA). C1q-binding class I HLA antibodies were measured using the LABScreen single antigen class I assay with added EDTA, and max MFIs were plotted for LCA− versus LCA+ subjects. Bars indicate mean and standard error. Groups were compared by unpaired t-test, ***p<0.0001.
To assess the complement-binding capability of anti-HLA antibodies present in these samples, three different assays were used. The first was the LABScreen Mixed (LSM), which does not distinguish between different HLA specificities of the antibody, but instead uses multiple beads, each coated with multiple HLA antigens to bind anti-HLA antibodies, followed by a detection step using C1q. The levels detected with this assay were plotted against and correlated with the levels of any anti-class I HLA IgG antibodies detected (Figure 1B), and while a significant correlation was observed (r2 = 0.5230, p<0.0001), the assay did not appear to detect much C1q binding antibody. The second and third assays used a single antigen detection screen (LS1A04), either with or without EDTA added to counteract the prozone phenomenon (when high concentrations of antibodies saturates antigen binding, preventing cross-linking and detection).33 This assay distinguishes between different class I HLA specificities by using beads coated with specific single antigens. The levels detected with each of these assays were also plotted against and correlated with the levels of any anti-class I HLA IgG antibodies detected (Figure 1C–D) with significant correlations observed (r2 = 0.2160, <0.0001, and r2 = 0.44477, <0.0001, respectively). All 3 assays were compared directly by plotting the maximum MFIs detected by each assay (Figure 1E). The LS1A04 assay with added EDTA appeared to be the most sensitive and had a clear bimodal distribution of MFIs, so data collected using this assay were used for the remainder of the analyses.
C1q-binding anti-HLA antibodies do not predict platelet refractoriness
As antibody complement-binding activity may be important for rejection of donor platelets, we next looked to see if we could detect complement binding anti-class I HLA antibodies missed by the LCA. C1q-binding anti-class I HLA MFIs were plotted comparing LCA− and LCA+ patients (Figure 1F). The majority (15/20) of the LCA+ samples had very high-level antibodies, and overall the levels were significantly higher among the LCA+ samples (p<0.0001) compared with the LCA− samples. There were, however, several LCA− samples with high or moderate level C1q-binding anti-class I HLA antibodies and 5 LCA+ samples with low or moderate levels of C1q-binding anti-class I HLA antibodies as well.
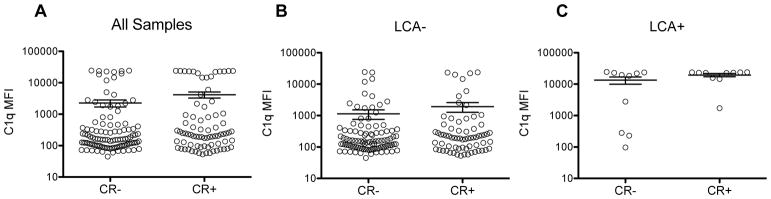
To determine if the newly detected complement-binding antibodies could account for some of the platelet refractoriness seen among the LCA− subjects, levels of detected C1q-binding anti-class I HLA antibodies were compared between refractory (CR+) and non-refractory (CR−) subjects (Figure 2). No significant differences were seen between the refractory and non-refractory subjects among either all subjects (Figure 2A), the LCA− (Figure 2B), or the LCA+ (Figure 2C) subjects.
Figure 2. C1q-binding class I HLA antibody levels not associated with platelet refractoriness.
C1q-binding anti-class I HLA antibodies were measured using the LABScreen single antigen class I assay with added EDTA, and max MFIs are plotted for non-clinically refractory (CR−) versus clinically refractory (CR+) subjects (A) among all subjects, (B) LCA− subjects or (C) LCA+ subjects. Bars indicate mean and standard error. Groups were compared by unpaired t-test.
Low to moderate C1q-binding and total anti-HLA antibodies are not associated with reduced CCIs
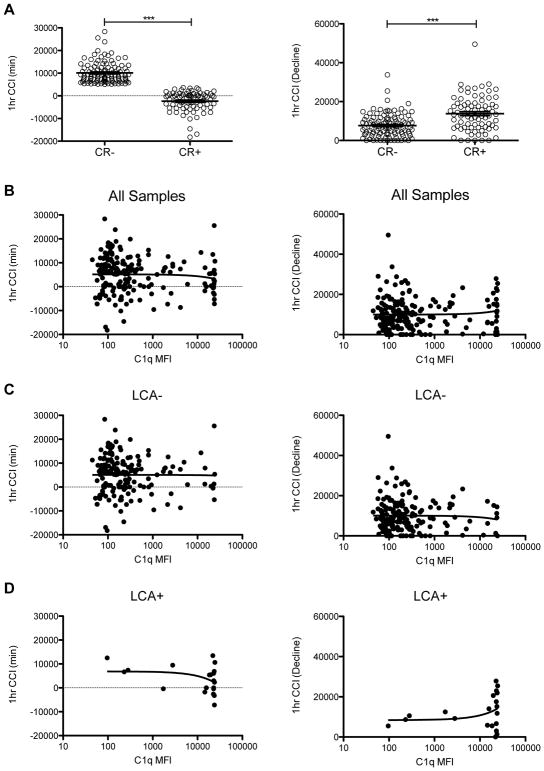
Low-to-moderate C1q-binding antibodies do not account for the platelet refractoriness seen in the LCA− TRAP subjects, but they could still cause some platelet loss resulting in poorer platelet CCIs that did not meet the trial definition of refractoriness. To assess this, the minimum 1 hour CCI measured for each subject was determined, along with a measure of the decline in CCIs. These values were compared between the CR+ and CR− subjects, with significantly lower minimum values and a significantly higher decline in CCI values compared to baseline CCI among the refractory subjects (Figure 3A). These values were next plotted against the level of detected C1q-binding anti-HLA antibodies for all subjects (Figure 3B), LCA− subjects (Figure 3C), and for LCA+ subjects (Figure 3D), and correlations were accessed. There was no significant correlation observed for any of these comparisons, though the sample size was very small for the LCA+ subjects. The same analysis was done using the 18–24 hour CCI to see if these antibodies might be more important in delayed platelet rejection (Figure S1). No significant correlations were observed for any of these comparisons.
Figure 3. C1q-binding class I HLA antibody levels not associated with reduced CCIs.
The minimum 1 hour CCI (lowest measured) and the decline in 1 hour CCI (earliest measured CCI - minimum) were determined for each subject. (A) These values were plotted for non-clinically refractory (CR−) versus clinically refractory (CR+) subjects, and groups were compared by unpaired t-test, ***p<0.0001. Correlation was evaluated between these measures of CCI and levels of measured C1q-binding anti-class I HLA antibodies for (B) all samples, (C) LCA− samples, and (D) LCA+ samples.
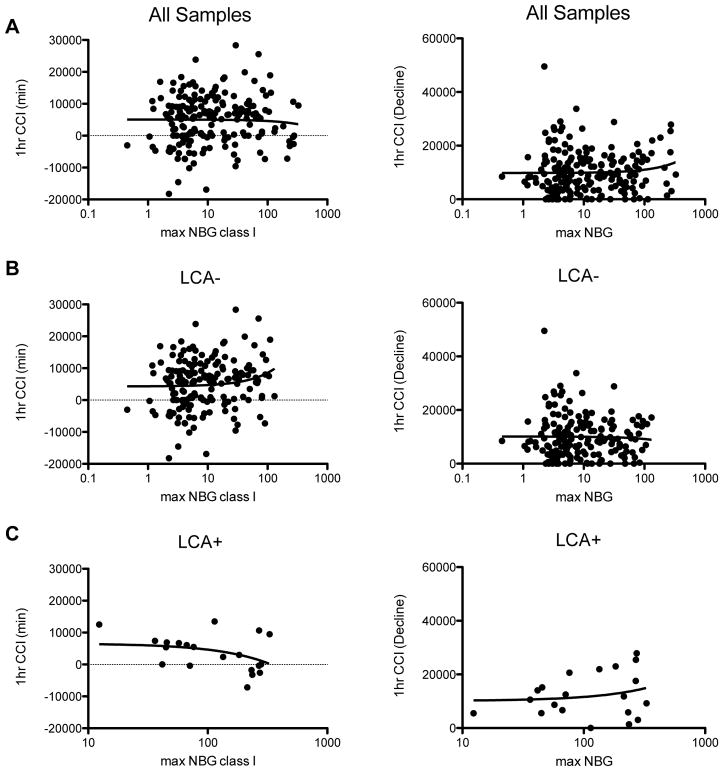
We next compared the minimum 1 hour CCI and the decline in 1 hour CCI with all anti-class I HLA antibody levels to determine if low-to-moderate level antibodies might predict more subtle platelet loss than what is picked up using our cutoff for platelet refractoriness. Correlation was assessed between minimum 1 hour CCI and the maximum normalized detected class I antibody and the decline in 1 hour CCI and the maximum normalized detected class I antibody for all subjects (Figure 4A), LCA− subjects (Figure 4B), and LCA+ subjects (Figure 4C). No significant correlations were observed for any of these comparisons. These analyses were also done using the 18–24 CCI values, with no significant correlations observed (Figure S2).
Figure 4. Total class I HLA antibody levels not associated with reduced CCIs.
The correlation between both the minimum 1 hour CCI and the decline in 1 hour CCI with total anti-class I HLA antibodies was evaluated for (A) all samples, (B) LCA− samples, and (C) LCA+ samples.
DISCUSSION
Allogeneic platelet transfusion can result in generation of an anti-HLA antibody response, which can in turn result in failure of subsequent platelet transfusions through antibody mediated complement lysis and opsonization. In the TRAP study, while the presence of LCA detected antibodies was associated with development of platelet refractoriness, 101 of 530 subjects were refractory in the absence of these antibodies.7 Low-to-moderate level anti-HLA antibodies detected with more sensitive bead-based assays among the LCA− TRAP subjects do not produce platelet refractoriness.32 Here, using these more sensitive bead-based assays, adapted specifically for C1q-binding anti-class I HLA antibody detection, we have assayed the TRAP samples for complement-binding antibodies to see if they might account for some of the platelet refractoriness among the LCA− TRAP subjects. We found that many of the LCA− samples were positive for C1q-binding antibodies using these more sensitive assays, but that these antibodies were also not associated with platelet refractoriness. Furthermore, neither the C1q-binding antibodies nor any anti-class I HLA IgG antibodies were associated with lower CCIs that did not meet the criteria for platelet refractoriness.
The new assay for C1q-binding antibodies was generally consistent with the original LCA results, with significantly higher levels of antibodies detected among the LCA+ individuals with very high level antibodies detected in 15/20 of these subjects (Figure 1). The remaining 5 samples with weaker signal might be the result of antigens better picked up by the LCA. Alternatively, this could be due to timing as we were only able to run a single sample for each subject in the current analysis, and while we selected samples based on peak total anti-class I HLA antibody response, this might not have been the peak of the complement-binding antibody response for all subjects. A wide range of C1q-binding antibody levels was seen among the LCA− samples as well, including some individuals with levels comparable to the high LCA+ samples. This confirms that the new assay was able to detect complement-binding antibodies missed by the initial LCA screen.
The levels of these newly detected C1q-binding assays did not differ between the refractory and non-refractory LCA− subjects, with a similar distribution seen in each group (Figure 2B). There was also no significant difference seen between the refractory and non-refractory LCA+ subjects, though this may be the result of small sample size; the 3 lowest signals were seen in the non-refractory group (Figure 2C).
One criticism of our earlier study was that lower level antibodies might cause moderate platelet loss, but not enough to reach the refractory threshold used (a CCI less than 5000 after 2 sequential transfusions with ABO-compatible platelets). We tested this hypothesis both for C1q-binding and any anti-class I HLA IgG antibodies and found no correlation between levels of antibody and either the 1 hour or 18–24 hour CCIs for the LCA− subjects (Figures 3C, 4B, S3C, and S4B). No significant correlations were seen among the LCA+ subjects either (Figure 3D, 4C, S3D, and S4C), though again, the sample sizes were small, and for the C1q-binding antibodies, the distribution was very tight (Figure 3D and S3D). The trends were, however, in the expected direction for the LCA+ subjects, that is, lower CCIs with increasing antibody MFIs.
Taken together, this work confirms that non-antibody mediated mechanisms are responsible for the refractoriness seen among the LCA− subjects. A number of non-immune factors have been shown to be associated with platelet refractoriness including splenomegaly, sepsis, disseminated intravascular coagulation (DIC), venoocclusive disease, and graft-versus-host disease.2,34,35 Among the TRAP cohort, increased risk of refractoriness was shown to be associated with heparin use, fever, bleeding, higher weight, and men, and additional factors such as infection and palpable spleen were associated significant reductions in CCIs.2
While these non-immune mediated mechanisms are known to result in platelet refractoriness, it is somewhat surprising that the presence of low-to-moderate level alloantibodies does not appear to contribute to platelet loss. One likely explanation for this is the antigen specificities of these lower level responses. We have observed in other studies that a wider range of antibody specificities is seen in patients with high levels of antibody.36 This makes some intuitive sense, as multiple allogeneic exposures would potentially boost levels of specific antibodies where there were overlapping alloantigens, as well as introduce new specificities with each exposure as unique alloantigens are seen. The greater the range of the specificities, the more likely that an antibody will be reactive against a subsequent new allogeneic donor resulting in platelet rejection. If this explanation is correct, low-level antibodies should be perfectly able to drive platelet rejection if the donor platelets happen to have HLA antigens matching the specificity of the antibodies. This is indeed what we have seen in mouse models, where very low levels of antigen specific antibody efficiently reject donor platelets (RPJ, manuscript submitted). In addition to increasing the level of antibodies, repeated exposure to the same alloantigens should increase the affinity of the antibodies produced, so higher level antibodies may also be associated with more potent antibodies.
Taken together, these data confirm that antibody-independent mechanisms are responsible for a large portion of the platelet transfusion refractory TRAP cases. Furthermore, the presence of anti-HLA antibodies, while associated with an increased risk of refractoriness when present at high levels, do not always result in poorer platelet transfusion outcomes.
Supplementary Material
Acknowledgments
Sources of support: This work was supported by NIH R01 HL-095470
This work was supported by NIH R01 HL-095470. This manuscript was prepared using TRAP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center.
Footnotes
Conflict of interest: The authors have no conflict of interest.
References
- 1.Howard JE, Perkins HA. The natural history of alloimmunization to platelets. Transfusion. 1978;18:496–503. doi: 10.1046/j.1537-2995.1978.18478251250.x. [DOI] [PubMed] [Google Scholar]
- 2.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–14. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itescu S, Tung TC, Burke EM, et al. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998;98:786–93. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 4.Massad MG, Cook DJ, Schmitt SK, et al. Factors influencing HLA sensitization in implantable LVAD recipients. The Annals of thoracic surgery. 1997;64:1120–5. doi: 10.1016/s0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 5.Moazami N, Itescu S, Williams MR, et al. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1998;17:876–80. [PubMed] [Google Scholar]
- 6.Tsau PH, Arabia FA, Toporoff B, et al. Positive panel reactive antibody titers in patients bridged to transplantation with a mechanical assist device: risk factors and treatment. ASAIO journal. 1998;44:M634–7. doi: 10.1097/00002480-199809000-00067. [DOI] [PubMed] [Google Scholar]
- 7.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–9. doi: 10.1056/NEJM199712253372601.
- 8.Andreu G, Dewailly J, Leberre C, et al. Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood. 1988;72:964–9. [PubMed] [Google Scholar]
- 9.Dutcher JP, Schiffer CA, Aisner J, et al. Alloimmunization following platelet transfusion: the absence of a dose-response relationship. Blood. 1981;57:395–8. [PubMed] [Google Scholar]
- 10.Fisher M, Chapman JR, Ting A, et al. Alloimmunisation to HLA antigens following transfusion with leucocyte-poor and purified platelet suspensions. Vox Sang. 1985;49:331–5. doi: 10.1111/j.1423-0410.1985.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 11.Murphy MF, Metcalfe P, Thomas H, et al. Use of leucocyte-poor blood components and HLA-matched-platelet donors to prevent HLA alloimmunization. Br J Haematol. 1986;62:529–34. doi: 10.1111/j.1365-2141.1986.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 12.Schiffer CA, Dutcher JP, Aisner J, et al. A randomized trial of leukocyte-depleted platelet transfusion to modify alloimmunization in patients with leukemia. Blood. 1983;62:815–20. [PubMed] [Google Scholar]
- 13.van Marwijk Kooy M, van Prooijen HC, Moes M, et al. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood. 1991;77:201–5. [PubMed] [Google Scholar]
- 14.Karpinski M, Pochinco D, Dembinski I, et al. Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol. 2004;15:818–24. doi: 10.1097/01.asn.0000115399.80913.b1. [DOI] [PubMed] [Google Scholar]
- 15.Jackman RP, Deng X, Bolgiano D, et al. Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion. 2014;54:672–80. doi: 10.1111/trf.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sintnicolaas K, van Marwijk Kooij M, van Prooijen HC, et al. Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: a randomized prospective study. Blood. 1995;85:824–8. [PubMed] [Google Scholar]
- 17.Sniecinski I, O’Donnell MR, Nowicki B, et al. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71:1402–7. [PubMed] [Google Scholar]
- 18.Clynes R, Ravetch JV. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995;3:21–6. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 19.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 20.Hed J. Role of complement in immune or idiopathic thrombocytopenic purpura. Acta paediatrica. 1998;424:37–40. doi: 10.1111/j.1651-2227.1998.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 21.Bobak DA, Gaither TA, Frank MM, et al. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. Journal of immunology. 1987;138:1150–6. [PubMed] [Google Scholar]
- 22.Terasaki PI, McClelland JD. Microdroplet Assay of Human Serum Cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 23.Phelan DL, Rodey GE, Anderson CB. The development and specificity of antiidiotypic antibodies in renal transplant recipients receiving single-donor blood transfusions. Transplantation. 1989;48:57–60. doi: 10.1097/00007890-198907000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Carrick DM, Johnson B, Kleinman SH, et al. Agreement among HLA antibody detection assays is higher in ever-pregnant donors and improved using a consensus cutoff. Transfusion. 2011;51:1105–16. doi: 10.1111/j.1537-2995.2010.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei R, Lee J, Chen T, et al. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60:1293–302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 26.Uboldi de Capei M, Pratico L, Curtoni ES. Comparison of different techniques for detection of anti-HLA antibodies in sera from patients awaiting kidney transplantation. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics. 2002;29:379–82. doi: 10.1046/j.1365-2370.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 27.Wahrmann M, Exner M, Haidbauer B, et al. [C4d]FlowPRA screening--a specific assay for selective detection of complement-activating anti-HLA alloantibodies. Hum Immunol. 2005;66:526–34. doi: 10.1016/j.humimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Fadeyi E, Adams S, Peterson B, et al. Analysis of a high-throughput HLA antibody screening assay for use with platelet donors. Transfusion. 2008;48:1174–9. doi: 10.1111/j.1537-2995.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes LB, Fabron A, Jr, Chiba AK, et al. Impact of using different laboratory assays to detect human leukocyte antigen antibodies in female blood donors. Transfusion. 2010;50:902–8. doi: 10.1111/j.1537-2995.2009.02523.x. [DOI] [PubMed] [Google Scholar]
- 30.Worthington JE, Robson AJ, Sheldon S, et al. A comparison of enzyme-linked immunoabsorbent assays and flow cytometry techniques for the detection of HLA specific antibodies. Hum Immunol. 2001;62:1178–84. doi: 10.1016/s0198-8859(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 31.Fontaine MJ, Kuo J, Chen G, et al. Complement (C1q) fixing solid-phase screening for HLA antibodies increases the availability of compatible platelet components for refractory patients. Transfusion. 2011;51:2611–8. doi: 10.1111/j.1537-2995.2011.03194.x. [DOI] [PubMed] [Google Scholar]
- 32.Jackman RP, Deng X, Bolgiano D, et al. Low-level HLA antibodies do not predict platelet transfusion failure in TRAP study participants. Blood. 2013;121:3261–6. doi: 10.1182/blood-2012-12-472779. quiz 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnaidt M, Weinstock C, Jurisic M, et al. HLA antibody specification using single-antigen beads--a technical solution for the prozone effect. Transplantation. 2011;92:510–5. doi: 10.1097/TP.0b013e31822872dd. [DOI] [PubMed] [Google Scholar]
- 34.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–60. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishida A, Handa M, Wakui M, et al. Clinical factors influencing posttransfusion platelet increment in patients undergoing hematopoietic progenitor cell transplantation--a prospective analysis. Transfusion. 1998;38:839–47. doi: 10.1046/j.1537-2995.1998.38998409004.x. [DOI] [PubMed] [Google Scholar]
- 36.Carrick DM, Norris PJ, Endres RO, et al. Establishing assay cutoffs for HLA antibody screening of apheresis donors. Transfusion. 2011;51:2092–101. doi: 10.1111/j.1537-2995.2010.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.