Abstract
The incidence of diabetes continues to rise among all ages and ethnic groups worldwide. Diabetic retinopathy is a complication of diabetes that affects the retinal neurovasculature causing serious vision problems, including blindness. Its pathogenesis and severity is directly linked to the chronic exposure to high glucose conditions. No treatments are currently available to stop the development and progression of diabetic retinopathy. To develop new and effective therapeutic approaches, it is critical to better understand how hyperglycemia contributes to the pathogenesis of diabetic retinopathy at the cellular and molecular levels. We propose alterations in O-GlcNAc modification of target proteins during diabetes contribute to the development and progression of diabetic retinopathy. The O-GlcNAc modification is regulated through hexosamine biosynthetic pathway. We showed this pathway is differentially activated in various retinal vascular cells under high glucose conditions perhaps due to their selective metabolic activity. O-GlcNAc modification can alter protein stability, activity, interactions, and localization. By targeting the same amino acid residues (serine and threonine) as phosphorylation, O-GlcNAc modification can either compete or cooperate with phosphorylation. Here we will summarize the effects of hyperglycemia-induced O-GlcNAc modification on the retinal neurovasculature in a cell-specific manner, providing new insight into the role of O-GlcNAc modification in early loss of retinal pericytes and the pathogenesis of diabetic retinopathy.
Keywords: Hyperglycemia, Hexosamine Biosynthetic Pathway, Pericytes, Posttranslational modification
Introduction
The worldwide prevalence of diabetes mellitus continues to rise and its complications continue to impact human health. Diabetic Retinopathy (DR) is a complication of diabetes, and remains the leading cause of vision loss in many developed countries [1]. In the US, an estimated 40% of people with Type 1 diabetes mellitus (T1DM) and 86% with Type 2 diabetes mellitus (T2DM) have DR. Of the affected diabetic individuals, 8% with T2DM and 42% with T1DM have a vision-threatening form of DR [2, 3]. Vision loss primarily occurs from either proliferation of new retinal blood vessels (proliferative diabetic retinopathy), or from increased permeability of retinal vessels (diabetic macular edema) [4].
Several processes are linked to the pathogenesis of DR, including imbalance in the retinal production of neuroprotective factors and extracellular glutamate accumulation [5], activation of protein kinase C [6], oxidative stress [7], polyol pathway activation [8], accumulation of advanced glycation end products (AGEs) [9], inflammation [10], mitochondrial dysfunction, and endoplasmic reticulum stress (ER) [11]. These processes are interrelated, and increased O-GlcNAc modification may be involved in the pathogenesis of DR by contributing to these mechanisms as discussed below.
O-GlcNAc modification is a unique type of post-translational modification (PTM), first described over 30 years ago [12]. Research on O-GlcNAc modification is increasing in parallel with the studies that link its dysregulation to various diseases including, cancer [13], Alzheimer [14], Parkinson [15], systemic lupus erythematosus [16], diabetes mellitus [17], and obesity [18]. O-GlcNAc modification is a protein glycosylation, yet this modification is unique from all other common forms of protein glycosylation due to its highly dynamic cycle, its specificity for Ser/Thr residues, and its ability to bind cytoplasmic and nuclear proteins. In this manner, O-GlcNAc modification has dynamics that are similar to phosphorylation. These PTMs may compete or cooperate, and regulate the function of various target proteins [19]. Thus, like phosphorylation O-GlcNAcylation is directly involved in the regulation of many cellular processes by modulating activity, interaction, degradation, and subcellular localization of target proteins [19].
In order to decode the pathogenesis of most diseases, the involvement of PTM must be taken into consideration. Imbalanced O-GlcNAc modification may involve the etiology of diabetes and the pathogenesis of various diabetes complications. In this review, we will further discuss the impact of increased O-GlcNAcylation on retinal vascular cell function, and its contribution to progression of DR. However, the mechanisms of how O-GlcNAcylation affects the pathogenesis of DR may be shared by other diabetes complications.
O-GlcNAc Modification and Its Impact in Diabetes
Hyperglycemia is a hallmark symptom of T1DM and T2DM. Overtime, the hyperglycemic environment becomes toxic and contributes to pancreatic β cell destruction and various systemic complications of diabetes, including diabetic retinopathy, nephropathy, neuropathy, cardiomyopathy, and atherosclerosis [20]. Hyperglycemia impairs retinal neurovasculature and initiates the pathogenesis of DR that can eventually progress to blindness [11]. Glucose also reacts non-enzymatically with various molecules and generates glycated products, which contribute to oxidative stress and inflammatory phenotypes associated with diabetes [21]. It is the accumulation of such products, coupled with chronic inflammation, that drives the pathogenesis of diabetes complications. Unfortunately, there are no efficient treatments available to counteract these hyperglycemia-mediated changes, beyond decreasing the systemic levels of glucose by insulin replacement, drugs, changing diet and/or life style. At best, it is a challenging task to achieve normal glucose levels during didabetes and mitigate the pathogenesis of DR.
O-GlcNAc modification is induced under various cellular stress conditions, including hyperglycemia. Increased O-GlcNAc modification is associated with the pathogenesis of diabetes and its complications, and involves the progress of insulin resistance and hyperglycemia-induced glucose toxicity. Pancreatic β-cells express abundant amounts of both O-linked β-N-acetylglucosamine transferase (OGT) and O-GlcNAcase (OGA), suggesting O-GlcNAc cycling is important in pancreatic β-cell function and survival under normal glucose conditions [22]. Increased O-GlcNAc modification contributes to increased apoptosis of β-cells under prolonged hyperglycemia [23]. This negative effect on β-cell survival appears to be due to dysregulation of key elements in the insulin signaling pathway including O-GlcNAc modification.
Glucosamine-induced O-GlcNAc modification results in the inhibition of tyrosine-phosphorylation of the insulin receptor (IR), insulin receptor substare-1 (IRS-1) and IRS-2, followed by impaired activation of the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and protein kinase B (PKB)/Akt survival signaling. PI3K/Akt inactivation results in reduced glycogen synthase kinase 3 (GSK-3) and forkhead box protein O1a (FOXO1a) inactivation and expression of the pro-apoptotic protein Bim. Furthermore, inhibition of O-GlcNAc modification by using specific OGT inhibitors prevented the inhibition of tyrosine-phosphorylation of IRS-1/IRS-2 and glucosamine induced apoptosis in β-cells [23]. In a similar manner, it has been reported that increased O-GlcNAc modification inhibits Akt1 activation by preventing its phosphorylation at Ser473 leading to the apoptosis of mouse pancreatic β-cells [24].
Hyperglycemia-induced O-GlcNAc modification is linked to insulin resistance in insulin sensitive tissues, such as skeletal muscle and adipose tissue [25, 26]. Key insulin signaling proteins, including IRS-1, IRS-2, Akt, pancreatic duodenal homeobox-1 (PDK1), and the p110 subunit of PI3K, are all targets of O-GlcNAcylation [27, 28]. Increased flux through the hexosamine biosynthetic pathway (HBP) and higher O-GlcNAcylation results in impairment of the PTM of insulin signaling proteins and inhibition of the glucose transporter 4 (GLUT-4) mediated glucose uptake in these tissues [26, 29]. Multiple studies support the role of increased O-GlcNAc modification in insulin resistance by using transgenic mouse models or cell lines overexpressing glutamine fructose-6-phosphate amidotransferase (GFAT) and OGT, glucosamine exposure, and pharmacological approach with inhibitors [28, 30–32]. However, a few studies that used specific OGA inhibitors were unable to confirm the induction of insulin resistance by increased O-GlcNAc modification [33, 34]. These data suggest that caution should be used when evaluating increased O-GlcNAc modification using different approaches. Substrate abundancy, genetic modifications of the regulator genes, and the selected pharmacological inhibitions may have other effects creating different outcomes. Ongoing studies to improve our understanding of the effects of O-GlcNAc modification are needed. However, there is considerable evidence to suggest a link between hyperglycemia-induced O-GlcNAc modification in pancreatic β-cell loss and insulin resistance in various tissues. In this manner, increased O-GlcNAc modification contributes to the worsening of hyperglycemia during diabetes.
Molecular Aspects and Regulation of O-GlcNAc Modification
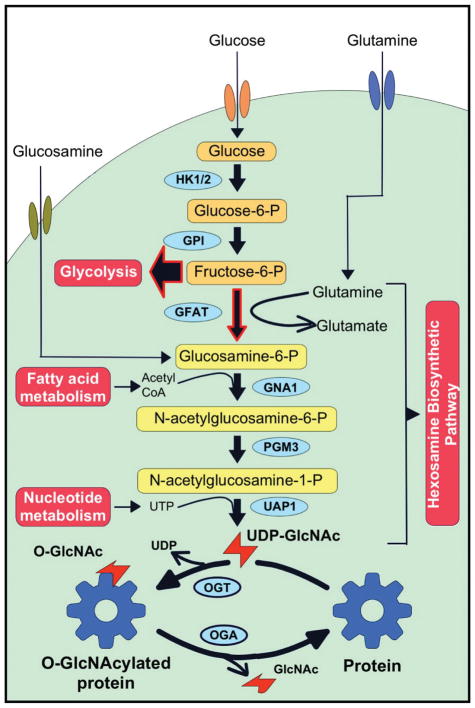
O-GlcNAc modification forms by the transfer of an uncharged acetylated hexosamine sugar, N-acetylglucosamine (GlcNAc) through a glycosyl-linkage to the hydroxyl group of serine and threonine residues of various proteins. Unlike other glycosylation modifications, O-GlcNAc modification does not continue to elongate. This modification is dynamically regulated by OGT and OGA [35–37]. OGT catalyzes the transfer of GlcNAc from a high-energy sugar donor, UDP-GlcNAc to Ser/Thr residues, and OGA hydrolyzes the GlcNAc from proteins. UDP-GlcNAc is the final product of HBP, which is further regulated by several metabolic pathways (Figure 1).
Figure 1. The hexosamine biosynthesis pathway and protein O-GlcNAcylation.
Hexosamine Biosynthetic Pathway (HBP) shares first two steps with glycolysis. Only 2–3% of Fructose-6-P enters to HBP, 97–98% to glycolysis. HBP requires glucose, glutamine, acetyl-CoA, and UTP. End-product of HBP, UDP-GlcNAc used by O-GlcNAc transferase (OGT). O-GlcNAc modification dynamically added to Ser/Thr residues by OGT and removed by O-GlcNAcase (OGA).
HBP shares the first two-steps involved in the glycolysis pathway. Glucose is phosphorylated to glucose-6-phosphate, and then metabolized to fructose-6-phosphate after entering the cell [38, 39]. Most of the fructose-6-phosphate is diverted to glycolysis, with only 2–3% of it used in the HBP. GFAT transfers an amino group from glutamine to fructose-6-phosphate and produces glucosamine-6-phosphate (Figure 1). Next, acetylation by glucosamine 6-phosphate N-acetyltransferase (Gnpnat1) is followed by the isomerization to N-acetyl-1-phosphate glucosamine. The final product of HBP, UDP-GlcNAc, is produced by adding the nucleoside to the sugar by UDP-N-acetylhexosamine pyrophosphorylase (UAP1) [38]. OGT catalyzes the addition of N-acetylglucosamine from UDP-GlcNAc to specific serine or threonine residues to form a β-glycosidic linkage [40]. OGA has hydrolase activity that removes N-acetylglucosamine from serine or threonine residues [35].
Phosphorylation and O-GlcNAc modification both regulate protein activity by targeting serine and threonine residues. More than 500 kinases phosphorylate their target proteins with distinct sequence specificity [91]. In contrast, only a single enzyme (OGT) governs the addition and another enzyme (OGA) the removing O-GlcNAc modification in mammalian cells [92]. Furthermore, O-GlcNAcylation does not display a strict amino acid consensus sequence, although serine or threonine residues flanked by proline and valine are the preferred sites of O-GlcNAcylation [93]. In this manner, it is important to understand the structure and target selectivity of OGT and OGA to unlock their possible therapeutic potentials.
The OGT gene is localized on the X chromosome, and its protein product is expressed in all mammalian tissues. OGT expression is higher in certain tissues including α/β pancreatic cells and brain neurons. O-GlcNAc modification and OGT expression is necessary for murine embryogenesis, such that germ line deletion of OGT is embryonic lethal [94]. Three alternative spliced variants of OGT have been identified: nucleocytoplasmic (nc), mitochondrial (m), and short (s) isoforms. These isoforms differ by the number of N-terminal 34 amino acid tetratricopeptide repeats (TPR). The longest and most abundant human ncOGT isoform (115 kDa) contains 13.5 TPR, and is encoded by all 23 exons of the OGT gene, whereas mOGT (103 kDa) has 9.5 and is encoded by exons 5 to 23, and sOGT (76 kDa) has only 2.5 TPR and encoded by exons 10 to 23 [38, 92]. The N-terminal TPR domain of OGT controls its interaction with both target and regulator proteins [95]. The C-terminus of OGT contains its catalytic domain, and this region of the protein is identical in all three OGT isoforms. However, sOGT may lack transferase activity [96–98]. Furthermore, sOGT may inhibit the activity of the other isoforms [99, 100], and this isoform protects against apoptosis under some stress conditions [100, 101]. In addition to these OGT isoforms, a novel extracellular/luminal glycosyltransferase, eOGT, has recently been reported [102], but this isoform has no apparent homology to any of other OGT isoforms. The function of eOGT is to catalyze the addition of O-β-GlcNAc monosaccharide residues to the extracellular domains of the Notch receptor [102].
A few mechanisms have been reported for the regulation of OGT binding and activity. OGT forms various substrate specific holoenzymes by dynamically interacting with many binding partners, such as mSin3A [103], Sp1 [104], TET2/3 [105], p38 MAPK [106], Trak1 (OIP106) [107], and MYPT1 [108]. These proteins interact with OGT through its TPR domain, and serve as adaptor or bridging proteins to recruit OGT directly to its targets. The concentration of UDP-GlcNAc regulates OGT affinity and activity as well [109]. OGT demonstrates differential affinity for specific target proteins, even on different Ser/Thr residues on the same peptide, dependent on variations in UDP-GlcNAc concentration [109, 110]. These findings highlight the importance of the intracellular location of UDP-GlcNAc production, and its transportation to specific locations in the cell, in the regulation of OGT. Moreover, the affinity of OGT for target proteins is further governed by its own posttranslational modifications [111].
O-GlcNAcase (OGA), previously called hexosaminidase C, is the only enzyme that cleaves O-GlcNAcylation [112–114]. Like OGT, OGA is highly conserved and ubiquitously present in tissues, with more abundancy in the pancreas, brain and skeletal muscle [35, 113, 115]. Two splice variants of OGA are identified in humans: long OGA (OGA-L) and short OGA (OGA-S). The OGA-L isoform is a 916-amino acid protein and has an acidic isoelectric point [113]. OGA-L is a bifunctional enzyme with two active domains; the N-terminal catalytic domain removes O-GlcNAc modification from its targets and the C-terminal Histone Acetyl Transferase (HAT) domain may be involved in histone regulation [116]. However, there is not yet a consensus on the role of the HAT domain in OGA-L [117], and it is commonly termed as a pseudo HAT domain [118]. The linker site in between N- and C-terminus carries caspase-3 target sequences, and this site is cleaved during programmed cell death by caspase-3 [117, 119]. These cleavage products appear at the same time as the cleavage of Poly (ADP-ribose) polymerase (PARP), which is a known caspase-3 substrate [117]. However, the caspase-3 cleavage of OGA-L has no effect on its O-GlcNAcase activity [117, 119]. The OGA-S isoform is a 677-amino acid protein, which differs from OGA-L in that it lacks the pseudo HAT domain [115]. OGA-S has lower hexosaminidase activity, and it localizes to the endoplasmic reticulum and lipid droplets, whereas OGA-L acts as a nucleocytoplasmic enzyme [120].
The regulation of OGA activity and substrate targeting are not well understood. Some possible mechanisms involved in OGA regulation include, post-translational modifications, the divergent subcellular localization of OGA isoforms, and its interaction with OGT and other proteins [121]. Despite current studies providing some information regarding target selection and regulation of OGT and OGA, we are far from understanding any specificity regarding the activation and targeting of OGA and OGT. Furthermore, progress is still needed in the methods to purify and identify O-GlcNAcylated proteins. Using conventional methods, such as pulling-down with wheat germ agglutinin (WGA) or specific antibodies against O-GlcNAc, requires a caution due to rapid cycling rate of this modification and that need to be protected from O-GlcNAcase and lysosomal hexosaminidases activities during enrichment steps. More advanced techniques are utilizing the specific labelling of O-GlcNAc sites with unnatural components, such as azide-modified GlcNAc [128, 129]. Using these chemical handles and “click-It chemistry” have increased the number of identified proteins as O-GlcNAcylation target. However, exposing cells to these modified components are not ideal in terms of biological relevance. Moreover, these components are in competition with natural glucose and determining the alteration in O-GlcNAc modification under different glucose conditions is not possible with these techniques. Thus, new robust techniques to enrich and analyze O-GlcNAc modification without chemical derivatization such as top down proteomics are needed.
Cell-Specific Effects of O-GlcNAc Modifications in DR
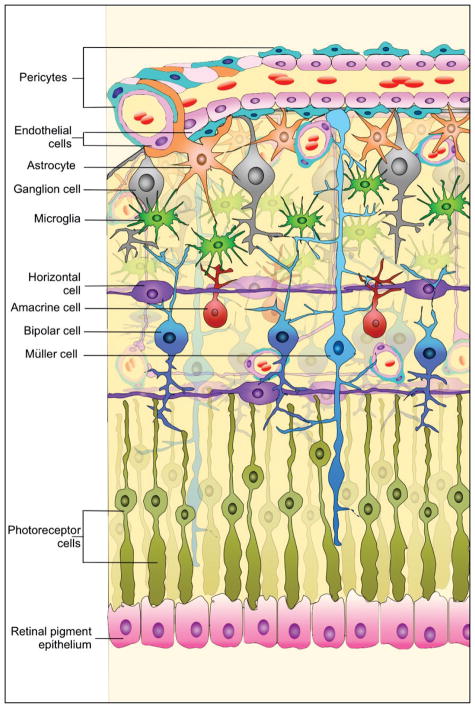
DR affects the retinal neurovasculature, a highly-organized network of retinal neurons, Müller cells, astrocytes (AC), endothelial cells (EC) and pericytes (PC) (Figure 2). These cells constitute the inner retinal blood-retinal barrier (BRB), and regulate nutrient flow and the tissue microenvironment to allow proper functions. Hyperglycemic conditions result in dysfunction of retinal neurovasculature, perhaps due to loss of retinal neurons, thickening of the retinal blood vessel basement membrane, loss of PC, disruption of the tight junctions, formation of non-perfused capillaries, ischemia, and stimulation of neovascular growth [4, 41]. Unfortunately, the newly formed blood vessels are abnormal, leaky, and interfere with vision, and if left untreated result in retina traction detachment and loss of vision [41,42]. Studies have indicated that the sensitivity of retinal vascular cells to hyperglycemia varies [43–45], and alterations in O-GlcNAc modification levels may regulate the fate of retinal vascular cells under high glucose conditions [46,47].
Figure 2. Schematic diagram of the retinal neurovasculature indicates the major cell types.
Single endothelial cell layer forms capillary lumen and constitutes the blood-retina barrier together with pericytes and astrocytes. This unit interacts with other cells and layers of retina.
O-GlcNAc Modification and Retinal Neuronal Cell Function
New imaging technologies indicated that loss of retinal neurons appears at least during the same period or even earlier than microvascular defects in the onset of DR [48]. Neural apoptosis is accompanied by glial dysfunction (reactive gliosis) and results in thinning of the retinal nerve fiber layer [49–51]. O-GlcNAcylation enzymes (OGT and OGA) are highly expressed in neuronal cells [52], and impaired O-GlcNAc cycling is involved in the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [53]. Notably, high glucose and glucosamine-induced HBP increase apoptosis in rat retina originated neuronal cells possibly via blocking the neuroprotective effect of insulin/Akt signaling pathways [54]. Another study reported retinal ganglion cell loss with increased O-GlcNAcylation of NF-κB p65 subunit in a diabetic mouse model [55]. According to preliminary studies and established contribution of impaired O-GlcNAc modification in neurodegenerative diseases, one can speculate that increased O-GlcNAc modification contributes to glucose toxicity in retinal neurons, as it does in pancreatic β-cell, another cell type with high levels of OGT and OGA as discussed above.
O-GlcNAc Modification and Retinal Astrocyte Function
Astrocytes (AC) and Müller cells are macroglia members of retinal neurovascular unit. Retinal AC originate from the optic nerve head and migrate into the retina along the optic nerve during vascular development [55]. Retinal AC provide mechanical and trophic support to retinal neurovasculature and to the inner BRB. Retinal AC are involved in neuro-protective processes, called reactive gliosis, which becomes activated under trauma events or neurodegeneration [56]. Upon dysfunction or excessive activation of the gliosis process, reactive gliosis itself may cause worsening of the primary pathology and is linked to activation of microglial cells and production of inflammatory mediators [57].
Reactive AC also secrete pro-inflammatory factors such as monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-2 alpha (MIP-2α), interleukin-1 beta (IL-1β), IL-6 and IL-8 [58]. These inflammatory factors secreted by AC also contribute to the inflammation that occurs during diabetes complications [59]. Incubation of AC with IL-1β promotes a proinflammatory and oxidative stress condition [60]. Notably, hyperglycemic conditions have no or minimal effects on the survival of retinal AC, however hyperglycemia does have a significant impact on AC proliferation, adhesion, and migration, as well as increases in their production of inflammatory cytokines and oxidative stress [46, 59]. Altered AC localization and secretion impair the homeostasis of the retina and inner BRB during diabetes.
In a recent study, we confirmed that high glucose does not affect the rate of apoptosis in retinal AC in culture [46]. Interestingly, total O-GlcNAc modification levels also did not change dramatically in retinal AC under high glucose conditions [47]. Thus, glucose uptake and/or fructose-6-phosphate enterance to HBP must be tightly controlled in retinal AC. However, Matthews and colleagues reported that glucosamine-induced HBP flux caused short-term phosphorylation of Akt and its activation likely leads to increased ER stress in a human astroglial cell line [61]. Furthermore, Mao et al. found that O-GlcNAc modification of p27 (Kip1) competes with its phosphorylation and inhibits AC migration and functional recovery after spinal cord injury [62]. Thus,
O-GlcNAc Modification and Retinal Endothelial Cell Function
Retinal EC constitute the lining of retinal vasculature, aligning as a single layer to form the retinal capillary wall (endothelium), just as in any other capillary or vessel. Retinal EC are the main component of the inner BRB. As a first cell layer in contact with blood, EC are responsible for the homeostasis of retina by controlling the transport of nutrients, metabolites, ions, fluids and many other exchanges in between blood and retina. Furthermore, EC are involved in angiogenesis, vasoregulation, fibrinolysis and coagulation [63].
Retinal EC are the first retinal cell types to encounter hyperglycemic conditions, however retinal EC are structurally stable during mild to moderate non-proliferative DR. As hyperglycemia prolongs and DR progresses to a more severe non-proliferative stage, the effects on retinal EC become detectable. During this stage, retinal EC loss is apparent and is accompanied by a thickened basement membrane, capillary occlusion and vasoconstriction. Subsequently, dilated capillaries and shunts between arteries and veins can also be identified. Upon the final stage of proliferative DR, retinal EC migrate, proliferate and new capillaries form from existing vessels. However, most of these new vessels are not properly reinforced and are prone to leakage. Angiogenesis can also extend to the vitreous body and form fibrotic tissue. Eventually, retinal detachment and vitreous hemorrhage cause severe vision impairment [42].
The dysfunction of retinal EC contributes to all of the later stages of DR. Thus, it is still unclear whether hyperglycemia directly affects retinal EC function. A few studies reported that high glucose may induce apoptosis in retinal EC by disrupting mitochondrial morphology [64] or a caspase-independent pathway [65]. However, others, including us, have been unable to detect any direct apoptotic effect of high glucose on retinal EC in culture [45, 46]. We showed O-GlcNAc modification was increased only moderately in retinal EC compared with retinal PC [47]. However, high glucose conditions did result in enhanced migration of retinal EC with minimal effect on their survival [46, 66]. Furthermore, the protective effect of increased O-GlcNAc modification against reactive oxygen species (ROS) was reported in human retinal EC [67] and cardiac cells [68]. Further studies are required to understand possible direct effects of hyperglycemia and induced O-GlcNAc modification on retinal EC function. However, impairment of the surrounding cells and microenvironment may have adverse effects on retinal EC as DR progresses, coupled with both hyperglycemia and increased O-GlcNAcylation.
During neovascularization, the basal lamina is digested by proteases that are released by other cells, including leukocytes. Moreover, hypoxia plays an important role and causes an imbalance between pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and anti-angiogenic proteins including pigment epithelium-derived factor and thrombospondin-1. Increased hypoxia-inducible factor 1 (HIF-1) expression stimulates the production of VEGF in surrounding cells. Thus, hypoxia induces the formation of new blood vessels by retinal EC migration and proliferation through the VEGF rich regions [68, 69].
To assess the impact of hypoxia on O-GlcNAc modification levels in the retina, we determined O-GlcNAc modification levels in retinas from mice subjected to oxygen-induced ischemic retinopathy (OIR). The mouse OIR model is commonly used to evaluate hypoxia-induced changes in the retinal vasculature and during proliferative retinal neovascularization. These studies demonstrated that O-GlcNAc modification is increased rapidly as a cytoprotective mechanism at the early stages of hypoxic conditions. Under strong and persisting hypoxia, elevated O-GlcNAc modification may contribute to release of angiogenic factors and lead to retinal EC proliferation and migration [47, 70].
EC also regulate vascular tone by secreting vasoactive factors, such as nitric oxide (NO). EC cannot produce enough NO and vasodilation is impaired under diabetic conditions [71–73]. Recent studies have linked O-GlcNAc modification to the inactivation of endothelial nitric oxide synthase (eNOS). eNOS activation requires phosphorylation of serine 1177 by the protein kinase Akt/PKB. Glucosamine, or high glucose-induced eNOS O-GlcNAc modification, resulted in decreased phosphorylation of serine 1177, leading to inhibited eNOS activity in bovine aortic EC [74], rat aorta [74], and human coronary artery EC [75]. Interestingly, reduction in NO enhances 26S proteasome functionality, which is associated with a reduction in O-GlcNAcylation of 26S proteasome regulatory subunit 4 homolog (Rpt2) and an increase in proteasome chymotrypsin-like activity [76]. This negative feedback loop may worsen the situation and create stress for EC survival during diabetes. In fact, 26S proteasome-mediated OGT reduction and its contribution to hypoxia-induced vascular endothelial inflammatory response has been reported [77]. Furthermore, hyperglycemia-induced intracellular adhesion molecule 1 (ICAM-1) expression is linked to O-GlcNAc modification of transcription factor specificity protein 1 (Sp1) in human umbilical vein endothelial cells and rat retinal capillary endothelial cells [78]. Increased O-GlcNAcylation of SP1 is associated with other pathways in DR, such as inducing VEGF-A production in retinal endothelium and pigment epithelium [79].
O-GlcNAc Modification and Retinal Pericyte Function
Pericytes envelop the capillary wall formed by EC and play an essential role in homeostasis, stability and contractility of capillaries in the retina [80, 81]. They are also important for maintaining inner BRB [81, 82]. The ratio of EC:PC is around 1:1 in the retinal vasculature, which is higher than any other organ [83, 84]. This high PC coverage provides strength to the vascular integrity in the retina and to the tight junctions in modulating the inner BRB [84]. Besides providing physical strength, PC produce important trophic factors for EC survival, such as VEGF [85] and angiopoietin-1 [86]. Moreover, PC and EC interaction is important in control of vascular growth by transforming growth factor-β1 (TGF-β1) mediated regulation of EC apoptosis [87, 88]. In this manner, PC integrity is crucial for the maintenance of retinal vasculature and its barrier characteristics.
Loss of retinal PC has been recognized as one of the earliest sign of DR [43, 44]. Pericyte loss is followed by capillary dilation, the formation of acellular capillaries and microaneurysms, thickening of the vascular basement membrane, finally neovascularization, vascular leakage and macular edema [89]. These cascades of events are linked to the loss and/or dysfunction of PC. However, the lack of successful treatments to prevent retinal PC loss in diabetes indicates that a better understanding of the selective sensitivity of PC to hyperglycemia is required.
We recently showed that retinal PC, but not retinal EC or AC, undergo apoptosis in response to chronic exposure to high glucose conditions in culture [46]. Glucose uptake studies indicated that only retinal PC show a significant increase in the level of intracellular glucose levels when exposed to high glucose conditions with a dramatic increase in their level of O-GlcNAc modification. O-GlcNAc levels increased only moderately in retinal EC, but not in retinal AC in the same study [47]. Furthermore, we showed that increased levels of O-GlcNAc modification using pharmacological agents, under normal glucose conditions, increased apoptosis of retinal PC, but not retinal EC or AC. Thus, increased O-GlcNAc modification in retinal PC under high glucose conditions may drive the apoptosis of retinal PC, contributing to the early vascular dysfunction and neovascularization during diabetes [47].
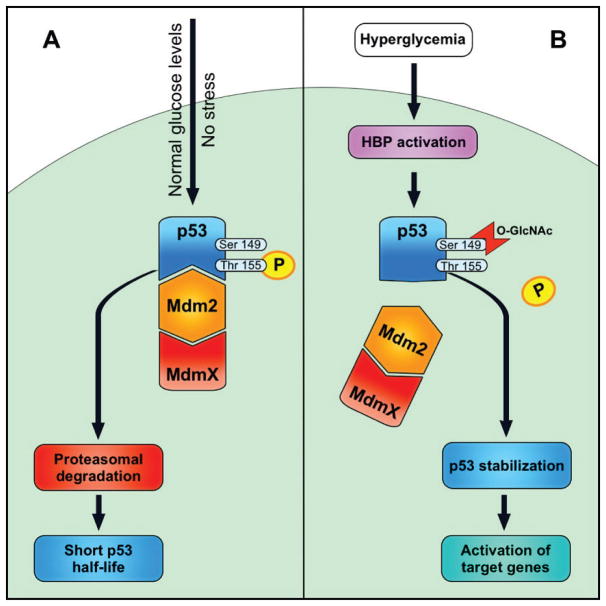
A key protein in modulation of apoptosis is p53, which is also a target of O-GlcNAc modification [46, 90]. Phosphorylation of p53 regulates its half-life in cells (Figure 3). Phosphorylation at Thr155 residue promotes p53 interaction with Mdm2 and thus, increases its degradation. However, O-GlcNAc modification at Ser149 increases p53 stabilization by preventing phosphorylation of Thr155 [90]. Recently, we reported that high glucose conditions resulted in increased levels of p53 protein in PC cultured under high glucose conditions, which was O-GlcNAc modified. Thus, no significant changes in mRNA level of p53 suggested that post-translational mechanisms impact the stability of p53 in PC cultured under high glucose conditions [46].
Figure 3. Modulation of p53 by O-GlcNAcylation and phosphorylation.
A: Normal glucose and no stress conditions, p53 phosphorylated at Thr155 and interacts with Mdm2, enters to proteasomal degradation.
B: Hyperglycemia activates HBP, p53 get O-GlcNAcylated at Ser149. This modification prevents Thr155 phosphorylation, weakens p53 – Mdm2 interaction, p53 stabilized and activates its target proteins and apoptotic processes.
We proposed that O-GlcNAc modification at Ser149 affects p53 phosphorylation and turnover through the ubiquitin mediated degradation pathway in retinal PC (Figure 3). Thus, we believe increased O-GlcNAc modification of p53 under high glucose conditions is responsible for increased levels of p53 and PC loss by apoptosis [46]. This is further supported with increased apoptosis of PC incubated with OGA inhibitor under normal glucose conditions. However, the importance of O-GlcNAc modification in p53 stabilization and retinal PC loss during diabetes awaits further confirmation.
Conclusions and Future Directions
There is accumulating data that indicate altered O-GlcNAc modifications are involved in etiology and complications of diabetes. Pancreatic β-cell and neuronal cells have a higher O-GlcNAc cycling rate, and any impairment in O-GlcNAcylation negatively affects their functions and survival [22, 49]. In addition, increased O-GlcNAc modification also has negative effects on these cells and contribute to glucotoxicity [23, 53]. Elevated O-GlcNAc modification also is linked to insulin resistance in insulin target tissues [28, 30]. Furthermore, hyperglycemia induced O-GlcNAc modification may be important in retinal neurovascular damage during diabetes [46, 47].
We are at the early stages of understanding the impact of O-GlcNAc modification on various biological functions and its contribution to various diseases. It is still unclear how O-GlcNAc modification is regulated in both target-specific as well as cell-specific manners. Recent studies on retinal vascular cells provided important insight regarding how the regulation of O-GlcNAc modification may differ in various cell types, even though they are all components of the same tissue. Under normal glucose conditions, retinal PC have the lowest total O-GlcNAcylation levels in comparison to retinal EC and AC, but only the PC O-GlcNAcylation level increases significantly under high glucose conditions [47]. Ongoing studies indicate that retinal PC and EC show differences in their glucose metabolism, such as glucose uptake, glycolysis rate and citrate levels. Thus, it is important to simultaneously evaluate O-GlcNAc modification and glucose metabolism in a particular cell type. Understanding the details of these pathways together may explain the differences in sensitivity of various target cells and/or tissues to hyperglycemia.
The O-GlcNAc modification-mediate p53 stabilization and its possible involvement in loss of PC in DR, is a good example of how ubiquitin-proteasome pathway may be involved in the pathogenesis of diabetes complications [46]. The O-GlcNAc modification may facilitate or compete with phosphorylation on Ser/Thr residues and alters protein targeting by ubiquitin-proteasome pathway, such as p53 [90], casein kinase II [122], Snail1 [123], delta-lactoferrin [124], keratins 8 and 18 [125]. In addition, O-GlcNAcylation also directly regulates proteins that are the members of ubiquitin-proteasome pathway such as 19S-regulatory subunit [126] and ubiquitin-activating enzyme E1 [127]. Interestingly, we identified 431 O-GlcNAc modified proteins in retinal PC by using a click-It chemistry/LC-MS approach and more than 100 of them have functions in protein synthesis and degradation, including E3 ubiquitin-protein ligase NEDD4, proteasome activator complex subunit 2, proteasome subunit beta type-8 and ubiquitin-conjugating enzyme E2 L3-like [46]. These findings give us an introduction to how closely O-GlcNAcylation and protein turnover are linked, and further studies are needed to understand the extent of this relationship.
The contribution of altered O-GlcNAc modification in diabetes and its complications cannot be ignored, and further studies are required to decode the full process. Further identification of O-GlcNAcylation targets and analyzing the effects of O-GlcNAc modification on their functions will help to understand the molecular signatures of DR, and will provide novel insight into distinct, but physiologically relevant, disease associated pathways including apoptosis, inflammation and metabolism. This knowledge can be extended to other target organs in diabetes including pancreas, kidney and cardiovascular system. Together these studies will generate important data for therapeutic translation and development of new therapies for diabetes and its complications.
Acknowledgments
The work in NS laboratory is supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, EY026078 and EY022883. NS is a recipient of RPE Stein Innovation Award.
References
- 1.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.Kempen JH, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 3.Roy MS, et al. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122(4):546–51. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353(8):839–41. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 5.Simo R, Hernandez C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61(2):187–96. doi: 10.1016/j.survophthal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Curtis TM, Stitt AW. Advanced glycation end products and diabetic retinopathy. Curr Med Chem. 2013;20(26):3234–40. doi: 10.2174/09298673113209990025. [DOI] [PubMed] [Google Scholar]
- 10.Semeraro F, et al. Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy S, et al. Mitochondrial dysfunction and endoplasmic reticulum stress in diabetic retinopathy: mechanistic insights into high glucose-induced retinal cell death. Curr Clin Pharmacol. 2013;8(4):278–84. doi: 10.2174/1574884711308040003. [DOI] [PubMed] [Google Scholar]
- 12.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259(5):3308–17. [PubMed] [Google Scholar]
- 13.Du J, et al. CALGB 150905 (Alliance): Rituximab broadens the anti-lymphoma response by activating unlicensed NK cells. Cancer Immunol Res. 2014;2(9):878–89. doi: 10.1158/2326-6066.CIR-13-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, et al. The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem. 2014;289(50):34472–81. doi: 10.1074/jbc.R114.601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groves JA, et al. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 2013;18(5):535–58. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsokos GC, Nambiar MP, Juang YT. Activation of the Ets transcription factor Elf-1 requires phosphorylation and glycosylation: defective expression of activated Elf-1 is involved in the decreased TCR zeta chain gene expression in patients with systemic lupus erythematosus. Ann N Y Acad Sci. 2003;987:240–5. doi: 10.1111/j.1749-6632.2003.tb06054.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Hart GW. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev Proteomics. 2013;10(4):365–80. doi: 10.1586/14789450.2013.820536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YR, et al. Obesity resistance and increased energy expenditure by white adipose tissue browning in Oga(+/−) mice. Dia betologia. 2015;58(12):2867–76. doi: 10.1007/s00125-015-3736-z. [DOI] [PubMed] [Google Scholar]
- 19.Hart GW, et al. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papatheodorou K, et al. Complicationsof Diabetes 2016. J Diabetes Res. 2016;2016:6989453. doi: 10.1155/2016/6989453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 22.Zraika S, et al. The hexosamine biosynthesis pathway regulates insulin secretion via protein glycosylation in mouse islets. Arch Biochem Biophys. 2002;405(2):275–9. doi: 10.1016/s0003-9861(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessandris C, et al. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. Faseb j. 2004;18(9):959–61. doi: 10.1096/fj.03-0725fje. [DOI] [PubMed] [Google Scholar]
- 24.Kang ES, et al. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp Cell Res. 2008;314(11–12):2238–48. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 25.McClain DA, et al. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 2002;99(16):10695–9. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelan SA, et al. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-Linked beta-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285(8):5204–11. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball LE, Berkaw MN, Buse MG. Identification of the major site of O-linked beta-N-acetylglucosamine modification in the C terminus of insulin receptorsubstrate -1. Mol Cell Proteomics. 2006;5(2):313–23. doi: 10.1074/mcp.M500314-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–9. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 29.Buse MG, et al. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 2002;283(2):E241–50. doi: 10.1152/ajpendo.00060.2002. [DOI] [PubMed] [Google Scholar]
- 30.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53(4):921–30. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 31.Vosseller K, et al. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002;99(8):5313–8. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, et al. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia. 2016;59(6):1287–96. doi: 10.1007/s00125-016-3919-2. [DOI] [PubMed] [Google Scholar]
- 33.Macauley MS, et al. Inhibition of O-GlcNAcase using a potent and cell-permeable inhibitor does not induce insulin resistance in 3T3-L1 adipocytes. Chem Biol. 2010;17(9):937–48. doi: 10.1016/j.chembiol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macauley MS, et al. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 2010;17(9):949–58. doi: 10.1016/j.chembiol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidasefrom rat spleen cytosol. J Biol Chem. 1994;269(30):19321–30. [PubMed] [Google Scholar]
- 36.Hart GW, et al. O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology. 1996;6(7):711–6. doi: 10.1093/glycob/6.7.711. [DOI] [PubMed] [Google Scholar]
- 37.Snow DM, Hart GW. Nuclear and cytoplasmic glycosylation. Int Rev Cytol. 1998;181:43–74. doi: 10.1016/s0074-7696(08)60416-7. [DOI] [PubMed] [Google Scholar]
- 38.Vogelpoel LT, et al. FcgammaRIIa cross-talk with TLRs, IL-1R, and IFNgammaR selectively modulates cytokine production in human myeloid cells. Immunobiology. 2015;220(2):193–9. doi: 10.1016/j.imbio.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Ngoh GA, et al. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107(2):171–85. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267(13):9005–13. [PubMed] [Google Scholar]
- 41.Ockrim Z, Yorston D. Managing diabetic retinopathy. Bmj. 2010;341:c5400. doi: 10.1136/bmj.c5400. [DOI] [PubMed] [Google Scholar]
- 42.Wong TY, et al. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 43.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–78. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 44.Hammes HP, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–12. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 45.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57(7):1952–65. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurel Z, et al. Identification of O-GlcNAc modification targets in mouse retinal pericytes: implication of p53 in pathogenesis of diabetic retinopathy. PLoS One. 2014;9(5):e95561. doi: 10.1371/journal.pone.0095561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurel Z, et al. Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol Vis. 2013;19:1047–59. [PMC free article] [PubMed] [Google Scholar]
- 48.van Dijk HW, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(7):3404–9. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu K, et al. Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem. 2004;89(4):1044–55. doi: 10.1111/j.1471-4159.2004.02389.x. [DOI] [PubMed] [Google Scholar]
- 50.Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86(7):725–8. doi: 10.1136/bjo.86.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dijk HW, et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53(6):2715–9. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wani WY, et al. O-GlcNAcylation and neurodegeneration. Brain Res Bull. 2016 doi: 10.1016/j.brainresbull.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura M, et al. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001;276(47):43748–55. doi: 10.1074/jbc.M108594200. [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, et al. Increased O-GlcNAcylation of NF-kappaB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy. Curr Eye Res. 2016;41(2):249–57. doi: 10.3109/02713683.2015.1006372. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332(6167):834–7. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- 56.de Hoz R, et al. Retinal Macroglial Responses in Health and Disease. Biomed Res Int. 2016;2016:2954721. doi: 10.1155/2016/2954721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94(4):1077–98. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 59.Shin ES, et al. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS One. 2014;9(7):e103148. doi: 10.1371/journal.pone.0103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malaplate-Armand C, et al. Astroglial CYP1B1 up-regulation in inflammatory/oxidative toxic conditions: IL-1beta effect and protection by N-acetylcysteine. Toxicol Lett. 2003;138(3):243–51. doi: 10.1016/s0378-4274(02)00417-4. [DOI] [PubMed] [Google Scholar]
- 61.Matthews JA, et al. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol Cell Biochem. 2007;298(1–2):109–23. doi: 10.1007/s11010-006-9358-5. [DOI] [PubMed] [Google Scholar]
- 62.Mao X, et al. O-GlcNAc glycosylation of p27(kip1) promotes astrocyte migration and functional recovery after spinal cord contusion. Exp Cell Res. 2015;339(2):197–205. doi: 10.1016/j.yexcr.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Alberts B, et al. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. [Google Scholar]
- 64.Trudeau K, et al. High glucose disrupts mitochondrial morphologyin retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol. 2010;177(1):447–55. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leal EC, et al. High glucose and oxidative/nitrosative stress conditions induce apoptosis in retinal endothelial cells by a caspase-independent pathway. Exp Eye Res. 2009;88(5):983–91. doi: 10.1016/j.exer.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295(6):C1647–57. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu GD, et al. The augmentation of O-GlcNAcylation reduces glyoxal-induced cell injury by attenuating oxidative stress in human retinal microvascular endothelial cells. Int J Mol Med. 2015;36(4):1019–27. doi: 10.3892/ijmm.2015.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones SP, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117(9):1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 69.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res. 2014;9(3):362–73. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu C, et al. O-GlcNAcylation under hypoxic conditions and its effects on the blood-retinal barrier in diabetic retinopathy. Int J Mol Med. 2014;33(3):624–32. doi: 10.3892/ijmm.2013.1597. [DOI] [PubMed] [Google Scholar]
- 71.Cohen RA. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog Cardiovasc Dis. 1995;38(2):105–28. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 72.Heygate KM, et al. Impaired endothelium-dependent relaxation in isolated resistance arteries of spontaneously diabetic rats. Br J Pharmacol. 1995;116(8):3251–9. doi: 10.1111/j.1476-5381.1995.tb15132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oyama Y, et al. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986;132(1):75–8. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- 74.Du XL, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108(9):1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Federici M, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106(4):466–72. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 76.Liu H, et al. Identification of nitric oxide as an endogenous inhibitor of 26S proteasomes in vascular endothelial cells. PLoS One. 2014;9(5):e98486. doi: 10.1371/journal.pone.0098486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H, et al. Proteasomal degradation of O-GlcNAc transferase elevates hypoxia-induced vascular endothelial inflammatory responsedagger. Cardiovasc Res. 2014;103(1):131–9. doi: 10.1093/cvr/cvu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, et al. O-GlcNAc modification of Sp1 mediates hyperglycaemia-induced ICAM-1 up-regulation in endothelial cells. Biochem Biophys Res Commun. 2017;484(1):79–84. doi: 10.1016/j.bbrc.2017.01.068. [DOI] [PubMed] [Google Scholar]
- 79.Donovan K, et al. O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregulation in retinal cells. Invest Ophthalmol Vis Sci. 2014;55(12):7862–73. doi: 10.1167/iovs.14-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelley C, et al. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987;104(3):483–90. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quaegebeur A, Segura I, Carmeliet P. Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron. 2010;68(3):321–3. doi: 10.1016/j.neuron.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 82.Ferland-McCollough D, et al. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci. 1987;28(7):1086–91. [PubMed] [Google Scholar]
- 84.Shepro D, Morel NM. Pericyte physiology. Faseb j. 1993;7(11):1031–8. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 85.Darland DC, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264(1):275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 86.Sundberg C, et al. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82(4):387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 87.Antonelli-Orlidge A, et al. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86(12):4544–8. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walshe TE, et al. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4(4):e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38(10):1203–6. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 90.Yang WH, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8(10):1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 91.Manning G, et al. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 92.Hanover JA, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409(2):287–97. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 93.Jochmann R, et al. Validation of the reliability of computational O-GlcNAc prediction. Biochim Biophys Acta. 2014;1844(2):416–21. doi: 10.1016/j.bbapap.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Shafi R, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97(11):5735–9. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iyer SP, Hart GW. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem. 2003;278(27):24608–16. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- 96.Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology. 2006;16(5):415–21. doi: 10.1093/glycob/cwj078. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, et al. A peptide panel investigation reveals the acceptor specificity of O-GlcNAc transferase. Faseb j. 2014;28(8):3362–72. doi: 10.1096/fj.13-246850. [DOI] [PubMed] [Google Scholar]
- 98.Ortiz-Meoz RF, et al. Microarray discovery of new OGT substrates: the medulloblastoma oncogene OTX2 is O-GlcNAcylated. J Am Chem Soc. 2014;136(13):4845–8. doi: 10.1021/ja500451w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrali SS, Marz P, Ozcan S. Ataxin-10 interacts with O-GlcNAc transferase OGT in pancreatic beta cells. Biochem Biophys Res Commun. 2005;337(1):149–53. doi: 10.1016/j.bbrc.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 100.Shin SH, Love DC, Hanover JA. Elevated O-GlcNAc-dependent signaling through inducible mOGT expression selectively triggers apoptosis. Amino Acids. 2011;40(3):885–93. doi: 10.1007/s00726-010-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fletcher BS, et al. Functional cloning of SPIN-2, a nuclear anti-apoptotic protein with roles in cell cycle progression. Leukemia. 2002;16(8):1507–18. doi: 10.1038/sj.leu.2402557. [DOI] [PubMed] [Google Scholar]
- 102.Matsuura A, et al. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J Biol Chem. 2008;283(51):35486–95. doi: 10.1074/jbc.M806202200. [DOI] [PubMed] [Google Scholar]
- 103.Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110(1):69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- 104.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17(5):2550–8. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deplus R, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. Embo j. 2013;32(5):645–55. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283(19):13009–20. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278(7):5399–409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 108.Cheung WD, et al. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008;283(49):33935–41. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274(45):32015–22. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 110.Rilla K, et al. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem. 2013;288(8):5973–83. doi: 10.1074/jbc.M112.443879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272(14):9308–15. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 112.Braidman I, et al. Characterisation of human N-acetyl-beta-hexosaminidase C. FEBS Lett. 1974;41(2):181–4. doi: 10.1016/0014-5793(74)81206-8. [DOI] [PubMed] [Google Scholar]
- 113.Gao Y, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276(13):9838–45. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 114.Overdijk B, et al. Isolation and further characterization of bovine brain hexosaminidase C. Biochim Biophys Acta. 1981;659(2):255–66. doi: 10.1016/0005-2744(81)90052-8. [DOI] [PubMed] [Google Scholar]
- 115.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283(3):634–40. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 116.Toleman C, et al. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279(51):53665–73. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 117.Butkinaree C, et al. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem. 2008;283(35):23557–66. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He Y, et al. Three-dimensional structure of a Streptomyces sviceus GNAT acetyltransferase with similarity to the C-terminal domain of the human GH84 O-GlcNAcase. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 1):186–95. doi: 10.1107/S1399004713029155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wells L, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277(3):1755–61. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 120.Keembiyehetty CN, et al. A lipid-droplet-targeted O-GlcNAcase isoform is a key regulator of the proteasome. J Cell Sci. 2011;124(Pt 16):2851–60. doi: 10.1242/jcs.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagel AK, Ball LE. O-GlcNAc transferase and O-GlcNAcase: achieving target substrate specificity. Amino Acids. 2014;46(10):2305–16. doi: 10.1007/s00726-014-1827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tarrant MK, et al. Regulation of CK2 by phosphorylation and O-GlcNAcylation revealedby semisynthesis. Nat Chem Biol. 2012;8(3):262–9. doi: 10.1038/nchembio.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park SY, et al. Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. Embo j. 2010;29(22):3787–96. doi: 10.1038/emboj.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hardiville S, et al. O-GlcNAcylation/phosphorylation cycling at Ser10 controls both transcriptional activity and stability of delta-lactoferrin. J Biol Chem. 2010;285(25):19205–18. doi: 10.1074/jbc.M109.080572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Srikanth B, Vaidya MM, Kalraiya RD. O-GlcNAcylation determines the solubility, filament organization, and stability of keratins 8 and 18. J Biol Chem. 2010;285(44):34062–71. doi: 10.1074/jbc.M109.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang F, et al. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115(6):715–25. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 127.Guinez C, et al. Protein ubiquitination is modulated by O-GlcNAc glycosylation. Faseb j. 2008;22(8):2901–11. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
- 128.Hahne H, et al. Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J Proteome Res. 2013;12(2):927–36. doi: 10.1021/pr300967y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zaro BW, et al. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci U S A. 2011;108(20):8146–51. doi: 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]