Abstract
Background and Objectives:
Endoscopic stenting is a minimally invasive treatment modality for patients with various gastrointestinal conditions. We evaluated the safety and efficacy of uncovered biodegradable stents for postoperative leaks and strictures in the upper gastrointestinal tract.
Methods:
This was a retrospective study of patients treated endoscopically with biodegradable stents from January 2010 through November 2017.
Results:
Thirteen patients were enrolled, 7 of whom were men. Their mean age was 46 (range, 21–82) years. The indications for stent placement were postoperative leakage and stricture in 9 and 4 patients, respectively. The primary diagnoses were obesity in 7 patients, gastric cancer in 5, and peptic ulcer in 1. The average time to stent placement after surgery was 35 (range, 17–125) and 166 (range, 153–185) days for patients with postoperative leakage and stricture, respectively. Stent insertion was successful at the first attempt in all patients. Complete resolution of the leak and stricture was achieved after stent application in 11 patients, for a clinical success rate of 85%. The mean follow-up duration was 50 (range, 24–76) months. There were no major complications.
Conclusions:
Compared to self-expanding metal and plastic stents, the main advantages of uncovered biodegradable stents are that they do not have to be removed and have a low migration rate. Our results suggest that these stents have promise for management of postoperative gastrointestinal complications. Further randomized trials with larger sample sizes are necessary to determine the role of biodegradable stents in the treatment algorithm.
Keywords: Biodegradable stent, Endoscopic stenting, Gastrointestinal complications, Postoperative leaks, Postoperative strictures
INTRODUCTION
Endoscopic stenting is used as a definitive treatment, a bridge to surgery, or a palliation of symptoms in patients with various gastrointestinal conditions, including benign or malignant strictures, obstructions, perforations, leaks, and fistulae.1,2 It is a minimally invasive technique and thus is typically suitable for patients who are malnourished and frequently have multiple comorbidities.2
Rigid plastic stents were formerly used for the palliation of malignant strictures related to esophageal cancer.1 The introduction of uncovered self-expanding metal stents (SEMS) in the early 1990s reduced the use of rigid stents1; however, problems such as mucosal hyperplastic reaction (over- or in-growth) with subsequent obstruction of the stent were encountered.3 Therefore, SEMS were fully covered with a silicone membrane to reduce ingrowth of granulation tissue.4 Unfortunately, covered stents are more apt to migrate, are less flexible, and have a shorter radial force. These problems prompted the development of partially covered metal stents with flared uncovered segments at both ends1,4,5 and self-expanding plastic stents (SEPS) to minimize hyperplastic tissue reactions.6 The need for removal is an important disadvantage of SEPS and SEMS.
To overcome these drawbacks, biodegradable stents (BSs) have been developed.7 Goldin et al8 used a poly l-lactide BS to treat a refractory benign esophageal stricture. The BS disintegrated 6 weeks after placement and obstructed the esophageal lumen. Since 2007, only polydioxanone (SX-ELLA biodegradable esophageal stent; Milady Horakove, Kralove, Czech Republic) BSs have been used for endoscopic placement.9
BSs with body diameters of 18 to 25 mm and lengths of 60 to 135 mm are available, typically with both ends flaring to 23–31 mm to reduce the migration rate. Stent integrity and radial force are maintained for 6–8 weeks after deployment, and complete hydrolytic degradation occurs 11–12 weeks after insertion.9 The biodegradation rate is dependent, not only on stent size and structure, but also in pH, temperature, tissue, and fluid. Acid-suppression therapy slows BS degradation.
We retrospectively investigated the safety and efficacy of the uncovered BS (UBS) for the management of upper gastrointestinal conditions (eg, postoperative leakage and stricture).
MATERIALS AND METHODS
This was a retrospective study of patients treated endoscopically with UBSs at Bakırköy Sadi Konuk Training and Research Hospital from January 2010 through November 2017. The study protocol was approved by the Ethics Committee of our institute. The demographic characteristics, indications for endoscopic stenting, primary diagnoses, previous surgical interventions, endoscopic procedure duration, stent type, procedural complications, time from surgery to stent placement, and outcomes were recorded.
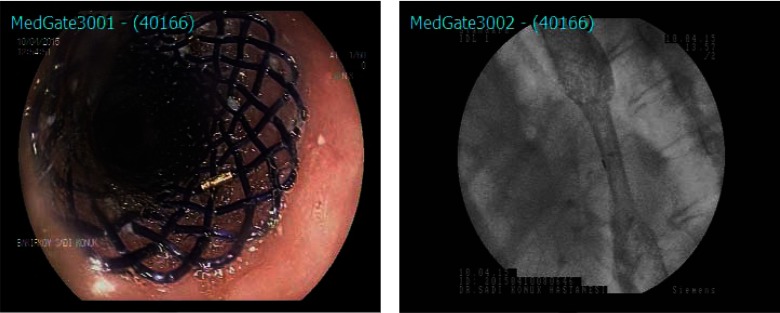
All endoscopic stenting procedures were performed by an experienced surgeon with the patient under sedation with intravenous propofol. Figure 1 shows the stent design. Polydioxanone UBSs were placed under fluoroscopic guidance in all patients. First, a guidewire was inserted through the endoscope to the distal part of the pathological segment. Second, a stent of a size appropriate for the patient's condition was implanted over the wire after withdrawal of the endoscope. The length of the stricture was measured. After placement, stent position and luminal patency were checked fluoroscopically, with administration of radio-opaque solution. Possible complications were explained to all patients, and written consent was obtained before each procedure.
Figure 1.
Endoscopic view of the uncovered bio-degradable stent (on the left). Flouroscopic image of the stent (on the right).
After stent placement, clinical, endoscopic, and radiologic outcomes and the timing of initiation of oral feeding were assessed according to the indication for endoscopic stenting. The stent positions were endoscopically checked in all patients at 2 and 6 month. In patients with gastric tumors, endoscopic controls were performed annually. No endoscopic follow-up was performed in patients who remained asymptomatic.
Technical success was defined as successful stent deployment. Clinical success was defined as healing of the postoperative leak or stricture and restoration of oral food intake after UBS application without other intervention.
RESULTS
This study involved 13 patients, 7 (54%) of whom were men. The ages of the patients ranged from 21 to 82 (mean, 46) years.
The indications for stent placement were postoperative leakage and stricture in 9 and 4 patients, respectively. The primary diagnoses were obesity in 7 patients, gastric cancer in 5, and peptic ulcer in 1. Postoperative proximal gastric leakage developed after sleeve gastrectomy in 5 patients. Three had anastomotic leakage after total gastrectomy. One patient with postoperative leakage from the gastroesophageal junction, who had undergone vagotomy and pyloroplasty secondary to massive bleeding associated with duodenal ulcer, presented with a tracheal-esophageal fistula.
UBSs were placed in 6 patients with postoperative leak after failure of 14 days of conservative treatment, which comprised parenteral nutrition, full fasting, and intravenous antibiotics. Three of the 9 patients with postoperative leakage had undergone unsuccessful interventions before UBS placement. One of them had undergone a mini gastric bypass because of proximal gastric leakage after sleeve gastrectomy. Endoscopic closure of anastomotic leakage using an over-the-scope clip device was attempted in the second patient. Placement of a self-expandable metal stent had been unsuccessful in the third patient.
The strictures were located at the proximal suture line after sleeve gastrectomy in 2 patients and at the anastomosis after total gastrectomy in 2 patients. These patients were able to tolerate liquids only. The median length of the strictures was 1 (range, 1–3) cm. All patients with stricture had multiple endoscopic dilation sessions before stent application. Regarding stent dimensions, 31 × 100-mm stents were placed in 6 patients, with 28 × 80-mm stents in 4, 23 × 80-mm stents in 2, and a 30 × 80-mm stent in 1. The mean procedure time was 20 (range, 15–30) min. In 2 patients with stricture, balloon dilation was necessary to advance the guidewire beyond the stenotic segment. Stent insertion was successful at the first attempt in all patients (technical success rate, 100%). No intraprocedural complications occurred.
The average time to stent placement after surgery was 35 (range, 17–125) and 166 (range, 153–185) days for patients with postoperative leakage and stricture, respectively.
After stent placement, all patients except those who underwent total gastrectomy received acid-suppression therapy to prevent reflux disease. Patients with postoperative leakage underwent a fluoroscopic check-up with oral administration of contrast medium 3 to 5 days after stent deployment. In 1 patient, ongoing contrast leakage through the bioabsorbable thread of the stent was detected during the check-up. This patient underwent deployment of a covered metallic stent over the previous stent. In another patient with chronic gastric fistula, total gastrectomy was performed 10 months after stent placement. Therefore, in 2 of 13 patients UBS placement did not resolve gastric leakage (clinical success rate, 85%). Except in those cases, no further procedure was required.
Patients with postoperative leakage started oral intake after persistent leakage was ruled out by clinical and radiologic evaluations. The mean duration of hospital stay after placement of a UBS in those patients was 10 (range, 7–16) days. Patients with stricture tolerated liquids immediately after stent placement and subsequently consumed solids. They were discharged later on the day of stent placement.
The mean follow-up duration was 50 (range, 24–76) months. Four patients with gastric cancer died. After stent placement, retrosternal pain, the sensation of having a foreign object in the body, and drooling occurred in all patients. Retrosternal pain was responsive to analgesics and ceased in 10–15 days. The foreign-object feeling and drooling lasted for several weeks. Aversion to water developed in 8 patients, lasted for 2 months, and resolved spontaneously. Six patients had nausea for a few days, which was treated with metoclopramide. As for endoscopic surveillance, partial and complete stent degradation, along with mild mucosal hypertrophy, were seen in all but 1 of the patients at the 2- and 6-month follow-ups, respectively. In 1 patient, mucosal hypertrophy was excessive, appearing as pseudopolyps, but they were asymptomatic and did not require intervention. Patient and surgical information is shown in Table 1.
Table 1.
Characteristics of the Patients and Surgical Procedures
| No. | Gender | Age | Cause of UBS Application | Primary Diagnosis | Previous Surgery | Pre-UBS Therapy | Post-UBS Therapy | Procedure Time (minutes) | Stent Type | Time to stent placement (days) | Duration of Hospital Stay After Stenting (days) | Follow-up (months) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | Proximal gastric leak | Obesity | Sleeve gastrectomy | – | – | 25 | 23×80 | 37 | 15 | 76 | RP, FOF, D, N, MH |
| 2 | M | 40 | Proximal gastric leak | Obesity | Sleeve gastrectomy | Metal stent | – | 25 | 31×100 | 25 | 8 | 64 | RP, FOF, D, N, MH |
| 3 | F | 43 | Proximal gastric leak | Obesity | Sleeve gastrectomy | – | – | 15 | 23×80 | 20 | 9 | 63 | RP, FOF, D, N, MH |
| 4 | F | 22 | Proximal gastric leak | Obesity | Sleeve gastrectomy | Mini gastric bypass | Total gastrectomy and R-Y esophagojejunostomy | 20 | 31×100 | 125 | 7 | 29 | RP, FOF, D, N |
| 5 | F | 21 | Proximal gastric leak | Obesity | Sleeve gastrectomy | OTSC | – | 30 | 31×100 | 27 | 7 | 38 | RP, FOF, D, RW, MH |
| 6 | M | 55 | Anastomotic leak | Gastric cancer | Total gastrectomy | – | Covered metal stent | 15 | 31×100 | 21 | 14 | 48 | RP, FOF, D, RW, MH |
| 7 | M | 71 | Anastomotic leak | Gastric cancer | Total gastrectomy | – | – | 15 | 31×100 | 18 | 8 | 36 | RP, FOF, D, RW, MH |
| 8 | F | 82 | Anastomotic leak | Gastric cancer | Total gastrectomy | – | – | 15 | 28×80 | 17 | 10 | 24 | RP, FOF, D, MH |
| 9 | F | 54 | Tracheal-esophageal fistula | Peptic ulcer | Vagotomy -pyloroplasty | – | – | 20 | 28×80 | 32 | 16 | 59 | RP, FOF, D, N, RW, MH |
| 10 | F | 31 | Stricture at proximal suture line | Obesity | Sleeve gastrectomy | Endoscopic dilatations | – | 20 | 28×80 | 153 | Same day | 63 | RP, FOF, D, N, RW, MH and Pseudopolyps |
| 11 | M | 44 | Stricture at proximal suture line | Obesity | Sleeve gastrectomy | Endoscopic dilatations | – | 15 | 30×80 | 160 | Same day | 57 | RP, FOF, D, RW, MH |
| 12 | M | 69 | Anastomotic stricture | Gastric cancer | Total gastrectomy | Endoscopic dilatations | – | 20 | 31×100 | 185 | Same day | 46 | RP, FOF, D, RW, MH |
| 13 | M | 39 | Anastomotic stricture | Gastric cancer | Total gastrectomy | Endoscopic dilatations | – | 15 | 28×80 | 166 | Same day | 54 | RP, FOF, D, RW, MH |
D, drooling; FOF, foreign object feeling; MH, mucosal hypertrophy; N, nausea; RP, retrosternal pain; RW, rejection of water.
DISCUSSION
We report our clinical experience with UBS for postoperative anastomotic or staple line leak, fistula, and stricture. Technical success was achieved in all patients. Eleven of the 13 patients achieved complete resolution of the leak and stricture after UBS deployment and symptom relief after stent degradation.
All surgical interventions have a risk of complications. Postoperative leaks can be fatal and occur after 1%–10% and 1%–3% of total and sleeve gastrectomies, respectively.10–14 Various factors, including technical errors, stapler misfiring, poor blood supply, and increased intragastric pressure, are related to postoperative leakage. Patients with unstable parameters require prompt reoperation for washout and drainage and possibly also debridement and suturing of the orifice, if appropriate. The optimal management of postoperative leaks is controversial because of the lack of standardized treatment protocols.15 Extensive nutritional support and intravenous antibiotic infusion are important components of management. Generally, enteral nutrition is preferred over total parenteral nutrition during the postoperative period because it provides a better response to infectious complications, allows better maintenance of bowel function, reduces hepatic steatosis, and costs less. Despite these advantages, during the early postoperative period, which is often associated with bowel hypoperistalsis, parenteral nutrition is generally used in patients with newly placed stents to decrease intraluminal secretions and bowel pressure. Before UBS application, we made conservative treatment with parenteral nutrition in all patients with leakage but it persisted. In stable patients in whom conservative therapy has failed, endoscopic stenting is a valid treatment option for acute leaks, together with adequate drainage.16 Stent placement can reduce the time from procedure to starting oral intake, morbidity, and duration of hospital stay compared with surgical intervention. Stents may also facilitate correction of the sleeve axis in cases of gastric twisting.17 There is no consensus as to which type of stent is optimal. Use of covered metal stents to stop benign upper gastrointestinal leaks, perforations, and fistulae has a success rate of 72%–79%.18,19 Alazmi et al17 found that metal and plastic stents have a 76% success rate for post–sleeve gastrectomy leaks. Simon et al20 reported that earlier endoscopic stent placement (<3 wk after leak diagnosis) accelerates healing in patients with gastric leakage after sleeve gastrectomy. BSs are used increasingly frequently, as they do not have to be removed and are effective for treating leaks after total and sleeve gastrectomy.1,20 In this study, UBSs promoted complete healing by inducing a fibrous reaction in 11 of 13 patients.
Postoperative strictures affect 2%–5% and 1%–3% of patients after total and sleeve gastrectomy, respectively.21,22 The following factors are associated with postoperative stricture development: technical errors, decreased vascularization, foreign-body reaction, inflammation after small anastomotic leaks, and radiotherapy. These strictures are complex and usually require multiple endoscopic dilations with bougies or balloons.21 However, several novel techniques have become available: incision of the stenosis with electrocautery, intralesional steroid injection, and stent placement.2,23 Van Hooft et al24 reported that BS placement was an effective 1-step treatment for postsurgical esophagogastric anastomotic strictures in 6 of 10 patients. By contrast, a recent meta-analysis of the clinical outcomes of plastic and metal stents compared to BSs in patients with benign esophageal strictures reported a pooled clinical success rate of 40.5%, which did not differ among the types of stent.25 Placement of UBSs was successful in our patients who had stricture, with no need for reintervention during a 50-month follow-up. BSs facilitate tissue remodeling, resulting in recovery of the stricture. Resolution of postoperative inflammation and the prolonged dilatory effects of the stent may be important factors for maintaining an adequate lumen.
In a case series, Ham and Kim26 reported a biodegradable stent migration rate of 0% to 22%. The uncovered design of BSs enables the stent to become embedded in the underlying tissue, which reduces the migration rate but causes reactive tissue hyperplasia, which can lead to severe stent stenosis and recurrent dysphagia. Karakan et al27 reported that 57% of their patients experienced tissue hyperplasia. To prevent this development, drug-eluting (such as rapamycin and paclitaxel) stents have been evaluated.28 In this study, mild mucosal hypertrophy occurred in all patients, except 1, whose mucosal hypertrophy was excessive and appeared as pseudopolyps; fortunately, the condition did not have clinical consequences. Thoracic pain, the sensation of having a foreign object in the body, perforation, and bleeding are other complications of BS placement.9 Retrosternal pain was a common finding after stent placement and was associated with gradual expansion of the stent. No stent migration occurred. Aversion to water was another complication of stent treatment, but the mechanism is unknown.
Although biodegradable stents are more expensive than other plastic or metal stents, they could be cost effective because there is no need for removal. Sometimes, repeated endoscopic procedures are necessary to remove other stents, resulting in loss of workforce, time, and money.
In conclusion, the optimal type of endoscopic stent for postsurgical upper gastrointestinal tract issues is unclear. The optimal stent would be easy to place and to retrieve, would use a small-calibre delivery mechanism, would not migrate, and would induce minimal tissue reaction. Our results indicate that the placement of UBSs for the management of postoperative leaks and strictures is technically simple, safe, and clinically effective. Larger randomized trials that compare the various types of stent are necessary to confirm our results.
Contributor Information
Osman Köneş, General Surgery Unit, Bakırköy Training and Research Hospital, Istanbul, Turkey..
Ebru Oran, General Surgery Unit, Bakırköy Training and Research Hospital, Istanbul, Turkey..
References:
- 1. Pavlides M, Gorard DA. Stents in gastrointestinal endoscopy. Ther Gastrointest Endosc. 2011;22:115–140. [Google Scholar]
- 2. Dabizzi E, Arcidiacono PG. Update on enteral stents. Curr Treat Options Gastroenterol. 2016;14:178–184. [DOI] [PubMed] [Google Scholar]
- 3. Vlavianos P, Zabron A. Clinical outcomes, quality of life, advantages and disadvantages of metal stent placement in the upper gastrointestinal tract. Curr Opin Support Palliat Care. 2012;6:27–32. [DOI] [PubMed] [Google Scholar]
- 4. Kang SG. Gastrointestinal stent update. Gut Liver. 2010;4:S10–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer A, Bausch D, Richter-Schrag HJ. Use of a specially designed partially covered self-expandable metal stent (PSEMS) with a 40-mm diameter for the treatment of upper gastrointestinal suture or staple line leaks in 11 cases. Surg Endosc. 2013;27:642–647. [DOI] [PubMed] [Google Scholar]
- 6. Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:931–938. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Li N, Li R, Li Y, Ruan L. Biodegradable intestinal stents: review. Prog Nat Sci. 2014;24:423–432. [Google Scholar]
- 8. Goldin E, Fiorini A, Ratan Y. A new biodegradable and self-expandible stent for benign esophageal strictures. Gastrointest Endosc. 1996;43:294. [Google Scholar]
- 9. Lorenzo-Zúñiga V, Moreno-de-Vega V, Marín I, Boix J. Biodegradable stents in gastrointestinal endoscopy. World J Gastroenterol. 2014;20:2212–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deguchi Y, Fukagawa T, Morita S, Ohashi M, Saka M, Katai H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J Surg, 2012;36:1617–1622. [DOI] [PubMed] [Google Scholar]
- 11. Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moon RC, Shah N, Teixeira AF, Jawad MA. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis. 2015;11:24–59. [DOI] [PubMed] [Google Scholar]
- 13. Nomura S, Sasako M, Katai H, Sano T, Maruyama K. Decreasing complication rates with stapled esophagojejunostomy following a learning curve. Gastric Cancer 2000;29:97–101. [DOI] [PubMed] [Google Scholar]
- 14. Durmush EK, Ermerak G, Durmush D. Short-term outcomes of sleeve gastrectomy for morbid obesity: does staple line reinforcement matter? Obes Surg, 2014;24:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willingham FF, Buscaglia JM. Endoscopic management of gastrointestinal leaks and fistula. Clin Gastroenterol Hepatol. 2015;13:1714–1721. [DOI] [PubMed] [Google Scholar]
- 16. Raimondo D, Sinagra E, Facella T, et al. Self-expandable metal stent placement for closure of a leak after total gastrectomy for gastric cancer: report on three cases and review of the literature. Case Rep Gastrointest Med. 2014:409283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alazmi W, Al-Sabah S, Ali DA, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28:3425–3428. [DOI] [PubMed] [Google Scholar]
- 18. Orive-Calzada A, Calderón-García Á, Bernal-Martínez A, et al. Closure of benign leaks, perforations, and fistulas with temporary placement of fully covered metal stents: a retrospective analysis. Surg Laparosc Endosc Percutan Tech. 2014;24–528–536. [DOI] [PubMed] [Google Scholar]
- 19. Van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon F, Siciliano I, Gillet A, Castel B, Coffin B, Msika S. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23:687–692. [DOI] [PubMed] [Google Scholar]
- 21. Akarsu C, Unsal MG, Dural AC, et al. Endoscopic balloon dilatation as an effective treatment for lower and upper benign gastrointestinal system anastomotic stenosis. Surg Laparosc Endosc Percutan Tech. 2014;25:138–142. [DOI] [PubMed] [Google Scholar]
- 22. Sarkhosh K, Birch DW, Sharma A, Karmali S. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon's guide. Can J Surg. 2013;56:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nijhawan S, Udawat HP, Nagar P. Aggressive bougie dilatation and intralesional steroids is effective in refractory benign esophageal strictures secondary to corrosive ingestion. Dis Esophagus. 2016;29:1027–1031. [DOI] [PubMed] [Google Scholar]
- 24. van Hooft JE, van Berge Henegouwen MI, Rauws EA, et al. Endoscopic treatment of benign anastomotic esophagogastric strictures with a biodegradable stent. Gastrointest Endosc. 2011;73:1043–1047. [DOI] [PubMed] [Google Scholar]
- 25. Fuccio L, Hassan C, Frazzoni L, Miglio R, Repici A. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy. 2016;48:141–148. [DOI] [PubMed] [Google Scholar]
- 26. Ham YH, Kim GH. Plastic and biodegradable stents for complex and refractory benign esophageal strictures. Clin Endosc. 2014;47:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karakan T, Utku OG, Dorukoz O, et al. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Dis Esophagus. 2013;26:319–322. [DOI] [PubMed] [Google Scholar]
- 28. Zhu YQ, Cui WG, Cheng YS, Chang J, Chen NW, Yan L. Evaluation of biodegradable paclitaxel-eluting nanofibre-covered metal stents for the treatment of benign cardia stricture in an experimental model. Br J Surg. 2013;100:784–793. [DOI] [PubMed] [Google Scholar]