Abstract
Purpose: To investigate the changes in intraocular pressure (IOP), aqueous flow, and outflow facility, as well as efficacy of IOP-lowering drugs before and after sexual development in rabbits.
Methods: Male Dutch-belted rabbits were studied at night between the ages of 8 and 44 weeks. During these times, body weight, testicular volume, and serum testosterone were measured to monitor sexual maturity. Ocular measurements included anterior chamber depth, central corneal thickness, IOP, aqueous flow, and outflow facility. Systemic acetazolamide or topical timolol, latanoprost, or saline were administered pre- and postpuberty to assess drug effects on these parameters.
Results: Body weight, testicular volume, and serum testosterone increased until 28 weeks of age. IOP increased during prepuberty (R2 = 0.49, P = 0.003), dropped significantly during puberty, rising again immediate postpuberty, and changing little thereafter. Postpuberty compared with prepuberty found higher IOP (P < 0.0001), slower aqueous flow (P = 0.008), lower outflow facility (not statistically significant, P = 0.07), increased central cornea thickness, and increased anterior chamber volume. Timolol lowered IOP both pre- and postpuberty, whereas, latanoprost and acetazolamide decreased IOP postpuberty only.
Conclusions: As male rabbits mature, the cornea thickens and the anterior chamber volume increases. At the same time, aqueous flow slows, yet, IOP increases. This suggests that decreased outflow facility and/or increased episcleral venous pressure might contribute to the puberty-related changes in IOP. Underdevelopment of tissues of the outflow pathways may contribute to the differences in drug efficacy in rabbits when young compared with after sexual maturity.
Keywords: : intraocular pressure, puberty, aqueous flow
Introduction
Intraocular pressure (IOP) is maintained by the flow of aqueous humor into and out of the anterior chamber of the eye, by the resistance to that flow and by the pressure in the drainage vessels. Aqueous humor dynamics (AHD) describe the characteristics of this fluid movement. When dysregulation of AHD occurs, this may lead to a harmful increase in IOP, a major risk factor for glaucoma.1,2 Management of IOP surgically or medically is the standard therapy for glaucoma;3 thus an understanding of AHD is advantageous in efforts to develop new treatments to protect the eye from an unhealthy pressure environment.
Ocular AHD vary daily and throughout life. Efficacy of IOP-lowering therapies can be affected by these variations. For example, in primates, timolol does not lower IOP at night4 but is efficacious during the day, latanoprost works better when given in the evening than morning,5 and children respond differently to IOP-lowering therapies than adults.6,7 Ocular changes occur throughout childhood as evidenced by eye growth8,9 and IOP increase.10–13 One study14 did note no change in IOP in children from 8 to 16 years old, but an IOP increase may have been found had younger and older ages been included. The changes in AHD that contribute to the normal IOP rise during development are not known. Numerous reports exist of topical hypotensive drugs working poorly in some children depending on age.13,15–17 While the size of the eye is increasing,8,9 logically AHD must adjust to maintain emmetropia and a healthy IOP.18 These earlier studies and the fact that puberty is a time of robust changes throughout the body lead to our hypothesis that changes in AHD during puberty affect efficacy of IOP-lowering drugs. The hypothesis is tested in rabbits treated with IOP-lowering drugs before and after puberty.
The rabbit was the animal of choice for this study for several reasons. The rabbit matures relatively rapidly, reaching adulthood within 30 weeks. Ocular measurements can be made noninvasively and repeatedly in each rabbit as it matures. A previously published study in rabbits has identified age-related changes in IOP, aqueous flow (Fa), and outflow facility (C).19 For the current study, the Dutch-belted rabbit was chosen over the more commonly used New Zealand white (NZW) rabbit because IOPs in the former normally run between 20 and 35 mmHg, which provide a better chance of detecting IOP changes during puberty than the NZW rabbit, which has normal IOPs in the low to midteens. Although children would be the best model to test the hypothesis, ethical concerns regarding research conducted in children and the regulatory requirements protecting children limit drug administration and detailed assessment of AHD in this vulnerable population.
Methods
Animals
Male Dutch-belted rabbits (Covance, Princeton, NJ) were studied from the ages of 8 to 44 weeks (n = 15). All animal experimentations were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee before the study started. Somatic growth and sexual maturation were monitored throughout the study by body weight, testicular volume, and serum testosterone levels. Testicular volume was calculated from dimensions measured by Vernier calipers.20 Serum testosterone levels were measured with an ELISA assay following the company's protocol.
Experimental design and ocular measurements
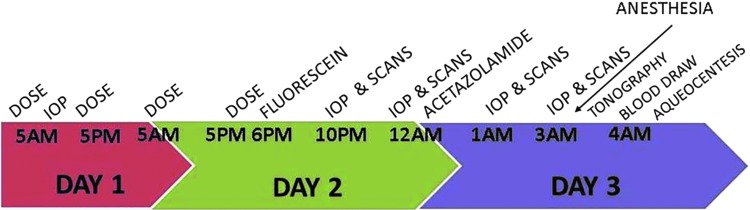
Measurements were made 6 times on each animal, 3 times following treatment with timolol, latanoprost, or saline in prepuberty, and all measurements were repeated postpuberty. Due to the time required to make all measurements, not all assessments could be collected in all animals while they were of the same age (Fig. 1). Therefore, the animals were divided into 3 groups of 5, and the measurements within groups were made when they were of the same age, but among groups, their ages may have differed by 6 weeks. At 5 am on the study day, baseline IOP measurements were made in the awake animal with a pneumatonometer (Classic 30, Reichert, DePew, NY). IOP measurements were made twice at each time interval and a third time if the results of the first 2 were more than 3 mmHg apart. If the 3 measurements were not similar, then the 2 that were within 3 mmHg were used. If all 3 were not similar, then a fourth measurement was made (very rare). The reported IOPs are the average of multiple measurements (most often 2 measurements). Biometric and AHD measurements began at 10 pm and ended at 5 am the following day. All corneal contact measurements were made on eyes anesthetized with 20 μL of 0.5% proparacaine. At 6 pm on the study day, three to four 10 μL drops of 10% fluorescein were applied to both eyes of the conscious animal. Starting at 10 pm, duplicate fluorophotometric scans (Fluorotron Master, OcuMetrics, Palo Alto, CA) were taken of the cornea and anterior chamber fluorescence at 45 min intervals for 2.5 h. Aqueous flow was calculated from these data.21 After each set of scans, 2 IOP measurements were collected and averaged. Acetazolamide (16 mg/kg) was given intramuscularly to slow aqueous flow and consequently lower IOP. Scans and IOP measurements were continued for 3 more sets. These data were used to calculate outflow facility (ratio of the change in aqueous flow to the change in IOP). At 4 am, 2-min tonography was performed on one or both eyes using the tonography setting on the pneumatonometer. Data were collected and stored digitally, and outflow facility was calculated as described previously.22
FIG. 1.
Timeline of experimental procedures. “Dose” signifies the dosing of either latanoprost, timolol, or saline. “Scan” signifies fluorophotometric scans of cornea and anterior chamber. Proparacaine was dosed topically before any measurement that required touching of the cornea (IOP, tonography). IOP, intraocular pressure.
Ultrasonic pachymetry (PacScan Series 300; Sonomed, Lake Success, NY) was used to measure central cornea thickness (CCT), while slit-lamp pachymetry was used to measure anterior chamber depth (ACD). Corneal diameter was measured by calipers. Anterior chamber volume was calculated from these measurements.23
Between the ages of 9 and 15 weeks, 25 μL of either sterile saline (control, EyeStream; Alcon, Ft. Worth, TX), timolol (0.5%; Alcon), or latanoprost (0.005%; Pfizer, New York, NY) eye drops were applied topically in random order for 2 days. Saline and timolol were dosed twice-daily at 5 am and 5 pm. Latanoprost was dosed at 5 am and saline at 5 pm. The 5 am dosing was at the beginning of the animal's sleep period (day), and drug effects were measured during the animal's active period (night). Latanoprost is most efficacious during the active period of humans,24 therefore, the latanoprost effect was expected to be better in rabbits during the night than day. All measurements were made on the third day. The second and third treatments and measurements were separated by 2 weeks. Animals were allowed to mature for the next 4 to 5 months. At 30–40 weeks of age, the treatments and measurements were repeated as before.
The measurements were made after dosing in random order with timolol, latanoprost, or saline. Measurements were made at night, during the animal's active period. This is the time in which timolol is most efficacious in rabbits.25 All measurements were made within an hour of the stated times in Fig. 1.
Statistics
Paired t-tests were used to identify differences pre- and postpuberty in AHD parameters, and to compare drug effects before and after puberty. Repeated measures ANOVA tests were used to examine changes in parameters over time. Spearman's correlations were used to identify relationships between age and the various parameters. P < 0.05 was considered statistically significant. Data are represented as mean ± SEM, unless otherwise noted.
Results
Puberty
Body weight increased from 0.81 ± 0.02 kg at 8 weeks until 2.00 ± 0.04 kg at 28 weeks. Weight correlated with age (R2 = 0.69, P < 0.001; Table 1). Testicular volume increased from 1.49 ± 0.2 mL at 12 weeks to 6.7 ± 0.42 mL at 28 weeks of age and remained stable thereafter (R2 = 0.56, P < 0.05; Table 1). Testicles were undescended before 12 weeks of age and thus, not measured. Serum testosterone increased between 12 and 38 weeks of age (R2 = 0.95, P < 0.0001, Table 1).
Table 1.
Parameters Measured to Monitor Sexual Development
| 8 | 10 | 12 | 17 | 20 | 28 | 35 | 38 | 42 | 44 | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (kg) | 0.8 ± 0.02 | 1.1 ± 0.03 | 1.6 ± 0.03 | 1.8 ± 0.03 | 1.9 ± 0.04 | 2.0 ± 0.04 | 2.0 ± 0.06 | 2.0 ± 0.05 | 2.0 ± 0.07 | 2.0 ± 0.05 | P < 0.01 |
| Testicular volume (mL) | NM | NM | 1.5 ± 0.2 | 2.0 ± 0.2 | 6.3 ± 0.6 | 6.7 ± 0.4 | 6.7 ± 0.4 | 5.7 ± 0.5 | 6.1 ± 0.9 | 5.2 ± 0.5 | P < 0.01 |
| Serum testosterone (ng/mL) | 2.9 ± 0.4 | 3.3 ± 0.6 | 3.3 ± 0.7 | 3.8 ± 0.9 | 4.5 ± 0.7 | 5.1 ± 0.9 | 5.1 ± 1.1 | 5.5 ± 0.9 | 5.5 ± 0.5 | 5.4 ± 0.7 | P < 0.01 |
P-value obtained from one-way ANOVA comparing means across ages.
Biometry
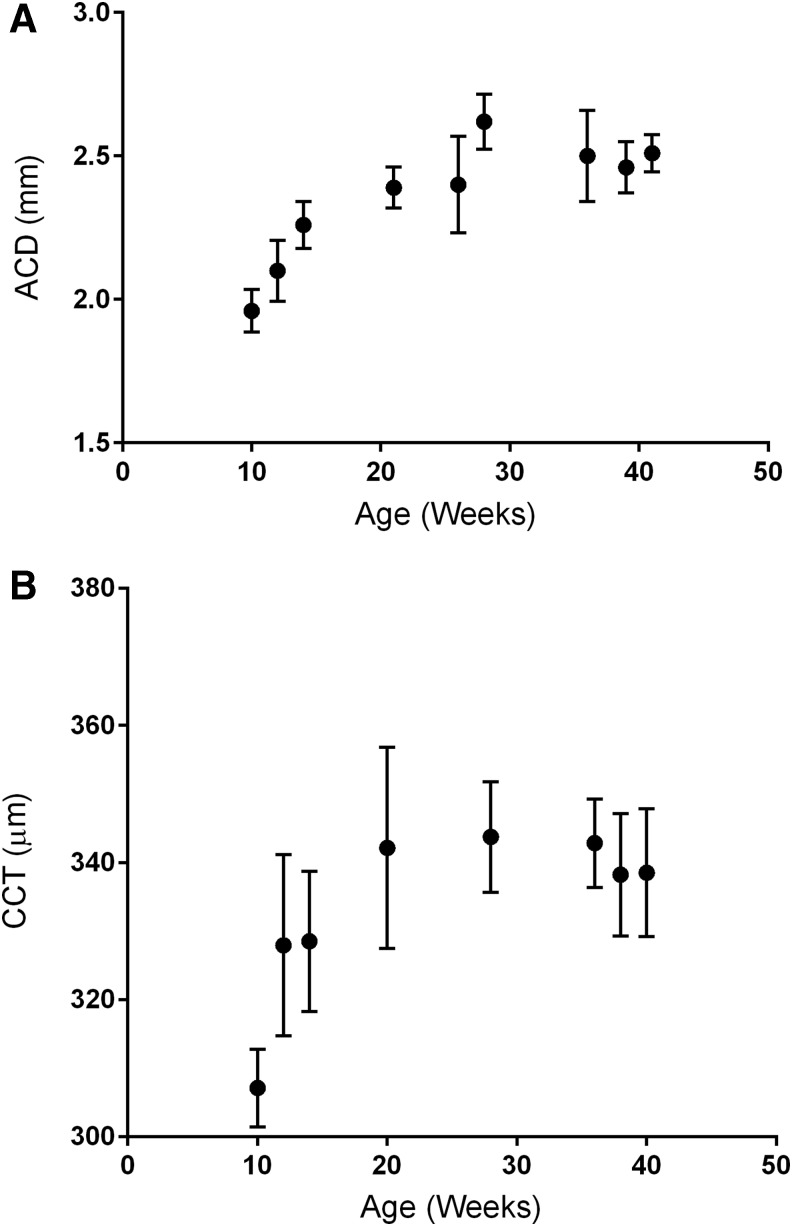
ACD increased from 9 to 27 weeks of age (Fig. 2A), and CCT increased from 9 to 20 weeks of age (Fig. 2B). Neither timolol nor latanoprost affected these parameters.
FIG. 2.
Biometric measurements of rabbits as they grew between the ages of 10 and 44 weeks. Data are mean ± SEM, n = 15, (A) Mean ACD R2 = 0.69, P = 0.005. (B) CCT measured by ultrasound pachymetry increases during maturation. R2 = 0.51, P = 0.04. ACD, anterior chamber depth; CCT, central corneal thickness.
IOP and AHD
Untreated
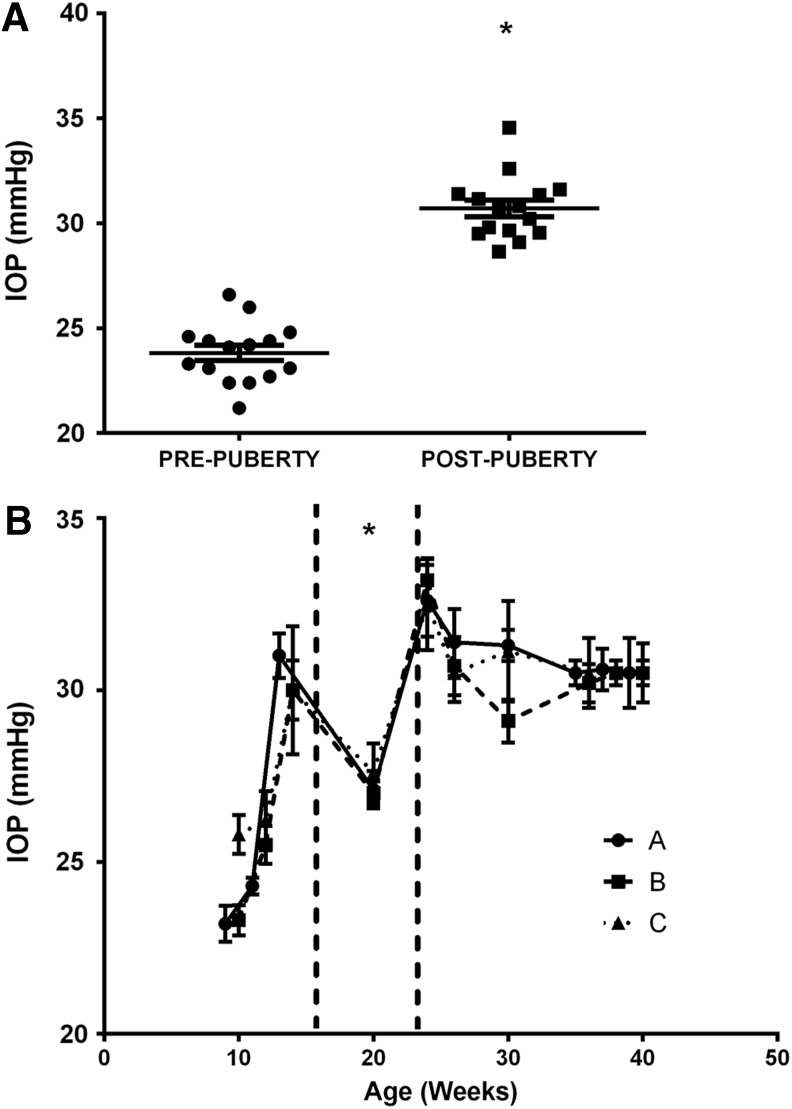
Mean untreated IOP postpuberty (ages 30–40 weeks 30.71 ± 0.39 mmHg) was higher than prepuberty (ages 9–15 weeks, 23.32 ± 0.36 mmHg; P < 0.0001, Fig. 3A). Baseline (untreated, 5 am) IOP from 9 to 15 weeks of age was positively correlated with age (R2 = 0.49, P = 0.0003). IOPs at week 20 were significantly less than IOPs at all other ages except week 12 (P < 0.001; Fig. 3B).
FIG. 3.
Untreated IOP measured by pneumatonometry in rabbits. (A) IOP was greater postpuberty (ages between 30 and 40 weeks) than prepuberty (ages between 9 and 15 weeks). Filled squares and circles indicate mean IOPs from both eyes of individual animals. *P < 0.0001 versus prepuberty, n = 15. (B) IOPs measured every 2 to 3 weeks from 10 to 42 weeks of age. Animals were divided into 3 groups A, B, and C, which consisted of 5 animals each. Animals within groups were the same age when measured but the different groups had to be measured at different ages because only 5 animals could be measured at a time. Dotted lines indicate the beginning and end of puberty. Data are mean ± SEM, n = 5. IOPs at 20 weeks were less than IOPs after 23 weeks of age (P < 0.001) and IOPs between 14–15 weeks of age.
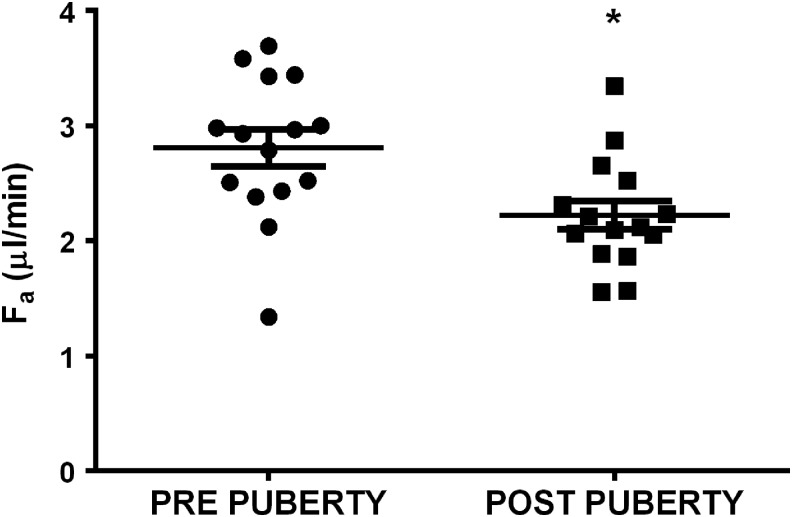
Aqueous flow rate was faster in untreated rabbits during prepuberty, 2.8 ± 0.2 compared with postpuberty, 2.2 ± 0.1 μL/min (P = 0.003, Fig. 4).
FIG. 4.
Mean aqueous flow (Fa) in rabbits postpuberty (ages 30–40 weeks) was less than prepuberty (ages 9–15 weeks). *P = 0.008 versus prepuberty. One data point represents an individual rabbit. Data are presented as mean ± SEM. N = 15.
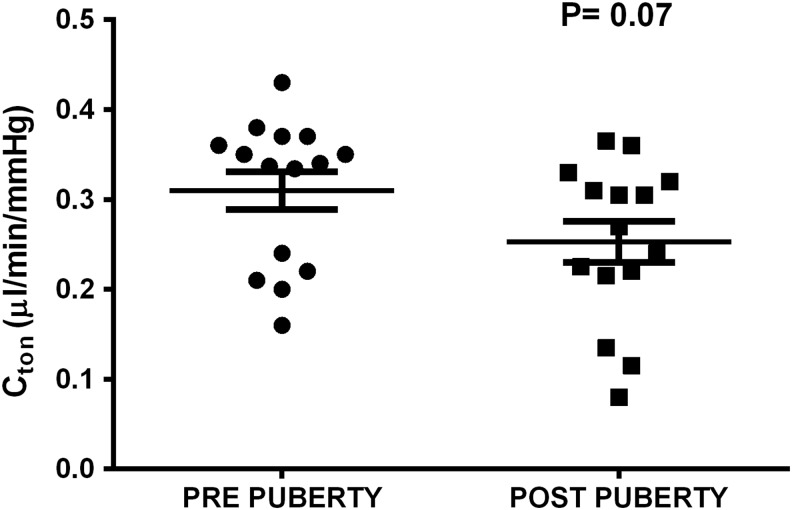
Tonographic outflow facility was not different when comparing pre- and postpuberty (P = 0.07; Fig. 5). Outflow facility by fluorophotometry could not be compared before and after puberty because acetazolamide (used to lower aqueous flow and IOP and calculate Cfl) did not work prepuberty in these rabbits.
FIG. 5.
Outflow facility by tonography (Cton) in rabbits showed a nonsignificant (P = 0.07) decrease when comparing postpuberty (30–40 weeks) with prepuberty (9–15 weeks). One data point represents an individual rabbit. Data are presented as mean ± SEM. N = 15.
Treated
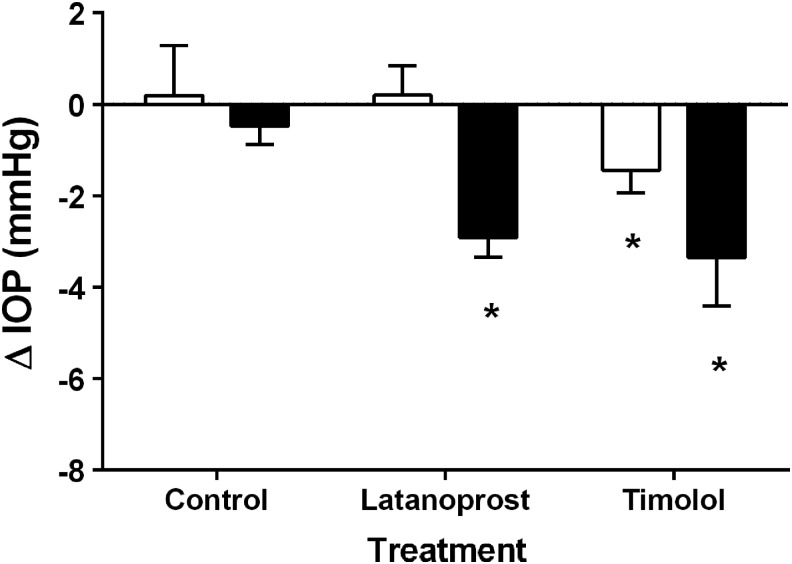
Treatment with saline (control) did not affect IOP at any age or any time of day (P > 0.05). During prepuberty, timolol reduced IOP from baseline (untreated 5 am reading) when measured at 10 pm (5 h after the last dose, P = 0.037) but not at 12 am (7 h after the last dose, P = 0.29). Postpuberty, timolol reduced IOP from baseline at 10 pm (P = 0.001) and 12 am (P = 0.001). This timolol-induced IOP change from baseline was significantly more postpuberty than prepuberty at 10 pm (P = 0.013) and 12 am (P = 0.041). Postpuberty, latanoprost reduced IOP from baseline when measured at 10 pm (P < 0.001) and 12 am (P < 0.05). There was no latanoprost effect prepuberty. The latanoprost-induced IOP change from baseline (untreated 5 am reading) was greater (P = 0.005) postpuberty (−2.94 ± 0.54 mmHg) than prepuberty (0.10 ± 0.72 mmHg; Fig. 6).
FIG. 6.
Change in intraocular pressure (IOP, at 10 pm) from topical treatment of timolol or latanoprost. The control group was treated with saline. White bars = prepuberty, black bars = postpuberty. N = 15 male Dutch-belted rabbits. *P < 0.05 versus prepuberty.
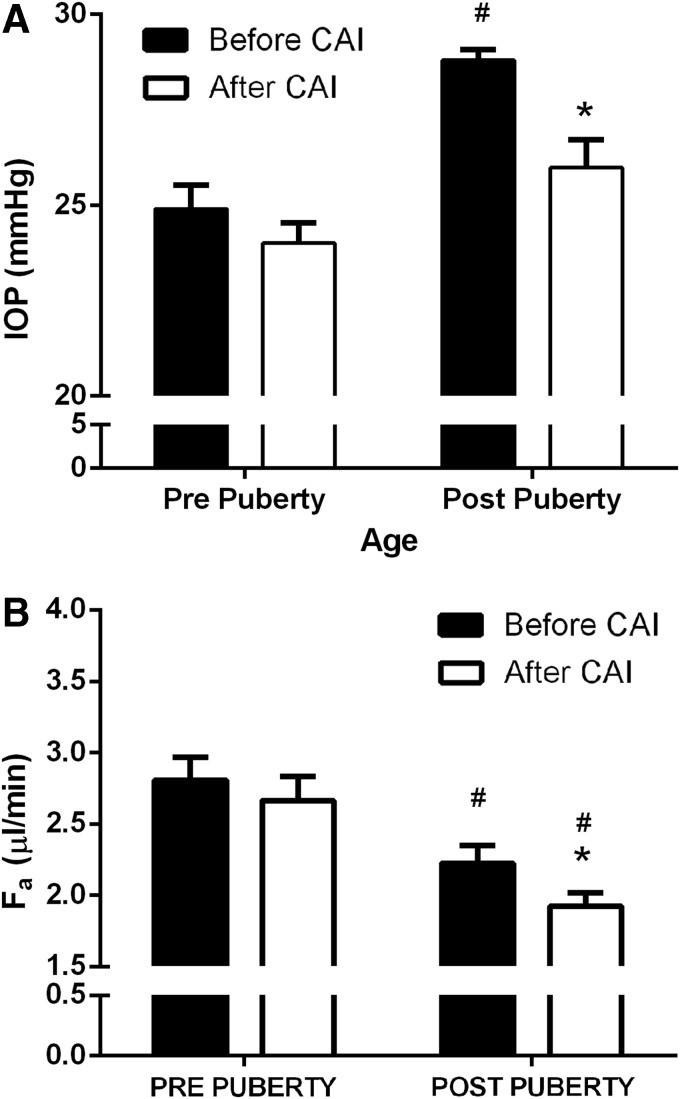
It was noted that IOP changes at 1–2 h following an intramuscular dose of acetazolamide were different when comparing pre- and postpuberty (Fig. 7). Acetazolamide caused a decrease in IOP postpuberty (P = 0.003), but not prepuberty (P > 0.05). Effects on IOP by acetazolamide are age dependent (P = 0.0024).
FIG. 7.
Effect of intramuscular acetazolamide (CAI), given at 12 am on 1 am IOP (A) and aqueous flow (Fa) (B). Black bars = before administration of CAI and white bars = after administration of CAI. N = 15 male Dutch-belted rabbits. *P < 0.05 versus before CAI (12 am). #P < 0.05 versus prepuberty. CAI, carbonic anhydrase inhibitor.
Aqueous flow among treatments was not different when comparing before (P = 0.24) and after puberty (P = 0.71). The effect of timolol on aqueous flow prepuberty approached significance (P = 0.09). Acetazolamide did not decrease aqueous flow before puberty, but did so postpuberty at 1–3 h postinjection (P < 0.05, Fig. 6B). The effect of acetazolamide on aqueous flow is age dependent (P < 0.0001).
Discussion
In the male Dutch-belted rabbits of the current study, testosterone levels started to rise at 10 weeks, and the testes were descended at 12 weeks of age. The cessation of somatic and testicular growth corresponded with the stabilization of serum testosterone at approximately 28 weeks of age; therefore, puberty was estimated to occur between 12 and 28 weeks of age. Other studies found that sexual maturity is reached in the male rabbit at or around 34 weeks.26,27 To understand the female response to drugs throughout development, sex hormones would need to be monitored more frequently and AHD measurements made more often than in the current study. The rate at which a rabbit matures would preclude the use of females for an AHD study of multiple IOP-lowering drugs.
As the rest of the body is reaching sexual maturity, the eye too is growing and changing.9 CCT is increasing gradually before puberty and begins to level off circa the time of puberty.28,29 Corneal diameter increases in humans between the ages of 1 and 13 years.30 This growth also is seen in chicks, cats, and dogs.31–33 In dogs, CCT increases starting at 6 weeks and reaches adult values at 24 weeks, which corresponds with the onset of puberty in these animals.32 In rabbits, the ACD and volume increase during puberty, probably due to an overall increase in globe size.8,9 Only a few studies have reported ACD or volumes in a normal pediatric population. One study of pediatric and adult patients reported a decline in ACD with aging.34 Another study found an increase in ACD in children between the ages of 2 and 14 years, yet, no difference in mean values between children and adults.35 Growth of the eye's gross structure could contribute to AHD changes in immature animals or humans.
Numerous studies report that IOP increases over time before puberty.29,36 The current study found IOP positively correlated with age; which agrees with conclusions made about age-related IOP changes in humans.10 Similar to prepubertal children, IOP was increasing in prepubertal rabbits. Children younger than 5 years of age have lower IOP than adults, but it increased consistently from birth.30,37
This study supports the idea that AHD changes as the eye advances through puberty, thus explaining how the IOP significantly differs during pre- and postpuberty. That IOP is higher during postpuberty than prepuberty cannot be explained by the aqueous flow reduction, which alone would decrease IOP. During the same time outflow facility decreased although not significantly. The methods used to evaluate outflow facility were selected because they are noninvasive, thus allowing for repeat measurements. However, they are not sensitive enough to detect subtle changes in outflow facility. Tonographic outflow facility was assessed with a pneumatonometer, in which a 10-gram weight was applied to the probe and placed on the cornea for 2 min, during which time >2000 IOPs were captured. These IOPs were imported into Excel, and outflow facility was calculated using previously published formulas and tables developed in NZW rabbits.22 We made no adjustments for species of rabbits, but there are some differences to consider. Dutch-belted rabbits have higher outflow facilities (0.26 ± 0.05 vs. 0.17 ± 0.01 μL/min/mmHg) and higher IOPs than NZW rabbits.22 The higher IOP is related to higher episcleral venous pressure.38 Uveoscleral outflow was not evaluated, but it may contribute to the pubertal changes in IOP. A recent, as yet unpublished study evaluated these parameters monthly in a separate group of Dutch-belted rabbits and found complex interplay among all parameters of AHD throughout puberty (Gulati et al., unpublished data presented at the 2015 ARVO annual meeting).
Our study tested the efficacy and AHD of two often prescribed IOP-lowering drugs in rabbits at 2 different ages. Latanoprost is a uveoscleral outflow stimulant that seems to work better in adults than children. On the contrary, timolol is an aqueous flow suppressant that reportedly works well at any age. Surprisingly, our results showed that neither drug worked well in the prepubertal rabbit. Knowing that timing of drug dosing and measurement collection could determine the outcome, we chose to situate the last dose of IOP-lowering drug at approximately 5 h before beginning measurements. This was the average time of peak efficacy based on latanoprost and timolol treatment in previous rabbit studies. Timolol is effective in the dark phase only in NZW rabbits between 4 and 6 h after topical dosing25 and in Japanese white rabbits between 1–7 h with the maximal effect at 2 h after dosing.39 Two days of dosing was chosen in our study because 5 days of dosing with timolol is reportedly no more effective at lowering IOP than fewer days of dosing.40 The last dose was applied during the light phase because topical administration of timolol reportedly is more effectively absorbed during the light phase than dark phase in pigmented rabbits.41 The ocular measurements were not measured during the day because timolol and latanoprost effects often are ineffective when measured at this time in rabbits.
Even with the carefully chosen dosing and measurement times, the timolol results in rabbits were not as expected. The IOP-lowering effect of timolol was twice as much when dosed postpuberty than when dosed prepuberty. It is possible that the discrepancy in efficacy may be due to the physiological development of the adrenergic system in the rabbit eye concomitant with sexual development. There is evidence that pediatric and adult adrenergic systems differ greatly.42 Investigations of psychiatric drug efficacy in children versus adults have shown considerable differences in the autonomic nervous system; namely that the sympathetic nervous system is not fully developed until puberty.42 Other works, which focus on adrenarche-associated changes as components of puberty, claim that the adrenal androgens are related to catecholamine production (adrenal medulla) on several different levels.43 The adrenergic regulation of AHD is verified by the hypotensive effect of β-blockers. If adrenergic function and regulation is indeed underdeveloped in children, it is reasonable that measurable differences in parameters of AHD were observed as the rabbits developed.
Latanoprost is not a first-line treatment in pediatric glaucoma because numerous reports have shown it to be ineffective in young children.15,16 One study reported that patients with juvenile open angle glaucoma did respond to latanoprost, but only if they were older than 9 years of age.6 This suggests that physiological changes during maturation of the eye occurs to allow a response to latanoprost at a later age. However, another study44 found that latanoprost caused a reduction in IOP across various ages younger than 18 years and with several different types of glaucoma. This study also reported that there were more responders in the older children compared with younger children. Two other studies reported a hypotensive effect with latanoprost, but these results were not stratified by age.11,45 Latanoprost reduces IOP in adults by increasing the rate of uveoscleral outflow,46,47 a relatively pressure-independent route of outflow through the interstitial spaces of the ciliary muscle. Differences in uveoscleral outflow could account for conflicting reports of efficacy in children. Although rabbits have significant differences in the structure of the uveoscleral outflow tissues and a slower rate of drainage through this pathway compared to humans,48 rabbits also exhibited an age-related difference in IOP efficacy when treated with latanoprost. Two studies reported a small but significant decrease in IOP in normotensive rabbits when dosed with latanoprost.40,49 Other studies reported inconsistent or no change in IOP following latanoprost treatment.15,16,50,51 Age of rabbits and time of measurements may contribute to these inconsistent findings. It is possible that immature rabbits have underdeveloped ciliary muscles causing less fractional outflow by the uveoscleral route, which may diminish a measurable effect on IOP by latanoprost.52,53 In addition, the interactions between the endocrine system and prostaglandin pathways are numerous.54–56 Reports of hormonal regulation of IOP57 together with the presence of local ocular steroidogenic machinery,58,59 hormone permeability across the blood–aqueous barrier,60 and local sex steroid hormone receptors61 support assumptions that pubertal endocrine changes could affect ocular prostaglandin metabolism.
Interestingly, the current study found an unexpected difference in efficacy of systemic carbonic anhydrase inhibitor (CAI; acetazolamide) with age, which has not been described elsewhere. The original reason for dosing with the CAI was to facilitate calculation of fluorophotometric outflow facility and not to examine the aging effects of CAIs on IOP-lowering efficacy. Nevertheless, these unexpected findings are worthy of discussion. A significant hypotensive effect by acetazolamide has been reported in children with glaucoma with untreated IOPs averaging 30 mmHg62,63; and the reduction in IOP by dorzolamide in children (27% reduction) is larger than reductions reported in adults.64 One may conclude that healthy rabbits do not predict outcomes in children with glaucoma. The lack of IOP effect in prepubertal rabbits may be specific to the Dutch-belted species as an earlier study19 in young NZW rabbits did report IOP reductions with acetazolamide. Our findings are reproducible because repeat of this experiment in a different group of Dutch-belted rabbits yielded the same lack of IOP effect of acetazolamide (Gulati, unpublished data presented at the 2015 ARVO annual meeting). When obtaining fluorophotometric data for outflow facility calculation in Dutch-belted rabbits, it would be advisable to use fully mature animals and make the measurements during the dark phase.
This study was conducted in healthy rabbits; hence, results have limited relevance to the maturing child with glaucoma. Many pediatric glaucomas are primary congenital glaucomas, which have significant differences from primary open angle glaucoma (POAG) in adults. In young children, maldevelopment of the anterior chamber angle (trabeculodysgenesis) is present and characterized by an absence of the ciliary body band due to the presence of translucent amorphous material that obscures the trabecular meshwork.65,66 The condition is associated with reduced aqueous humor outflow facility and elevated IOP. In children, treatment for elevated IOP is usually surgical,67 with procedures such as goniotomy, trabeculotomy, trabeculectomy, or combined trabeculotomy and trabeculectomy.7 In adults, treatment for POAG usually starts with topical IOP-lowering drugs.
Effects of testosterone on IOP have been reported but without consistent findings.57 No rise or fall in IOP of normal or glaucomatous human eyes was found after intramuscular injections of testosterone.68 New Zealand red rabbits showed no changes in IOP after topical treatment with 1% testosterone.69 Men with gonadal dysplasia do not have different IOPs than those with normal testicular function.70 However, in mice, a daily systemic injection of testosterone was reported to increase IOP; likewise bilateral castration in male rabbits caused a decrease in IOP.57 Thus far, no role for endogenous androgens in regulation of AHD has been identified.
This is the first description of developmentally dependent differences in ocular hypotensive drug treatment, which include assessment of AHD. Direct evidence of lack of IOP effect of commonly prescribed ocular hypotensive drugs in young rabbits raises the possibility of substantial differences in AHD and drug efficacy in children compared with adults. This possibility can be strengthened only after a similar study is done in primates. Evidence presented in this study of age-related differences in specific parameters of AHD warrants consideration by investigators who use rabbits for ocular studies. Age and time of day or night of measurements always should be considered when interpreting results from past and future animal studies. Careful designing and execution of animal experiments, taking into account these factors, would facilitate the development of effective treatments for glaucoma.
Acknowledgments
Supported by Research to Prevent Blindness (University of Nebraska Medical Center and Case Western Reserve University).
Author Disclosure Statement
C.B. Toris is receiving research support from 4 companies that are developing new glaucoma treatments, Novartis, Santen, Ivantis, and Nicox. No competing financial interests exist for the other authors.
References
- 1.Gordon M.O., Beiser J.A., Brandt J.D., et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120:714–720; discussion 829–730, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S., Pascolini D., Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 82:844–851, 2004 [PMC free article] [PubMed] [Google Scholar]
- 3.Prum B.E., Jr., Lim M.C., Mansberger S.L., et al. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 123:P112–P151, 2016 [DOI] [PubMed] [Google Scholar]
- 4.McCannel C.A., Heinrich S.R., and Brubaker R.F. Acetazolamide but not timolol lowers aqueous humor flow in sleeping humans. Graefes Arch. Clin. Exp. Ophthalmol. 230:518–520, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Alm A., and Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology. 102:1743–1752, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Black A.C., Jones S., Yanovitch T.L., et al. Latanoprost in pediatric glaucoma—pediatric exposure over a decade. J. AAPOS 13:558–562, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Turach M.E., Aktan G., and Idil A. Medical and surgical aspects of congenital glaucoma. Acta Ophthalmol. Scand. 73:261–263, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Igbinedion B.O., and Ogbeide O.U. Measurement of normal ocular volume by the use of computed tomography. Niger. J. Clin. Pract. 16:315–319, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Wang D., Ding X., Liu B., et al. Longitudinal changes of axial length and height are associated and concomitant in children. Invest. Ophthalmol. Vis. Sci. 52:7949–7953, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Dusek W.A., Pierscionek B.K., and McClelland J.F. Age variations in intraocular pressure in a cohort of healthy Austrian school children. Eye (Lond). 26:841–845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sihota R., Tuli D., Dada T., et al. Distribution and determinants of intraocular pressure in a normal pediatric population. J. Pediatr. Ophthalmol. Strabismus. 2006;43:14–18; quiz 36–17 [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Blasco T., Gonzalez-Meijome J.M., and Montes-Mico R. Age-related changes in the human visual system and prevalence of refractive conditions in patients attending an eye clinic. J. Cataract. Refract. Surg. 34:424–432, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Chang L., Ong E.L., Bunce C., et al. A review of the medical treatment of pediatric glaucomas at Moorfields Eye Hospital. J. Glaucoma. 22:601–607, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Heidary F., Gharebaghi R., Wan Hitam W.H., et al. Central corneal thickness and intraocular pressure in Malay children. PLoS One. 6:e25208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enyedi L.B., Freedman S.F., and Buckley E.G. The effectiveness of latanoprost for the treatment of pediatric glaucoma. J. AAPOS. 3:33–39, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Enyedi L.B., and Freedman S.F. Latanoprost for the treatment of pediatric glaucoma. Surv. Ophthalmol. 47 Suppl 1:S129–S132, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Boger W.P., 3rd, and Walton D.S. Timolol in uncontrolled childhood glaucomas. Ophthalmology. 88:253–258, 1981 [DOI] [PubMed] [Google Scholar]
- 18.De Rousseau C.J., and Bito L.Z. Intraocular pressure of rhesus monkeys (Macaca mulatta). II.Juvenile ocular hypertension and its apparent relationship to ocular growth. Exp. Eye Res. 32:407–417, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Zhao M., Hejkal J.J., Camras C.B., et al. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Invest. Ophthalmol. Vis. Sci. 51:3145–3151, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Paufler S.K., Van Vleck L.D., and Foote R.H. Estimation of testicular size in the live rabbit. Int. J. Fertil. 14:188–191, 1969 [PubMed] [Google Scholar]
- 21.Yablonski M.E., Zimmerman T.J., Waltman S.R., et al. A fluorophotometric study of the effect of topical timolol on aqueous humor dynamics. Exp. Eye Res. 27:135–142, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Langham M.E., and Edwards N. A new procedure for the measurement of the outflow facility in conscious rabbits. Exp. Eye Res. 45:665–672, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Erickson K.A., Gonnering R.S., Kaufman P.L., et al. The cynomolgus monkey as a model for orbital research. III. Effects on ocular physiology of lateral orbitotomy and isolation of the ciliary ganglion. Curr. Eye Res. 3:557–564, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Gulati V., Fan S., Zhao M., et al. Diurnal and nocturnal variations in aqueous humor dynamics of patients with ocular hypertension undergoing medical therapy. Arch. Ophthalmol. 130:677–684, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Gregory D.S. Timolol reduces IOP in normal NZW rabbits during the dark only. Invest. Ophthalmol. Vis. Sci. 31:715–721, 1990 [PubMed] [Google Scholar]
- 26.Skinner J.D. Puberty in the male rabbit. J. Reprod. Fertil. 14:151–154, 1967 [DOI] [PubMed] [Google Scholar]
- 27.Macari M., and Machado C.R. Sexual maturity in rabbits defined by the physical and chemical characteristics of the semen. Lab. Anim. 12:37–39, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Pediatric Eye Disease Investigator G., Bradfield Y.S., Melia B.M., et al. Central corneal thickness in children. Arch. Ophthalmol. 129:1132–1138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakalar Y.B., Keklikci U., Unlu K., et al. Distribution of central corneal thickness and intraocular pressure in a large population of Turkish school children. Ophthalmic. Epidemiol. 19:83–88, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Duckman R.H., and Fitzgerald D.E. Evaluation of intraocular pressure in a pediatric population. Optom. Vis. Sci. 69:705–709, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Montiani-Ferreira F., Cardoso F., and Petersen-Jones S. Postnatal development of central corneal thickness in chicks of Gallus gallus domesticus. Vet. Ophthalmol. 7:37–39, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Montiani-Ferreira F., Petersen-Jones S., Cassotis N., et al. Early postnatal development of central corneal thickness in dogs. Vet. Ophthalmol. 6:19–22, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Moodie K.L., Hashizume N., Houston D.L., et al. Postnatal development of corneal curvature and thickness in the cat. Vet. Ophthalmol. 4:267–272, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Bhardwaj V., and Rajeshbhai G.P. Axial length, anterior chamber depth-a study in different age groups and refractive errors. J. Clin. Diagn. Res. 7:2211–2212, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchiya A.K., Tanaka K., Sakurada I., et al. Ultrasound biomicroscopic measurement of anterior chamber biometry between before and after pupil dilation in children. Eur. J. Ophthalmol. 18:532–539, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Sauer A., Abry F., Blavin J., et al. [Sedated intraocular pressure and corneal thickness standards in children from birth to 10 years of age]. J. Fr. Ophtalmol. 34:238–242, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Goethals M., and Missotten L. Intraocular pressure in children up to five years of age. J. Pediatr. Ophthalmol. Strabismus. 20:49–51, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Kiel J.W., and Kopczynski C.C. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J. Ocul. Pharmacol. Ther. 31:146–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akaishi T., Ishida N., Shimazaki A., et al. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. J. Ocul. Pharmacol. Ther. 21:436–444, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Dinslage S., McLaren J., and Brubaker R. Intraocular pressure in rabbits by telemetry II: effects of animal handling and drugs. Invest. Ophthalmol. Vis. Sci. 39:2485–2489, 1998 [PubMed] [Google Scholar]
- 41.Ohdo S., Zhu J., and Lee V.H. Light-dark variations in ocular timolol concentrations following topical solution instillation in the pigmented rabbit. Life Sci. 51:2025–2031, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Murrin L.C., Sanders J.D., and Bylund D.B. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem. Pharmacol. 73:1225–1236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weise M., Eisenhofer G., and Merke D.P. Pubertal and gender-related changes in the sympathoadrenal system in healthy children. J. Clin. Endocrinol. Metab. 87:5038–5043, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Maeda-Chubachi T., Chi-Burris K., Simons B., et al. Impact of age, diagnosis, and history of glaucoma surgery on outcomes in pediatric patients treated with latanoprost. J. Glaucoma. 22:614–619, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Maeda-Chubachi T., Chi-Burris K., Simons B.D., et al. Comparison of latanoprost and timolol in pediatric glaucoma: a phase 3, 12-week, randomized, double-masked multicenter study. Ophthalmology. 118:2014–2021, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Toris C.B., Camras C.B., and Yablonski M.E. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 100:1297–1304, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Ziai N., Dolan J.W., Kacere R.D., et al. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Arch. Ophthalmol. 111:1351–1358, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Bill A. Uveoscleral drainage of aqueous humor: physiology and pharmacology. Prog. Clin. Biol. Res. 312:417–427, 1989 [PubMed] [Google Scholar]
- 49.Paschalis E.I., Cade F., Melki S., et al. Reliable intraocular pressure measurement using automated radio-wave telemetry. Clin. Ophthalmol. 8:177–185, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishii K., Tomidokoro A., Nagahara M., et al. Effects of topical latanoprost on optic nerve head circulation in rabbits, monkeys, and humans. Invest. Ophthalmol. Vis. Sci. 42:2957–2963, 2001 [PubMed] [Google Scholar]
- 51.Orihashi M., Shima Y., Tsuneki H., et al. Potent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbits. Biol. Pharm. Bull. 28:65–68, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Prince J., Diesem C., and Eglitis I. The Rabbit in Eye Research. Springfield, IL: Charles C. Thomas; 1964 [Google Scholar]
- 53.Nilsson S.F. The uveoscleral outflow routes. Eye (Lond). 11:149–154, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Frungieri M.B., Gonzalez-Calvar S.I., Parborell F., et al. Cyclooxygenase-2 and prostaglandin F2 alpha in Syrian hamster Leydig cells: Inhibitory role on luteinizing hormone/human chorionic gonadotropin-stimulated testosterone production. Endocrinology. 147:4476–4485, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Lee P.Y., Podos S.M., and Severin C. Effect of prostaglandin F2 alpha on aqueous humor dynamics of rabbit, cat, and monkey. Invest. Ophthalmol. Vis. Sci. 25:1087–1093, 1984 [PubMed] [Google Scholar]
- 56.Whittle W.L., Patel F.A., Alfaidy N., et al. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol. Reprod. 64:1019–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Kass M.A., and Sears M.L. Hormonal regulation of intraocular pressure. Surv. Ophthalmol. 22:153–176, 1977 [DOI] [PubMed] [Google Scholar]
- 58.Coca-Prados M., and Escribano J. New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Prog. Retin. Eye Res. 26:239–262, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Rocha E.M., Wickham L.A., da Silveira L.A., et al. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br. J. Ophthalmol. 84:76–84, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toda R., Kawazu K., Oyabu M., et al. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J. Pharm. Sci. 100:3904–3911, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Gupta P.D., Johar K., Sr., Nagpal K., et al. Sex hormone receptors in the human eye. Surv. Ophthalmol. 50:274–284, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Ott E.Z., Mills M.D., Arango S., et al. A randomized trial assessing dorzolamide in patients with glaucoma who are younger than 6 years. Arch. Ophthalmol. 123:1177–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Coppens G., Stalmans I., Zeyen T., et al. The safety and efficacy of glaucoma medication in the pediatric population. J. Pediatr. Ophthalmol. Strabismus. 46:12–18, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Portellos M., Buckley E.G., and Freedman S.F. Topical versus oral carbonic anhydrase inhibitor therapy for pediatric glaucoma. J. AAPOS. 2:43–47, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Azuara-Blanco A., Spaeth G.L., Araujo S.V., et al. Ultrasound biomicroscopy in infantile glaucoma. Ophthalmology. 104:1116–1119, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Tawara A., and Inomata H. Developmental immaturity of the trabecular meshwork in juvenile glaucoma. Am. J. Ophthalmol. 98:82–97, 1984 [DOI] [PubMed] [Google Scholar]
- 67.Yu Chan J.Y., Choy B.N., Ng A.L., et al. Review on the Management of Primary Congenital Glaucoma. J. Curr. Glaucoma Pract. 9:92–99, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avasthi P., and Luthra M.C. Effect of sex hormones on intraocular pressure. Int. Surg. 48:350–355, 1967 [PubMed] [Google Scholar]
- 69.Knepper P.A., Collins J.A., and Frederick R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Invest. Ophthalmol. Vis. Sci. 26:1093–1100, 1985 [PubMed] [Google Scholar]
- 70.Madroszkiewicz M., Niebroj T.K., Malecka A., et al. [Tonometric and tonographic examinations in men with disturbed function of the gonads]. Klin. Oczna. 41:393–396, 1971 [PubMed] [Google Scholar]