Abstract
Thirty-seven commercial aldehydes containing aliphatic chains and aromatic rings as well as heteroaromatic rings were evaluated for their inhibitory activities against Chinese amaranth (Amaranthus tricolor L.) and barnyardgrass (Echinochloa crus-galli (L.) Beauv). Polysorbate 80 (Tween® 80) was used as a surfactant and the research was preliminarily conducted at 400 μM of all aldehydes. Among these aldehydes, (E)-cinnamaldehyde (7) showed the greatest inhibitory effect on seed germination, shoot and root growth of Chinese amaranth by 54.55%, 75.53%, and 85.13% respectively. Similarly, (E)-crotonaldehyde (5), a related α,β-unsaturated aldehyde, inhibited the germination and seedling growth of the tested species at a high percentage. Apart from these two unsaturated aldehydes, no other aliphatic aldehydes had a harmful effect on Chinese amaranth. In terms of benzaldehyde (6), it had no effect on the tested plant; however, many of its derivatives displayed some inhibitory activity. Furthermore, for the ten common heteroaromatic aldehydes, picolinaldehyde (32) had a high inhibitory effect on Chinese amaranth which closely related to the effect of (E)-crotonaldehyde (5) and (E)-cinnamaldehyde (7), whereas, other heteroaromatic aldehydes showed lower effects. In the case of a monocot plant, barnyardgrass, no tested aldehydes reduced seed germination, however, (E)-cinnamaldehyde (7), 2,4,6-trimethoxybenzaldehyde (16) and 4-(dimethylamino)benzaldehyde (24) could inhibit the seedling growth of the plant with low to moderate levels. The herbicidal effects of the most active aldehydes were then further investigated in order to find the minimum concentration of these aldehydes suppressing the germination and growth of the tested plants. At concentrations as low as 50–100 μM some aldehydes could inhibit the seedling growth of the tested species. The structure-activity relationship (SAR) study reported here demonstrates the chemical clues governing the inhibitory activity of aldehydes which could be utilized in the development of highly effective herbicides in the near future.
Keywords: inhibitory, herbicidal, allelopathy, aldehydes, Chinese amaranth, barnyardgrass
1. Introduction
Weeds are a major problem on the yield of agricultural crops. They compete with crop plants for water, nutrients, space or even sunlight, causing the crop plants to grow slowly or even die [1,2]. Therefore, in order to achieve high crop production and yields, it is crucially important to control these unwanted species. In general, weed management practices vary widely depending upon climatic and environmental conditions as well as weed species. Overall, the main methods of weeding are manual, mechanical, chemical and biological controls respectively [3,4,5]. Among those methods, chemical control is one of the most popular procedures. Moreover, the use of natural compounds or allelochemicals for weeding has been studied extensively [3,6,7,8,9,10,11,12,13,14,15]. Allelochemicals are substances released from plants or microorganisms to control the growth of other plants or other small organisms [14,16]. Usually, it is believed that natural products or naturally-occurring compounds are safer than synthetic chemicals, comparably easy to decompose and environmentally friendly [12,13,17]. Also, they could be conveniently used in a form of both pure chemical and crude extract. Xanthoxyline and (±)-odorine are two examples of such allelochemicals which were successfully isolated in our research group from Makhwaen (Zanthoxylum limonella Alston) fruits and Prayong (Aglaia odorata Lour.) leaves [18,19]. Both natural compounds inhibited well the seed germination and seedling growth of tested plants, Chinese amaranth, and barnyardgrass. In addition to those examples mentioned above, there are numerous reports showing the diverse groups of allelochemicals or natural products which are used as herbicides [6,13,14,20,21,22,23,24]. For examples, fatty acids, essential oil, amino acids, peptides, alkaloids, flavonoids and phenolics etc. These compounds could inhibit seed germination and seedling growth of tested weeds, algae and microorganisms. Although, the herbicidal properties of these chemicals have been studied for many years, a thorough investigation of allelopathic potentials of each specific chemical class is still required. Commonly, purification and identification of bioactive natural products are time-consuming and quite expensive, especially, with the limited natural resources [9,14]. Frequently, only minute quantities of allelochemicals are obtained. Moreover, several of them have complex chemical structures which lead to expensive, long and difficult synthetic procedures to access. Their structure-activity relationship (SAR) studies, therefore, are difficult to accomplish.
In this study we are interested in investigating the herbicidal activities of a variety of aldehydes (both aliphatic and aromatic aldehydes (Figure 1) as well as heteroaromatic aldehydes (Figure 2)) on seed germination and seedling growth of two tested plants, Chinese amaranth (Amaranthus tricolor L.) and barnyardgrass (Echinochloa crus-galli (L.) Beauv). Both species were chosen as representatives of dicot and monocot plants, respectively. We selected an aldehyde chemical class since there are several reports indicating that natural and synthetic aldehydes [25,26,27,28,29,30,31,32,33,34,35,36] or crude extracts containing aldehydes [37,38,39,40,41,42,43,44] could interrupt the germination, growth, and development of plants, algae, and microorganisms. Furthermore, numerous aldehydes are commercially available in pure form which can be directly purchased. Positive results of the current research could be applied in the development of new and highly reactive herbicides.
Figure 1.
Aliphatic and aromatic aldehydes used in this study.
Figure 2.
Heteroaromatic aldehydes used in this study.
2. Results and Discussion
2.1. Inhibitory Effects of Thirty-Seven Aldehydes on Germination and Seedling Growth of Chinese Amaranth
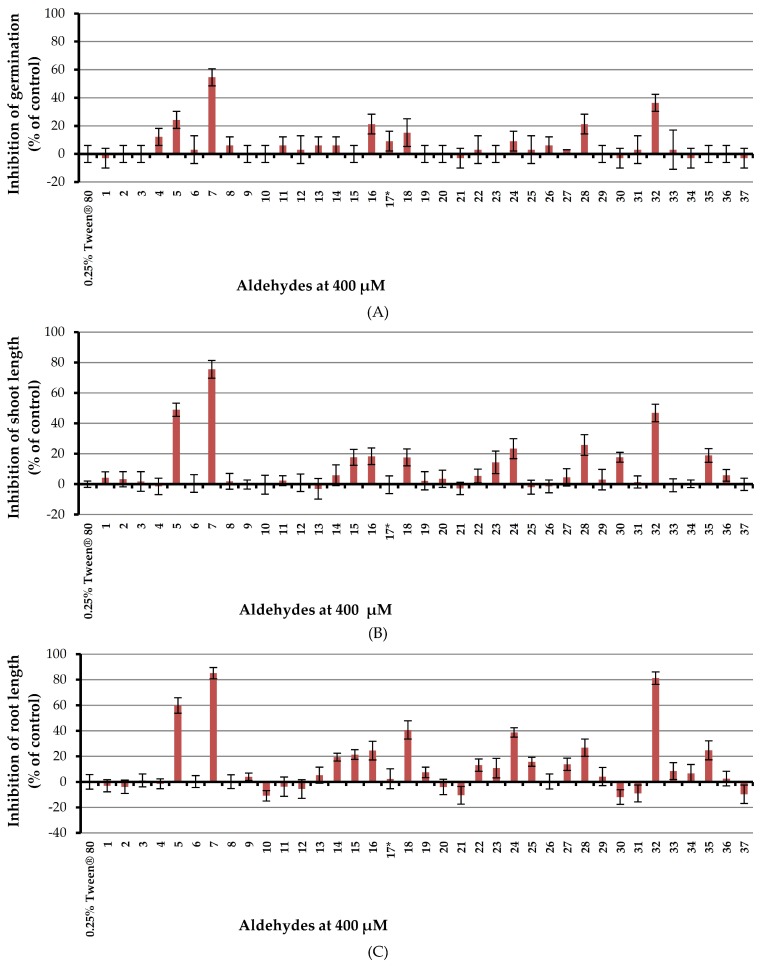
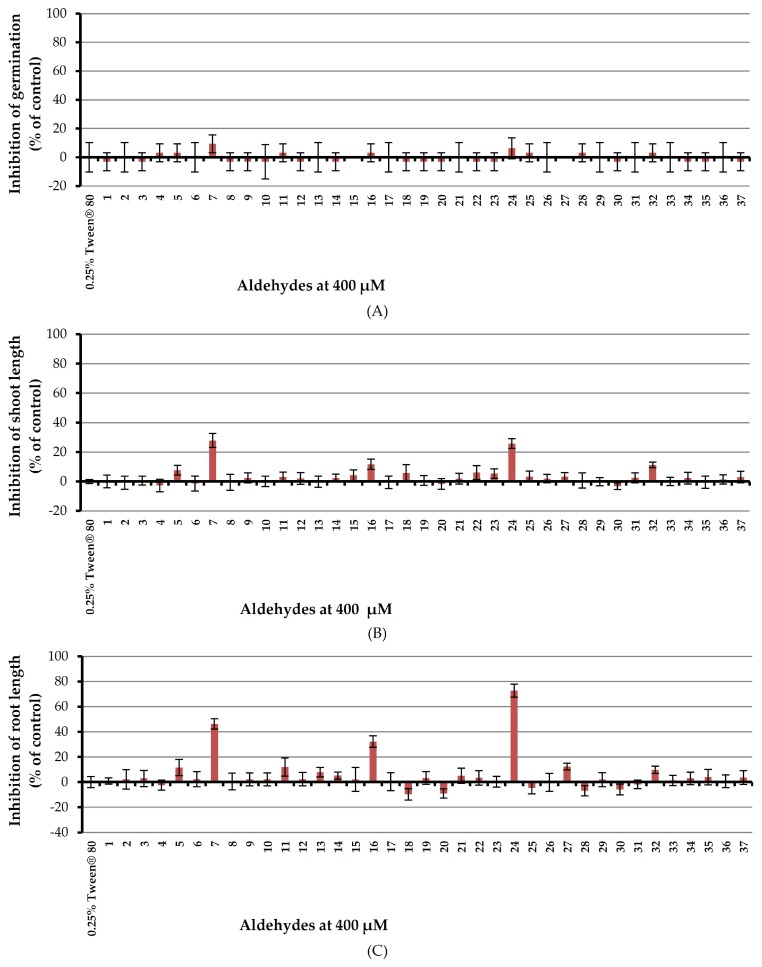
In the preliminary investigation, Chinese amaranth was selected as a representative dicotyledon. According to our previous study [45] 0.25% (v/v) aqueous solution of Tween® 80 (Sigma-Aldrich, Singapore) was used as a surfactant. A basic structure-activity relationship (SAR) study was performed by using three groups of aldehydes namely; aliphatic, aromatic and heteroaromatic respectively. The results revealed that (Figure 3), four aliphatic aldehydes containing 2–6 carbon atoms (acetaldehyde (1), propionaldehyde (2), butyraldehyde (3) and hexanal (4)) had no effect on germination and seedling growth of Chinese amaranth. Fortunately, unsaturated aliphatic aldehyde such as (E)-crotonaldehyde (5) could inhibit seed germination, shoot, and root elongation by 24.24, 48.89, and 59.88% respectively. In comparison with butyraldehyde (3) which is similarly composed of four carbon atoms, (E)-crotonaldehyde (5) showed much higher inhibitory activity. Moreover, the size of a substituent attached to an α,β-unsaturated part could possibly determine the inhibitory effect of the compound. Accordingly, (E)-cinnamaldehyde (7) showed a greater herbicidal effect against Chinese amaranth than (E)-crotonaldehyde (5) did. At a concentration of 400 μM, (E)-cinnamaldehyde (7) inhibited seed germination, shoot and root length of the tested plant by 54.55, 75.53, and 85.13% respectively, which indicates the most reactive chemical among all aldehydes.
Figure 3.
Inhibitory effects of aqueous solutions of thirty-seven aldehydes (at 400 µM) on seed germination (A), shoot length (B), and root length (C) of Chinese amaranth. A 0.25% (v/v) aqueous solution of Tween® 80 was used as a control. * Result from a previous study [45], the effect of vanillin (17) on Chinese amaranth.
In terms of aromatic aldehydes, both benzaldehyde (6) and its derivatives bearing alkyl substituents (aldehydes 8–9) and halogen substituents (aldehydes 10–13) had no inhibitory effect on Chinese amaranth. However, for the derivatives containing methoxy-substituent (compounds 14–16) it turned out that position and number of the substituents affected the activity which meta-position (m-anisaldehyde 15) expressed higher effect than para-position (p-anisaldehyde 14) and tri-substituted (2,4,6-trimethoxybenzaldehyde 16) had a greater effect than monosubstituted (aldehydes 14–15).
For derivatives having hydroxyl-substituents (compounds 17–19), the para-position (4-hydroxybenzaldehyde 18) seemed to have greater effect of herbicidal potential than the ortho-position (salicylaldehyde 19). Nevertheless, vanillin (17) containing both a para-hydroxyl group and a meta-methoxy group had very low phytotoxicity against the tested plant. 3-Nitrobenzaldehyde (20), likewise, had no effect on the tested plant. Next, the effect of a carboxyl substituent (compound 21–23) was investigated and it appeared that the substitution on the chain outside the benzene ring (aldehyde 23) had more effect than the substitution on the ring (aldehydes 21–22). Among aldehydes with amino-substituent (compounds 24–27), 4-(dimethylamino)benzaldehyde (24) exhibited a moderate inhibitory effect on the tested plant. In the case of heteroaromatic aldehydes 28–37, picolinaldehyde (32) inhibited seed germination, shoot and root growths of Chinese amaranth by 36.36, 46.87, and 81.28% respectively. The derivatives 28, 30 and 35 showed some activities, but other heteroaromatics had no effect.
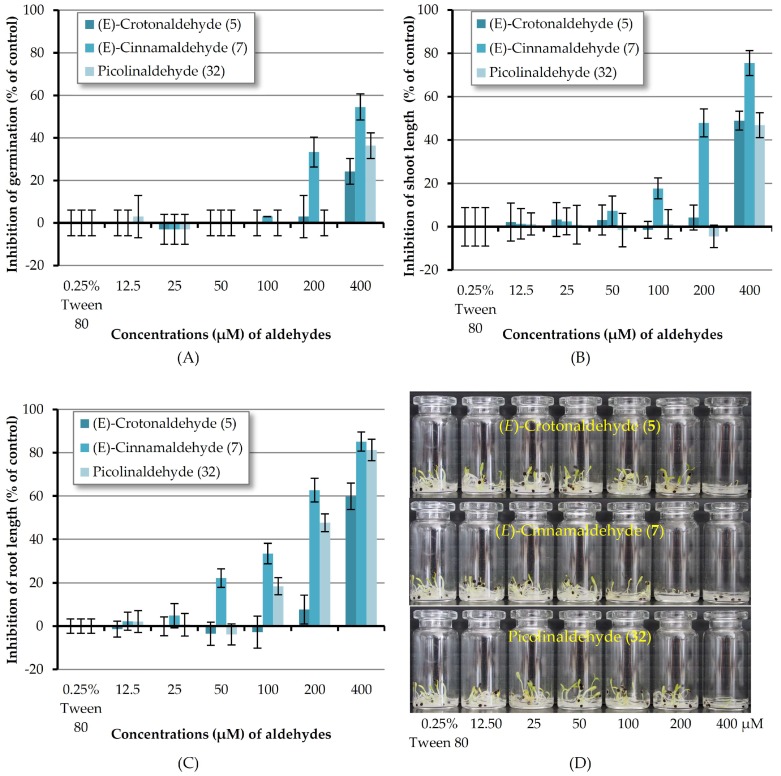
According to the results mentioned above, (E)-crotonaldehyde (5), (E)-cinnamaldehyde (7) and picolinaldehyde (32) are the three most reactive chemicals towards Chinese amaranth. In order to know the minimum molar concentration at which all three compounds could suppress the germination and seedling growth of the tested dicot, these substances were then investigated at concentrations of 12.5–400 μM (Figure 4). It revealed that at 200 μM only (E)-cinnamaldehyde (7) could inhibit seed germination of the tested plant. Aldehyde 7 at the other concentrations and aldehydes 5 and 12 at 12.5–200 μM showed no effect. In terms of shoot length, although at 400 μM, aldehyde 5 and 32 could inhibit shoot growth, at 12.5–200 μM these two substances had no adverse effect on shoot length. (E)-Cinnamaldehyde (7), however, could inhibit shoot length at the concentrations as low as 50 μM. In the case of root development, at concentrations lower than 400 μM, (E)-crotonaldehyde (5) had no effect on root growth. Picolinaldehyde (32) could inhibit root length at the concentrations down to 100 μM. At concentrations of 50, 100 and 200 μM, the most reactive substance, (E)-cinnamaldehyde (7), inhibited root length by 22.18, 33.52, and 62.69% respectively.
Figure 4.
Inhibitory effects of aqueous solutions of three aldehydes (at 12.5–400 µM) on seed germination (A), shoot length (B), and root length (C) of Chinese amaranth. A 0.25% (v/v) aqueous solution of Tween® 80 was used as a control. (D) Chinese amaranth in small vials.
The results stated above reveal the importance of an unsaturated structure on the herbicidal activity of aldehydes. This is consistent with numerous reports on the effects of some polyunsaturated aldehydes (PUAs) on diatoms, planktons, and algae [31,46,47,48,49,50,51,52]. For example, Casotti and coworkers [52] investigated the effects of three diatom-produced PUAs, 2E,4E-decadienal, 2E,4E-octadienal and 2E,4E-heptadienal, on six phytoplankton. The result showed that the reduction of growth rate of tested plankton was concentration-dependent and species-specific. Also, the longer-chained aldehydes had stronger effects on the plankton growth than the shorter-chained aldehydes which is in agreement with our result that (E)-cinnamaldehyde (7) showed greater adverse effect than (E)-crotonaldehyde (5). Vaughn and Spencer [53] examined the inhibitory effect of some naturally-occurring aromatic aldehydes and thymol on potato tuber sprouting and found that most tested compounds inhibited sprouting of tubers exposed up to 10 days. Moreover, direct application of 1% cinnamaldehyde (7) and 10% benzaldehyde (6) completely suppressed sprouting for 14 days after treatment without apparent tuber damage. Apart from PUAs, some unsaturated aldehydes are also found in reactive fractions of plant crude extracts. For instance, Song and coworkers [42] reported the allelopathic effects of crude extracts from the green peel of Juglans mandshurica Maxim. on three plants; Brassica chinesis, Raphanus sativus, and Medicago sativa. It was revealed that the alcohol extract and its ethyl acetate soluble fraction showed good inhibitory activity. After GC-MS analysis of the extracts it uncovered that aside from a major allelochemical component, juglone, some aldehydes such as 4 butoxybenzaldehyde, 5-(hydroxymethyl)-2-furancarboxaldehyde and 4-hydroxy-2-methoxycinnamaldehyde were also found in the active fractions.
Others have shown that (E)-cinnamaldehyde (7) also has antimicrobial activity [26,35,54,55]. For example, Zhang and coworkers [35] studied the structure-activity relationships (SAR) of cinnamaldehyde (7) and eugenol derivatives against two plant pathogenic fungi, Rhizoctonia solani and Fusarium oxysporum. It displayed that many derivatives showed good activities against both fungi. Interestingly, the fungicidal potential of cinnamaldehyde derivatives could be related to conjugated double bond and the length of CH chain outside the ring. Moreover, the authors suggested that the presence of the lipophilic part would be influent on the toxicity of phenylpropenes.
Regarding detrimental effects of heteroaromatic aldehydes, among those tested substances, furfural (28) and picolinaldehyde (32) seemed to have the greatest inhibition on Chinese amaranth. The reason behind this is still unclear; however, the allelopathic effects of natural products containing heteroaromatic parts have been extensively documented [56,57,58,59,60,61,62,63,64,65,66,67]. For examples of pyridyl and furanyl bearing compounds, Rizvi and coworkers [64] investigated the alellopathic activity of a pyridine containing alkaloid, nicotine, on maize (Zea mays) and rice (Oryza sativa) and found that nicotine adversely affected the germination, radicle and plumule length and seedling vigor of rice. On the other hand, it favorably affected the growth of maize by increasing the height, specific leaf weight, and chlorophyll content. Komai and coworkers [61] isolated a plant growth inhibitor, perilla ketone, from Egoma plant (Perilla frutescens var. japonica) and investigated its inhibitory effect on lettuce (Lactuca sativa L. c.v. new york.) and large crabgrass (Digitaria adsendens Henr.). Apparently, this ketone inhibited the radicle elongation of the tested plants at concentrations of 50–100 ppm. However, it did not inhibit seed germination of lettuce. In 2017, Chahal and coworkers [62] determined the chemical compositions of root and rhizosphere soil extracts of allelopathic plant, Marigold (Tagetes patula L.) using GC-MS analysis method. Twenty-five and twenty-seven compounds were identified in the two fractions. 5-Hydroxymethylfurfural was one of the major components in the methanol root extract which comprised of 21.81%. Eventually, the authors suggested that those leached compounds would be responsible for the allelopathic potential of this plant.
2.2. Effects of Tween® 80 Surfactant on Germination and Seedling Growth of Barnyardgrass
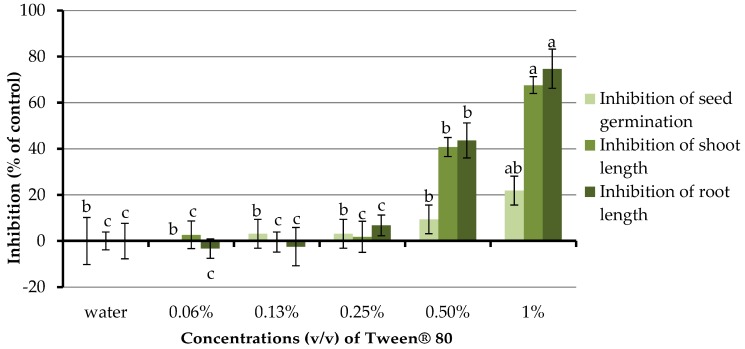
Although Tween® 80 helps emulsifying organic compound in an aqueous solution, it could also affect the germination and growth of plants depending upon the applied concentrations [45]. Therefore, the minimum quantity of this surfactant that influences the germination and growth of barnyardgrass have to be evaluated. The concentrations of the aqueous solution of Tween® 80 being investigated were 0.06–1.0% and distilled water was used as a control treatment (Figure 5). Clearly, at concentrations of 0.06–0.25% Tween® 80 had no significant effect on germination and seedling growth of barnyardgrass. However, at concentrations of 0.5 and 1.0%, this surfactant highly inhibited the germination and development of barnyardgrass. Therefore, Tween® 80 at a concentration of 0.25% was chosen to use as a proper surfactant in the next section.
Figure 5.
Effects of Tween® 80 surfactant (at concentrations of 0.06–1.00% (v/v)) on the germination and growth of barnyardgrass. Distilled water was used as a control treatment. Means with the same letters in the graph are not significantly different at p ≤ 0.05 level.
2.3. Inhibitory Effects of Thirty-Seven Aldehydes on Germination and Seedling Growth of Barnyardgrass
Inhibitory effect of aldehydes at 400 μM on monocotyledon plant, barnyardgrass, was investigated by utilizing an aqueous solution of Tween® 80 as a surfactant (Figure 6).
Figure 6.
Inhibitory effects of aqueous solutions of thirty-seven aldehydes (at 400 µM) on seed germination (A), shoot length (B), and root length (C) of barnyardgrass. A 0.25% (v/v) aqueous solution of Tween® 80 was used as a control.
Results showed that all tested aldehydes had no effect on seed germination of barnyardgrass. In terms of shoot growth, (E)-cinnamaldehyde (7) and 4-(dimethylamino)benzaldehyde (24) moderately inhibited shoot length by 27.83 and 25.80% respectively but other aldehydes showed very low or no harmful effects. For root growth, (E)-cinnamaldehyde (7), 2,4,6-trimethoxybenzaldehyde (16), and 4-(dimethylamino) benzaldehyde (24) inhibited root growth of the tested plant by 46.20, 32.21 and 72.77% respectively but other aldehydes, again, had very low or no effects.
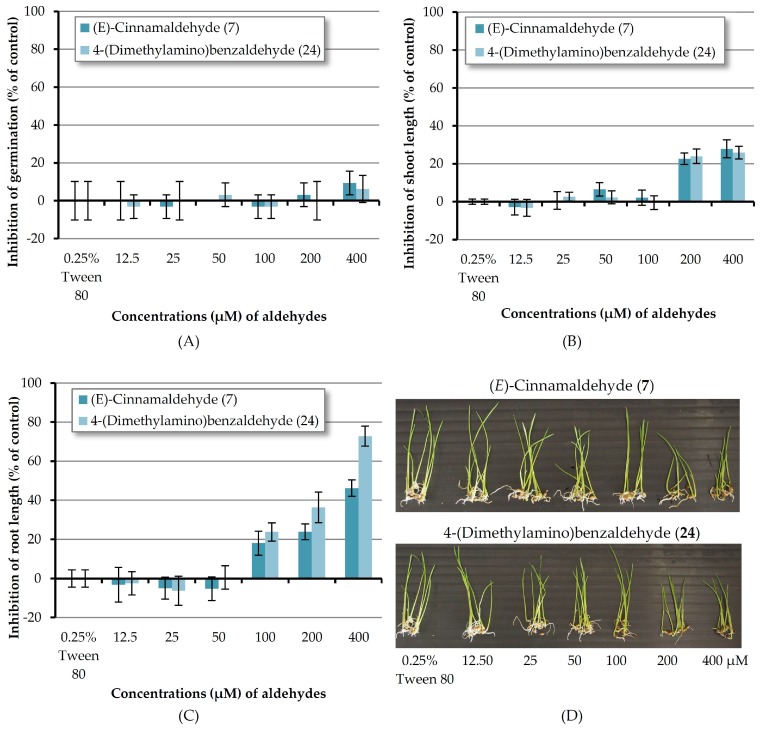
As mentioned above, (E)-cinnamaldehyde (7) and 4-(dimethylamino)benzaldehyde (24) are the most reactive chemicals toward barnyardgrass. In order to know the minimum concentration that the two compounds could inhibit the germination and seedling growth of a monocot plant; these two substances were then tested at concentrations of 12.5–400 μM (Figure 7). It revealed that at 200 μM aldehydes 7 and 24 inhibited shoot growth by 26.62 and 23.93% respectively but at other lower concentrations, both compounds showed no effect. In the case of root length, both chemicals could inhibit root growth at the concentrations down to 100 μM. Compound 7 and compound 24 at the concentration of 200 μM inhibited root length by 23.81 and 36.31% respectively and at the concentration of 100 μM inhibited root elongation by 18.00 and 23.72% respectively. However, at lower concentrations both compounds showed no inhibition.
Figure 7.
Inhibitory effects of aqueous solutions of two aldehydes (at 12.5–400 µM) on seed germination (A), shoot length (B), and root length (C) of barnyardgrass. A 0.25% (v/v) aqueous solution of Tween® 80 was used as a control. (D) Shoot and root growth of barnyardgrass.
The inhibitory effect of those thirty-seven aldehydes on dicot and monocot seeds has been unclosed in the present study. A comparison between the two species of plants showed that this group of chemicals tends to exhibit a stronger effect on Chinese amaranth than barnyardgrass. This is in agreement with our previous work [18] that we investigated the allelopathic effect of Makhwaen fruits on germination and growth of Chinese amaranth and barnyardgrass and eventually led us to isolate an active phenolic, xanthoxyline. After evaluation of the allelopathic activity of this compound on the tested plants we found that the germination of the dicot plant was totally inhibited at a concentration of 2,500 μM. However, at the same applied concentration, this compound showed a lower inhibitory effect on barnyardgrass. Furthermore, in the present study, most aldehydes affected root growth more than shoot growth and all of the tested compounds had no effect on seed germination of barnyardgrass. Similarly, in 2016, Gauda and coworkers [30] conducted a study on the herbicidal activity of a variety of monoterpenes against barnyardgrass. They found that, generally, these monoterpenes were more effective against seedling growth than seed germination of the plant. Besides, the inhibition of root development by all compounds was greater than that of shoot growth. Our results here indicated that 4-(dimethylamino)benzaldehyde (24) exhibited the greatest detrimental effect on the root length of barnyardgrass but this substance had a low inhibitory effect on the germination and seedling growth of Chinese amaranth. Also, the herbicidal potentials of the tested aldehydes relied on the applied concentrations. These suggested that the inhibitory effect of these chemicals is species-specific and concentration dependent.
3. Experimental
3.1. Chemicals
Tween® 80, acetaldehyde (1), propionaldehyde (2), butyraldehyde (3), (E)-crotonaldehyde (5), (E)-cinnamaldehyde (7), o-tolualdehyde (8), cuminaldehyde (9), 2-(trifluoromethyl)benzaldehyde (10), 2-fluorobenzaldehyde (11), 2,4-dichlorobenzaldehyde (12), 3-bromobenzaldehyde (13), p-anisaldehyde (14), m-anisaldehyde (15), 2,4,6-trimethoxybenzaldehyde (16), vanillin (17), 3-nitrobenzaldehyde (20), 2-formylbenzoic acid (21), 4-formylbenzoic acid (22), 2-(2-formylphenoxy) acetic acid (23), 4-(dimethylamino)benzaldehyde (24), 4-((2-hydroxyethyl)(methyl)amino) benzaldehyde (25), 4-(bis(2-hydroxyethyl)amino)benzaldehyde (26), furfural (28), 1H-pyrrole- 2-carbaldehyde (29), thiophene-2-carbaldehyde (30), 1-methyl-1H-pyrrole-2-carbaldehyde (31), picolinaldehyde (32), 2-bromonicotinaldehyde (33), oxazole-4-carbaldehyde (34), thiazole-2-carbaldehyde (35), 1H-indole-3-carbaldehyde (36) and 1H-pyrrolo[2,3-b]pyridine-2-carbaldehyde (37) were purchased from Sigma-Aldrich (Singapore). Hexanal (4), benzaldehyde (6) and 4-hydroxybenzaldehyde (18) were purchased from Fluka (Buchs, Switzerland). Salicylaldehyde (19) and 4-morpholinobenzaldehyde (27) were purchased from Tokyo Chemical Industry (TCI, Tokyo, Japan). All compounds were reagent grade and used without further purification.
3.2. Preparation of Aqueous Solutions of Tween® 80 at Concentrations of 0.06–1.00% (v/v)
As previously described [45], to a 100 mL-beaker, 1 mL of Tween® 80 surfactant and 40 mL of distilled water were added. The mixture was well mixed by continuous stirring at room temperature for about 10 min. Then, the clear solution was transferred to a 100 mL–volumetric flask. Adjust the volume of the flask by adding distilled water, and followed by inverting the flask many times to obtain a 1% (v/v) Tween® 80 stock solution. Other required concentrations were prepared by a dilution method to afford the aqueous solutions of Tween® 80 at 0.50, 0.25, 0.13, and 0.06% (v/v) respectively.
3.3. Preparation of Aqueous Solutions of Aldehydes at 400 μM
Into a 100 mL-beaker, forty micromoles of a pure aldehyde and 0.25 mL of Tween® 80 were added. The mixture was blended until it became clear (or no solid sample remains). To the mixture, 40 mL of distilled water was added, and the mixture was continuously stirred for 10 min. This thoroughly mixed solution was transferred to a 100 mL-volumetric flask and the volume of the flask was adjusted with distilled water to obtain a 400 μM of a pure aldehyde which contained 0.25% (v/v) of Tween® 80 surfactant.
3.4. Preparation of Aqueous Solutions of Aldehydes at 400, 200, 100, 50, 25 and 12.5 μM
The 400 μM stock solutions of aldehydes 5, 7, 24 and 32 were prepared as described in Section 3.3. Aqueous solutions of compounds 5, 7, 24 and 32 at concentrations of 200, 100, 50, 25 and 12.5 μM were prepared by diluting the stock solutions with 0.25% (v/v) aqueous solution of Tween® 80.
3.5. Tested Plants
Seeds of Chinese amaranth and barnyardgrass were used in the assessment of the herbicidal activity. Chinese amaranth seeds were purchased from Thai Seed & Agriculture Co. Ltd., Bangkok, Thailand, and barnyardgrass seeds were collected from rice fields in Phitsanulok Province, Thailand, in August 2016. The seed germination tests of both species were found to be >80%.
3.6. Seed Germination and Seedling Growth Bioassay
As previously described [45], to a small glass vial (4.5 cm × 2 cm) lined with germination paper, 0.5 mL of an aqueous solution of aldehydes were added. Ten seeds of a tested plant were then placed on the germination paper. The vials were sealed with Parafilm® (in order that the solution does not dry out) and maintained at 28–30 °C in a growth chamber (cool white 840 Climacell 707, Munich, Germany). The chamber was set with a 12/12 h dark/light photoperiod, a light intensity of 100 µmol m−2·s−1, and around 80% of relative humidity. An aqueous solution of Tween® 80 at a concentration of 0.25% (v/v) was used as a control experiment. The treatments and control group were replicated four times. After 7 days, numbers of seed germination were counted, shoot length and root length were measured, and percentages of inhibition were calculated as follows:
| (1) |
3.7. Statistical Analysis
For the effect of Tween® 80 on seed germination and seedling growth of barnyardgrass, a completely randomized design (CRD) was used. Data were subjected to the analysis of variance and comparisons were made between treatments at probability level p ≤ 0.05 using Tukey’s studentized range test.
4. Conclusions
In the present SAR study, the allelopathic effects of a variety of aldehydes were investigated by using Chinese amaranth as a representative of dicot plant and barnyardgrass as a representative of monocot plant. Factors determining the reactivity of those aldehyde allelochemicals were found to be unsaturation of structures, type, number and position of substituents and concentrations of aldehydes. Most aliphatic aldehydes had no allelopathic effect but α,β-unsaturated compounds showed supreme activity, especially the most reactive aldehyde, (E)-cinnamaldehyde (7) which could inhibit both dicot and monocot species. Aromatic aldehydes with methoxy-, hydroxyl- and alkylamino- substituents in the right position could also inhibit seed germination and seedling growth of the tested plants. Regarding heteroaromatic aldehydes, picolinaldehyde (32) impressively effected the germination and growth of the dicot plant in comparison with other related chemicals. Interestingly, 4-(dimethylamino)benzaldehyde (24) showed chemical clues suggesting a species-specific compound. Obviously, this substance highly inhibited root growth of barnyardgrass. Further research is still needed to find a mode of actions of these reactive aldehydes and also to develop potential natural product based herbicides in agrochemical industry.
Acknowledgments
We would like to thank King Mongkut’s Institute of Technology Ladkrabang (KMITL) for providing the funding (grant number KREF165906 (New Lecturer Mentoring Program)) and the department of chemistry KMITL for laboratory facilities.
Author Contributions
The listed authors contributed to this work as described in the following: Nawasit Chotsaeng conceived, designed and conducted the experiments and wrote the paper. Patchanee Charoenying and Chamroon Laosinwattana contributed in the discussion of the corresponding data and gave advice on the manuscript preparation. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Baucom R.S., Holt J.S. Weeds of agricultural importance: Bridging the gap between evolutionary ecology and crop and weed science. New Phytol. 2009;184:741–743. doi: 10.1111/j.1469-8137.2009.03077.x. [DOI] [PubMed] [Google Scholar]
- 2.Hager A. Illinois Agronomy Handbook. 24th ed. University of Illinois at Urbana-Champaign, College of Agriculture, Cooperative Extension Service; Urbana-Champaign, IL, USA: 2009. Weed management; p. 224. [Google Scholar]
- 3.Bàrberi P. Weed management in organic agriculture: Are we addressing the right issues? Weed Res. 2002;42:177–193. doi: 10.1046/j.1365-3180.2002.00277.x. [DOI] [Google Scholar]
- 4.Bayer D.E. Weed management. In: Luh B.S., editor. Rice: Volume I. Production/Volume II. Utilization. Springer; Boston, MA, USA: 1991. pp. 287–309. [Google Scholar]
- 5.Tu M., Hurd C., Randall J.M. Weed Control Methods Handbook: Tools & Techniques for Use in Natural Areas. The Nature Conservancy; Arlington, VA, USA: 2001. p. 219. [Google Scholar]
- 6.Dayan F.E., Cantrell C.L., Duke S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Jabran K., Mahajan G., Sardana V., Chauhan B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015;72:57–65. doi: 10.1016/j.cropro.2015.03.004. [DOI] [Google Scholar]
- 8.Khanh T. Role of allelochemicals for weed management in rice. Allelopath. J. 2007;19:85–96. [Google Scholar]
- 9.Macías F.A., Molinillo J.M., Varela R.M., Galindo J.C. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007;63:327–348. doi: 10.1002/ps.1342. [DOI] [PubMed] [Google Scholar]
- 10.Reigosa M.J., Pedrol N., González L. Allelopathy: A Physiological Process with Ecological Implications. Springer Science & Business Media; Dordrecht, The Netherlands: 2006. p. 637. [Google Scholar]
- 11.Sangeetha C., Baskar P. Allelopathy in weed management: A critical review. Afr. J. Agric. Res. 2015;10:1004–1015. [Google Scholar]
- 12.Singh H., Batish D.R., Kohli R. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Crit. Rev. Plant Sci. 2003;22:239–311. doi: 10.1080/713610858. [DOI] [Google Scholar]
- 13.Soltys D., Krasuska U., Bogatek R., Gniazdowska A. Allelochemicals as bioherbicides—Present and perspectives. In: Price A.J.K., Jessica A., editors. Herbicides—Current Research and Case Studies in Use. Intech; Rijeka, Croatia: 2013. p. 662. [Google Scholar]
- 14.Vyvyan J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron. 2002;58:1631–1646. doi: 10.1016/S0040-4020(02)00052-2. [DOI] [Google Scholar]
- 15.Cheng F., Cheng Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015 doi: 10.3389/fpls.2015.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice E.L. Allelopathy. 2nd ed. Acadamic Press; New York, NY, USA: 1984. p. 422. [Google Scholar]
- 17.Duke S.O., Romagni J.G., Dayan F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000;19:583–589. doi: 10.1016/S0261-2194(00)00076-4. [DOI] [Google Scholar]
- 18.Charoenying P., Teerarak M., Laosinwattana C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hort. 2010;125:411–416. doi: 10.1016/j.scienta.2010.04.045. [DOI] [Google Scholar]
- 19.Teerarak M., Charoenying P., Laosinwattana C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant. 2012;34:1277–1285. doi: 10.1007/s11738-011-0923-5. [DOI] [Google Scholar]
- 20.Fernández-Aparicio M., Bernard A., Falchetto L., Marget P., Chauvel B., Steinberg C., Morris C.E., Gibot-Leclerc S., Boari A., Vurro M., et al. Investigation of amino acids as herbicides for control of Orobanche minor parasitism in red clover. Front. Plant Sci. 2017 doi: 10.3389/fpls.2017.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haig T. Allelochemicals in plants. In: Zeng R.S., Mallik A.U., Luo S.M., editors. Allelopathy in Sustainable Agriculture and Forestry. Springer; New York, NY, USA: 2008. pp. 63–104. [Google Scholar]
- 22.Li Z.-H., Wang Q., Ruan X., Pan C.-D., Jiang D.-A. Phenolics and plant allelopathy. Molecules. 2010;15:8933–8952. doi: 10.3390/molecules15128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popa V.I., Dumitru M., Volf I., Anghel N. Lignin and polyphenols as allelochemicals. Ind. Crops Prod. 2008;27:144–149. doi: 10.1016/j.indcrop.2007.07.019. [DOI] [Google Scholar]
- 24.Laosinwattana C., Teerarak M., Charoenying P. Effects of Aglaia odorata granules on the seedling growth of major maize weeds and the influence of soil type on the granule residue's efficacy. Weed Biol. Manag. 2012;12:117–122. doi: 10.1111/j.1445-6664.2012.00444.x. [DOI] [Google Scholar]
- 25.Abd-Alla M.A., El-Mougy N.S., Abd-El-Kader M.M., Abd-El-Kareem F., El-Gamal N.G., El-Mohamedy R.S. Aldehydes compounds for controlling black scurf disease of potato (Solanum tubrosum L.) under field conditions. Int. J. Agric. For. 2013;3:34–39. [Google Scholar]
- 26.Alla M.A., El-Sayed H.Z., Riad S. Control of rhizopus rot disease of apricot fruits (Prunus armeniaca L.) by some plant volatiles aldehydes. Res. J. Agric. Biol. Sci. 2008;4:424–433. [Google Scholar]
- 27.Bradow J.M., Connick W.J. Seed-germination inhibition by volatile alcohols and other compounds associated with Amaranthus palmeri residues. J. Chem. Ecol. 1988;14:1633–1648. doi: 10.1007/BF01012528. [DOI] [PubMed] [Google Scholar]
- 28.Connick W.J., Jr., Bradow J.M., Legendre M.G. Identification and bioactivity of volatile allelochemicals from amaranth residues. J. Agric. Food Chem. 1989;37:792–796. doi: 10.1021/jf00087a049. [DOI] [Google Scholar]
- 29.Gallardo M.T., Martin B.B., Martin D.F. Inhibition of water fern Salvinia minima by cattail (Typha domingensis) extracts and by 2-chlorophenol and salicylaldehyde. J. Chem. Ecol. 1998;24:1483–1490. doi: 10.1023/A:1020955615868. [DOI] [Google Scholar]
- 30.Gouda N.A.A., Saad M.M.G., Abdelgaleil S.A.M. Pre and post herbicidal activity of monoterpenes against barnyard grass (Echinochloa crus-galli) Weed Sci. 2016;64:191–200. doi: 10.1614/WS-D-15-00045.1. [DOI] [Google Scholar]
- 31.Lavrentyev P.J., Franzè G., Pierson J.J., Stoecker D.K. The effect of dissolved polyunsaturated aldehydes on microzooplankton growth rates in the Chesapeake Bay and Atlantic Coastal Waters. Mar. Drugs. 2015;13:2834–2856. doi: 10.3390/md13052834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masi M., Meyer S., Pescitelli G., Cimmino A., Clement S., Peacock B., Evidente A. Phytotoxic activity against Bromus tectorum for secondary metabolites of a seed-pathogenic Fusarium strain belonging to the F. tricinctum species complex. Nat. Prod. Res. 2017;31:2768–2777. doi: 10.1080/14786419.2017.1297445. [DOI] [PubMed] [Google Scholar]
- 33.Patterson D.T. Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max) Weed Sci. 1981;29:53–59. [Google Scholar]
- 34.Siddiqui I.A., Shaukat S.S. Effect of phenolic acids and an aromatic aldehyde on infectivity of Meloidogyne javanica and colonization by Pseudomonas aeruginosa in mungbean. Nematol. Medit. 2002;30:119–123. [Google Scholar]
- 35.Xie Y., Huang Q., Wang Z., Cao H., Zhang D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017;97:388–394. doi: 10.1016/j.indcrop.2016.12.043. [DOI] [Google Scholar]
- 36.Choi G.-H., Ro J.-H., Park B.-J., Lee D.-Y., Cheong M.-S., Lee D.-Y., Seo W.-D., Kim J.H. Benzaldehyde as a new class plant growth regulator on Brassica campestris. J. Appl. Biol. Chem. 2016;59:159–164. doi: 10.3839/jabc.2016.029. [DOI] [Google Scholar]
- 37.Fujii Y. Dish Pack Method: A New Bioassay for Volatile Allelopathy. Centre for Rural Social Research, Charles Sturt University; New South Wales, Australia: 2005. pp. 493–497. [Google Scholar]
- 38.Iacobellis N.S., Lo Cantore P., Capasso F., Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005;53:57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 39.Jones C.D., Woods K.E., Setzer W.N. A chemical ecological investigation of the allelopathic potential of Lamium amplexicaule and Lamium purpureum. Open J. Ecol. 2012;2:167–177. doi: 10.4236/oje.2012.24020. [DOI] [Google Scholar]
- 40.Li Z., Jiang L., Tang R., Xiong F., Tang X., Jiang J., He L., Zhong R., Han Z. Identification and allelopathy of chemical compositions of peanut root exudates. J. South China Agr. Univ. 2015;36:48–53. [Google Scholar]
- 41.Pacioni G. Effects of Tuber metabolites on the rhizospheric environment. Mycol. Res. 1991;95:1355–1358. doi: 10.1016/S0953-7562(09)80384-5. [DOI] [Google Scholar]
- 42.Sun M.-L., Song Z.-Q., Fang G.-Z. Allelopathy and chemical components of extract from green peel of Juglans mandshurica Maxim. Chem. Ind. Forest Prod. 2008;28:45–49. [Google Scholar]
- 43.Yun K.W., Kil B.S., Park J.S. Identification of naturally occurring, chemicals from Artemis princeps var. orientalis. Allelopathy J. 1994;1:95–104. [Google Scholar]
- 44.Kanchiswamy C.N., Malnoy M., Maffei M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015 doi: 10.3389/fpls.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chotsaeng N., Laosinwattana C., Charoenying P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules. 2017;22:1841. doi: 10.3390/molecules22111841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfman C. Bachelor’s Thesis. The University of Akron; Akron, OH, USA: 2017. The effects of polyunsaturated aldehydes on pelagic microbial food webs in the Chesapeake Bay area and Atlantic Coastal Waters. [Google Scholar]
- 47.Hansen E., Eilertsen H.C. Do the polyunsaturated aldehydes produced by Phaeocystis pouchetii (Hariot) Lagerheim influence diatom growth during the spring bloom in Northern Norway? J. Plankton Res. 2006;29:87–96. doi: 10.1093/plankt/fbl065. [DOI] [Google Scholar]
- 48.Ianora A., Bentley M.G., Caldwell G.S., Casotti R., Cembella A.D., Engström-Öst J., Halsband C., Sonnenschein E., Legrand C., Llewellyn C.A. The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field. Mar. Drugs. 2011;9:1625–1648. doi: 10.3390/md9091625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leflaive J., Ten-Hage L. Chemical interactions in diatoms: Role of polyunsaturated aldehydes and precursors. New Phytol. 2009;184:794–805. doi: 10.1111/j.1469-8137.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 50.Pichierri S., Pezzolesi L., Vanucci S., Totti C., Pistocchi R. Inhibitory effect of polyunsaturated aldehydes (PUAs) on the growth of the toxic benthic dinoflagellate Ostreopsis cf. ovata. Aquat. Toxicol. 2016;179:125–133. doi: 10.1016/j.aquatox.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 51.Ribalet F., Bastianini M., Vidoudez C., Acri F., Berges J., Ianora A., Miralto A., Pohnert G., Romano G., Wichard T. Phytoplankton cell lysis associated with polyunsaturated aldehyde release in the Northern Adriatic Sea. PLoS ONE. 2014;9:e85947. doi: 10.1371/journal.pone.0085947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribalet F., Berges J.A., Ianora A., Casotti R. Growth inhibition of cultured marine phytoplankton by toxic algal-derived polyunsaturated aldehydes. Aquat. Toxicol. 2007;85:219–227. doi: 10.1016/j.aquatox.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Vaughn S.F., Spencer G.F. Naturally-occurring aromatic compounds inhibit potato tuber sprouting. Am. Potato J. 1993;70:527–533. doi: 10.1007/BF02846753. [DOI] [Google Scholar]
- 54.Liang D., Xing F., Selvaraj J.N., Liu X., Wang L., Hua H., Zhou L., Zhao Y., Wang Y., Liu Y. Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and Aflatoxin B1 biosynthesis in Aspergillus flavus. J. Food Sci. 2015;80:2917–2924. doi: 10.1111/1750-3841.13144. [DOI] [PubMed] [Google Scholar]
- 55.Marei G., Abdelgaleil S. Antifungal potential and biochemical effects of monoterpenes and phenylpropenes on plant pathogenic fungi. Plant Protect. Sci. 2018;54:9–16. [Google Scholar]
- 56.Adiv S., Ahronov-Nadborny R., Carmeli S. New aeruginazoles, a group of thiazole-containing cyclic peptides from Microcystis aeruginosa blooms. Tetrahedron. 2012;68:1376–1383. doi: 10.1016/j.tet.2011.12.045. [DOI] [Google Scholar]
- 57.Barto E.K., Hilker M., Müller F., Mohney B.K., Weidenhamer J.D., Rillig M.C. The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE. 2011;6:e27195. doi: 10.1371/journal.pone.0027195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berry J. Marine and freshwater microalgae as a potential source of novel herbicides. In: Kortekamp A., editor. Herbicides and Environment. InTech; Shanghai, China: 2011. pp. 705–734. [Google Scholar]
- 59.Bravo H.R., Iglesias M.J., Copaja S.V., Argandoña V.H. Phytotoxicity of indole alkaloids from cereals. Rev. latinoamer. Quím. 2010;38:123–129. [Google Scholar]
- 60.Jüttner F., Todorova A.K., Walch N., von Philipsborn W. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry. 2001;57:613–619. doi: 10.1016/S0031-9422(00)00470-2. [DOI] [PubMed] [Google Scholar]
- 61.Komai K., Hamada M., Iwamura J., Shindo T. Allelopathic substances in egoma, Perilla frutescens var. japonica. Mem. Fac. Agric. Kinki Univ. 1989;22:23–29. [Google Scholar]
- 62.Kumar A., Chahal K., Kataria D. Comparison of chemical composition of root and rhizosphere soil extracts of Tagetes patula L.: GC-MS analysis. Asian J. Chem. 2017;29:797–800. doi: 10.14233/ajchem.2017.20307. [DOI] [Google Scholar]
- 63.Ramalakshmi S., Muthuchelian K. Studies on cytotoxic, phytotoxic and volatile profile of the bark extract of the medicinal plant, Mallotus tetracoccus (Roxb.) Kurz. Afr. J. Biotechnol. 2013;12:6176–6184. [Google Scholar]
- 64.Rizvi S., Mishra G., Rizvi V. Allelopathic effects of nicotine on maize I. Its possible importance in crop rotation. Plant Soil. 1989;116:289–291. doi: 10.1007/BF02214562. [DOI] [Google Scholar]
- 65.Xuan T.D., Tawata S., Khanh T.D. Herbicidal activity of mimosine and its derivatives. In: Price A.J.K., Jessica A., editors. Herbicides—Advances in Research. InTech; Rijeka, Croatia: 2013. pp. 299–312. [Google Scholar]
- 66.Zhang H., Peng Y., Zhang S., Cai G., Li Y., Yang X., Yang K., Chen Z., Zhang J., Wang H., et al. Algicidal effects of Prodigiosin on the harmful algae Phaeocystis globosa. Front. Microbiol. 2016 doi: 10.3389/fmicb.2016.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Q., Wang S.-Q., Tang H.-Y., Li X.-J., Zhang L., Xiao J., Gao Y.-Q., Zhang A.-L., Gao J.-M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain—Many compounds method. J. Agric. Food Chem. 2013;61:11447–11452. doi: 10.1021/jf403200g. [DOI] [PubMed] [Google Scholar]