Abstract
Background
Brucellosis is a disease with significant public and economic implications but strategies for controlling this disease remain problematic.
Objectives
This study sought to determine the sero-prevalence of brucellosis in prolonged fever patients and to identify modifiable risk factors for the infection in humans in post conflict Northern Uganda.
Methods
The study employed a cross-sectional method among prolonged fever patients who had visited selected health facilities in the study districts in Northern Uganda. Sero-prevalence of brucellosis was calculated for i-ELISA IgG/IgM. A structured questionnaire was used to obtain data on possible risk factors for brucellosis. Associations between sero-prevalence and risk factors were measured using the Odds Ratio.
Results
Brucellosis was confirmed in 18.7% of the 251 patients that tested positive for the disease, with the rapid Brucella Plate Agglutination Test, and ages 10–84 years (median age 47+0.86). Sex (p = 0.001; OR 3.79; 95% CI 1.75 – 8.24), rearing livestock (p < 0.005; OR 8.44; 95% CI 2.84–25.03) and consumption of unpasteurised milk (p = 0.023; OR 2.57; 95% CI 1.14–5.80) were factors associated with brucellosis.
Conclusion
Control of brucellosis in animals, training and sensitisation of the community on brucellosis is needed to stimulate action on human brucellosis control.
Keywords: Brucellosis, human, fever, prevalence, Uganda, zoonosis
Introduction
Brucellosis is a widespread zoonosis of both public health and economic concern1–3. The global burden of human brucellosis remains enormous with more than 500,000 new infections occurring annually worldwide4. Brucellosis is caused by organisms of genus Brucella with six pathogenic species of which four are known to cause human disease5. B. melitensis cause the most severe human disease, followed by B. suis, B. abortus and B. canis6. In humans brucellosis is transmitted by direct or indirect contact with infected animals and their products7. Inhalation of infectious aerosols and ingestion of raw milk or unpasteurized dairy products or meat from an infected animal is the most frequent route of infection8,9. Major symptoms for brucellosis in humans include; undulant fever, weight loss, back pain, fatigue and night sweats1.
Diagnosis of brucellosis is impossible without laboratory confirmation involving a combination of methods10. Blood culture for Brucellae isolation remains the gold standard11 but may not provide a positive result for all patients12. Nucleic acid amplification can provide rapid detection and confirmation of Brucella13 but standardization of extraction methods, infrastructure, equipment and expertise are still lacking6. Bacteriological and genomic limitations make serology the most practical and useful tool for brucellosis diagnosis11. The serological tests like Rose Bengal Plate Agglutination Test (RBPT), standard tube agglutination test (STAT), Enzyme Linked Immuno-Sorbent Assay (ELISA) and fluorescence polarization assay (FPA) among others have been applied in human brucellosis diagnosis11,13,14. However, STAT remains the most popular serological test used14 and titres of 1:160 and above are considered diagnostic13. Nevertheless, STAT has limitations making ELISA to be most acceptable for diagnosing human brucellosis11,15,16. Mantur and others11 found ELISA to be more sensitive than STAT in detecting brucellosis in both acute and chronic cases while sensitivity and specificity of ELISA was reported to be 71.3% and 100% respectively. Araj and others15 reported sensitivities of 100% and 91% for IgM and IgG ELISAs, respectively, and a specificity of 100% for both assays. In addition, Asaad and other workers16 reported sensitivities of ELISA IgM and IgG to be 85.2% and 96.3%,respectively and specificity of 100% for each..
Human brucellosis occurs widely in Uganda8,17,18. A recent study carried out in Gulu district in Northern Uganda reported individual animal and herd level sero-prevalence of bovine brucellosis to be 6% and 19%, respectively. Restocking and communal grazing were the major risk factors associated with the disease19. Northern Uganda is under rehabilitation with promotion of livestock rearing and re-stocking. To date, no study has described human brucellosis in post conflict Northern Uganda, a region that witnessed a total breakdown in socio-economic infrastructure, governance and services. The aim of this study was to estimate the sero-prevalence of human brucellosis in prolonged fever patients and identify modifiable risk factors for the infection in post-conflict Northern Uganda. This study considered ELISA IgG/IgM which is reported as the most specific serology test against SAT.
Methods
Ethics and consent to participate statement
Ethical approval was obtained from Gulu University Research Council (Ref No: GU/IRC/02/07/13) and the Uganda National Council of Science and Technology (UNCST) Reference No: HS 1442. Written consent/assent was sought from all individuals before enrolment.
The study was carried out in post-conflict areas of Northern Uganda, in the districts of Apac, Gulu, Lira and Pader covering up to nine percent of the country's 236,040 Km2 area size.
Study design and enrolment of study patients
A cross-sectional study was conducted from March 2014 to February 2015. Study patients were selected from 17 out of the 244 public and private health facilities in the study area20–23. The health facilities were purposively selected basing on capacity to test for brucellosis and willingness to participate in the study. The study targeted patients with fever, headache, joint pain, malaise, backache, fatigue and loss of appetite visiting study health facilities. Medical personnel in the out patient department (OPD) identified suspects and tested them with the rapid Brucella Plate Agglutination Test (BPAT). The sample size for study subjects was calculated using the formula for cross sectional survey as described before24 at 5% desired precision and 95% confidence interval with prevalence of human brucellosis in Uganda estimated at 18%8.
Laboratory investigation
To determine the sero-prevalence of brucellosis in prolonged fever patients, STAT 1: 160 was used as the standard serological test against SAT 1: 320, iELISA IgG, iELISA IgM and iELISA IgG/IgM. Only BPAT positive samples from consented subjects were further investigated. All subjects were briefed on the objectives of the study, and signed a consent form. Cephalic vein blood was collected into sterile silicon-coated tubes without anticoagulant for serum harvesting after 24 hours at room temperature. The serum was transported on ice to Makerere University, College of Veterinary Medicine, Animal Resources and Biosecurity (CoVAB) and kept at -20°C. Samples were analysed using STAT (Remel kit, Europe) and i-ELISA (Vircell S.L, Santa Fe, and Granada, Spain) according to the manufacturers' guidelines. To improve sensitivity, results for i-ELISA, were interpreted in parallel, with any IgG or IgM positive test reported as positive for i-ELISA as described previously17. A structured questionnaire was used to obtain data on possible risk factors for human brucellosis. Information collected from study subjects included age, gender, district of origin, education level, rearing livestock, knowledge on brucellosis transmission and practice of consumption of unpasteurized milk/milk products.
Statistical analysis
Sero-prevalence of brucellosis in humans was calculated for both STAT and i-ELISA (IgG and IgM). Associations between sero-prevalence of human brucellosis and modifiable risk factors were assessed with a Chi-square test and Odds Ratio at 95% confidence interval using SPSS, (SPSS Inc. Chicago, USA Version 16.0). Variables with p ≤ 0.1 at univariable analysis were included in a multivariable logistic regression model. The backward selection procedure was used to identify factors for the final models. Goodness of fit for the final model was assessed using Hosmer and Lemeshow goodness of fit test.
Results
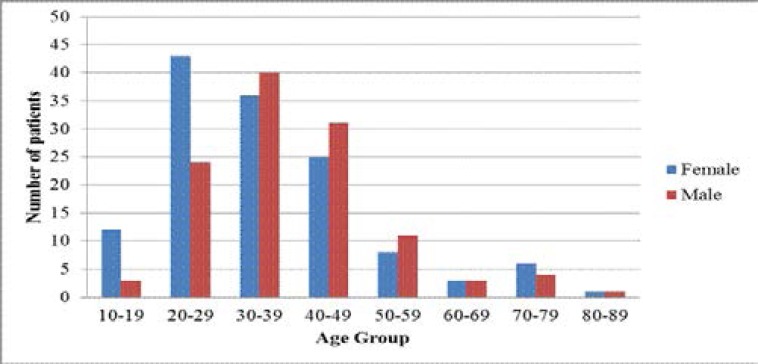
A total of 251 prolonged fever patients aged 10–84 years (median age 47.0± 0.86) were enrolled in the study (Fig 1).
Figure 1.
Distribution of study patients by gender and age group
Up to 207 study subjects were from government and 44 from private health facilities. All serological tests showed significant (p<0.05) differences in brucellosis sero-positivity between health facilities, sex and keeping livestock. In addition brucellosis sero-positivity differed significantly (p<0.05) with consumption of milk and milk products basing on STAT 1:160, 1:320 and i-ELISA IgM, but did not differ basing on i-ELISA IgG. There was no difference in sero-positivity with education level (p>0.05) basing on all the four serological tests (Table 1).
Table 1.
Sero-prevalence of brucella among prolonged fever patients
| Test Results (%,p-value), n=251 | ||||||
| Variable | Category | Frequency | STAT 1:160 | STAT1:320 | i-ELISA IgG | i-ELISA IgM |
| Health facility | Government | 207 | 32.7(0.000)* | 17.5(0.026)* | 12.7(0.005)* | 10.8(0.039)* |
| Private | 44 | 2.0 | 1.2 | 0.0 | 0.4 | |
| Gender | Male | 117 | 19.9(0.012)* | 11.2 (0.048)* | 10.8(0.000)* | 8.4(0.001)* |
| Female | 134 | 14.7 | 7.5 | 1.9 | 2.8 | |
| Age category of patients |
Children | 8 | 0.8(0.887) | 0.0(0.0507) | 0.0(0.722) | 0.0(0.361) |
| Youth | 188 | 26.3 | 14.3 | 9.6 | 7.6 | |
| Adult | 41 | 6.0 | 3.6 | 2.4 | 2.4 | |
| Elderly | 14 | 1.6 | 0.8 | 0.8 | 1.2 | |
| Educational | ≤Primary | 152 | 22.7(0.242) | 11.2(0.878) | 7.6(0.883) | 8.0(0.212) |
| level | > Primary | 99 | 12.0 | 7.5 | 5.1 | 3.2 |
| Keep livestock | Yes | 145 | 29.9(0.000)* | 16.3(0.000)* | 11.1(0.000)* | 10.4(0.000)* |
| No | 106 | 4.8 | 2.4 | 1.6 | 0.8 | |
| Knowledge Transmission |
No | 231 | 31.5(0.601) | 16.7(0.453) | 11.6(0.753) | 9.2(0.040)* |
| Yes | 20 | 3.2 | 2.0 | 1.1 | 2.0 | |
| Consume pasteurised Milk/milk products |
No | 151 | 27.1(0.000)* | 17.1(0.000)* | 9.6(0.066) | 9.2(0.012)* |
| Yes | 100 | 7.6 | 1.6 | 3.1 | 2.0 | |
5% level of significance
Basing on the parallel interpretation of either positive i-ELISA IgG or IgM or both tests, brucellosis sero-prevalence differed significantly (p<0.05) with gender, rearing livestock and consumption of unpasteurised milk/milk products (Table 2).
Table 2.
Relationship between the hypothesised risk factors for human brucellosis and sero-positivity based on i-ELISA IgG/IgM.
| Variable | Category | % test positive (n=251) |
χ2 | df | p-value |
| Gender | Male | 14.3 | 20.89 | 1 | <0.005* |
| Female | 4.4 | ||||
| Age group | < 30years | 5.2 | 3.15 | 1 | 0.076 |
| > 30 years | 13.5 | ||||
| Education level | ≤Primary | 12.0 | 0.26 | 1 | 0.611 |
| > Primary | 6.7 | ||||
| Keep livestock | Yes | 17.1 | 26.95 | 1 | <0.005* |
| No | 1.6 | ||||
| Knowledge on | Not Know | 14.3 | 1.82 | 1 | 0.178 |
| Transmission | Knowledgeable | 3.4 | |||
| Consume milk | Unpasteurised | 14.7 | 8.32 | 1 | 0.004* |
| and milk products | Pasteurised | 4.0 |
Significant at 5% level
Gender (OR 3.79; 95% CI: 1.75–8.24), livestock rearing (OR 8.44; 95% CI: 2.84–25.03) and consumption of unpasteurised milk (OR 2.57; 95% CI: 1.14–5.80) were significantly associated with brucellosis. The model explained 30.9% (NagelkerkeoR2) of the variance in brucellosis sero-prevalence and correctly classified 83.3% of the cases. The Odds of being brucella positive in males with prolonged fever were four times the Odds in females. Livestock rearing was highly associated with brucella sero-positivity and consumption of unpasteurised milk and milk products increased the likelihood of testing brucella sero-positive (Table 3).
Table 3.
Multivariable logistic regression of factors associated with human brucellosis in post-conflict northern Uganda
| Variable | Category | % Sero- positivity (n=251) |
Odds Ratios(95%CI) |
p-values |
| Gender | Male | 14.3 | 3.79(1.75–8.24) | 0.001* |
| Female | 4.4 | |||
| Age group | < 30 years | 5.2 | 1.73 (0.80–3.75) | 0.164 |
| > 30 years | 13.5 | |||
| Keep livestock | Yes | 17.1 | 8.44(2.84–25.03) | 0.005* |
| No | 1.6 | |||
| Consumption of | Unpasteurised | 14.7 | 2.57(1.14–5.80) | 0.023* |
| Milk/milk products | Pasteurised | 4.0 |
5% level of significance
Discussion
This is the first study to document the sero-prevalence of human brucellosis among patients with prolonged fever in Northern Uganda. The study showed sero-prevalence of human brucellosis in Northern Uganda to be 18.7% with i- ELISA and 34.7% with STAT (1:160). However, sero-positivity with STAT titres (1>1:320) was 18.7%, an indication of active infection. This is not surprising since study subjects had come to seek medical attention with symptoms relating to brucellosis. This finding is comparable to a recent study17 which reported STAT sero-prevalence results not to be different (p<0.05) from that for c-ELISA. Findings here support the use of ELISA tests for confirming brucellosis in low-income countries where brucellosis is endemic. This study showed men to be four times likely to test positive for brucellosis than women. In the current study, milking and grazing animals is a man's role thus men are more in contact with animals than women. This finding is supported by reports of epidemiological studies in other parts of the world where human brucellosis is considered an occupational disease25. In addition, reports show no sex-wise discrimination of brucellosis infection between male and female as both have equal susceptibility if provided with exposure to potential risk factors26. Nevertheless, the current study contradicts studies elsewhere which reported high prevalence of brucellosis among females than males25,27.
This study clearly shows livestock rearing (p<0.005) to be strongly associated with human brucellosis. Generally, livestock harbour brucellosis in a sub-clinical state and farm families are at high risk of exposure to the disease through contact with fluids of affected livestock or consumption of their unpasteurized products. This study is in agreement with Corbel6 who pointed out that when animals are kept in close proximity to human habitat, the practice can result in brucella infection as people are in constant contact with infective materials. This study has also shown brucellosis sero-positivity to be highly associated with consumption of unpasteurised milk/milk products. This finding parallels another study28 which reported that significantly (p<0.02) more sero-positive humans with a history of raw milk. This calls for increased awareness among the communities in post-conflict Northern Uganda about the dangers associated with drinking raw milk, among others. Since this study dealt with only suspected brucellosis patients and did not cover all health facilities, results need to be interpreted cautiously. However, the study has provided important information to support brucellosis control programs.
Conclusion and recommendations
Findings in this study show clearly that the sero-prevalence of human brucellosis among patients with prolonged fever is high (18.7%). Rearing livestock and consumption of unpasteurised milk/milk products were factors associated with brucellosis. Strategies to control animal brucellosis and raising awareness about the consumption of unpasteurised milk and milk products are necessary to reduce the incidence of human infection in Northern Uganda. Innovative methods to sensitize the community on brucellosis and cost effective methods to control animal brucellosis are recommended.
Acknowledgements
We thank Gulu University ECART (Enhancing Capacity for Agricultural Research and Training), Netherlands Organization for Capacity Building in Higher Education (NUFFIC) and the International Centre for Development Oriented Research in Agriculture (ICRA) for the financial support. We thank all participants in health facilities and veterinary departments in the study districts, as well, the personnel in Makerere University, College of Veterinary Medicine, Animal Resources and Biosecuritycentral diagnostic.
Authors' contributions
HMN was involved in the development of the concept, study design, data collection, analysis, and writing of the paper. JE and DOO helped to develop the concept, supervised the study, and critically revised the paper. GWN participated in the study design and data interpretation, revised the paper and approved the final version. JMK participated in the acquisition of the data, revised the paper and approved the final version. DO and JN participated in the development of the concept study design and the drafting of the paper. All authors read and approved the final version of the paper.
Conflict of interest
None.
References
- 1.Sauret JM, Vilissova N. Human brucellosis. J Am Board Fam Pract [Internet] 2002;15(5):401–406. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12350062. [PubMed] [Google Scholar]
- 2.Purcell Bret K, Hoover David L, Friedlander AM. Chapter 9, Brucellosis. Med Asp Biol Warf. 2007:185–198. PubMed. [Google Scholar]
- 3.OIE, author. Bovine Brucellosis. OIE Terr Man. 2009 May;:1–35. 2009. [Google Scholar]
- 4.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos E V. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. doi: 10.1016/S1473-3099(06)70382-6. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Moreno E, Cloeckaert A, Moriyón I. Brucella evolution and taxonomy. Vet Microbiol. 2002;90(1–4):209–227. doi: 10.1016/s0378-1135(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 6.Brucellosis in humans and animals Brucellosis in humans and animals.
- 7.Kunda J, Fitzpatrick J, French N, Kazwala R, Kambarage D, Mfinanga GS, et al. Quantifying risk factors for human brucellosis in Rural Northern Tanzania. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0009968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabukenya I, Kaddu-Mulindwa D, Nasinyama GW. Survey of Brucella infection and malaria among Abattoir workers in Kampala and Mbarara Districts, Uganda. BMC Public Health [Internet]. BMC Public Health. 2013;13(1):901. doi: 10.1186/1471-2458-13-901. PubMed. Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyebambe PS. Case Reports Acute brucella meningomyeloencephalo - spondylosis in a teenage male. Afr Health Sci. 2005;5(1):69–72. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 10.Mantur BG, Mulimani MS, Bidari LH, Akki AS, Tikare N V. Bacteremia is as unpredictable as clinical manifestations in human brucellosis. Int J Infect Dis. 2008;12(3):303–307. doi: 10.1016/j.ijid.2007.09.004. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Mantur B, Parande A, Amarnath S, Patil G, Walvekar R, Desai A, et al. ELISA versus conventional methods of diagnosing endemic brucellosis. Am J Trop Med Hyg. 2010;83(2):314–318. doi: 10.4269/ajtmh.2010.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casañas MC, Queipo-Ortuño MI, Rodriguez-Torres A, Orduña A, Colmenero JD, Morata P. Specificity of a Polymerase Chain Reaction Assay of a Target Sequence on the 31-Kilodalton Brucella Antigen DNA Used to Diagnose Human Brucellosis. J Clin Microbiol. 2001;20:127–131. doi: 10.1007/pl00011242. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Mantur B G, Amarnath SK SR. Review of clinical and laboratory features of human brucellosis. Vet Microbiol. 2007;25(3):188–202. doi: 10.4103/0255-0857.34758. PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Al-Anazi K, Al-Jasser A. Brucellosis: A Global Re-emerging Zoonosis History, Epidemiology, Microbiology, Immunology and Genetics. EsciencecentralOrg [Internet] 2014:1–10. Available from: http://esciencecentral.org/ebooks/bacterial-mycotic-infections/pdf/brucellosis-3.pdf%5Cnhttp://esciencecentral.org/ebooks/bacterial-mycotic-infections/pdf/brucellosis1.pdf. [Google Scholar]
- 15.Araj GF, Kattar MM, Fattouh LG, Bajakian KO, Kobeissi SA. Evaluation of the PANBIO Brucella immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays for diagnosis of human brucellosis. Clin Diagn Lab Immunol. 2005;12(11):1334–1335. doi: 10.1128/CDLI.12.11.1334-1335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asaad AM, Alqahtani JM. J Infect Public Health [Internet] 2. Vol. 5. King Saud Bin Abdulaziz University for Health Sciences; 2012. Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia; pp. 189–194. Available from: http://dx.doi.org/10.1016/j.jiph.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Nasinyama G, Ssekawojwa E, Opuda J, Grimaud P, Etter E BA. Brucella sero-prevalence and modifiable risk factors among predisposed cattle keepers and consumers of un-pasteurized milk in Mbarara and Kampala districts , Uganda Abstract. Afr Health Sci. 2014;14(4):790–796. doi: 10.4314/ahs.v14i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutanda L. Selected laboratory tests in febrile patients in Kampala, Uganda. East Afr Med J. 1998;75(2):68–72. PubMed. [PubMed] [Google Scholar]
- 19.Mugizi DR, Boqvist S, Nasinyama GW, Waiswa C, Ikwap K, Rock K, et al. Prevalence of and factors associated with Brucella sero-positivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J Vet Med Sci. 2015:1–8. doi: 10.1292/jvms.14-0452. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apac statistical abstract. Apac district local government statistical abstract. UBOS; 2012. Jun, [Google Scholar]
- 21.Gulu statistical abstract. The republic of Uganda Gulu district local government. UBOS; 2013. Jun, [Google Scholar]
- 22.Lira statistical abstract. The republic of Uganda Lira district local governmentpa. UBOS; 2012. Jun, [Google Scholar]
- 23.Pader statistical abstract. Higher local government statistical abstract. Pader district P. O Box 1 , Pader-Uganda: UBOS; 2009. Jun 1, [Google Scholar]
- 24.Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol from Bed to Bench. 2013;6(1):14–17. [PMC free article] [PubMed] [Google Scholar]
- 25.Sayyad S, Malak MM, Miri BA, Gharib SM, VA The Prevalence Rate of Human Brucellosis in Sanandaj County, West of Iran. Life Sci J. 2014;11(3s):23–25. [Google Scholar]
- 26.Yohannes M, Gill JP. Seroepidemiological survey of human brucellosis in and around Ludhiana, India. Emerg Health Threats J. 2011;4:1–7. doi: 10.3402/ehtj.v4i0.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makita K, Fèvre EM, Waiswa C, Kaboyo W, Eisler MC, WSC Spatial epidemiology of hospital-diagnosed brucellosis in Kampala, Uganda. Int J Health Geogr BioMed Central Ltd. 2011;10(1):52. doi: 10.1186/1476-072X-10-52. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed MO, Elmeshri SE, Abuzweda AR, Blauo M, Abouzeed YM, Ibrahim A, et al. Seroprevalence of brucellosis in animals and human populations in the western mountains region in Libya , December 2006 — January 2008. Euro surveill. 2010;15(30):1–4. [PubMed] [Google Scholar]