Abstract
Background
Quorum sensing is a cell-to-cell communication system in bacteria that controls the production of virulence factors. Serratia marcescens is a causative agent of hospital-acquired infections that shows high resistance to antibiotics. This makes the treatment of these infections difficult. Quorum sensing regulates the production of virulence factors of S. marcescens such as prodigiosin, protease, swimming and swarming motilities and formation of biofilms. Inhibition of quorum sensing may be an alternative to antibiotic treatment to avoid emergence of resistance.
Objectives
Testing the ability of glyceryl trinitrate to inhibit quorum sensing and virulence factors of Serratia marcescens.
Methods
The inhibiting activities of sub-inhibitory concentration of glyceryl trinitrate against the quorum-sensing regulated violacein pigment in Chromobacterium violaceum CV026 was performed to evaluate the anti-quorum sensing effect of glyceryl trinitrate. The anti-virulence activity was assessed against prodigiosin, protease, biofilm formation in addition to swimming and swarming motilities.
Results
Glyceryl trinitrate at at a concentration of 0.25 mg/ml produced significant inhibitory effects against violacein (67.01%), prodigiosin (82.67%), protease, swimming (36.72%) and swarming (79.31%) motilities and biofilm formation (87.83%).
Conclusion
Glyceryl trinitrate is a quorum sensing and virulence inhibitor that may be useful in treatment of nosocomial infections caused by Serratia marcescens.
Keywords: Serratia marcescens, quorum sensing, virulence, glyceryl trinitrate
Introduction
Serratia marcescens is a nosocomial pathogen that can lead to many nosocomial infections such as those affecting urinary tract, respiratory tract in addition to wound infections. It has an arsenal of virulence factors that aid in escape from the immune system and causing these infections such as prodigiosin, proteases, biofilm and its ability to swim and swarm1–3. These virulence factors are controlled by a mechanism of intercellular communication termed quorum sensing. Quorum sensing enables bacterial cells to monitor their numbers and respond by coordinating the expression of genes including virulence genes2–4. Some strains of Serratia marcescens cause infections that are hard to treat. The underlying reason is their high resistance to many categories of antibiotics involving fluoquinolones, aminoglycosides and β-lactams5,6. The high virulence and resistance of Serratia marcescens necessitates seeking a new treatment strategy. It is supposed that quorum sensing inhibition is one useful approach that can help in treating infections without exerting stress on the growth of bacteria to avoid the evolution of antibiotic resistance. Furthermore, it can enhance the immune clearance of the pathogens7,8.
Glyceryl trinitrate (GTN) is an anti-hypertensive that has anti-microbial properties9,10. It could inhibit the growth of Candida albicans and Pseudomonas aeruginosa planktonic growth10,11. Moreover, GTN exerted antibiofilm activity against Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa and candida albicans12. In our previous study, GTN was found to inhibit quorum sensing in Pseudomonas aeruginosa11. GTN is an FDA approved drug that can be used topically for anal fissures at concentrations that can reach 0.4%13.
This study aimed to investigate the potential anti-quorum sensing and anti-virulence activities of GTN against the nosocomial pathogen Serratia marcescens.
Materials and methods
Media and chemicals
Tryptone soya broth, Mueller Hinton broth and Mueller Hinton agar were the products of Oxoid (Hampshire, UK). Luria-Bertani (LB) broth and LB agar were purchased from Lab M Limited (Lancashire, United Kingdom).Glyceryl trinitrate was obtained from POHL-Boskamp, Gmbh&Co., Hohenlockstedt, Germany (Stock solution of 1 mg/ml). Other chemicals were of pharmaceutical grade.
Bacterial Strains
The Serratia marcescens isolate in this study was used in a previous study14. It is a clinical one obtained from an Intensive Care Unit patient admitted to Zagazig University Hospital by endotracheal aspiration. The MALDI-TOFF apparatus at the Clinical Pathology Department, Faculty of Medicine, Zagazig University was used for identification of this isolate. The biosensor strain Chromobacterium violaceum CV026 was obtained from the Department of Microbiology, Faculty of Pharmacy, Ain Shams University.
Determination of Minimum Inhibitory Concentration (MIC)
The agar dilution method was used in determination of the minimum inhibitory concentration of GTN according to the Clinical Laboratory and Standards Institute Guidelines (CLSI)15. The tested strain was incubated overnight in tryptone soya broth (TSB) and the suspension was diluted with Mueller-Hinton broth in order to prepare a suspension with a turbidity approximating that of 0.5McFarland Standard. The suspension was further diluted with sterile saline (1:10). By using a micropipette, a standardized inoculum (approximately 104 CFU per spot) was spotted on the surface of Mueller-Hinton agar plates containing different GTN concentrations and control plate without GTN. The MIC of GTN was the lowest concentration that inhibits growth on the plate after incubation at 37°C for 20 h.
Effect of GTN on bacterial growth
The effect of sub-inhibitory concentration of GTN on the growth of the tested strain of S. marcescens was detected according to Nalca et al.16 overnight culture from S. marcescens was prepared in LB broth and adjusted to 0.5 McFarland Standard. The prepared suspension was used to inoculate LB broth containing 0.25 mg/ml of GTN and control LB broth without GTN so that the final inoculum is approximately 1×106 CFU/ml. After overnight incubation at 37 °C, the optical densities of both cultures were measured at 600 nm by using Biotek Spectrofluorimeter (Biotek, USA).
Violacein inhibition assay
To determine the quorum sensing inhibiting activity of GTN, the biosensor strain Chromobacterium violaceum CV026 was used according to Choo et al17. A suspension of the tested strain with OD600 of 1 was prepared from overnight culture. Aliquots of 100 µl of bacterial suspension were added to the wells of a 96-well microtiter plate to which aliquots of 100 µl of LB broth with N-hexanoyl homoserinelactone in the presence and absence of 0.25 mg/ml of GTN were delivered. The plate was incubated at 28°C for 16 h and was dried at 60°C. The purple pigment violacein was extracted by addition of aliquots of 100 µl of DMSO to the wells and incubation at 30°C with shaking. DMSO was used as negative control and the absorbance was measured at 590 nm using Biotek Spectrofluorimeter (Biotek, USA).
Biofilm inhibition assay
The tested strain was reported as a strong biofilm forming isolate14. To determine the ability of GTN to inhibit biofilm formation, the modified method of Abraham et al.18 was used. A suspension of S. marcescens strain was prepared from overnight culture in tryptone soya broth (TSB) and its optical density was adjusted to OD600 of 0.4 (1×108 CFU/ ml) was added. Aliquot of 10µl of the suspension was added to 1 ml amounts of fresh TSB with and without 0.25 mg/ml of GTN. Aliquots of 100 µl of TSB with and without GTN were delivered into the wells of 96 wells microtiter plate and incubated at 28 °C for 24 h. The planktonic cells were aspirated and the wells were washed three times with distilled water and left to dry. The attached cells were fixed with methanol for 20 minutes and stained with crystal violet (1%) for 20 minutes. The wells were washed and the attached dye was eluted by 33% glacial acetic acid. The absorbance was measured at 590 nm using Biotek Spectrofluorimeter (Biotek, USA). The percentage of biofilm inhibition was calculated using the following formula
% of biofilm inhibition = [OD600 control- OD600 in presence of ambroxol]/ OD600 control
Microscopic visualization of biofilm inhibition by the light microscope and scanning electron microscope
In order to analyze biofilm inhibition, the method of Sakar et al.19 was followed with some modification. The biofilm of the tested strain of Serratia marcescens was formed on glass cover slips placed in polystyrene petri plates in the presence and absence of 0.25 mg/ml of GTN. The plates were incubated for 24 h at 28°C, the cover slips were washed with water three times and stained with crystal violet (1%) for 20 minutes. The cover slips were examined after staining under the light microscope at a 400X magnification. For scanning electron microscopic (SEM) analysis, biofilms formed on glass cover slips were fixed by glutaraldehyde (2.5%) for 120 minutes. After washing with distilled water, the cover slips were dehydrated by increasing concentrations of ethanol (50%, 60%, 70%, 80%, 90% and 100%) for 30 seconds. The samples were gold coated after critical-point drying and examined under scanning electron microscope20.
Swimming and swarming motilities assay
The ability of GTN to block the swimming and swarming motilities was detected according to Matsuyama et al.21 For swimming assay, LB agar plates containing 0.3% agar with and without 0.25 mg/ml GTN were prepared. Overnight culture of S.marcescens in LB broth was prepared and 5µl of the suspension was inoculated into the center of the plates. Swarming LB gar plates with 0.5% agar containing 0.25% mg/ml of GTN and control plates were point inoculated with 5µl of the prepared suspension. The plates were incubated at 28°C for 20 h. The zones of swimming and swarming were measured. The experiment was made in triplicates and the results were averaged.
Prodigiosin inhibition assay
The production of prodigiosin by S. marcescens was quantified in the presence and absence of GTN. The tested strain was grown overnight in LB broth and adjusted to OD600 of 0.4 and inoculated in 2 ml fresh LB broth and incubated at 28°C for 18 h. The cells were collected by centrifugation at 13000 rpm for 10 minutes. To extract prodigiosin, acidified ethanol (4% 1M HCl in ethanol) was used. The absorbance was measured at 534 nm using Biotek Spectrofluorimeter (Biotek, USA) and the degree of inhibition was determined. The experiment was made in triplicate and the results were averaged22.
Protease assay
To assay the inhibitory activity of GTN on protease production, the qualitative skim milk agar method was used23. Skim milk LB agar plates (5% skim milk) with and without GTN (0.25 mg/ml) were prepared. The plates were surface inoculated, each with 5 µl of overnight culture of S. marcescens in LB broth and incubated at 28°C for 20h. The diameter of the clear zones surrounding the growth were measured.
Statistical analysis
The inhibitory activities of GTN on quorum sensing and virulence factors of Serratia marcescens were compared by t test, Graph Pad Prism 5. P values <0.05 were considered statistically significant.
Results
Determination of MIC
Glyceryl trinitrate could not inhibit the growth of Serratia marcescens isolate at 0.5 mg/ml which is the maximum available concentration (stock solution of GTN is of concentration of 1 mg/ml), so MIC is equal to or higher than 1 mg/ml. the concentration selected to test the anti-quorum sensing and anti-virulence activities of GTN is 0.25 mg/ml which is equivalent to ¼ MIC or less.
Determination of the effect of GTN on bacterial growth
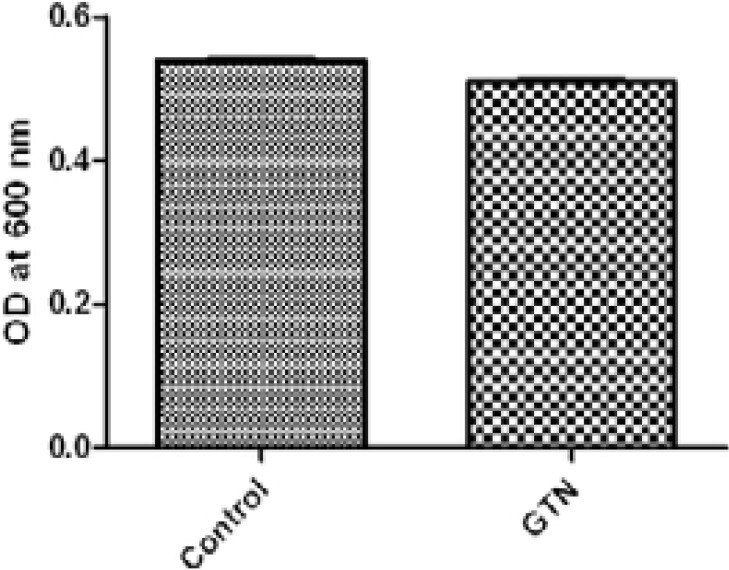
To determine the effect of GTN on growth, the optical densities of the bacterial suspension at 600 nm following overnight incubation in LB broth were measured in the presence or absence of GTN. No statistically significant difference was found between the turbidities of the bacterial suspension with or without GTN indicating the lack of the effect of GTN on growth (Fig. 1).
Figure 1.
Effect of GTN on growth of Serratia marcescens. No statistically significant difference between OD600 of the GTN treated and untreated cultures after overnight incubation in LB broth.
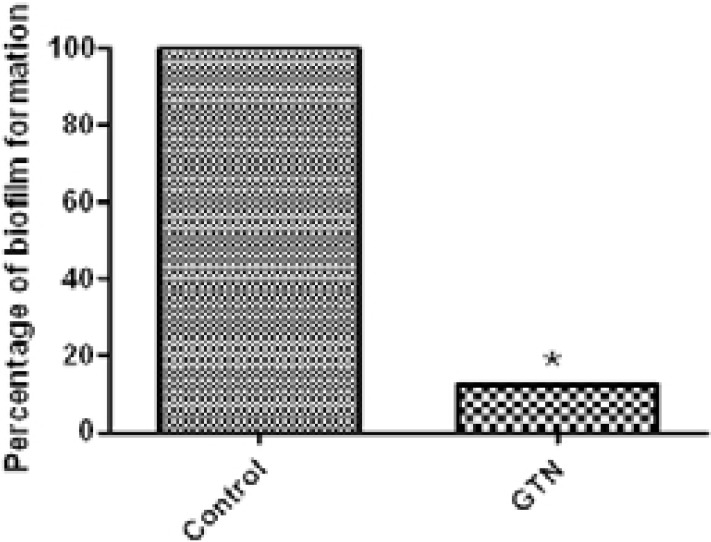
Assessment of biofilm inhibition
The biofilm formation was quantified to show the ability of GTN to interfere with biofilm production. GTN at 0.25 mg/ml produced statistically significant reduction in biofilm biomass (p<0.05). The percentage of biofilm inhibition reached 87.83% (Fig. 2).
Figure 2.
Biofilm inhibition of S. marcescens by GTN.*, significant P< 0.05.
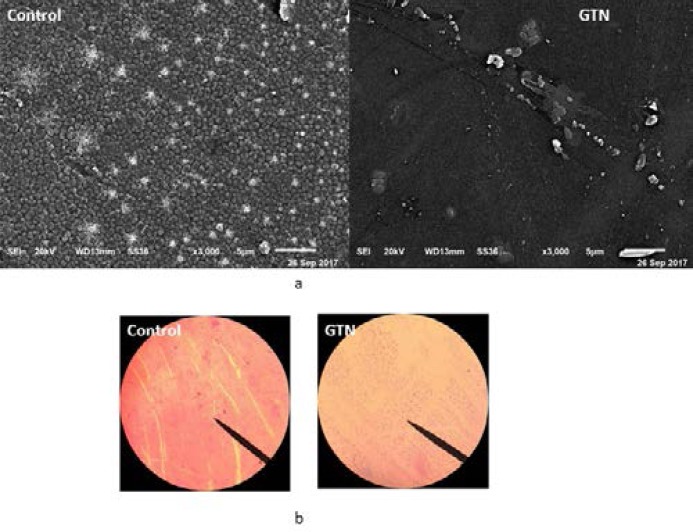
To further explore the biofilm inhibiting activity of GTN, light microscopic examination of biofilms formed on glass cover slips in the presence and absence of GTN was performed. In the presence of GTN, both the thickness and surface coverage were markedly decreased (Fig. 3). Furthermore, the SEM images clearly illustrated the biofilm inhibiting activity of GTN. In GTN treated sample, very few scattered cells were observed as compared to the untreated sample that showed the complex biofilm structure with aggregated cells.
Figure 3.
Microscopic analysis of biofilm inhibition by GTN (a) SEM images and (b) Light microscopic images of GTN treated and untreated biofilms.
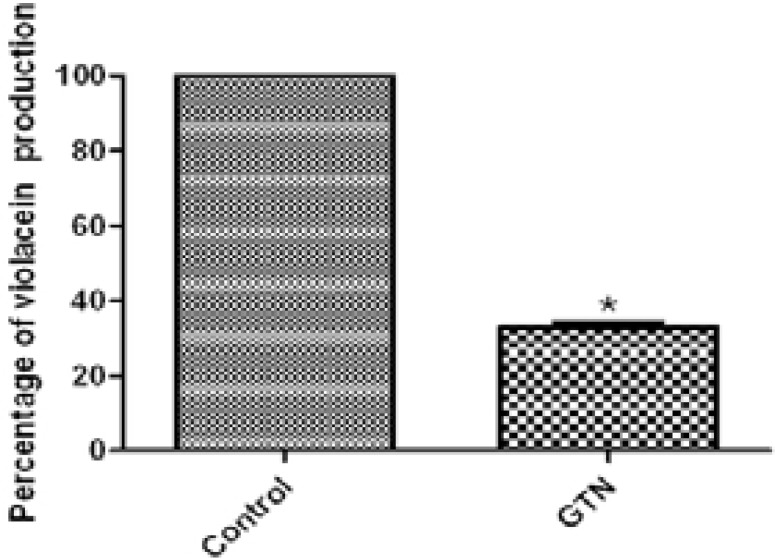
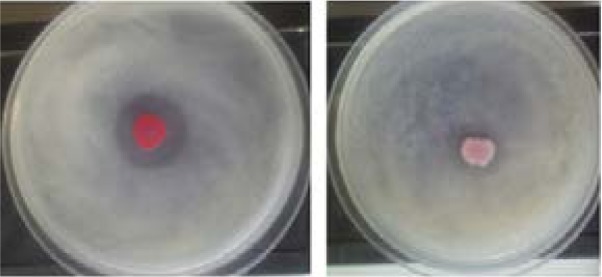
Inhibition of violacein production
To prove the quorum sensing inhibition by GTN, the production of violacein pigment in the biosensor strain Chromobacterium violaceum CV026 that is controlled by quorum sensing mechanism was monitored. GTN significantly diminished violacein production (67.01%). This confirms that GTN is a quorum sensing inhibitor (Fig. 4).
Figure 4.
Inhibition of violacein pigment of Chromobacterium violaceum CV026 by GTN. *, significant P< 0.05.
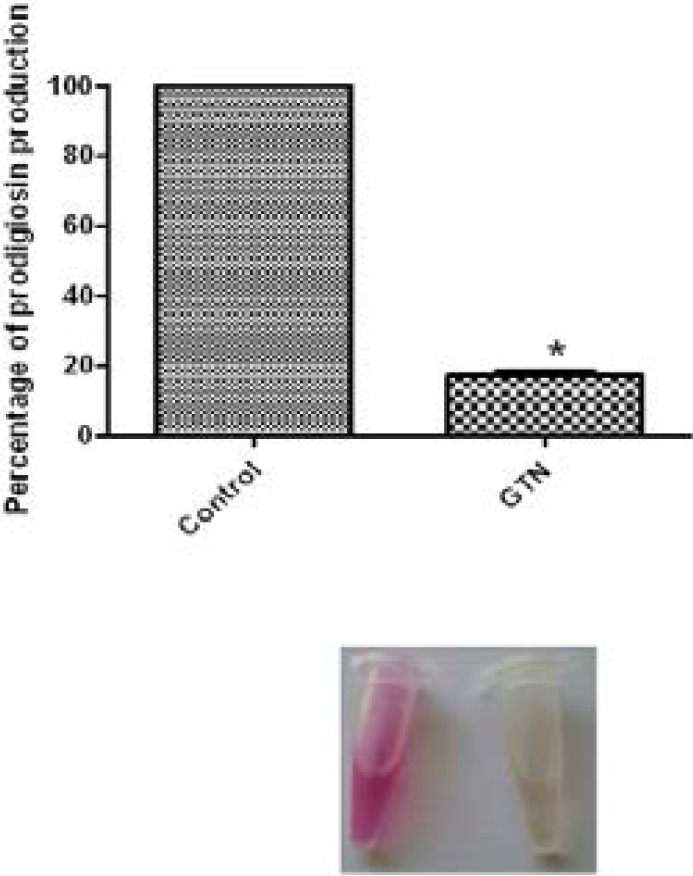
Prodigiosin inhibition assay
Prodigiosin is another quorum-sensing controlled pigment that is produced by S. marcescens. GTN showed a significant ability to inhibit prodigiosin production. The inhibition percentage achieved was 82.67% (Fig 5).
Figure 5.
Inhibition of prodigiosin pigment of Serratia marcescens by GTN. *, significant P< 0.05.
Inhibition of Protease Production
The skim milk agar method was used for the qualitative assay of protease inhibition. GTN could interfere with protease production as shown by decreasing the clear zone of proteolysis (Fig. 6).
Figure 6.
Inhibition of protease production by the skim milk agar method.
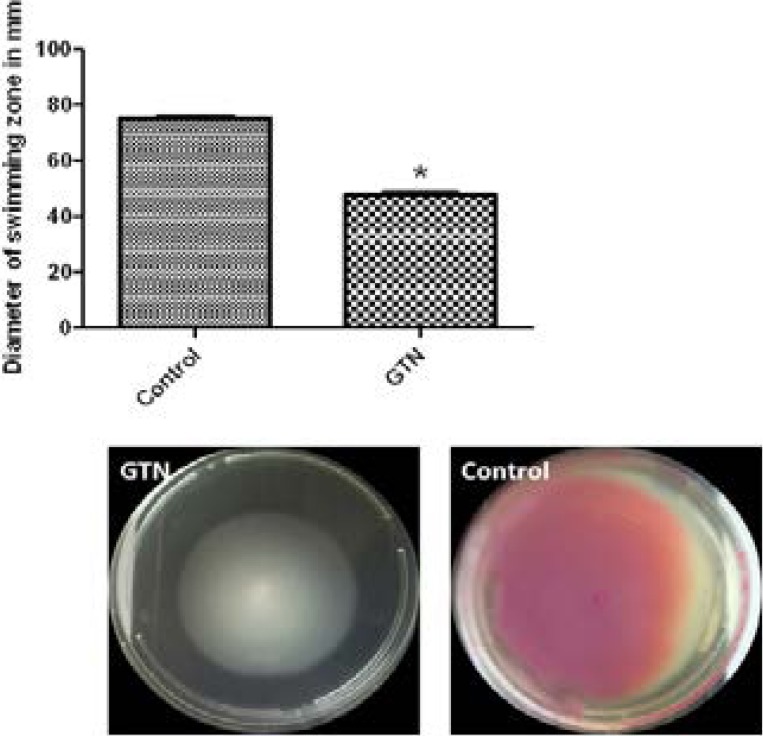
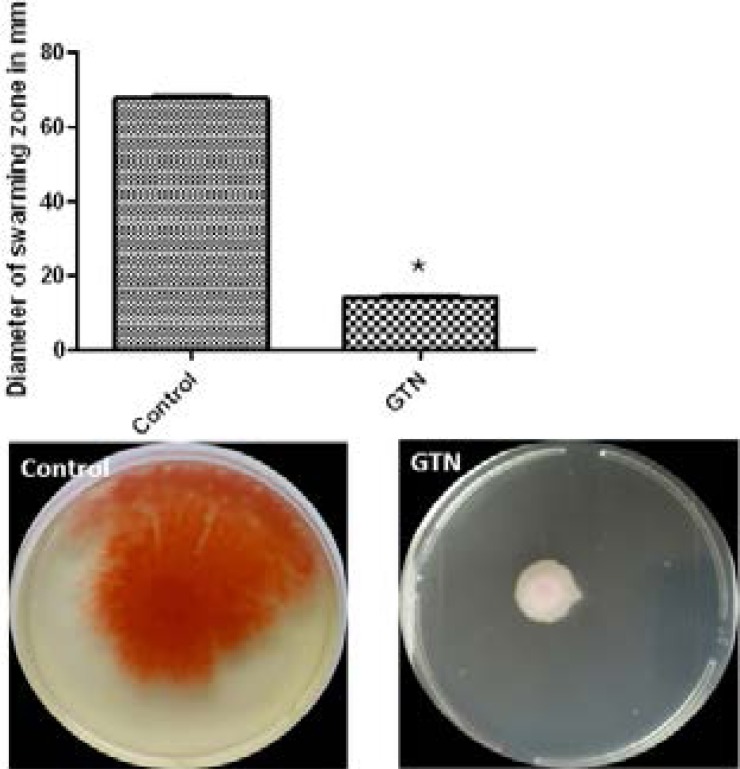
Inhibition of swimming and swarming
Swimming and swarming motility are important for adhesion and biofilm formation. In the presence of GTN, swimming motility was reduced by 36.72%, while swarming motility was decreased to the level of 79.31% (Fig. 7&8).
Figure 7.
Inhibition of swimming motility by GTN. *, significant P< 0.05.
Figure 8.
Inhibition of swarming motility by GTN. *, significant P< 0.05.
Discussion
Serratia marcescens is a nosocomial bacterium that is considered the seventh most common pathogen that causes nosocomial pneumonia and the tenth most common one that is responsible for hospital acquired blood stream infections24.
This study was performed to detect the possible quorumsensing mediated virulence factors inhibition by sub-inhibitory concentration of glyceryl trinitrate in Serratia marcescens.
The underlying reason for targeting quorum sensing is to bypass the stress on bacterial growth that leads to emergence of resistance and finding another way to treat infection in the light of the high antibiotic resistance of Serratia marcescens25. Quorum sensing is a key regulator of the pathogenicity in many bacteria. Inhibition of quorum sensing may be beneficial in attenuation of virulence and the treatment of infections caused by resistant strains of bacteria26,27.
The tripyrrole red prodigiosin pigment is synthesized under the control of quorum sensing in S. marcescens21. GTN caused remarkable reduction in prodigiosin pigment. Protease is involved in the pathogenicity of S. marcescens. GTN reduced the production of protease28.
In this study, GTN showed high ability to inhibit biofilm. Biofilms render the infection difficult to treat due to the high resistance to antibiotics and immunity of the host29. The ability of Serratia marcescens to swim and swarm affects adhesion and biofilm formation. As a result, interference with swarming could affect biofilm production30,31. GTN blocked swarming motility.
The inhibition of virulence factors production of Serratia marcescens may be due to interference with quorum sensing. This hypothesis was proved by the ability of GTN to cause significant reduction in the production of the quorum-sensing regulated violacein pigment in the biosensor strain C. violaceum CV026. Moreover, GTN was previously reported as a quorum sensing inhibitor in Pseudomonas aeruginosa PAO1 strain11.
GTN has the advantage of FDA approval that makes GTN clinical application possible. This is very important because most quorum sensing inhibitors have toxic effects that hinder their use in treatment. Serratia marcescens causes surgical wounds infections and urinary tract infections. GTN can be used topically in the treatment of surgical site infections or as a catheter lock solutions for catheter associated urinary tract infections32.
Conclusion
The emergence of antibiotic resistance makes it obligatory to seek for new therapeutic options for infection control. GTN may be a useful agent in this approach because of its anti-quorum sensing and anti-virulence activities against S. marcescens. Targeting quorum sensing regulated virulence poses no growth pressure on bacteria and hence the resistance is much less likely to develop.
Conflict of interests
There is no conflict of interest
References
- 1.Hejazi A, Falkiner FR. Serratia marcescens. Journal of Medical Microbiology. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 2.Rice SA, Koh KS, Queck SY, Labbate M, Lam KW, Kjelleberg S. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. Journal of Bacteriology. 2005;187:3477–3485. doi: 10.1128/JB.187.10.3477-3485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarah JC, Neil RW, Abigail KPH, David RS, George PCS. Metabolic and regulatory engineering of Serratia marcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology. 2006;152:1899–1911. doi: 10.1099/mic.0.28803-0. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford ST, Bassler BL. Bacterial Quorum Sensing: its role in virulence and possibilties of its control. Cold Spring Harbor Prespectives in Medicine. 2012;2(11):a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stock I, Burak S, Sherwood KJ, Gruger T, Wiedemann B. Natural anti-microbial susceptibilities of strains of 'unusual’ Serratia species: S. ficaria, S. fonticola, S. odorifera, S. plymuthica and S. rubidaea. Journal of Antimicrobial Chemotherapy. 2003;51:865–885. doi: 10.1093/jac/dkg156. [DOI] [PubMed] [Google Scholar]
- 6.Traub WH. Antibiotic susceptibility of Serratia marcescens and Serratia liquefaciens. Chemotherapy. 2000;46:315–321. doi: 10.1159/000007304. [DOI] [PubMed] [Google Scholar]
- 7.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of anti-virulence therapies. Nature Reviews Microbiology. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nature Reviews Drug Discovery. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 9.Gorfine SR. Treatment of benign anal disease with topical nitroglycerin. Diseases of the Colon and Rectum. 1995;38:453–457. doi: 10.1007/BF02148842. [DOI] [PubMed] [Google Scholar]
- 10.Palmeira-de-Oliveira A, Ramos AR, Gaspar C, et al. In vitro anti-Candida activity of lidocaine and nitroglycerin: alone and combined. Infectious Diseases in Obstetrics and Gynecology. 2012 doi: 10.1155/2012/727248. Article ID 727248, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbas HA, Shaldam MA. Glyceryl trinitrate is a novel inhibitor of quorum sensing in Pseudomonas aeruginosa. African Health Sciences. 2016;16(4):1117–1125. doi: 10.4314/ahs.v16i4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenblatt J, Reitzel RA, Raad I. Caprylic acid and glyceryl trinitrate combination for eradication of biofilm. Antimicrobial Agents and Chemotherapy. 2015;59:1786–1788. doi: 10.1128/AAC.04561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton C, Wellington K, Easthope SE. 0.4% nitroglycerin ointment: in the treatment of chronic anal fissure pain. Drugs. 2006;66:343–349. doi: 10.2165/00003495-200666030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Abbas HA, Hegazy WA. Targeting the virulence factors of Serratia marcescens by ambroxol. Roumanian Archives of Microbiology and Immunology. 2017;76 In press. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute, author. Methods for dilution anti-microbial susceptibility tests for bacteria that grow aerobically; Approvated standard, CLSI Document M07-A9. 3. Vol. 32. Wayne, PA, USA: 2012. [Google Scholar]
- 16.Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Haussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrobial Agents and Chemotherapy. 2006;50:1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Letters in Applied Microbiology. 2006;42(6):637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 18.Abraham SVPI, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Archives of Medical Research. 2011;42:658–668. doi: 10.1016/j.arcmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar R, Chaudhary SK, Sharma A, Yadav KK, Nema NK, Sekhoacha M, et al. Antibiofilm activity of Marula- A study with the standardized bark extract. Journal of Ethnopharmacology. 2014;154(1):170–175. doi: 10.1016/j.jep.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Gowrishankar S, Poornima B, Pandian SK. Inhibitory efficacy of cyclo(Lleucyl-L-prolyl) from mangrove rhizosphere bacterium-Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Research in Microbiology. 2014;165(4):278–289. doi: 10.1016/j.resmic.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. Journal of Bacteriology. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morohoshi T, Shiono T, Takidouchi K, Kato M, Kato N, Kato J, et al. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acyl homoserine lactone. Applied and Environmental Microbiology. 2007;73:6339–6344. doi: 10.1128/AEM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov Metal The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology. 2001;147:2517–2528. doi: 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- 24.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clinical Infectious Diseases. 2010;51(1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 25.Coulthurst SJ, Williamson NR, Harris AKP, Spring DR, Salmond GPC. Metabolic and regulatory engineering of Serratia marcescens: mimicking phage mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology. 2006;152:1899–1911. doi: 10.1099/mic.0.28803-0. [DOI] [PubMed] [Google Scholar]
- 26.Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. Journal of Clinical Investigation. 2003;112:1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.March JC, Bentley WE. Quorum sensing and bacterial cross-talk in biotechnology. Current Opinion in Biotechnology. 2004;15:495–502. doi: 10.1016/j.copbio.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Wei JR, Lai HC. N-acylhomoserine lactone-dependent cell-to-cell communication and social behavior in the genus Serratia. International Journal of Medical Microbiology. 2006;296:117–124. doi: 10.1016/j.ijmm.2006.01.033. 2006. [DOI] [PubMed] [Google Scholar]
- 29.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 284:1318–1322. doi: 10.1126/science.284.5418.1318. 199. [DOI] [PubMed] [Google Scholar]
- 30.Shanks RMQ, Lahr RM, Stella NA, Arena KE, Brothers KM, Kwak DH, et al. A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS. PLoS One. 2013;8(3):e57634. doi: 10.1371/journal.pone.0057634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soo PC, Horng YT, Chang YL, Tsai WW, Jeng WY, Lu CC, et al. ManA is regulated by RssAB signaling and promotes motility in Serratia marcescens. Research in Microbiology. 2014;165:21–29. doi: 10.1016/j.resmic.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Galloway WR, Hodgkinson JT, Bowden S, Welch M, Spring DR. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends in Microbiology. 2012;20:449–458. doi: 10.1016/j.tim.2012.06.003. [DOI] [PubMed] [Google Scholar]