Abstract
Sequence-specific detection of nucleic acids has been intensively studied in the field of molecular diagnostics. In particular, the detection and analysis of single-nucleotide polymorphisms (SNPs) is crucial for the identification of disease-causing genes and diagnosis of diseases. Sequence-specific hybridization probes, such as molecular beacons bearing the fluorophore and quencher at both ends of the stem, have been developed to enable DNA mutation detection. Interestingly, DNA mutations can be detected using fluorescently labeled oligonucleotide probes with only one fluorophore. This review summarizes recent research on single-labeled oligonucleotide probes that exhibit fluorescence changes after encountering target nucleic acids, such as guanine-quenching probes, cyanine-containing probes, probes containing a fluorophore-labeled base, and microenvironment-sensitive probes.
Keywords: DNA, nucleic acids chemistry, oligonucleotides, fluorescent probes, single-nucleotide polymorphism, fluorophore
1. Introduction
Since the post-genome era, there has been a growing need for faster and more accurate detection methods for nucleic acids. In particular, the interest in single-nucleotide polymorphisms (SNPs), which involve a variation of a single nucleotide at a specific location in the genome, is increasing, so typing an SNP using a DNA probe is very important [1,2,3,4]. Several methods for SNP typing have been developed in efforts to establish an ideal typing system that exhibits very sensitive and specific behavior for the target DNA, without costly and time-consuming steps. Among these methods, molecular beacon (MB) probes have been widely used for the detection of SNPs, for the real-time detection of nucleic acids, for the quantification of polymerase chain reactions (PCRs), for isothermal amplification, as DNA microarray–immobilized probes, and as antisense probes for detecting RNA in vivo [5,6,7,8].
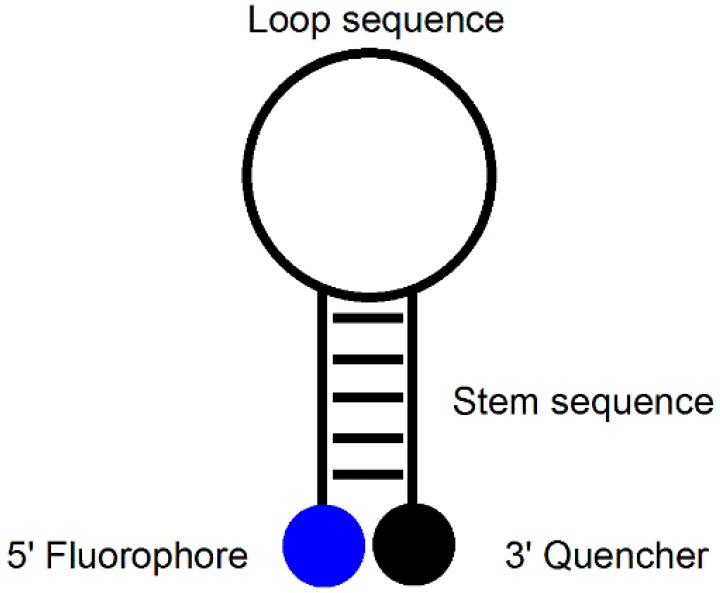
An MB probe is an oligonucleotide that forms a hairpin-like stem-loop structure tagged with a fluorophore at its 5′-terminus and a quencher at its 3′-terminus (Figure 1). The hairpin loop consists of approximately 15–25 nucleotides (nt) that are complementary to the target DNA, and the terminal stems are composed of 5–7 nt that are complementary to each other [9,10,11]. When the MB probe exists in the form of a hairpin structure, the fluorescence disappears because the quencher is positioned close to the fluorophore. When the MB probe meets its target DNA, however, the hairpin structure is opened, and fluorescence is restored as a result of the separation of the fluorophore and quencher units. The advantage of MB probes over single-dye probes (linear probes) is that they provide high signal-to-noise ratios and high degrees of single-base mismatch discrimination. Despite these attractive features, MB probes have a disadvantage in that they require a specific target sequence of 15–25 nt to separate the fluorophore and quencher upon dimerization with the target. In addition, because the hairpin must be opened, the rate of dimer formation is slower than that of a corresponding linear probe. In addition, both the fluorophore and quencher units are required, adding to the cost of preparation.
Figure 1.
MB probe.
If fluorescent hairpin- and linear-oligonucleotides could be made to function in a manner similar to that of MB probes, but without the need for quencher units, such probes would be simpler and cheaper to prepare. Furthermore, quencher-free DNA probes could then be prepared with the fluorophore positioned anywhere along the sequence, not only at the end of the stem. Examples of systems capable of fluorescence-based sequence detection, discrimination of alleles, and DNA quantification using oligonucleotide probes containing only fluorophore units (i.e., without quenchers) have been reported. This article reviews recent progress in the development of single-labeled DNA probes that can sense the presence of a specific nucleic acid through a change in fluorescence intensity.
2. Guanine-Quenching Probes
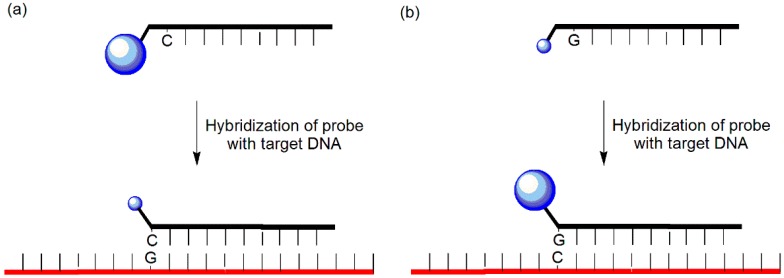
Fluorescence quenching between a guanosine residue and a fluorophore is commonly exploited because guanine bases function as energy acceptors [12,13,14]. Two types of probes have been designed using this principle (Figure 2). First, quenching of the fluorophores such as fluorescein, BODIPY, 6-carboxyfluorescein, and tetramethyl-6-carboxyrhodamine can be achieved by placing one or more adjacent cytosine residues next to the fluorophore unit of the probe [15,16,17]; hybridization induces a decrease in the fluorescence of the fluorophore close to one or more guanine residues in the complementary strand. Second, dequenching can be induced by placing one or more guanine residues next to the fluorescein unit in the probe [18]; the internal quenching is relaxed upon hybridization with the target DNA, thereby enhancing the emission.
Figure 2.
(a) Guanine-quenching probe and (b) Guanine-dequenching probe.
3. Cyanine-Containing Probes
As mentioned above, the interaction of fluorophores with nucleobases usually leads to the quenching of fluorescence. However, some cyanines such as thiazole orange (TO) and oxazole yellow (YO) are notable exceptions because they are powerful reagents for nucleic acid staining. In attempts to develop fluorescence “turn-on” probes, oligonucleotides containing these dyes have been developed [19,20,21,22,23,24,25].
The Ishiguro group designed a YO-linked DNA probe [19]. A YO moiety was inserted at an internal cytidine, C*, of a 13mer, 5′-CTCGC*GGGGGCTG-3′, complementary to the 5′-terminus non-coded region of hepatitis C virus RNA (Figure 3a). The YO-linked DNA probe recognized the complementary DNA or RNA and increased the fluorescence by the intercalation of the YO moiety into the target DNA or RNA.
Figure 3.
(a) YO-linked probe and (b) TO-linked probe.
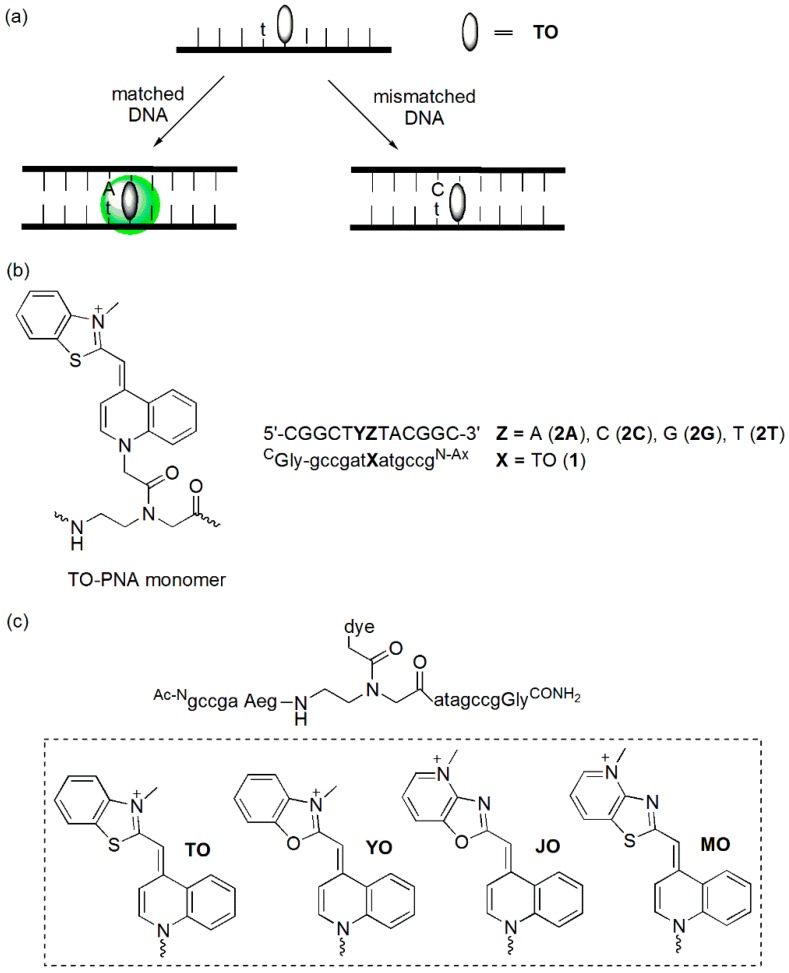
The Asseline group reported a TO-linked DNA probe (Figure 3b) [20]. The fluorescence intensities of the mismatched duplexes were greater than those of the corresponding matched duplexes. The highest discrimination factor, which was the fluorescence intensity ratio between the mismatched duplexes and the perfectly matched ones, was obtained when TO was linked in the position adjacent to A (discrimination factor >4) and to T (discrimination factor >3).
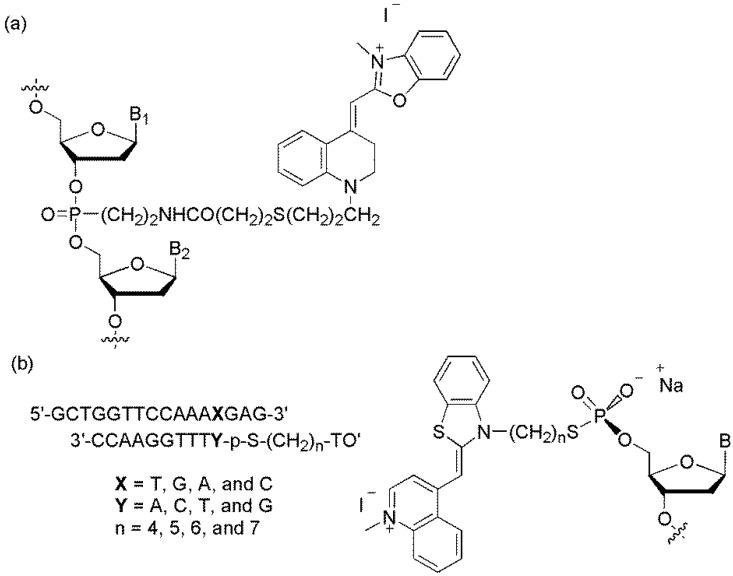
The Seitz group developed forced intercalation probes (FIT probes, Figure 4a), TO-linked PNA (peptide nucleic acid) probes, in which TO was utilized as a fluorescent base surrogate [21,22,23]. When these probes were hybridized to the matched DNA target, hybridization-induced fluorescence enhancement was observed. TO fluorescence was sensitive to the structural disturbance of PNA/DNA caused by an adjacent base mismatch (Figure 4b). The emission of a duplex (1/2C, Y = C) containing a C/T mismatch was reduced 3.4- and 11-fold relative to that of a matched duplex (1/2A, Y = A) when measured at 25 °C and 61 °C, respectively. The discrimination factor at 61 °C was higher because the thermal stability of the mismatch duplex was 9 K lower compared to that of the matched duplex.
Figure 4.
(a) Principle of FIT-probes; (b) TO-PNA conjugate; and (c) TO-, YO-, MO-, and JO-PNA conjugates.
Other cyanine dyes such as oxazole yellow (YO), thiazolopyridine (MO), and oxazolopyridine (JO) have also been tested (Figure 4c) [24]. The TO-PNA probe was able to discriminate the a/T matches from a/A and a/G mismatches with 3-fold selectivity. However, it was not able to discriminate a/C mismatches. This type of mismatch could be easily discriminated by the YO–PNA probe. However, the YO-PNA probe was not able to discriminate an a/A mismatch. Discrimination of the match/mismatch at 60 °C increased to 4- to 6-fold for the TO–PNA probe and to 7-fold for the YO-PNA probe. A glycerol-TO nucleotide-containing DNA probe has also been developed [25]. Emission of the matched duplex was at least 5.6-fold higher than those of mismatched duplexes at 25 °C. It was proposed that the increase in the available space introduced by mismatched base-pairs reduced the emission of the “TO-nucleobase”.
4. Probes Containing a Fluorescent Nucleobase Analog
The classical fluorescent nucleobase consists of an adenine analog 2-aminopurine that maintains complementarity to thymine but also wobble pairs with cytosine. It has a high fluorescent quantum yield but shows reduced fluorescence following its interaction with the base when inserted into the oligonucleotide [26]. Thus, fluorescent base analogs that are structurally similar to native nucleobases, capable of pairing with Watson-Crick pairs, and applicable as SNP probes are being developed.
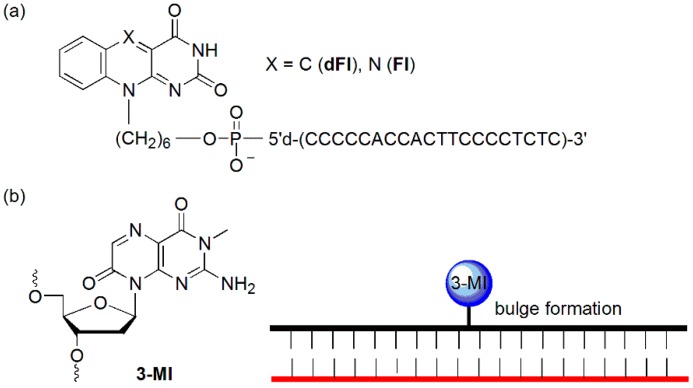
The Fontecave group demonstrated that flavin (Fl) and deazaflavin (dFl) were able to hybridize to a matched target DNA both in the solution and on a solid surface and that the hybridization could be detected because of the dramatic quenching of the matched target DNA (Figure 5a) [27]. Significantly decreased quenching of fluorescence could be detected when mismatched targets were hybridized. This effect probably resulted from a combination of the increased distance between the dye and target and reduced amounts of the hybridized probe at equilibrium, making it possible to detect mutations in solution and on a solid support.
Figure 5.
(a) Oligonucleotide conjugates of 5-deazaflavin (dFl) and flavin (Fl) and (b) single-base bulge formation of a 3-MI-containing oligonucleotide.
The Hawkins group reported 3-MI-containing hybridization probe (Figure 5b) [28]. Following the formation of a matched duplex, a bulge formed at the sites of 3-MI, leading to an increase of up to 27-fold in fluorescence intensity. They demonstrated that the bulge-formation technique could be used to detect positive PCR products using an HIV-1 detection system.
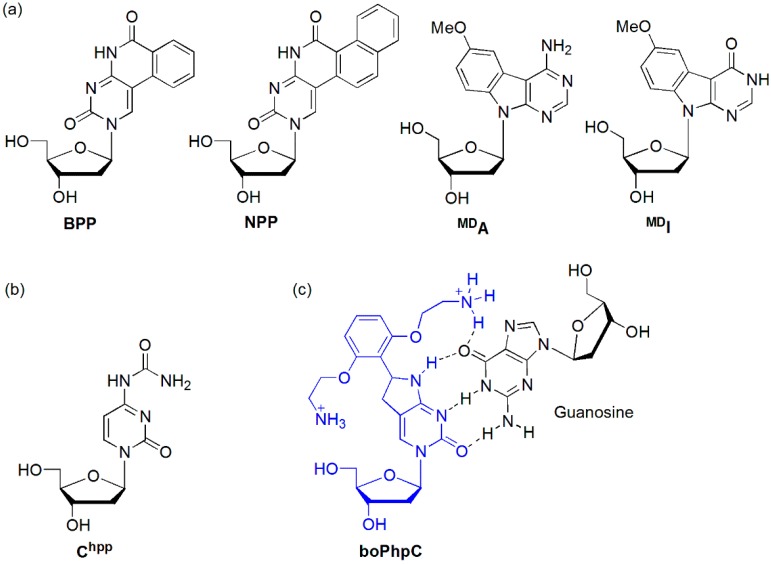
The Saito group designed benzopyridopyrimidine (BPP) as an effective degenerate base, forming stable base pairs in the Watson-Crick pairing mode for BPP/G and in the wobble mode for BPP/A (Figure 6a) [29]. However, the fluorescence behavior of the BPP-containing oligodeoxynucleotide (ODN) [ODN(BPP)] was strongly dependent on the purine bases opposite BPP. The fluorescence quantum yield (ΦF) of ODN(BPP)/ODN(G) (i.e., when BPP met G, ΦF = 0.0018) was approximately 20-fold less than that observed for ODN(BPP)/ODN(A) (ΦF = 0.035). Therefore, BPP-containing ODN is an effective probe for typing the A/G SNP. BPP-containing ODN was also applied for the detection of a single nucleotide alteration in RNA [30]. ODN(BPP)/RNA(A) exhibited a pale blue fluorescence that was clearly distinguished from the very weak emission observed for ODN(BPP)/RNA(G). Naphthopyridopyrimidine (NPP) also could clearly distinguish the nucleobases opposite NPP, particularly between the A and G bases [31]. The fluorescence quantum yield of ODN(NPP)/ODN(G) (ΦF = 0.007) was approximately 14-fold less than that observed for ODN(NPP)/ODN(A) (ΦF = 0.096). For the other mismatched duplexes ODN(NPP)/ODN(C) and ODN(NPP)/ODN(T), the emissions were weak compared with that for ODN(NPP)/ODN(A) (ΦF = 0.021 and 0.051, respectively). In addition, methoxybenzodeazaadenine (MDA) and methoxybenzodeazainosine (MDI) emitted strong fluorescence only when the base on the complementary strand was C and T, respectively [32].
Figure 6.
(a) BPP, NPP, MDA, and MDI, (b) Chpp, and (c) boPhpC.
The Sekine group synthesized a bicyclic 4-N-carbamoyldeoxycytidine derivative (Chpp) as the geometrically locked nucleoside and inserted it at the central position of a 13mer ODN (5′-CGCAAT ChppTAACGC-3′) (Figure 6b) [33]. They found that Chpp forms stable base pairs with not only the complementary guanine base but also the adenine base. Although the emission intensity was very similar to that of the single-stranded ODN (ssODN) containing Chpp when the Chpp base faced a mismatch base A, the emission of the duplex containing a Chpp/G base pair was significantly reduced.
The Hudson group synthesized [bis-ortho-(aminoethoxy)phenyl]pyrrolocytosine (boPhpC), capable of additional hydrogen bonding with guanine, and a boPhpC-containing PNA (GTAGAT CXCT-Lys, X = boPhpC) (Figure 6c) [34]. This PNA exhibited a significant increase in affinity toward matched DNA and RNA (ΔTm = +11.5 and +10.0 relative those of natural PNA/DNA and PNA/RNA duplexes, respectively) presumably due to an additional hydrogen bond to guanine. Interestingly, duplex formation with mismatch DNAs showed a sharp decrease in Tm (ΔTm ≥ −13.5 °C). The discrimination was equal to or greater than that of cytosine itself. However, upon duplex formation, the quantum yield of the matched PNA/DNA duplex dramatically decreased by ca. 50% compared to that observed for a single-stranded PNA.
The Tor group designed a fluorescent nucleoside, 7-aminoquinazoline-2,4-(1H,3H)-dione, which contains an electron-rich ring fused into an electron-deficient pyrimidine, and incorporated it into the central position (X) of ODN (5′-GCGATGXGTAGCG-3′) [35]. When this nucleoside formed duplexes with A, T, and C, a quenched emission was observed, but when it met with G, a fluorescence increase of approximately 2-fold was observed. Therefore, this nucleoside sensed mismatched pairings by displaying a G-specific fluorescence enhancement, a characteristic not observed in other fluorescent nucleosides.
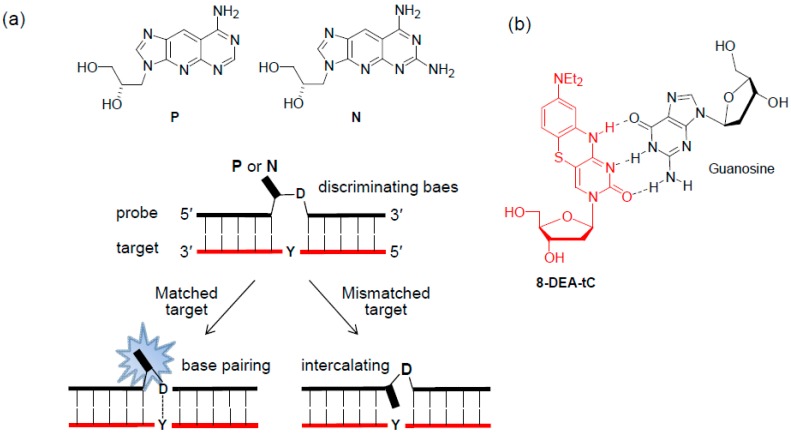
The Ueno group reported a fluorescent tricyclic base-linked acyclonucleoside, P and N. (Figure 7a) [36,37]. When a discriminating base D of the probe was complementary to the DNA target base Y, the base pairs between D and Y caused the fluorescent analog to flip outside of the DNA helix, enhancing the fluorescence intensity of the analogs. However, when the target base Y was mismatched with the discriminating base D, the analogs intercalated into the DNA helix resulted in a decrease in the emission of the analogs. The results of the fluorescence experiment for the RNA targets were similar to those observed for the DNA targets.
Figure 7.
(a) Tricyclic base-linked acyclonucleoside, P and N, and (b) tricyclic cytidine analog 8-DEA-tC.
The Purse group designed the tricyclic cytidine analog 8-DEA-tC and incorporated it into the X of ODN, 5′-CGCANXN′TCG-3′ (N and N′ = A, T, G, or C) (Figure 7b) [38]. The ssODNs were up to 5-fold brighter than the 8-DEA-tC nucleoside and showed further emission increases in all sequences of up to 4-fold when forming a matched duplex with the complementary DNA target. However, mispairing 8-DEA-tC with A resulted in an emission increase of less than 2-fold.
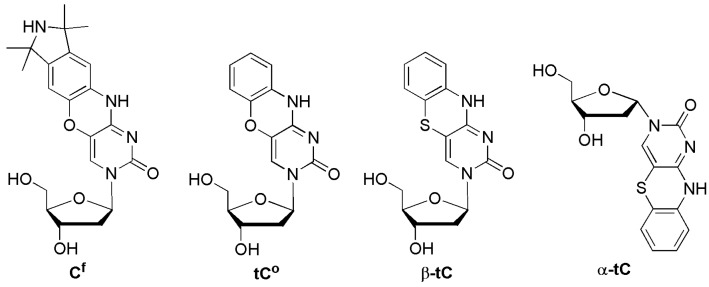
The Sigurdsson group designed the ssODN 5′-GACCTCGCfATCGTG-3′ containing another cytidine analog Cf. Mispairing Cf with A resulted in the most fluorescent among all duplexes and was similar to that of the single strand (Figure 8) [39]. The fluorescence emission of the mismatched duplexes Cf/C and Cf/T was between that of Cf/A and the matched duplex Cf/G. They also investigated the SNP typing of α- and β-anomers of phenotiazine (tC), which is a highly fluorescent DNA base analog that forms Watson-Crick base pairs with guanine [40] and acts as an i-motif probe [41]. Neither anomer of tC was suitable for SNP detection because they were unable to detect mismatches in any tested sequence [42]. However, phenoxazine (tCo) was able to detect mismatches for some flanking sequences, but tCo did not perform as good as the Cf probe.
Figure 8.
Tricyclic cytidine analogs, Cf, tCo, β-tc, and α-tc.
5. Probes Containing a Fluorophore-Labeled Base
5.1. HyBeacon Probes
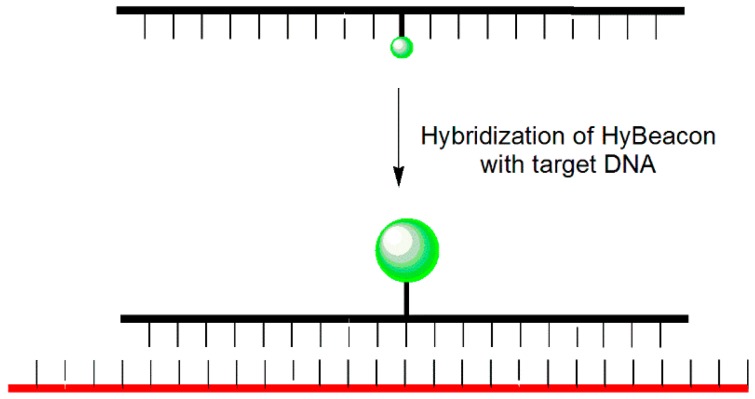
HyBeacon probes are linear oligonucleotides containing a fluorophore-labeled uracil base at an internal position of the oligonucleotide (no quencher moiety) and a 3′-phosphate or octanediol to prevent PCR extension (Figure 9) [43]. The fluorescent dyes 6-carboxyfluorescein, tetrachloro-6-carboxyfluorescein, and hexachloro-6-carboxyfluorescein can be attached to the 5-position uracil base through novel linkage chemistries. Hybridization of HyBeacons with complementary target DNA increases the fluorescence intensity. HyBeacon probes can be integrated into real-time PCR analysis to detect the presence and monitor the accumulation of specific DNA targets. In addition, sequence differences as small as a single nucleotide can be detected and differentiated by measuring the melting temperature.
Figure 9.
HyBeacon probe.
5.2. Probes Containing a Nucleobase-Labeled Fluorophore with an Acetylene Group
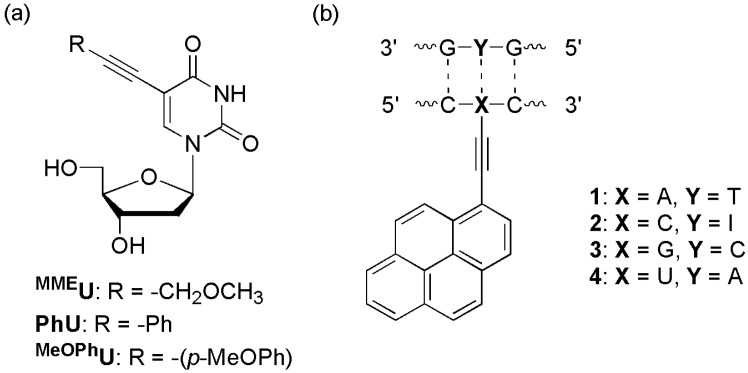
The Hudson group synthesized structurally simple 5-phenylethynyl derivatives of uracil, MMEU, PhU, and MeOPhU, which are intrinsically fluorescent, and incorporated them into the X (5′-CGCAATXTAACGC-3′) (Figure 10a) [44]. These ODNs exhibited 6-, 2-, and 6-fold increases in fluorescence intensity in the presence of complementary DNA, respectively. Although there was no significant reduction of fluorescence in the presence of mismatched DNAs, these fluorescent nucleosides were responsive to their local structure/environment and therefore have potential use in SNP probes.
Figure 10.
(a) 5-Phenylethynyluridine and (b) pyrenylethynyl derivatives.
The Wagenknecht group designed 1-ethynylpyrene-labeled pyrimidines and purines (Figure 10b) [45]. A strong new absorption band appeared at ~420 nm (1 and 3) or ~400 nm (2 and 4) following DNA-duplex formation. This absorption band may originate from the ground-state interaction of the 1-ethynylpyrene moiety with the adjacent base pairs (C/G). When a complementary DNA hybridized, the fluorescence intensity increased up to 40-fold relative to ssODN (in case of 3). This difference in fluorescence intensity was highest when the duplex was excited at ~420 nm (1 and 3) and ~400 nm (2 and 4).
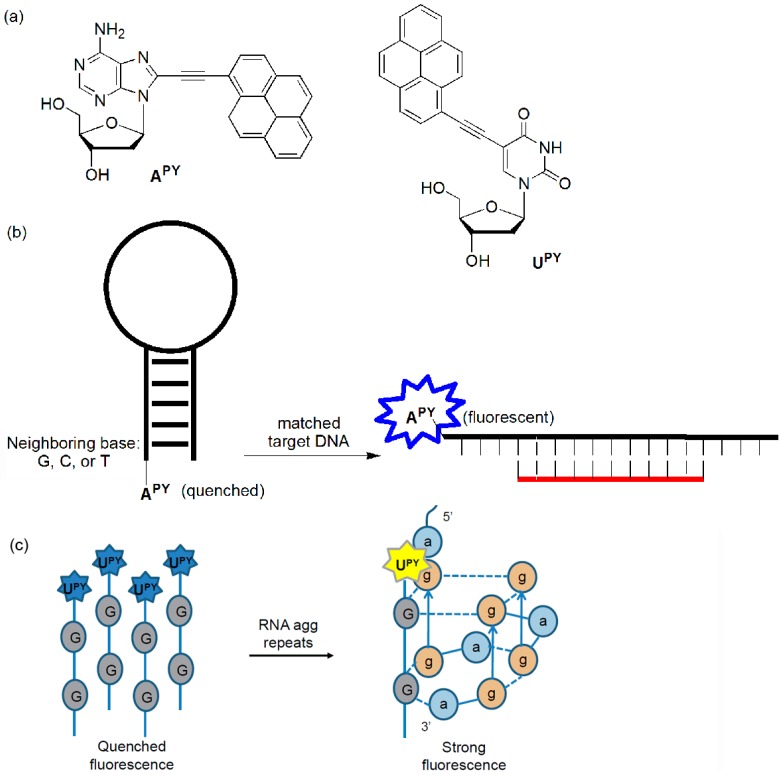
The Kim group incorporated pyrene-labeled deoxyadenosine (APY) into the 5′-end of hairpins and examined the quenching effect of the neighboring bases (Figure 11). The fluorescence could be quenched through photoinduced electron transfer (PET) from the fluorophore to neighboring C, T, and G bases, but not to the A moiety. Their quenched emissions were recovered when they met the matched targets [46]. They also developed a sensitive system for detecting AGG trinucleotide repeats through the formation of intermolecular G-quadruplexes in the presence of added K+ ions using a ssODN (5′-UPYGGTT-3′) (Figure 11c) [47]. When this probe interacted with the RNA target sequence (5′-aggaggagga-3′), very strong fluorescence enhancements were observed (44.7-fold increase in fluorescence). In the presence of the DNA target sequence (5′-AGGAGGAGGA-3′), a large increase in fluorescence was also observed (35.0-fold).
Figure 11.
(a) APY and UPY, (b) APy-labeled probe, and (c) probe for agg trinucleotide repeats.
The Hrdlicka group found that introduction of LNA nucleotides as direct neighbors into UPY improved the discrimination of SNPs [48]. They synthesized a ssODN, 5′-GTGNUPyN TGC-3′ (N = DNA nucleotide, A or LNA nucleotide, a). Hybridization of LNA-free ssODN with complementary DNA and RNA resulted in approximately 1.3- and 2.7-fold increases in emission relative to ssODN at 460 nm, respectively. Higher relative increases were observed when LNA nucleotides were incorporated as direct neighbors (approximately 2.0- and 4.5-fold increases for a duplex with complementary DNA and RNA, respectively). In contrast, mismatched duplexes were consistently less emissive than matched duplexes when using ssODN bearing the flanking LNA nucleotides. This strongly suggests that flanking LNA nucleotides can be used to produce probes with greater diagnostic potential. However, APY was also investigated in the same way, but its ability as a SNP probe was very limited.
We have developed quencher-free MB probes that incorporate a 2-ethynylfluorene derivative covalently attached to a 2′-deoxyuridine residue (UF) (Figure 12). Although fluorene (FL) derivatives provide a high fluorescence yield, they do not significantly affect the stability of the DNA duplexes because they have small volumes [49,50,51,52,53,54,55]. When an FL derivative was introduced at the C-5 position of deoxyuridine, the DNA containing it had little effect on the stability of the double-stranded DNA (dsDNA) formed upon hybridization with the complementary DNA [56,57,58,59]. In addition, an acetylene bridge provided strong electronic bonding between the uridine moiety and the FL derivative.
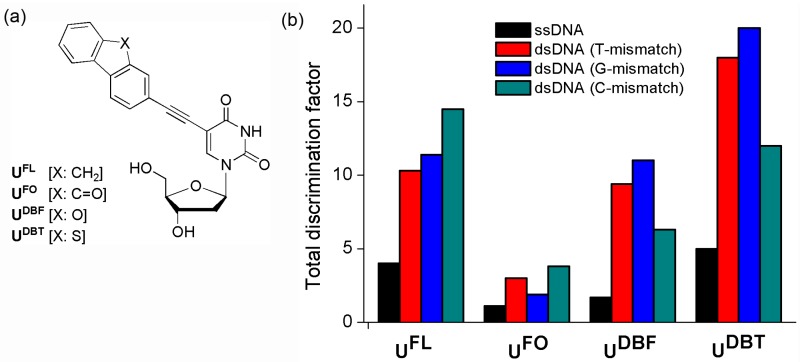
Figure 12.
(a) 2′-Deoxyuridines labeled with FL derivatives and (b) Total discrimination factors for the OND4(UF) probes relative to those of ssDNAs and mismatched dsDNAs.
Deoxyuridine derivatives were labeled with FL units [60,61,62] as well as several FL derivatives: 2-ethynyl-9H-fluoren-9-one (FO) [63], dibenzofuran (DBF) [64], and dibenzothiophene (DBT) [64]. FL, FO, DBF, and DBT have dramatically different photophysical properties because different atoms connect the two aromatic rings. To examine the effect of the flanking bases (FBs) on the emission properties, we modified such ODNs mainly at the bases flanking the UF units. These ODNs were 15mers containing a UF residue at the central position (Table 1).
Table 1.
Quencher-free linear beacons.
| ODN 1 | Sequence 2 | ODN 1 | Sequence 2 |
|---|---|---|---|
| ODN1(UF) | 5′-d(TGGACTAUFATCAATG)-3′ | ODN1′(N) | 3′-d(ACCTGATNTAGTTAC)-3′ |
| ODN2(UF) | 5′-d(TGGACTTUFTTCAATG)-3′ | ODN2′(N) | 3′-d(ACCTGAANAAGTTAC)-3′ |
| ODN3(UF) | 5′-d(TGGACTGUFGTCAATG)-3′ | ODN3′(N) | 3′-d(ACCTGACNCAGTTAC)-3′ |
| ODN4(UF) | 5′-d(TGGACTCUFCTCAATG)-3′ | ODN4′(N) | 3′-d(ACCTGAGNGAGTTAC)-3′ |
1 UF is UFL, UFO, UDBF, or UDBT. 2 Underlined bases are the FBs of the UF units.
In particular, ODN4(UFL) bearing C-FBs produced more efficient fluorescence ON/OFF systems than did other ODNs having other combinations of FBs [62]. The intensities of the fluorescence of the matched dsDNA formed between ODN4(UFL) and ODN4′(A) were increased 4.0-, 10.3-, 11.4-, and 14.5-fold over those of ODN4(UFL) and the mismatched dsDNAs with T-, G-, and C-mismatched targets, respectively. However, hybridization of the ODN4(UFO) bearing C-FBs with the matched target ODN4′(A) resulted in only a 1.1-fold increase in emission intensity relative to that of ssODN4(UFO); its total discrimination factors for the recognition of A/T, A/G, and A/C single-base mismatches were 3.0, 1.9, and 3.8, respectively [63]. The DBF- and DBT-labeled deoxyuridines UDBF and UDBT, respectively, were also introduced at the central positions of ODNs [64]. “Turn-on” responses to the matched targets were observed when the UDBF and UDBT units of ODNs bearing pyrimidine-FBs were positioned opposite to the four natural nucleobases. The total discrimination factors of the ODN2(UDBF) probe bearing T-FBs for recognition of A/T, A/G, and A/C single-base mismatches were 3.2, 7.0, and 2.8, respectively; those of the ODN2(UDBT) probe were 2.6, 4.8, and 2.3, respectively. Interestingly, the fluorescence emissions of ODN4(UDBF) and ODN4(UDBT) bearing C-FBs decreased greatly upon hybridization with all the mismatched targets, leading to total discrimination factors of 9.4 (T), 6.3 (C), and 11 (G) for the ODN4(UDBF) probe and 18 (T), 12 (C), and 20 (G) for the ODN4(UDBT) probe. In comparison, the fluorescence intensity ratios of matched dsDNAs bearing C-FBs to ssDNAs were 4.0 for FL, 1.1 for FO, 1.7 for DBF, and 5.0 for DBT (Figure 12b). Therefore, ODN4(UDBT) bearing C-FBs represent a very efficient fluorescent “turn-on” system and are more sensitive than any other quencher-free MB system.
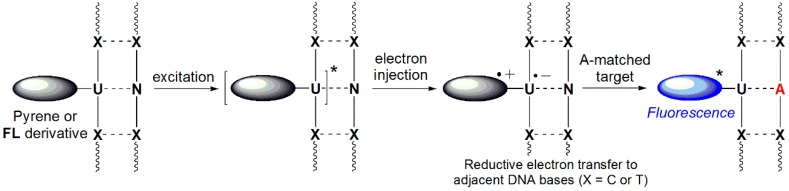
Based on data from established studies on the reducibility of nucleobases, excitation of ODNs containing 2′-deoxyuridine units labeled with pyrene or fluorene derivatives results in electron injection into DNA, producing pyrenyl- or fluorenyl-radical cations and uracyl radical anions. Photoexcited pyrene and FL derivatives are capable of reducing only their adjacent pyrimidine bases (C or T), resulting in substantial quenching of emissions through base-to-base electron transfer [65,66,67,68,69,70]. Therefore, efficient quenching of ssDNAs containing a pyrene or FL derivative can be obtained by placing C or T nucleobases adjacent to the pyrene or FL derivative. This quenching is, however, inhibited when encountering a matched target, resulting in the intrinsic emission intensity of the pyrene or FL derivative (Figure 13). In addition, ODN4(UF) bearing C-FBs exhibited significantly decreased fluorescence emission when hybridized with all mismatched targets relative to that of the ssODN4(UF). This dramatic quenching may have resulted from the close proximity of the FL derivative to the two guanine units that were the complementary bases of the C-FBs and served as internal quenchers [12,13,14]. That is, ODN4(UF) bearing C-FBs could effectively discriminate mismatched targets by decreasing the fluorescence intensity as a result of the guanine bases acting as internal quenchers as well as photoinduced charge transfer to the C-FBs.
Figure 13.
Working mechanism of quencher-free linear beacon systems.
5.3. BDF Probes
The Saito group devised a novel strategy using pyrene-labeled base-discriminating fluorescent (BDF) oligonucleotides as probes for the discrimination of single-base alterations. The concept is based on the fluorescence change of the BDF base, which can be used to clearly distinguish the type of base in the opposite strand.
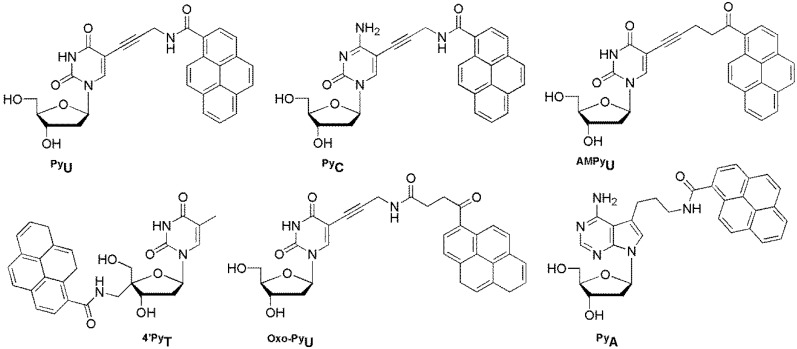
PyU and PyC showed unique fluorescence properties depending on the nature of the base of the complementary strand and showed a large increase in fluorescence by distinguishing between A and G opposite the BDF base, respectively (Figure 14) [71,72]. Other pyrene-labeled pyrimidines, AMPyU, 4′PyT, and Oxo-PyU, can also be used to efficiently discriminate A in a target DNA opposite the BDF base by displaying enhanced emission. Such clear fluorescence changes are very useful for SNP genotyping [73,74,75]. However, pyrene-labeled 7-deaza-2′-deoxyadenosine PyA exhibited increased emission when the bases opposite PyA were mismatched bases, and fluorescence intensity was completely quenched when a matched T was encountered [76].
Figure 14.
BDF probes.
6. Microenvironment-Sensitive Probes
Fluorescent nucleosides have been employed as microenvironment-sensitive probes, utilizing their high sensitivity to changes in polarity, viscosity, and surrounding pH. DNA or RNA containing this nucleoside has been studied for the selective detection of target DNA/RNA, abasic sites, and so on.
6.1. Probes Containing a Heterocycle-Conjugated Pyrimidine
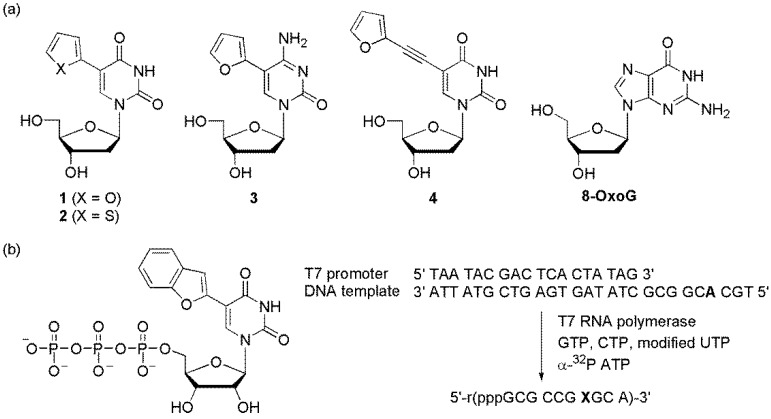
The Tor group designed furan-modified dU (1), thiophene-modified dU (2), furan-modified dC (3), and an extended ethynylfuran dU (4) as viscosity-sensitive fluorescent molecular probes, also referred to as molecular rotors (Figure 15a) [77,78]. Viscous media impeded the free rotation of these heterocycles to provide a structurally robust environment. This resulted in an increase in fluorescence intensity while reducing the contribution of the nonradiative decay pathway. Particularly, when the ODN containing furan-modified dU (1) was hybridized and the abasic site was located on the opposite side of 1, a significant emission enhancement was observed compared to that of the matched duplex. Although the exact structure around the abasic site is not clear, it is believed that the intrahelical vacant but confined space between the neighboring base pairs effectively limited the free rotation of the furan-uracil single bond, resulting in an increase in fluorescence. The thiophene-modified dU (2) was demonstrated to be an efficient probe for the detection of G, 8-oxoG, and its transverse mutation product T by providing significantly different emission intensities [79].
Figure 15.
(a) Furan- or thiophene-modified pyrimidines and (b) incorporation of ribonucleoside triphosphate.
The Srivatsan group synthesized benzofuran-labeled uridine and incorporated it into oligoribonucleotides using T7 RNA polymerase to produce fluorescent oligoribonucleotide constructs (Figure 15b) [80]. Abasic site-containing duplexes were constructed by hybridizing an RNA transcript to custom DNA and RNA oligonucleotides that contained an abasic-site surrogate. The abasic site-containing duplex (RNA/DNA) showed nearly a 4-fold higher emission than that of the perfect duplex. However, an RNA/RNA duplex that possessed an abasic site opposite the modified uracil showed slightly increased emission compared to that of the matched RNA-RNA duplex. In addition, they designed benzofuran-labeled 2′-deoxyuridine and demonstrated that it can discriminate an abasic site in a model depurinated sarcin/ricin RNA motif of a eukaryotic 28S rRNA [81].
6.2. ESF Probes
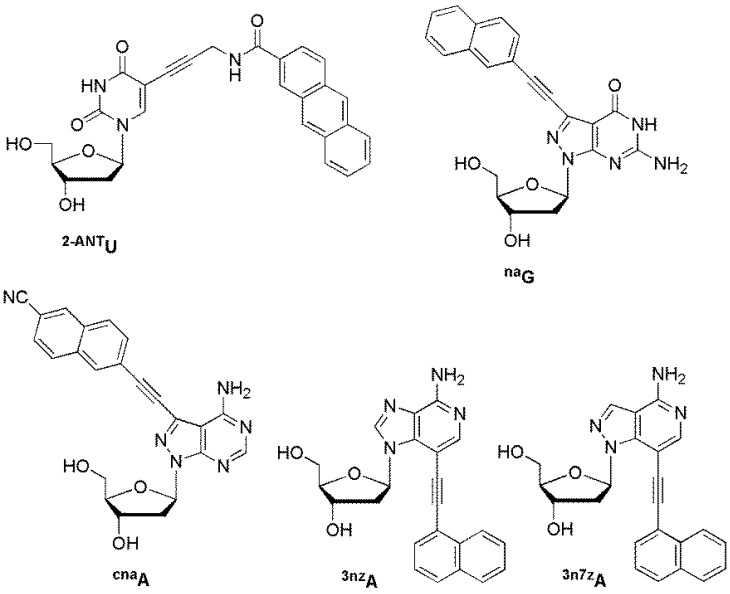
The Saito group developed DNA probes containing an environmentally sensitive fluorescent (ESF) nucleoside. These probes could sense a target DNA through significant changes in fluorescence wavelength and intensity. A strong emission band appeared in polar environments. Both the emission intensity and emission wavelength of 2-anthracenecarboxamide-labeled 2′-deoxyuridine 2-ANTU were significantly affected by solvent polarity (Figure 16) [82]. When a probe containing 2-ANTU was hybridized with DNA targets, strong fluorescence was observed only for its perfectly matched target. C7-naphthylethynylated 8-aza-7-deaza-2′-deoxyguanosine naG exhibited very weak fluorescence and emitted at a longer wavelength of 418 nm in polar solvents such as methanol [83]. Interestingly, the emission maximum was red-shifted by 29 nm to 409 nm compared to that of other mismatched targets only when naG hybridized with a matched target DNA. The fluorescence changes caused by the differences in the opposite bases of target DNAs can be attributed to changes in the local environment around the ESF bases 2-ANTU and naG.
Figure 16.
ESF probes.
cnaA, 3nzA, and 3n7zA displayed significant environmentally sensitive fluorescence properties, which originate from the coplanar and non-coplanar conformers of the dye moiety and nucleobase. ODN probes containing cnaA, 3nzA, and 3n7zA were used to sense a matched sequence and an abasic site in the target DNA through significant changes in emission wavelength and intensity [84,85,86].
6.3. pH-Sensitive Probes
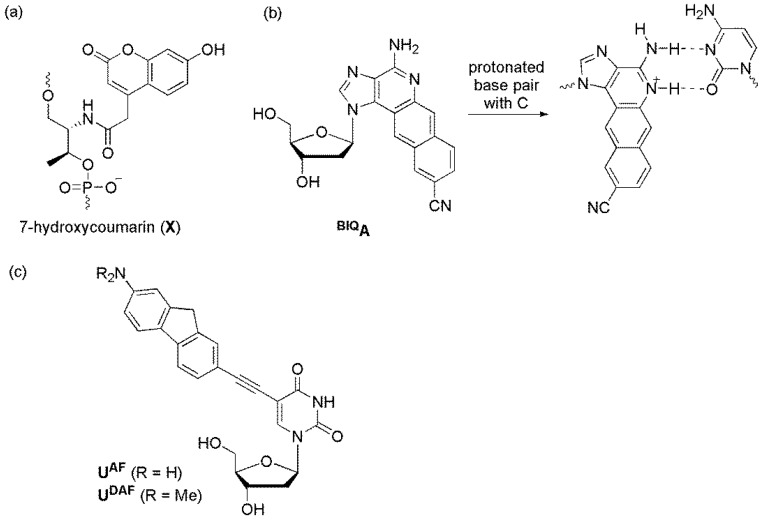
The Asanuma group designed a quencher-free MB by introducing 7-hydroxycoumarin (X) into the stem region (Figure 17a) [87]. X showed quenched fluorescence upon protonation. The pKa of X in a single strand was 8.8. However, it exceeded 10 in the DNA duplex because of the anionic and hydrophobic microenvironment inside the duplex. Without the target at pH 8, the stem region formed double strands, and the fluorescence was quenched. However, when the target was added, the MB opened, and the dye was deprotonated, resulting in the fluorescence of the formed duplex being 10-fold higher than that observed for MB itself.
Figure 17.
pH-sensitive probes. (a) 7-hydroxycoumarin; (b) BIQA; and (c) UAF and UDAF.
The Saito group reported BIQA, which exhibited large changes in absorption and fluorescence spectra upon the protonation/deprotonation of the N5 positions of BIQA. SNP identification of a C/T (mutant/wild type) sequence of the breast cancer type 1 gene was examined using a DNA probe containing BIQA at pH 7.5 (Figure 17b) [88]. When the opposite base of BIQA was thymine, an intense emission band was observed. In contrast, when the opposite base was cytosine, fluorescence was significantly reduced as protonation occurred.
We synthesized 2′-deoxyuridine derivatives UAF and UDAF labeled with 2-aminofluorene and 2-dimethylaminofluorene, respectively (Figure 17c) [89]. The pKa values of UAF and UDAF were 4.27 and 4.66, respectively, and their fluorescence increased under acidic conditions. Thus, ODNs containing UAF and UDAF exhibited distinct pH-sensitive emission behaviors upon hybridization with matched and mismatched targets [62]. ODNs bearing UAF and UDAF as a fluorescent nucleotide and C- or T-FBs, especially ODN4 bearing UDAF and C-FBs, clearly discriminated their A-matched targets, with increased emissions, under slightly acidic conditions (pH 6.5 and 6.0). However, ODN4 bearing UFL and C-FBs allowed for efficient SNP typing, regardless of the tested pH value (pH 5.5–8.0).
Table 2 summarizes the characteristics and the fluorophore used for the single-labeled probes discussed above.
Table 2.
Single-labeled oligonucleotide probes.
| No. | Probes | Notable Features | Fluorophores Used | Ref. |
|---|---|---|---|---|
| 1 | Guanine-quenching probes | Utilization the quenching effect of adjacent guanosine. | Fluorescein, BODIPY, 6-carboxyfluorescein, and tetramethyl-6-carboxyrhodamine | [15,16,17,18] |
| 2 | Cyanine-containing probes | Interaction of cyanine derivatives with nucleobases leads to the enhancement of fluorescence | Thiazole orange (TO), oxazole yellow (YO), thiazolopyridine (MO), and oxazolopyridine (JO) | [19,20,21,22,23,24,25] |
| 3 | Probes containing a fluorescent nucleobase analog | Utilization of fluorescent base analogs that are structurally similar to native nucleobases, capable of pairing with Watson-Crick pairs, and applicable as SNP probes | Flavin (Fl), deazaflavin (dFl), 3-methyl isoxanthopterin (3-MI), etc. | [27,28,29,30,31,32,33,34,35,36,37,38,39] |
| 4 | HyBeacon | HyBeacon probes can be integrated into real-time PCR analysis to detect specific DNA targets | 6-Carboxyfluorescein, tetrachloro-6-carboxyfluorescein, and hexachloro-6-carboxyfluorescein | [43] |
| 5 | Nucleobase-labeled fluorophore with an acetylene group | Probes for SNP detection, trinucleotide repeats, etc. have been developed | Pyrene and fluorene derivatives | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] |
| 6 | BDF probes | Clearly distinguish the type of base on the opposite strand of the BDF base | Pyrene | [71,72,73,74,75,76] |
| 7 | Probes containing a heterocycle-conjugated pyrimidine | Efficient probes for an abasic site, 8-oxoG, etc. | Furan-, thiophene-, or benzofuran-modified pyrimidines | [77,78,79,80,81] |
| 8 | ESF probes | DNA probes containing an environmentally sensitive fluorescent nucleoside | Pyrene and naphthalene derivatives | [82,83,84,85,86] |
| 9 | pH-sensitive probes | DNA probes exhibit pH-sensitive emission behaviors | 7-hydroxycoumarin, benzo[g]imidazo[4,5-c]quinoline (BIQA), and 2′-deoxyuridine labeled with 2-dimethylaminofluorene (UDAF) | [62,87,88,89] |
7. Conclusions
Recently, single nucleotide polymorphism (SNP) detection techniques have evolved to become automated, efficient, and relatively inexpensive. In particular, DNA probes labeled with a single fluorophore are attractive because they are simple and inexpensive to fabricate. The following systems have been developed for this purpose: (1) A single fluorophore is connected to the ODN, and a change in fluorescence following duplex formation with the target nucleic acid is caused by the quenching effect of the guanine base adjacent to the fluorophore; (2) Cyanines, such as thiazole orange and oxazole yellow, have a property in which their fluorescence increases when intercalated into a nucleic acid duplex. This property of a cyanine dye can be used to selectively recognize matched and mismatched targets; (3) Fluorescent nucleobase analogs with structures similar to those of native nucleobases and capable of Watson-Crick base pairing have been developed and used for SNP typing; (4) Probes bearing a fluorescently labeled base exhibit dramatic emission changes after detecting a specific sequence; (5) Fluorescent nucleosides that are sensitive to polarity, viscosity, and surrounding pH have been designed and applied to SNP typing. All of these single-labeled nucleosides can be inserted at any position of the DNA according to the sequence to be designed. In addition, the biomolecules or nanoparticles can be attached to the 5′- or 3′-end of these oligonucleotide probes, increasing their applicability. Finally, no quencher is required and only a single fluorophore is included, making it possible to build simple and inexpensive probe systems. Therefore, this type of probe system has the potential to become a powerful tool for the detection of single nucleotide variations.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03034920).
Supplementary Materials
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Vignal A., Milan D., SanCristobal M., Eggen A. A review on SNP and other types of molecular markers and their use in animal genetics. Genet. Sel. Evol. 2002;34:275–305. doi: 10.1186/1297-9686-34-3-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobrino B., Brion M., Carracedo A. SNPs in forensic genetics: A review on SNP typing methodologies. Forensic Sci. Int. 2005;154:181–194. doi: 10.1016/j.forsciint.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Venkatesan N., Seo Y.J., Kim B.H. Quencher-free molecular beacons: A new strategy in fluorescence based nucleic acid analysis. Chem. Soc. Rev. 2008;37:648–663. doi: 10.1039/b705468h. [DOI] [PubMed] [Google Scholar]
- 4.Krasheninina O.A., Novopashina D.S., Apartsin E.K., Venyaminova A.G. Recent advances in nucleic acid targeting probes and supramolecular constructs based on pyrene-modified oligonucleotides. Molecules. 2017;22:2108. doi: 10.3390/molecules22122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang X., Mi Y., Li J.J., Beck T., Schuster S., Tan W. Molecular beacons: Fluorogenic probes for living cell study. Cell Biochem. Biophys. 2002;37:71–81. doi: 10.1385/CBB:37:2:071. [DOI] [PubMed] [Google Scholar]
- 6.Tan L., Li Y., Drake T.J., Moroz L., Wang K., Li J., Munteanu A., Chaoyong J.Y., Martinez K., Tan W. Molecular beacons for bioanalytical applications. Analyst. 2005;130:1002–1005. doi: 10.1039/b500308n. [DOI] [PubMed] [Google Scholar]
- 7.Huang K., Marti A.A. Recent trends in molecular beacon design and applications. Anal. Bioanal. Chem. 2012;402:3091–3102. doi: 10.1007/s00216-011-5570-6. [DOI] [PubMed] [Google Scholar]
- 8.Kuang T., Chang L., Peng X., Hu X., Gallego-Perez D. Molecular beacon nano-sensors for probing living cancer cells. Trends Biotechnol. 2017;35:347–359. doi: 10.1016/j.tibtech.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi S., Kramer F.R. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 10.Piatek A.S., Tyagi S., Pol A.C., Telenti A., Miller L.P., Kramer F.R., Alland D. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat. Biotechnol. 1998;16:359–363. doi: 10.1038/nbt0498-359. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi S., Bratu D.P., Kramer F.R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 12.Seidel C.A.M., Schulz A., Sauer M.H.M. Nucleobase-specific quenching of fluorescent dyes. 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies. J. Phys. Chem. 1996;100:5541–5553. doi: 10.1021/jp951507c. [DOI] [Google Scholar]
- 13.Luo G.H., Zheng L., Zhang X.Y., Zhang J., Nilsson-Ehle P., Xu N. Genotyping of single nucleotide polymorphisms using base-quenched probe: A method does not invariably depend on the deoxyguanosine nucleotide. Anal. Biochem. 2009;386:161–166. doi: 10.1016/j.ab.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.A., Hwang G.T. A linear beacon system featuring an internal deoxyguanine quencher allows highly selective detection of single base mismatches. Bull. Korean Chem. Soc. 2010;31:2011–2014. doi: 10.5012/bkcs.2010.31.7.2011. [DOI] [Google Scholar]
- 15.Crockett A.O., Wittwer C.T. Fluorescein-labeled oligonucleotides for real-time PCR: Using the inherent quenching of deoxyguanosine nucleotides. Anal. Biochem. 2001;290:89–97. doi: 10.1006/abio.2000.4957. [DOI] [PubMed] [Google Scholar]
- 16.Kurata S., Kanagawa T., Yamada K., Torimura M., Yokomaku T., Kamagata Y., Kurane R. Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY® FL-labeled probe or primer. Nucleic Acids Res. 2001;29:E34. doi: 10.1093/nar/29.6.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang D.S., Zhai K., Wang L.Z. Multiplexed DNA detection with a composite molecular beacon based on guanine-quenching. Analyst. 2013;138:5318–5324. doi: 10.1039/c3an01060k. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn C.P., Elenitoba-Johnson K.S. Hybridization-induced dequenching of fluorescein-labeled oligonucleotides: A novel strategy for PCR detection and genotyping. Am. J. Pathol. 2003;163:29–35. doi: 10.1016/S0002-9440(10)63627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiguro T., Saitoh J., Yawata H., Otsuka M., Inoue T., Sugiura Y. Fluorescence detection of specific sequence of nucleic acids by oxazole yellow-linked oligonucleotides. Homogeneous quantitative monitoring of in vitro transcription. Nucleic Acids Res. 1996;24:4992–4997. doi: 10.1093/nar/24.24.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asseline U., Chassignol M., Aubert Y., Roig V. Detection of terminal mismatches on DNA duplexes with fluorescent oligonucleotides. Org. Biomol. Chem. 2006;4:1949–1957. doi: 10.1039/b602262f. [DOI] [PubMed] [Google Scholar]
- 21.Köhler O., Seitz O. Thiazole orange as fluorescent universal base in peptide nucleic acids. Chem. Commun. 2003:2938–2939. doi: 10.1039/B308299G. [DOI] [PubMed] [Google Scholar]
- 22.Köhler O., Venkatrao D., Jarikote D.V., Seitz O. Forced intercalation probes (FIT probes): Thiazole orange as a fluorescent base in peptide nucleic acids for homogeneous single-nucleotide-polymorphism detection. ChemBioChem. 2005;6:69–77. doi: 10.1002/cbic.200400260. [DOI] [PubMed] [Google Scholar]
- 23.Jarikote D.V., Krebs N., Tannert S., Röder B., Seitz O. Exploring base-pair-specific optical properties of the DNA stain thiazole orange. Chem. Eur. J. 2007;13:300–310. doi: 10.1002/chem.200600699. [DOI] [PubMed] [Google Scholar]
- 24.Bethge L., Jarikote D.V., Seitz O. New cyanine dyes as base surrogates in PNA: Forced intercalation probes (FIT-probes) for homogeneous SNP detection. Bioorg. Med. Chem. 2008;16:114–125. doi: 10.1016/j.bmc.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 25.Bethge L., Singh I., Seitz O. Designed thiazole orange nucleotides for the synthesis of single labelled oligonucleotides that fluoresce upon matched hybridization. Org. Biomol. Chem. 2010;8:2439–2448. doi: 10.1039/c000697a. [DOI] [PubMed] [Google Scholar]
- 26.Jean J.M., Hall K.B. 2-aminopurine fluorescence quenching and lifetimes: Role of base stacking. Proc. Natl. Acad. Sci. USA. 2001;98:37–41. doi: 10.1073/pnas.98.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dueymes C., Décout J.L., Peltié P., Fontecave M. Fluorescent deazaflavin-oligonucleotide probes for selective detection of DNA. Angew. Chem. Int. Ed. 2002;41:486–489. doi: 10.1002/1521-3773(20020201)41:3<486::AID-ANIE486>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins M.E., Balis F.M. Use of pteridine nucleoside analogs as hybridization probes. Nucleic Acids Res. 2004;32:E62. doi: 10.1093/nar/gnh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto A., Tainaka K., Saito I. Clear distinction of purine bases on the complementary strand by a fluorescence change of a novel fluorescent nucleoside. J. Am. Chem. Soc. 2003;125:4972–4973. doi: 10.1021/ja034090u. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto A., Tainaka K., Saito I. Detection of A/G single nucleotide alteration in RNA using base-discriminating fluorescent oligodeoxynucleotides. Chem. Lett. 2003;32:684–685. doi: 10.1246/cl.2003.684. [DOI] [Google Scholar]
- 31.Okamoto A., Tainaka K., Saito I. Synthesis and properties of a novel fluorescent nucleobase, naphthopyridopyrimidine. Tetrahedron Lett. 2003;44:6871–6874. doi: 10.1016/S0040-4039(03)01740-4. [DOI] [Google Scholar]
- 32.Okamoto A., Tanaka K., Fukuta T., Saito I. Design of base-discriminating fluorescent nucleoside and its application to T/C SNP typing. J. Am. Chem. Soc. 2003;125:9296–9297. doi: 10.1021/ja035408l. [DOI] [PubMed] [Google Scholar]
- 33.Miyata K., Tamamushi R., Ohkubo A., Taguchi H., Seio K., Santa T., Sekine M. Synthesis and properties of a new fluorescent bicyclic 4-N-carbamoyldeoxycytidine derivative. Org. Lett. 2006;8:1545–1548. doi: 10.1021/ol053125n. [DOI] [PubMed] [Google Scholar]
- 34.Wojciechowski F., Hudson R.H.E. Fluorescence and hybridization properties of peptide nucleic acid containing a substituted phenylpyrrolocytosine designed to engage guanine with an additional H-bond. J. Am. Chem. Soc. 2008;130:12574–12575. doi: 10.1021/ja804233g. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Maxson T., Tor Y. Fluorescent nucleoside analogue displays enhanced emission upon pairing with guanine. Org. Biomol. Chem. 2010;8:5053–5055. doi: 10.1039/c0ob00413h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa K., Hattori M., Ohki T., Kitamura Y., Kitade Y., Ueno Y. Nucleic acid probe containing fluorescent tricyclic base-linked acyclonucleoside for detection of single nucleotide polymorphisms. Bioorg. Med. Chem. 2012;20:16–24. doi: 10.1016/j.bmc.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Hattori M., Ohki T., Yanase E., Ueno Y. Fluorescence detection of single nucleotide polymorphisms using nucleic acid probe containing tricyclic base-linked acyclonucleoside. Bioorg. Med. Chem. Lett. 2012;22:253–257. doi: 10.1016/j.bmcl.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Burns D.D., Teppang K.L., Lee R.W., Lokensgard M.E., Purse B.W. Fluorescence turn-on sensing of DNA duplex formation by a tricyclic cytidine analogue. J. Am. Chem. Soc. 2017;139:1372–1375. doi: 10.1021/jacs.6b10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cekan P., Sigurdsson S.T. Single base interrogation by a fluorescent nucleotide: Each of the four DNA bases identified by fluorescence spectroscopy. Chem. Commun. 2008:3393–3395. doi: 10.1039/b801833b. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelmsson L.M., Holmén A., Lincoln P., Nielson P.E., Norden B. A highly fluorescent DNA base analogue that forms Watson-Crick base pairs with guanine. J. Am. Chem. Soc. 2001;123:2434–2435. doi: 10.1021/ja0025797. [DOI] [PubMed] [Google Scholar]
- 41.Bielecka P., Juskowiak B. Fluorescent sensor for pH monitoring based on an i-motif–switching aptamer containing a tricyclic cytosine analogue (tC) Molecules. 2015;20:18511–18525. doi: 10.3390/molecules201018511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardarsson H., Kale A.S., Sigurdsson S.T. Structure-function relationships of phenoxazine nucleosides for identification of mismatches in duplex DNA by fluorescence spectroscopy. ChemBioChem. 2011;12:567–575. doi: 10.1002/cbic.201000478. [DOI] [PubMed] [Google Scholar]
- 43.French D.J., Archard C.L., Brown T., McDowell D.G. HyBeacon™ probes: A new tool for DNA sequence detection and allele discrimination. Mol. Cell. Probes. 2001;15:363–374. doi: 10.1006/mcpr.2001.0384. [DOI] [PubMed] [Google Scholar]
- 44.Hudson R.H.E., Ghorbani-Choghamarani A. Oligodeoxynucleotides incorporating structurally simple 5-alkynyl-2′-deoxyuridines fluorometrically respond to hybridization. Org. Biomol. Chem. 2007;5:1845–1848. doi: 10.1039/B705805E. [DOI] [PubMed] [Google Scholar]
- 45.Mayer E., Valis L., Wagner C., Rist M., Amann N., Wagenknecht H.-A. 1-Ethynylpyrene as a tunable and versatile molecular beacon for DNA. ChemBioChem. 2004;5:865–868. doi: 10.1002/cbic.200300845. [DOI] [PubMed] [Google Scholar]
- 46.Seo Y.J., Ryu J.H., Kim B.H. Quencher-free, end-stacking oligonucleotides for probing single-base mismatches in DNA. Org. Lett. 2005;7:4931–4933. doi: 10.1021/ol0518582. [DOI] [PubMed] [Google Scholar]
- 47.Park Y., Kim K.T., Kim B.H. G-quadruplex formation using fluorescent oligonucleotides as a detection method for discriminating AGG trinucleotide repeats. Chem. Commun. 2016;52:12757–12760. doi: 10.1039/C6CC06566J. [DOI] [PubMed] [Google Scholar]
- 48.Kaura M., Hrdlicka P.J. Locked nucleic acid (LNA) induced effect on the hybridization and fluorescence properties of oligodeoxyribonucleotides modified with nucleobase-functionalized DNA monomers. Org. Biomol. Chem. 2015;13:7236–7247. doi: 10.1039/C5OB00860C. [DOI] [PubMed] [Google Scholar]
- 49.Panozzo S., Stephan O., Vial J.C. Interplay between carrier dynamics and structure of poly(alkylthiophene) and poly(dialkylfluorene) films. J. Appl. Phys. 2003;94:1693–1698. doi: 10.1063/1.1591052. [DOI] [Google Scholar]
- 50.Jozefowicz M. Determination of reorganization energy of fluorenone and 4-hydroxyfluorenone in neat and binary solvent mixtures. Spectrochim. Acta Part A. 2007;67:444–449. doi: 10.1016/j.saa.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen D.D., Trunk J., Nakhimovsky L., Spanget-Larsen J. Electronic transitions of fluorene, dibenzofuran, carbazole, and dibenzothiophene from the onset of absorption to the ionization threshold. J. Mol. Spectrosc. 2010;264:19–25. doi: 10.1016/j.jms.2010.08.009. [DOI] [Google Scholar]
- 52.Cho H.Y., Woo S.K., Hwang G.T. Synthesis and photophysical study of 2′-deoxyuridines labeled with fluorene derivatives. Molecules. 2012;17:12061–12071. doi: 10.3390/molecules171012061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M.J., Seo Y., Hwang G.T. Synthesis and photophysical properties of 2′-deoxyguanosine derivatives labeled with fluorene and fluorenone units: Toward excimer probes. RSC Adv. 2014;4:12012–12017. doi: 10.1039/c3ra47383j. [DOI] [Google Scholar]
- 54.Dziuba D., Pospisil P., Matyasovsky J., Brynda J., Nachtigallova D., Rulisek L., Pohl R., Hof M., Hocek M. Solvatochromic fluorene-linked nucleoside and DNA as color-changing fluorescent probes for sensing interactions. Chem. Sci. 2016;7:5775–5785. doi: 10.1039/C6SC02548J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M.J., Hwang G.T. Fluorescent oligonucleotides containing a 2-ethynylfluorene- or 2-ethynylfluorenone-labeled 2′-deoxyguanosine unit: Fluorescence changes upon duplex formation. Bull. Korean Chem. Soc. 2016;37:1290–1297. doi: 10.1002/bkcs.10856. [DOI] [Google Scholar]
- 56.Hurley D.J., Tor Y. Ru(II) and Os(II) nucleosides and oligonucleotides: Synthesis and properties. J. Am. Chem. Soc. 2002;124:3749–3762. doi: 10.1021/ja0123103. [DOI] [PubMed] [Google Scholar]
- 57.Hwang G.T., Seo Y.J., Kim S.J., Kim B.H. Fluorescent oligonucleotide incorporating 5-(1-ethynylpyrenyl)-2′-deoxyuridine: Sequence-specific fluorescence changes upon duplex formation. Tetrahedron Lett. 2004;45:3543–3546. doi: 10.1016/j.tetlet.2004.03.071. [DOI] [Google Scholar]
- 58.Kottysch T., Ahlborn C., Brotzel F., Richert C. Stabilizing or destabilizing oligodeoxynucleotide duplexes containing single 2′-deoxyuridine residues with 5-alkynyl substituents. Chem. Eur. J. 2004;10:4017–4028. doi: 10.1002/chem.200306044. [DOI] [PubMed] [Google Scholar]
- 59.Seo Y., Hwang G.T. Quencher-free linear beacon systems with dual fluorene-labeled deoxyuridines. Bull. Korean Chem. Soc. 2011;32:4129–4132. doi: 10.5012/bkcs.2011.32.11.4129. [DOI] [Google Scholar]
- 60.Hwang G.T., Seo Y.J., Kim B.H. A highly discriminating quencher-free molecular beacon for probing DNA. J. Am. Chem. Soc. 2004;126:6528–6529. doi: 10.1021/ja049795q. [DOI] [PubMed] [Google Scholar]
- 61.Ryu J.H., Seo Y.J., Hwang G.T., Lee J.Y., Kim B.H. Triad base pairs containing fluorene unit for quencher-free SNP typing. Tetrahedron. 2007;63:3538–3547. doi: 10.1016/j.tet.2006.10.091. [DOI] [Google Scholar]
- 62.Lee J.W., Son Y.-S., Hwang J.Y., Park Y., Hwang G.T. pH-Responsive quencher-free molecular beacon systems containing 2′-deoxyuridine units labeled with fluorene derivatives. Org. Biomol. Chem. 2017;15:7165–7172. doi: 10.1039/C7OB01703K. [DOI] [PubMed] [Google Scholar]
- 63.Ryu J.H., Heo J.Y., Bang E.K., Hwang G.T., Kim B.H. Quencher-free linear beacon systems containing 2-ethynylfluorenone-labeled 2′-deoxyuridine units. Tetrahedron. 2012;68:72–78. doi: 10.1016/j.tet.2011.10.092. [DOI] [Google Scholar]
- 64.Lee J., Cho H.Y., Hwang G.T. Highly efficient quencher-free molecular beacon systems containing 2-ethynyldibenzofuran- and 2-ethynyldibenzothiophene-labeled 2′-deoxyuridine units. ChemBioChem. 2013;14:1353–1362. doi: 10.1002/cbic.201300240. [DOI] [PubMed] [Google Scholar]
- 65.Amann N., Pandurski E., Fiebig T., Wagenknecht H.A. Electron injection into DNA: Synthesis and spectroscopic properties of pyrenyl-modified oligonucleotides. Chem. Eur. J. 2002;8:4877–4883. doi: 10.1002/1521-3765(20021104)8:21<4877::AID-CHEM4877>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Daublain P., Thazhathveetil A.K., Wang Q., Trifonov A., Fiebig T., Lewis F.D. Dynamics of photochemical electron injection and efficiency of electron transport in DNA. J. Am. Chem. Soc. 2009;131:16790–16797. doi: 10.1021/ja905140n. [DOI] [Google Scholar]
- 67.Tainaka K., Fujitsuka M., Takada T., Kawai K., Majima T. Sequence dependence of excess electron transfer in DNA. J. Phys. Chem. B. 2010;114:14657–14663. doi: 10.1021/jp1024685. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka M., Elias B., Barton J.K. DNA-mediated electron transfer in naphthalene-modified oligonucleotides. J. Org. Chem. 2010;75:2423–2428. doi: 10.1021/jo1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaballah S.T., Vaught J.D., Eaton B.E., Netzel T.L. Charge-transfer excited state dynamics in DNA hairpins substituted with an ethylenylpyrenyl-du electron source and halo-du traps. J. Phys. Chem. B. 2005;109:5927–5934. doi: 10.1021/jp044968j. [DOI] [PubMed] [Google Scholar]
- 70.Gaballah S.T., Collier G., Netzel T.L. Charge transfer excited-state dynamics in DNA duplexes substituted with an ethynylpyrenyldeoxyuridine electron source and a fluorodeoxyuridine electron trap. J. Phys. Chem. B. 2005;109:12175–12181. doi: 10.1021/jp044457x. [DOI] [PubMed] [Google Scholar]
- 71.Okamoto A., Kanatani K., Saito I. Pyrene-labeled base-discriminating fluorescent DNA probes for homogeneous SNP typing. J. Am. Chem. Soc. 2004;126:4820–4827. doi: 10.1021/ja039625y. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto A., Tainaka K., Ochi Y., Kanatani K., Saito I. Simple SNP typing assay using a base-discriminating fluorescent probe. Mol. Biosyst. 2006;2:122–126. doi: 10.1039/b515923g. [DOI] [PubMed] [Google Scholar]
- 73.Saito Y., Miyauchi Y., Okamoto A., Saito I. Synthesis and properties of novel base-discriminating fluorescent (BDF) nucleosides: A highly polarity-sensitive fluorophore for SNP typing. Tetrahedron Lett. 2004;45:7827–7831. doi: 10.1016/j.tetlet.2004.09.003. [DOI] [Google Scholar]
- 74.Dohno C., Saito I. Discrimination of single-nucleotide alterations by G-specific fluorescence quenching. ChemBioChem. 2005;6:1075–1081. doi: 10.1002/cbic.200400325. [DOI] [PubMed] [Google Scholar]
- 75.Bag S.S., Kundu R., Matsumoto K., Saito Y., Saito I. Singly and doubly labeled base-discriminating fluorescent oligonucleotide probes containing oxo-pyrene chromophore. Bioorg. Med. Chem. Lett. 2010;20:3227–3230. doi: 10.1016/j.bmcl.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 76.Saito Y., Miyauchi Y., Okamoto A., Saito I. Base-discriminating fluorescent (BDF) nucleoside: Distinction of thymine by fluorescence quenching. Chem. Commun. 2004:1704–1705. doi: 10.1039/b405832a. [DOI] [PubMed] [Google Scholar]
- 77.Greco N.J., Tor Y. Simple fluorescent pyrimidine analogues detect the presence of DNA abasic sites. J. Am. Chem. Soc. 2005;127:10784–10785. doi: 10.1021/ja052000a. [DOI] [PubMed] [Google Scholar]
- 78.Sinkeldam R.W., Wheat A.J., Boyaci H., Tor Y. Emissive nucleosides as molecular rotors. ChemPhysChem. 2011;12:567–570. doi: 10.1002/cphc.201001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greco N.J., Sinkeldam R.W., Tor Y. An emissive C analog distinguishes between G, 8-oxog, and T. Org. Lett. 2009;11:1115–1118. doi: 10.1021/ol802656n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanpure A.A., Srivatsan S.G. A microenvironment-sensitive fluorescent pyrimidine ribonucleoside analogue: Synthesis, enzymatic incorporation, and fluorescence detection of a DNA abasic site. Chem. Eur. J. 2011;17:12820–12827. doi: 10.1002/chem.201101194. [DOI] [PubMed] [Google Scholar]
- 81.Tanpure A.A., Srivatsan S.G. Synthesis and photophysical characterisation of a fluorescent nucleoside analogue that signals the presence of an abasic site in RNA. ChemBioChem. 2012;13:2392–2399. doi: 10.1002/cbic.201200408. [DOI] [PubMed] [Google Scholar]
- 82.Saito Y., Motegi K., Bag S.S., Saito I. Anthracene based base-discriminating fluorescent oligonucleotide probes for SNPs typing: Synthesis and photophysical properties. Bioorg. Med. Chem. 2008;16:107–113. doi: 10.1016/j.bmc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 83.Saito Y., Suzuki A., Okada Y., Yamasaka Y., Nemoto N., Saito I. An environmentally sensitive fluorescent purine nucleoside that changes emission wavelength upon hybridization. Chem. Commun. 2013;49:5684–5686. doi: 10.1039/c3cc42605j. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki A., Nemoto N., Saito I., Saito Y. Design of an environmentally sensitive fluorescent 8-aza-7-deaza-2′-deoxyadenosine derivative with dual fluorescence for the specific detection of thymine. Org. Biomol. Chem. 2014;12:660–666. doi: 10.1039/C3OB41757C. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki A., Yanaba T., Saito I., Saito Y. Molecular design of an environmentally sensitive fluorescent nucleoside, 3-deaza-2′-deoxyadenosine derivative: Distinguishing thymine by probing the DNA minor groove. ChemBioChem. 2014;15:1637–1643. doi: 10.1002/cbic.201402078. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki A., Saito M., Katoh R., Saito Y. Synthesis of 8-aza-3,7-dideaza-2′-deoxyadenosines possessing a new adenosine skeleton as an environmentally sensitive fluorescent nucleoside for monitoring the DNA minor groove. Org. Biomol. Chem. 2015;13:7459–7468. doi: 10.1039/C5OB00862J. [DOI] [PubMed] [Google Scholar]
- 87.Kashida H., Yamaguchi K., Hara Y., Asanuma H. Quencher-free molecular beacon tethering 7-hydroxycoumarin detects targets through protonation/deprotonation. Bioorg. Med. Chem. 2012;20:4310–4315. doi: 10.1016/j.bmc.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 88.Siraiwa S., Suzuki A., Katoh R., Saito Y. Design and synthesis of a novel fluorescent benzo[g]imidazo[4,5-c]quinoline nucleoside for monitoring base-pair-induced protonation with cytosine: Distinguishing cytosine via changes in the intensity and wavelength of fluorescence. Org. Biomol. Chem. 2016;14:3934–3942. doi: 10.1039/C6OB00494F. [DOI] [PubMed] [Google Scholar]
- 89.Lee J.W., Son Y.S., Lee J.Y., Kim M.H., Woo S.K., Lee K.C., Lee Y.J., Hwang G.T. pH-Sensitive fluorescent deoxyuridines labeled with 2-aminofluorene derivatives. Tetrahedron. 2016;72:5595–5601. doi: 10.1016/j.tet.2016.07.049. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.