Abstract
Agarwood, a highly precious non-timber fragrant wood of Aquilaria spp. (Thymelaeaceae), has been widely used in traditional medicine, religious rites, and cultural activities. Due to the inflated demanding and depleted natural resources, the yields of agarwood collected from the wild are shrinking, and the price is constantly rising, which restricts agarwood scientific research and wide application. With the sustainable planting and management of agarwood applied, and especially the artificial-inducing methods being used in China and Southeast Asian countries, agarwood yields are increasing, and the price is becoming more reasonable. Under this condition, illuminating the scientific nature of traditional agarwood application and developing new products and drugs from agarwood have become vitally important. Recently, the phytochemical investigations have achieved fruitful results, and more than 300 compounds have been isolated, including numerous new compounds that might be the characteristic constituents with physiological action. However, no one has focused on the new compounds and presented a summary until now. Alongside phytochemical advances, bioactivity screening and pharmacological investigation have also made a certain progress. Therefore, this review discussed the new compounds isolated after 2010, and summarized the pharmacological progress on agarwood and Aquilaria plants.
Keywords: agarwood, Aquilaria plants, chemical constitutes, bioactive compounds, pharmacological function
1. Introduction
Agarwood, known as chenxiang in Chinese and called aloeswood, agalloch, eaglewood, jinkoh, gaharu, or kanankoh in different regions, is a highly valuable non-timber fragrant wood of Aquilaria spp. (Thymelaeaceae) [1,2,3,4]. There are 31 species of Aquilaria found worldwide in Indonesia, Malaysia, China, India, Philippines, Cambodia, Vietnam, Laos, Thailand, Papua New Guinea, and Singapore [5,6], among which 19 species can produce agarwood after being attacked by physical force [7,8], insects [9], or bacteria/fungi infection [10,11,12]. Agarwood is used for incense, perfume, traditional medicine, and other products in the world market. In traditional Chinese medicine, agarwood is used as a qi-regulating drug and carminative medicine to relieve gastric problems, coughs, rheumatism, and high fever. It can promote qi circulation to relieve pain, warm the middle energizer to arrest vomiting, and regulate respiration to relieve asthma [13]. In traditional Arabian medicine, agarwood essential oil is used for aromatherapy. Simultaneously, agarwood has also been widely used for centuries as incense in Buddhist, Hindu, and Islamic ceremonies.
With the increasing demand for agarwood, the population of Aquilaria species is declining rapidly in the wild, and all species of Aquilaria have been placed on the Appendix II list of the Convention on International Trade in Endangered Species of Wild Fauna and Flora since 2004 [1]. In response to this situation, sustainable planting and management of agarwood with artificial methods are arising, and the agarwood yield is increasing. As a result, agarwood no longer needs to be obtained from wild natural resources, enabling its wider application and investigation, especially on pharmaceutical study. Based on the phytochemical studies, a number of new compounds have been isolated and identified from agarwood and Aquilaria plants. However, there is no literature concentrating on the new compounds, even though earlier literature have summarized the chemical constituents of agarwood and related plants [6,14,15]. Therefore, this review discussed the new compounds isolated after 2010, and summarized the pharmacological progress on agarwood and Aquilaria plants.
2. Results and Discussion
2.1. New Compounds from Agarwood and Aquilaria Plants
The chemical constitutes of agarwood originating from the genus Aquilaria, include 2-(2-phenylethyl)-4H-chromen-4-one derivatives, terpenoids, flavonoids etc., in which 2-(2-phenylethyl)-4H-chromen-4-one derivatives and sesquiterpenes are the two predominant constituents in agarwood. There have been 154 new compounds (Table 1) isolated from agarwood and genus Aquilaria trees since 132 compounds were summarized in June 2010 [14].
Table 1.
Chemical constituents of agarwood originating from the genus Aquilaria.
| No. | Compound Class and Name | Source or Origin | Extraction * | Ref. |
|---|---|---|---|---|
| 2-(2-Phenylethyl)chromones | ||||
| 1 | 7-Hydroxy-6-methoxy-2-[2-(3′-hydroxy-4′-ethoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 2 | 6,7-Dimethoxy-2-[2-(4′-hydroxy-3′-methoxyphenyl) ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 3 | 6,7-Dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 4 | 6-Hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 5 | 6,8-Dihydroxy-2-[2-(3′-hydroxy4′-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 6 | 6-Hydroxy-2-[2-(4′-hydroxy-3′-methoxyphenyl)ethenyl]chromone | A. sinensis (China) | EtOH | [16] |
| 7 | 6-Hydroxy-7-methoxy2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 8 | 6,7-Dimethoxy-2-[2-(3′-hydroxy-4′-xyphenyl)ethyl]chromone | A. sinensis (China) | EtOH | [16] |
| 9 | 5,6,7,8-Tetrahydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one | A. sinensis (China) | EtOH–H2O | [17] |
| 10 | 8-Chloro-6-hydroxy-2-(2-phenylethyl)chromen-4-one | A. sinensis (China) | EtOH–EtOAc | [18] |
| 11 | 8-Chloro-6-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromen-4-one | A. sinensis (China) | EtOH–EtOAc | [18] |
| 12 | Rel-(5R,6S,7R)-5,6,7,8-Tetrahydro-5,6,7-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 13 | Rel-(5R,6S,7R)-5,6,7,8-Tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran4-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 14 | 7-Hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 15 | Rel-(1aR,2R,3R,7bS)-1α,2,3,7β-Tetrahydro-2,3-dihydroxy-5-[2-(4-methoxyphenyl)ethyl]-7H-oxireno[f] [1]benzopyran-7-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 16 | Rel-(1aR,2R,3R,7bS)-1α,2,3,7β-Tetrahydro-2,3-dihydroxy-5-(2-phenylethyl)-7H-oxireno[f] [1]benzopyran-7-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 17 | Rel-(1aR,2R,3R,7bS)-1α,2,3,7β-Tetrahydro-2,3-dihydroxy-5-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-7H-oxireno[f] [1]benzopyran-7-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 18 | Rel-(5R,6S,7S,8R)-8-Chloro-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 19 | Rel-(5R,6S,7S,8R)-8-Chloro-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | A. malaccensis (Laos) | EtOH–n-BuOH | [19] |
| 20 | 6-Hydroxy-7-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 21 | 6-Hydroxy-2-[2-(3,4-dimethoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 22 | 6,8-Dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 23 | 8-Chloro-6-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 24 | 5-Methoxy-6-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 25 | (R)-6,7-Dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 26 | (S)-6,7-Dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone | A. sinensis (China) | EtOH–EtOAc | [20] |
| 27 | 6-Methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [8] |
| 28 | 5-Hydroxy-6-methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [8] |
| 29 | 5,6-Epoxy-7β-hydroxy-8β-methoxy-2-(2-phenylethyl)chromone | A. sinensis (China) | EtOH–EtOAc | [8] |
| 30 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-{6-methoxy-2-[2-(3‴-methoxy-4‴-hydroxypheny)ethyl]chromonyl-7-oxy}chromone | A. sinensis (China) | EtOH–EtOAc | [21] |
| 31 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-5,6,7-trihydroxy-5,6,7,8-Tetrahydro-8-{2-[2-(4‴-methoxyphenyl)ethyl]chromonyl-6-oxy}chromone | A. sinensis (China) | EtOH–EtOAc | [21] |
| 32 | (5S,6R,7S,8R)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone | A. sinensis (China) | EtOH–EtOAc | [21] |
| 33 | (5R,6R,7R,8S)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone | A. sinensis (China) | EtOH–EtOAc | [21] |
| 34 | Aquilarone A | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 35 | Aquilarone B | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 36 | Aquilarone C | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 37 | Aquilarone D | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 38 | Aquilarone E | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 39 | Aquilarone F | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 40 | Aquilarone G | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 41 | Aquilarone H | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 42 | Aquilarone I | A. sinensis (China) | EtOH–CHCl3 | [22] |
| 43 | 5-Hydroxy-7-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–CH2Cl2 | [23] |
| 44 | 5,8-Dihydroxy-6-methoxy-2-(2-phenylethyl)chromone | A. sinensis (China) | EtOH–CH2Cl2 | [23] |
| 45 | 5α,6α-Epoxy-7β,8α,30-trihydroxy-40-methoxy-2-(2-phenylethyl)chromone | A. sinensis (China) | EtOH–CH2Cl2 | [23] |
| 46 | 6-Methoxy-2-[2-(20,30,40-trihydroxy)phenyl)ethyl]chromone | A. sinensis (China) | EtOH–CH2Cl2 | [23] |
| 47 | 5-Hydroxy-6,7-dimethoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | A. sinensis (China) | EtOH–EtOAc | [24] |
| 48 | (5R,6R,7R,8S)-8-Chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | A. sinensis (China) | EtOH–EtOAc | [24] |
| 49 | (5S,6S,7S,8S)-8-Chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone | A. sinensis (China) | EtOH–EtOAc | [24] |
| 50 | (5R,6R,7R,8R)-8-Chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | A. sinensis (China) | EtOH–EtOAc | [24] |
| 51 | (5R,6S,7S)-5,6,7-Trihydroxy-2-(4-hydroxy-3-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | A. sinensis (China) | EtOH–EtOAc | [24] |
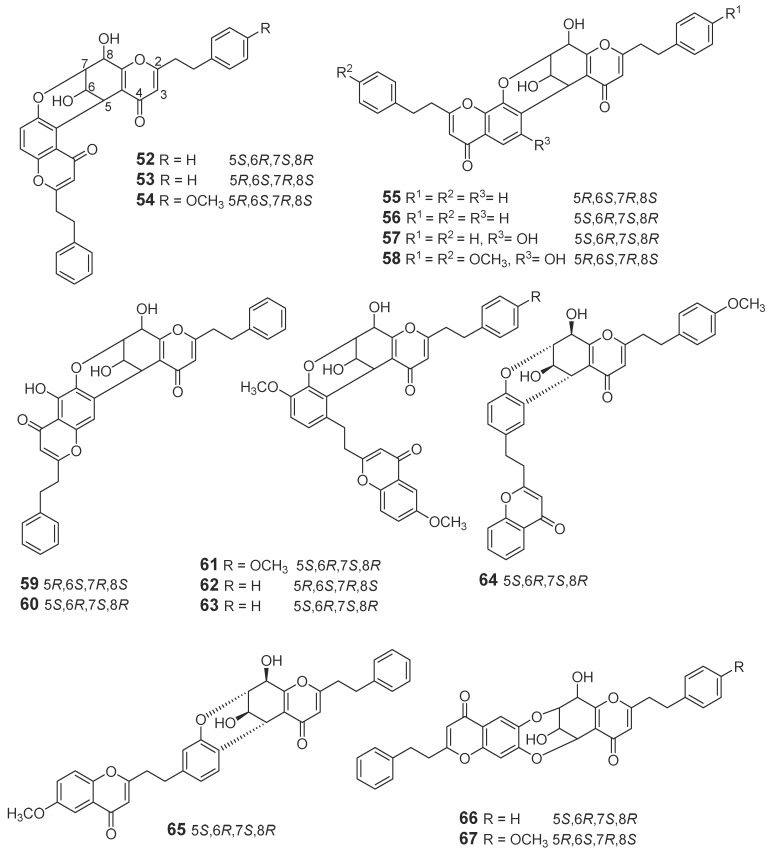
| 52 | (5S,6R,7S,8R) Aquisinenone A | A. sinensis (China) | EtOH–EtOAc | [25] |
| 53 | (5R,6S,7R,8S) Aquisinenone A | A. sinensis (China) | EtOH–EtOAc | [25] |
| 54 | (−)-4′-Methoxyaquisinenone A | A. sinensis (China) | EtOH–EtOAc | [25] |
| 55 | (5R,6S,7R,8S) Aquisinenone B | A. sinensis (China) | EtOH–EtOAc | [25] |
| 56 | (5S,6R,7S,8R)Aquisinenone B | A. sinensis (China) | EtOH–EtOAc | [25] |
| 57 | (−)-6″-Hydroxyaquisinenone B | A. sinensis (China) | EtOH–EtOAc | [25] |
| 58 | (+)-6″-Hydroxy-4′,4‴-dimethoxyaquisinenone B | A. sinensis (China) | EtOH–EtOAc | [25] |
| 59 | (5R,6S,7R,8S)Aquisinenone C | A. sinensis (China) | EtOH–EtOAc | [25] |
| 60 | (5S,6R,7S,8R)Aquisinenone C | A. sinensis (China) | EtOH–EtOAc | [25] |
| 61 | (−)-Aquisinenone D | A. sinensis (China) | EtOH–EtOAc | [25] |
| 62 | (5R,6S,7R,8S)4′-Demethoxyaquisinenone D | A. sinensis (China) | EtOH–EtOAc | [25] |
| 63 | (5S,6R,7S,8R)4′-Demethoxyaquisinenone D | A. sinensis (China) | EtOH–EtOAc | [25] |
| 64 | (+)-Aquisinenone E | A. sinensis (China) | EtOH–EtOAc | [25] |
| 65 | (−)-Aquisinenone F | A. sinensis (China) | EtOH–EtOAc | [25] |
| 66 | (−)-Aquisinenone G | A. sinensis (China) | EtOH–EtOAc | [25] |
| 67 | (+)-4′-Methoxyaquisinenone G | A. sinensis (China) | EtOH–EtOAc | [25] |
| 68 | Tetrahydrochromone A | A. sinensis (China) | EtOH–EtOAc | [26] |
| 69 | Tetrahydrochromone B | A. sinensis (China) | EtOH–EtOAc | [26] |
| 70 | Tetrahydrochromone C | A. sinensis (China) | EtOH–EtOAc | [26] |
| 71 | Tetrahydrochromone D | A. sinensis (China) | EtOH–EtOAc | [26] |
| 72 | Tetrahydrochromone E | A. sinensis (China) | EtOH–EtOAc | [26] |
| 73 | Tetrahydrochromone F | A. sinensis (China) | EtOH–EtOAc | [26] |
| 74 | Tetrahydrochromone G | A. sinensis (China) | EtOH–EtOAc | [26] |
| 75 | Tetrahydrochromone H | A. sinensis (China) | EtOH–EtOAc | [26] |
| 76 | Tetrahydrochromone I | A. sinensis (China) | EtOH–EtOAc | [26] |
| 77 | Tetrahydrochromone J | A. sinensis (China) | EtOH–EtOAc | [26] |
| 78 | Tetrahydrochromone K | A. sinensis (China) | EtOH–EtOAc | [26] |
| 79 | Tetrahydrochromone L | A. sinensis (China) | EtOH–EtOAc | [26] |
| 80 | Tetrahydrochromone M | A. sinensis (China) | EtOH–EtOAc | [26] |
| 81 | 7-Hydroxyl-6-methoxy-2-(2-phenylethyl)chromone | A. sinensis (China) | EtOH–EtOAc | [27] |
| 82 | Qinanone A | A. sinensis (China) | EtOH–Et2O | [28] |
| 83 | Qinanone B | A. sinensis (China) | EtOH–Et2O | [28] |
| 84 | Qinanone C | A. sinensis (China) | EtOH–Et2O | [28] |
| 85 | Qinanone D | A. sinensis (China) | EtOH–Et2O | [28] |
| 86 | Qinanone E | A. sinensis (China) | EtOH–Et2O | [28] |
| 87 | Qinanone G | A.sinensis (China) | EtOH–Et2O | [28] |
| 88 | 2-(2-Hydroxy-2-phenylethyl)-4H-chromen-4-one | A. filaria (Japan) | EtOH–MeOH | [29] |
| Terpenoids | ||||
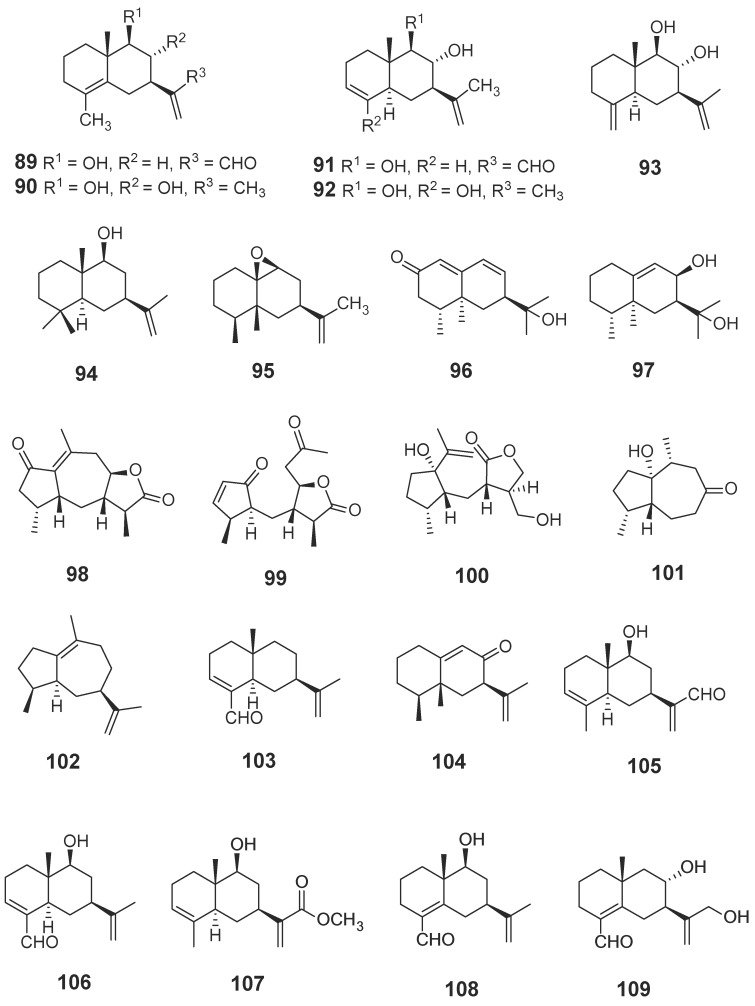
| 89 | (+)-9β-Hydroxyeudesma-4,11(13)-dien-12-al | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 90 | (+)-Eudesma-4,11(13)-dien-8α,9β-diol | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 91 | (+)-8α-Hydroxyeudesma-3,11(13)-dien-14-al | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 92 | (+)-Eudesma-3,11(13)-dien-8α,9β-diol | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 93 | (+)-Eudesma-4(14),11(13)-dien-8α,9β-diol | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 94 | (4R,5R,7S,9S,10S)-(−)-Eudesma-11(13)-en-4,9-diol | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 95 | (+)-9β,10β-Epoxyeremophila-11(13)-en | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 96 | (+)-11-Hydroxyvalenc-1(10),8-dien-2-one | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 97 | (−)-Eremophila-9-en-8β,11-diol | A.sinensis (China) | EtOH–petroleum ether | [30] |
| 98 | 1,10-Dioxo-4H-5H-7H-11H-1,10-secoguaia-2(3)-en-12,8-olide | A. sinensis (China) | EtOH | [31] |
| 99 | 1-Hydroxy-4H-5H-7H-11H-8,9-secoguaia-9(10)-en-8,12-olide | A. sinensis (China) | EtOH | [31] |
| 100 | 1-Hydroxy-4α,10α-dimethyl-5H-octahydro-azulen-8-one | A. sinensis (China) | EtOH | [31] |
| 101 | 1α-Hydroxy-4α,10α-dimethyl-5βH-octahydro-azulen-8-one | A. sinensis (China) | EtOH | [31] |
| 102 | 4-Hydroxyl-baimuxinol | A. sinensis (China) | EtOH–Et2O | [32] |
| 103 | 7β-H-9(10)-ene-11,12-Epoxy-8-oxoeremophilane | A. sinensis (China) | EtOH–Et2O | [32] |
| 104 | 7α-H-9(10)-ene-11,12-Epoxy-8-oxoeremophilane | A. sinensis (China) | EtOH–Et2O | [32] |
| 105 | (5S,7S,9S,10S)-(+)-9-Hydroxy-selina-3,11-dien-12-al | A. sinensis (China) | EtOH–EtOAc | [33] |
| 106 | (5S,7S,9S,10S)-(−)-9-Hydroxy-selina-3,11-dien-14-al | A. sinensis (China) | EtOH–EtOAc | [33] |
| 107 | (5S,7S,9S,10S)-(+)-9-Hydroxy-eudesma-3,11(13)-dien-12-methyl ester | A. sinensis (China) | EtOH–EtOAc | [33] |
| 108 | (7S,9S,10S)-(+)-9-Hydroxy-selina-4,11-dien-14-al | A. sinensis (China) | EtOH–EtOAc | [33] |
| 109 | (7S,8S,10S)-(+)-8,12-Dihydroxy-selina-4,11-dien-14-al | A. sinensis (China) | EtOH–EtOAc | [33] |
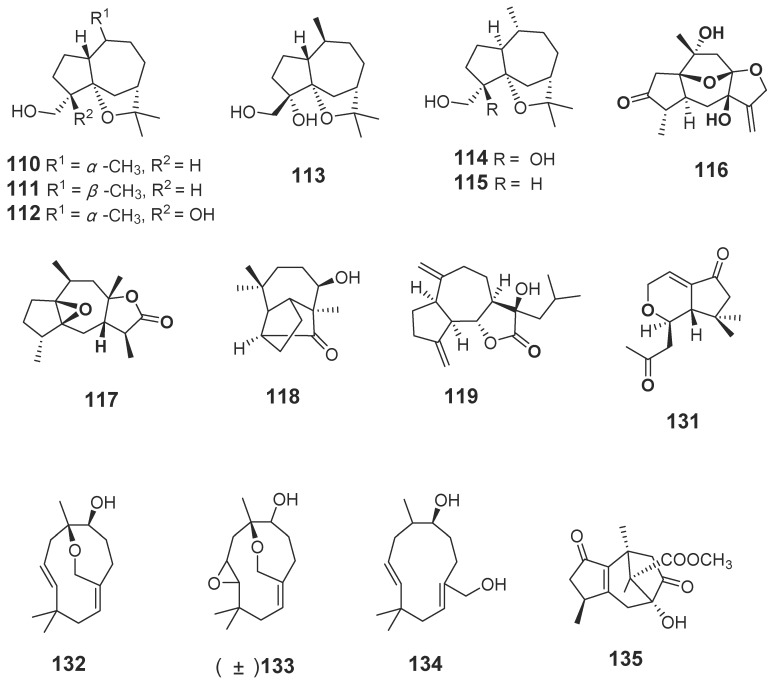
| 110 | Qinanol A | A. sinensis (China) | EtOH–Et2O | [34] |
| 111 | Qinanol B | A. sinensis (China) | EtOH–Et2O | [34] |
| 112 | Qinanol C | A. sinensis (China) | EtOH–Et2O | [34] |
| 113 | Qinanol D | A. sinensis (China) | EtOH–Et2O | [34] |
| 114 | Qinanol E | A. sinensis (China) | EtOH–Et2O | [34] |
| 115 | Qinanol F | A. sinensis (China) | EtOH–Et2O | [34] |
| 116 | 3-oxo-7-Hydroxylholosericin A | A. sinensis (China) | EtOH–EtOAc | [35] |
| 117 | 1,5,8,12-Diepoxy-guaia-12-one | A. sinensis (China) | EtOH–EtOAc | [35] |
| 118 | (+)-8β-Hydroxy-longicamphenylone | A. sinensis (China) | EtOH–petroleum ether | [37] |
| 119 | 11β-Hydroxy-13-isopropyl-dihydrodehydrocostus lactone | A. sinensis (China) | EtOH–petroleum ether | [37] |
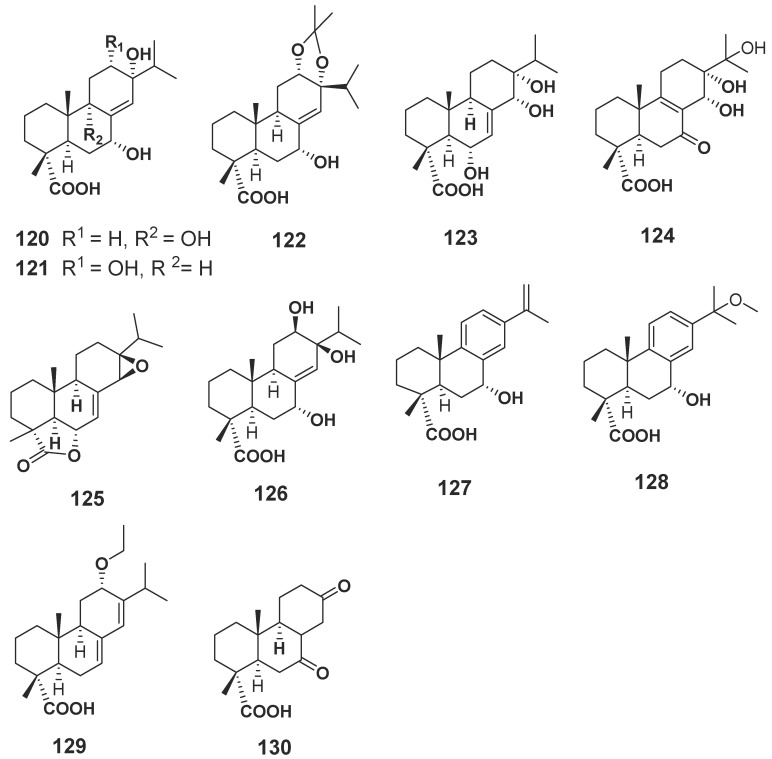
| 120 | Aquilarabietic acid A | A. sinensis (China) | EtOH | [38] |
| 121 | Aquilarabietic acid B | A. sinensis (China) | EtOH | [38] |
| 122 | Aquilarabietic acid C | A. sinensis (China) | EtOH | [38] |
| 123 | Aquilarabietic acid D | A. sinensis (China) | EtOH | [38] |
| 124 | Aquilarabietic acid E | A. sinensis (China) | EtOH | [38] |
| 125 | Aquilarabietic acid F | A. sinensis (China) | EtOH | [38] |
| 126 | Aquilarabietic acid G | A. sinensis (China) | EtOH | [38] |
| 127 | Aquilarabietic acid H | A. sinensis (China) | EtOH | [38] |
| 128 | Aquilarabietic acid I | A. sinensis (China) | EtOH | [38] |
| 129 | Aquilarabietic acid J | A. sinensis (China) | EtOH | [38] |
| 130 | Aquilarabietic acid K | A. sinensis (China) | EtOH | [38] |
| 131 | Aquilarin B | A. sinensis (China) | EtOH–EtOAc | [39] |
| 132 | Aquilanol A | A. malaccensis (Laos) | EtOH–Et2O | [36] |
| 133 | Aquilanol B | A. malaccensis (Laos) | EtOH–Et2O | [36] |
| 134 | Daphnauranol D | A. malaccensis (Laos) | EtOH–Et2O | [36] |
| 135 | Chamaejasmone E | A. malaccensis (Laos) | EtOH–Et2O | [36] |
| 136 | Aquilacallane A | A. sinensis (China) | EtOH–EtOAc | [40] |
| 137 | Aquilacallane B | A. sinensis (China) | EtOH–EtOAc | [40] |
| 138 | Aquimavitalin | A. malaccensis (Taiwan) | EtOH–EtOAc | [41] |
| 139 | 12-O-(2′E,4′E)-6-oxohexa-2′,4′-Dienoylphorbol-13-acetate | A. malaccensis (Taiwan) | EtOH–EtOAc | [42] |
| 140 | 12-Deoxy-13-O-acetylphorbol-20-(9′Z)-octadecenoate | A. malaccensis (Taiwan) | EtOH–EtOAc | [42] |
| 141 | 12-O-(2′E,4′E)-6′,7′-(erythro)-dihydroxytetradeca-2′,4′-dienoylphorbol-13-acetate. | A. malaccensis (Taiwan) | EtOH–EtOAc | [42] |
| 142 | 12-O-(2′E,4′E)-6′,7′-(threo)-dihydroxytetradeca-2′,4′-dienoylphorbol-13-acetate. | A. malaccensis (Taiwan) | EtOH–EtOAc | [42] |
| Flavonoids | ||||
| 143 | 4′-O-Geranyltricin | A. sinensis (Taiwan) | EtOH–EtOAc | [27] |
| 144 | 3′-O-Geranylpolloin | A. sinensis (Taiwan) | EtOH–EtOAc | [27] |
| 145 | Aquisiflavoside | A. sinensis (China) | EtOH–n-BuOH | [43] |
| 146 | Aquilarisinin | A. sinensis (China) | EtOH–n-BuOH and EtOAc | [44] |
| 147 | Aquilarisin | A. sinensis (China) | EtOH–n-BuOH and EtOAc | [44] |
| 148 | Aquilarixanthone | A. sinensis (China) | EtOH–n-BuOH and EtOAc | [44] |
| 149 | Hypolaetin 5-O-β-D-glucuronopyranoside | A. sinensis (China) | EtOH–n-BuOH and EtOAc | [44] |
| 150 | 7-β-D-Glucoside of 5-O-methylapigenin | A. sinensis (China) | EtOH–n-BuOH | [45] |
| Others | ||||
| 151 | Aquilarinoside A | A. sinensis (China) | EtOH–n-BuOH | [45] |
| 152 | Aquilarin A | A. sinensis (China) | EtOH–EtOAc | [46] |
| 153 | (9S) Megastigma-4,7-diene-2,3,9-triol-9-O-β-D-glucopyranoside | A. sinensis (China) | EtOH–n-BuOH | [47] |
| 154 | (9S) Megastigma-4(13),7-diene-3,6,9-triol-9-O-β-D-glucopyranoside | A. sinensis (China) | EtOH–n-BuOH | [47] |
* The first one or two solvents used to extract before the separation on columns. Ethanol: EtOH; ethyl acetate: EtOAc; n-butyl alcohol: n-BuOH; diethyl ether: Et2O; chloroform: CHCl3; and dichloromethane: CH2Cl2.
2.1.1. 2-(2-Phenylethyl)chromones
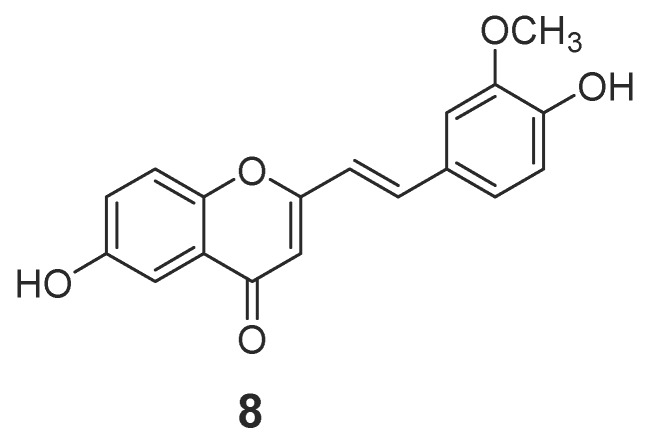
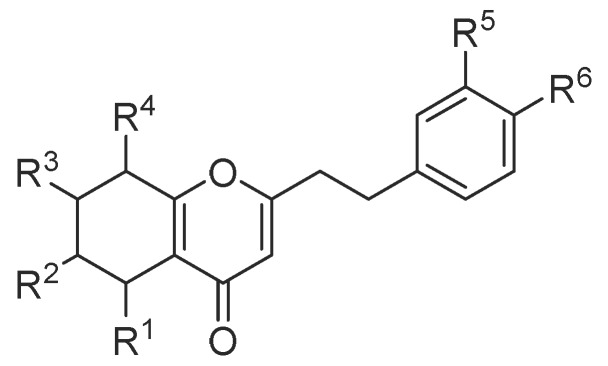
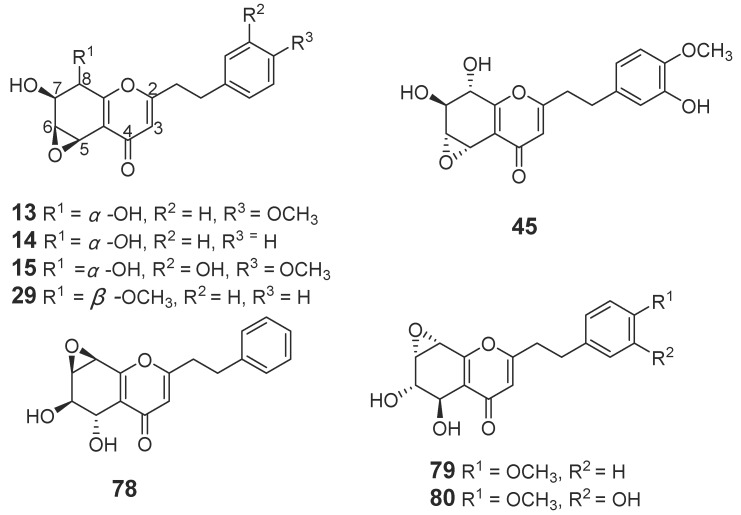
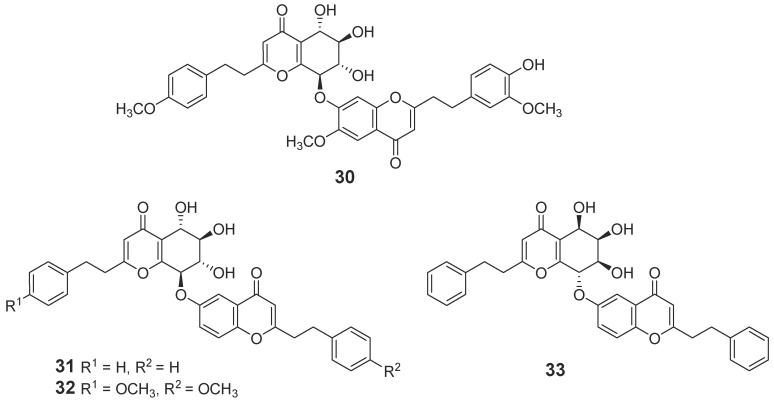
In total, 88 new 2-(2-phenylethyl)chromone compounds (1–88) have been isolated from agarwood and genus Aquilaria plants (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). Yang et al. [16] carried out a bioassay-guided isolation strategy from A. sinensis, resulting in seven new 2-(2-phenylethl)chromone derivatives (1–7) and a new 2-(2-phenylethenyl)chromone (8) being obtained from an ethanol (EtOH) extract. The investigation of EtOH extract obtained another three 2-(2-phenylethl)chromones (9–11) [17,18] and eight derivatives (12–19) from different fractions [19]. Liao et al. [20] reported seven new 2-(2-phenylethyl)chromone derivatives (20–26), including a chlorinated one (23) from the ethyl acetate (EtOAc) fraction of artificial agarwood (A. sinensis). The EtOAc fraction also contained three 2-(2-phenylethyl)chromones (27–29) [8] and four new bi-phenylethylchromones (30–33) [21]. A phytochemical investigation of a resinous wood (A. sinensis) led to the isolation of nine new 2-(2-phenylethyl)chromone derivatives, aquilarones A–I (34–42) from a chloroform (CHCl3) fraction [22]. Liu et al. found four new 2-(2-phenylethyl)chromone derivatives (43–46) from Chinese agarwood produced via the whole-tree agarwood-inducing technique [23]. Huo et al. gained five new 2-(2-phenylethyl)chromone derivatives (47–51) [24] and sixteen dimeric 2-(2-phenylethyl)chromones (52–67) from the resinous wood of A. sinensis [25]. Liao et al. isolated thirteen 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (68–80) [26] from the artificial agarwood of A. sinensis. Additionally, one 2-(2-phenylethyl)chromone compound (81) was isolated from the stem bark EtOH extract of A. sinensis [27].
Figure 1.
Structures of chromones identified in agarwood.
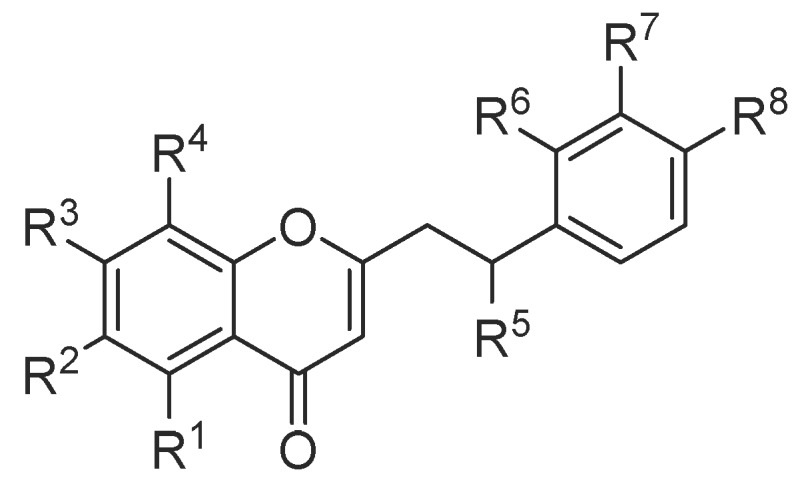
| NO. | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 1 | H | OH | OCH3 | H | H | H | OH | OCH3 |
| 2 | H | OCH3 | OCH3 | H | H | H | OH | OCH3 |
| 3 | H | OCH3 | OH | H | H | H | OH | OCH3 |
| 4 | H | OCH3 | OCH3 | H | H | H | OCH3 | OH |
| 5 | H | OH | OH | H | H | H | H | OCH3 |
| 6 | H | OH | OCH3 | H | H | H | H | OH |
| 7 | H | OH | H | OH | H | H | OH | OCH3 |
| 9 | OH | OH | OH | OH | H | H | OH | OCH3 |
| 10 | H | OH | H | Cl | H | H | H | H |
| 11 | H | OH | H | Cl | H | H | H | OCH3 |
| 12 | H | OCH3 | OH | H | H | H | H | OCH3 |
| 20 | H | OH | OCH3 | H | H | H | H | OCH3 |
| 21 | H | OH | H | H | H | H | OCH3 | OCH3 |
| 22 | H | OH | H | OH | H | H | H | OCH3 |
| 23 | H | OH | H | Cl | H | H | OH | OCH3 |
| 24 | OCH3 | OH | H | H | H | H | OH | OCH3 |
| 25 | H | OCH3 | OCH3 | H | α-OH | H | H | H |
| 26 | H | OCH3 | OCH3 | H | β-OH | H | H | H |
| 27 | H | OCH3 | H | H | H | H | OH | OCH3 |
| 28 | OH | OCH3 | H | H | H | H | OH | OCH3 |
| 40 | H | OH | OCH3 | H | H | H | OCH3 | OH |
| 41 | H | OCH3 | H | H | H | H | H | OH |
| 42 | H | OH | H | H | H | H | OH | OCH3 |
| 43 | OH | H | OCH3 | H | H | H | H | OCH3 |
| 44 | OH | OCH3 | H | OH | H | H | H | H |
| 46 | H | OCH3 | H | H | H | OH | OH | OH |
| 47 | OH | OCH3 | OCH3 | H | H | H | H | OCH3 |
| 81 | H | OCH3 | OH | H | H | H | H | H |
| 82 | H | H | H | H | H | H | OH | OCH3 |
| 83 | H | H | H | H | H | H | OCH3 | OH |
| 84 | H | H | H | H | H | OH | H | OCH3 |
| 85 | H | H | H | H | H | H | H | OH |
| 86 | H | H | H | H | H | H | OH | H |
| 87 | H | H | H | H | H | OH | H | H |
| 88 | H | H | H | H | OH | H | H | H |
Figure 2.
Structure of 2-(2-phenylethenyl)chromone identified in agarwood.
Figure 3.
Structures of 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones identified in agarwood.
| NO. | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 16 | α-OH | β-OH | β-OH | α-Cl | H | OCH3 |
| 17 | α-OH | β-OH | β-OH | α-Cl | OH | OCH3 |
| 18 | α-OH | α-OH | β-OH | H | H | H |
| 19 | α-OH | α-OH | β-OH | H | H | OCH3 |
| 34 | α-OH | α-OH | α-OH | β-OH | OH | OCH3 |
| 35 | α-OH | α-OH | α-OH | β-OH | H | H |
| 36 | α-OH | α-OH | α-OH | β-OH | H | OCH3 |
| 37 | α-OH | β-OH | α-OH | β-OH | OH | OCH3 |
| 38 | α-OH | β-OH | β-OH | α-OH | OH | OCH3 |
| 39 | α-OH | β-OH | β-OH | α-OH | H | OH |
| 48 | β-OH | β-OH | β-OH | α-Cl | H | OCH3 |
| 49 | α-OH | α-OH | α-OH | α-Cl | H | H |
| 50 | β-OH | β-OH | β-OH | β-Cl | H | OCH3 |
| 51 | β-OH | β-OH | β-OH | H | OCH3 | OH |
| 68 | α-OCH3 | β-OH | β-OH | α-OH | H | OCH3 |
| 69 | β-OCH3 | α-OH | α-OH | β-OH | H | OCH3 |
| 70 | α-OCH3 | β-OH | β-OH | α-OH | OH | OCH3 |
| 71 | α-OCH3 | β-OH | β-OH | α-Cl | H | OCH3 |
| 72 | α-OH | β-OH | β-OH | α-Cl | H | OCH3 |
| 73 | α-OCH3 | α-OH | α-OH | β-OH | H | OCH3 |
| 74 | β-OCH3 | β-OH | β-OH | α-OH | H | OCH3 |
| 75 | α-OCH3 | α-OH | α-OH | β-OH | OH | OCH3 |
| 76 | α-OCH3 | α-OH | α-OH | β-Cl | H | OCH3 |
| 77 | α-OCH3 | α-OH | α-OH | β-Cl | OH | OCH3 |
Figure 4.
Structures of 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones with epoxide identified in agarwood.
Figure 5.
Structures of dimeric 2-(2-phenylethyl)chromones identified in agarwood.
“Qi-Nan” is regarded as the highest quality agarwood, valued for its mysterious oriental odor that can be smelt without burning, unlike other kinds of agarwood. The investigation of EtOH extract of high-quality Chinese agarwood “Qi-Nan” (A. sinensis) obtained seven new 2-(2-phenylethl)chromone derivatives (82–88) [28,29].
2.1.2. Terpenoids
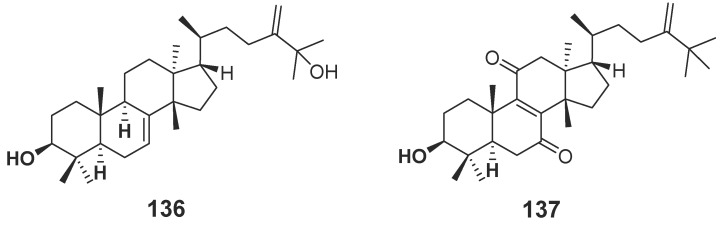
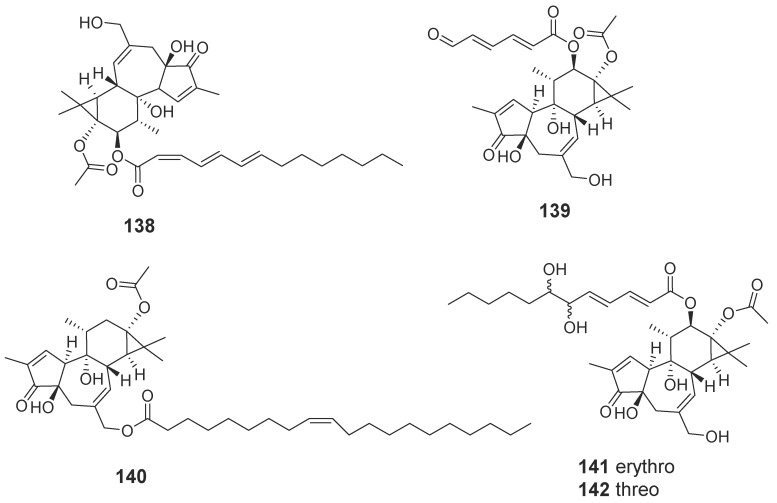
Terpenoids are compounds derived from mevalonic acid, whose basic carbon frame is characterized by having two or more isoprene units. Terpenoids, including sesquiterpenes and diterpenes, are the main components of agarwood. The EtOH extract of agarwood was isolated and, as a result, a total of 34 new sesquiterpenes (89–117, 131–135) (Figure 6) were gained [30,31,32,33,34,35,36], in which nine compounds (102–104, 110–115) were identified from “Qi-Nan” [32,34]. The isolation of a petroleum ether fraction obtained two new sesquiterpene derivatives (118, 119) (Figure 6) [37], and eleven new diterpenoids (120–130) (Figure 7) were identified from EtOH extract [38]. Additionally, many new terpenoids have also been found in other parts of genus Aquilaria plants. Peng et al. [39] isolated a novel degraded sesquiterpene, named aquilarin B (131) (Figure 6) from the EtOH extract of the fresh stem (A. sinensis) and Cheng et al. [40] got two new tirucallane triterpenoids (136–137) (Figure 8) from the leaves of A. sinensis. Furthermore, aquimavitalin (138) and four new phorbol esters (139–142) were isolated from an A. malaccensis seeds ethanolic extract [41,42] (Figure 9).
Figure 6.
Structures of sesquiterpenes identified in agarwood.
Figure 7.
Structures of diterpenes identified in agarwood.
Figure 8.
Structures of tirucallane triterpenoids from Aquilaria sinensis.
Figure 9.
Structures of phorbol esters from Aquilaria malaccensis.
2.1.3. Flavonoids
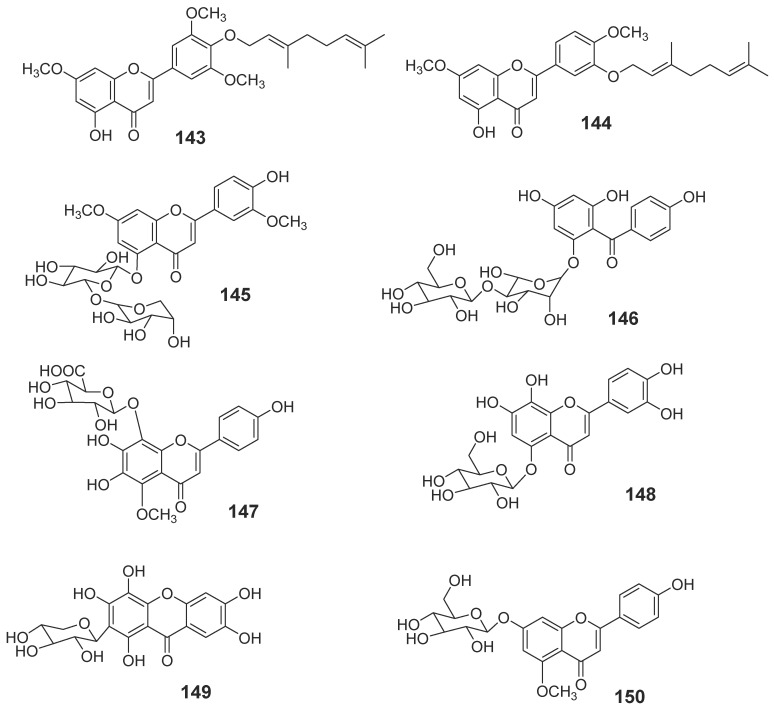
Flavonoids consist of a large group of polyphenolic compounds with a benzo-γ-pyrone structure, which is ubiquitously present in plants; there is no exception for the genus Aquilaria plants. Two new flavones (143, 144) were obtained from the EtOAc fraction of stem bark (A. sinensis) [27] (Figure 10). Another six new flavonoids (145–150) were isolated from the leaves of A. sinensis [43,44,45] (Figure 10).
Figure 10.
Structures of flavonoids from Aquilaria malaccensis.
2.1.4. Others
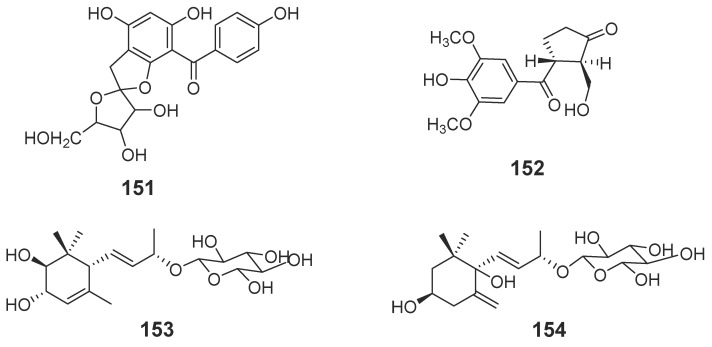
Compounds 151–154 are included here, as they do not belong to any of the above classes [45,46,47] (Figure 11).
Figure 11.
Structures of compounds from agarwood.
2.2. Pharmacological Activity of Fraction and Components from Agarwood and Aquilaria Trees
2.2.1. Neural Activity
Agarwood has been traditionally used as a medicine for tranquilizing and reducing excitement in China, Southeast Asia, and the Middle East for centuries. Modern pharmacological studies have demonstrated that agarwood has an active effect on the nervous system [48,49]. Okugawa et al. [50] determined that a benzene extract of A. malaccensis agarwood reduced spontaneous motility, prolonged hexobarbiturate-induced sleeping time, and decreased rectal temperature, whereas petroleum ether, chloroform, or water extracts did not have that effect. A further bio-guided isolation of a benzene extract found that jinkoh-eremol and agarospirol were the main active constituents [51,52]. Takemoto et al. [53] reported that agarwood essential oil sedated mice through vapor inhalation, in which the main volatile constituents were benzylacetone, α-gurjunene, and (+)-calarene. As benzylacetone had a sedative effect, a number of derivatives were synthesized and assessed for a sedative effect. The results demonstrated that benzylacetone-like compounds had sedative activities, and their intensities varied depending on the functional group in the carbon chain, the substituent in the benzene ring, and their combinations [54]. Our recent studies showed that both the ethanol extract and essential oil of agarwood, induced by the whole-tree agarwood inducing technique in A. sinensis trees, had a sedative-hypnotic effect, where its potential mechanism is related to regulating the gene expression of GABAA receptors and potentiating the GABAA receptor function [55,56]. Agarofuran, derived from agarwood essential oil, was reported to have anxiolytic and anti-depression activity in mice [49]. To explore a potential drug for treating anxiety and depression, a series of agarofuran-like derivatives were synthesized and the activity screened, among which, buagafuran was an effective compound for anti-anxiety and anti-depression, with low toxicity and a high safety coefficient [49,57]. The potential mechanism might be through modulating central neurotransmitters, such as dopamine [58]. A metabolic study showed that buagafuran could be transformed to hydroxy metabolite and carbonyl one in a human liver microsome, where carbonyl metabolite was the main one [59]. Until now, phase II clinical trials are being conducted on buagafuran. Furthermore, many other activity screening results have also shown that compounds from agarwood have an effect on neural activity. Compound 7 (10 µg/mL) showed neural protective activity against both glutamate-induced and corticosterone-induced neurotoxicity in PC12 pheochromocytoma and human U251 glioma cells [16]. Compounds 118 and 119 exhibited potent anti-depressant activity in vitro by inhibiting [3H]-5-HT reuptake in rat synaptosomes [37]. Compound 120 demonstrated remarkable antidepressant activity in vitro, by inhibiting norepinephrine reuptake in rat brain synaptosomes [38]. Simultaneously, seventeen new 2-(2-phenylethyl)chromones, including compounds 22, 27–29, 31–33, 68, 69, 78–80, 82–86, and eleven new terpenoids, such as 103–105 and 110–117, had acetylcholinesterase inhibitive effect [8,20,21,26,28,32,33,34,35]. Above all, neural activity of agarwood is one of the most studied aspects with many active compounds and a promising drug candidate found, which will sustain it as a research hotspot in the future.
2.2.2. Gastrointestinal Regulation
Pharmacological studies showed that agarwood and the leaves of A. sinensis trees have a gastrointestinal regulating effect. Our studies demonstrated that the agarwood ethanol extract significantly improved intestinal peristalsis, enhanced gastric emptying, and inhibited gastric ulcer [60]. Li et al. reported that the ethanol extract of agarwood and A. sinensis leaves enhanced intestinal propulsion [61]. Kakina et al. reported that leaves of A. sinensis trees induced laxation via acetylcholine receptors on loperamide-induced constipation in mice [62]. The acetone extract of A. sinensis leaves had a laxative effect without causing diarrhea, in which genkwanin 5-O-β-primeveroside was the active constituent, whereas the methanol extract did not have the laxative effect [63]. The ethanol extract of A. sinensis leaves had a laxative effect without causing diarrhea in a rat model of low-fiber diet-induced constipation [64]. Mangiferin and genkwanin 5-O-primeveroside were the two major bioactive compounds [65]. Additionally, benzylacetone, an active compound from essential oil, had the effect of enhancing appetite [66,67]. Even though agarwood on alleviating abdominal discomfort has been widely used for centuries, the gastrointestinal regulating effect, especially on a specific disease, is not completely clear.
2.2.3. Antibacterial and Antifungal
The original use of agarwood was for anticorrosive deodorization in ancient China, as well as Southeast Asian countries. In Thailand, agarwood has been used for a long time as a traditional treatment for infectious diseases such as diarrhea and skin diseases [68]. Chen et al. [69] found that agarwood essential oil derived from A. sinensis, regardless of whether it originated from artificial or natural agarwood, had inhibitive activities towards Bacillus subtilis and Staphylococcus aureus [69]. Extracts of agarwood (A. crassna), isolated by water distillation, supercritical fluid carbon dioxide, and supercritical fluid carbon dioxide with ethanol as the co-solvent, showed antimicrobial activities against S. aureus and Candida albicans, but were not against Escherichia coli [70]. Sirilak et al. [68] found that an aqueous extract of A. crassna leaves possessed an in vitro antibacterial action against Staphylococcus epidermidis, causing bacterial cells to swell and distort, inhibiting the biofilm formation, and leading to cell wall rupture. An ethyl acetate soluble fraction of ethanol extract from A. crassna exhibited stronger antifungal (Fusarium solani) activity than ethanol extract [10]. Additionally, many other compounds had an antibacterial activity, such as compound 27, exhibiting inhibitory effect against S. aureus [8], compound 105 and 107 against both S. aureus and R. solanacearum, and compound 109 against S. aureus [33]. Even though the antibacterial/antifungal effect of agarwood is definite, the inhibited microbial species are not completely known. Therefore, antibacterial spectrum investigation of agarwood should be carried out.
2.2.4. Anti-Inflammatory
Agarwood essential oil has an anti-inflammatory function, significantly reducing the skin thickness, ear weight, oxidative stress, and pro-inflammatory cytokines production in the 12-O-tetradecanoylphorobol-13 acetate (TPA)-induced mouse ear inflammation model [71]. The ethanol extract of agarwood also inhibited ear edema induced by xylene, and peritoneal inflammation induced by low concentrative acetic acid in mice [72]. Linalool and the corresponding acetate derivate play a major role in anti-inflammatory activity [73]. An in silico molecular docking study suggests that 10-epi-γ-eudesmol, jinkoh-eremol, and agarospirol were preferentially more active than other identified compounds, with strong binding affinity to major anti-inflammatory receptors [71]. Furthermore, many other activity screening results have shown that compounds from agarwood exhibited a potent inhibitory activity against inflammation. Compounds 34–42, 43, 48–51, 52–56, 58, 61–63, 95, 99, and 145 showed significant inhibition of NO production [22,23,24,25,30,31,43]. Compound 150 showed inhibition activity against polymorphonuclear neutrophil respiratory burst stimulated by phorbol 12-myristate 13-acetate [45]. Compounds 81 and 144 exhibited inhibition of superoxide anion generation [27], and inversely, compounds 139–142 exerted enhancing activity on superoxide anion generation [42]. At the same time, compounds 81, 139, and 144 showed potent inhibitory activity on elastase release [27,42]. As we all know, inflammation has a close relationship with other diseases, such as immunopathy, metabolic disorders, and neoplasms, so the anti-inflammatory effect of agarwood, in a certain degree, portends the extensive pharmacological activities of agarwood.
2.2.5. Analgesic Effect
Wang et al. [74] found that chloroform extracts of agarwood prolonged the pain threshold induced by hot plate, and reduced the times of writhing reactions. Jinkoh-eremol and agarospirol may be the active compounds, and jinkoh-eremol’s analgesic effect could be blocked by naloxone (a opioid antagonist), whereas agarosporol was weakly effected by naloxone [51]. At the same time, jinkoh-eremol and agarospirol could inhibit D2 receptor binding and 5-HT2A receptor binding [51]. Additionally, compound 138 showed strong inhibitory activity in A23178- and antigen-induced degranulation assay, with IC50 values of 1.7 nM and 11 nM, respectively [41].
2.2.6. Antiasthma
The antiasthma effect of agarwood has been traditionally used in China, and can be found in the latest Chinese Pharmacopoeia [13]. However, to our knowledge, only one study found that an ethanol extract of agarwood and A. sinensis leaves could inhibit asthma induced by histamine phosphate in guinea pig [75].
2.2.7. Cytotoxicity
Agarwood essential oil possesses anticancer activity towards MCF-7 breast cancer cells [76] and HCT 116 colorectal carcinoma cells [77,78,79]. β-Caryophyllene, isolated from the essential oil of A. crassna, exhibited selective anti-proliferative effects against colorectal cancer cells (IC50 19 μM) and induced apoptosis via nuclear condensation and fragmentation pathways. Additionally, β-caryophyllene also showed potent inhibition of clonogenicity, migration, invasion, and spheroid formation in colon cancer cells [80]. Additionally, other activity screening results showed that compounds from agarwood exhibited cytotoxic activity [81], whereas compound 88 suppressed tumor promotion at noncytotoxic concentrations [29].
2.2.8. Anti-Diabetes
Mei et al. [82] found that the ethanol extracts of both agarwood and A. sinensis leaves alleviated diabetes induced by mesoxyalyurea in mice. The methanol extract of A. sinensis leaves possessed the fast blood glucose activity in rat and glucose uptake transportation by rat adipocytes [83]. Iriflophenone 3-C-β-glucoside decreased the fasting blood glucose levels in streptozocin-induced diabetic mice, and enhanced glucose uptake into adipocytes [84]. Compounds 146–149 isolated from agarwood had an inhibitive effect on α-glucosidase [44].
2.2.9. Antioxidation
The essential oil of agarwood had a protective effect against oxidative damage induced by hydrogen peroxide (H2O2) in PC12 cells [85]. The aqueous extract of A. crassna leaves had radical scavenging capacities determined by 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS), ferric reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) scavenging assays [68]. A methanol extract of A. crassna leaves was also found to have anti-oxidative activities [86]. The 100% (v/v) ethanol extract exhibited the highest DPPH radical scavenging activity among the 0% to 100% (v/v) ethanol extracts isolated from A. crassna young leaves [87]. β-Caryophyllene displayed strong antioxidant effects determined by the DPPH and FRAP scavenging methods [80]. Other compounds 28, 35, and 144, isolated from agarwood, also showed an anti-oxidative effect [8,22,27].
2.2.10. Others
A methanol extract of A. crassna leaves significantly reduced fever (rectal temperature) induced by baker’s yeast at five and six hours after subcutaneous injection in rat [86]. The aqueous extract of A. malaccensis leaves was effective on Trypanosoma evansi with an IC50 value 36.29 ± 1.32 μg/mL, whereas the ethanol extract was relatively weak (IC50 = 128.63 ± 6.70 μg/mL) [88]. An ethyl acetate extract of A. crassna showed an anti-ischemic effect by attenuation of P38-MAPK activation [89].
3. Conclusions
Among the 154 new compounds identified from Aquilaria plants, 2-(2-phenylethyl)-4H-chromen-4-one derivatives and sesquiterpenes account for 57% and 35%, respectively, where most of the new compounds, accounting for 89%, were isolated from A. sinensis. Generally, agarwood originating from different Aquilaria plants share some common compounds, but still have several different compounds [14]. In addition, there are at least 19 species of Aquilaria plants producing agarwood, which means that large quantities of new compounds need to be explored in agarwood and Aquilaria plants. The chemical components of agarwood are diverse and complex, contributing to the diversity of bioactivity and pharmacology, including neural activity, gastrointestinal regulation, antibacterial, anti-inflammation, and cytotoxicity. Based on the specific disease and target, illuminating the active ingredients and compounds of agarwood should be carried out, which may not only contribute to the understanding of the scientific nature of the traditional agarwood application, but also benefit the new drug research and agarwood product development.
Acknowledgments
This work was supported by Science & Technology Programs from Hainan Province of China (No. ZDKJ2016004), the Central Organization Department Plan of the Leading Talents Project of Science and Technology Innovation (No. 99950534), the National Natural Science Foundation of China (Nos. 81403055 and 81303312), Program for Creative Research Groups of Hainan Provincial Natural Science Foundation (No. 2017CXTD022) and the 2016 annual Graduate Innovation Fund Project of PUMC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.CITES Amendments to appendices I and II of CITES; Proceedings of the Thirteenth Meeting of the Conference of the Parties 2004; Bangkok, Thailand. 2 October 2004. [Google Scholar]
- 2.CITES Government of India Hosted Asian Regional Workshop on the Management of Wild and Planted Agarwood Taxa. [(accessed on 5 May 2015)]; Available online: https://www.Cites.Org/eng/2015_india_agarwood_workshop.
- 3.CITES Report on NDF of Agarwood for Sustainability Harvest in Indonesia. [(accessed on 29 May 2015)]; Available online: https://cites.Org/sites/default/files/ndf_material/agarwood_in_indonesia_ndf%5b1%5d.Pdf.
- 4.Liu Y., Wei J., Gao Z., Zhang Z., Lyu J. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017;9:22–30. doi: 10.1016/S1674-6384(17)60072-8. [DOI] [Google Scholar]
- 5.The IUCN Red List of Threatened Species. [(accessed on 17 December 2017)]; Version 2017-3. Available online: www.Iucnredlist.Org.
- 6.Kalra R., Kaushik N. A review of chemistry, quality and analysis of infected agarwood tree (Aquilaria sp.) Phytochem. Rev. 2017;16:1045–1079. doi: 10.1007/s11101-017-9518-0. [DOI] [Google Scholar]
- 7.Liu Y., Chen H., Yang Y., Zhang Z., Wei J., Meng H., Chen W., Feng J., Gan B., Chen X., et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules. 2013;18:3086–3106. doi: 10.3390/molecules18033086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Cai C.H., Dong W.H., Guo Z.K., Wang H., Mei W.L., Dai H.F. 2-(2-phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia. 2014;98:117–123. doi: 10.1016/j.fitote.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Kalita J., Bhattacharyya P.R., Boruah H.P.D., Unni B.G., Lekhak H., Nath S.C. Association of zeuzera conferta walker on agarwood formation in Aquilaria malaccensis Lamk. Asian J. Plant Sci. Res. 2015;5:4–9. [Google Scholar]
- 10.Novriyanti E., Santosa E., Syafii W., Turjaman M., Sitepu I.R. Antifungal activity of wood extract of Aquilaria crassna Pierre ex Lecomte against agarwood-inducing fungi, Fusarium solani. J. For. Res. 2010;7:155–165. [Google Scholar]
- 11.Mohamed R., Jong P.L., Kamziah A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. For. Res. 2014;25:201–204. doi: 10.1007/s11676-013-0395-0. [DOI] [Google Scholar]
- 12.Peng C.S., Osman M.F., Bahar N., Nuri E.A.K., Zakaria R., Rahim K.A. Agarwood inducement technology: A method for producing oil grade agarwood in cultivated Aquilaria malaccensis Lamk. J. Agrobiotechnol. 2015;6:1–16. [Google Scholar]
- 13.National Pharmacopoeia Committee . Pharmacopoeia of the People’s Republic of China. Volume 1. Chinese Medical Science and Technology Press; Beijing, China: 2015. pp. 185–186. 2015 Version. [Google Scholar]
- 14.Chen H.Q., Wei J.H., Yang J.S., Zhang Z., Yang Y., Gao Z.H., Sui C., Gong B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012;9:236–250. doi: 10.1002/cbdv.201100077. [DOI] [PubMed] [Google Scholar]
- 15.Hashim Y.Z., Kerr P.G., Abbas P., Mohd S.H. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016;189:331–360. doi: 10.1016/j.jep.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Qiao L., Xie D., Yuan Y., Chen N., Dai J., Guo S. 2-(2-phenylethyl)chromones from Chinese eaglewood. Phytochemistry. 2012;76:92–97. doi: 10.1016/j.phytochem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Dai H.F., Liu J., Zeng Y.B., Han Z., Wang H., Mei W.L. A new 2-(2-phenylethyl)chromone from Chinese eaglewood. Molecules. 2009;14:5165–5168. doi: 10.3390/molecules14125165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y.H., Liu J.M., Lu H.X., Wei Z.X. Two new 2-(2-phenylethyl)chromen-4-ones from Aquilaria sinensis (Lour.) Gilg. Helv. Chim. Acta. 2012;95:951–954. doi: 10.1002/hlca.201100442. [DOI] [Google Scholar]
- 19.Wu B., Kwon S., Hwang G.S., Park J.H. Eight new 2-(2-phenylethyl)chromone (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta. 2012;95:1657–1665. doi: 10.1002/hlca.201200069. [DOI] [Google Scholar]
- 20.Liao G., Mei W.L., Dong W.H., Li W., Wang P., Kong F.D., Gai C.J., Song X.Q., Dai H.F. 2-(2-phenylethyl)chromone derivatives in artificial agarwood from Aquilaria sinensis. Fitoterapia. 2016;110:38–43. doi: 10.1016/j.fitote.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Xiang P., Mei W., Chen H., Kong F., Wang H., Liao G., Zhou L., Dai H. Four new bi-phenylethylchromones from artificial agarwood. Fitoterapia. 2017;120:61–66. doi: 10.1016/j.fitote.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Chen D., Xu Z., Chai X., Zeng K., Jia Y., Bi D., Ma Z., Tu P. Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW264.7 cells. Eur. J. Org. Chem. 2012;27:5389–5397. doi: 10.1002/ejoc.201200725. [DOI] [Google Scholar]
- 23.Liu Y.Y., Chen D.L., Wei J.H., Feng J., Zhang Z., Yang Y., Zheng W. Four new 2-(2-phenylethyl)chromone derivatives from Chinese agarwood produced via the whole-tree agarwood-inducing technique. Molecules. 2016;21:1433. doi: 10.3390/molecules21111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo H.X., Gu Y.F., Sun H., Zhang Y.F., Liu W.J., Zhu Z.X., Shi S.P., Song Y.L., Jin H.W., Zhao Y.F., et al. Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia. 2017;118:49–55. doi: 10.1016/j.fitote.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Huo H.X., Zhu Z.X., Song Y.L., Shi S.P., Sun J., Sun H., Zhao Y.F., Zheng J., Ferreira D., Zjawiony J.K., et al. Anti-inflammatory dimeric 2-(2-phenylethyl)chromones from the resinous wood of Aquilaria sinensis. J. Nat. Prod. 2017 doi: 10.1021/acs.jnatprod.7b00919. [DOI] [PubMed] [Google Scholar]
- 26.Liao G., Mei W.L., Kong F.D., Li W., Yuan J.Z., Dai H.F. 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry. 2017;139:98–108. doi: 10.1016/j.phytochem.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang S.L., Hwang T.L., Chung M.I., Sung P.J., Shu C.W., Cheng M.J., Chen J.J. New flavones, a 2-(2-phenylethyl)-4H-chromen-4-one derivative, and anti-inflammatory constituents from the stem barks of Aquilaria sinensis. Molecules. 2015;20:20912–20925. doi: 10.3390/molecules201119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang D.L., Mei W.L., Zeng Y.B., Guo Z.K., Zhao Y.X., Wang H., Zuo W.J., Dong W.H., Wang Q.H., Dai H.F. 2-(2-phenylethyl)chromone derivatives in Chinese agarwood “qi-nan” from Aquilaria sinensis. Planta Med. 2013;79:1329–1334. doi: 10.1055/s-0033-1350647. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki A., Miyake K., Saito Y., Rasyid F.A., Tokuda H., Takeuchi M., Suzuki N., Ichiishi E., Fujie T., Goto M., et al. Phenylethylchromones with in vitro antitumor promoting activity from Aquilaria filaria. Planta Med. 2017;83:300–305. doi: 10.1055/s-0042-110858. [DOI] [PubMed] [Google Scholar]
- 30.Huo H.X., Zhu Z.X., Pang D.R., Li Y.T., Huang Z., Shi S.P., Zheng J., Zhang Q., Zhao Y.F., Tu P.F., et al. Anti-neuroinflammatory sesquiterpenes from Chinese eaglewood. Fitoterapia. 2015;106:115–121. doi: 10.1016/j.fitote.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H., Peng Q., Han Z., Yang L., Wang Z. Three new sesquiterpenoids and one new sesquiterpenoid derivative from Chinese eaglewood. Molecules. 2016;21:281. doi: 10.3390/molecules21030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D.L., Wang H., Guo Z.K., Li W., Mei W.L., Dai H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘qi-nan’ from Aquilaria sinensis. Phytochem. Lett. 2014;8:121–125. doi: 10.1016/j.phytol.2014.03.003. [DOI] [Google Scholar]
- 33.Li W., Cai C.H., Guo Z.K., Wang H., Zuo W.J., Dong W.H., Mei W.L., Dai H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia. 2015;100:44–49. doi: 10.1016/j.fitote.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Yang D.L., Li W., Dong W.H., Wang J., Mei W.L., Dai H.F. Five new 5,11-epoxyguaiane sesquiterpenes in agarwood “qi-nan” from Aquilaria sinensis. Fitoterapia. 2016;112:191–196. doi: 10.1016/j.fitote.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Li W., Liao G., Dong W.H., Kong F.D., Wang P., Wang H., Mei W.L., Dai H.F. Sesquiterpenoids from Chinese agarwood induced by artificial holing. Molecules. 2016;21:274. doi: 10.3390/molecules21030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma C.T., Eom T., Cho E., Wu B., Kim T.R., Oh K.B., Han S.B., Kwon S.W., Park J.H. Aquilanols A and B, macrocyclic humulene-type sesquiterpenoids from the agarwood of Aquilaria malaccensis. J. Nat. Prod. 2017;80:3043–3048. doi: 10.1021/acs.jnatprod.7b00462. [DOI] [PubMed] [Google Scholar]
- 37.Yang L., Qiao L.R., Zhang J.J., Dai J.G., Guo S.X. Two new sesquiterpene derivatives from Chinese eaglewood. J. Asian Nat. Prod. Res. 2012;14:1054–1058. doi: 10.1080/10286020.2012.704910. [DOI] [PubMed] [Google Scholar]
- 38.Yang L., Qiao L., Ji C., Xie D., Gong N.B., Lu Y., Zhang J., Dai J., Guo S. Antidepressant abietane diterpenoids from Chinese eaglewood. J. Nat. Prod. 2013;76:216–222. doi: 10.1021/np3006925. [DOI] [PubMed] [Google Scholar]
- 39.Peng K., Mei W.L., Zhao Y.X., Tan L.H., Wang Q.H., Dai H.F. A novel degraded sesquiterpene from the fresh stem of Aquilaria sinensis. J. Asian Nat. Prod. Res. 2011;13:951–955. doi: 10.1080/10286020.2011.598860. [DOI] [PubMed] [Google Scholar]
- 40.Cheng J.T., Han Y.Q., He J., De Wu X., Dong L.B., Peng L.Y., Li Y., Zhao Q.S. Two new tirucallane triterpenoids from the leaves of Aquilaria sinensis. Arch. Pharm. Res. 2013;36:1084–1089. doi: 10.1007/s12272-013-0088-4. [DOI] [PubMed] [Google Scholar]
- 41.Korinek M., Wagh V.D., Lo I.W., Hsu Y.M., Hsu H.Y., Hwang T.L., Wu Y.C., Cheng Y.B., Chen B.H., Chang F.R. Antiallergic phorbol ester from the seeds of Aquilaria malaccensis. Int. J. Mol. Sci. 2016;17:398. doi: 10.3390/ijms17030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagh V.D., Korinek M., Lo I.W., Hsu Y.M., Chen S.L., Hsu H.Y., Hwang T.L., Wu Y.C., Chen B.H., Cheng Y.B., et al. Inflammation modulatory phorbol esters from the seeds of Aquilaria malaccensis. J. Nat. Prod. 2017;80:1421–1427. doi: 10.1021/acs.jnatprod.6b01096. [DOI] [PubMed] [Google Scholar]
- 43.Yang X.B., Feng J., Yang X.W., Zhao B., Liu J.X. Aquisiflavoside, a new nitric oxide production inhibitor from the leaves of Aquilaria sinensis. J. Asian Nat. Prod. Res. 2012;14:867–872. doi: 10.1080/10286020.2012.701209. [DOI] [PubMed] [Google Scholar]
- 44.Feng J., Yang X.W., Wang R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72:242–247. doi: 10.1016/j.phytochem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Qi J., Lu J.J., Liu J.H., Yu B.Y. Flavonoid and a rare benzophenone glycoside from the leaves of Aquilaria sinensis. Chem. Pharm. Bull. (Tokyo) 2009;57:134–137. doi: 10.1248/cpb.57.134. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q.H., Peng K., Tan L.H., Dai H.F. Aquilarin A, a new benzenoid derivative from the fresh stem of Aquilaria sinensis. Molecules. 2010;15:4011–4016. doi: 10.3390/molecules15064011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J., Xia F., Wang S., Wang K.Y., Chen J.M., Tu P.F. Structural elucidation of two new megastigmane glycosides from the leaves of Aquilaria sinensis. Chin. J. Nat. Med. 2015;13:290–294. doi: 10.1016/S1875-5364(15)30016-9. [DOI] [PubMed] [Google Scholar]
- 48.Chinese Academy of Medical Sciences the Institute of Materia Medica . Modern Research of Chinese Herbal Medicine. Volume 3. China Union Medical University Press; Beijing, China: 2010. pp. 156–158. Version 1. [Google Scholar]
- 49.Guo J., Wang W., Fang H., Liu Q., Zhang W. United State Patent: Agarofuan Derivatives, Their Preparation, Pharmaceutical Composition Containing Them and Their Use as Medicine. 6486201b1. U.S. Patent. 2002 Nov 26;
- 50.Okugawa H., Ueda R., Matsumoto K., Kawanishi K., Kato A. Effects of agarwood extracts on the central nervous system in mice. Planta Med. 1993;59:32–36. doi: 10.1055/s-2006-959599. [DOI] [PubMed] [Google Scholar]
- 51.Okugawa H., Ueda R., Matsumoto K., Kawanishi K., Kato K. Effects of sesquiterpenoids from “oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2a receptors in rat brain. Phytomedicine. 2000;7:417–422. doi: 10.1016/S0944-7113(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 52.Okugawa H., Ueda R., Matsumoto K., Kawanishi K., Kato A. Effect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Planta Med. 1996;62:2–6. doi: 10.1055/s-2006-957784. [DOI] [PubMed] [Google Scholar]
- 53.Takemoto H., Ito M., Shiraki T., Yagura T., Honda G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008;62:41–46. doi: 10.1007/s11418-007-0177-0. [DOI] [PubMed] [Google Scholar]
- 54.Miyosh T., Ito M., Kitayama T., Isomori S., Yamashit F. Sedative effects of inhaled benzylacetone and structural features contributing to its activity. Biol. Pharm. Bull. 2013;36:1474–1481. doi: 10.1248/bpb.b13-00250. [DOI] [PubMed] [Google Scholar]
- 55.Wang S., Wang C., Peng D., Liu X., Wu C., Guo P., Wei J. Agarwood essential oil displays sedative-hypnotic effects through the GABAergic system. Molecules. 2017;22:2190. doi: 10.3390/molecules22122190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S., Zhou Y., Ma F., Zhang Q., Liu Y., Gong B., Guo P., Wei J. Effect of agarwood produced by whole-tree agarwood-inducing technique on hypnotic and spontaneous activity inhibition of mice. J. Int. Pharm. Res. 2016;43:1082–1087. [Google Scholar]
- 57.Liu Q., Wang D., Li C., Lv D. The synthensis and centrol nervous system activity of agarofuran. Chin. J. Med. Chem. 2003;13:125–130. [Google Scholar]
- 58.Zhang Y., Wang W., Zhang J. Effects of novel anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine neurotransmitters in rats. Eur. J. Pharmacol. 2004;504:39–44. doi: 10.1016/j.ejphar.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 59.Li N., Zhang J., Zhou T. Metabolic research of new antidepression drug AF-5 and its metabolin in human liver microsomes in vitro. Yao Xue Xue Bao. 2001;36:528–531. [PubMed] [Google Scholar]
- 60.Liu Y., Wang S., Zhou Y., Zhang Q., Ma F., Gong B., Guo P., Wei J. Effect of agarwood extracts produced by the whole-tree agarwood-inducing technique on gastrointestinal motility and gastric ulcer. J. Int. Pharm. Res. 2016;43:1076–4081. [Google Scholar]
- 61.Li H., Jiang Z., Mei Q. Comparasion the intestinal propulsion effect of agarwood leaves and resion. Asia Pac. Tradit. Med. 2013;9:24–25. [Google Scholar]
- 62.Kakino M., Izuta H., Ito T., Tsuruma K., Araki Y., Shimazawa M., Oyama M., Iinuma M., Hara H. Agarwood induced laxative effects via acetylcholine receptors on loperamide-induced constipation in mice. Biosci. Biotechnol. Biochem. 2010;74:1550–1555. doi: 10.1271/bbb.100122. [DOI] [PubMed] [Google Scholar]
- 63.Hara H., Ise Y., Morimoto N., Shimazawa M., Ichihashi K., Ohyama M., Iinuma M. Laxative effect of agarwood leaves and its mechanism. Biosci. Biotechnol. Biochem. 2008;72:335–345. doi: 10.1271/bbb.70361. [DOI] [PubMed] [Google Scholar]
- 64.Kakino M., Tazawa S., Maruyama H., Tsuruma K., Araki Y., Shimazawa M., Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement. Altern. Med. 2010;10:68. doi: 10.1186/1472-6882-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito T., Kakino M., Tazawa S., Watara T., Oyama M., Maruyama H. Quantification of polyphenols and pharmacological analysis of water and ethanol-based extracts of cultivated agarwood leaves. J. Nutr. Sci. Vitaminol. 2012;58:136–142. doi: 10.3177/jnsv.58.136. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa K., Ito M. Appetite-enhancing effects of trans-cinnamaldehyde, benzylacetone and 1-phenyl-2-butanone by inhalation. Planta Med. 2016;82:84–88. doi: 10.1055/s-0035-1558087. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa K., Ito M. Appetite-enhancing effects: The influence of concentrations of benzylacetone and trans-cinnamaldehyde and their inhalation time, as well as the effect of aroma, on body weight in mice. Biol. Pharm. Bull. 2016;39:794–798. doi: 10.1248/bpb.b15-00937. [DOI] [PubMed] [Google Scholar]
- 68.Kamonwannasit S., Nantapong N., Kumkrai P., Luecha P., Kupittayanant S., Chudapongse N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013;12:20. doi: 10.1186/1476-0711-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H., Yang Y., Xue J., Wei J., Zhang Z., Chen H. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules. 2011;16:4884–4896. doi: 10.3390/molecules16064884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wetwitayaklung P., Thavanapong N., Pharmacognosy D.O., Charoenteeraboon J. Chemical constituents and antimicrobial activity of essential oil and extracts of heartwood of Aquilaria crassna obtained from water distillation and supercritical fluid carbon dioxide extraction. Silpakorn Univ. Sci. Technol. J. 2009;3:25–33. [Google Scholar]
- 71.Yadav D.K., Mudgal V., Agrawal J., Maurya A.J., Bawankule D.U., Chanotiya C.S., Khan F., Thul S.T. Molecular docking and ADME studies of natural compounds of agarwood oil for topical anti-inflammatory activity. Curr. Comput. Aided Drug Des. 2013;9:360–370. doi: 10.2174/1573409911309030012. [DOI] [PubMed] [Google Scholar]
- 72.Lin Z., Li H., Mei Q. Comparative study on antiinflammatory of agarwood leaves and resion. Chin. Ache. Tradit. Chin. Med. 2013;31:548–549. [Google Scholar]
- 73.Peana A.T., D’Aquila P.S., Panin F., Serra G., Pippia P., Moretti M.D. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 74.Wang J., Xu X., Liang Y. Comparative study on analgesic effects of different years of Chinese eaglewood. Hainan Med. J. 2014;25:2188–2190. [Google Scholar]
- 75.Wu X., Li H., Mei Q., Lin Z. Comparative study on preventing asthma effects of agarwood leaves and resion. Pharm. Today. 2013;23:346–347. [Google Scholar]
- 76.Hashim Y., Phirdaous A., Azura A. Screening of anticancer activity from agarwood essential oil. Pharmacogn. Res. 2014;6:191–194. doi: 10.4103/0974-8490.132593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dahham S.S., Hassan L.E., Ahamed M.B., Majid A.S., Majid A.M., Zulkepli N.N. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna) BMC Complement. Altern. Med. 2016;16:236. doi: 10.1186/s12906-016-1210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahham S., Tabana Y., Sandai D., Ahmed M. In vitro anticancer and antiangiogenic activity of essential oils extracts from agarwood Aquilaria crassna. Med. Aromat. Plants. 2016;5:256–268. doi: 10.4172/2167-0412.1000256. [DOI] [Google Scholar]
- 79.Ibrahim A., Rawi S., Abdul M., Rahman N.N.A., Salah K.M.A., AbKadir M.O. Separation and fractionation of Aquilaria malaccensis oil using supercritical fluid extraction and tthe cytotoxic properties of the extracted oil. Procedia Food Sci. 2011;1:1953–1959. doi: 10.1016/j.profoo.2011.09.287. [DOI] [Google Scholar]
- 80.Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B., Ezzat M.O., Majid A.S., Majid A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J., Wu J., Zhao Y.X., Deng Y.Y., Mei W.L., Dai H.F. A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin. Chem. Lett. 2008;19:934–936. doi: 10.1016/j.cclet.2008.05.034. [DOI] [Google Scholar]
- 82.Mei Q., Li H., Lin Z., Wu X., Liang L. Comparative study on antidiabetes of agarwood leaves and resion. Lishizhen Med. Mater. Med. Res. 2013;24:1606–1607. [Google Scholar]
- 83.Pranakhon R., Aromdee C. Antihyperglycemic activity of agarwood leaf extracts in STZ-induced diabetic rats and glucose uptake enhancement activity in rat adipocytes. Songklanakarin J. Sci. Technol. 2011;33:405–410. [Google Scholar]
- 84.Pranakhon R., Aromdee C., Pannangpetch P. Effects of iriflophenone 3-c-β-glucoside on fasting blood glucose level and glucose uptake. Pharmacogn. Mag. 2015;11:82–89. doi: 10.4103/0973-1296.149711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong L., Li L., Lin L., Chen D., Wu J. Protective effect of lignum Aquilaria resionatum essential oil on H2O2-induced oxidative damage of PC12 cells. Tradit. Chin. Drug Res. Clin. Pharm. 2014;25:28–32. [Google Scholar]
- 86.Sattayasai J., Bantadkit J., Aromdee C., Lattmann E., Airarat W. Antipyretic, analgesic and anti-oxidative activities of Aquilaria crassna leaves extract in rodents. J. Ayurveda Integr. Med. 2012;3:175–179. doi: 10.4103/0975-9476.104427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tay P., Tan C., Abas F., Yim H., Ho C. Assessment of extraction parameters on antioxidant capacity, polyphenol content, epigallocatechin gallate (EGCG), epicatechin gallate (ECG) and iriflophenone 3-c-β-glucoside of agarwood (Aquilaria crassna) young leaves. Molecules. 2014;19:12304–12319. doi: 10.3390/molecules190812304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dyary H.O., Arifah A.K., Sharma R.S., Rasedee A., Mohd-Aspollah M.S., Zakaria Z.A., Zuraini A., Somchit M.N. Antitrypanosomal screening and cytotoxic effects of selected medicinal plants. Trop. Biomed. 2014;31:89–96. [PubMed] [Google Scholar]
- 89.Suwannasing C. Anti-ischemic effect of ethyl acetate extract of Aquilaria crassna by attenuation of p38-MAPK activation. J. Appl. Pharm. Sci. 2012;2:26–30. doi: 10.7324/JAPS.2012.21005. [DOI] [Google Scholar]