Abstract
In this study, a series of di-O-caffeoylquinic acids (di-COQs) were systematically investigated for their antioxidant and cytoprotective effects towards •OH-damaged bone marrow-derived mesenchymal stem cells (bmMSCs). Five di-COQs were measured using a set of antioxidant assays. The results show that adjacent 4,5-Di-O-caffeoylquinic acid (4,5-COQ) and 3,4-di-O-caffeoylquinic acid (3,4-COQ) always gave lower IC50 values than did non-adjacent di-COQs. In the Fe2+-chelating assay, 4,5-COQ and 3,4-COQ presented greater UV-Vis spectra and darker colors than did non-adjacent di-COQs. In the UPLC-ESI-MS/MS analysis, no corresponding radical adduct formation (RAF) peak was found in the reaction products of di-COQs with PTIO•. In the MTT assay, all di-COQs (especially 1,5-COQ, 1,3-COQ, and 4,5-COQ) dose-dependently increased the cellular viabilities of •OH-damaged bmMSCs. Based on this evidence, we conclude that the five antioxidant di-COQs can protect bmMSCs from •OH-induced damage. Their antioxidant mechanisms may include electron-transfer (ET), H+-transfer, and Fe2+-chelating, except for RAF. Two adjacent di-COQs (4,5-COQ and 3,4-COQ) always possessed a higher antioxidant ability than the non-adjacent di-COQs (1,3-COQ, 1,5-COQ, and 3,5-COQ) in chemical models. However, non-adjacent 1,3-COQ and 1,5-COQ exhibited a higher cytoprotective effect than did adjacent di-COQs. These differences can be attributed to the relative positions of two caffeoyl moieties and, ultimately, to the conformational effect from the cyclohexane skeleton.
Keywords: conformational effect, caffeoylquinic acids, antioxidant, cytoprotective effect
1. Introduction
The activity of synthetic and natural antioxidants is derived from the molecular phenolic moiety, but it can be affected by structural factors such as hydrogen-bonding [1], the amount of phenolic –OHs [2,3], O-methylation [4], glycosidation [5], anisylation [6], and heterocycles [6]. Even the 6″-OH group of the sugar residue in flavonoid glycosides could alter the antioxidant levels [7].
However, these structural factors are limited to molecular “structure” and are not involved in the molecular “conformation.” From the perspective of organic chemistry [8], molecular conformation derives from the σ-bond free rotation, which can alter the spatial relative position of the moieties (or atoms). A representative example is the cyclohexane molecule, which can give two distinctive conformations: chair conformation and boat conformation. The chair conformation has been proven to be preferential; in it, the axial bond (a bond) and equatorial bond (e bond) are alternately arrayed. The difference between the a bond and e bond results in the chemical characteristics of the moieties (or atoms), which has been called the conformational effect. Recently, the conformational effect has been found to change some of the chemical properties, such as red-shifted emission, of luciferin [9]. It is hypothesized that, if an antioxidant moiety is attached to the cyclohexane skeleton and occupies different bond types (a/e bonds), its antioxidant potential may be distinctive. However, such conformational effects towards antioxidant ability have not, to our knowledge, yet been reported.
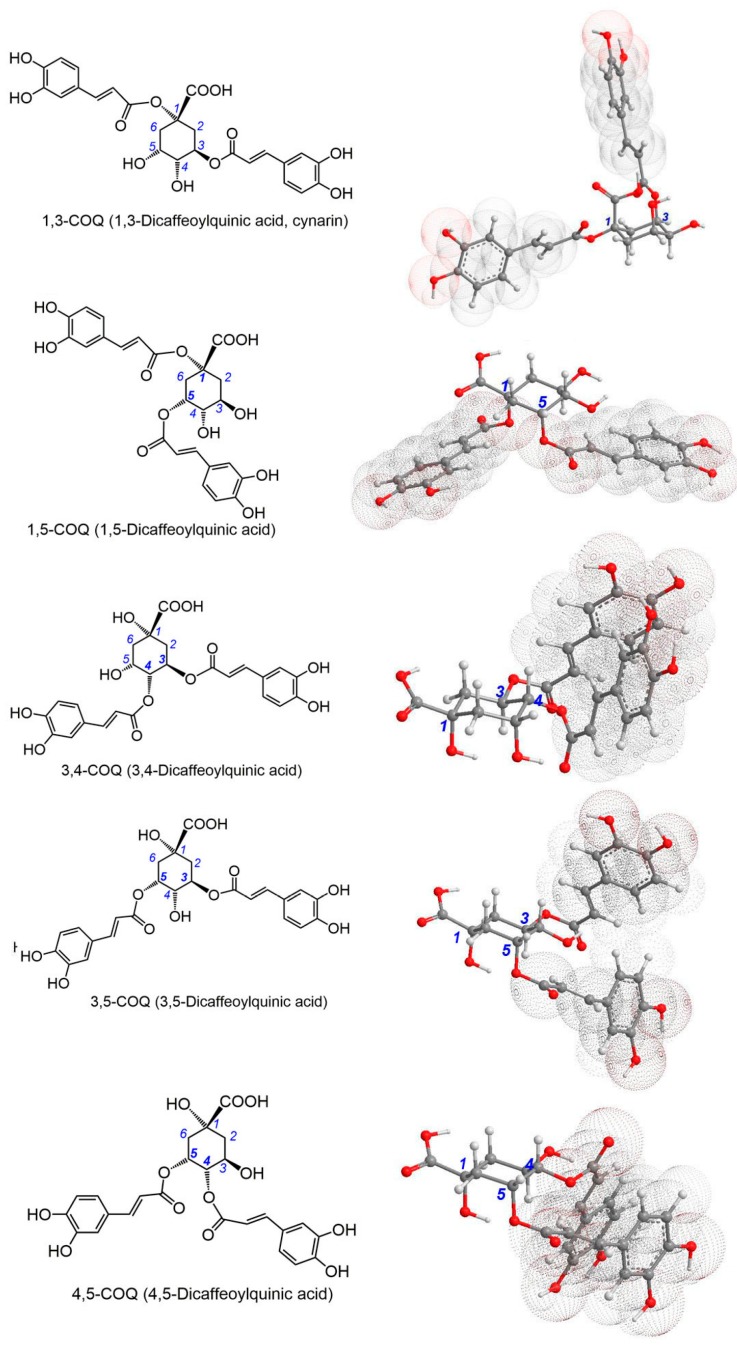
In this study, five di-O-caffeoylquinic acids (di-COQ), which are distributed in different plants [10,11,12,13,14], were selected as references for the conformational effect investigation. As shown in Figure 1, the five di-COQs comprise 1,3-di-O-caffeoylquinic acid (1,3-COQ), 1,5-di-O-caffeoylquinic acid (1,5-COQ), 3,4-di-O-caffeoylquinic acid (3,4-COQ), 3,5-di-O-caffeoylquinic acid (3,5-COQ), and 4,5-di-O-caffeoylquinic acid (4,5-COQ). In the five molecules, the caffeoyl moiety, with antioxidant potential, is attached to hexacyclic quinic acid; thus, these five acids may be the ideal reference for such an investigation.
Figure 1.
Structures (left) and preferential conformation-based ball-stick models (right) of five di-O-caffeoylquinic acids (di-COQs). The ball-stick models were created in Chem3D Pro 14.0. The screenshots from models are from the same perspective; i.e., C-1 was deposited on the right end, and –COOH is upward. The three-dimensional perspective animations are shown in Video S1–5. However, the relative degree of crowd for caffeoyl moieties remains unchanged.
It is worth noting that some of these compounds have already been explored for their antioxidant ability using a DPPH•-scavenging assay, ABTS•+-scavenging assay, anti-low-density lipoprotein (LDL) oxidation assay [11,15], and cellular assay [13]. However, each of these studies was based on the plant origin: Hung focused on antioxidants from Dipsacus asper, Zhang only explored antioxidants in Lonicera japonica, and Wan was only engaged in the phytochemical work of Chrysanthemum coronarium [14]. Thus, for di-COQs, these works are non-systematical. For instance, the phytochemical work of Wan lacks 1,3-COQ [14] and is irrelevant to the antioxidant study, and Hung’s work lacks two important members: 1,3-COQ and 1,5-COQ. However, some mono-O-caffeoylquinic acids (e.g., chlorogenic acid) and flavonoids were involved in these studies [10,11,14]. Hence, these works are non-comparative and cannot be used to analyze the structure–activity relationship of the di-COQs antioxidant family.
The present study, however, used five di-COQs for comparative study, based on chemical and cellular models. The cellular model is based on oxidatively stressed bone marrow-derived mesenchymal stem cells (bmMSCs). bmMSCs are considered a highly promising cell type candidate for cell-based tissue transplantation engineering and regeneration, but they are limited by their lower cellular viability derived from oxidative stress [16]. Our study will also provide new information about the di-COQs family regarding bmMSCs transplantation engineering.
2. Results and Discussion
Iron overload can induce oxidative stress to severely damage cells, which can cause a series of diseases (including neurodegeneration), resulting from the ability of iron (particularly Fe2+) to promote the generation of ROS [17]. A typical example is the Fenton reaction, which can produce •OH radicals. Thus, iron chelation has now been developed as a therapy for these diseases [18,19], and the iron chelation level of a natural antioxidant is regularly evaluated using colorimetric methods and UV-Vis spectra analysis [20,21]. However, UV-Vis spectra analysis is considered direct evidence of an iron chelating reaction [22,23].
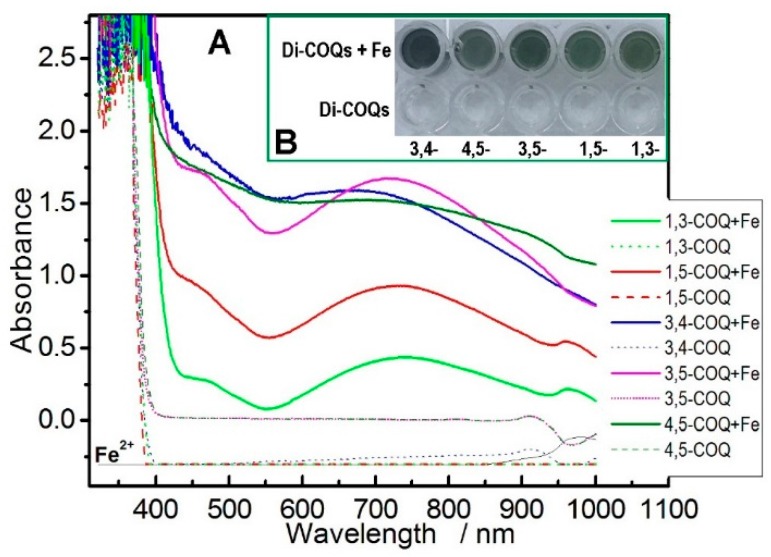
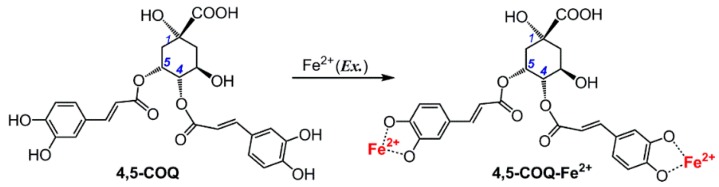
In the present study, we used UV-Vis spectra to analyze the Fe2+-chelating ability of the five di-COQs. As shown in Figure 2, after incubation with Fe2+, each of the five di-COQs gave rise to an absorption maximum around 750 nm and a green product mixture, suggesting that an Fe2+-chelating reaction between Fe2+ and each of the di-COQs occurs. Therefore, each of the di-COQs may undergo an Fe2+-chelating approach to reduce the oxidative stress from ROS (especially •OH). A typical Fe2+-chelating reaction could be proposed, as shown in Figure 3. Since Fe2+-chelating can indirectly release oxidative stress, it is sometimes called the indirect antioxidant mechanism.
Figure 2.
(A) UV-Vis spectra of the five di-COQs and their chelating products with excess Fe2+. (B) The colors of complexes, resulting from the product mixtures, were taken by a camera.
Figure 3.
The proposed chelating reaction of 4,5-COQ (4,5-di-O-caffeoylquinic acid) with excessive Fe2+ (the reaction formula is proposed based on previous studies [11,22,23,24]).
Correspondingly, radical-scavenging is termed a direct antioxidant mechanism. In this study, five di-COQs were observed to dose-dependently scavenge various radicals in chemical models, including PTIO•, DPPH•, and ABTS+• radicals (Figure S1). PTIO• is an oxygen-centered radical, whereas both DPPH• and ABTS+• are nitrogen-centered radicals. The ability of the five di-COQs to scavenge the three radicals implies that they can scavenge not only ROS but also reactive nitrogen species (RNS, e.g., ONOO− and NO) in cells and may undergo a direct antioxidant approach to reduce the oxidative stress.
Furthermore, these free radical-scavenging reactions are mediated by different antioxidant pathways. PTIO• scavenging at pH 4.5 is an electron transfer (ET) pathway [25]. The effectiveness of the five di-COQs with PTIO• scavenging at pH 4.5 shows that they can undergo ET to exert their antioxidant action, which is further supported by evidence from the FRAP assay, a mere ET process [26].
However, PTIO• scavenging at pH 7.4 was proven to be an H+-transfer pathway [27]. Since the five di-COQs can efficiently scavenge PTIO• at pH 7.4, this implies that a H+-transfer may play a role in their antioxidant action. It is worth noting that the so-called ET or H+-transfer is a unidirectional process, where the antioxidant donates an electron or H+ to the radical rather than the antioxidant accepting an electron or H+ from the radical [21,27,28].
Unlike the FRAP assay (or PTIO• scavenging at pH 4.5), DPPH•-scavenging and ABTS+•-scavenging assays are mediated via complicated pathways. DPPH•-scavenging includes multiple hydrogen atom transfer (HAT)-based pathways [27], and ABTS+• is scavenged through multiple ET-based pathways [26]. The DPPH•-scavenging and ABTS+•-scavenging by the five di-COQs indicated that their antioxidant actions may also be fulfilled via multiple antioxidant pathways, including H+-transfer, ET, and HAT.
It should be noted that the radical adduct formation (RAF) may also occur in the radical-scavenging action of phenolic antioxidants [28,29]. However, no RAF product peak was observed in the UPLC-ESI-MS/MS spectra of the di-COQs reaction products with PTIO•. By comparison, chlorogenic acid (a mono-O-caffeoylquinic acid) generated a peak at m/z 708, which is the value of the chlorogenic acid–chlorogenic acid dimer (Figure S2). This suggests that the five di-COQs cannot mediate RAF to exert the antioxidant action. The inactivity of di-COQs in the RAF pathway is presumed to be from steric hindrance, although this presumption needs further identification. Therefore, the evidence from the chemical models indicated that as natural antioxidants, di-COQs may undergo multiple antioxidant pathways (including H+-transfer, ET, or HAT, but not RAF) to exert their antioxidant action.
From the perspective of quantitative analysis, the IC50 values of the five di-COQs were different from each other (Table 1), which indicates that there are differences in the relative antioxidant levels. In general, adjacent di-COQs (4,5-COQ and 3,4-COQ) always possess higher levels than do non-adjacent di-COQs (1,3-COQ, 1,5-COQ, and 3,5-COQ). Interestingly, the relative levels are similar to the anti-inflammatory activities [30].
Table 1.
The IC50 values of five di-COQs in various antioxidant assays.
| Compounds | PTIO•-Scavenging (pH 4.5, mg/mL, mM) | PTIO• Scavenging (pH 7.4, mg/mL, mM) | FRAP (μg/mL, μM) | DPPH•-Scavenging (μg/mL, μM) | ABTS+•-Scavenging (μg/mL, μM) |
|---|---|---|---|---|---|
| 1,3-COQ | 47.2 ± 1.6 | 57.7 ± 1.0 | 3.4 ± 0.2 | 2.9 ± 0.1 | 3.6 ± 0.0 |
| (91.4 ± 3.6 e) | (111.8 ± 2.0 c) | (6.5 ± 0.4 c) | (5.7 ± 0.3 b) | (6.9 ± 0.1 c) | |
| 1,5-COQ | 35.5 ± 2.8 | 63.0 ± 7.6 | 3.3 ± 0.1 | 4.7 ± 0.6 | 3.5 ± 0.0 |
| (68.7 ± 5.4 d) | (121.9 ± 14.8 c) | (6.4 ± 0.2 c) | (9.2 ± 1.1 c) | (6.7 ± 0.1 c) | |
| 3,4-COQ | 19.1 ± 0.4 | 22.3 ± 6.2 | 2.6 ± 0.1 | 2.9 ± 0.5 | 3.2 ± 0.0 |
| (37.0 ± 0.5 c) | (43.1 ± 12.0 b) | (5.1 ± 0.2 b) | (5.7 ± 0.9 b) | (6.2 ± 0.1 b) | |
| 3,5-COQ | 55.9 ± 2.6 | 60.2 ± 2590 | 3.4 ± 0.0 | 3.2 ± 0.5 | 3.6 ± 0.1 |
| (108.0 ± 5.1 f) | (116.5 ± 5.0 c) | (6.7 ± 0.1 c) | (6.1 ± 0.9 b) | (7.0 ± 0.3 c) | |
| 4,5-COQ | 4.3 ± 0.3 | 23.3 ± 0.3 | 2.6 ± 0.1 | 1.7 ± 0.9 | 2.8 ± 0.0 |
| (8.3 ± 0.3 b) | (45.2 ± 0.6 b) | (3.4 ± 2.9 a) | (3.4 ± 1.8 a) | (5.4 ± 0.1 a) | |
| Trolox | 33.4 ± 0.5 | 26.8 ± 1.5 | 16.4 ± 2.9 | 6.2 ± 0.0 | 6.9 ± 0.1 |
| (0.1 ± 0.0 a) | (0.1 ± 0.0 a) | (31.7 ± 5.5 d) | (12.0 ± 0.1 d) | (13.4 ± 0.2 d) |
The IC50 value (in μg/mL unit) was defined as the final concentration of 50% radical inhibition or relative reducing power, calculated by linear regression analysis, and expressed as the mean ± SD (n = 3). The linear regression was analyzed by Origin 6.0 professional software. The IC50 value was also expressed in μM/mM unit. The IC50 value in the μM/mM unit, with different superscripts (a, b, c, d, e, or f) in the same diagram, are significantly different (p < 0.05). Trolox is the positive control. 1,3-COQ: 1,3-di-O-caffeoylquinic acid; 1,5-COQ: 1,5-di-O-caffeoylquinic acid; 3,4-COQ: 3,4-di-O-caffeoylquinic acid; 3,5-COQ: 3,5-di-O-caffeoylquinic acid; 4,5-COQ: 4,5-di-O-caffeoylquinic acid. The dose-response curves are listed in Figure S1.
As shown in Figure 1 (right), 4,5-COQ and 3,4-COQ contain two adjacent caffeoyl moieties and belong to adjacent di-COQs. Caffeoyl moieties are attached to the chair conformation-preferred hexacyclic skeleton, where the a and e bonds are alternately arrayed [9]. In 4,5-COQ, two caffeoyl moieties present a trans-configuration; in 3,4-COQ, however, two caffeoyl moieties display a cis-configuration. Despite having two adjacent caffeoyl moieties in a trans-configuration and an e bond, they are still very crowded. The degree of crowd increases the molecular energy, thereby elevating the redox potential. Thus, in the redox-based antioxidant assays, 4,5-COQ and 3,4-COQ, which contain two adjacent caffeoyl moieties, are always more effective than are the three non-adjacent di-COQs (1,3-COQ, 1,5-COQ, and 3,5-COQ). In each of the three non-adjacent di-COQs, two caffeoyl moieties are distant from each other, regardless of the a/e bonds and trans-/cis- configurations. The distance effectively releases the crowd to decrease the molecular energy and redox reactivity. Correspondingly, the antioxidant potential has been lowered.
The difference between adjacent and non-adjacent di-COQs can also be observed in the Fe2+-chelating assay. As shown in Figure 2A, compared by three non-adjacent di-COQs, both 4,5-COQ and 3,4-COQ gave stronger peaks in the UV spectra when treated by excessive Fe2+. In the aspect of complex color, 4,5-COQ and 3,4-COQ also yielded a darker color than did the three non-adjacent di-COQs (Figure 2B). These results imply that adjacent 4,5-COQ and 3,4-COQ also displayed a higher Fe2+-chelating ability than did the three non-adjacent di-COQs.
In each member of the di-COQs family, the ligand for the Fe2+-chelating reaction is the caffeoyl moiety [11]. As shown in Figure 1 (right), two caffeoyl moieties in 3,4-COQ and 4,5-COQ molecules stretch out of the skeleton on the same side, in which they can surround excessive Fe2+ to participate in the chelating reaction. In contrast, two caffeoyl moieties in non-adjacent di-COQs (especially 1,3-COQ and 1,5-COQ) extended from two directions and can hardly surround excessive Fe2+ for joint chelation. As a result, 3,4-COQ and 4,5-COQ are more effective Fe2+-chelators than are the three non-adjacent di-COQs. It should be noted that the dihydroxyl groups in the quinic acid ring cannot chelate metals to form a stable ringed complex, such as the 4,5-dihydroxyl groups in 1,3-COQ and the 3,4-dihydroxyl groups in 1,5-COQ [21,23,31]. In a word, two adjacent di-COQs (4,5-COQ and 3,4-COQ) always possess a higher antioxidant ability than the three non-adjacent di-COQs (1,3-COQ, 1,5-COQ, and 3,5-COQ) in chemical models.
In the cellular model, their relative cytoprotective levels exhibit only small changes. As shown in Table 2, all five di-COQs could concentration-dependently enhance the viability percentages of •OH-treated bmMSCs in the MTT assay. Furthermore, 1,3-COQ, 1,5-COQ, and 4,5-COQ gave higher viability percentages than did the other compounds. Generally speaking, two non-adjacent di-COQs (1,3-COQ and 1,5-COQ) exhibit a higher cytoprotective effect than adjacent di-COQs. It is hypothesized that 1,3-COQ and 1,5-COQ exhibited a better cytoprotective effect due to their molecular shapes. In 1,3-COQ and 1,5-COQ, two caffeoyl moieties extend from the molecule in different directions, resulting in a long and narrow structure, which may help them freely cross the cytomembrane to the nucleus. However, the above hypothesis requires further study. In short, these differences among the five di-COQs in terms of antioxidant or cytoprotective effects may result from the conformation of the coral cyclohexane skeleton.
Table 2.
The viability percentages of five di-COQs towards •OH-damaged bmMSCs in the MTT assay.
| Compounds | Control | Model | 10 μg/mL | 30 μg/mL | 50 μg/mL | 100 μg/mL |
|---|---|---|---|---|---|---|
| 1,3-COQ | 100% | 12.67% | 17.03% * | 20.23% * | 23.97% * | 47.15% * |
| 1,5-COQ | 100% | 12.67% | 19.09% * | 21.65% * | 24.90% * | 44.93% * |
| 3,4-COQ | 100% | 12.67% | 13.02% | 13.97% | 15.53% * | 21.15% * |
| 3,5-COQ | 100% | 12.67% | 13.06% | 16.21% * | 19.55% * | 23.38% * |
| 4,5-COQ | 100% | 12.67% | 14.14% | 21.78% * | 22.72% * | 42.04% * |
Experiments were performed with 3 different batches of cells and each batch was tested in triplicate. The Fenton reagent (FeCl2 plus H2O2) was used to generate •OH radicals. These data represent the mean ± SD (n = 3). * p < 0.05 vs. model. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. 1,3-COQ: 1,3-di-O-caffeoylquinic acid; 1,5-COQ: 1,5-di-O-caffeoylquinic acid; 3,4-COQ: 3,4-di-O-caffeoylquinic acid; 3,5-COQ: 3,5-di-O-caffeoylquinic acid; 4,5-COQ: 4,5-di-O-caffeoylquinic acid.
3. Materials and Methods
3.1. Chemicals and Animals
1,3-Di-O-caffeoylquinic acid (CAS 30964-13-7, 97%), 1,5-di-O-caffeoylquinic acid (CAS 19870-46-3, 97%), 3,4-di-O-caffeoylquinic acid (CAS 14534-61-3, 97%), 3,5-di-O-caffeoylquinic acid (CAS 2450-53-5, 97%), and 4,5-di-O-caffeoylquinic acid (CAS 57378-72-0, 97%) were obtained from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). The 1,1-diphenyl-2-picryl-hydrazl radical (DPPH•), (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid (Trolox), 2,4,6-tripyridyltriazine (TPTZ), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Shanghai Trading Co. (Shanghai, China). (NH4)2ABTS [2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt)] was obtained from Amresco Chemical Co. (Solon, OH, USA). The 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical (PTIO•) was from TCI Chemical Co. (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin were purchased from Gibco (Grand Island, NY, USA). CD44 and Proteinase K were purchased from Wuhan Boster Co., Ltd. (Wuhan, China). All other reagents were of analytical grade.
Sprague–Dawley (SD) rats of 4 weeks of age were obtained from the Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). The protocol of this experiment was performed under the supervision of the Institutional Animal Ethics Committee in Guangzhou University of Chinese Medicine (Approval number 20170306A).
3.2. UV-Vis Spectra Analysis of Fe2+-Chelating with di-COQs
This method was based on the previous study [20]. Briefly, 100 μL of a methanolic solution of di-COQs (3 mg/mL) was added to 400 μL of an aqueous solution of FeCl2·4H2O (10 mg/mL). The solution was then mixed vigorously. Subsequently, the resulting mixture was incubated at room temperature for 30 min, and the spectrum was obtained using a UV-Vis spectrophotometer (Jinhua 754 PC, Shanghai, China) from 200–1000 nm. Then, 200 μL of the supernatant was transferred to a 96-well plate and photographed using a camera.
3.3. PTIO•-Scavenging Assay
The PTIO•-scavenging assay was conducted based on our method [32]. In brief, 80 μL of an aqueous PTIO• solution (0.1 mM) was mixed with 20 μL of phosphate buffer (pH 4.5, 7.4) containing sample (5 mg/mL) at the indicated concentrations. The mixture was maintained at 37 °C for 2 h, and the absorbance was measured at 560 nm on a microplate reader (Multiskan FC, Thermo Scientific, Shanghai, China). The PTIO• inhibition percentage was calculated as follows:
| (1) |
where A0 indicates the absorbance of the blank and A indicates the absorbance of the sample.
3.4. FRAP Assay
The FRAP assay was established by Benzie and Strain [33]. In the present study, the FRAP reagent was prepared freshly by mixing 10 mM TPTZ, 20 mM FeCl3, and 0.25 M acetate buffer (pH 3.6) at 1:1:10. The sample solution (x = 1–9 μL, 0.1 mg/mL) was added to (20 − x) μL of 95% ethanol followed by 80 μL of FRAP reagent. After incubation at ambient temperatures for 30 min, the absorbance was measured at 595 nm using distilled water as the blank. The relative reducing power of the sample was calculated using the formula:
| (2) |
where Amax is the maximum absorbance, and Amin is the minimum absorbance in the test. A is the absorbance of sample.
3.5. DPPH•-Scavenging Assay
DPPH• radical-scavenging activity was determined as previously described [34]. Briefly, 80 μL of DPPH• solution (0.1 mol/L) was mixed with the indicated concentrations of sample (0.05 mg/mL, 2–10 μL) dissolved in methanol. The mixture was maintained at room temperature for 30 min, and the absorbance was measured at 519 nm on a microplate reader. The percentage of DPPH• scavenging activity was calculated based on the formula presented in Section 3.3.
3.6. ABTS•+-Scavenging Assay
The ABTS•+-scavenging activity was evaluated according to the method [24]. The ABTS•+ was produced by mixing 0.2 mL of (NH4)2ABTS (7.4 mmol/L) with 0.35 mL of potassium persulfate (2.6 mmol/L). The mixture was kept in the dark at room temperature for 12 h to allow completion of radical generation and then diluted with distilled water (about 1:20), so that its absorbance at 734 nm was measured on a microplate reader. To determine the scavenging activity, the test sample (x = 1–9 μL, 0.1 mg/mL) was added to (20 − x) μL of distilled water followed by 80 μL of ABTS•+ reagent, and the absorbance at 734 nm was measured 3 min after the initial mixing, using distilled water as the blank. The percentage inhibition of the samples was calculated based on the formula listed in Section 3.3.
3.7. UPLC-ESI-Q-TOF-MS/MS Analysis of Reaction Products of di-COQs and Chlorogenic Acid with PTIO•
This method was based on the previous study [27]. The methanol solution of di-COQs was mixed with a solution of PTIO• radical in methanol at a molar ratio of 1:2, and the resulting mixture was incubated for 24 h at room temperature. The product mixture was then filtered through a 0.22 μm filter and analyzed using a UPLC-ESI-Q-TOF-MS/MS system equipped with a C18 column (2.0 mm i.d. × 100 mm, 2.2 μm, Shimadzu Co., Kyoto, Japan). The mobile phase was used for the elution of the system and consisted of a mixture of methanol (Phase A) and water (Phase B). The column was eluted at a flow rate of 0.3 mL/min with the following gradient elution program: 0–10 min, 60%–100% A; 10–15 min, 100% A. The sample injection volume was set at 1 μL for the separation of the different components. Q-TOF-MS/MS analysis was performed on a Triple TOF 5600plus Mass spectrometer (AB SCIEX, Framingham, MA, USA) equipped with an ESI source, which was run in the negative ionization mode. The scan range was set at 100–2000 Da. The system was run with the following parameter: ion spray voltage: −4500 V; ion source heater: 550 °C; curtain gas (CUR, N2): 30 psi; nebulizing gas (GS1, Air): 50 psi; Tis gas (GS2, Air): 50 psi. The declustering potential (DP) was set at −100 V, whereas the collision energy (CE) was set at −40 V with a collision energy spread (CES) of 20 V. The RAF products were quantified by extracting corresponding formula (e.g., [C43H36N5O18−H]− for di-COQs-DPPH•) from the Total Ion Chromatogram, integrating the corresponding peak. We used chlorogenic acid as a positive control to repeat the above experiments, instead of di-COQs.
3.8. Cytoprotective Effect towards •OH-Damaged bmMSCs (MTT Assay)
The bmMSCs were cultured according to our previous report [35] with slight modifications. In brief, bone marrow was obtained from the femur and tibia of rat. The marrow samples were diluted with DMEM (low glucose) containing 10% FBS. The bmMSCs were prepared by gradient centrifugation at 900 g for 30 min on 1.073 g/mL Percoll. The prepared cells were detached by treatment with 0.25% trypsin and passaged into cultural flasks at 1 × 104/cm2. The bmMSCs at Passage 3 were evaluated for cultured cell homogeneity.
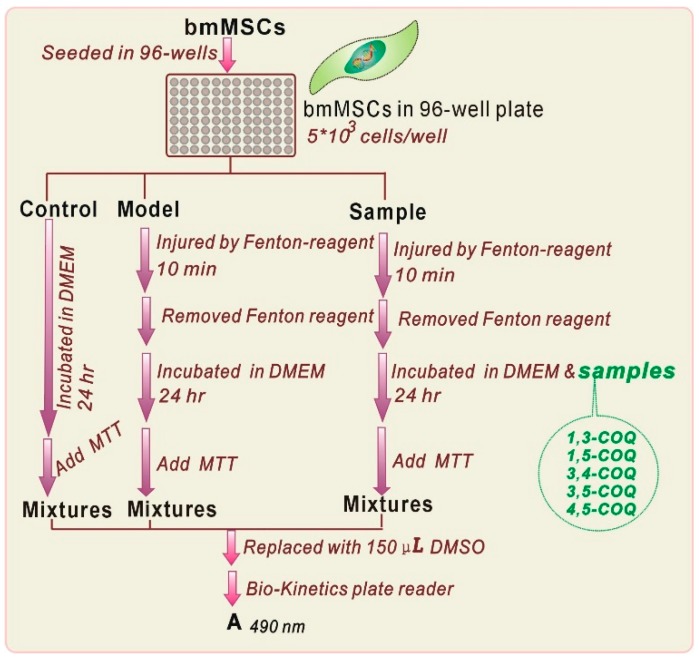
The MTT assay was used to evaluate cytoprotective effect of di-COQs towards bmMSCs [34,36,37]. The experimental protocol is briefly illustrated in Figure 4.
Figure 4.
Experimental procedures for the MTT assay. (PE-1420 Bio-Kinetics reader: Bio-Kinetics Corporation, Sioux Center, IA, USA. Each test was repeated in five independent wells. MTT was used at 5 mg/mL (in PBS), and the addition volume was 20 μL. The addition of Fenton reagent was conducted by injection of FeCl2 (100 μM) followed by H2O2 (50 μM).
3.9. Statistical Analysis
Each experiment was performed in triplicate and the data were recorded as mean ± SD (standard deviation). The dose response curves were plotted using Origin 6.0 professional software (OriginLab, Northampton, MA, USA). The IC50 value was defined as a final concentration of 50% radical inhibition (or relative reducing power) [38]. Statistical comparisons were made by one-way ANOVA to detect significant difference using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) for Windows. p < 0.05 was considered to be statistically significant.
4. Conclusions
Five antioxidant di-COQs can protect bmMSCs from •OH-induced damage. Their antioxidant mechanisms may include ET, H+-transfer, and Fe2+-chelating, except for RAF. However, the antioxidant (or cytoprotective) levels are different among them. These differences can be attributed to the positions of the two caffeoyl moieties and, ultimately, to the conformational effect from the cyclohexane skeleton.
Acknowledgments
This work was supported by the Guangdong Science and Technology Project (2017A030312009, 2017A050506043) and the National Nature Science Foundation of China (81573558).
Abbreviations
The following abbreviations are used in this manuscript:
| 1,3-COQ | 1,3-di-O-caffeoylquinic acid |
| 1,5-COQ | 1,5-di-O-caffeoylquinic acid |
| 3,4-COQ | 3,4-di-O-caffeoylquinic acid |
| 3,5-COQ | 3,5-di-O-caffeoylquinic acid |
| 4,5-COQ | 4,5-di-O-caffeoylquinic acid |
| ABTS | [2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt)] |
| bmMSCs | bone marrow-derived mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH• | (1,1-diphenyl-2-picryl-hydrazl) |
| ET | electron transfer |
| FBS | fetal bovine serum |
| FRAP | ferric reducing antioxidant power |
| HAT | hydrogen atom transfer |
| PTIO• | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| RAF | radical adduct formation |
| SD | standard deviation |
| TPTZ | 2,4,6-tris(2-pyridyl-s-triazine) |
| Trolox | [(±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid] |
Supplementary Materials
The following are available online. Video S1–5: Animations of 1,3-COQ, 1,5-COQ, 3,4-COQ, 3,5-COQ, and 4,5-COQ. Figure S1: Dose response curves of five di-COQs in various assays. Figure S2: Original Data of UPLC-ESI-MS/MS analysis.
Author Contributions
Xican Li, Xiaohua Jiang, and Dongfeng Chen conceived and designed the experiments; Yueying Li, Hong Xie, and Xiaojun Zhao performed the antioxidant experiments; Ke Li performed the MTT assay; Yulu Xie analyzed the data; Xican Li wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Liliana M. Intramolecular hydrogen bonding and conformational preferences of arzanol—An antioxidant acylphloroglucinol. Molecules. 2017;22:1294. doi: 10.3390/molecules22081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Chen D., Mai Y., Wen B., Wang X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi) Nat. Prod. Res. 2012;26:1050–1053. doi: 10.1080/14786419.2010.551771. [DOI] [PubMed] [Google Scholar]
- 3.Wang T., Zeng G., Li X., Zeng H. In vitro studies on the antioxidant and protective effect of 2-substituted- 8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010;75:214–222. doi: 10.1111/j.1747-0285.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 4.Heim K.E., Tagliaferro A.R., Bobily D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 5.Li X.C., Liu J.J., Lin J., Wang T.T., Huang J.Y., Lin Y.Q., Chen D.F. Protective effects of dihydromyricetin against •OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules. 2016;21:604. doi: 10.3390/molecules21050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G., Li X., Zeng H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009;67:974–982. [Google Scholar]
- 7.Li X.C., Jiang Q., Wang T.T., Liu J.J., Chen D.F. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH group. Molecules. 2016;21:1246. doi: 10.3390/molecules21091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham Solomons T.W., Fryhle C.B. Organic Chemistry. 8th ed. Chemical Industry Press; Beijing, China: 2004. p. 167. [Google Scholar]
- 9.Berraud-Pache R., Navizet I. QM/MM calculations on a newly synthesised oxyluciferin substrate: New insights into the conformational effect. Phys. Chem. Chem. Phys. 2016;18:27460–27467. doi: 10.1039/C6CP02585D. [DOI] [PubMed] [Google Scholar]
- 10.Cho J.Y., Kim J.Y., Lee Y.G., Lee H.J., Shim H.J., Lee J.H., Kim S.J., Ham K.S., Moon J.H. Four New Dicaffeoylquinic Acid Derivatives from Glasswort (Salicornia herbacea L.) and Their Antioxidative Activity. Molecules. 2016;21:1097. doi: 10.3390/molecules21081097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung T.M., Na M., Thuong P.T., Su N.D., Sok D., Song K.S., Seong Y.H., Bae K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall Tran. J. Ethnopharmacol. 2006;108:188–192. doi: 10.1016/j.jep.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Ma J., Li N., Li X. Caffeoylquinic acid derivatives from leaves of Lonicera japonica. Zhongguo Zhong Yao Za Zhi. 2009;34:2346–2348. [PubMed] [Google Scholar]
- 13.Yu B., Li J.L., Guo B.B., Fan H.M., Zhao W.M., Wang H.Y. Chlorogenic acid analogues from Gynura nepalensis protect H9c2 cardiomyoblasts against H2O2-induced apoptosis. Acta Pharmacol. Sin. 2016;37:1413–1422. doi: 10.1038/aps.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan C., Li S., Liu L., Chen C., Fan S. Caffeoylquinic acids from the aerial parts of Chrysanthemum coronarium L. Plants. 2017;6:10. doi: 10.3390/plants6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Du N., Wen L., Zhu H., Liu F., Wang X., Du J., Li S. An efficient method for the preparative isolation and purification of flavonoid glycosides and caffeoylquinic acid derivatives from leaves of lonicera japonica thunb. Using high speed counter-current chromatography (HSCCC) and prep-HPLC guided by DPPH-HPLC experiments. Molecules. 2017;22:229. doi: 10.3390/molecules22020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denu R.A., Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell. Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivars D., Orero M.T., Javier K., Díaz-Vico L., García-Giménez J.L., Mena S., Tormos C., Egea M., Pérez P.L., Arrizabalaga B., et al. Oxidative imbalance in low/intermediate-1-risk myelodysplastic syndrome patients: The influence of iron overload. Clin. Biochem. 2017;50:911–917. doi: 10.1016/j.clinbiochem.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Chiueh C.C. Iron overload, oxidative stress, and axonal dystrophy in brain disorders. Pediatr. Neurol. 2001;25:138–147. doi: 10.1016/S0887-8994(01)00266-1. [DOI] [PubMed] [Google Scholar]
- 19.Murillo-Ortiz B., Ramírez Emiliano J., Hernández Vázquez W.I., Martínez-Garza S., Solorio-Meza S., Albarrán-Tamayo F., Ramos-Rodríguez E., Benítez-Bribiesca L. Impact of oxidative stress in premature aging and iron overload in hemodialysis patients. Oxid. Med. Cell. Longev. 2016;2016:1578235. doi: 10.1155/2016/1578235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Li X., Lin J., Li Y., Wang T., Jiang Q., Chen D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016;16:423. doi: 10.1186/s12906-016-1383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Han W., Mai W., Wang L. Antioxidant activity and mechanism of Tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013;8:787–789. [Google Scholar]
- 22.Přemysl M., Kateřina M., Tomáš F., Libuše Z., Luděk J., Paolo B., Ilaria P.S., Radomír H., Luciano S. In vitro analysis of iron chelating activity of flavonoids. Org. Biochem. 2011;105:693–701. doi: 10.1016/j.jinorgbio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Mira L., Fernandez M.T., Santos M., Rocha R., Florêncio M.H., Jennings K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 24.Li X.C., Mai W.Q., Chen D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014;61:383–391. doi: 10.1002/jccs.201300396. [DOI] [Google Scholar]
- 25.Goldstein S., Russo A., Samuni A. Reactions of PTIO and Carboxy-PTIO with •NO, •NO2, and O2. Biol. Chem. 2003;278:50949–50955. doi: 10.1074/jbc.M308317200. [DOI] [PubMed] [Google Scholar]
- 26.Gülçin İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 27.Lin J., Li X., Chen L., Lu W., Chen X., Han L., Chen D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014;35:1633–1638. doi: 10.5012/bkcs.2014.35.6.1633. [DOI] [Google Scholar]
- 28.Li X., Gao Y., Li F., Liang A., Xu Z., Bai Y., Mai W., Han L., Chen D. Maclurin protects against Hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014;219:221–228. doi: 10.1016/j.cbi.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Osman A.M., Wong K.K.Y., Fernyhough A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem. Biophys. Res. Commun. 2006;34:6321–6329. doi: 10.1016/j.bbrc.2006.05.118. [DOI] [PubMed] [Google Scholar]
- 30.Song Y.L., Wang H.M., Ni F.Y., Wang X.J., Zhao Y.W., Huang W.Z., Wang Z.Z., Xiao W. Study on anti-inflammatory activities of phenolic acids from Lonicerae Japonicae Flos. Chin. Tradit. Herb. Drugs. 2015;46:490–495. [Google Scholar]
- 31.Jiang Q., Li X., Tian Y., Lin Q., Xie H., Lu W., Chi Y., Chen D. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect mesenchymal stem cells from •OH–treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017;17:242. doi: 10.1186/s12906-017-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) Radical-scavenging: A New and simple antioxidant assay in vitro. Agric. Food Chem. 2017;65:6288–6297. doi: 10.1021/acs.jafc.7b02247. [DOI] [PubMed] [Google Scholar]
- 33.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Wei G., Wang X., Liu D., Deng R., Li H., Zhou J., Li Y., Zeng H., Chen D. Targeting of the Shh pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012;35:1328–1335. doi: 10.1248/bpb.b12-00265. [DOI] [PubMed] [Google Scholar]
- 35.Chen D., Li X., Xu Z., Liu X., Du S., Li H., Zhou J., Zeng H., Hua Z. Hexadecanoic acid from Buzhong Yiqi decoction induces proliferation of bone marrow mesenchymal stem cells. J. Med. Food. 2010;13:967–970. doi: 10.1089/jmf.2009.1293. [DOI] [PubMed] [Google Scholar]
- 36.Li X., Hu Q., Jiang S., Li F., Lin J., Han L., Hong Y., Lu W., Gao Y., Chen D. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism: A chemistry study. J. Saudi Chem. Soc. 2015;19:454–460. doi: 10.1016/j.jscs.2014.06.004. [DOI] [Google Scholar]
- 37.Li X., Han L., Li Y., Zhang J., Chen J., Lu W., Zhao X., Lai Y., Chen D., Wei G. Protective effect of sinapine against hydroxyl radical-induced damage to mesenchymal stem cells and possible mechanisms. Chem. Pharm. Bull. 2016;64:319–325. doi: 10.1248/cpb.c15-00850. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Wu X., Huang L. Correlation Between Antioxidant Activities and Phenolic Contents of Radix Angelicae Sinensis (Danggui) Molecules. 2009;14:5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.