Abstract
Diosmetin, a plant flavonoid, has been shown to exert promising effects on prostate cancer cells as an anti-proliferative and anticancer agent. In this study, using western blot analysis for protein expression and flow cytometry for cell cycle analysis, we determined that the treatment of the LNCaP and PC-3 prostate cancer cells with diosmetin resulted in a marked decrease in cyclin D1, Cdk2 and Cdk4 expression levels (these proteins remain active in the G0-G1 phases of the cell cycle). These changes were accompanied by a decrease in c-Myc and Bcl-2 expression, and by an increase in Bax, p27Kip1 and FOXO3a protein expression, which suggests the potential modulatory effects of diosmetin on protein transcription. The treatment of prostate cancer cells with diosmetin set in motion an apoptotic machinery by inhibiting X-linked inhibitor of apoptosis (XIAP) and increasing cleaved PARP and cleaved caspase-3 expression levels. On the whole, the findings of this study provide an in-depth analysis of the molecular mechanisms responsible for the regulatory effects of diosmetin on key molecules that perturb the cell cycle to inhibit cell growth, and suggest that diosmetin may prove to be an effective anticancer agent for use in the treatment of prostate cancer in the future.
Keywords: prostate cancer cells, diosmetin, growth inhibition
Introduction
The most common and slow-growing cancer in males is prostate cancer. The prerequisites for any type of cancer, including prostate cancer are increased cell viability and cell survival, traits which are inherited through the dysregulation of cell cycle events. Cyclin-dependent kinases (Cdks) and their modulatory cyclin partners are the key molecules of cell cycle regulation. The phosphorylation of specific kinase regulates Cdks and cyclin proteins (1). Additionally, c-Myc activation promotes the induction of cyclin D1 transcription through Cdk-specific kinase (2). Activated cyclin D1 forms complexes with Cdk4 and Cdk6, and these complexes act in the early G1 phase of the cell cycle; conversely, cyclin E forms complexes with Cdk2 and act in the late G1 phase and S phase of the cell cycle (3). Much attention has been paid towards the identification of novel agents with the ability to modulate the cell cycle by altering Cdks and acting as Cdk inhibitors in human cancers (4,5). There is a need for the development of effective treatment agents with less to no toxicity. The majority of the anticancer agents are associated with side-effects or drug-related toxicities, which limits their uses (6,7). Anticancer strategies should ideally involve the use of an effective agent with the ability to modulate cell cycle regulatory molecules without affecting normal cells. Diosmetin is an O-methylated flavone (3′,5,7-trihydroxy-4′-methoxyflavone) abundantly present in legumes and olive leaves, and has shown to have potential for use as an anticancer agent (8–10). Diosmetin selectively induces apoptosis and inhibits cancer cell growth without affecting normal cells (10,11). It has been suggested that diosmetin exerts growth inhibitory effects through various signal transduction pathways, which have relevance in cancer (12–14). Some studies have suggested that diosmetin exerts anti-carcinogenic effects on cancer cells through the induction of apoptosis (9,10,14). Recently in an in vivo model system, diosmetin treatment was shown to delay acute myeloid leukemia tumor progression (15). Various kinases and proteins have been reported as the potential sites at which diosmetin funtions, and these include the c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and AKT signaling pathways, as well as the p53/p21 pathways (9,10,16). Diosmetin-induced cell growth arrest has been shown to be associated with observed a marked decrease in cyclin activity and Cdk levels, with an increase in the levels of p21Cip1/p53 and Cdk inhibitor expressions (16). Moreover, it has been suggested that diosmetin exerts anticancer effects on hepatocellular carcinoma cells, partly through the p53 enzyme, which regulates cytochrome P450 CYP1A (9). However, the role of diosmetin in prostate cancer has not yet been fully elucidated. Thus, the present study aimed to investigate the role of diosmetin in the cell cycle machinery and the induction of the apoptosis of prostate cancer cells.
Material and methods
Reagents and cell lines
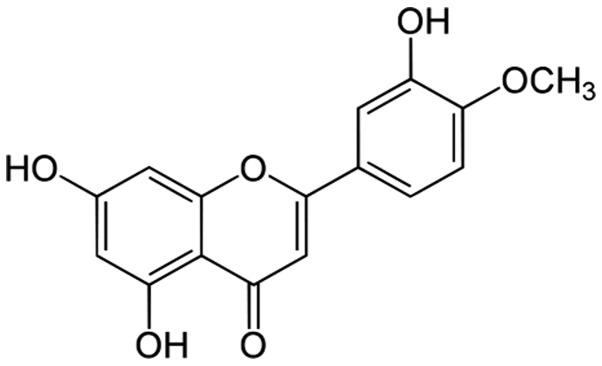
Diosmetin (>99% purity; chemical structure shown in Fig. 1), 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The prostate cancer cells (LNCaP and PC-3) were purchased from the American Type Culture Collection (Manassas, VA, USA). The RPMI-1640 medium used to culture the cells, phosphate-buffered saline (PBS) and trypsin were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The reagents used for western blot analysis were purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Figure 1.
Structure of diosmetin; 3′,5,7-trihydroxy-4′-methoxyflavone.
Cell culture and treatment
Both human prostate androgen-dependent cancer cells (LNCaP) and human prostate androgen-independent cancer cells (PC-3) were cultured in RPMI-1640 medium with 10 and 5% fetal bovine serum, respectively. The cells were treated with diosmetin at various concentrations (5–80 µM) for various periods of time (24, 48 and 72 h). The cells were grown at 37°C with 5% CO2/95% air and were passaged every 48 h using trypsin-ethylene-diaminetetra-acetic acid (EDTA).
Cell viability assay (MTT assay)
The PC-3 or LNCaP cells were seeded into 96-well flat-bottomed plates at 25% confluency. After 24 h of normal growth, the medium was carefully removed without disturbing the cells and the cells were treated with diosmetin at various concentrations (0, 2.5, 5, 10, 20, 40 and 80 µM) and sterile dimethyl sulfoxide (DMSO) used as a vehicle control. Each drug dosage was repeated 12 times within the 96-well plate. After the cells were treated for various periods of time (24, 48 and 72 h), the treatment was terminated. MTT was dissolved in sterile DMSO at 5 mg/ml and a working solution was made of 1 ml from stock MTT:4 ml media. Since MTT is light-sensitive, the procedure was performed in the presence of very little light. The working solution was added to the cells followed by incubation at 37°C for 3 h to form formazan crystals, which are purple-colored. Subsequently, the MTT solution was removed and the cells/crystals were solubilized with DMSO and shaken for 10 min. The results were read at 540 nm using a microplate reader with a built-in UV/VIS spectrophotometer (FLUOstar Omega Microplate Reader, BMG Labtech, Cary, NC, USA). IC50 values were determined to the concentration of diosmetin to reduce by 50% the growth of the treated cells in 24 h. Data were analyzed to determine the percentage of cells unaffected by treatment and the standard deviation between the 12 different trials.
Cell cycle analysis (flow cytometry)
The untreated and treated LNCaP and PC-3 cells were fixed by using cold 100% methanol and stored at −20°C indefinitely. The cells were then removed from the methanol and added to RNAse solution that included sodium azide, EDTA and PBS. This was followed by incubation at 37°C for 15–30 min and chilling at 4°C for 10 min. PI was then used to stain the cells, since it intercalates into the major groove of double-stranded DNA and produces a highly-fluorescent adduct that can be excited at 488 nm with broad emission centered around 600 nm (17).
Western blot analysis
Total cell lysate was prepared using lysis buffer containing 10 µg/ml phenylmethylsulfonyl fluoride (PMSF), 20 µg/ml protease inhibitor cocktail (PIC), 20 µg/ml sodium fluoride and 10 µg/ml sodium orthovanadate (Na3VO4). The protein concentration in the cell lysate was estimated using Lowry protein assay and 25 µg of protein was aliquoted to run a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a 4–20% Tri-glycine gel. Data from the gel were transferred onto a nitrocellulose membrane and incubated with the anti-cyclin D1 (sc-450), anti-Cdk2 (sc-6248), anti-Cdk4 (sc-260), anti-p27 (sc-393380), anti-GAPDH (sc-166545), anti-Bax (sc-493), anti-Bcl2 (sc-7382), anti-c-Myc (sc-40), anti-cyclin E (sc-248) were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Additionally anti-XIAP (#2042), anti-FOXO3a (#12829), anti-cleaved caspase-3 (#9661), anti-cleaved PARP (#5625) procured from Cell Signaling Technology (Danvers, MA, USA) and anti-β-actin (#A1978) were procured from Sigma-Aldrich. Secondary antibodies used were anti-mouse (#HAF007) anti-rabbit (#HAF008) from R&D Systems (Minneapolis, MN, USA). All antibodies were diluted to 1:1,000 dilution of stock solution of 1 mg/ml. Densitometric analysis of the protein bands were performed using the Kodak 2000R imaging system digitalized scientific software program.
Statistical analysis
Each experiment was performed at least in triplicate. The results are expressed as the means ± standard deviation. Statistical analysis was performed using analysis of variance (ANOVA) with a post hoc Fisher’s LSD test between the treated and the control groups. As there were multiple groups for the time points and diosmetin concentrations, we therefore also applied Kruskal-Wallis tests and the normality test to confirm that there was no difference. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
Prostate cancer cell growth inhibition by diosmetin
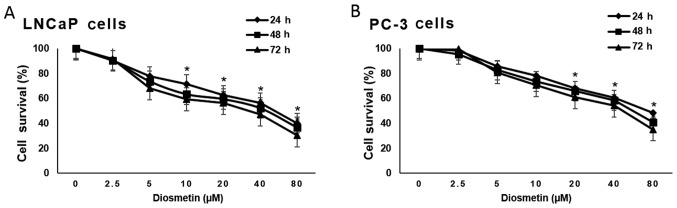
Treatment of the prostate cancer cells, LNCaP (androgen-responsive) and PC-3 cells (androgen-refractory), with diosmetin exerted diosmetin dose-dependent (0–80 µM) inhibitory effects on cell growth, compared to vehicle (DMSO-treated) controls by MTT assay. The magnitude of cell growth inhibition was higher in the LNCaP cells compared to the PC-3 cells. Diosmetin exerted clear dose-dependent inhibitory effects on prostate cancer cell growth. Moreover, we observed that diosmetin also suppressed prostate cancer cell growth in a time-dependent manner; the effects were more noticeable at 48 and 72 h of treatment than at 24 h (Fig. 2).
Figure 2.
Human prostate cancer cell growth inhibition by diosmetin. Asynchronous (A) LNCaP and (B) PC-3 cells were treated with diosmetin at increasing concentrations and exposure times and later subjected to MTT assay. Values are represented as the means ± SD from 3 independent experiments. *P<0.05 for time points vs. vehicle (DMSO) controls.
Cell cycle arrest following diosmetin treatment
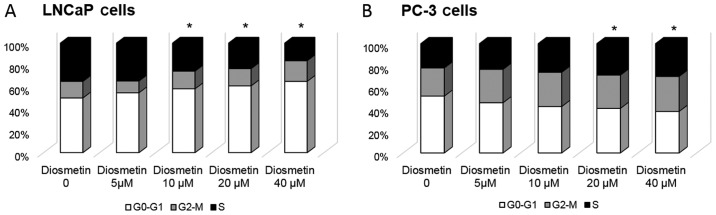
Using flow cytometric analysis, we assessed whether the cell growth inhibitory effects induced by diosmetin are mediated through alterations in the cell cycle. As we observed the marked inhibitory effects of diosmetin on cell growth, we further treated the asynchronous LNCaP and PC-3 cells with various concentrations (0–40 µM) of diosmetin for 24 h to determine the DNA content at various stages of the cell cycle. The vehicle (DMSO-treated) control LNCaP cells represented ~50% of the cells that were in the G0-G1 phase of the cell cycle. Following treatment with increasing concentrations of diosmetin, we observed that the cell number in the G0-G1 phase increased, evidenced by an increased accumulation of cells in the G0-G1 phase. Treatment with diosmetin at 5 µM resulted in 55% of the cells being arrest in the G0-G1 phase of the cell cycle, whereas treatment with 10 µM diosmetin further increased the number of cells in the G0-G1 phase to 58%. At the concentrations of 20 and 40 µM, diosmetin increased the number of cells in the G0-G1 phase to 61 and 65%, respectively (Fig. 3A). In the PC-3 cells, we observed evident S phase arrest following diosmetin treatment. Treatment of the PC-3 cells with diosmetin at 5 µM resulted in 24% of the cells being arrest at the S phase; at the concentrations of 10, 20 and 40 µM, diosmetin led to 26, 29 and 30% of the cells being arrested at the S phase compared to the vehicle-treated controls (22%) (Fig. 3B). The dose-dependent increase in the S phase cell population of the PC-3 cells induced by diosmetin was associated with a concomitant decrease in the number of cells in the G0-G1 phase, whereas opposite effects were observed in the LNCaP cells. These differences may be due to the different origin of the cell lines, as the LNCaP cells are androgen-sensitive and the PC-3 cells are androgen refractory.
Figure 3.
DNA content cell cycle analysis profiling of (A) LNCaP and (B) PC-3 cells following diosmetin treatment. These cells were initially treated with the vehicle (DMSO-treated) or with increasing doses of diosmetin for 24 h, stained with dye (propidium iodide) and analyzed by flow cytometry. Cell percentages were calculated using cell fit computer software in G0-G1, S and G2-M phases and values were plotted as averages from 3 different sets of experiments performed in duplicate. *P<0.05 for G0-G1 phase vs. control.
Alterations in cell cycle regulatory molecules following diosmetin dose-response treatment
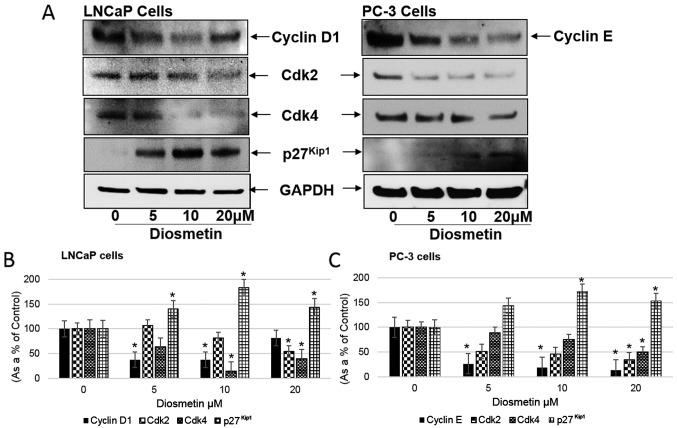
Diosmetin induced the downregulation of cyclin D1, cyclin E, Cdk2 and Cdk4 levels. Diosmetin at concentrations of 10 and 20 µM suppressed the growth of the LNCaP and PC-3 cells. These effective diosmetin concentrations were also able to decrease Cdk expression levels in the LNCaP cells at IC50 ± SD 10±2 µM and whereas, in PC-3 cells it was 18±5 µM. These results suggest that diosmetin may be responding more like a Cdk inhibitor; however, other mechanisms may be involved. We further evaluated the diosmetin dose-response effect on the expression levels of cyclin D1, cyclin E, Cdk2 and Cdk4, operating in the G1 and S phase of the cell cycle machinery by protein western blot analysis (Fig. 4A). A decreased cyclin D1 expression was observed in the LNCaP cells following treatment with diosmetin (37.61% at 5 µM and 37.35% at 10 µM). In the PC-3 cells, cyclin E levels also decreased following treatment with diosmetin (26.34% at 5 µM, 19.07% at 10 µM and 13.95% at 20 µM). We further observed that the expression levels of Cdk2 and Cdk4 significantly decreased following diosmetin treatment of the prostate cancer cells. In the LNCaP cells, diosmetin treatment resulted in a decreased Cdk2 expression (81.74% at 10 µM and 54.49% at 20 µM). Similarly in the PC-3 cells, Cdk2 expression also decreased following treatment with diosmetin (50.88% at 5 µM, 45.56% at 10 µM and 34.61% at 20 µM). Moreover, in the LNCaP cells following diosmetin treatment, Cdk4 expression decreased (63.96% at 5 µM, 14.79% at 10 µM and 40.07% at 20 µM); a decrease in Cdk4 expression was also observed in the PC-3 cells (89.28% at 5 µM, 75.15% at 10 µM and 50% at 20 µM) (Fig. 4B and C). The Cdk2 and Cdk4 expression levels were decreased compared with the controls.
Figure 4.
Dose-response effects of diosmetin on cell cycle regulatory molecules. (A) LNCaP and PC-3 cells; both asynchronous cells were exposed to increasing concentrations (0, 5, 10 and 20 µM) of diosmetin for 24 h. Total cell lysates were prepared and using SDS-PAGE, lysates proteins were resolved and then subjected to western blot analysis for the evaluation of cyclin D1, cyclin E, Cdk2, Cdk4 and p27Kip1. Lanes marked with ‘0’ are the DMSO control (0.2%)-treated cells. For protein band density, densitometric analysis was performed and proteins expression levels were correlated to express relative controls (B and C). For loading control blots were stripped and reprobed with GAPDH. *P<0.05 for diosmetin concentration vs. vehicle controls.
Additionally p27Kip1 expression in the LNCaP cells increased following diosmetin dose-response treatment (67.33% at 5 µM, 143.33% at 10 µM and 109.77% at 20 µM). Similarly in the PC-3 cells, p27Kip1 expression increased (143.54% at 5 µM, 171.77% at 10 µM and 153.22% at 20 µM) compared to the vehicle-treated controls (Fig. 4B and C).
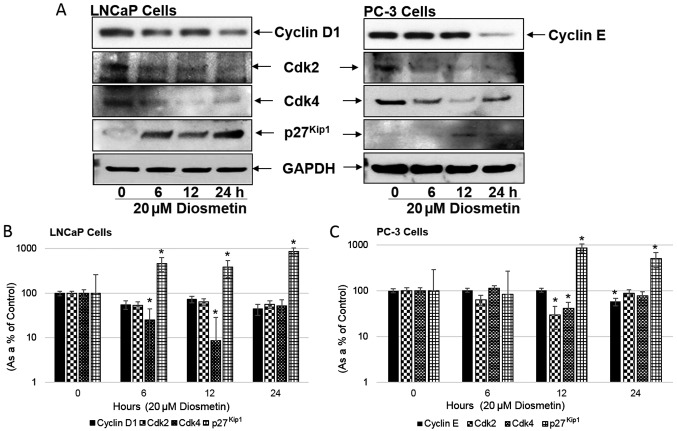
Modulation of cell cycle regulatory molecules following diosmetin time-response treatment
We then examined the diosmetin time-dependent treatment effects on the cell cycle modulatory molecules, cyclin D1, cyclin E, Cdk2, Cdk4 and p27Kip1 (Fig. 5A). Diosmetin time-response treatment (20 µM) of the LNCaP cells led to a decrease in cyclin D1 expression compared to the controls (6 h, 55.33%; 12 h, 72.88%; and 24 h, 44.16%); in the PC-3 cells, a decrease was also observed in the cyclin E levels (57.92% at 24 h). Both the Cdk2 and Cdk4 levels were also markedly decreased in these cells treated with diosmetin. In the LNCaP cells, diosmetin treatment resulted in a decreased Cdk2 expression (53.72% at 6 h, 64.52% at 12 h and 56.93% at 24 h). Similarly in the PC-3 cells, a decrease was observed in Cdk2 expression (63.39% at 6 h and 29.76% at 12 h). Moreover in the LNCaP cells treated with diosmetin, Cdk4 expression decreased (25.18% at 6 h, 8.77% at 12 h and 51.57% at 24 h). In the PC-3 cells, a decrease was also observed in Cdk4 expression (40.54% at 12 h and 77.97% at 24 h) (Fig. 5B and C). Similar to the diosmetin dose-response decrease in the levels of cyclins, diosmetin time-response treatment also decreased the expression levels of Cdk2 and Cdk4 compared to the controls.
Figure 5.
Time-response effects of diosmetin on cell cycle regulatory molecules. (A) LNCaP and PC-3 cells; both asynchronous cells were exposed to increasing times (0, 6, 12 and 24 h) of diosmetin (20 µM). Total cell lysates were prepared and using SDS-PAGE, lysates proteins were resolved and then subjected to western blot analysis for the evaluation of cyclin D1, cyclin E, Cdk2, Cdk4 and p27Kip1. Lanes marked ‘0’ are the DMSO control (0.2%)-treated cells. For protein band density, densitometric analysis was performed and protein expression levels were correlated to express relative controls (B and C). For loading control blots were stripped and reprobed with GAPDH. *P<0.05 for time points vs. vehicle controls.
Additionally p27Kip1 expression in the LNCaP cells increased significantly following diosmetin 20 µM time-response treatment, by ~4-fold at 6 h, >3-fold at 12 h and ~9-fold at 24 h. Similarly in the PC-3 Cells, p27Kip1 expression increased (83.33% at 6 h, >8-fold at 12 h and 5-fold at 24 h) compared to the vehicle-treated controls.
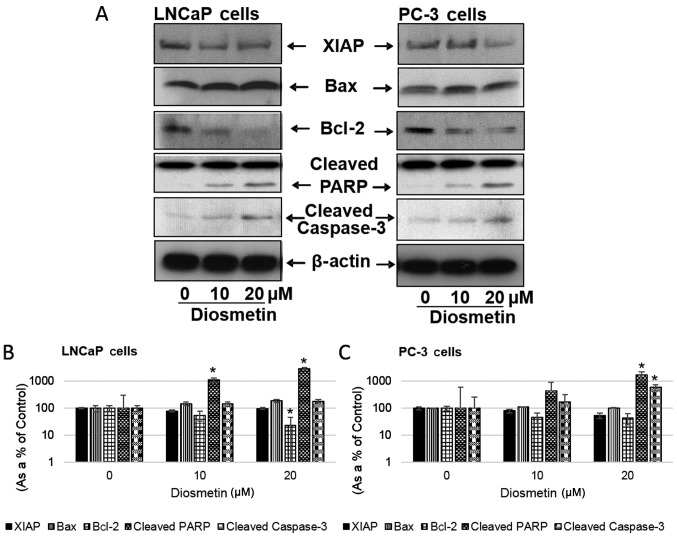
Diosmetin induces the apoptotic response in prostate cancer cells
Treatment of the LNCaP and PC-3 cells with increasing concentrations of diosmetin altered the levels of the apoptosis-related molecules, Bax, Bcl-2, cleaved caspase-3 and cleaved PARP (Fig. 6A). Diosmetin dose-response treatment of the LNCaP cells increased Bax expression 1.5-fold at 10 µM and ~2-fold at 20 µM. In the PC-3 cells Bax expression also increased (110.33% at 10 µM and 103.29% at 20 µM). Conversely, in the LNCaP cells, Bcl-2 expression levels decreased (54.92% at 10 µM and 23.11% at 20 µM) and similarly, Bcl-2 expression levels were also decreased in the PC-3 cells (46.21% at 10 µM and 42.96% at 20 µM). The levels of the apoptosis inducers, cleaved caspase-3 and cleaved PARP, were also markedly increased in the cells following diosmetin dose-response treatment. In the LNCaP cells, diosmetin treatment resulted in increased cleaved caspase-3 expression (144.37% at 10 µM and 143.37% at 20 µM). Similarly, in the PC-3 cells, the expression of cleaved caspase-3 increased (1.5-fold at 10 µM and >6-fold at 20 µM). Furthermore, in the LNCaP cells treated with diosmetin, cleaved PARP expression increased (10-fold at 10 µM and 30-fold at 20 µM), whereas in the PC-3 cells the expression increased, 4-fold at 10 µM and >17-fold at 20 µM.
Figure 6.
Dose-response effects of diosmetin on apoptotic and anti-apoptotic molecules. (A) LNCaP and PC-3 cells were exposed to two concentrations of diosmetin (10 and 20 µM) for 24 h. Total cell lysates were prepared and using SDS-PAGE, lysates proteins were resolved and then subjected to western blot analysis for the evaluation of XIAP, Bax, Bcl-2, cleaved PARP and cleaved caspase-3. Lanes marked ‘0’ are the DMSO control (0.2%)-treated cells. For protein band density, densitometric analysis was performed and protein expressions levels were correlated to express relative controls (B and C). For loading control blots were stripped and reprobed with GAPDH. *P<0.05 for diosmetin concentration vs. vehicle controls.
X-linked inhibitor of apoptosis protein (XIAP) expression decreased following diosmetin dose-response treatment in both the LNCaP and PC-3 cells. The diosmetin-treated LNCaP cells exhibited a decreased expression of XIAP (78.94% at 10 µM and 97.52% at 20 µM); similarly in the PC-3 cells, we observed a decrease in XIAP expression (79.78% at 10 µM and 54.19% at 20 µM) (Fig. 6B and C).
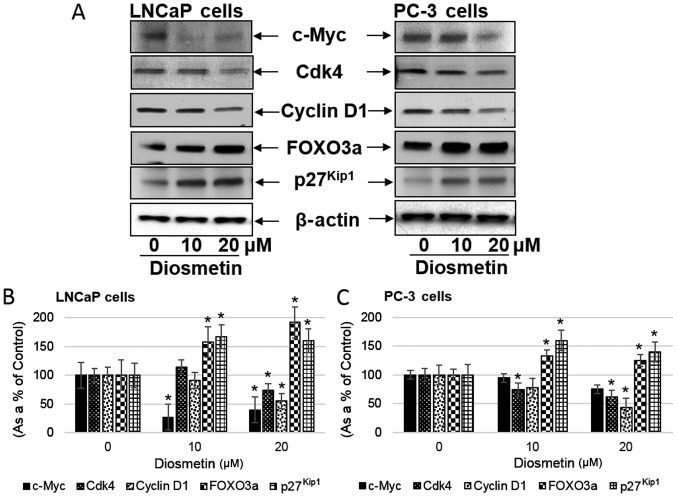
Diosmetin treatment altered proposed mechanistic molecules
c-Myc overexpression in prostate cancer patients predicts more aggressive disease progression and biochemical recurrence (18). In this study, we observed that diosmetin dose-response treatment of the LNCaP and PC-3 cells significantly decreased c-Myc protein expression. Following treatment of the LNCaP cells with diosmetin, c-Myc expression decreased (~27.16% at 10 µM and 39.43% at 20 µM); a decrease in c-Myc expression was also observed in the PC-3 cells (94.77% at 10 µM and 74.97% at 20 µM) (Fig. 7). A previous study on cancer cell lines suggested that c-Myc downregulates the FOXO3a-dependent activation of the p27Kip1 promoter. On the p27Kip1 promoter, a functional association was observed between FOXO3a and c-Myc at the proximal Forkhead binding element (19). In this study, we observed that following diosmetin treatment, c-Myc expression decreased, while FOXO3a expression increased. The expression of p27Kip1, downstream of FOXO3a, increased significantly following diosmetin treatment. In the LNCaP cells, p27Kip1 expression increased (166.96% at 10 µM and 160.06% at 20 µM), and also increased in the PC-3 cells (159.86% at 10 µM and 139.91% at 20 µM) (Fig. 7B and C). Moreover, in the LNCaP cells, FOXO3a expression also increased (157.82% at 10 µM and 191.69% at 20 µM). Similarly in the PC-3 cells, FOXO3a expression increased (133.27% at 10 µM and 124.68% at 20 µM). Additionally, the expression levels of cyclins and Cdks decreased following diosmetin dose-response treatment. In the LNCaP cells following diosmetin treatment, a decreased Cyclin D1 expression was observed (~54.82% at 20 µM), whereas, in the PC-3 cells cyclin E expression decreased (42.99% at 20 µM). Furthermore Cdk4 expression decreased to 73.6% at 20 µM in the LNCaP cells, whereas in the PC-3 cells Cdk4 expression decreased to 61.09% at 20 µM.
Figure 7.
Dose-response effects of diosmetin on signaling pathways in human prostate cancer cells. (A) LNCaP and PC-3 cells were exposed to two concentrations of diosmetin (10 and 20 µM) for 24 h. Total cell lysates were prepared and using SDS-PAGE, lysates proteins were resolved and then subjected to western blot analysis for the evaluation of c-Myc, Cdk4, cyclin D1, FOXO3a and p27Kip1. Lanes marked ‘0’ are the DMSO control (0.2%)-treated cells. For protein band density, densitometric analysis was performed and protein expression levels were correlated to express relative controls (B and C). For loading control blots were stripped and reprobed with GAPDH. *P<0.05 for diosmetin concentration vs. vehicle controls.
Discussion
Various factors play a role in prostate cancer progression; diet and obesity are one of them. Both are associated with an increased risk of high-grade tumors (20,21). A recent study suggested that psychosocial stress also enhances prostate cancer incidence and progression (22). Moreover, epigenetic information harbored on Y-chromosomes may play a significant role in prostate cancer progression (23). Numerous preventive approaches delay the progression of prostate cancer. The development of an effective chemopreventive agent depends on its evaluation with available biomarkers that would predict the potential effets of the agent in prostate cancer. To the best of our knowledge, this is the first study on prostate cancer cells treated with diosmetin, which exhibited growth inhibitory and cytotoxic effects on androgen-sensitive (LNCaP) and androgen refractory (PC-3) cells. The data presented herein imply that these effects of diosmetin are due to the induction of prostate cancer cell arrest the at G1 and S phases of the cell cycle followed by the activation of apoptotic machinery, as evidenced by the increase in cleaved caspase-3 expression.
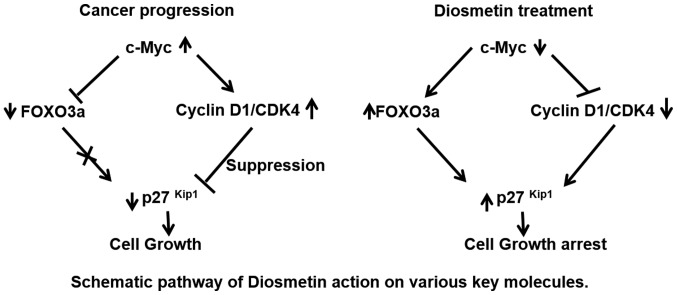
In mammalian cells, alterations in cell cycle machinery result in changes in cell viability and cell growth. Studies have demonstrated that an association exists between cell cycle dysregulation and cancer; cell cycle inhibition is one of the mechanisms that controls the growth of cancer (24,25). It has recently been suggested that diosmetin exerts a substantial growth inhibitory effect on prostate carcinoma cells by altering the G1/S phase of the cell cycle. In eukaryotes, cell cycle mechanisms are controlled by the orchestrated role of protein kinase complexes. Each complex contains at least a catalytic subunit, Cdks and a potential partner known as cyclins (26). Cyclin E and cyclin D are the major players which lead to G1/S phase cell cycle arrest. Both these cyclins in association with Cdk2, Cdk4 or Cdk6 lead to Rb gene phosphorylation and cell growth, whereas hyper-phosphorylated Rb further leads to the release of the E2F complex. This free E2F from Rb then activates c-Myc, which results in cell cycle progression and cell viabilty (27). It has been suggested that c-Myc silenced cells significantly exhibited reduced levels of Cdk4-cyclin D1 and CDK6-cyclin D1 complexes during the G0 to S phase of the cell cycle transition (2). In this study, we observed that the diosmetin dose-response treatment of prostate cancer cells decreased c-Myc, cyclin D1 and Cdk4 expression levels. This suggests that diosmetin has the potential to modulate upstream target c-Myc to alter the downstream cell cycle regulatory cascade of molecules. There is evidence to suggest that the Forkhead family member, FOXO3a, negatively regulates c-Myc (28). Forkhead family transcription factor Fox ‘O’ regulates the cell cycle, cellular differentiation, growth, tumor suppression pathways and metabolism (29). We hypothesized that the diosmetin-mediated upregulation of FOXO3a expression could lead to cell growth inhibition and apoptosis; these events were mediated by the perturbation of the G1/S phase of the cell cycle (Fig. 8). Chip sequencing data suggest an inverse correlation between c-Myc and FOXO3a (30). Another study suggested that the FOXO3a-induced increase in miRNA levels alters c-Myc mRNA translation (31). It has also been suggested FOXO3a plays a significant role in prostate cancer suppression. The overexpression of cyclin D has been found in various types of cancer, as well as cell lines derived from tumors, which is linked with uncontrolled cell growth (32). Cyclin D overexpression in cells represents a shorter G1 phase with a rapid transition into the S phase (33). Based on current and other studies (16), this provides evidence that diosmetin has the ability to arrest cancer cell growth, and may thus be a potential candidate for chemopreventive and therapeutic strategies in prostate cancer.
Figure 8.
Schematic diagram of the proposed cell growth arrest signaling pathways mediated by diosmetin in human prostate cancer cells. Overexpression of c-Myc, Cdk4 and cyclin D1 in prostate cancer cells drives cell growth. Diosmetin treatment inhibits the expression of c-Myc and its downstream molecules, cyclin D1 and Cdk4, conversely increasing FOXO3a and p27Kip1 expression levels to arrest cell growth. Moreover, diosmetin treatment potentiates the apoptotic machinery by modulating cleaved caspase-3 and cleaved PARP in prostate cancer cells.
Cdk inhibitors (CKIs), such as p21Cip/p27Kip and the INK4 families of proteins inhibit cyclin-Cdk complexes to modulate cell cycle progression (34). Similarly, this study demonstrated that diosmetin acts as a CKI in the G1 and S phases of the cell cycle in the LNCaP and PC-3 cells, respectively. Moreover, we observed a marked upregulation of p27Kip1 expression in the LNCaP and PC-3 cells following diosmetin treatment, which represented G1 and S phase cell cycle arrest. This may be one of the molecular mechanisms through which diosmetin suppresses the viability of prostate cancer cells.
A potent suppressor of the apoptotic pathway is the Bcl-2 protein, which regulates apoptosis by the permeabilization of the mitochondrial membrane (35). Bcl-2 is found to be overexpressed in ~50% of all human tumors (36). Moreover, Bcl-2 forms a heterodimer with Bax (pro-apoptotic member), and this interaction abolishes the pro-apoptotic functions of Bax protein. Thus, it has been proposed that the ratio between Bax/Bcl-2 will determine whether the cell will undergo apoptosis or not. In both the LNCaP and PC-3 prostate cancer cells, we observed a significant decrease in Bcl-2 protein expression following diosmetin treatment. Conversely, diosmetin treatment of these cells increased Bax protein expression. Thus, this suggested that diosmetin treatment altered the Bax to Bcl-2 ratio in favor of apoptosis.
In this study, we present experimental findings that diosmetin induces the apoptosis of prostate cancer cells by inhibiting cell growth at the G1 and S phase of the cell cycle. Furthermore, detailed studies are warranted to determine the effects of diosmetin on various human prostate cancer cells and in different signaling pathways. In vivo studies are also important to determine the tumor growth inhibitory effects of diosmetin in prostate cancer models. Pharmacokinetic studies using healthy volunteers have suggested a 26–43 h-long plasma elimination half-life (37,38) and an excellent correlation has been observed between diosmetin and glucuronide metabolites (39). Moreover, based on a published study, the diosmetin concentrations used in vitro in the present study are within clinically relevant concentrations (40). Therefore, our findings suggest that diosmetin has potential to be developed as a possible agent for use in the treatment of prostate cancer.
Acknowledgments
Not applicable.
Funding
This study was supported by NIH grants R21 CA190921 funded to SS.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
CO was involved in the conception, design and drafting of the manuscript. AOK assisted in the acquisition of the data, analysis and interpretation of the data, and in the critical revision of the manuscript and statistical analysis. II assisted in the acquisition of the data. NB was involved in the conception of the study and also provided technical support. EW assisted in the critical revision of the manuscript and interpretation of the data. SS was involved in the conception and design of the study, and in the analysis and interpretation of the data, drafting of the manuscript, as well as in the critical revision of the manuscript for important intellectual content, obtaining funding, administrative and technical support and overall supervision.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateyak MK, Obaya AJ, Sedivy JM. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/MCB.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 4.Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: A few unresolved issues. Oncogene. 2005;24:2787–2795. doi: 10.1038/sj.onc.1208611. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 6.Benson C, Kaye S, Workman P, Garrett M, Walton M, de Bono J. Clinical anticancer drug development: Targeting the cyclin-dependent kinases. Br J Cancer. 2005;92:7–12. doi: 10.1038/sj.bjc.6602229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sausville EA. Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med. 2002;8(Suppl 4):S32–S37. doi: 10.1016/S1471-4914(02)02308-0. [DOI] [PubMed] [Google Scholar]
- 8.Androutsopoulos V, Wilsher N, Arroo RR, Potter GA. Bioactivation of the phytoestrogen diosmetin by CYP1 cytochromes 450. Cancer Lett. 2009;274:54–60. doi: 10.1016/j.canlet.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Jia K, Yang Y, Hao S, Lu C, Xu F, Feng D, Zhu R. Diosmetin induces cell apoptosis by regulating CYP1A1/CYP1A2 due to 53 activation in HepG2 cells. Protein Pept Lett. 2017;24:406–412. doi: 10.2174/0929866524666170227123557. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Yan Y, Xiao L, Dai S, Zeng S, Qian L, Wang L, Yang X, Xiao Y, Gong Z. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS One. 2017;12:e0175977. doi: 10.1371/journal.pone.0175977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21:1525–1528. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Ren H, Liu B, Zhang Q, Li M, Zhu R. Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Oncol Lett. 2016;12:4385–4392. doi: 10.3892/ol.2016.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrajón-Catalán E, Taamalli A, Quirantes-Piné R, Roldan-Segura C, Arráez-Román D, Segura-Carretero A, Micol V, Zarrouk M. Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line. J Pharm Biomed Anal. 2015;105:156–162. doi: 10.1016/j.jpba.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Ciolino HP, Dankwah M, Yeh GC. Resistance of MCF-7 cells to dimethylbenz(a)anthracene-induced apoptosis is due to reduced CYP1A1 expression. Int J Oncol21: 2002:385–391. [PubMed] [Google Scholar]
- 15.Roma A, Rota SG, Spagnuolo PA. Diosmetin induces apoptosis of acute myeloid leukemia cells. Mol Pharm. 2018;15:1353–1360. doi: 10.1021/acs.molpharmaceut.7b01151. [DOI] [PubMed] [Google Scholar]
- 16.Androutsopoulos VP, Spandidos DA. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J Nutr Biochem. 2013;24:496–504. doi: 10.1016/j.jnutbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–311. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 18.Hawksworth D, Ravindranath L, Chen Y, Furusato B, Sesterhenn IA, McLeod DG, Srivastava S, Petrovics G. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010;13:311–315. doi: 10.1038/pcan.2010.31. [DOI] [PubMed] [Google Scholar]
- 19.Chandramohan V, Mineva ND, Burke B, Jeay S, Wu M, Shen J, Yang W, Hann SR, Sonenshein GE. c-Myc represses FOXO3a-mediated transcription of the gene encoding the p27(Kip1) cyclin dependent kinase inhibitor. J Cell Biochem. 2008;104:2091–2106. doi: 10.1002/jcb.21765. [DOI] [PubMed] [Google Scholar]
- 20.Ferro M, Terracciano D, Buonerba C, Lucarelli G, Bottero D, Perdonà S, Autorino R, Serino A, Cantiello F, Damiano R. The emerging role of obesity, diet and lipid metabolism in prostate cancer. Future Oncol. 2017;13:285–293. doi: 10.2217/fon-2016-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Cobelli O, Terracciano D, Tagliabue E, Raimondi S, Galasso G, Cioffi A, Cordima G, Musi G, Damiano R, Cantiello F, et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol Oncol. 2015;33:201.e1–201.e8. doi: 10.1016/j.urolonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri A, Bimonte S, Palma G, Luciano A, Rea D, Giudice A, Scognamiglio G, La Mantia E, Franco R, Perdonà S. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro an in vivo. Int J Oncol. 2015;47:527–534. doi: 10.3892/ijo.2015.3038. [DOI] [PubMed] [Google Scholar]
- 23.Patel R, Khalifa AO, Isali I, Shukla S. Prostate cancer susceptibility and growth linked to Y chromosome genes. Front Biosci (Elite Ed) 2018;10:423–436. doi: 10.2741/e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16:36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandal T. Molecular aspects of the mammalian cell cycle and cancer. Oncologist. 2002;7:73–81. doi: 10.1634/theoncologist.7-1-73. [DOI] [PubMed] [Google Scholar]
- 26.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong P, Maddali MV, Srimani JK, Thélot F, Nevins JR, Mathey-Prevot B, You L. Division of labour between Myc and G1 cyclins in cell cycle commitment and pace control. Nat Commun. 2014;5:4750. doi: 10.1038/ncomms5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peck B, Ferber EC, Schulze A. Antagonism between FOXO and MYC regulates cellular powerhouse. Front Oncol. 2013;3:96. doi: 10.3389/fonc.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarrado-Castellarnau M, de Atauri P, Cascante M. Oncogenic regulation of tumor metabolic reprogramming. Oncotarget. 2016;7:62726–62753. doi: 10.18632/oncotarget.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eijkelenboom A, Mokry M, de Wit E, Smits LM, Polderman PE, van Triest MH, van Boxtel R, Schulze A, de Laat W, Cuppen E. Genome-wide analysis of FOXO3 mediated transcription regulation through RNA polymerase II profiling. Mol Syst Biol. 2013;9:638. doi: 10.1038/msb.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19:968–979. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller A, Odze R, Jenkins TD, Shahsesfaei A, Nakagawa H, Inomoto T, Rustgi AK. A transgenic mouse model with cyclin D1 overexpression results in cell cycle, epidermal growth factor receptor, and p53 abnormalities. Cancer Res. 1997;57:5542–5549. [PubMed] [Google Scholar]
- 34.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- 36.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cova D, De Angelis L, Giavarini F, Palladini G, Perego R. Pharmacokinetics and metabolism of oral diosmin in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1992;30:29–33. [PubMed] [Google Scholar]
- 38.Spanakis M, Kasmas S, Niopas I. Simultaneous determination of the flavonoid aglycones diosmetin and hesperetin in human plasma and urine by a validated GC/MS method: In vivo metabolic reduction of diosmetin to hesperetin. Biomed Chromatogr. 2009;23:124–131. doi: 10.1002/bmc.1092. [DOI] [PubMed] [Google Scholar]
- 39.Silvestro L, Tarcomnicu I, Dulea C, Attili NR, Ciuca V, Peru D, Rizea Savu S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography-mass spectrometry and ion mobility mass spectrometry. Anal Bioanal Chem. 2013;405:8295–8310. doi: 10.1007/s00216-013-7237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quintieri L, Palatini P, Moro S, Floreani M. Inhibition of cytochrome P450 2C8-mediated drug metabolism by the flavonoid diosmetin. Drug Metab Pharmacokinet. 2011;26:559–568. doi: 10.2133/dmpk.DMPK-11-RG-048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.