Abstract
Objective
Various materials are used by otolaryngologists for vocal cord injections in the management of vocal cord paralysis. An ideal injection material should be long-term effective, readily available, cheap, easy to prepare, have no donor morbidity, easy to use, biocompatible, resistant to resorption or migration, and easy to extract during revision. In this study, we aimed to see the histopathological effects of hyaluronic acid (HYA) and platelet-rich plasma (PRP) injections into the vocal cords of New Zealand rabbits.
Methods
PRP was injected into the right vocal cords of twelve rabbits, which was prepared from their serum (PRP group). HYA was injected into the left vocal cords of first six rabbits (numbered 1–6) (HYA group), and the left vocal cords of the other six rabbits (numbered 7–12) were followed with no intervention (control group). Two months later, histomorphological findings in the vocal cords were assessed by two experienced pathologists in seven parameters: chronic inflammation, mucosal atrophy, necrosis, neovascularization, fibrosis, foreign body reaction, and muscular atrophy. They were scored double-blinded as negative (0), mild (+1), moderate (+2), and severe (+3). Fisher’s chi-square test was used to evaluate any statistical significance among the three groups.
Results
Chronic inflammation, mucosal atrophy, necrosis, foreign body reaction, and muscular atrophy parameters were scored as “0” for each preparate by both pathologists. For neovascularization and fibrosis, a stasistically significant difference was seen among the three groups (p<0.05). Neovascularization was increased in the PRP and HYA groups compared with the control group. No significant difference was observed in fibrosis when the groups were compared separately. After two months, two of the six vocal cords injected with HYA revealed HYA; however, none of the PRP-injected vocal cords showed PRP.
Conclusion
HYA and PRP can be safely injected into vocal cords. Our findings show that HYA is a biocompatible and safe injection material for clinical use. Only two of the six vocal cords showed HYA at the end of two months, suggesting that HYA is a short-term effective material. Similarly, PRP was also shown to be a short-term effective material and can be used in patients for testing purpose before using a long-term effective material. The advantages of PRP are that it is inexpensive, readily available, and completely inert as it is prepared from the subject itself.
Keywords: Vocal cord, hyaluronic acid, platelet-rich plasma, vocal cord paralysis
Introduction
Different kinds of materials are used by otolaryngologists for injection in the management of vocal cord paralysis. The first material used for this purpose was paraffin applied by Brüning in 1911 (1). Later in 1962 Arnold described the polytetrafluoroethylene (PTFE) injection which has been since commonly used for treating glottic incompetence (2–4). While chronic inflammation and fibrosis that develop after injection give the impression of a mass, this response can sometimes take an uncontrolled path and form giant-cell granuloma (teflonoma) (5, 6). Issues associated with the use of teflon has brought about a search for other injection materials since the early 1990s.
Ideally, the injection material should be long-acting, readily available and easy to prepare, not cause donor-site morbidity, be affordable, easy to use, tissue compatible and of similar characteristics with the vocal cord, be resistant to resorption or migration and easy to extract during revision. Until today, collagen-based products, autologous adipose tissue, carboxymethyl cellulose, calcium hydroxylapatite and hyaluronic acid (HA) have been the short- or long-acting materials widely used in clinical practice. Materials like embryonic stem cells, various growth factors, micronized dermis, platelet-rich plasma (PRP) are currently in experimentation phase. In this study, we investigated the histopathological effects of HA and PRP on the vocal cords of rabbits in a controlled comparative study.
Methods
The study was initiated after approval was obtained from the Ankara Animal Experimentation Local Ethics Committee (2015.02.23). Twelve New Zealand rabbits aged 3 to 5 years and weighing 4 to 6 kg were used in the study. All animals were treated in adherence to the conditions set forth in the Guide for the Care and Use of Laboratory Animals (www.nap.edu/catalog/5140.html).
Rabbits were anesthetized using 5mg/kg intramuscular xylazin HCL, 45mg/kg ketamin. Vocal cords of the rabbits were exposed with the help of a speculum placed on the mouth and a 0-degree endoscope (Storz endoscope 0 degree, 2.7 mm, Tuttlingen, Germany) was inserted (Figure 1).
Figure 1.
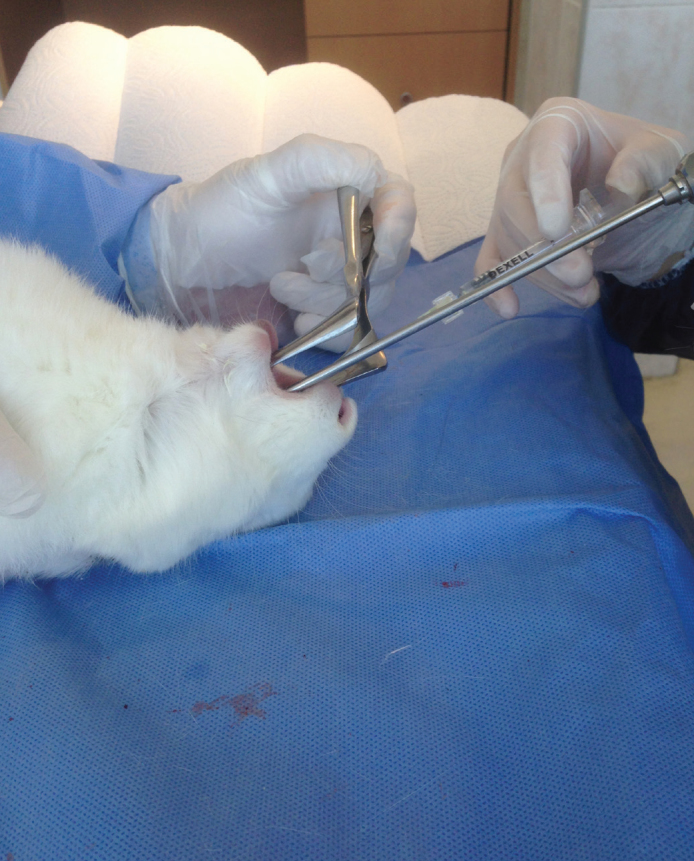
Exposition of rabbit vocal cord using a speculum and a 0-degree endoscope
PRP prepared from their own serum was injected into the right vocal cord of all 12 rabbits (Group 1). Of these 12, the left vocal cord of six rabbits were injected with HA (Restylane, Q-Med AB, Uppsala, Sweden) (Group 2) and the remaining six rabbits (rabbits 7 to 12) were monitored as control (Group 3) without injection to the vocal cord.
The rabbits were monitored for two months under conditions compliant with the guidelines of the ethics committee and without any problems. At the end of the two months the rabbits were sacrificed and the larynx of each was excised. Larynx tissues were fixed in 10% neutrally buffered formalin for 72 hours and vertically sectioned in two parts as left and right. The larynx was sampled in the coronal plane on both sides and sectioned into 5-mm slices after routine tissue processing. Samples were stained with Hematoxylin Eosin and Masson’s Trichrome (MSC). Histomorphologic findings were evaluated using seven different parameters, namely: chronic inflammation, mucosal atrophy, necrosis, neovascularization, fibrosis, foreign body reaction, and muscular atrophy. Fibrosis was examined using MSC staining. All parameters were scored double blind by two experienced pathologists as follows: 0 negative (−), mild (+1), moderate (+2), and severe (+3). To achieve statistically significant results, parameters that had been scored as ‘0’ and ‘1’ were recorded as ‘0’, and parameters that had been scored as ‘2’ and ‘3’ were recorded as ‘1’ for statistical comparison. Fisher’s Chi-square analysis was conducted to see whether there were any significant differences among the three groups, and chi-square test was performed to compare two groups at a time in parameters that showed significant differences.
Preparation of platelet-rich plasma
Under general anesthesia, 4cc blood was collected from the central ear artery of each rabbit into a sterile tube filled with 0.6cc anticoagulant citrate dextrose solution. The collected blood sample was placed in the centrifugal drum and centrifuged at 1500rpm for 15 minutes to separate the blood into three layers with the lowermost layer containing the red blood cells, the middle layer acellular plasma, and the upper layer plasma. The upper plasma layer was collected into a syringe and immediately injected into the targeted vocal cord.
Results
Scores recorded by the two observers for the parameters on each of the preparates were identical for ‘0’ and ‘1’, and no differences were found between the reduced ‘0’ and ‘1’ scores. These scores are given in Table 1 (dichotomous values). Chronic inflammation, mucosal atrophy, necrosis, foreign body reaction, muscular atrophy parameters were scored as ‘0’ for each preparate by both observers. Statistically significant differences were found among the three groups in two of the parameters: neovascularization and fibrosis (p<0.05). Compared to Group 3 (control group), neovascularization was seen to have significantly increased in Group 1 (PRP group) and in Group 2 (HA group). No significant differences were found in two-group comparisons for fibrosis.
Table 1.
Histopathological evaluation scores for each vocal cord by the two pathologists
| Group Nr | Mucosal atrophy | Chronic inflammation | Necrosis | Neovascularization | Fibrosis | Foreign body reaction | Gland atrophy | Muscular atrophy |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Group 1: Platelet-rich plasma injection; Group 2: Hyaluronic acid injection; Group 3: Control group
While injection material was observed in two of the six vocal cords injected with HA, residual material was not observed in any of the 12 vocal cords injected with PRP. Samples of histopathological results of all groups are given in Figures 2–4.
Figure 2.
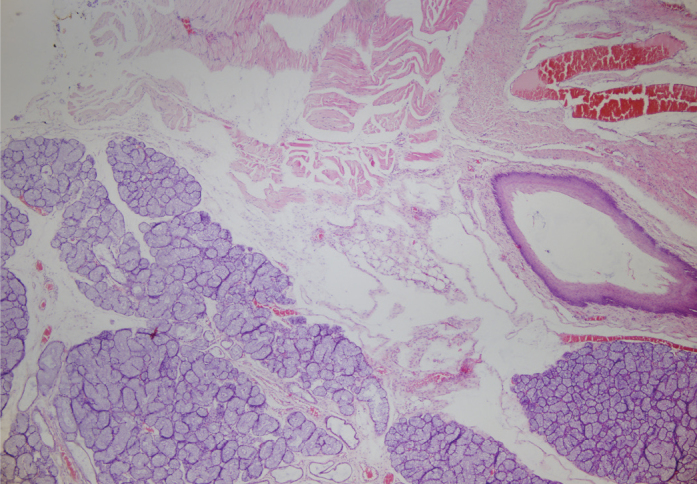
Hematoxylin eosin staining of 10th right vocal cord, ×40
Figure 3.
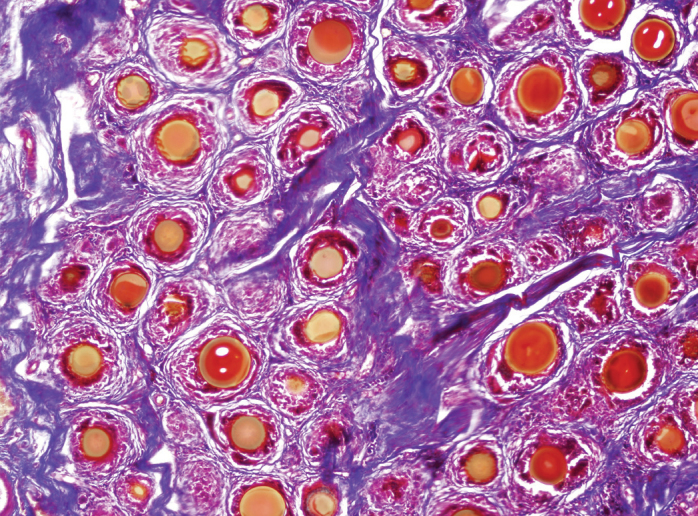
Hematoxylin eosin staining of 4th left vocal cord, ×100
Figure 4.
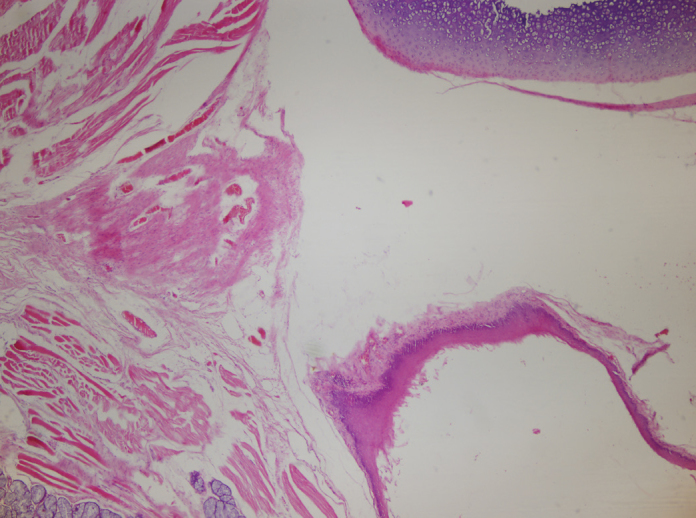
Hematoxylin eosin staining of 10th left vocal cord (control), ×40
Discussion
Phonation is key for effective communication and possible with fully functioning vocal cords. Functional and morphologic changes in the vocal cords due to reasons like vocal cord traumas, recurrent laryngeal nerve traumas during surgery, or radiotherapy can cause dysphonia. Medialization of a paralytic vocal cord can enhance the life quality of patients. Ideal injection materials are being explored in experimental studies for the purposes of clinical use.
Whereas the canine is the most suitable subject model for studies conducted with animals in that their larynx size is similar to that of humans, the high cost of this model and the need to use as many subjects as possible, as well as the need for long-term monitoring for increased reliability has limited its usage in studies (7, 8). To date New Zealand rabbits have been used in many phonosurgical studies (2, 9, 10).
That HA is readily available and easy to administer, can be preserved at room temperature, is biocompatible, causes less immune response, is non-carcinogenic, does not cause infection has made it a commonly used material in both clinical practice and animal model studies (11–16). In this study, when examined two months after injection, chronic inflammation, mucosal atrophy, necrosis, foreign body reaction or muscular atrophy in the vocal cords was not observed in any of the groups including the HA group. Compared to the control group, only increased neovascularization was observed in the vocal cords injected with HA. These results are consistent with those of the other studies reported on HA injection and demonstrate that HA can be used in clinical practice as a biocompatible and safe injection material (11, 12). That HA was identified in only two of the six vocal cords at the end of the two months demonstrated that HA can be used as a short-acting material.
Platelet-rich plasma is essentially a concentrate of autologous platelets and rich in PDGF, TGFb1, VEGF and EGF growth factors produced by these platelets (17). There are studies demonstrating that each of these polypeptides has various roles in accelerated wound healing, enhanced immune response, increased angiogenesis, and enhanced bone regeneration (18–20).
There are also clinical studies which demonstrate the wound healing ability of PRP (18, 21–23). In animal model studies, histologic evaluations have shown PRP to enhance fibroblastic and endothelial cell formation, increase neovascularization and improve granulation tissue formation (18, 24–27). An animal model study has histologically demonstrated the effectiveness of PRP injection in vocal cord wound healing (28). In our study, no chronic inflammation, mucosal atrophy, necrosis, foreign body reaction or muscular atrophy was identified in any of the vocal cords two months after PRP injection. With respect to the other parameters, neovascularization and fibrosis, no significant differences were observed between the PRP and HA groups. These results demonstrate that HA, like PRP, is a biocompatible and safe material. Neovascularization was observed to have increased in the PRP group vs. the control group. This result is consistent with other studies that have shown PRP to increase neovascularization (18, 24–27).
At the end of two months no residual material was observed in any of the 12 vocal cords injected with PRP. This result shows that PRP is a short-acting material and can be a safe option in short-term administrations for testing purposes before deciding on a long-acting material. The benefits of PRP are its affordability, availability, and that it does not cause immune response since is obtained from the organism itself.
Conclusion
Hyaluronic acid and PRP are short-acting, reliable and biocompatible materials that can be clinically used in vocal cord injections. Apart from the absence of unfavorable outcomes such as foreign body reaction, inflammation, necrosis, their ease of access and administration make these two materials preferable options. Further studies conducted with more subjects and using other injection materials can further orient future clinical practices.
Acknowledgement
The authors thank to Assist. Prof. Aslıhan Alhan for her help in statistical analysis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ankara University Animal Studies Research Committe (Date: 11/02/2015 Number: 2015-2-26).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Design - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Supervision - S.K.Ö., F.T., E.T., E.A., H.D., K.B; Resource - S.K.Ö, F.T., E.T., E.A., H.D., K.B.; Materials - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Data Collection and/or Processing - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Analysis and/or Interpretation - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Literature Search - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Writing - S.K.Ö., F.T., E.T., E.A., H.D., K.B.; Critical Reviews - S.K.Ö., F.T., E.T., E.A., H.D., K.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Brünings W. Über eine neue Behandlungsmethode der Rekurrenslahmung. Verh Dtsch Laryngol. 1911;18:93–151. [Google Scholar]
- 2.Arnold GE. Vocal rehabilitation of paralytic dysphonia. I. Cartilage injection into a paralyzed vocal cord. Arch Otolaryngol. 1955;62:1–17. doi: 10.1001/archotol.1955.03830010003001. https://doi.org/10.1001/archotol.1955.03830010003001. [DOI] [PubMed] [Google Scholar]
- 3.Rubin HJ. Misadventures with injectable polytef (Teflon) Arch Otolaryngol. 1975;101:114–6. doi: 10.1001/archotol.1975.00780310036010. https://doi.org/10.1001/archotol.1975.00780310036010. [DOI] [PubMed] [Google Scholar]
- 4.Siegler J. Rehabilitation of voice after recurrent laryngeal nerve paralysis using teflon suspension. J Laryngol Otol. 1967;81:1121–9. doi: 10.1017/s0022215100068134. https://doi.org/10.1017/S0022215100068134. [DOI] [PubMed] [Google Scholar]
- 5.Malizia AA, Jr, Reiman HM, Myers RP, Sande JR, Barham SS, Benson RC, Jr, et al. Migration and granulomatous reaction after periurethral injection of polytef (Teflon) JAMA. 1984;251:3277–81. https://doi.org/10.1001/jama.251.24.3277. [PubMed] [Google Scholar]
- 6.Stone JW, Arnold GE, Stephens CB. Intracordal polytef (Teflon) injection. Histologic study of three further cases. Arch Otolaryngol. 1970;91:568–74. doi: 10.1001/archotol.1970.00770040798014. https://doi.org/10.1001/archotol.1970.00770040798014. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao TY, Liu CM, Luschei ES, Titze IR. The effect of cricothyroid muscle action on the relation between subglottal pressure and fundamental frequency in an in vivo canine model. J Voice. 2001;15:187–93. doi: 10.1016/S0892-1997(01)00020-0. https://doi.org/10.1016/S0892-1997(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 8.Cox KA, Alipour F, Titze IR. Geometric structure of the human and canine cricothyroid and thyroarytenoid muscles for biomechanical applications. Ann Otol Rhinol Laryngol. 1999;108:1151–8. doi: 10.1177/000348949910801210. https://doi.org/10.1177/000348949910801210. [DOI] [PubMed] [Google Scholar]
- 9.Flint PW, Corio RL, Cummings CW. Comparison of soft tissue response in rabbits following laryngeal implantation with hydroxylapatite, silicone rubber, and teflon. Ann Otol Rhinol Laryngol. 1997;106:399–407. doi: 10.1177/000348949710600508. https://doi.org/10.1177/000348949710600508. [DOI] [PubMed] [Google Scholar]
- 10.Caballero M, Bernal-Sprekelsen M, Calvo C, Farrè X, Quintó L, Alòs L. Polydimethylsiloxane versus polytetrafluoroethylene for vocal fold medialization: histologic evaluation in a rabbit model. J Biomed Mater Res B Appl Biomater. 2003;67:666–74. doi: 10.1002/jbm.b.10061. https://doi.org/10.1002/jbm.b.10061. [DOI] [PubMed] [Google Scholar]
- 11.Perazzo PS, de Duprat AC, Lancelotti C, Donati F. A study of the histological behavior of a rabbit vocal fold after a hyaluronic acid injection. Braz J Otorhinolaryngol. 2007;73:171–8. doi: 10.1016/S1808-8694(15)31063-6. https://doi.org/10.1016/S1808-8694(15)31063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perazzo PS, de Duprat AC, Lancellotti CL. Histological behavior of the vocal fold after hyaluronic acid injection. J Voice. 2009;23:95–8. doi: 10.1016/j.jvoice.2007.05.006. https://doi.org/10.1016/j.jvoice.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Hallén L, Testad P, Sederholm E, Dahlqvist A, Laurent C. DiHA (dextranomers in hyaluronan) injections for treatment of insufficient closure of the vocal folds: early clinical experiences. Laryngoscope. 2001;111:1063–7. doi: 10.1097/00005537-200106000-00025. https://doi.org/10.1097/00005537-200106000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Manna F, Dentini M, Desideri P, De Pità O, Mortilla E, Maras B. Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (hylaform from rooster combs and restylane from streptococcus) used for soft tissue augmentation. J Eur Acad Dermatol Venereol. 1999;13:183–92. https://doi.org/10.1111/j.1468-3083.1999.tb00881.x. [PubMed] [Google Scholar]
- 15.Olenius M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthetic Plast Surg. 1998;22:97–101. doi: 10.1007/s002669900172. https://doi.org/10.1007/s002669900172. [DOI] [PubMed] [Google Scholar]
- 16.Hertegård S, Hallén L, Laurent C, Lindström E, Olofsson K, Testad P, et al. Cross-linked hyaluronan used as augmentation substance for treatment of glottal insufficiency: safety aspects and vocal fold function. Laryngoscope. 2002;112:2211–9. doi: 10.1097/00005537-200212000-00016. https://doi.org/10.1097/00005537-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Karataylı Özgürsoy S, Tunçkaşık ME, Tunçkaşık F, Akıncıoğlu E, Doğan H, Kocatürk S. Platelet-Rich Plasma Application for Acute Tympanic Membrane Perforations. J Int Adv Otol. 2017;13:195–9. doi: 10.5152/iao.2016.2533. https://doi.org/10.5152/iao.2016.2533. [DOI] [PubMed] [Google Scholar]
- 18.Sommeling CE, Heyneman A, Hoeksema H, Verbelen J, Stillaert FB, Monstrey S. The use of platelet-rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2013;66:301–11. doi: 10.1016/j.bjps.2012.11.009. https://doi.org/10.1016/j.bjps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Chang SH, Hsu YM, Wang YJ, Tsao YP, Tung KY, Wang TY. Fabrication of pre-determined shape of bone segment with collagen-hydroxyapatite scaffold and autogenous platelet-rich plasma. J Mater Sci Mater Med. 2009;20:23–31. doi: 10.1007/s10856-008-3507-1. https://doi.org/10.1007/s10856-008-3507-1. [DOI] [PubMed] [Google Scholar]
- 20.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. https://doi.org/10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009;40:801–5. doi: 10.1016/j.injury.2008.05.002. https://doi.org/10.1016/j.injury.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8:307–12. doi: 10.1111/j.1742-481X.2011.00797.x. https://doi.org/10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet. 1990;170:56–60. [PubMed] [Google Scholar]
- 24.Pietramaggiori G, Kaipainen A, Czeczuga JM, Wagner CT, Orgill DP. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006;14:573–80. doi: 10.1111/j.1743-6109.2006.00164.x. https://doi.org/10.1111/j.1743-6109.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 25.Pietramaggiori G, Scherer SS, Mathews JC, Alperovich M, Yang HJ, Neuwalder J, et al. Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen. 2008;16:218–25. doi: 10.1111/j.1524-475X.2008.00362.x. https://doi.org/10.1111/j.1524-475X.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 26.Pietramaggiori G, Scherer SS, Mathews JC, Gennaoui T, Lancerotto L, Ragno G, et al. Quiescent platelets stimulate angiogenesis and diabetic wound repair. J Surg Res. 2010;160:169–77. doi: 10.1016/j.jss.2008.09.010. https://doi.org/10.1016/j.jss.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takikawa M, Nakamura S, Nakamura S, Nambu M, Ishihara M, Fujita M, et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J Biomed Mater Res B Appl Biomater. 2011;97:373–80. doi: 10.1002/jbm.b.31824. https://doi.org/10.1002/jbm.b.31824. [DOI] [PubMed] [Google Scholar]
- 28.Woo SH, Jeong HS, Kim JP, Koh EH, Lee SU, Jin SM, et al. Favorable vocal fold wound healing induced by platelet-rich plasma injection. Clin Exp Otorhinolaryngol. 2014;7:47–52. doi: 10.3342/ceo.2014.7.1.47. https://doi.org/10.3342/ceo.2014.7.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]


