Abstract
Objective
The apnea-hypopnea index (AHI) does not provide information about the apnea depth and length. We aimed to evaluate the correlation of the oxygen desaturation index (ODI) with AHI and the subjective symptoms because it is known that hypoxia plays an important role in morbidity and complications of obstructive sleep apnea syndrome (OSAS).
Methods
We reviewed the data of patients who applied to our clinic between 2010 and 2014 and underwent polysomnography (PSG) with a diagnosis of suspected sleep apnea. The demographic and anthropometric data of the patients were recorded. Epworth sleepiness scale (ESS) and values of AHI and ODI were analyzed in PSG.
Results
A total of 321 patients were divided into four groups, according to AHI as follows: 82 (25.5%) common snoring, 77 (24%) mild obstructive sleep apnea (OSA), 71 (22.1%) moderate OSA, and 91 (28.3%) severe OSA. A strong correlation was detected between AHI and ODI (p<0.005 and r=0.904) in all patient groups. There was a positive correlation between AHI and ESS (p<0.05 and r=0.435), but the correlation of ESS with ODI was stronger than that with AHI (p<0.05 and r=0.504)
Conclusion
The subjective symptoms of sleep apnea syndrome seem to be closely related to oxygen desaturations. Hypoxia during apnea periods of OSA is important; therefore, we suggest that ODI is as valuable as AHI in diagnosing and grading the OSAS.
Keywords: Obstructive sleep apnea, apnea-hypopnea index, oxygen desaturation index, polysomnography, Epworth sleepiness scale
Introduction
Sleep is a major component of our lives, and an average person spends approximately one third of their life asleep. Sleep-related breathing disorders (SRBD) are the most frequent type of sleep disorders, with a prevalence between 2% and 4% (1). The obstructive sleep apnea syndrome (OSAS) is the most common type of SRBD, with a prevalence of 90%–95%. (2).
Respiratory arrest during sleep causes CO2 retention and hypoxemia; consequently, the autonomic and hemodynamic response mechanism is triggered (1). Reportedly, hypoxia events during obstructive sleep apnea (OSA) are closely related to cardiovascular pathologies such as stroke, arrhythmia, and coronary artery disease (3–5).
Full-night polysomnography (PSG) is the only diagnostic method to quantify SRBD and is considered the gold standard for the diagnosis of OSAS (6). Measurement of the number of apnea and hypopnea events per hour, which is called the apnea-hypopnea index (AHI), is used to grade OSA (7). The cardiovascular and cerebrovascular morbidity and mortality rates are increased in patients with AHI higher than 20. However, AHI lower than 20 does not eliminate increased morbidity in OSAS (8).
The most important problem regarding AHI is that the morphology of abnormal respiratory events (i.e., duration and depth) is not taken into account; thus, AHI is a quantitative parameter, and the physiological stress levels of different patients with similar AHI levels can be very different (4). In fact, when the depth and duration of the apnea attacks increase, AHI may paradoxically fall (4) (Figure 1). Therefore, we believe that additional parameters are required to evaluate OSAS.
Figure 1. a, b.
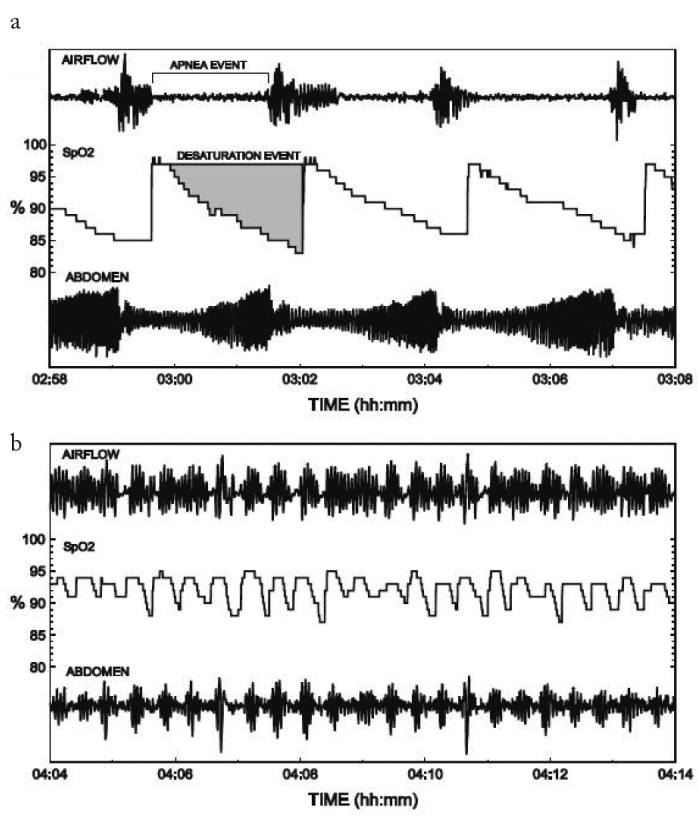
PSG images of two patients showing the apnea episodes. (a) It is observed that apnea attacks of the patient were longer and oxygen desaturations were deeper, but attacks were not so frequent (AHI 21.0), (b) It is observed that the patient had shorter apneas not with desaturation and apnea attacks, and they repeated more frequently (AHI 48.5).
Desaturation episodes are one of the main reasons for the development of complications associated with OSAS. The average number of desaturation episodes per hour can be measured using PSG and is called the oxygen desaturation index (ODI). Desaturation episodes are generally described as a decrease in the mean oxygen saturation of ≥4% (over the last 120 seconds) that lasts for at least 10 seconds. An ODI>5 is a good predictor for AHI>5 with an accuracy of 87%, an ODI>15 for AHI>15 with an accuracy of 84%, and an ODI>30 for AHI>30 with an accuracy of 93.7%. A cut-off value of the ODI>10 has high sensitivity (93.3%) to detect moderate and severe OSA. ODI has a prognostic value because the complications and mortality of OSA are related to nocturnal hypoxia (6).
Taking into consideration the importance of oxygen desaturation events in the pathophysiology of OSA, we aimed to study the relationship between ODI and AHI and the subjective symptoms of OSAS.
Methods
We reviewed the data of patients who were referred to the Ear, Nose, and Throat Unit of our Sleep Diagnostics Department between January 2010 and June 2014. Patients were examined and underwent PSG and were asked to complete our OSA Department patient evaluation forms. Subjects with other sleep disorders, upper respiratory resistance syndrome, and comorbid respiratory diseases such as chronic obstructive pulmonary disease (COPD) were excluded from the study. Subjects younger than 18 years, with incomplete data, who had a total sleeping time of less than 4 hours in PSG reports, and who underwent PSG testing at other centers were also excluded.
Sleep and respiratory scores were attributed in line with the 2009 manual of the American Academy of Sleep Medicine. Apnea was defined as the absence of nasal airflow for at least 10 seconds, whereas hypopnea was defined as a decrease in nasal airflow of 30% for at least 10 seconds, a 4% decrease in oxygen saturation, or arousal. A decrease of at least 4% in the mean oxygen saturation value during sleep was considered to be desaturation, while the mean desaturation value in an hour was represented by ODI.
Patients were divided into four groups according to AHI, a commonly used indicator for the diagnosis and grading of sleep apneas. AHIs of <5, 5–14, 15–29, and ≥30 were referred to as common snoring, mild OSA, moderate OSA, and severe OSA, respectively. ODI was graded into three groups: mild (5–14), moderate (15–29), and severe (≥30) OSA. Patients with an ODI<5 were graded as having no oxygen disturbance.
The validated Turkish version of the Epworth sleepiness scale (ESS) was used to evaluate the excessive daytime sleepiness (EDS) scores of the patients. ESS was categorized into three groups: no daytime sleepiness (ESS≤8), mild or moderate EDS (9–14), and severe EDS (15–24).
The relationships between OSA grade and demographic range, anthropometric features, other important parameters of PSG [ODI, lowest oxygen saturation (SaO2), mean SaO2], and ESS scores were evaluated.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences 22.0 (IBM Corp.; Armonk, NY, USA) software for the analysis of quantitative data. Spearman analyses were used for the correlation analysis. The Kappa concordance test was used for the concordance analysis.
Informed consent was obtained from all patients, and the study was approved by the Institutional Ethics Committee of Bakırköy Dr Sadi Konuk Training and Research Hospital (2014/12/01; Date: 09/15/2014).
Results
During a retrospective review of the data, 321 patients were found to be eligible and were enrolled in the study. The demographic and anthropometric characteristics of the patients are given in Table 1. The male/female ratio of OSA was found to be 4.1:1, and the mean patient age was 48.5 (range, 23.0–82.0) years, but these findings do not reflect the general population.
Table 1.
Demographic and anthropometric characteristics of patients
| Median | Min. | Max. | Mean. | ±SD./n-% | |
|---|---|---|---|---|---|
| Age | 48 | 23 | 82 | 48.5 | ±11.7 |
| Female | 62 | 19.3% | |||
| Male | 259 | 80.7% | |||
| Weight (kg) | 86 | 52 | 143 | 89 | ±15.5 |
| Height (cm) | 172 | 80 | 200 | 171.7 | ±9.9 |
| BMI | 29.4 | 16 | 57.6 | 30.1 | ±5.1 |
AHI: apnea-hypopnea index; ESS: Epworth sleepiness scale; OSA: obstructive sleep apnea; BMI: body mass index; SD: standard deviation
According to AHI results of these patients, 82 patients (25.5%) were classified as experiencing common snoring, 77 patients (24%) were classified with mild OSA, 71 patients (22.1%) were classified with moderate OSA, and 91 patients (28.3%) were classified with severe OSA. The range of OSA grades according to each parameter (AHI, ESS, and ODI) is presented in Table 2.
Table 2.
Range of grading according to ESS, AHI, and ODI
| Severity | Number | % | |
|---|---|---|---|
| AHI | CS (<5) | 82 | 25.5 |
| Mild OSA (5–14) | 77 | 24.0 | |
| Moderate OSA (15–29) | 71 | 22.1 | |
| Severe OSA (≥30) | 91 | 28.3 | |
| ESS | Normal (≤8) | 148 | 46.1 |
| Mild/Moderate (9–14) | 100 | 31.1 | |
| Severe (15–24) | 73 | 22.7 | |
| ODI | CS (<5) | 64 | 19.9 |
| Mild OSA (5–14) | 90 | 28 | |
| Moderate OSA (15–29) | 68 | 21. 2 | |
| Severe OSA (≥30) | 99 | 30.8 |
AHI: apnea-hypopnea index; ESS: Epworth sleepiness scale; ODI: oxygen desaturation index; CS: common snoring; OSA: obstructive sleep apnea
None of the parameters (AHI, ODI, and ESS) showed a significant correlation with age or height (p>0.05). However, there were positive correlations with body mass index and weight (p<0.05) and negative correlations with the lowest SaO2 (p<0.05), and these were found to be statistically significant.
There was no significant correlation between AHI and ESS (p>0.05) in patients who had normal AHI scores or mild OSA. However, there was a significant positive correlation between AHI and ESS values in patients with moderate or severe OSA (p<0.05) (Table 3). In all OSA grades, in relation to AHI, there was a significant positive correlation between AHI and ODI (p<0.05) (Table 4). Significant positive correlations were found between AHI and both ESS and ODI (p<0.05). There was a positive correlation between ODI and ESS, and this correlation was higher than that between AHI and ESS (r=0.504 and r=0.435, respectively) (Table 5).
Table 3.
AHI and ESS correlation according to OSA grades
| ESS | |||||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | ||
| AHI | r | 0.189 | 0.066 | 0.270 | 0.207 |
| p | 0.090 | 0.568 | 0.023 | 0.049 | |
AHI: apnea-hypopnea index; ESS: Epworth sleepiness scale; OSA: obstructive sleep apnea
Table 4.
AHI and ODI correlation according to the OSA grades
| ODI | |||||
|---|---|---|---|---|---|
| Normal | Mild OSA | Moderate OSA | Severe OSA | ||
| AHI | R | 0.411 | 0.589 | 0.600 | 0.739 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | |
AHI: apnea-hypopnea index; ODI: oxygen desaturation index; OSA: obstructive sleep apnea
Table 5.
The comparison of the correlations between AHI-ESS and ODI-ESS
| ESS | ODI | ESS | ||||
|---|---|---|---|---|---|---|
| AHI | r | 0.435 | 0.904 | ODI | r | 0.504 |
| p | 0.000 | 0.000 | p | 0.000 | ||
AHI: apnea-hypopnea index; ESS: Epworth sleepiness scale; ODI: oxygen desaturation index; OSA: obstructive sleep apnea
Kappa analysis showed a significant concordance of 72.3% (Table 6) between AHI and ODI (p<0.05).
Table 6.
Concordance test according to AHI and ODI
| AHI | Coherence | Kappa | p | |||||
|---|---|---|---|---|---|---|---|---|
| CS | Mild OSA | Moderate OSA | Severe OSA | |||||
| ODI | CS | 54 | 8 | 2 | 0 | 72.3% | 0.629 | 0.000 |
| Mild OSA | 25 | 53 | 12 | 0 | ||||
| Moderate OSA | 2 | 12 | 44 | 10 | ||||
| Severe OSA | 1 | 4 | 13 | 81 | ||||
AHI: apnea-hypopnea index; CS: common snoring; ODI: oxygen desaturation index; OSA: obstructive sleep apnea
Discussion
Obstructive sleep apnea is a common health problem and is characterized by episodic obstructions in the upper airway, nocturnal hypoxemia, and sleep defects. EDS is one of the most common symptoms of OSAS (5). This situation affects the cognitive functions and work performance of patients (5, 9). However, not all patients with OSA complain of daytime sleepiness. On the contrary, the EDS complaints of some OSA patients may be very severe (10, 11).
Mediano et al. (5) determined that patients with daytime sleepiness had shorter sleep latencies, increased sleeping efficiency, and worse nocturnal oxygenation compared to patients without daytime sleepiness. They found that the most important indicator of daytime sleepiness in OSA patients was nocturnal hypoxemia (5). According to another study by Roure et al. (12), OSA patients with daytime sleepiness slept longer and more effectively compared to those without daytime sleepiness, but their nocturnal oxygenation and sleep fragmentation were worse. Hence, Roure et al. (12) emphasized the importance of nocturnal hypoxemia for OSA. Different methods have been developed for the interpretation of EDS. Among them, the most commonly used method is ESS (12, 13).
A significant positive correlation was found between AHI values and ESS values when comparing, without distinguishing between AHI levels. However, when the values were compared after distinguishing according to severity, ESS and AHI values of patients with mild OSA and common snoring were not found to be correlated, although there was a positive correlation between patients with moderate and severe OSA. Kulkas et al (4) stated that AHI was not a sufficient parameter for diagnosis or prognosis. Therefore, they conducted research on 160 patients and developed a new parameter to measure the amount of desaturation. In their study, which examined an average of 183 months of follow-up results, they demonstrated that patients with the same AHI values may have OSAs of different severities and thus, different prognoses (4).
Otero et al. (7) evaluated several respiratory-related parameters and their potential to assist the diagnosis of OSA. By calculating the combined percentage of apnea, hypopnea, and desaturation duration from the total sleep time, they claimed that they could diagnose OSA with increased accuracy compared to AHI (7).
As mentioned above, if one accepts that hypoxia is an important factor for the development of different complications and the pathogenesis of OSA, AHI scores alone are insufficient to grade the severity of OSA, and hypoxia events should not be ignored during the diagnosis and classification of OSA.
Another parameter used for increasing the diagnosing and grading the quality of PSG is ODI. ODI is the mean number of desaturation events that occur within an hour. Several studies have reported a strong correlation between AHI and ODI (1, 14). ODI can be measured with nocturnal oximetry (NO), which consists of pulse oximetry and a saturation-recording device. The main principle of NO is to calculate and record desaturation events during night sleep. The use of pulse oximetry is cost effective, easy to apply, and non-invasive; therefore, many authors have recommended it as a screening test for OSA (14–16).
Fietze et al. (17) conducted PSG on OSA patients and measured their ODI values taken over seven consecutive nights with NO. They found a high consistency across different nights and a correlation with AHI. Nuber et al. (18) claimed that the combined use of nocturnal pulse oximetry and ESS can double the accuracy of the diagnosis. The cut-off value of ODI in the diagnosis of OSA remains controversial. There is also conflict regarding the threshold value of ODI for OSA. Although some authors state that the lower limit should be ≥5, ≥10, or ≥15, the most commonly used limit and the one we used in this study was ≥5 (6, 17, 19).
Our study separately assessed each group according to AHI and found that ESS values of the patients linked to common snoring and mild OSA were not correlated to AHI, whereas ESS values of the moderate and severe OSA groups were positively correlated. In line with the literature, we found that there was a high positive correlation between AHI and ODI among all patient groups (p<0.05 and r=0.904) (Table 4, 5) (6). Perhaps the most important finding was that there was a positive correlation between ODI and ESS (p<0.05 and r=0.435) (Table 3, 5), and it was noticeable that the positive correlation between ODI and ESS levels was clearer than that for AHI (p<0.05 and r=0.504) (Table 5). Furthermore, the cohesion rate of our study was found to be 72.3% according to all three parameters of AHI and ODI criteria. Although AHI and ODI were correlated and the cohesion rate was high, different conclusions could be drawn from the same patient depending on each criterion. For example, as indicated in Table 6, only 44 (61.9%) of the 71 patients who were considered to have mild OSA according to AHI were consistent with the criteria for mild OSA, 12 (16.9%) with the criteria for light OSA, two (2.81%) with the criteria for common snoring, and 13 (18.3%) with the criteria for severe OSA, according to ODI. In light of the finding that there was a stronger correlation between ESS and ODI and the change in OSA severity as assessed by ODI, ODI should not be ignored while determining the severity of the OSA.
However, the use of ODI as part of the OSA assessment has some disadvantages. First, it is not suitable for cases with respiratory disease or a disease that causes hypercapnia; therefore, comorbidities need to be thoroughly investigated for a definitive diagnosis in OSA patients. Another disadvantage is that ODI alone cannot indicate the origin of hypoxaemia. However, when the saturation record is closely examined, it is possible to distinguish apnea from hypopnea, central apnea from obstructive apnea, and even Cheyne-Strokes respiration according to the morphology of the saturation fluctuations (15) (Figure 2). Factors such as anemia, hypotension, peripheral vascular disorders, obesity, COPD, and frequent movement during sleep can cause misleading results in pulse oximetry (15). Hence, ODI should be assessed alongside the other parameters of PSG.
Figure 2.
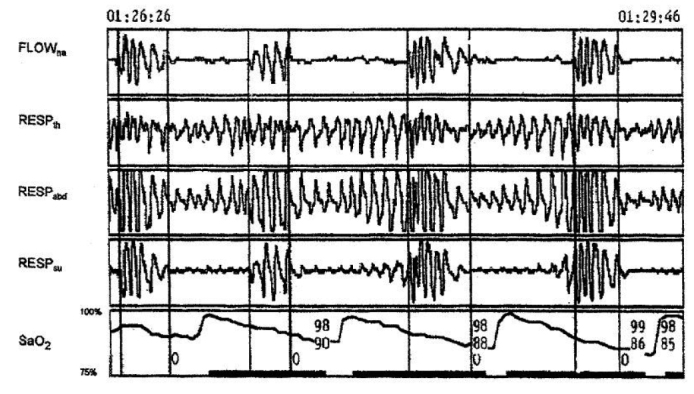
Respiratory patterns during a 3-minute time period for a patient with OSA syndrome showing obstructive apneas with typical saw-tooth morphology of the pulse oximetry curve
Flowna=Nasal and oral airflow, Respth=Thoracic respiratory effort, Respabd=Abdominal respiratory effort, Respsum=Thoracic and abdominal respiratory effort total, O=Obstructive apnea.
Furthermore, assessment of ODI together with objective tests to measure daytime sleepiness and quality of life may increase the evidence level of these types of studies.
Conclusion
Polysomnography is accepted as a gold standard examination method for the diagnosis of OSA. OSA is an important public health problem around the world and still has many unknown features. Consideration should be given to the fact that falls in oxygen saturation play an important role in the development of OSA complications.
We suggest that ODI, an indicator of the number of oxygen desaturations developing together with apnea and hypopnea during sleep, can be a significant parameter in addition to AHI for the diagnosis of OSA. We state that ODI should not be neglected during the PSG assessment of OSA patients and that this assessment criterion is at least as important as AHI. We believe that a new OSA evaluation approach, taking into account ODI along with AHI, should be utilized.
Acknowledgement
The authors thank to PSG Technician Metin İlkılıç for his assistance in the data collection.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Bakırköy Dr. Sadi Konuk Training and Research Hospital (2014/12/01; Date: 09/15/2014).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Z.M.Y.; Design - Z.M.Y., D.T., İ.S.; Supervision - Z.M.Y., İ.S.; Resource - S.G., D.T.; Materials - D.T., İ.S.; Data Collection and/or Processing - S.G., D.T.; Analysis and/or Interpretation - Z.M.Y., D.T.; Literature Search - S.G., D.T.; Writing - Z.M.Y., S.G., D.T.; Critical Reviews - İ.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–20. doi: 10.1164/rccm.200411-1564OC. https://doi.org/10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer JT. Early diagnosis of sleep related breathing disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2008;7:1–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension - an Update. Hypertension. 2014;63:203–9. doi: 10.1161/HYPERTENSIONAHA.113.00613. https://doi.org/10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput. 2013;51:697–708. doi: 10.1007/s11517-013-1039-4. https://doi.org/10.1007/s11517-013-1039-4. [DOI] [PubMed] [Google Scholar]
- 5.Mediano O, Barceló A, de la Pe-a M, Gozal D, Agustí A, Barbé F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30:110–3. doi: 10.1183/09031936.00009506. https://doi.org/10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 6.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114:993–1000. doi: 10.1213/ANE.0b013e318248f4f5. https://doi.org/10.1213/ANE.0b013e318248f4f5. [DOI] [PubMed] [Google Scholar]
- 7.Otero A, Félix P, Presedo J, Zamarrón C. An evaluation of indexes as support tools in the diagnosis of sleep apnea. Ann Biomed Eng. 2012;40:1825–34. doi: 10.1007/s10439-012-0536-1. https://doi.org/10.1007/s10439-012-0536-1. [DOI] [PubMed] [Google Scholar]
- 8.Jean-Louis G, Brown CD, Zizi F, Ogedegbe G, Boutin-Foster C, Gorga J, et al. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Rev Cardiovasc Ther. 2010;8:995–1005. doi: 10.1586/erc.10.55. https://doi.org/10.1586/erc.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Spuy I, Karunanayake CP, Dosman JA, McMullin K, Zhao G, Abonyi S, et al. Determinants of excessive daytime sleepiness in two First Nation communities. BMC Pulm Med. 2017;17:192. doi: 10.1186/s12890-017-0536-x. https://doi.org/10.1186/s12890-017-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramar K, Olson EJ. Management of common sleep disorders. Am Fam Physician. 2013;88:231–8. [PubMed] [Google Scholar]
- 11.Castiglioni P, Lombardi C, Di Rienzo M, Lugaresi E, Montagna P, Cortelli P, et al. What are the causes of excessive daytime sleepiness in patients with sleep-disordered breathing? Eur Respir J. 2008;32:526–7. doi: 10.1183/09031936.00043308. https://doi.org/10.1183/09031936.00043308. [DOI] [PubMed] [Google Scholar]
- 12.Roure N, Gomez S, Mediano O, Duran J, Peña Mde L, Capote F, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9:727–31. doi: 10.1016/j.sleep.2008.02.006. https://doi.org/10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Izci B, Ardic S, Firat H, Sahin A, Altinors M, Karacan I. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath. 2008;12:161–8. doi: 10.1007/s11325-007-0145-7. https://doi.org/10.1007/s11325-007-0145-7. [DOI] [PubMed] [Google Scholar]
- 14.Torre-Bouscoulet L, Castorena-Maldonado A, Ba-os-Flores R, Vázquez-García JC, Meza-Vargas MS, Pérez-Padilla R. Agreement between oxygen desaturation index and apnea-hypopnea index in adults with suspected obstructive sleep apnea at an altitude of 2240 m. Arch Bronconeumol. 2007;43:649–54. doi: 10.1016/s1579-2129(07)60150-5. https://doi.org/10.1157/13112962. [DOI] [PubMed] [Google Scholar]
- 15.Andrés-Blanco AM, Álvarez D, Crespo A, Arroyo CA, Cerezo-Hernández A, Gutiérrez-Tobal GC, et al. Assessment of automated analysis of portable oximetry as a screening test for moderate-to-severe sleep apnea in patients with chronic obstructive pulmonary disease. PLoS One. 2017;12:e0188094. doi: 10.1371/journal.pone.0188094. https://doi.org/10.1371/journal.pone.0188094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CM, Kang CH, Su MC, Lin HC, Huang EY, Chen CC, et al. Usefulness of desaturation index for the assessment of obstructive sleep apnea syndrome in children. Int J Pediatr Otorhinolaryngol. 2013;77:1286–90. doi: 10.1016/j.ijporl.2013.05.011. https://doi.org/10.1016/j.ijporl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Fietze I, Dingli K, Diefenbach K, Douglas NJ, Glos M, Tallafuss M, et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J. 2004;24:987–93. doi: 10.1183/09031936.04.00100203. https://doi.org/10.1183/09031936.04.00100203. [DOI] [PubMed] [Google Scholar]
- 18.Nuber R, Vavrina J, Karrer W. Predictive value of nocturnal pulse oximetry in sleep apnea screening. Schweiz Med Wochenschr Suppl. 2000;116:120–2. [PubMed] [Google Scholar]
- 19.Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, et al. Screening for obstructive sleep apnea in adults: An evidence review for the US preventive services task force [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2017. Jan, Report No: 14-05216-EF-1. [PubMed] [Google Scholar]


