Abstract
Post-operative microwave (MW) hyperthermia has been applied as an important adjuvant therapy to enhance the efficacy of traditional cancer treatment. A better understanding of the molecular mechanisms of MW hyperthermia may provide guided and further information on clinical hyperthermia treatment. In this study, we examined the effects of MW hyperthermia on non-small cell lung carcinoma (NSCLC) cells in vitro, as well as the underlying mechanisms. In order to mimic clinical treatment, we developed special MW heating equipment for this study. Various NSCLC cells (H460, PC-9 and H1975) were exposed to hyperthermia treatment using a water bath or MW heating system. The results revealed that MW hyperthermia significantly inhibited cell growth compared with the water bath heating system. Furthermore, MW hyperthermia increased the production of reactive oxygen species (ROS), decreased the levels of mitochondrial membrane potential (MMP) and induced caspase-3 dependent apoptosis. It also induced G2/M phase arrest through the upregulation of the expression of phosphorylated (p-) ataxia telangiectasia mutated (ATM), p-checkpoint kinase 2 (Chk2) and p21, and the downregulation of the expression of cdc25c, cyclin B1 and cdc2. On the whole, the findings of this study indicate that the exposure of NSCLC cells to MW hyper-thermia promotes caspase-3 dependent apoptosis and induces G2/M cell cycle arrest via the ATM pathway. This preclinical study may help to provide laboratory-based evidence for MW hyperthermia treatment in clinical practice.
Keywords: microwave hyperthermia, water bath, non-small cell lung cancer, cell cycle, apoptosis
Introduction
Non-small cell lung carcinomas (NSCLCs) comprise approximately 85% of lung cancers and have an overall 5-year survival rate of 17.1% (1). The gold standard for the treatment of lung cancer is surgical resection, combined with chemotherapy and radiation therapy (2). Even though some molecular targeting drugs [such as tyrosine kinase inhibitors (TKIs)] have been applied to the treatment of patients with NSCLC with epidermal growth factor receptor (EGFR) mutations (3,4), the overall survival of patients with this disease remains discouraging. Therefore, the development of novel therapeutic strategies is required to improve the clinical outcomes.
Recently, post-operative hyperthermia has been applied as an important adjuvant therapy to enhance the efficacy of traditional chemotherapy and/or radiotherapy (5,6). As past technologies have not been able to deliver heat to tumor sites effectively and homogeneously without damaging the surrounding non-tumor tissue, the development of hyperthermia is relatively outdated in modern medical research. Recent novel techniques (such as computer modeling and non-invasive thermometry) which directly apply heat treatment, led us to focus on hyperthermia again. Numerous clinical data have suggested that microwave (MW) ablation can improve the oncologic outcomes of patients with hepatocellular carcinoma (7), colorectal liver metastases (8) and recurrent colorectal lung metastases (9). MW hyperthermia (temperature usually ranges between 42 and 45°C) has been widely employed in clinical trials of superficial tumors, including head and neck cancer, or breast cancer recurrences to the chest wall (10–13). It can also be used effectively in conjunction with radiation therapy and chemotherapy, and promotes body's immune response to fight against the target tumor (14,15). MW therapy is able to kill cancer cells through thermal as well as non-thermal effects (16). According to previous studies, MW hyperthermia has been shown to be a promising alternative non-invasive treatment strategy for various types of cancer, including lung cancer (17,18). A better understanding of the molecular mechanisms underlying its effects may provide further information on clinical hyperthermia treatment, to a certain extent; however, the precise mechanisms through which MW hyperthermia affects NSCLC remain undetermined.
In parallel to clinical research, several aspects of heat action have been examined in numerous preclinical studies. Due to the lack of special experimental devices, and the fact that clinical hyperthermia devices are difficult to be applied in preclinical studies, the water bath is the common heating system used in vitro and in vivo in experimental studies (19,20). When using a water bath to study hyperthermia, the temperature is the only indicator being evaluated; the non-thermal effects of MW are not considered. Additionally, differences in the biological effects induced by various heat treatments (including a water bath and MW hyperthermia) under isothermal conditions are not yet clear. In order to resolve this issue, we developed a special heating equipment (21) which can provide MW irradiation under certain temperatures. In this study, using this system, we investigated the mechanisms underlying the effects of MW hyperthermia on NSCLC cells in vitro.
Therefore, the aim of this study was to investigate the effects on NSCLCs induced by two types of heating systems (water bath and MW hyperthermia) under different temperature conditions and to explore the potential underlying mechanisms. We found that MW hyperthermia induced markedly more potent cytotoxic effects, increased reactive oxygen species (ROS) production, promoted mitochondrial dysfunction and promoted G2/M checkpoint arrest, thereby inducing the apoptosis of NSCLCs to a greater degree than the water bath heating system.
Materials and methods
Cells and cell culture
The human NSCLC cell lines, H460 (EGFR wild-type), PC-9 (exon 19-deletion EGFR mutant) and H1975 (T790M + L858R EGFR mutant) and the normal human lung epithelial cell line, BEAS-2B, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The NSCLC cell lines were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (Gibco, NY, USA) and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere. The BEAS-2B cells were maintained in DMEM supplemented with 5% FBS. Primary astrocyte cultures were separated from the cerebral cortex of 1-day-old post-natal Sprague-Dawley rats as previously described (22,23), with slight modifications. The 1-day-old post-natal rats were purchased from the Animal Center of the Zhejiang Academy of Medical Sciences. All the animals were raised at a specific pathogen-free level (SPF) laboratory at the Animal Center of the Zhejiang Academy of Medical Sciences. After purchase, the rats were sacrificed immediately to obtain primary astrocytes. The animals were anaesthetized with pentobarbital (40 mg/kg, intraperitoneal injection) and then decapitated. The whole brains were dissected under sterile conditions. The globulin, striatum, hippocampus and basal brain tissue were removed; cerebral cortex was collected, freed from adherent meninges. All experimental procedures were performed according to the National Institute of Health Guild for the Care and Use of Laboratory Animals and were in accordance with the Experimental Animal Welfare Ethics Committee of Zhejiang Academy of Medical Sciences (2018–045). The tissue was then washed in phosphate-buffered saline (PBS), cut into small fragments and digested with trypsin. Cell suspensions were centrifuged at 150 × g for 10 min and precipitation was re-suspended in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Grand Island, NY, USA) supplemented with 25 mM glucose, 10% FBS, 2 mM glutamine and 1% penicillin/streptomycin. The cells were seeded on poly-D-lysine-coated flasks, and grown in medium for the first 24 h. The medium was replaced every 3 days. After 12–14 days, the confluent cultures were shaken overnight to minimized microglial contamination. The number of glial fibrillary acidic protein (GFAP)-positive cells in these cultures was >95% (data not shown).
MW hyperthermia system
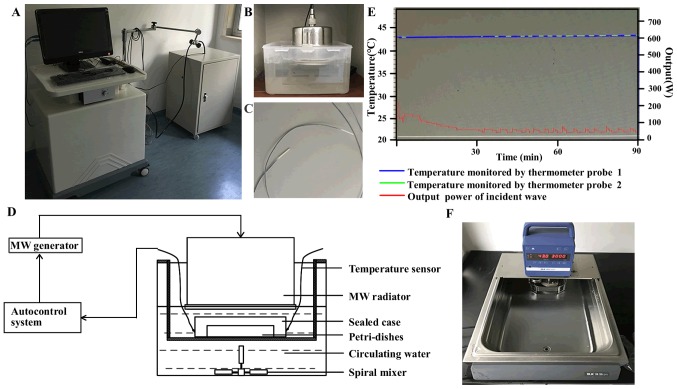
A novel MW applicator (21) was developed for the experimental hyperthermia treatment of cancer in vitro, which was equipped with a 433 MHz MW generator and temperature auto-control system (Fig. 1). The MW hyperthermia device consists of an auto-control system (computer), 2 fiber-optic thermometer probes, a MW generator and a MW radiator. The MW radiator is connected to a MW generator via a flexible cable and enclosed in a circulating water bath used as a protection against overheating of the cells. During MW hyperthermia treatment, the temperature of the cultured cells was measured with a temperature sensor under the dishes, and the temperature of surrounding circulating water was monitored via another sensor probe. Compared with a common thermistor thermometer, these thermometer probes used in our device can avoid the effects of electromagnetic waves, such as MW and allow for the measurement of temperatures within ±0.2°C of accuracy. The temperature was automatically controlled by a decrease/increase in the power output to maintain the temperature at the set value. Cell culture dishes or plates are positioned in the sealed box under the MW radiator. The MW heating system in vitro model is schematically illustrated in Fig. 1. Following initial irradiation, the temperatures and MW output reached a plateau with an output value of approximately 50 W, and the maximum output value of MW was set at 200 W.
Figure 1.
Schematic principles of the microwave hyperthermia device. (A) Photographic appearance of the microwave hyperthermia device: The novel irradiation system consisted of a (B) 433 MHz microwave generator and computer control system with MW radiator and (C) a temperature sensor. (D) Schematic representation of the MW hyperthermia device. Petri dishes or plates were positioned under the radiator and exposed to microwave radiation at 433 MHz. (E) Changes in temperature and output of microwave. Blue line indicates the temperature of the cultured cells measured using thermometer probe 1 under the Petri dishes. Green line indicates the temperature of the surrounding circulating water measured by thermometer probe 2. The red line indicates the output of the incident wave. (F) Photographic appearance of the water bath system.
Hyperthermia treatment
The cells were seeded in culture plates prior to treatment. For MW hyperthermia treatment, the cell culture dishes or plates were exposed to MW irradiation at the indicated temperatures for the indicated periods of time. For water bath treatments, the cell culture dishes or plates were immersed in a circulating water bath (IKA group, Staufen, Germany) at 43°C for 60 min or 90 min (Fig. 1F). For recovery following hyperthermia treatment, the cells were placed in an incubator at 37°C until further analysis.
Cell viability assay (cell counting kit-8 assay)
The inhibitory effects on tumor cell viability observed following treatment with the MW hyperthermia device or water bath were determined by CCK-8 assay. The NSCLC cells, H460, PC-9 and H1975, were seeded in 96-well plates at 1×104 cells/well. The cells were exposed to different temperatures (moderate hyperthermia) for 60 or 90 min. In the negative control group, the cells were incubated at 37°C in a CO2 incubator instead of MW irradiation or the water bath, and then incubated in a CO2 incubator. After 6, 12 or 24 h of treatment, 10 µl of CCK-8 solution (MedChem Express, Princeton, NJ, USA) were added to each well, and the cells were cultured for a further 1–3 h at 37°C. Cell viability in each group was measured at 450 nm using a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA). The cells were also pre-treated with or without the caspase-3 inhibitor, Ac-DEVD-CHO (Selleckchem, Houston, TX, USA), for 3 h prior to MW hyperthermia (43°C for 90 min) for 24 h. Cell viability in each group was then measured.
Apoptosis detection by flow cytometry
For each cell line, 2×105 cells/well were seeded in a 6-well plate. Following hyper-thermia treatment with MW or the water bath system, the cells were harvested and then stained with the Annexin V-FITC Apoptosis kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's instructions and analyzed using a flow cytometer (BD Biosciences). Annexin V+/PI− cells were considered early apoptotic and Annexin V+/PI+ cells were considered late apoptotic. The cells were also pre-treated with or without Ac-DEVD-CHO (50 µM) for 3 h prior to MW hyperthermia (43°C for 90 min) for 24 h.
Caspase-3 activity assay
Caspase-3 activity was determined using a Caspase-3 Activity Assay kit (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer's instructions. Briefly, the cells were treated with heat using a water bath or MW hyperthermia device and collected. The cells were washed with PBS twice. Lysis buffer was added by incubation on ice for 20 min. Cell lysates were centrifuged at 12,000 × g for 15 min at 4°C, and the protein concentration of the supernatants was determined using the Bradford protein assay kit (Beyotime Institute of Biotechnology). This was followed by incubation at 37°C for 1–2 h and the addition of Ac-DEVD-pNA (10 µl) and mixing with the cell lysates. The activity of caspase-3 was then quantified at 405 nm with a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific). The cells were also pre-treated with or without Ac-DEVD-CHO (50 µM) for 3 h prior to MW hyperthermia (43°C for 90 min) for 24 h.
Measurement of intracellular ROS generation
The detection of intracellular ROS generation following treatment was performed using the fluorescence probe, 2′,7′- dichlorodihydrofluorescein diacetate (DCFH-DA, Beyotime Institute of Biotechnology). The cells were seeded in a 35-mm culture plate (Thermo Fisher Scientific) at a density of 5×105 cells/ml in a volume of 1 ml. The cells were treated with 43°C (moderate hyperthermia) with MW or a water bath for 90 min. After 6 h of treatment, the cells were pre-loaded with 10 µM DCFH-DA in FBS-free RPMI-1640 medium for 20 min. After washing 3 times for 10 min with PBS, the cells were mounted under a fluorescenc microscope (Olympus BX61; Olympus, Tokyo, Japan) at an excitation of 488 nm and emission of 525 nm. Using the Image-Pro Plus program, the mean fluorescence intensity of the images was assessed and normalized to obtain relative ratios that were compared between the experimental groups.
Detection of mitochondrial membrane potential (MMP)
MMP was determined using the fluorescent probe, JC-1 (Beyotime Institute of Biotechnology), according to the instructions of the manufacturer. JC-1 is a cationic dye. Under normal conditions, the mitochondrial membrane exhibits red fluorescence; when MMP is depolarized, red fluorescence turns into green fluorescence. Briefly, following treatment, the cells were incubated with 5 µg/ml JC-1 staining solution for 20 min. After washing 2 times with PBS, the cells were observed under a confocal fluorescence microscope (Leica Microsystems AG, Mannheim, Germany). At least 10 visual fields in each were analyzed. All experiments were repeated at least 3 times.
Cell cycle analyses by flow cytometry
In order to obtain the distribution of cells in different phases of the cell cycle, the cells were fixed with 70% ice cold ethanol, stored overnight at −20°C, and subsequently stained using the cycle test plus DNA reagent kit according to the manufacturer's instructions (BD Biosciences). The samples were finally analyzed using a flow cytometer (BD Biosciences).
Western blot analysis
Following the different treatments, total protein was extracted using RIPA lysing buffer. Lysates were centrifuged at 15,000 × g for 10 min at 4°C. Protein was quantified using a BCA protein kit (Thermo Fisher Scientific). Equal amounts (40 µg) of tissue lysates were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The PVDF membranes were blocked with 5% non-fat milk at room temperature for 1–2 h and then incubated with specific primary antibodies at 4°C overnight. The following antibodies were used: anti-ataxia telangiectasia mutated (ATM; sc-377293, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylated (p-)ATM (13050S, 1:1,000, Cell Signaling Technology, Danvers, MA, USA), anti-p-checkpoint kinase 2 (Chk2; 2197S, 1:1,000, Cell Signaling Technology), anti-p21 (ab109520, 1:1,000, Abcam, Cambridge, MA, USA), anti-cdc 25c (4688S, 1:1,000, Cell Signaling Technology), anti-cyclin B1 (12231S, 1:1,000, Cell Signaling Technology), anti-cdc 2 (28439S, 1:1,000, Cell Signaling Technology), anti-β-actin (sc-47778, 1:1,000, Santa Cruz Biotechnology). After washing with TBST, the membranes were incubated with secondary antibodies (anti-rabbit IgG, sc-2357, 1:5,000 and anti-mouse IgG, sc-2005, 1:5,000 from Santa Cruz Biotechnology) at room temperature for a further 2 h. The protein bands were visualized using the ECL system (Beyotime Institute of Biotechnology). Images were captured using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). All experiments were repeated at least 3 times.
Statistical analysis
All analyses represented at least in triplicate experiments in vitro. Data are presented as the means ± SEM. The student t-test was used for single-group comparisons, and one-way ANOVA followed by Tukey's post hoc test was used for multiple comparisons. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
MW hyperthermia treatment inhibits tumor cell viability
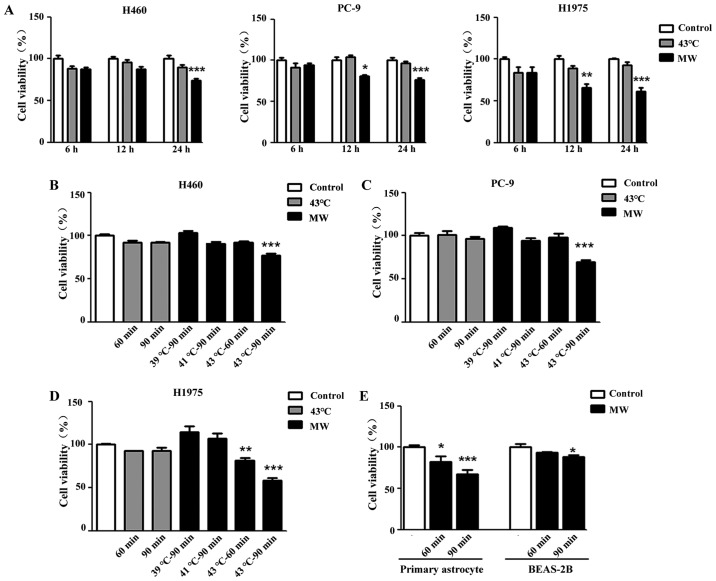
To examine the inhibitory effect of hyperthermia on tumor cell growth, CCK-8 assay was used to evaluate cell viability. All 3 different NSCLC cells (H460, PC-9 and H1975) were treated with MW hyperthermia or a water bath, while the control cell monolayers were maintained at 37°C. Following 90 min of heat treatment and a subsequent 6 h of incubation, heat treatment using a water bath or MW did not affect cell viability, while 12 h of incubation attenuated the viability of the PC-9 and H1975 cells treated with MW hyperthermia (Fig. 2A). After of 24 h pf incubation, cell viability was decreased significantly by MW hyperthermia treatment in all 3 cell lines (Fig. 2A). As shown in Fig. 2B–D, the mean value of percentage cell viability based upon the control (100%) was 77.35±1.89% (MW at 43°C for 90 min, P<0.001) in the H460 cell line, 69.75±1.82% (MW at 43°C for 90 min, P<0.001) in the PC-9 cell line, and 81.84±2.93% (MW at 43°C for 60 min, P<0.01) and 57.88±3.42% (MW at 43°C for 90 min, P<0.001) in the H1975 cell line. Compared with the control group, no significant differences were observed in the 3 cell lines treated with the water bath. Furthermore, we examined the side-effects of MW hyperthermia on some normal cells (murine primary astrocyte and human lung epithelial cells, BEAS-B2). In primary astrocytes, cell viability decreased as the irradiation time and temperature increased (MW at 43°C for 90 min, P<0.001). As for the normal lung epithelial cells, the BEAS-2B cells were the least sensitive to MW irradiation in the experiments shown in Fig. 2E. MW hyperthermia did not affect the viability of the BEAS-2B cells treated with 43°C mild hyperthermia for 60 min, although it induced 11.85% cell death with 43°C MW hyperthermia for 90 min (P<0.05).
Figure 2.
Microwave (MW) hyperthermia inhibits growth in different cell lines. (A-D) NSCLC cells (1×104/well) were seeded in a 96-well plate and were exposed to the water bath or MW hyperthermia for different periods of time. Following incubation for 6, 12 or 24 h, cell viability was determined by CCK-8 assay. (E) Primary astrocytes (1×104/well) and human normal lung epithelial cells, BEAS-2B cells (1×104/well) were seeded in a 96-well plate and exposed to MW hyperthermia for 60 or 90 min. Following incubation for 24 h, cell viability was determined by CCK-8 assay. Data are expressed as the means ± SEM of 3 independent experiments. *P<0.05 vs. the control group, **P<0.01 vs. the control group, ***P<0.01 vs. the control group.
MW hyperthermia treatment induces caspase-3-dependent apoptosis
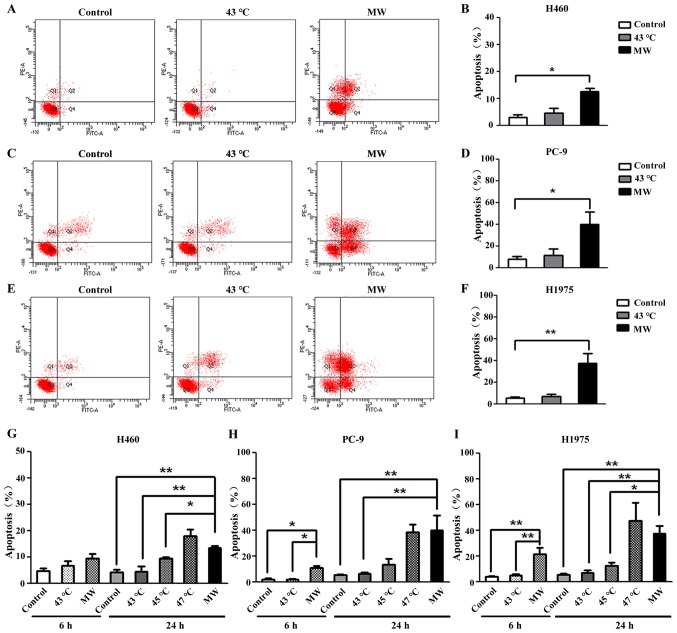
In order to determine the type of cell death induced by MW hyperthermia, the effects of heat treatment on apop-tosis were examined. Annexin V-positive and PI-negative cells represent early apoptotic cells, and double-positive cells are defined as late apoptotic cells. For the H460 cells, the total number of apoptotic cells (including early apoptotic and late apoptotic cells) increased significantly (3.89-fold compared to the control) following treatment with MW at 43°C for 90 min (Fig. 3A and B). For the PC-9 cells, a 5.56-fold increase in the total number of apoptotic cells was observed following treatment with MW at 43°C for 90 min (Fig. 3C and D). For the H1975 cells, an 8-fold increase in the total number of apoptotic cells was observed following treatment with MW at 43°C for 90 min (Fig. 3E and F). The mean value of percentage apoptosis of the cells treated with MW hyperthermia was 12.45±1.20%, 40.00±11.22% and 37.70±9.17% in the H460, PC-9 and H1975 cells, respectively. No significant differences were observed between the control groups and water bath-treated groups in all 3 NSCLC cell lines. These results suggested that the enhanced the inhibitory effects on cancer cell survival were associated with cell apoptosis in the cells treated with MW hyperthermia. Increasing the temperature of the water bath proportionally increased the percentages of apoptotic cells, which were 4.54±1.87%, 9.30±0.70%, 17.83±2.62% in the H460 cells (Fig. 3G), 6.33±1.24%, 13.26±4.71% and 38.30±5.89% in the PC-9 cells (Fig. 3H), and 7.06±1.83%, 12.10±2.95% and 47.43±13.87% in the H1975 cells (Fig. 3I) at 43, 45 and 47°C, respectively.
Figure 3.
Microwave (MW) hyperthermia promotes apoptosis. H460, PC-9 and H1975 cells were exposed to a water bath or MW hyperthermia at 43°C for 90 min, then allowed to recover at 37°C until 24 h. (A, C and E) Representative images of flow cytometry results. (B, D and F) Quantitative analyses of Annexin-FITC positive H460, PC-9 and H1975 cells are shown. (G-I) Quantitative analyses of Annexin-FITC positive H460, PC-9 and H1975 cells subjected to different treatments are shown. *P<0.05 vs. the control group, **P<0.01 vs. the control group.
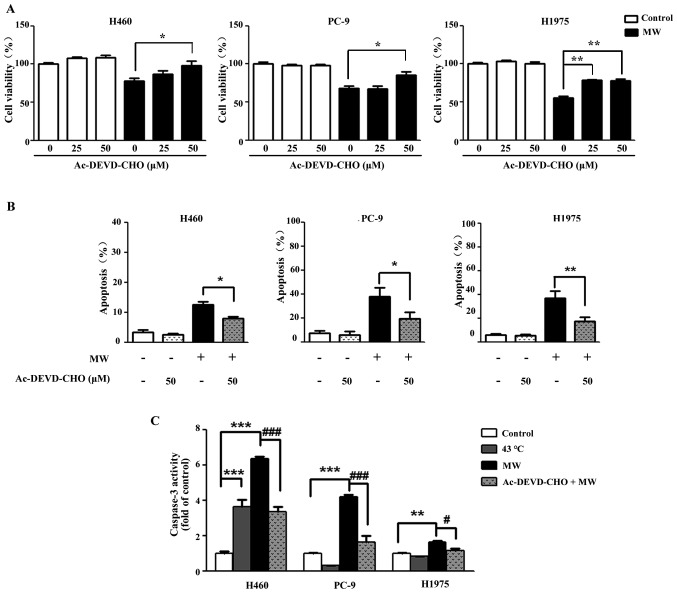
To examine whether caspase-3 activation is involved in the apoptosis triggered by MW hyperthermia, the cells were treated with Ac-DEVD-CHO, a caspase-3 specific inhibitor for 3 h prior to MW hyperthermia. Following MW treatment in the presence or absence of Ac-DEVD-CHO, cell viability was examined at 24 h. As shown in Figure 4A, Ac-DEVD-CHO markedly attenuated MW hyperthermia-induced cell death compared with the cells treated with MW alone. Furthermore, the apoptosis induced by MW was partially reversed by Ac-DEVD-CHO (Fig. 4B). Compared with each control group, the level of caspase-3 activation was markedly increased in the MW hyperthermia group (8.52-fold in the H460 cells, 4.14-fold in the PC-9 cells and 1.64-fold in the H1975 cells). Furthermore, we found that Ac-DEVD-CHO significantly attenuated the increase in caspase-3 production compared with the cells treated with MW hyperthermia alone for 24 h (Fig. 4C). These results indicated that caspase-3 was involved in the apoptosis induced by MW hyperthermia.
Figure 4.
Microwave (MW) hyperthermia triggers cell death through caspase-3-mediated apoptosis. H460, PC-9 and H1975 cells were treated with or without Ac-DEVD-CHO for 3 h prior to MW hyperthermia (43°C for 90 min), then allowed to recover at 37°C until 24 h. (A) Cell viability was determined by CCK-8 assay. Data are expressed as the means ± SEM of 3 independent experiments. *P<0.05 vs. the control group, **P<0.01 vs. the control group. (B) Quantitative analyses of Annexin-FITC positive H460, PC-9 and H1975 cells are shown. *P<0.05 vs. the control group, **P<0.01 vs. the control group. (C) Caspase-3 activity following treatment was analyzed. Data are expressed as the means ± SEM of 3 independent experiments. **P<0.01 vs. the control group, ***P<0.001 vs. the control group; #P<0.05 vs. MW treatment group, ###P<0.001 vs. MW treatment group.
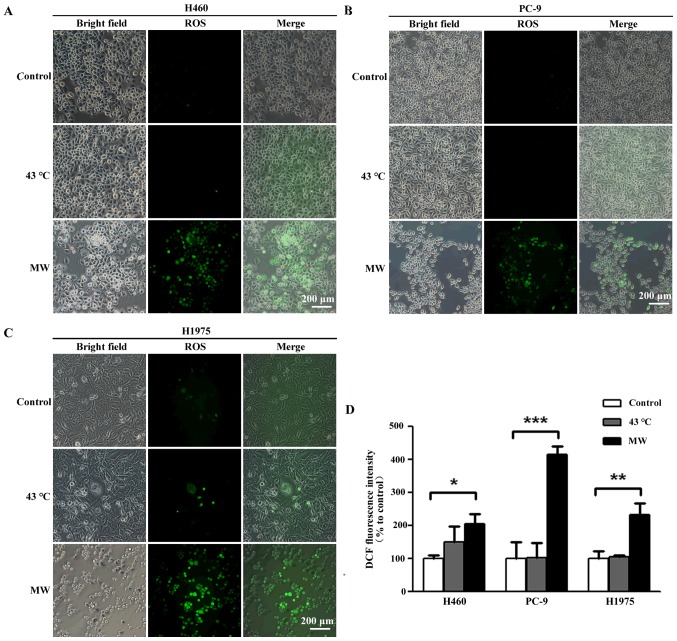
MW hyperthermia treatment increases ROS levels
To investigate whether oxidative stress contributes to the apoptosis induced by MW hyperthermia, we measured intracellular ROS production using a DCFDA fluorescence probe, an indicator of total cellular ROS. ROS production increased 6 h following MW hyperthermia (2.07-fold in the H460 cells, 2.31-fold in the H1975 cells and 4.41-fold in the PC-9 cells; Fig. 5), while heat-treatment using a water bath induced a slight, yet insignificant, increase in ROS levels (1.49-fold in the H460 cells, 1.04-fold in the H1975 cells and 1.01-fold in the PC-9 cells), compared to each control group. Taken together, our results demonstrated that oxidative stress contributed to the apoptosis of NSCLC cells induced by MW hyperthermia.
Figure 5.
Microwave (MW) hyperthermia treatment increases reactive oxygen species (ROS) levels. Cells were seeded onto 6-well plate at 5×105/well and then exposed to the water bath or MW radiation at 43°C for 90 min. Following incubation for 6 h, cells were pre-loaded with DCFH-DA and then mounted under a fluorescence microscope. (A-C) Representative photographs from DCF fluorescence staining in the H460, PC-9 and H1975 cells, respectively. Scale bars, 200 µm. (D) Quantitative analyses of the mean fluorescence intensity. *P<0.05 vs. the control group in H460 cells, ***P<0.001 vs. the control group in PC-9 cells, **P<0.01 vs. the control group in H1975 cells.
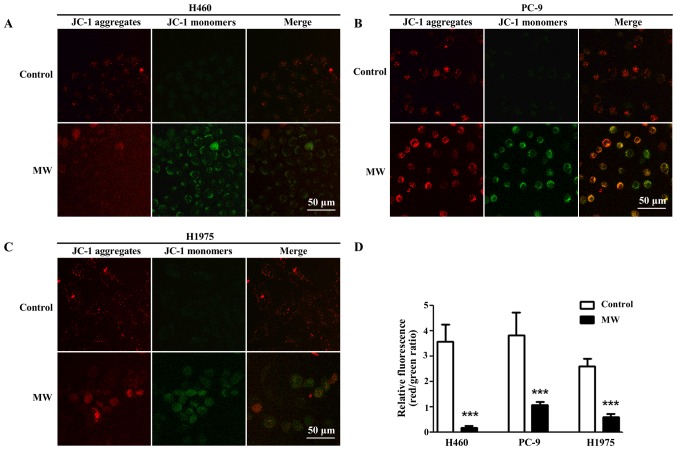
MW hyperthermia treatment decreases MMP in NSCLC cells
To determine whether the change in MMP was induced by MW hyperthermia, JC-1 staining was performed. JC-1 is an ideal fluorescent probe commonly used to detect MMP. In normal healthy cells, JC-1 accumulates in the mitochondria with red fluorescence, while in apoptotic cells, it depolarizes MMP, and JC-1 is diffused in the cytosol with green fluorescence. Thus, the transition from red fluorescence to green fluorescence of JC-1 staining suggests mitochondrial depolarization and apoptosis. We found that MMP was decreased in the NSCLC cells following treatment with MW hyperthermia (Fig. 6).
Figure 6.
Microwave (MW) hyperthermia treatment alters mitochondrial membrane potential (MMP). Cells were seeded onto 6-well plate at 2×105/well and then exposed to a water bath or MW radiation at 43°C for 90 min. Following incubation for 6 h, MMP was examined by JC-1 staining with a confocal microscope. (A-C) Representative photographs from DCF fluorescence staining in the H460, PC-9 and H1975 cells, respectively. Scale bars, 50 µm. (D) Quantitative analyses of relative fluorescence are shown. ***P<0.001 compared to each control group.
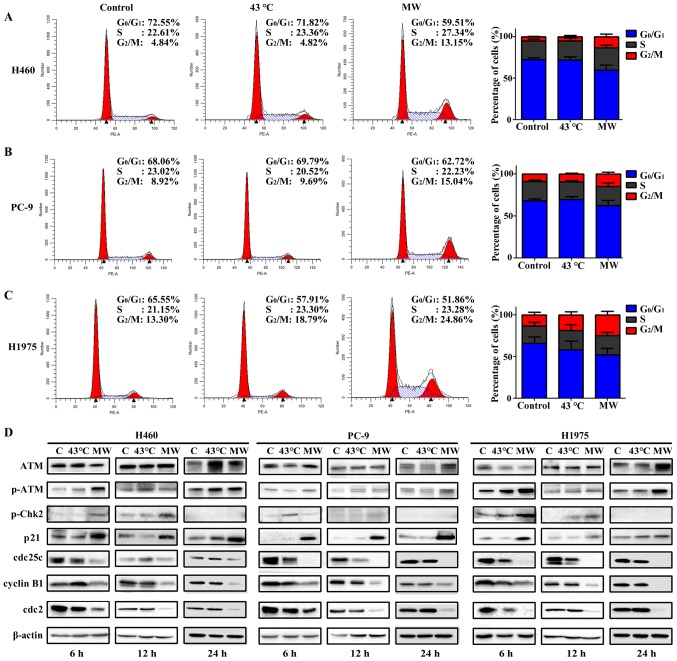
MW hyperthermia treatment induces G2/M arrest
The cells were treated with MW hyperthermia or a water bath, and the cell cycle distributions were analyzed by flow cytometry. As shown in Fig. 7A–C, the water bath treatment had no significant effect on cell cycle distribution, while MW hyperthermia treatment markedly induced G2/M phase arrest, indicated by the was significant increase in the G2/M phase cell population compared with that of the other groups. Compared with the control group, the proportion of cells in the G2/M phase increased with MW hyperthermia treatment (2.72-fold for the H460 cells, 1.88-fold for the PC-9 cells and 2.10-fold for the H1975 cells; Fig. 7A–C).
Figure 7.
Microwave (MW) hyperthermia induces G2/M arrest. Cells were seeded onto 6-well plate at 1×106/well and then exposed to a water bath or MW at 43°C for 90 min. The cells were cultivated for 24 h, cell cycle distribution were analyzed by flow cytometry. (A-C) The cell cycle distributions following treatment of H460, PC-9 and H1975 cells. (D) Western blot analysis of ATM, p-ATM, p-Chk2, p21, cdc25c, cyclin B1 and cdc2 in NSCLCs cells. β-actin expression was included as an internal control. Values were the means of triplicate analyses.
To examine the effects of MW hyperthermia on the cell cycle in more detail, the cell cycle regulator proteins for cell transiting through the G2/M phase were examined by western blot analysis. In all cell lines, ATM protein expression was not altered considerably, while the phosphorylation of ATM was increased in the MW hyperthermia group at 6 and 12 h, when compared with the control group. p-Chk2 protein expression was increased in the MW group at 6 and 12 h, but was not detected at 24 h. We speculated that Chk2 phosphorylation was detected transiently following MW exposure, which in turn rapidly activated downstream signaling pathways. In the PC-9 cells, Chk2 phosphorylation was increased slightly in the cells treated with the water bath at 6 h, compared with the control group. The expression of p21 was markedly increased in the MW hyperthermia group at 6, 12 and 24 h in all cell lines. The expression levels of cdc25c, cdc2 and cyclin B1 in the MW hyperthermia group were markedly decreased at 6, 12 and 24 h in the various NSCLC lines. The protein expression levels of regulators of the G2/M checkpoint were not markedly altered with water bath treatment (Fig. 7D). Our results indicated that the ATM signaling pathway may play a critical role in G2/M arrest induced by MW hyperthermia.
Discussion
In this study, we examined the effects of MW hyperthermia (433 MHz) on different NSCLC cells in vitro using a self-developed device. Under the isothermal conditions (43°C for 90 min), MW hyperthermia inhibited cell survival and increased the cell apoptotic rates to a greater extent than water bath treatment in vitro, and induced G2/M cell cycle arrest. The mechanisms involved may be related to the activation of the ATM pathway.
Over the past few decades, scientists have developed a large number of equipment and techniques for use in clinical hyperthermia (24). Previous studies have indicated the positive effects of hyperthermia treatments in clinical trials (25); however, the potential underlying mechanisms remain unclear. The goal of thermal biological research is to disclose the mechanisms responsible for the thermal effects, in order to further reveal the mechanisms of thermal radiosensitization and chemosensitization. In preclinical research, water baths are the most common heating system used to investigate hyperthermia, due to the lack of special thermal therapy instruments. It is difficult to explain the antitumor mechanisms of hyperthermia in the clinical setting. Water baths exert thermal effects alone, while MW hyperthermia therapies also exert non-thermal effects on cancer cells. A specifically designed device is needed that will mimic the clinical treatment on heating cancer cells in vitro. Electromagnetic heating devices operating from 8–915 MHz have been proposed for the hyperthermia treatment of cancer (24,26). To mimic the clinical conditions, we developed a novel MW applicator for the experimental hyperthermia treatment of cancer in vitro, which was equipped with a 433 MHz (ISM frequency in Europe: a frequency allocated for industry, science and medicine) (27) MW generator (Fig. 1). Using this novel MW system, we found that the viability of the H460, PC-9 and H1975 cells was decreased by MW hyperthermia, but not by the water bath at 43°C. These results indicated that the thermal-tolerant cells could be killed by MW hyperthermia. Caspase-3 was found to be involved in MW hyperthermia-induced apoptosis. Our results are consistent with those of another study in that MW hyperthermia induced cell apoptosis (28). The efficacy of hyperthermia depends on the treatment temperature and duration in cancer cells (29). Furthermore, we found that increasing the temperature of the water bath resulted in similar apoptotic rates to MW hyperthermia at 43°C (Fig. 3G–I). Pawlik et al reported that heat-treated cells at 43.5 and 45°C exhibited marked apoptotic phenomena (30). This indicated that the degree of temperature was one of the essential parameters in inducing apoptosis, but most importantly, MW may play a key role in the antitumor effects. Our results indirectly confirmed that MW hyperthermia exerted non-thermal effects on cancer cell death. Asano et al reported that normothermic MW irradiation induced cell death via heat-independent apoptosis (31). The potential mechanisms underlying these non-thermal effects of MW warrant further investigation. These results indicated that our novel MW device may be applied to preclinical studies in thermal biology, which is difficult to achieve when employing typically used water bath device. Moreover, we found that long-term exposure to MW (90 min) may have some side-effects on primary astrocytes and normal lung epithelial cells, BEAS-2B cells (Fig. 2E). Our results indicated that local treatment with MW hyperthermia in the clinical setting may be beneficial for eliminating the damage to normal surrounding tissue. On the whole, our results provide give a clear explanation of the distinct cell-killing effect of MW hyperthermia and the intrinsic molecular mechanisms using this novel device.
Hyperthermia may enhance the production of intracellular (ROS) (32,33). Hyperthermia-induced oxidative stress is crucial in the initiation of apoptotic cell death (28,34). Therefore, we speculated that ROS may play a role in cell apoptosis induced by MW hyperthermia. The results revealed that the accumulation of ROS was observed in the MW-treated NSCLC cells. It has been recognized that ROS can damage a variety of cellular components, leading to DNA damage, mitochondrial dysfunction and apoptosis. The excessive accumulation of ROS can open the mitochondrial permeability transition pore, releasing pro-apoptotic proteins, and finally activating the caspase cascade and inducing apoptosis (35). Furthermore, we found that MMP was depolarized in all NSCLC cell lines following treatment with MW hyperthermia. It is well known that depolarized MMP is an indicator of mitochondrial dysfunction during apoptosis (36), and it is an early event that coincides with caspase activation. The level of caspase-3 activation was markedly increased in the MW hyperthermia group (8.52-fold in the H460 cells, 4.14-fold in the PC-9 cells and 1.64-fold in the H1975 cells; Fig. 4C). The caspase-3 specific inhibitor, Ac-DEVD-CHO, significantly attenuated the increase in caspase-3 activity compared with MW hyperthermia alone. Our data suggested that ROS accumulation and MMP depolarization contributed to the MW hyperthermia-induced caspase-3-dependent apoptosis of human NSCLC cells.
There is accumulating evidence to indicate that hyper-thermia inhibits cell growth via inducing apoptosis and/or cell cycle checkpoint activation in tumor cells (37,38). Cell cycle checkpoints are important for regulating cell growth. In the present study, the results indicated that MW hyperthermia was found to arrest the cell cycle of NSCLC cells at the G2/M phase. Furthermore, we focused on investigating the potential molecular mechanisms of MW hyperthermia-induced G2/M checkpoint arrest. It is well known that cell cycle progression requires the precise expression and activation of several cyclins proteins and cyclin-dependent kinases (39). The ATM plays a key role in the activation of cell cycle checkpoints (40). The ATM pathway responds not only to DNA double-strand breaks, but also to damage induced by various types of stress (41–43). p21 is an important member of the family of cyclin kinase inhibitors, mediated G2/M cell cycle arrest (44). When DNA damage occurs, activated ATM induces Chk2 phosphorylation and p21 activation, consequently regulating cdc25c. Chk2 can induce G2/M cell cycle arrest by decreasing the protein expression of cdc25c (45). It is well known that in the G2/M checkpoint, cdc2 and cyclin B1 are the important regulators, and the activated cyclin B1/cdc 2 complex can trigger the transition from the G2 to the M phase (46,47). In the present study, we found that MW hyperthermia activated ATM and Chk2, further regulating the protein expression levels of p21, cdc25c, cyclin B1 and cdc2. Our results indicated that the mechanisms responsible for the effects of MW hyperthermia on G2/M phase arrest may be related to the regulation of the ATM-Chk2/p21-cdc25c signaling pathway.
Of note, we found that MW hyperthermia treatment induced a significantly higher proportion of apoptotic NSCLC cells with EGFR mutations (40.00±11.22% in the PC-9 cells with exon19 deletion in the EGFR gene, 37.70±9.17% in the H1975 cells with L858R/T790M double mutations in the EGFR gene) than in the H460 cells with wild-type EGFR (12.45±1.20%) (Fig. 3B, D and F). Thus, we hypothesized that the sensitivity of the cells to MW may differ among cells with different EGFR gene types. It has been reported that EGFR mutation-positive NSCLC is associated with the regression of patient tumors to intrapleural perfusion with hyperthermic chemotherapy complete treatment in clinical trials, and that the mechanisms involved may be related to the decreased protein level of EFGR (48). Our results provide experimental evidence to support the thesis that EGFR mutation-positive NSCLC cells may be more sensitive towards hyperthermia. These results indicate that MW hyperthermia may be one of the most promising adjuvant therapies against NSCLC tumors with mutations in the EGFR gene.
In conclusion, in this study, we report for the first time, at least to the best of our knowledge, that the exposure of NSCLC cells to a specially designed MW device (433 MHz) increases ROS production, mitochondrial dysfunction, promotes caspase-3-dependent cell apoptosis and induces G2/M cell cycle arrest by regulating the ATM signaling pathway. Heat treatment under a water bath did not have the same effect. Our results demonstrated that the MW hyperthermia device specially designed by our group may be a good tool for hyperthermia research in vitro.
Acknowledgments
The authors would like to thank the Experimental Animal laboratory of the Zhejiang Academy of Medical Sciences for assisting with the culture of astrocytes.
Funding
This study was supported by grants from the National Natural Science Foundation (81602555), Zhejiang Medical and Health Technology project of Zhejiang (2018247705). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YYZ, SLM and SRZ designed the experiments; SLM and SRZ were involved in project administration; YYZ, SLM and SRZ wrote and edited the manuscript; YYZ, QW, ZBW, JJZ, LCZ, and YY performed experiments and analyzed the data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures were performed according to the National Institute of Health Guild for the Care and Use of Laboratory Animals and were in accordance with the Experimental Animal Welfare Ethics Committee of Zhejiang Academy of Medical Sciences (2018-045).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YL, Sequist LV, Tan EH, Geater SL, Orlov S, Zhang L, Lee KH, Tsai CM, Kato T, Barrios CH, et al. Afatinib as first-line treatment of older patients with EGFR mutation-positive non-small-cell lung cancer: Subgroup analyses of the LUX-lung 3, LUX-lung 6, and LUX-lung 7 trials. Clin Lung Cancer. 2018;S1525-7304(18):30051–2. doi: 10.1016/j.cllc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, et al. European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) European Society for Hyperthermic Oncology (ESHO) Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta NR, Rogers S, Ordóñez SG, Puric E, Bodis S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:31–40. doi: 10.3109/02656736.2015.1099746. [DOI] [PubMed] [Google Scholar]
- 7.Han JB, Kong FW, Ding H, Zhang YF, Liu JM, Wei Q, Hu L, Zhao L, Xu CJ, Yi YX. Hepatectomy combined with microwave ablation of the spleen for treatment of hepatocellular carcinoma complicated with splenomegaly: A retrospective study. Mol Clin Oncol. 2017;6:204–208. doi: 10.3892/mco.2016.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulou LS, Thanos L, Ziakas PD, Merikas E, Achimastos A, Gennatas C, Syrigos KN. Thermal ablation may improve outcomes in patients with colorectal liver metastasis: A case-control study. J BUON. 2017;22:673–678. [PubMed] [Google Scholar]
- 9.Bäcklund M, Freedman J. Microwave ablation and immune activation in the treatment of recurrent colorectal lung metastases: A case report. Case Rep Oncol. 2017;10:383–387. doi: 10.1159/000468982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherar MD, Liu FF, Newcombe DJ, Cooper B, Levin W, Taylor WB, Hunt JW. Beam shaping for microwave waveguide hyperthermia applicators. Int J Radiat Oncol Biol Phys. 1993;25:849–857. doi: 10.1016/0360-3016(93)90315-M. [DOI] [PubMed] [Google Scholar]
- 11.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, González González D, et al. International Collaborative Hyperthermia Group Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-X. [DOI] [PubMed] [Google Scholar]
- 12.Kouloulias V, Triantopoulou S, Vrouvas J, Gennatas K, Ouzounoglou N, Kouvaris J, Karaiskos P, Aggelakis P, Antypas C, Zygogianni A, et al. Combined chemoradiotherapy with local microwave hyperthermia for treatment of T3N0 laryngeal carcinoma: A retrospective study with long-term follow-up. Acta Otorhinolaryngol Ital. 2014;34:167–173. [PMC free article] [PubMed] [Google Scholar]
- 13.Kouloulias V, Triantopoulou S, Efstathopoulos E, Platoni K, Kouvaris J, Uzunoglou N, Antypas C, Karaiskos P, Aggelakis P, Vrouvas J, et al. Microwave hyperthermia in conjunction with radiotherapy in superficial tumours: Correlation of thermal parameters with tumour regression. West Indian Med J. 2013;62:752–757. doi: 10.7727/wimj.2012.328. [DOI] [PubMed] [Google Scholar]
- 14.Agostinelli E, Belli F, Dalla Vedova L, Marra M, Crateri P, Arancia G. Hyperthermia enhances cytotoxicity of amine oxidase and spermine on drug-resistant LoVo colon adenocarcinoma cells. Int J Oncol. 2006;28:1543–1553. [PubMed] [Google Scholar]
- 15.Li L, Wang W, Pan H, Ma G, Shi X, Xie H, Liu X, Ding Q, Zhou W, Wang S. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J Transl Med. 2017;15:23. doi: 10.1186/s12967-017-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano M, Sakaguchi M, Tanaka S, Kashimura K, Mitani T, Kawase M, Matsumura H, Yamaguchi T, Fujita Y, Tabuse K. Effects of normothermic conditioned microwave irradiation on cultured cells using an irradiation system with semiconductor oscillator and thermo-regulatory applicator. Sci Rep. 2017;7:41244. doi: 10.1038/srep41244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomised data. Int J Hyperthermia. 2010;26:612–617. doi: 10.3109/02656736.2010.487194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallory M, Gogineni E, Jones GC, Greer L, Simone CB., II Therapeutic hyperthermia: The old, the new, and the upcoming. Crit Rev Oncol Hematol. 2016;97:56–64. doi: 10.1016/j.critrevonc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9:e87979. doi: 10.1371/journal.pone.0087979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao F, Ye Y, Zhang Y, Yang J. Water bath hyperthermia reduces stemness of colon cancer cells. Clin Biochem. 2013;46:1747–1750. doi: 10.1016/j.clinbiochem.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Wu ZB, Ma SL, Zhu J, Long JP, Li XD, Yao XY, Lu HQ, Zhang YM, Chen SM, Jing SS. An experimental device for heating tumor cell. 2015205415415. CN Patent. Filed July 23, 2015; issued December 2, 2015.
- 22.Wang R, Zhang X, Zhang J, Fan Y, Shen Y, Hu W, Chen Z. Oxygen-glucose deprivation induced glial scar-like change in astrocytes. PLoS One. 2012;7:e37574. doi: 10.1371/journal.pone.0037574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levison SW, McCarthy KD. Astroglia in culture. In: G Banker, K Goslin., editors. Nerve Cell Culture. MIT Press; Cambridge, MA: 1991. pp. 309–336. [Google Scholar]
- 24.Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21:731–744. doi: 10.1080/02656730500331868. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen CM, Oei AL, Chin KWTK, Crezee J, Bel A, Westermann AM, Buist MR, Franken NAP, Stalpers LJA, Kok HP. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat Oncol. 2017;12:75. doi: 10.1186/s13014-017-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rietveld PJ, van Putten WL, van der Zee J, van Rhoon GC. Comparison of the clinical effectiveness of the 433 MHz Lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys. 1999;43:681–687. doi: 10.1016/S0360-3016(98)00443-X. [DOI] [PubMed] [Google Scholar]
- 27.Paulides MM, Bakker JF, Chavannes N, Van Rhoon GC. A patch antenna design for application in a phased-array head and neck hyperthermia applicator. IEEE Trans Biomed Eng. 2007;54:2057–2063. doi: 10.1109/TBME.2007.895111. [DOI] [PubMed] [Google Scholar]
- 28.Xing F, Zhan Q, He Y, Cui J, He S, Wang G. 1800MHz microwave induces p53 and p53-mediated caspase-3 activation leading to cell apoptosis in vitro. PLoS One. 2016;11:e0163935. doi: 10.1371/journal.pone.0163935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JF, Yan XM, Lan B, Lei YR, Li XH, Gao S, Guo YF, Guo F. Molecular mechanisms of synergistic induction of apoptosis by the combination therapy with hyperthermia and cisplatin in prostate cancer cells. Biochem Biophys Res Commun. 2016;479:159–165. doi: 10.1016/j.bbrc.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 30.Pawlik A, Nowak JM, Grzanka D, Gackowska L, Michalkiewicz J, Grzanka A. Hyperthermia induces cytoskeletal alterations and mitotic catastrophe in p53-deficient H1299 lung cancer cells. Acta Histochem. 2013;115:8–15. doi: 10.1016/j.acthis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, Tanaka S, Sakaguchi M, Matsumura H, Yamaguchi T, Fujita Y, Tabuse K. Normothermic microwave irradiation induces death of HL-60 cells through heat-independent apoptosis. Sci Rep. 2017;7:11406. doi: 10.1038/s41598-017-11784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS One. 2013;8:e75044. doi: 10.1371/journal.pone.0075044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katschinski DM, Boos K, Schindler SG, Fandrey J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J Biol Chem. 2000;275:21094–21098. doi: 10.1074/jbc.M001629200. [DOI] [PubMed] [Google Scholar]
- 34.Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int J Mol Sci. 2014;15:17380–17395. doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H, Kim S, Choi BH, Park MT, Lee J, Jeong SY, Choi EK, Lim BU, Kim C, Park HJ. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int J Hyperthermia. 2011;27:698–707. doi: 10.3109/02656736.2011.608217. [DOI] [PubMed] [Google Scholar]
- 36.Gu Y, Chen T, Fu S, Sun X, Wang L, Wang J, Lu Y, Ding S, Ruan G, Teng L, et al. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med. 2015;13:35. doi: 10.1186/s12967-015-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed K, Tabuchi Y, Kondo T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis. 2015;20:1411–1419. doi: 10.1007/s10495-015-1168-3. [DOI] [PubMed] [Google Scholar]
- 38.Furusawa Y, Iizumi T, Fujiwara Y, Zhao QL, Tabuchi Y, Nomura T, Kondo T. Inhibition of checkpoint kinase 1 abrogates G2/M checkpoint activation and promotes apoptosis under heat stress. Apoptosis. 2012;17:102–112. doi: 10.1007/s10495-011-0660-7. [DOI] [PubMed] [Google Scholar]
- 39.Roskoski R., Jr Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol Res. 2016;107:249–275. doi: 10.1016/j.phrs.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Kurz EU, Lees-Miller SP. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst) 2004;3:889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Shaltiel IA, Krenning L, Bruinsma W, Medema RH. The same, only different - DNA damage checkpoints and their reversal throughout the cell cycle. J Cell Sci. 2015;128:607–620. doi: 10.1242/jcs.163766. [DOI] [PubMed] [Google Scholar]
- 42.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 43.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 44.Beecken WD, Ringel EM, Babica J, Oppermann E, Jonas D, Blaheta RA. Plasmin-clipped beta(2)-glycoprotein-I inhibits endothelial cell growth by down-regulating cyclin A, B and D1 and up-regulating p21 and p27. Cancer Lett. 2010;296:160–167. doi: 10.1016/j.canlet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Yin H, Jiang M, Peng X, Cui H, Zhou Y, He M, Zuo Z, Ouyang P, Fan J, Fang J. The molecular mechanism of G2M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget. 2016;7:35592–35606. doi: 10.18632/oncotarget.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et al. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev Cell. 2014;29:217–232. doi: 10.1016/j.devcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng YM, Tsai CC, Hsu YC. Sulforaphane, a dietary isothiocyanate, induces G(2)/M arrest in cervical cancer cells through cyclin B1 downregulation and GADD45beta/CDC2 association. Int J Mol Sci. 2016;17:1530. doi: 10.3390/ijms17091530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Zhan C, Ke J, Xue Z, Zhang A, Xu K, Shen Z, Yu L, Chen L. EGFR kinase domain mutation positive lung cancers are sensitive to intrapleural perfusion with hyperthermic chemotherapy (IPHC) complete treatment. Oncotarget. 2016;7:3367–3378. doi: 10.18632/oncotarget.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.