Abstract
Stryphnodendron spp., popularly known as “barbatimão”, is the native Brazilian tree most often employed to treat wounds and infections. The aim of the present study was to highlight the importance of S. adstringens, as well as other Stryphnodendron species recognized as “barbatimão”, to human health, depicting the relevance of ethnopharmacological knowledge to scientific evidence for uses, related chemical compounds, development of pharmaceutical formulations, and the establishment of toxicity parameters. For this purpose, the literature databases PubMed, Scielo, Lilacs, CAPES Thesis and Google Scholar were searched until 2017. It was observed that stem bark was the primary part of the plant used, mainly as a decoction, for wound healing and treatment of infectious and inflammatory disorders. Confirmed biological activities, including wound healing, anti-inflammatory, antioxidant, and antimicrobial activities, were related to the presence of compounds from tannin class, mostly proanthocyanidins. Toxicity parameters for stem bark were inconclusive, but toxicity was observed to a significant extent when seeds were ingested by cattle or other animals. Due to these important and confirmed biological activities, government policy encourages the phytotherapic use of S. adstringens, and some formulations with stem bark extracts were developed and patented. Furthermore, antiprotozoal, hypoglycemic and antiviral activities were identified as promising.
Keywords: ethnopharmacology, medicinal plant, biological activity, tannin, catechin, wound healing
1. Introduction
Plants are a source of molecules with a wide variety of applications, and humanity has learned to harness their benefits and to recognize their toxic effects throughout history. Ethnopharmacological uses of plants represent part of each culture around the world, so it is no wonder that isolated active molecules are being used in standard preparations [1,2]. Today, natural drugs, including plants and their derivatives, are one of the biggest sources of approved medicines [2]. Brazil has extensive biodiversity, and many exotic species have been introduced, reflecting a rich folk medicine with the influence of native, African, and European peoples [3,4]. In that sense, government policies were established over the years to protect Brazilian biodiversity and to stimulate drug development and phytotherapy in the country’s health system [5,6,7].
In Brazil, one of the native trees commonly used is known as “barbatimão”, from which stem barks are prepared as decoctions or infusions with the main purpose of wound and infection healing [8,9]. On the other hand, the broad beans are also recognized as an abortive for cattle when they are eaten in the field [10,11]. Other popular names of these trees include “barbatimão-verdadeiro”, “barba-de-timão”, “chorãozinho-roxo” and “casca-da-virgindade”. The scientific name of the species is Stryphnodendron adstringens (Mart.) Coville, with accepted synonyms Acacia adstringens Mart., Mimosa barbadetimam Vell., Mimosa virginalis Arruda, Stryphnodendron barbatimam Mart., and Stryphnodendron barbadetiman (Vell.) Mart. [12,13]. However, other Stryphnodendron species are also popularly known as “barbatimão” as S. obovatum Benth. (synonym of S. rotundifolium Mart.), S. polyphyllum Mart., and S. rotundifolium Mart.. Other species such as Abarema cochliocarpos (Gomes) Barneby & Grimes, Pithecellobium cocliocarpum (Gomez) Macbr., and Dimorphandra mollis Benth. can be mistakenly referred in the literature as “barbatimão” but they are recognized as “falso-barbatimão” which could be freely translated as “fake-barbatimão”. There are 42 species in the Stryphnodendron genus presently, and they are disseminated in the Neotropical region, from Costa Rica in Central America to the south of Brazil, with the majority of species in Brazil present either in the rainforest or in the Brazilian savanna (“Cerrado”) [14]. Phylogenetic analysis of the Stryphnodendron genus showed very short internal branches but clustered together savanna species as S. adstringens and S. rotundifolium, whereas species from the rainforest such as S. polyphyllum formed a different cluster, indicating rapid evolution and morphological diversification in the group. Abarema, Pithecellobium and Dimorphandra were not included in this study as their genera were not closely related to Stryphnodendron [15].
Thus, data compilation reviewing the studies already done with Stryphnodendron spp. barks will contribute to the phytotherapeutic use of “barbatimão” in the health care system and to the development of efficient formulations. In addition, this data compilation will also highlight the gaps still present in literature in order to elucidate the safe use of the barks in a sustainable way and to create a future in which the active molecules of the extract can be utilized as new drugs or new drug prototypes. Furthermore, by stimulating the conservation of the species and disseminating its benefits to the scientific community, more drugs can be developed for treatment of wounds as well as infectious and inflammatory disorders.
In that sense, the aim of the present work is to review the existing information available about the benefits of S. adstringens and other “barbatimão” species from the Stryphnodendron genus to human health, thereby demonstrating the relevance of folk knowledge to scientifically-proven biological activities, formulations developed, toxicity parameters, and the main compounds involved.
2. Data Collection Methodology
The scientific names of the Stryphnodendron genus referred to as “barbatimão” were selected for search in databases. Those names comprised S. adstringens, A. adstringens, M. barbadetimam, M. virginalis, S. barbatimam, S. barbadetiman, S. discolor, S. obovatum, S. polyphyllum, and S. rotundifolium. Searches were performed in the following databases: Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/), Scielo (http://www.scielo.br/), Lilacs (http://lilacs.bvsalud.org/), and the CAPES Brazilian Thesis databank (http://bancodeteses.capes.gov.br/banco-teses/#!/) up to 5 November, 2017. Complementary information was searched in Google (http://www.google.com).
3. General Aspects on Literature about Stryphnodendron
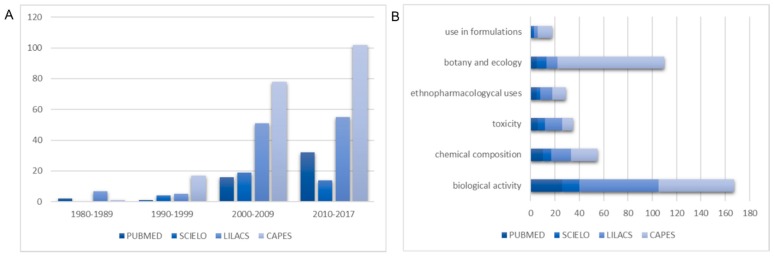
A survey of the databases PubMed, Scielo and Lilacs identified about 200 papers covering “barbatimão” species (S. adstringens, S. obovatum, S. polyphyllum and S. rotundifolium, including synonyms), and more than 200 Master and PhD Theses in Brazil were found in CAPES. After the year 2000, the number of publications about Stryphnodendron spp. known as “barbatimão” increased about 10-fold and twice in journals indexed in PubMed after 2010 (Figure 1A). Most of the publications were related to the biological properties of the species, mainly for the treatment of internal and external infectious and inflammatory diseases, but also for wound and ulcerative wound healing related to the astringent and cicatrization properties, highlighting the ethnopharmacological attributes of the species. Demonstrating the ethnopharmacological use of a plant requires well-conducted scientific studies correlating the plant results with a well-established standard [1]. Attention should be payed to studies of biological activity with inconclusive data, which, while not confirming ethnopharmacological use, can be a step in the right direction [16]. To that end, the Brazilian Pharmacopeia and Government have amassed the efforts of recognized scientific institutions and internationally published papers to enable proper identification of “barbatimão” species, their active chemical constituents, and their therapeutic uses according to the proven efficacy of the species [17,18,19].
Figure 1.
Number of publications in the searched databases according year (A) and research area (B).
Chemical composition and standardization of methods to analyze the barks constitution are expressive. Although there are some studies related to plant toxicity of “barbatimão” species for cattle, there are few studies focusing on toxicity levels and safety in humans. Different medicinal topical products derived from bark extracts are being developed, and their activity has been demonstrated (Figure 1B).
4. Botanical Features and Sustainable Management Aspects
Plants from genus Stryphnodendron belongs to the Fabaceae Lindl. family, which comprises more than 200 genera [12]. Trees of the genus are small to medium sized evergreens, with an unbranched trunk, and the stem generally has tortuous and thick rust-colored bark [13,20]. S. adstringens trees have a low and round top, while S. polyphyllum has a large top, and the top of S. rotundifolium is round and diffuse [20].
S. polyphyllum has the shortest petiole at 3–6.5 cm, which contrasts with S. adstringens at 6.5–9 cm and S. rotundifolium at 2.5–10 cm. Species are more easily differentiated by their bipinnate compound leaves; S. adstringens has 5–7 pairs of leaflets in opposite insertion with 5–6 pairs of second order leaflets alternately inserted, limb is asymmetrical ovoid, sometimes elliptical, with 1.5–3.5 × 1–2.5 cm emarginated or round apex, flat margin narrowly thickening; card form limb, glabrous, concolor, visible veins immersed in the limb in the superior face and salient in the inferior face. On the other hand, leaflets of S. polyphyllum shows higher number of pairs of leaflets (11–18, although less are present in leaflets close to the branch apex) in opposite insertion, also with higher number of pairs of second order leaflets (14–23) and insertion subopposite; limb is slightly asymmetrical, oblong (sometimes distal pairs are obovate and rarely proximal pairs are elliptical to oval), with 3–8 × 1.5–4 mm, mostly round apex but, sometimes, apex is obtuse, margin is revolute; card form limb, pubescent, subconcolor, darker superior face with invisible veins immersed in the limb and slightly visible in the inferior face [20,21,22]. S. rotundifolium has 6–13 pairs of leaflets in opposite insertion in distal pairs and subopposite in proximal pairs, 5–12 pairs of second order leaflets with alternate insertion (except distal pairs have opposite insertion), asymmetric to symmetric limb, generally orbicular, ovoid, elliptical or elliptical-ovoid (distal pairs are obovate) with 7–18 × 6–13 mm, asymmetrical apex generally retuse to emarginate, but sometimes round, sub-revolute margin, slightly thickening; card form limb, darker superior face with slightly visible immersed veins and central vein sometimes ridged, which is salient in the inferior face where the other veins are also slightly seen [20,21,22].
Inflorescence of these species are simple thyrse type. S. adstringens has snowy to yellowish inflorescences, rarely pinkish, geminate to ternate spikes, sometimes isolated, with 10–11 cm of length; S. polyphyllum has them in pink to reddish color, generally isolated spikes with 8–11 cm; while S. rotundifolium shows similar color of inflorescence as S. adstringens but 2–3 spikes of 9–18 cm of length [20]. Flowers are hermaphrodite (but S. adstringens and S. rotundifolium can rarely show male and female flowers), calyx and corolla are campanulate. S. adstringens has snowy to yellowish flowers, corolla of 5 mm of length; S. polyphyllum has reddish flowers, corolla of 3–3.5 mm and, S. rotundifolium presents snowy, light green to yellowish flowers also with 3–3.5 mm corolla [20,23]. They present seeds (8–10, 7–8 and 5–15, respectively in S. adstringens, S. polyphyllum, and S. rotundifolium) that are located in broad bean without salience [20,23]. Nucoide legume of S. adstringensis straight, with rounded apex and base, S. polyphyllum and S. rotundifolium have it straight but it is also rarely found slightly curved [20]. There are no significant macro- and microscopic differences between these species’ barks, which are commonly sold in free markets and therefore other assays could be done for identification, as tannin content [21,24].
Attempts of conservation and domestication of these species in order to keep their chemical composition and biological properties include: germination [25,26,27], micropropagation [28,29], callus culture [30,31], and genetic studies [32,33]. That is important since agricultural expansion eliminates native specimens and is an ecological concern that compels the sustainable management of these trees [34]. Even more, barks are extracted in a disorderly way from the trees for medicinal purposes and this exploitation reduces the regeneration process and the density of specimens, corroborating need for sustainable management, domestication, and conservation of the species [35,36,37].
5. Ethnopharmacological Uses
Traditional medicine is an important source of medicinal information, and over time humans have learned from Nature which plants, animals, and other elements could be used to help their survival. The wide biodiversity of flora in Brazil has provided an abundance of ethnopharmacologically important plants, and the use of “barbatimão” is consistently reported. It is noteworthy that the reports of the utilization of medicinal plants, including “barbatimão”, occurs mainly among adults with limited access to health care and is related to familiar traditional uses in addition to vendors in public markets or descendant of indigenous and maroon people [8,24,38,39,40].
Almost all collected papers about ethnopharmacological uses of “barbatimão” reported the treatment of wounds, followed by infections. Treatments for female genitourinary conditions and uterine disorders, and gastric ulcers were also frequently reported. Treatment of cancer was addressed in some ethnopharmacological surveys, as well as treatments of hemorrhage, diabetes, and pain. The most common preparations were infusions, macerations, and decoctions of the barks. S. adstringens and S. rotundifolium were the species most often observed. Cicatrizing, astringent, anti-inflammatory, and antimicrobial properties of the barks were the main attributes that characterized the folk choice of “barbatimão” and they were demonstrated scientifically [9,41,42]. Oral and topical were mentioned as the preferred routes of administration (Table 1).
Table 1.
Overview of ethnopharmacological reports in literature about “barbatimão” uses.
| Species | Part of Plant | Medicinal Use | Form of Preparation and Administration | Reference |
|---|---|---|---|---|
| SA | Stem bark | Uterine infection, ovary inflammation, wound healing, ulcer, cicatrizing, anti-inflammatory, hygiene, sore throat and itch | Baths | [43] |
| SA | Stem bark | Ulcerous wounds | Macerated, used as bath | [44] |
| SA | Stem bark | Not mentioned | Topical use | [45] |
| SA | Stem bark | Wound healing | Decoction, infusion or macerated, for external or internal uses | [8] |
| SA | Stem bark | Wound, chilblain, diabetes, prostate problems, inflammation, gastritis, liver diseases, dental inflammation, pain in general | Not mentioned | [39] |
| SA | Stem bark | Leucorrhea, wound healing, ulcer and vaginal discharge | Not mentioned | [46] |
| SA | Stem bark | Urinary infection | Oral | [47] |
| SA | Stem bark | Wound healing | Tea, infusion, bottleful, powder | [48] |
| SR | Stem bark and seeds | Diuretic, anti-diarrheic, ulcer, cicatrizing, chilblain, astringent, for gums | Macerated in water | [49] |
| SR | Stem bark | Wounds, inflammation, gastritis and ulcer, vaginal inflammation, pain, infection, prostate disorders, sexually transmitted diseases, rheumatism, hypertension, dermatitis, burns, menopause, postpartum healing, renal calculi, influenza, lung diseases | Immersion in water for oral or topical administration | [50] |
| SR | Stem bark, roots and leaves | Inflammation, vaginal discharge, urinary infection, uterine lesions | Decoction and infusion | [51] |
| SR | Stem bark | General wound healing, ulcer, general inflammation, headache, gastritis, cancer, fever, leg, body, stomach and belly pain, cough, cuts, scabs, flu, sore throat, heart, childbirth inflammation, blood pressure, blood disorder, kidneys, lung inflammation, sinus and urinary infection, excessive menstruation, itch, vaginal discharge, stanch blood from cuts, skin allergy, swelling, tightening the vagina for sexual intercourse | Not mentioned | [36] |
| SR | Stem bark and roots | Backache | Macerated for oral administration | [52] |
| SR | Stem bark | Wound, uterus and skin inflammation, wound healing, genital disease and cancer | Immersion in water or decoction is prepared for oral and topical administration and baths | [40] |
| SR | Stem bark | Ulcer, wound healing, venereal disease, hemorrhage, diabetes, anthelmintic, high blood pressure, anemia, cancer, liver disease | Infusion and tincture | [24] |
SA: S. adstringens. SR: S. rotundifolium.
One of the Brazilian policies related to the development of more economically accessible health treatments has been to stimulate the phytotherapy in the primary healthcare system [53], and “barbatimão” was one of the ethnopharmacologically-used plants included in the list of medicinal plants in the system [8]. Owing to this phytotherapeutic policy and the extensive ethnopharmacological use, the quality control, correct identification [54,55,56] and conservation of the species are essential requirements to ensure accurate treatment, to avoid toxicity or dose dilution with impurities [43]. The Brazilian Pharmacopeia includes one monograph for correct identification of S. adstringens barks, purity and dose assays based in its high tannin content [17], which can vary throughout the year in the different species [57,58]. Another ongoing concern is agricultural advance into habitat areas of these species [44].
Besides the stimulus for the national phytotherapeutic use of medicinal plants, ethnopharmacology has a cultural value and shelters an important source of molecules with biological activities and those are good reasons for more pharmacological studies and plant conservation practices.
6. Chemical Composition
6.1. Metabolites Identified in Stryphnodendron Species
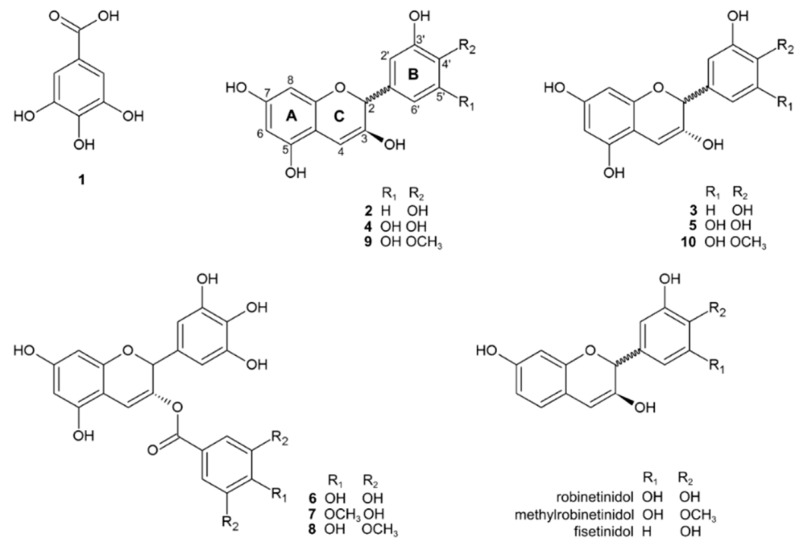
The stem bark of “barbatimão” is the main utilized part of the plant and, therefore, its chemical composition was extensively studied focusing on its secondary metabolites, which led to the identification of high phenolic or tannic contents and elucidation of the main low- or high-weight tannins present in aqueous, hydroalcoholic, and acetone:water extracts [59,60,61,62]. Some extracts of “barbatimão” were also prepared using propylene glycol and water as solvent for use in pharmaceutical formulations. In those cases, a higher content of tannins was obtained with 80% propylene glycol [63]. Chromatographic methods are mainly used for the isolation and identification of the presence of a variety of polyphenolic compounds, especially hydrolysable and condensed (as the proanthocyanidins, prodelphinidins, prorobinetinidins, and profisetenidins) tannins in extracts and fractions of “barbatimão” [64]. The compounds identified in the barks are presented in Table 2. The basic skeletal structure of hydrolysable tannins and proanthocyanidins are illustrated in Figure 2.
Table 2.
Compounds identified in the stem bark of Stryphnodendron species known as “barbatimão”.
| Number | Compound Name | Species | Reference |
|---|---|---|---|
| 1 | Gallic acid | SA, SP, SR | [54,56,60,62,64,65,66] |
| 2 | Catechin | SA, SR | [56,60,65,67] |
| 3 | Epicatechin | SA | [67] |
| 4 | Gallocatechin | SA, SP, SR | [54,56,62,64,65,67,68] |
| 5 | Epigallocatechin | SA, SP, SR | [54,56,62,64,65,66,67,68] |
| 6 | Epigallocatechin 3-O-gallate | SA, SR | [56,61,64,65,66,68] |
| 7 | Epigallocatechin 3-O-methylgallate | SA | [66] |
| 8 | Epigallocatechin 3-O-(3,5-dimethyl)gallate | SA | [54,68] |
| 9 | 4′-O-methylgallocatechin | SA, SP | [54,64,68] |
| 10 | 4′-O-methylepigallocatechin | SA | [66] |
| 11 | 4′-O-methylepigallocatechin-3-O-gallate | SA | [64] |
| 12 | Epigallocatechin 3-O-(3-methoxy-4-hydroxy)benzoate | SA | [54,68] |
| 13 | Gallocatechin-(4α→8)-epigallocatechin 3-O-(4-hydroxy)benzoate | SA | [54,68] |
| 14 | Epigallocatechin-(4β→8)-epigallocatechin 3-O-(4-hydroxy)benzoate | SA | [54,68] |
| 15 | Gallocatechin-(4β→8)-epigallocatechin 3-O-gallate | SA | [68] |
| 16 | Epigallocatechin-(4β→8)-gallocatechin | SA, SP | [54,68] |
| 17 | Epigallocatechin-(4β→8)-epigallocatechin | SA | [64,66,68] |
| 18 | Epigallocatechin-(4β→6)-epigallocatechin | SA | [68] |
| 19 | Epigallocatechin-(4β→8)-epigallocatechin3-O-gallate | SA | [68] |
| 20 | Epigallocatechin 3-O-gallate-(4β→8)-epigallocatechin 3-O-gallate | SA | [64,68] |
| 21 | 4′-O-methylepigallocatechin 3-O-gallate-epigallocatechin 3-O-gallate | SA | [64] |
| 22 | Epigallocatechin-epigallocatechin 3-O-gallate | SA | [64,66,68] |
| 23 | 4′-O-methylepigallocatechin-epigallocatechin | SA | [64] |
| 24 | 4′-O-methylepigallocatechin-4′-O-methylepigallocatechin | SA | [66] |
| 25 | Robinetinidol | SA | [66] |
| 26 | Robinetinidol-(4α→8)-epigallocatechin | SA | [66,69] |
| 27 | Robinetinidol-(4β→8)-epigallocatechin | SA | [69] |
| 28 | Robinetinidol-4′-O-methylepigallocatechin | SA | [66,69] |
| 29 | Robinetinidol-(4β→8)-epigallocatechin-3-O-gallate | SA | [69] |
| 30 | Robinetinidol-(4α→8)-epigallocatechin-3-O-gallate | SA | [69] |
| 31 | Robinetinidol-(4α→6)-gallocatechin | SA | [69] |
| 32 | Robinetinidol-(4α→6)-epigallocatechin | SA | [69] |
| 33 | Robinetinidol-[4β→6(8)]-gallocatechin | SA | [69] |
| 34 | Robinetinidol-(4α→8)-gallocatechin | SA | [69] |
| 35 | 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylgallocatechin | SA | [54] |
| 36 | 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylepigallocatechin | SA | [54] |
| 37 | 4′-O-methylgallocatechin-(4α→8)-4′-O- methylgallocatechin | SA | [54,70] |
| 38 | 4′-O-methylrobinetinidol-(4β→6)-4′-O-methylgallocatechin | SP | [54] |
| 39 | Fisetinidol-(4α→8)-gallocatechin | SP | [54] |
| 40 | Fisetinidol-(4β→8)-gallocatechin | SP | [54] |
| 41 | Polymer of 2114 Da of molecular weight with 6 monomers of flavan-3-ols and one galoil group consisting of prodelphinidin and prorobinetinidin units with configuration 2,3-cis and 2,3-trans | SA | [42] |
| 42 | Caffeic acid | SR | [60] |
| 43 | Rutin | SR | [60] |
SA: S. adstringens. SP: S. polyphyllum. SR: S. rotundifolium.
Figure 2.
General chemical structure of gallic acid and proanthocyanidins identified in the bark of Stryphnodendron spp. known as “barbatimão”. Numbers indicate the respective molecule in Table 2, with respective substituent (R). Dimers and the polymer identified are repetition of these monomers according to their names in the corresponding carbon positions.
Nevertheless, polar extract of the leaves of S. rotundifolium also showed a high polyphenol content but not as high as that found in the bark. Gallic acid, catechin, caffeic acid, and rutin are examples of polyphenols identified in the leaves [60]. Previously, prodelphinidins, gallic acid, and flavonols were also described in the leaves of S. adstringens and S. polyphyllum [62], as well as saponins and cumarins [71]. The tannins in the leaves were identified as gallic acid, epicatechin-(4β→8)-catechin, and epicatechin 3-O-gallate [59]. Moreover, galactomannans were extracted from the seeds of S. adstringens [72].
Lopes et al. [54] observed that S. adstringens and S. rotundifolium differ by the presence of epigallocatechin 3-O-(3,5-dimethyl)gallate and epigallocatechin-3-O-(3-methoxy-4-hydroxy)-benzoate and are chemically related as they both contain the 5-deoxyproanthocyanidins-B-ring trihydroxylated (prodelphinidins), whereas S. polyphyllum shows the presence of the dihydroxylated form (profisetenidins). However, S. adstringens is the most studied “barbatimão” species and, so, S. polyphyllum and S. rotundifolium tannin composition descriptions may be incomplete.
Therefore, the chemical composition of the Stryphnodendron spp. includes a wide variety of tannins particularly of the condensed class with diverse forms, and it was possible to identify the proanthocyanidins as monomers, dimers, and polymers (Table 2). Those molecules have interesting properties and interactions with protein among other organic compounds, which is the basis of the biological activities of the bark [73].
6.2. Extraction and Analysis of Tannin Metabolites of Stryphnodendron Species
Because of their polarity, the most commonly described solvent for extracting tannins from the bark of Stryphnodendron spp. is the polar system consisting of acetone:water at a 7:3 (v/v) ratio [42,54,62,66,67,68,69,70]. Subsequently, the extracts were dried, partitioned with water and ethyl acetate, and then the ethyl acetate fraction was subjected to column chromatography (CC) using Sephadex LH-20. The subfractions generated by CC were chromatographically analyzed using multi-layer coil counter current chromatography (MLCCC), high-pressure liquid chromatography (HPLC), or CC until purification and identification of the tannins.
Thus, gallic acid was obtained using HPLC. The condensed tannin 4′-O-methylgallocatechin was separated using HPLC from an MLCCC (ethyl acetate:n-propanol:water, 140:8:80, v/v/v) subfraction using an isocratic methanol:acetonitrile:water (15:5:80, v/v/v) system, and identified using electron ionization mass spectrometry (EI-MS) and the acetylated form was identified using proton nuclear magnetic resonance (1H-NMR) [68]. Gallocatechin and epigallocatechin were separated after a second MLCCC, by HPLC (same isocratic system). Epigallocatechin 3-O-gallate in the same subfraction from the second MLCCC was purified using CC [68]. Purification of epigallocatechin 3-O-(3,5-dimethyl)gallate and epigallocatechin 3-O-(3-methoxy-4-hydroxy)benzoate required subjecting the subfraction obtained using the multi-layer method to MLCCC, generating a subfraction that was submitted to CC, followed by HPLC (using the same isocratic methanol:acetonitrile:water system), from which the compounds in the subfraction were acetylated, semi-purified using thin layer chromatography (TLC). Furthermore, a new HPLC procedure with a normal phase column (hexane:ethyl acetate, 55:45, v/v) provided the best separation of the compounds [68]. Epigallocatechin-(4β→8)-gallocatechin and epigallocatechin-(4β→8)-epigallocatechin were purified from the subfractions where they were concentrated using MLCCC and isocratic HPLC. However, epigallocatechin-(4β→8)-epigallocatechin-3-O-gallate was separated using MLCCC and HPLC with an isocratic system of methanol:water (3:1, v/v) [68]. In comparison, purification of epigallocatechin-(4β→8)-epigallocatechin 3-O-(4-hydroxy)benzoate was slightly different, using HPLC with an isocratic system of methanol:water at 21:79 (v/v) ratio [68]. In contrast, subfractions containing epigallocatechin 3-O-gallate-(4β→8)-epigallocatechin 3-O-gallate and epigallocatechin-(4β→6)-epigallocatechin were subjected to MLCCC and HPLC using a gradient system of methanol:water at 25–30%. Gallocatechin-(4β→8)-epigallocatechin 3-O-gallate and gallocatechin-(4α→8)-epigallo-catechin 3-O-(4-hydroxy)benzoate were purified from the subfrations following the same strategy, except that the gradient used for the HPLC were 24–27% and 28–30%, respectively [68]. Compounds were identified using EI-MS and 1H-NMR [68].
Prorobinetinidins were further purified from the ethyl acetate fraction by first subjecting it to CC using Sephadex LH-20, and then main subfractions were subjected to MLCCC (ethyl acetate:n-propanol:water, 35:2:2, v/v) and HPLC (reverse-phase C18). Therefore, robinetinidol-(4β→8)-epigallocatechin and robinetinidol-(4α→8)-gallocatechin were purified using HPLC and a methanol:acetonitrile:water (3:1:16, v/v/v) system, while robinetinidol-(4α→8)-epigallocatechin was purified using a methanol:water (21:79, v/v) system. The other prorobinetinidins were purified using a gradient system of methanol:water at 28–30% (robinetinidol-[4β→6(8)]-gallocatechin and robinetinidol-(4β→8)-epigallocatechin-3-O-gallate) and 25–30% (robinetinidol-(4α→6)-gallocatechin, robinetinidol-(4α→6)-epigallocatechin, and robinetinidol-(4α→8)-epigallocatechin-3-O-gallate) [69]. Subjecting different subfractions to MLCCC allowed the purification of 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylepigallocatehin, 4′-O-methylgallocatechin, 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylgallocatehin, gallocatechin, and epigallocatechin [54].Purification of the dimer 4′-O-methylgallocatechin-(4α→8)-4′-O-methylgallocatechin followed the same purification path: after extraction with acetone:water, the subfraction was partition using CC with ethyl acetate and water, and one of the subfractions from the MLCCC process produced the molecule that was identified by chemical shifts using 1H-NMR and 13C-NMR [54,70].
Metabolites from S. polyphyllum were similarly separated and analyzed, and after the extraction, partitioning, and CC, gallic acid was identified and the subfractions were subjected to other chromatographic purification procedures. Subsequently, 4′-O-methylrobinetinidol-(4β→6)-4′-O-methylgallocatechin was obtained from a second CC process using Sephadex LH-20 and gallocatechin, epigallocatechin, and 4′-O-methylgallocatechin were obtained from different subfractions following the MLCCC. A reverse and a normal-phase HPLC were used to obtain epigallocatechin-(4β→8)-gallocatechin. After the MLCCC procedure, the subfractions were subjected to a normal-phase column HPLC process, which produced profisetenidins fisetinidol-(4β→8)-gallocatechin and fisetinidol-(4β→8)-gallocatechin [54].
The water fraction from the partitioning step was subjected to CC using a Sephadex LH-20 column, which generated a subfraction rich in a polymer, identified using electrospray ionization (ESI)-MS and 13C-NMR, as a 2114 Da polymer with six monomers of flavan-3-ols and one galoil group consisting of prodelphinidin and prorobinetinidin units [42].
After identifying the purified compounds from the barks of Stryphnodendron species, the presence of the compounds was evaluated in studies using straight strategies. HPLC with reverse-phase column was used to analyze the organic phase of the fraction obtain from the liquid-liquid partitioning of ethanolic extracts from the barks of S. adstringens and S. rotundifolium using ethyl acetate:butanol:i-propanol:water (3.5:0.5:1.0:4.5, v/v/v/v), which was characterized by the presence of gallic acid, gallocatechin, epigallocatechin, catechin, and epigallocatechin 3-O-gallate [56,65]. The ethanolic extract of S. adstringens bark, which was partitioned with ethanol:i-propanol:n-butanol (42:12:6, v/v/v) and analyzed using ultraperformance liquid chromatography (UPLC) coupled to ESI-MS, was chemically characterized by the presence of gallic acid, gallocatechin, epigallocatechin, methylepigallocatechin 3-O-gallate, and the dimers of epigallocatechin, epigallocatechin 3-O-gallate, methylepigallocatechin3-O-gallate and epigallocatechin 3-O-gallate, epigallocatechin and epigallocatechin 3-O-gallate, 4′-O-methylgallocatechin and, methylepigallocatechin and epigallocatechin [64]. Analysis of the extract of S. adstringens obtained with acetone:water showed the characteristic chemical composition when subjected to MS and tandem MS (MS/MS): gallic acid, robinetinidol, epigallocatechin, 4′-O-methylepigallocatechin, epigallocatechin 3-O-gallate, epigallo-catechin-O-methylgallate, robinetinidol-epigallocatechin, robinetinidol-4′-O-methylepigallocatechin, epigallocatechin-epigallocatechin, 4′-O-methylepigallocatechin-4′-O-methylepigallocatechin, and epigallocatechin-epigallocatechin 3-O-gallate.
As summarized here, the purification of condensed tannins from Stryphnodendron was a particularly laborious task, involving several fractionations using suitable chromatographic methods. However, the chemical fingerprint of the bark can currently be obtained in a straightforward manner. To confirm the identity of “barbatimão” species, the Brazilian Pharmacopeia refers to the extract preparation with the acetone: water mixture and the characteristic presence of gallic acid, epigallocatechin, and 4′-O-methylgallocatechin [17].
7. Correlated Biological Activities
According to previous reports, the efficacy of the main ethnopharmacological uses of the bark of “barbatimão” were confirmed for the treatment of wounds, gastric wounds, and infectious and inflammatory disorders. The activity of the bark extract on other disorders such as cancer, pain, diabetes, blood pressure, and as a diuretic were evaluated but the results obtained were insufficient to scientifically prove its usefulness for these indications. Other activities not correlated to the ethnopharmacological applications such as anti-protozoal and antiviral (not against influenza), were also analyzed with promising preliminary results. For those activities, improved, established methods identified the compounds present in the plant sample tested, which could be associated with the confirmed activity (Table 3), paying more attention to the epigallocatechin 3-O-gallate and proanthocyanidin polymer of 2114 Da. A general description of the biological activities tested for in “barbatimão” from the Stryphnodendron genus is presented as follows.
Table 3.
Ethnopharmacological uses scientifically studied and correlated compounds (identified by their numbers in Table 2).
| Ethnopharmacological Use | Scientifically Observed? | Related Compound |
|---|---|---|
| Wound healing | Yes | 1, 5–7, 9, 17, 22, 24–28 |
| Gastric ulcer | Yes, but toxicity was observed | 1, 5–7, 9, 17, 22, 24–28 |
| Anti-inflammatory | Yes | 1, 4–6, 9, 11, 17, 20–23 |
| Against pain | Yes—peripheral antinociception | 6 and 41 |
| Cancer | Antioxidant activity has been extensively evaluated, but anticancer activity is not conclusive | 1, 2, 6, 42 and 43 |
| Antimicrobial-oral and genitourinary infections | Yes—against gram positive bacteria and Candida species | 1 and 41 |
7.1. “Barbatimão” Bark Promotes Wound Healing
The most common ethnopharmacological use stated for “barbatimão” stem bark is wound healing. Studies on animals and humans using only the extract or a pharmaceutical formulation in which it was incorporated, confirmed this biological activity. One of the earliest studies reported the wound healing activity of S. adstringens bark decoction at 1% on incisions in mice [74], which was also reported in similar studies performed subsequently [75,76,77]. Then, ointments containing the “barbatimão” bark extract were prepared and the wound healing activity was analyzed in rats and in humans. An ointment containing 10% of the aqueous bark extract was used to treat cutaneous wounds on rats and showed complete epithelization after 14 days with better inflammation and neovascularization process recovery than that of the control group treated with a physiological solution [78]. Subsequently, a 3% aqueous extract showed potential angiogenic activity, corroborating the previously observed neovascularization, which is an important step in the wound healing process [79,80]. Another ointment with 3% of the extract also healed patients with cutaneous wounds of decubitus position [77,81]. Based on those results, a topical preparation was developed with a composition of 1–6% total phenols from a dry extract of S. adstringens or S. polyphyllum and a patent was administered to the preparation [82]. An ointment containing 1% of a lyophilized ethyl acetate fraction (obtained from an acetone:water extract) of the bark of S. adstringens was similarly tested against wounds in rats, and its topical application stimulated epithelization [83].
In addition to reepithelization, the production of collagen fibers was also observed in wounds of diabetic rats treated with a gel formulation with a 1% acetone:water extract [66], and similar results were obtained with a 10% glycolic extract [84]. Further characterization of the acetone:water extract of the bark of S. adstringens indicated it contained almost 40% total polyphenol content and hydrolysable tannin gallic acid, prorobinetinidins robinetinidol, robinetinidol-epigallocatechin, robinetinidol-4′-O-methylepigallocatechin, prodelphinidin epigallocatechin, epigallocatechin 3-O-gallate, 4′-O-methyl-epigallocatechin, epigallocatechin-O-methylgallate, epigallocatechin-epigallo-catechin, 4′-O-methyl-epigallocatechin-4′-O-methyl-epigallocatechin, and epigallocatechin-epigallo-catechin 3-O-gallate [66]. It is interesting to note that the reepithelialization and angiogenesis effects have been described for epigallocatechin 3-O-gallate, which is one of the characteristic tannin metabolite in Strpynhodendron acetone:water bark extract [85,86].
Ointments containing 2.5% lyophilized acetonic extract or the ethyl acetate fraction from the barks of S. rotundifolium and S. polyphyllum were also tested on rat wounds and were shown to induce reepithelization. However, the epidermal growth was faster with the extract of S. polyphyllum and the ethyl acetate fraction of S. rotundifolium than with the other fractions. In that case, the first extract contained approximately 50% total phenolic content and 25% tannin content while the second fraction contained 89% and 36% phenolic and tannin contents, respectively [87]. The authors suggested that the differences were likely attributable to the presence of a variety of prodelphinidins, prorobinetinidins, and profisetenidins in S. polyphyllum although higher phenolic and tannin contents were observed in S. rotundifolium, whereas only prodelphinidins were observed in this last species. Prodelphinidins, prorobinetinidins, and a polymer of these two condensed tannins were also described in the bark of S. adstringens, indicating the diversity of its tannin composition that significantly corroborated the wound healing activity reviewed here. Therefore, the diverse condensed tannin contents could be more useful for wound healing than the content of a few structures, as was also observed for S. adstringens.
Treatment of gastric ulcer wounds with the bark of “barbatimão” has been widely reported as well and this application has been demonstrated using animal models (Table 3). Lesions induced by acute stress or acidified ethanol were significantly reduced after treatment with aqueous and butanolic fractions (obtained from an acetonic bark extract) of S. adstringens [88]. These observations indicate a concentration of specific polyphenols since the proanthocyanidins previously described were identified in the extract with wound healing activity. Another study showed the reduction of ulcer lesions induced by ethanol and hypothermic restraint-stress in rats pre-treated with an acetonic fraction (obtained from a methanolic extract) from S. adstringens [89]. The fraction also decreased the gastric secretory volume and elevated pH, showing an antisecretory effect [89]. However, both studies reported that the fraction did not have activity on indomethacin- or acetic acid-induced ulcers that could be caused by the indomethacin-induced inhibition of prostaglandin biosynthesis and not a potent antisecretory activity by the extract, as observed with cimetidine [88,89]. Tannins interact with proteins and, therefore, it has been proposed that the complex forms a protective layer for the recovery of the stomach endothelium and inhibition of the H+/K+ ATPase by hydrolysable tannin has also been observed, which reduces acid secretion and supports the results of S. adstringens on gastric ulcer treatment [90,91,92]. Although impressive results have been reported, the scientific studies of the internal use of these extracts against gastric ulcer were performed in rat models. Furthermore, one study reported a toxic effect that was comprehensively characterized [88], and should be considered.
7.2. Anti-inflammatory Activity of “barbatimão” and Correlation to Antinociception
Another claimed ethnopharmacological property of the barks of these species is anti-inflammation. The acetonic fraction of S. adstringens inhibited rat paw edema with reduction of the exudate volume and migration of leukocytes. In addition, inhibition of paw edema was observed in a model with induced arthritis and decreased vascular permeability mediated by intraperitoneal administration of acetic acid in mice [93]. Antiedema results were similarly observed with a 1% solution of S. adstringens [94]. The aqueous and organic (ethanol:isopropanol:butanol) fractions (obtained from an ethanolic bark extract) of S. adstringens reduced the accumulation of neutrophils in the joint cavity of rats with arthritis induced by lipopolysaccharide (LPS) but this effect was not mediated by a decrease in C-X-C motif chemokine ligand 1 (CXCL1), the major chemokine recruiter of neutrophils [64]. The same study showed that tumor necrosis factor (TNF)-α production decreased in human monocytes THP-1 cells stimulated with LPS, which supports the anti-inflammatory claim for S. adstringens [64]. The organic fraction reduced neutrophil accumulation considerably more than the dexamethasone control did. Phytochemical evaluation of the organic fraction identified the presence of gallic acid and 11 different monomers and dimers of prodelphinidins, including epigallocatechin 3-O-gallate [64]. The attenuation of inflammatory process by some of those tannins, especially to epigallocatechin 3-O-gallate, was previously described [95,96,97], indicating a correlation between the anti-inflammatory activity of the stem bark of S. adstringens and the prodelphinidin constituents.
The use of “barbatimão” against pain could be related to its anti-inflammatory property. One study evaluated the aqueous and ethyl acetate fractions of the acetonic extract of S. adstringens against three pain models but an antinociceptive effect was only observed in the acetic acid- and formalin-induced writhing models [98]. It is interesting to note that the extract and aqueous fraction reduced the number of writhing compared to the saline control in two models of pain induced by inflammatory process. Specifically, 1) acetic acid induces capillary permeability, liberating substances that cause pain in nerve ends [99] and 2) the late phase of the formalin test, which is mediated by an inflammatory process, is reported as a return of the nociception minutes after formalin injection, and is used to elucidate pain and analgesia mechanisms [100,101]. The aqueous fraction contained concentrated levels of the proanthocyanidin polymer of 2114 Da, whereas the dimers where found in the ethyl acetate fraction, indicating that the peripheral antinociceptive effect of S. adstringens is partly due to the polymer of the condensed tannin [98]. Interestingly, epigallocatechin 3-O-gallate was also associated with antinociception in bone cancer due to the attenuation of inflammation by the reduction of TNF-α expression [96].
7.3. Antioxidant Property Might Mediate Claimed Anticancer Activity
The reactive oxygen species (ROS) scavenging activity of the bark extract of S. adstringens prepared with 50% and 70% ethanol, acetone:water (7:3, v/v), and chloroform was comparatively evaluated based on the reduction of the reagent 1,1-diphenyl-2-picrylhydrazyl (DPPH) to DPPHH. Polar extracts showed scavenging capacity as high (95%) as that of the controls rutin (97%), gallic acid (97%), and vitamin C (98%) at the same concentration. However, the chloroformic extract, which showed irrelevant levels of total phenolic content, exhibited <75% scavenging capacity. TLC plates stained with DPPH presented a spot representing reduction at same retention time as that of the tannin spots [102]. The scavenging capacity of the acetonic extract, aqueous and ethyl acetate fractions, and CC subfractions of the stem bark of S. rotundifolium was similarly evaluated using TLC stained with DPPH and all samples reduced the free radicals [103]. Subsequently, the DPPH scavenging potential of hydroalcoholic bark extracts and aqueous extracts of the barks and leaves of S. rotundifolium was measured and both bark extracts showed better activity than that of vitamin C, whereas the leaf extract showed comparable scavenging capacity to that of the control at higher concentrations [60]. According to the authors, the bark extract contents of gallic acid, catechin, caffeic acid, and rutin were higher than those in the leaf extract, which explained the different antioxidant activity of the samples.
Comparison of the acetonic extract and ethyl acetate fraction of S. rotundifolium and S. polyphyllum revealed the greater scavenger capacity of the second species, similar to the results of the wound healing potential comparison [87]. Therefore, the proanthocyanidin constituent of the barks of Stryphnodendron spp. and the potent scavenging capacity of the extracts, justify their ethnopharmacological use as anticancer agent; however, scientific studies need to be performed to prove that application and establish the best extract, compound, and concentration for use.
Furthermore, an ethyl acetate fraction obtained from the acetone:water extract of the leaves of S. adstringens was evaluated for antioxidant and anticancer potential. It showed good antioxidant activity by reducing iron, inhibiting protein oxidation and scavenging DPPH at same 50% radical inhibition concentration as vitamin C. The fraction did not show cytotoxicity for non-cancerous rat primary bone marrow but was cytotoxic to MCF-7 and MDA-MB-435 human breast cancer cell lines, and altered their morphology in addition to inducing DNA cleavage, apoptosis, and autophagy [59]. A tannin, epigallocatechin 3-O-gallate, present in the leaf fraction has already been designated as an anticarcinogenic compound [104]. The oxidation of epigallocatechin 3-O-gallate by O2‒ species was observed in the galloyl group, thereby modulating ROS production, which in combination with the inhibition of nuclear factor-κB, activation of mitogen-activated kinases, and inhibition of DNA methyltransferases, suggests prodelphinidin is a strong anticancer agent [104,105]. S. adstringens saline extract also reduced the technetium-99 m (used in nuclear medicine) labeling of red blood cells probably because of the redox or chelating properties of tannins [106].
7.4. Antimicrobial Activity Corroborates Oral and Genitourinary Use
It is notable that the ethnopharmacological use of Stryphnodendron spp. for infection treatment has been scientifically demonstrated mostly against gram-positive bacteria and pathogenic yeasts, as described in Table 4.
Table 4.
Reports of antimicrobial activity of different extracts or fractions of "barbatimão".
| Species | Extracts/Fraction | Microorganisms | Reference |
|---|---|---|---|
| SA | Hydroalcoholic and acetone:water extracts from the bark | Staphylococcus aureus | [41,102,107,108,109] |
| SA | Hydroalcoholic bark extract | Staphylococcus epidermidis; Enterococcus faecalis; Streptococcus salivarius; Streptococcus sanguinis; Streptococcus mitis; Streptococcus mutans; Streptococcus sobrinus; Lactobacillus casei | [41,102,110] |
| SA | Ethanolic and hexanic bark extracts | Candida albicans; Streptococcus mutans; Aggregatibacter actinomycetemcomitans | [111] |
| SA | Propylene glycol | S. aureus; S. epidermidis; S.mutans; C. albicans; C. tropicalis; C. glabrata | [112] |
| SA | Hydroalcoholic bark extract | Mycobacterium tuberculosis | [113] |
| SP | Ethyl acetate fraction | S. aureus; Bacillus subtilis | [87] |
| SR | Ethyl acetate fraction | S. aureus | [87] |
| SA | Polymer-rich subfraction | C. albicans; C. tropicalis, Cryptococcus neoformans | [42,114,115] |
| SA | Hexanic leaf extract | Trychophyton rubrum | [116] |
| SA | Methanolic extract and tannin fraction from the bark | Pythium insidiosum | [117] |
SA: S. adstringens. SP: S. polyphyllum. SR: S. rotundifolium.
Interestingly, the hydroalcoholic extract (containing tannin pyrogallates, flavones, flavonols, xanthones, chalcones, aurones, flavononols, and flavonones) showed synergistic activity with the antibiotics gentamicin, kanamycin, amikacin, and neomycin against Escherichia coli and S. aureus [118]. In contrast, a saline extract of the seeds of S. rotundifolium did not inhibit S. aureus, E. coli, and Pseudomonas aeruginosa [119]. Hence, the antibacterial activity of gallic acid and different proanthocyanidins [120,121] could justify the use of the stem bark of Stryphnodendron spp. in disorders as sore throat and dental and urinary infections.
On the other hand, the antifungal activity of the “barbatimão” species is more significant and different studies have attempt to elucidate the mechanism involved in the Candida spp virulence, biofilm inhibition, as well as the main active compound [42,114,122]. The propylene glycol extract of S. adstringens inhibited the formation of C. albicans biofilm on acrylic resins [123]. To elucidate the main active compound, Ishida et al. [42] performed bioguided extract fractionation according to the growth inhibition of C. albicans, which yielded a group of subfractions from the aqueous partition and the ethyl acetate fraction contained the polymer of 2114 Da with six units of flavan-3-ol and one galloyl group. This subfraction had similar inhibitory activity to that of nystatin, but showed fungistatic effect. Moreover, it decreased the Candida spp. virulence effect of adherence and filamentous formation in C. albicans, and enhanced the yeast phagocytosis by macrophage. A similar activity was reported for the polymer-rich subfraction against C. tropicalis, and biofilm reduction was also observed following pretreatment of planktonic cells with the subfraction or treatment during the adherence and matrix formation [114]. Further, the subfraction decreased the metabolic activity of sessile and dispersed cells during biofilm formation and maturation, indicating that the subfraction penetrated the biofilm matrix [124]. These findings are very important to developing strategies for avoiding the contamination of implanted medical devices and dissemination of the infection by dispersion cells [125].
Genitourinary disorders, especially in women, are among the most ethnopharmacological uses of “barbatimão”, since the widespread and recurrent vulvoginal candidiasis infection [126] can be treated with the stem bark of Stryphnodendron spp. Furthermore, the proven activity of the stem bark against Candida could contribute to the standardization of the extract and pharmaceutical formulations to effectively combat the pathogen.
In addition, the polymer-rich fraction inhibited the growth of Cryptococcus neoformans despite its fungistatic effect [115] and the hexanic leaf extract of S. adstringens inhibited the growth of different clinical isolates of the dermatophyte Trychophyton rubrum [116]. These findings could explain the folkloric use of this fraction for itching, chilblain, and dermatitis and is extremely important since both fungi present concerns of resistance to antifungals [127,128].
The antimicrobial activities of “barbatimão” could be related to the activity of the tannin content. Scalbert [129] proposed three different tannin antimicrobial mechanisms of action. These are inhibition of enzymes of the microorganism (correlating with protein interaction); microorganism deprivation of nutrients such as metals (explained by the complexation with tannin hydroxyl groups), and inhibition of oxidative phosphorylation (due to the antioxidant property). In addition, it is noteworthy that the compound epigallocatechin 3-O-gallate also exhibits antimicrobial activity. It has shown antibacterial (mainly against gram-positive bacteria), antibiofilm, and anticandidal activities, which enhances the understanding of the Stryphnodendron species antimicrobial activity [130].
7.5. Effect on Diabetes, Blood Pressure, and Diuresis
This review found only one study on the use of Stryphnodendron spp. in diabetes. Low concentrations (1.86 and 0.61 µg/mL) of the ethanolic extract of the bark of S. adstringens inhibited the enzymes α-amylase and α-glucosidase, which indicates its potential blood glucose-lowering effects [131]. Moreover, the enzymatic inhibition likely corresponds to the general interaction of tannins with proteins, but a more specific interaction with the active site could also be possible [73,131]. Further studies on diabetes would be important since epigallocatechin 3-O-gallate also improved the insulin sensitivity of rats and myocytes [132]. Another study [107] reported the reduction of blood pressure in dogs with normal arterial pressure following treatment with 8.2–12.3 mg/kg of acetone:water extract of the bark of S. adstringens and the fractions obtained with ethyl acetate, butanol, and water.
Analysis of the bark of S. adstringens and S. rotundifolium as dry powder on water renal excretion in mice revealed an antidiuretic effect, whereas the dry powder of the seeds of S. rotundifolium induced the claimed diuretic effect with no electrolyte excretion changes [133,134].
However, the data of the activities described in this section are currently inconclusive and further detailed analysis must be performed to ensure the proper use of extracts in the phytotherapy of diabetes, hypertension, and for diuresis. The evaluation of the toxicity of these extracts is an important topic that needs to be summarized, particularly with the allegedabortive effects of the seeds, which are described later.
7.6. Anthelmintic Activity and Other Promising Activities
The claimed folkloric anthelmintic activity of the bark was tested against Schistosoma mansoni using the acetone:water extracts of S. adstringens and S. polyphyllum, which both showed a faster larvicidal activity against miracidia and cercariae forms than the control treatment did [135]. This finding supports the validity of this reported application of the extracts. In addition, extracts of the barks and leaves of S. adstringens and S. polyphyllum showed molluscicidal activity against Biomphalaria glabrata, the snail intermediate host of S. mansoni [136,137,138].
Studies of the ctivity of Stryphnodendron spp. against protozoans commenced when the acetonic extract of the bark of S. adstringens was shown to inhibit the cell growth of Herpetomonas samuelpessoai. It is a non-pathogenic trypanosomatid used as an experimental model because it shares antigens with Trypanosoma cruzi, causing immune response and is susceptible to the same drugs used to treat T. cruzi [139]. Subsequently, inhibitory concentrations of the acetonic extract as well as the aqueous and ethyl acetate fractions were determined and ultrastructural alterations in cells were observed using transmission electron microscopy, in addition to decreased activity of the mitochondrial enzyme succinate cytochrome c reductase [140]. The extract of S adstringens (100 µg/mL) showed weak growth inhibition of the pathogenic protozoan Leishmania amazonensis in the promastigote and amastigote form and moderate inhibition of T. cruzi in the epimastigote form [141]. In an in vivo study, the ethanolic extract decreased the number of f T. cruzi parasites in the blood of inoculated mice [142]. The ethanolic extracts of the barks of S. adstringens and S. polyphyllum also exhibited trypanocidal activity, reducing the number of parasites in infected mice faster than the control group mice [143].
Then, an ethanolic extract of the bark of S. rotundifolium and its aqueous and organic fractions were tested against promastigote forms of L. amazonensis, which they highly inhibited and this effect was potent with the tannins isolated from the organic phase. Gallic acid exhibited the best inhibition (50% of promastigote inhibition at 1.7 µg/mL), followed by the epigallocatechin 3-O-gallate [65]. The hydroalcoholic extract of S. rotundifolium was also tested against L. brasiliensis and L. infantum promastigotes and T. cruzi epimastigotes and the mortality rates at 1000 µg/mL were of 56, 45, and 82%, respectively [144]. This observation indicates that the best activity of S. rotundifolium was achieved with the organic fraction and gallic acid was the most active compound against L. amazonensis. These results show the promising antiprotozoal activity of Stryphnodendron species, especially their tannin content, and further analysis should be carried out to determine the inhibitory agents, appropriate concentrations, and in vivo activity.
Ethnopharmacology data indicates the use of the stem bark of S. rotundifolium against influenza and the antiviral activity of S. adstringens bark was assayed using the aqueous and ethyl acetate fractions against poliovirus 1 (P-1) and bovine herpesvirus 1 (BHV-1) in HEp-2 cells. Both extracts inhibited the viral replication and the prodelphinidins catechin, epicatechin, gallocatechin, and epigallocatechin were identified in the ethyl acetate fraction [67].
Taking together, these results indicate the antiviral activity of the extract and its effective wound healing activity and, therefore, it was formulated and patented as an ointment with different “barbatimão” species for used against the human papillomavirus (HPV), particularly in the prevention of cervical cancer [145].
Another ointment was formulated using the aqueous extract of S. adstringens, and was found to reduce the hemorrhagic and myotoxic effects in mice caused by the venom of Bothrops pauloensis, and these effects were claimed to be mediated by an interaction between the tannins and the toxic proteins of the venom [146].
The bark and leaf extracts of S. rotundifolium and S. adstringens had little or no inhibitory effects on Rhipicephalus microplus (tick) [147], Rhopalosiphummaidis (corn aphid) [148] and Diabrotica speciose (the larva of this insect perforates potatoes) [149].
Therefore, the anthelmintic folkloric uses of this species has also been confirmed, but requires further detailed investigation. Among the activities not mentioned in ethnopharmacological surveys, the molluscicidal, antiviral, and antiprotozoal activity should receive more attention to confirm the promising results reported here to facilitate the standardization process for the use of the bark for these applications.
8. Cytotoxicity and Toxicology
As seen, stem bark of “barbatimão” from Stryphnodendron spp. has diverse uses in folk medicine and some of them were confirmed by scientific studies. Therefore, it is very important to ensure the safety of the active extracts and products obtained of the species in this genus.
In that sense, cytotoxic effects against mammalian cell lines is a recurrent property analyzed. The acetonic extract of the barks of S. adstringens did not cause hemolysis of sheep erythrocytes [141] neither did the aqueous and ethyl acetate fractions and subfractions. The same samples were not cytotoxic at 50% (50% cytotoxic concentration, CC50) of Vero and macrophages J774G8 cells up to 100 µg/mL and showed hemagglutinant effect above 500 µg/mL [42]. Likewise, the ethanolic extract, aqueous, and organic fractions of S. rotundifolium and the isolated compounds gallic acid, gallocatechin, epigallocatechin, catechin, and epigallocatechin 3-O-gallate showed CC50 against macrophages at 100-300 µg/mL and did not cause lysis of human red blood cells [65]; the hydroalcoholic extract of S. rotundifolium showed CC50 against fibroblast cells at 190.24 µg/mL [144]. According to all these reports, it is important to pay attention to the active concentration of samples to each biological activity and evaluate whether the cytotoxic concentration is smaller to indicate safe use. For example, ethyl acetate subfraction polymer-rich has anti-Candida activity bellow 10 µg/mL, whereas antibacterial and anti-protozoal activities are achieved close to 1000 µg/mL.
Cytotoxicity by genotoxic effects of ethanolic extracts of S. adstringens was evaluated but no DNA damage was observed, for instance, no mutagenicity activity was observed with the Ames test using Salmonella typhimurium strains [150], or by somatic mutation and recombination test or by chromosome damage in germ cells of Drosophila melanogaster, or even by mutation in larvae or in adult male of the same insect [151]. Otherwise, it was shown that aqueous extract and fraction and hydroalcoholic extract of the leaves of S. adstringens exhibited antigenotoxic effects by reducing DNA damage and micronuclei formation in bone marrow cells from rats treated with the genotoxic cyclophosphamide [152].
Acute toxicity and 50% lethal dose (LD50) are somewhat different among the extracts of S. adstringens. The LD50 of hydroalcoholic bark extract was considered high, at the concentration of 250 µg/mL, when administrated intraperitoneally in mice for 14 days [153]. An alcoholic extract of S. adstringens showed LD50 of 250 mg/kg also intraperitoneally in mice, did not show skin primary irritation or ocular alterations in rabbits [145]. No behavioral change was observed in rats receiving acetonic extract, and it did not show high LD50 (2699 mg/kg) though daily oral dose of 800 mg/kg for 30 days decreased the animals’ weight, promoted thymic involution and increased glucose and aspartate aminotransferase levels in their plasma [154]. Those effects could reflect alterations in cell metabolism and it was observed that the extract impaired mitochondrial oxidative phosphorylation and liver metabolism by increasing oxygen consumption [155]. In opposition, a methanolic extract did not show acute toxicity or histological alterations in liver and kidney of treated rats, neither showed hepatic or renal dysfunctions in rabbits [117].
However, when evaluating genotoxicity and acute toxicity of the polymer-rich subfraction obtained from the ethyl acetate fraction of the barks of S. adstringens, a safer sample was detected. In fact, it is possible to see a cytoprotective effect against mutagenicity, low cytotoxicity against Artemia salina [156], a LD50 higher than 3000 mg/kg in mice and chronic toxicity evaluation for 90 days with 200 mg/kg of the subfraction did not show biochemical, hematological, and histopathological effects [157]. Meanwhile, epigallocatechin 3-O-gallate caused hepatocyte disfunction and damage to mitochondria at high doses [158] and embryonic cytotoxicity [159].
Although broad beans of Stryphnodendron spp. are not used ethnopharmacologically, it is worth pointing out their abortive effect in animals. To confirm the information from farmers about cow abortion after consuming the beans from S. rotundifolium, a study administered 5 g/kg/day for 9 to 26 days. In fact, four out of seven cows had abortion and the behavior of others was abnormal, with decreased activity and appetite, salivation, difficulty in getting up, unstable gait, muscular tremors, and loss of weight [160]. Evaluation of toxic dose showed death of bovines with 60 g/kg of broad beans administered once and death from 10 g/kg on repeated days (for 8 days). Other diverse poisoning symptoms were also observed as well as diverse histopathological alterations [161,162]. Seed extracts of S. adstringens and S. polyphyllum showed abortive effect on female rats, corroborating to the precaution needed with the trees on farms with animals [11]. A congruent evaluation of cytotoxic and toxic effects and doses correlated with the different biological activities is still required for ensuring the safe use of the barks of “barbatimão” as well as the use of isolated compounds. No teratogenic or neurotoxic activity has been reported for the extracts in animals or humans.
9. Conclusions
Ethnoknowledge is still one of the most important tools for drug discovery, and phytotherapeutic treatments are easily accessible to the majority of the population. Ethnopharmacological uses of “barbatimão” from the genus Stryphnodendron genus were confirmed for different purposes, which would help guide the best approach for handling the stem bark extracts by the general population and would stimulate the development of pharmaceutical formulations. Topical use of the bark extract as a wound-healing agent, in order to take advantage of its antimicrobial action, is a well-established application of Stryphnodendron spp. Unfortunately, there is still insufficient scientific data about the correlation between dosage and pharmacological and toxicological parameters with regard to other activities, which can be explored as long as the mechanism of action of the bioactive compounds. A better understanding of those parameters could also motivate further studies on tannin from “barbatimão” aid the development of a drug that may be made available worldwide. It is also important to bear in mind the need to conserve these plants by adopting appropriate harvesting of the barks.
Acknowledgments
This study was supported by PADC-UNESP.
Author Contributions
All authors contributed substantially to this research. Tatiana M. Souza-Moreira and Geisiany M. Queiroz-Fernandes were responsible for the bibliographic search, analysis, and manuscript writing; Rosemeire C. L. R. Pietro was mainly responsible for conceiving the manuscript and critical revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Heinrich M., Heinrich M. Ethnopharmacology: Quo vadis? Challenges for the future. Rev. Bras. Farmacogn. 2014;24:99–102. doi: 10.1016/j.bjp.2013.11.019. [DOI] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 3.Medeiros P.M., Ferreira Júnior W.S., Ramos M.A., Silva T.C., Ladio A.H., Albuquerque U.P. Why do people use exotic plants in their local medical systems? A systematic review based on Brazilian local communities. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0185358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomazzoni M.I., Negrelle R.R.B., Centa M.L. Popular phytotherapy: The instrumental search as therapy. Texto Contexto Enferm. 2006;15:115–121. doi: 10.1590/S0104-07072006000100014. [DOI] [Google Scholar]
- 5.Santos R.L., Guimaraes G.P., Nobre M.S.C., Portela A.S. Analysis about phytotherapy as an integrating practice in the Brazilian Unified Health System (UHS) Rev. Bras. Plantas Med. 2011;13:486–491. doi: 10.1590/S1516-05722011000400014. [DOI] [Google Scholar]
- 6.Nogueira R.C., Cerqueira H.F., Soares M.B. Patenting bioactive molecules from biodiversity: The Brazilian experience. Expert Opin. Ther. Pat. 2010;20:145–157. doi: 10.1517/13543770903555221. [DOI] [PubMed] [Google Scholar]
- 7.Da Saúde M., De Ciência S., Estratégicos T.I., Estratégicos F.I. Programa Nacional de Plantas Medicinais e Fitoterápicos. Ministério da Saúde; Brasília, Brazil: 2009. p. 136. [Google Scholar]
- 8.Nascimento M.W.A., Veríssimo R.C.S.S., Bastos M.L.A., Bernardo T.H.L. Medicinal plants indications from herbal healers for wound treatment. Rev. Eletrôn. Enferm. 2016;18:1–10. [Google Scholar]
- 9.Passaretti T., Guarnieri A.P., Filipini R., Alves B.C.A., Fonseca F.L.A. Effective use of barbatiman (Stryphnodendron barbatiman) in the healing process of lesions: A literature review. ABCS Health Sci. 2016;41:51–54. [Google Scholar]
- 10.Riet-Correa F., Medeiros R.M.T., Schild A.L. A review of poisonous plants that cause reproductive failure and malformations in the ruminants of Brazil. J. Appl. Toxicol. 2011;32:245–254. doi: 10.1002/jat.1754. [DOI] [PubMed] [Google Scholar]
- 11.Bürger M.E., Ahlert N., Baldisserotto B., Langeloh A., Schirmer B., Foletto R. Analysis of the abortive and/or infertilizing activity of Stryphnodendron adstringens (Mart. Coville) Braz. J. Vet. Res. Anim. Sci. 1999;36 doi: 10.1590/S1413-95961999000600003. [DOI] [Google Scholar]
- 12.Souza V.C., Lima A.G. Flora Do Brasil—Stryphnodendron adstringens (Mart.) Coville. [(accessed on 5 November 2017)]; Available online: http://reflora.jbrj.gov.br/reflora/listaBrasil/FichaPublicaTaxonUC/FichaPublicaTaxonUC.do?id=FB19133.
- 13.Lorenzi H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas Do Brasil. 2nd ed. Instituto Plantarum; Nova Odessa, Brazil: 2000. p. 384. [Google Scholar]
- 14.Occhioni E.M.L. Considerações taxonômicas no gênero Stryphnodendron Mart. (Leguminosae-Mimosoideae) e distribuição geográfica das espécies. Acta Bot. Bras. 1990;4:153–158. [Google Scholar]
- 15.Simon M.F., Pastore J.F.B., Souza A.F., Borges L.M., Scalon V.R., Ribeiro P.G., Santos-Silva J., Souza V.C., Queiroz L.P. Molecular Phylogeny of Stryphnodendron (Mimosoideae, Leguminosae) and Generic Delimitations in the Piptadenia Group. Int. J. Plant Sci. 2015;177:44–59. doi: 10.1086/684077. [DOI] [Google Scholar]
- 16.Gertsch J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 2009;122:177–183. doi: 10.1016/j.jep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Agência Nacional de Vigilancia Sanitária . Farmacopeia Brasileira. 5th ed. Agência Nacional de Vigilancia Sanitária; Brasília, Brazil: 2010. [(accessed on 5 November 2017)]. Available online: http://www.anvisa.gov.br/hotsite/cd_farmacopeia/index.htm. [Google Scholar]
- 18.Agência Nacional de Vigilância Sanitária . Formulário de Fitoterápicos da Farmacopeia Brasileira. Agência Nacional de Vigilância Sanitária; Brasília, Brazil: 2011. [(accessed on 3 January 2018)]. p. 126. Available online: http://www.anvisa.gov.br/hotsite/farmacopeiabrasileira/conteudo/Formulario_de_Fitoterapicos_da_Farmacopeia_Brasileira.pdf. [Google Scholar]
- 19.Agência Nacional de Vigilância Sanitária . Memento Fitoterápico da Farmacopeia Brasileira. Agência Nacional de Vigilância Sanitária; Brasília, Brazil: 2016. [(accessed on 3 January 2018)]. p. 115. Available online: http://portal.anvisa.gov.br/documents/33832/2909630/Memento+Fitoterapico/a80ec477-bb36-4ae0-b1d2-e2461217e06b. [Google Scholar]
- 20.Scalon V.R. Ph.D. Thesis. Universidade de São Paulo; São Paulo, Brazil: 2007. Revisão Taxonômica de Stryphnodendron Mart. (Leguminosae-Mimosoideae) [Google Scholar]
- 21.Sanches A.C.C., Lopes G.C., Toledo C.E.M., Sacramento L.V.S., Sakuragui C.M., Mello J.C.P. Estudo morfológico comparativo das cascas e folhas de Stryphnodendron adstringens, S. polyphyllum e S. obovatum—Leguminosae. Lat. Am. J. Pharm. 2007;26:362–368. [Google Scholar]
- 22.Lima L.C.P., Garcia F.C.P., Sartori Â.L.B. As Leguminosae arbóreas das florestas estacionais do Parque Estadual do Itacolomi, Minas Gerais, Brasil. Rodriguésia. 2010;61:441–466. doi: 10.1590/2175-7860201061308. [DOI] [Google Scholar]
- 23.Ortiz P.L., Arista M., Oliveira P.E., Talavera S. Pattern of Flower and Fruit Production in Stryphnodendron adstringens, an Andromonoecious Legume Tree of Central Brazil. Plant Biol. 2003;5:592–599. doi: 10.1055/s-2003-44720. [DOI] [Google Scholar]
- 24.Bitu V.C.N., Matias E.F., Lima W.P., Costa Portelo A., Coutinho H.D., Menezes I.R. Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. J. Ethnopharmacol. 2015;172:265–272. doi: 10.1016/j.jep.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Meira M.R., Nobre D.A.C. Avaliação da qualidade de sementes de barbatimão oriundas de três locais no Norte de Minas Gerais. Rev. Ciênc. Agrár. 2014;37:50–58. [Google Scholar]
- 26.Martins C.C., Camara A.T.R., Machado C.G., Nakagawa J. Methods of breaking dormancy for seeds of Stryphnodendron. Acta Sci. Agron. 2008;30:381–385. [Google Scholar]
- 27.Martins C.C., Martins C.C., Machado C.G., Nakagawa J. Temperature and substrate for germination test of Stryphnodendron adstringens (Mart) Coville (Leguminosae) Rev. Árvore. 2008;32:633–639. doi: 10.1590/S0100-67622008000400004. [DOI] [Google Scholar]
- 28.Nicioli P.M., Paiva R., Nogueira R.C., Santana J.R.F., Silva L.C., Silva D.P.C., Porto J.M.P. Adjustment of the process of micropropagation of Stryphnodendron adstringens (Mart.) Coville. Cienc. Rural. 2008;38:685–689. doi: 10.1590/S0103-84782008000300014. [DOI] [Google Scholar]
- 29.Castro A.H.F., Paiva R., Alvarenga A.A., Castro E.M., Vitor S.M.M., Fernandes A.M. Cultivo in vitro e aspectos da anatomia foliar de barbatimão [Stryphnodendronadstringens (Mart.) Coville Fabaceae Papilionoideae] Plant Cell Cult. Micropropag. 2007;3:61–68. [Google Scholar]
- 30.Castro A.H.F., Paiva R., Alvarenga A.A., Vitor S.M.M. Callogenesis and contents of total phenols and tannins in barbatimão [Stryphnodendron adsrtingens (Mart.) Coville] Ciênc. Agrotec. 2009;33:385–390. doi: 10.1590/S1413-70542009000200004. [DOI] [Google Scholar]
- 31.Castro A.H.F., Lima M.M., Paiva R., Alvarenga A.A., Sóter M.O. Curva de crescimento, atividade da fenilalanina amônialiase e teores de fenóis e taninos totais em calos de Stryphnodendron adstringens (Mart.) Coville (Fabaceae-Mimosoideae) Plant Cell Cult. Micropropag. 2008;4:99–104. [Google Scholar]
- 32.Glasenapp J.S., Martins E.R., Casali V.W.D., Cruz C.D., Barbosa P.B. Characterization of diversity and genetic structure in natural populations of Stryphnodendron adstringens (Mart.) Coville by means of allozyme markers. Rev. Bras. Plantas Med. 2014;16:216–224. doi: 10.1590/S1516-05722014000200008. [DOI] [Google Scholar]
- 33.Mendonça P., Bertoni B.W., Amui S.F., Giuliatti S., Corrêa V.S.C., França S.C., Pereira A.N.S. Genetic diversity of Stryphnodendron adstringens (Mart.) Coville determined by AFLP molecular markers. Biochem. Syst. Ecol. 2017;41:16–20. doi: 10.1016/j.bse.2011.12.007. [DOI] [Google Scholar]
- 34.Meira M.R., Cabacinha C.D. Sustainable Management of Barbatimão in the Northern Minas Gerais State. Floresta Ambient. 2016;23:61–69. doi: 10.1590/2179-8087.041213. [DOI] [Google Scholar]
- 35.Borges Filho H.C., Felfili J.M. Evaluation of exploitation levels of barbatimão bark [Stryphnodendron adstringens (Mart.) Coville] in Distrito Federal, Brazil. Rev. Árvore. 2003;27:735–745. [Google Scholar]
- 36.Feitosa I.S., Albuquerque U.P., Monteiro J.M. Knowledge and extractivism of Stryphnodendron rotundifolium Mart. in a local community of the Brazilian Savanna, Northeastern Brazil. J. Ethnobiol. Ethnomed. 2014;10 doi: 10.1186/1746-4269-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feitosa I.S., Sobral A., Monteiro J.M., Araujo E.L., Albuquerque U.P. Impact of collection on bark regeneration from Stryphnodendron rotundifolium Mart. in northeastern Brazil. Environ. Monit. Assess. 2017;189 doi: 10.1007/s10661-017-5908-4. [DOI] [PubMed] [Google Scholar]
- 38.Santos P.V., Sebastiani R. Medicinal plants used for university students in São Paulo City, São Paulo State. J. Health Sci. Inst. 2011;29:11–15. [Google Scholar]
- 39.Santana B.F., Voeks R.A., Funch L.S. Ethnomedicinal survey of a maroon community in Brazil’s Atlantic tropical forest. J. Ethnopharmacol. 2016;181:37–49. doi: 10.1016/j.jep.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro D.A., Oliveira L.G., Macedo D.G., Menezes I.R., Costa J.G., Silva M.A., Lacerda S.R., Souza M.M. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014;155:1522–1533. doi: 10.1016/j.jep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 41.Souza T.M., Moreira R.R.D., Pietro R.C.L.R., Isaac V.L.B. Avaliação da atividade anti-séptica de extrato seco de Stryphnodendron adstringens (Mart.) Coville e de preparação cosmética contendo este extrato. Rev. Bras. Farmacogn. 2007;17:71–75. doi: 10.1590/S0102-695X2007000100015. [DOI] [Google Scholar]
- 42.Ishida K., Mello J.C., Cortez D.A., Dias Filho B.P., Ueda-Nakamura T., Nakamura C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006;58:942–949. doi: 10.1093/jac/dkl377. [DOI] [PubMed] [Google Scholar]
- 43.Nunes G.P., Silva M.F., Resende U.M., Siqueira J.M. Medicinal plants from herb sellers operating in downtown Campo Grande, Mato Grosso do Sul, Brazil. Rev. Bras. Farmacogn. 2003;13:83–92. doi: 10.1590/S0102-695X2003000200004. [DOI] [Google Scholar]
- 44.Macedo M., Ferreira A.R. Plantas medicinais usadas para tratamentos dermatológicos, em comunidades da Bacia do Alto Paraguai, Mato Grosso. Rev. Bras. Farmacogn. 2004;14:40–44. doi: 10.1590/S0102-695X2004000300016. [DOI] [Google Scholar]
- 45.Botelho N.M., Brito N.B., Silva N.M. The use of medicinal plants by canal da Visconde community. Rev. Para. Med. 2014;28:63–69. [Google Scholar]
- 46.Fenner R., Betti A.H., Mentz L.A., Rates S.M.K. Plants with potencial antifungal activity employed in Brazilian folk medicine. Rev. Bras. Cienc. Farm. 2006;42:369–394. doi: 10.1590/S1516-93322006000300007. [DOI] [Google Scholar]
- 47.Souza C.M.P., Brandão D.O., Silva M.S.P., Palmeira A.C., Simões M.O.S., Medeiros A.C.D. Use of medicinal plants with antimicrobial activity by users of the Public Health System in Campina Grande—Paraíba, Brazil. Rev. Bras. Plantas Med. 2013;15:188–193. doi: 10.1590/S1516-05722013000200004. [DOI] [Google Scholar]
- 48.Souza D.R., Rodrigues E.C.A.M.S. Medicinal plants: Traditional healers’ indications for the treatment of wounds. Rev. Bras. Promoç. Saúde. 2016;29:197–203. [Google Scholar]
- 49.Ustulin M., Figueiredo B.B., Tremea C., Pott A., Pott V.J., Bueno N.R., Castilho R.O. Commercialized medicinal plants in the Mercado Municipal of Campo Grande-MS. Rev. Bras. Farmacogn. 2009;19:805–813. doi: 10.1590/S0102-695X2009000500026. [DOI] [Google Scholar]
- 50.Oliveira D.R., Ferreira Júnior W.S., Bitu V.C.N., Pinheiro P.G., Menezes C.D., Almino Brito Junior F.E., Albuquerque U.P., Kerntopf M.R., Coutinho H.D.M., Fachinetto R., et al. Ethnopharmacological study of Stryphnodendron rotundifolium in two communities in the semi-arid region of northeastern Brazil. Rev. Bras. Farmacogn. 2014;24:124–132. doi: 10.1016/j.bjp.2014.03.003. [DOI] [Google Scholar]
- 51.Oliveira D.R., Brito Júnior F.E., Sampaio L.A., Torres J.C., Ramos A.G.B., Nunes A.A. Ethnopharmacological usage of medicinal plants in genitourinary infections by residents of Chapada do Araripe, Crato, Ceará—Brazil. Rev. Bras. Promoç. Saúde. 2012;25:278–286. [Google Scholar]
- 52.Melo N.D.P., Ribeiro S.C., Barros A.B. Etnoconhecimento de pequenos agricultores tradicionais sobre plantas medicinais no tratamento de dores provocadas pelo trabalho. Braz. J. Occup. Ther. 2016;24:563–574. [Google Scholar]
- 53.Figueredo C.A., Gurgel I.G.D., Gurgel Junior G.D. The National Policy on Medicinal Plants and Phytotherapy: Building, perspectives and challenges. Physis. 2014;24:381–400. doi: 10.1590/S0103-73312014000200004. [DOI] [Google Scholar]
- 54.Lopes G.C., Machado F.A.V., Toledo C.E.M., Sakuragui C.M., Mello J.C.P. Chemotaxonomic significance of 5-deoxyproanthocyanidins in Stryphnodendron species. Biochem. Syst. Ecol. 2009;36:925–931. doi: 10.1016/j.bse.2008.10.004. [DOI] [Google Scholar]
- 55.Lopes G.C., Sanches A.C.C., Toledo C.E.M., Isler A.C., Mello J.C.P. Determinação quantitativa de taninos em três espéciesde Stryphnodendron por cromatografia líquida de alta eficiência. Braz. J. Pharm. Sci. 2009;45:135–143. doi: 10.1590/S1984-82502009000100017. [DOI] [Google Scholar]
- 56.Nascimento A.M., Guedes P.T., Castilho R.O., Vianna-Soares C.D. Stryphnodendron adstringens (Mart.) Coville (Fabaceae) proanthocyanidins quantitation by RP-HPLC. Braz. J. Pharm. Sci. 2013;49:549–558. doi: 10.1590/S1984-82502013000300016. [DOI] [Google Scholar]
- 57.Santos S.C., Costa W.F., Batista F., Santos L.R., Ferri P.H., Ferreira H.D., Seraphin J.C. Seasonal variation in the content of tannins in barks of barbatimão species. Rev. Bras. Farmacogn. 2006;16:552–556. doi: 10.1590/S0102-695X2006000400019. [DOI] [Google Scholar]
- 58.Jacobson T.K.B., Garcia J., Santos S.C., Duarte J.B., Farias J.G., Kliemann H.J. Influência de fatores edáficos na produçãode fenóis totais e taninos de duas espécies debarbatimão (Stryphnodendron sp.) Pesqui. Agropecu. Trop. 2005;35:163–169. [Google Scholar]
- 59.Sabino A.P.L., Eustáquio L.M.S., Miranda A.C.F., Biojone C., Mariosa T.N., Gouvêa C.M.C.P. Stryphnodendron adstringens (“Barbatimão”) Leaf Fraction: Chemical Characterization, Antioxidant Activity, and Cytotoxicity Towards Human Breast Cancer Cell Lines. Appl. Biochem. Biotechnol. 2017 doi: 10.1007/s12010-017-2632-z. [DOI] [PubMed] [Google Scholar]
- 60.Costa J.G.M., Leite G.O., Dubois A.F., Seeger R.L., Boligon A.A., Athayde M.L., Campos A.R., Rocha J.B.T. Antioxidant Effect of Stryphnodendron rotundifolium Martius Extracts from Cariri-Ceará State (Brazil): Potential Involvement in Its Therapeutic Use. Molecules. 2012;17:934–950. doi: 10.3390/molecules17010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sousa J.N., Pedroso N.B., Borges L.L., Oliveira G.A., Paula J.R., Conceicao E.C. Optimization of Ultrasound-assisted extraction of polyphenols, tannins and epigallocatechin gallate from barks of Stryphnodendron adstringens (Mart.) Coville bark extracts. Pharmacogn. Mag. 2014;10:S318–S323. doi: 10.4103/0973-1296.133287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos S.C., Costa W.F., Ribeiro J.P., Guimaraes D.O., Ferri P.H., Ferreira H.D., Seraphin J.C. Tannin composition of barbatimao species. Fitoterapia. 2002;73:292–299. doi: 10.1016/S0367-326X(02)00081-3. [DOI] [PubMed] [Google Scholar]
- 63.Ardisson L., Godoy J.S., Ferreira L.A.M., Stehmann J.R., Brandão M.G.L. Preparation and characterization of propylene glicol extracts obtained from dried stem barks of Stryphnodendron adstringens (Mart.) Coville (barbatimão) Rev. Bras. Farmacogn. 2002;12:27–34. [Google Scholar]
- 64.Henriques B.O., Corrêa O., Azevedo E.P.C., Pádua R.M., Oliveira V.L.S., Oliveira T.H.C., Boff D., Dias A.C.F., Souza D.G., Amaral F.A., et al. In Vitro TNF-α Inhibitory Activity of Brazilian Plants and Anti-Inflammatory Effect of Stryphnodendron adstringens in an Acute Arthritis Model. Evid. Based Complement. Altern. Med. 2016 doi: 10.1155/2016/9872598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro T.G., Nascimento A.M., Henriques B.O., Chavez-Fumagalli M.A., Franca J.R., Duarte M.C., Lage P.S., Andrade P.H., Lage D.P., Rodrigues L.B., et al. Antileishmanial activity of standardized fractions of Stryphnodendron obovatum (Barbatimao) extract and constituent compounds. J. Ethnopharmacol. 2015;165:238–242. doi: 10.1016/j.jep.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 66.Pinto S.C., Bueno F.G., Panizzon G.P., Morais G., Santos P.V., Baesso M.L., Leite-Mello E.V., Mello J.C. Stryphnodendron adstringens: Clarifying Wound Healing in Streptozotocin-Induced Diabetic Rats. Planta Med. 2015;81:1090–1096. doi: 10.1055/s-0035-1546209. [DOI] [PubMed] [Google Scholar]
- 67.Felipe A.M., Rincao V.P., Benati F.J., Linhares R.E., Galina K.J., Toledo C.E., Lopes G.C., Mello J.C., Nozawa C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 2006;29:1092–1095. doi: 10.1248/bpb.29.1092. [DOI] [PubMed] [Google Scholar]
- 68.Mello J.C.P., Petereit F., Nahrstedt A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry. 1996;41:807–813. doi: 10.1016/0031-9422(95)00686-9. [DOI] [Google Scholar]
- 69.Mello J.C.P., Petereit F., Nahrstedt A. Prorobinetinidins from Stryphnodendron adstringens. Phytochemistry. 1996;42:857–862. doi: 10.1016/0031-9422(95)00953-1. [DOI] [Google Scholar]
- 70.Mello J.C.P., Petereit F., Nahrstedt A. A dimeric proanthocyanidin from Stryphnodendron adstringens. Phytochemistry. 1999;51:1105–1107. [Google Scholar]
- 71.Oliveira A.L.S., Figueiredo A.D.L. Prospecção Fitoquímica das Folhas de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae) Rev. Bras. Biocienc. 2007;5:384–386. [Google Scholar]
- 72.Salvalaggio Mde O., Freitas R.A., Franquetto E.M., Koop H.S., Silveira J.L. Influence of the extraction time on macromolecular parameters of galactomannans. Carbohydr. Polym. 2015;116:200–206. doi: 10.1016/j.carbpol.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 73.Adamczyk B., Simon J., Kitunen V., Adamczyk S., Smolander A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. Chem. Open. 2017;6:610–614. doi: 10.1002/open.201700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panizza S., Rocha A.B., Gecchi R., Souza e Silva R.A.P. Stryphnodendron barbadetiman (Vellozo) Martius: Teor em Tannino na casca e sua propriedade cicatrizante. Rev. Ciênc. Farm. 1988;10:101–106. [Google Scholar]
- 75.Silva C., Vilella H. Ação do Stryphnodendron barbadetiman sobre a cicatrização: Estudo experimental em ratos. HB Cient. 1996;3:77–79. [Google Scholar]
- 76.Jorge Neto J., Fracasso J.F., Camargo Neves C.L., Santos L.E., Banuth V.L. Tratamento de ulcera varicosa e lesoes de pele com Calendula officinalis L. e/ou com Stryphnodendron barbadetiman (Vellozo) Martius. Rev. Cienc. Farm. 1996;17:181–186. [Google Scholar]
- 77.Eurides D., Mazzanti A., Belleti M.E., Silva L.A.F., Fioravante M.C.S., Troncoso Neto N.S., Campos V.A., Lemos R.C., Silvestrini Junior P.L. Morfologia e Morfometria da Reparação Tecidual de Feridas Cutâneas de Camundongos Tratadas com Solução Aquosa de Barbatimão (Stryphynodendron barbatiman Martius) Revista da Faculdade de Zootecnia Veterinária e Agronomia. 1995/1996;2/3:30–40. [Google Scholar]
- 78.Coelho J.M., Antoniolli A.B., Nunes e Silva D., Carvalho T.M., Pontes E.R., Odashiro A.N. Effects of silver sulfadiazine, ipe roxo (Tabebuia avellanedae) extract and barbatimao (Stryphnodendron adstringens) extract on cutaneous wound healing in rats. Rev. Col. Bras. Cir. 2010;37:45–51. doi: 10.1590/S0100-69912010000100010. [DOI] [PubMed] [Google Scholar]
- 79.Chaves D.A., Lemes S.R., Araujo L.A., Sousa M.A.M., Freitas G.B., Lino-Junior R.S., Mrue F., Melo-Reis P.R. Angiogenic activity of the aqueous solution of Barbatimão (Stryphnodendron adstringens) Rev. Bras. Plantas Med. 2016;18:524–530. doi: 10.1590/1983-084X/15_093. [DOI] [Google Scholar]
- 80.Bauer S.M., Bauer R.J., Velazquez O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc. Endovasc. Surg. 2005;39:293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 81.Minatel D.G., Pereira A.M.S., Chiaratti T.M., Pasqualin L., Oliveira J.C.N., Couto L.B., Lia R.C.C., Cintra J.M., Bezzon M.F.A., Franca S.C. Estudo clínico para validação da eficácia de pomada contendo barbatimão (Stryphnodendron adstringens (Mart.) Coville) na cicatrização de ulceras de decúbito. Rev. Bras. Med. 2010;67:250–256. [Google Scholar]
- 82.Franca S.D.C., Oliveira J.C.N., Pasqualin L., Couto L.B., Lia R.C.C. Composition for Topic Use Containing an Extract of Stryphnodendron, Its Preparation as Well as Its Application. CN1878560 B. Eur. Pat. 2010 Dec 29;
- 83.Hernandes L., Pereira L.M.S., Palazzo F., Mello J.C.P. Wound-healing evaluation of ointment from Stryphnodendron adstringens (barbatimão) in rat skin. Braz. J. Pharm. Sci. 2010;46:431–436. doi: 10.1590/S1984-82502010000300005. [DOI] [Google Scholar]
- 84.Rodrigues D.F., Mendes F.F., Menezes L.B., Carvalho W.L., Sá S., Silva J.A., Souza L.A., Silva L.A.F. Treatment of excisional wound in rabbits with barbatiman extracts associated with autologous bone marrow mononuclear cells. Arq. Bras. Med. Vet. Zootec. 2017;69:1243–1250. doi: 10.1590/1678-4162-9301. [DOI] [Google Scholar]
- 85.Kim H.L., Lee J.H., Kwon B.J., Lee M.H., Han D.W., Hyon S.H., Park J.C. Promotion of full-thickness wound healing using epigallocatechin-3-O-gallate/poly (lactic-co-glycolic acid) membrane as temporary wound dressing. Artif. Organs. 2014;38:411–417. doi: 10.1111/aor.12190. [DOI] [PubMed] [Google Scholar]
- 86.Kim H., Kawazoe T., Han D.W., Matsumara K., Suzuki S., Tsutsumi S., Hyon S.H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008;16:714–720. doi: 10.1111/j.1524-475X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 87.Lopes G.C., Sanches A.C.C., Nakamura C.V., Dias Filho B.P., Hernandes L., Mello J.C.P. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J. Ethnopharmacol. 2005;99:265–272. doi: 10.1016/j.jep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 88.Audi E.A., Toledo D.P., Peres P.G., Kimura E., Pereira W.K., Mello J.C.P., Nakamura C., Alves-do-Prado W., Cuman R.K., Bersani-Amado C.A. Gastric antiulcerogenic effects of Stryphnodendron adstringens in rats. Phytother. Res. 1999;13:264–266. doi: 10.1002/(SICI)1099-1573(199905)13:3<264::AID-PTR443>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 89.Martins D.T., Lima J.C., Rao V.S. The acetone soluble fraction from bark extract of Stryphnodendron adstringens (Mart.) Coville inhibits gastric acid secretion andexperimental gastric ulceration in rats. Phytother. Res. 2002;16:427–431. doi: 10.1002/ptr.928. [DOI] [PubMed] [Google Scholar]
- 90.Murakami S., Muramatsu M., Otomo S. Inhibitory effect of tannic acid on gastric H+, K(+)-ATPase. J. Nat. Prod. 1992;55:513–516. doi: 10.1021/np50082a022. [DOI] [PubMed] [Google Scholar]
- 91.Vasconcelos P.C., Kushima H., Andreo M., Hiruma-Lima C.A., Vilegas W., Takahira R.K., Pellizzon C.H. Studies of gastric mucosa regeneration and safety promoted by Mouriri pusa treatment in acetic acid ulcer model. J. Ethnopharmacol. 2008;115:293–301. doi: 10.1016/j.jep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Jesus N.Z., Souza Falcao H., Gomes I.F., Almeida Leite T.J., Morais Lima G.R., Barbosa-Filho J.M., Tavares J.F., da Silva M.S., Athayde-Filho P.F., Batista L.M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012;13:3203–3228. doi: 10.3390/ijms13033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lima J.C.S., Martins D.T.O., Souza P.T. Experimental evaluation of stem bark of Stryphnodendron adstringens (Mart.) Coville for antiinflammatory activity. Phytother Res. 1998;12:218–220. doi: 10.1002/(SICI)1099-1573(199805)12:3<218::AID-PTR220>3.0.CO;2-4. [DOI] [Google Scholar]
- 94.Coutinho H., Pinto D.S., Ribeiro J.E.G., Friedman H. Anti-edema action of the Stryphnodendron barbadetiman (Barbatimão) 1 per cent in comparison with clorhexidin 0, 12 per cent. Rev. Odonto Ciênc. 2004;19:201–206. [Google Scholar]
- 95.Wang T., Zhou H., Xie H., Mu Y., Xu Y., Liu J., Zhang X. Epigallocatechin-3-gallate inhibits TF and TNF-α expression induced by the anti-β2GPI/β2GPI complex in human THP-1 cells. Int. J. Mol. Med. 2014;33:994–1002. doi: 10.3892/ijmm.2014.1635. [DOI] [PubMed] [Google Scholar]
- 96.Crouvezier S., Powell B., Keir D., Yaqoob P. The effects of phenolic components of tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine. 2001;13:280–286. doi: 10.1006/cyto.2000.0837. [DOI] [PubMed] [Google Scholar]
- 97.Uchiyama Y., Suzuki T., Mochizuki K., Goda T. Dietary Supplementation with a Low Dose of (−)-Epigallocatechin-3-Gallate Reduces Pro-Inflammatory Responses in Peripheral Leukocytes of Non-Obese Type 2 Diabetic GK Rats. J. Nutr. Sci. Vitaminol. 2013;59:541–547. doi: 10.3177/jnsv.59.541. [DOI] [PubMed] [Google Scholar]
- 98.Melo J.O., Endo T.H., Bersani-Amado L.E., Svidzinski A.E., Baroni S., Mello J.C.P., Bersani-Amado C.A. Effect of Stryphnodendron adstringens (barbatimão) bark on animal models of nociception. Rev. Bras. Cienc. Farm. 2007;43:465–469. doi: 10.1590/S1516-93322007000300015. [DOI] [Google Scholar]
- 99.Amico-Roxas M., Caruso A., Trombadore S., Scifo R., Scapagnini U. Gangliosides antinociceptive effects in rodents. Arch. Int. Pharmacodyn. Ther. 1984;272:103–117. [PubMed] [Google Scholar]
- 100.Tjolsen A., Berge O.G., Hunskaar S., Rosland J.H., Hole K. The formalin test: An evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 101.Coderre T.J., Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Souza T.M., Severi J.A., Silva V.Y.A., Santos E., Pietro R.C.L.R. Bioprospecção de atividade antioxidante e antimicrobiana da casca de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae) Rev. Ciênc. Farm. Básica Apl. 2009;28:221–226. [Google Scholar]
- 103.Sanches A.C.C., Lopes G.C., Nakamura C.V., Dias Filho B.P., Mello J.C.P. Antioxidant and antifungal activities of extracts and condensed tannins from Stryphnodendron obovatum Benth. Rev. Bras. Cienc. Farm. 2005;41:101–107. doi: 10.1590/S1516-93322005000100012. [DOI] [Google Scholar]
- 104.Min K.J., Kwon T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014;3:16–24. doi: 10.1016/j.imr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Losada-Echeberria M., Herranz-Lopez M., Micol V., Barrajon-Catalan E. Polyphenols as Promising Drugs against Main Breast Cancer Signatures. Antioxidants (Basel) 2017;6 doi: 10.3390/antiox6040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costa T.E.M.M., Dias A.P.M., Capriles P.V.S.Z., Oliveira M.B.N., Amorim E.L.C., Lima C.S.A., Bernardo-Filho M. Effect of barbatimão [Stryphnodendron adstringens (Mart.) Coville] infusion on the labling of blood elements with technetium-99m. Rev. Bras. Farmacogn. 2002;12:7–9. doi: 10.1590/S0102-695X2002000300004. [DOI] [Google Scholar]
- 107.Audi E.A., Toledo C.E.M., Santos F.S., Bellanda P.R., Alves-Do-Prado W., Ueda-Nakamura T., Nakamura C.V., Sakuragui C.M., Bersani-Amado C.A., Mello J.C.P. Biological Activity and Quality Control of Extractand Stem Bark From Stryphnodendron adstringens. Acta Farm. Bonaerense. 2004;23:328–333. [Google Scholar]
- 108.Pinho L., Souza P.N.S., Macedo Sobrinho E., Almeida A.C., Martins E.R. Antimicrobial activity of hydroalcoholic extracts from rosemary, peppertree, barbatimão and erva baleeira leaves and from pequi peel meal. Cienc. Rural. 2012;42:326–331. doi: 10.1590/S0103-84782012005000003. [DOI] [Google Scholar]
- 109.Eller S., Feitosa V.A., Arruda T.A., Antunes R.M.P., Catão R.M.R. Interactive study of the antimicrobial activity of plant extracts. Rev. Ciênc. Farm. Básica Apl. 2015;36:131–136. [Google Scholar]
- 110.Soares S.P., Vinholis A.H.C., Casemiro L.A., Silva M.L.A., Cunha W.R., Martins C.H.G. Antibacterial activity of the crude hydroalcoholic extract of Stryphnodendron adstringens on dental caries microorganisms. Rev. Odonto Ciênc. 2008;23:141–144. [Google Scholar]
- 111.Pereira E.M., Gomes R.T., Freire N.R., Aguiar E.G., Brandao M., Santos V.R. In vitro antimicrobial activity of Brazilian medicinal plant extracts against pathogenic microorganisms of interest to dentistry. Planta Med. 2011;77:401–404. doi: 10.1055/s-0030-1250354. [DOI] [PubMed] [Google Scholar]
- 112.Oliveira J.R., Castro V.C., Gracas Figueiredo Vilela P., Camargo S.E., Carvalho C.A., Jorge A.O., Oliveira L.D. Cytotoxicity of Brazilian plant extracts against oral microorganisms of interest to dentistry. BMC Complement. Altern. Med. 2013;13 doi: 10.1186/1472-6882-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oliveira D.G., Prince K.A., Higuchi C.T., Santos A.C.B., Lopes L.M.X., Simões M.J.S., Leite C.Q.F. Antimycobacterial activity of some Brazilian indigenous medicinal drinks. Rev. Ciênc. Farm. Básica Apl. 2007;28:165–169. [Google Scholar]
- 114.Morey A.T., Souza F.C., Santos J.P., Pereira C.A., Cardoso J.D., Almeida R.S., Costa M.A., Mello J.C.P., Nakamura C.V., Pinge-Filho P., et al. Antifungal Activity of Condensed Tannins from Stryphnodendron adstringens: Effect on Candida tropicalis Growth and Adhesion Properties. Curr. Pharm. Biotechnol. 2016;17:365–375. doi: 10.2174/1389201017666151223123712. [DOI] [PubMed] [Google Scholar]
- 115.Ishida K., Rozental S., Mello J.C.P., Nakamura C.V. Activity of tannins from Stryphnodendron adstringens on Cryptococcus neoformans: Effects on growth, capsule size and pigmentation. Ann. Clin. Microbiol. Antimicrob. 2009;8 doi: 10.1186/1476-0711-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melo e Silva F., Paula J.E., Espindola L.S. Evaluation of the antifungal potential of Brazilian Cerrado medicinal plants. Mycoses. 2009;52:511–517. doi: 10.1111/j.1439-0507.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- 117.Trolezi R., Azanha J.M., Paschoal N.R., Chechi J.L., Dias Silva M.J., Fabris V.E., Vilegas W., Kaneno R., Fernandes Junior A., Bosco S.M. Stryphnodendron adstringens and purified tannin on Pythium insidiosum: In vitro and in vivo studies. Ann. Clin. Microbiol. Antimicrob. 2017;16 doi: 10.1186/s12941-017-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oliveira D.R., Brito F.E., Jr., Bento E.B., Matias E.F., Sousa A.C., Costa J.G., Coutinho H.D., Kerntopf M.R., Menezes I.R. Antibacterial and modulatory effect of Stryphnodendron rotundifolium. Pharm. Biol. 2011;49:1265–1270. doi: 10.3109/13880209.2011.589857. [DOI] [PubMed] [Google Scholar]
- 119.Vasconcelos M.C.A., Rodovalho N.C.M., Pott A., Pott V.J., Ferreira A.M.T., Arruda A.L.A., Marques M.C.S., Castilho R.O., Bueno N.R. Avaliação de atividades biológicas das sementes de Stryphnodendron obovatum Benth. (Leguminosae) Rev. Bras. Farmacogn. 2004;14:121–127. doi: 10.1590/S0102-695X2004000200005. [DOI] [Google Scholar]
- 120.Mayer R., Stecher G., Wuerzner R., Silva R.C., Sultana T., Trojer L., Feuerstein I., Krieg C., Abel G., Popp M., et al. Proanthocyanidins: Target Compounds as Antibacterial Agents. J. Agric. Food Chem. 2008;56:6959–6966. doi: 10.1021/jf800832r. [DOI] [PubMed] [Google Scholar]
- 121.Nakamura K., Ishiyama K., Sheng H., Ikai H., Kanno T., Niwano Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food. Chem. 2015;63:7707–7713. doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- 122.Glehn E.A.V., Rodrigues G.P.S. Etest to confirm the action potential of plant hydroglycol extracts on Candida sp. (Berkhout) Rev. Bras. Plantas Med. 2012;14:435–438. doi: 10.1590/S1516-05722012000300002. [DOI] [Google Scholar]
- 123.Oliveira J.R., Vilela P.G.G., Oliveira F.E., Belato K.K., Carvalho C.A.T., Jorge A.O.C., Oliveira L.D. Antifungal effect of plant extracts on Candida albicans biofilm on acrylic resin. Braz. Dent. Sci. 2013;16 doi: 10.14295/bds.2013.v16i3.909. [DOI] [Google Scholar]
- 124.Luiz R.L., Vila T.V., Mello J.C.P., Nakamura C.V., Rozental S., Ishida K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015;15 doi: 10.1186/s12906-015-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uppuluri P., Chaturvedi A.K., Srinivasan A., Banerjee M., Ramasubramaniam A.K., Kohler J.R., Kadosh D., Lopez-Ribot J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donders G.G., Sobel J.D. Candida vulvovaginitis: A store with a buttery and a show window. Mycoses. 2017;60:70–72. doi: 10.1111/myc.12572. [DOI] [PubMed] [Google Scholar]
- 127.Seebacher C., Bouchara J.P., Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–352. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 128.Lin X., Heitman J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 129.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- 130.Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013;168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Souza P.M., Sales P.M., Simeoni L.A., Silva E.C., Silveira D., Magalhaes P.O. Inhibitory activity of alpha-amylase and alpha-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012;78:393–399. doi: 10.1055/s-0031-1280404. [DOI] [PubMed] [Google Scholar]
- 132.Keske M.A., Ng H.L., Premilovac D., Rattigan S., Kim J.A., Munir K., Yang P., Quon M.J. Vascular and Metabolic Actions of the Green Tea Polyphenol Epigallocatechin Gallate. Curr. Med. Chem. 2015;22:59–69. doi: 10.2174/0929867321666141012174553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silva Neto C.R., Lopes R.A., Contrera M.G.D., Pozetti G.L. Efeitos antagonicos de plantas medicinais na diurese do rato. Pesqui. Homeopat. 1987;4:17–21. [Google Scholar]
- 134.Silva Neto C.R., Lopes R.A., Rossi E., Contrera M.G.D. Excrecao renal de agua, sodio e potassio em animais submetidos a sobrecarga aquosa de barbatimao: Stryphodendron obovatum. Pesqui. Homeopat. 1988;5:9–20. [Google Scholar]
- 135.Vinaud M.C., Santos S.C., Ferri P.H., Lino Júnior R.S., Bezerra J.C.B. Avaliação da atividade larvicida de plantas fitoterápicas do cerrado do gênero Stryphnodendron spp. sobre miracídios e cercárias de Schistosoma mansoni. Rev. Patol. Trop. 2005;34:137–143. [Google Scholar]
- 136.Mendes N.M., Pereira J.P., Souza C.P., Oliveira M.L.L. Ensaios preliminares em laboratório para verificar a ação moluscicida de algumas espécies da flora brasileira. Rev. Saúde Pública. 1984;18:348–354. doi: 10.1590/S0034-89101984000500003. [DOI] [PubMed] [Google Scholar]
- 137.Bezerra J.C., Silva I.A., Ferreira H.D., Ferri P.H., Santos S.C. Molluscicidal activity against Biomphalaria glabrata of Brazilian Cerrado medicinal plants. Fitoterapia. 2002;73:428–430. doi: 10.1016/S0367-326X(02)00121-1. [DOI] [PubMed] [Google Scholar]
- 138.Vinaud M.C., Lino Junior R.S., Bezerra J.C.B. Activity of Stryphnodendron polyphyllum, a plant from the brazilian savannah, against hemocytes of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. J. Trop. Pathol. 2008;37:237–246. [Google Scholar]
- 139.Holetz F.B., Ueda-Nakamura T., Dias Filho B.P., Cortez D.A.G., Mello J.C.P., Nakamura C.V. Effect of plant extracts used in folk medicine on cell growth and differentiation of Herpetomonas samuelpessoai (Kinetoplastida, Trypanosomatidae) cultivated in defined medium. Acta Sci. 2002;24:657–662. [Google Scholar]
- 140.Holetz F.B., Ueda-Nakamura T., Dias Filho B.P., Mello J.C.P., Morgado-Diaz J.A., Toledo C.E., Nakamura C.V. Biological effects of extracts obtained from Stryphnodendron adstringens on Herpetomonas samuelpessoai. Mem. Inst. Oswaldo Cruz. 2005;100:397–401. doi: 10.1590/S0074-02762005000400010. [DOI] [PubMed] [Google Scholar]
- 141.Luize P.S., Tiuman T.S., Morello L.G., Maza P.K., Ueda-Nakamura T., Dias Filho B.P., Cortez D.A.G., Mello J.C.P., Nakamura C.V. Effects of medicinal plant extracts on growth of Leishmania (L.) amazonensis and Trypanosoma cruzi. Rev. Bras. Cienc. Farm. 2005;41:85–94. doi: 10.1590/S1516-93322005000100010. [DOI] [Google Scholar]
- 142.Herzog-Soares J.D.A., Alves R.K., Isac E., Bezerra J.C.B., Gomes M.H., Santos S.C., Ferri P.H. Atividade tripanocida in vivo de Stryphnodendron adstringens (barbatimão verdadeiro) e Caryocar brasiliensis (pequi) Rev. Bras. Farmacogn. 2002;12:1–2. doi: 10.1590/S0102-695X2002000300001. [DOI] [Google Scholar]
- 143.Herzog-Soares J.D.A., Isac E., Castro A.M., Bezerra J.C.B. Bioactivity of Stryphnodendron adstringens, S. polyphyllum, Caryocar brasiliense, plants from brazilian’s savannah on the Trypanosoma cruzi in vivo. Biosci. J. 2006;22:113–118. [Google Scholar]
- 144.Vandesmet V.C.S., Felipe C.F.B., Kerntopf M.R., Rolon M., Vega C., Coronel C., Barbosa A.G.R., Coutinho H.D.M., Menezes I.R.A. The use of herbs against neglected diseases: Evaluation of in vitro leishmanicidal and trypanocidal activity of Stryphnodendron rotundifolium Mart. Saudi J. Biol. Sci. 2017;24:1136–1141. doi: 10.1016/j.sjbs.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lins Neto M.A.F., Caetano L.C., Peixoto Neto P.A.S., Silva Z.P. Pharmaceutical Composition Using Stryphnodendron Extracts for Treating HPV Infections. 9023405 B2. U.S. Patent. 2015 May 5;
- 146.Lucena M.N., Mendes M.M., Brandeburgo M.I.H. Avaliação da Estabilidade da Pomada à Base de Stryphnodendron adstringens (Mart.) Conville e Sua Eficacia na Neutralização dos Efeitos Locais Induzidos Pela Peçonha de Bothrops pauloensis. [(accessed on 5 November 2017)];Horiz. Cient. 2009 3:1. Available online: https://ssl4799.websiteseguro.com/swge5/seg/cd2009/PDF/IC2009-0169.pdf. [Google Scholar]
- 147.Valente P.P., Amorim J.M., Castilho R.O., Leite R.C., Ribeiro M.F. In vitro acaricidal efficacy of plant extracts from Brazilian flora and isolated substances against Rhipicephalus microplus (Acari: Ixodidae) Parasitol. Res. 2014;113:417–423. doi: 10.1007/s00436-013-3670-2. [DOI] [PubMed] [Google Scholar]
- 148.Bichuette M.E., Varanda E.M., Barosela J.R. Effects of ester fractions from leaf epicuticular waxes of Bauhinia rufa (Steud.) Bong. and Stryphnodendron adstringens (Mart.) Coville from cerrado on the aphid Rhopalosiphum maidis (Fitch.) Braz. J. Bot. 1998;21:101–104. [Google Scholar]
- 149.Barbosa F.S., Leite G.L.D., Martins E.R., D’avila V.A., Cerqueira V.M. Medicinal plant extracts on the control of Diabrotica speciosa (Coleoptera: Chrysomelidae) Rev. Bras. Plantas Med. 2013;15:142–149. doi: 10.1590/S1516-05722013000100020. [DOI] [Google Scholar]
- 150.Vilar J.B., D’Oliveira M.I.P., Santos S.C., Chen L.C. Cytotoxic and genotoxic investigation on barbatimão [Stryphnodendron adstringens (Mart:) Coville, 1910] extract. Braz. J. Pharm. Sci. 2010;46:687–694. doi: 10.1590/S1984-82502010000400010. [DOI] [Google Scholar]
- 151.Sousa N.C., Carvalho S., Spano M.A., Graf U. Absence of genotoxicity of a phytotherapeutic extract from Stryphnodendron adstringens (Mart.) Coville in somatic and germ cells of Drosophila melanogaster. Environ. Mol. Mutagen. 2003;41:293–299. doi: 10.1002/em.10151. [DOI] [PubMed] [Google Scholar]
- 152.Santos Filho P.R., Ferreira L.A., Gouvêa C.M.C.P. Protective action against chemical-induced genotoxicity and free radical scavenging activities of Stryphnodendron adstringens (“barbatimão”) leaf extracts. Rev. Bras. Farmacogn. 2011;21:1000–1005. doi: 10.1590/S0102-695X2011005000176. [DOI] [Google Scholar]
- 153.Almeida A.C., Sobrinho E.M., Pinho L., Souza P.N.S., Martins E.R., Duarte E.R., Santos H.O., Brandi I.V., Cangussu A.S., Costa J.P.R. Acute toxicity of leaf hydroalcoholic extracts of Lippia sidoides, Myracroduon urundeuva, Stryphnodendron adstringens and of Caryocar brasilliense administered by intraperitoneal route. Cienc. Rural. 2010;40:200–203. doi: 10.1590/S0103-84782009005000230. [DOI] [Google Scholar]
- 154.Rebecca M.A., Ishii-Iwamoto E.L., Grespan R., Cuman R.K., Caparroz-Assef S.M., Mello J.C.P., Bersani-Amado C.A. Toxicological studies on Stryphnodendron adstringens. J. Ethnopharmacol. 2002;83:101–104. doi: 10.1016/S0378-8741(02)00219-2. [DOI] [PubMed] [Google Scholar]
- 155.Rebecca M.A., Ishii-Iwamoto E.L., Kelmer-Bracht A.M., Caparroz-Assef S.M., Cuman R.K., Pagadigorria C.L., Mello J.C.P., Bracht A., Bersani-Amado C.A. Effect of Stryphnodendron adstringens (barbatimao) on energy metabolism in the rat liver. Toxicol. Lett. 2003;143:55–63. doi: 10.1016/S0378-4274(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 156.Costa M.A., Ishida K., Kaplum V., Koslyk E.D., Mello J.C.P., Ueda-Nakamura T., Dias Filho B.P., Nakamura C.V. Safety evaluation of proanthocyanidin polymer-rich fraction obtained from stem bark of Stryphnodendron adstringens (BARBATIMAO) for use as a pharmacological agent. Regul. Toxicol. Pharmacol. 2010;58:330–335. doi: 10.1016/j.yrtph.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 157.Costa M.A., Mello J.C.P., Kaneshima E.N., Ueda-Nakamura T., Dias Filho B.P., Audi E.A., Nakamura C.V. Acute and Chronic Toxicity of an Aqueous Fraction of the Stem Bark of Stryphnodendron adstringens (Barbatimao) in Rodents. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/841580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kucera O., Mezera V., Moravcova A., Endlicher R., Lotkova H., Drahota Z., Cervinkova Z. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/476180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fan Y.C., Chan W.H. Epigallocatechin gallate induces embryonic toxicity in mouse blastocysts through apoptosis. Drug Chem. Toxicol. 2014;37:247–254. doi: 10.3109/01480545.2013.838778. [DOI] [PubMed] [Google Scholar]
- 160.Tokarnia C.H., Brito M.F., Driemeier D., Costa J.B.D., Camargo A.J.R. Aborto em vacas na intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae) Pesqui. Vet. Bras. 1998;18:35–38. doi: 10.1590/S0100-736X1998000100006. [DOI] [Google Scholar]
- 161.Brito M.F., Tokarnia C.H., Peixoto P.V., Silva H.K., Nogueira M. Intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae) em bovinos. 1. Caracterização do quadro clínico. Pesqui. Vet. Bras. 2001;21:9–17. doi: 10.1590/S0100-736X2001000100004. [DOI] [Google Scholar]
- 162.Brito M.F., Tokarnia C.H., Peixoto P.V. Intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae) em bovinos. 2. Achados anátomo e histopatológicos. Pesqui. Vet. Bras. 2001;21:61–71. doi: 10.1590/S0100-736X2001000200004. [DOI] [Google Scholar]