Abstract
The present study aimed to characterize the chemical composition of pyroligneous acid (PA) obtained from slow pyrolysis of the clone GG100 of Eucalyptus urophylla × Eucalyptus grandis. The efficiency of extraction of organic compounds by using different solvents—dichloromethane (DCM), diethyl ether (DE) and ethyl acetate (EA)—was evaluated. Wood discs were collected and carbonized at a heating rate of 1.25 °C/min until 450 °C. Pyrolysis gases were trapped and condensed, yielding a crude liquid product (CLP), which was refined to obtain pure PA. Then liquid–liquid extraction was carried out. Each extracted fraction was analyzed by GC-MS and the chemical compounds were identified. Experimental results showed that a larger number of chemical compounds could be extracted by using DCM and EA in comparison to diethyl ether DE. A total number of 93 compounds were identified, with phenolic compounds being the major group, followed by aldehydes and ketones, furans, pyrans and esters. Higher contents of guaiacol, phenol, cresols and furfural seem to explain the antibacterial and antifungal activity shown by PA, as reported previously in the literature. Experimental data indicated that the organic phase extracted from GG100 PA consists of a mixture of compounds similar to liquid smokes regularly used in the food industry.
Keywords: pyroligneous acid, wood vinegar, Eucalyptus GG100 clone, GC-MS characterization
1. Introduction
The forest sector is important to Brazilian agribusiness, contributing to the production of goods and services and generation of employment opportunities. A chain of direct and indirect providers contributes to a flourishing forest sector that continuously requires raw materials, power, water and heavy machinery, banking and communication facilities, etc. Currently, Brazil has 7.8 million hectares of planted forests, of which nearly 5.6 million hectares correspond to eucalyptus forests, with 1.1 million hectares dedicated exclusively to the production of firewood for making charcoal [1]. In 2015, Brazilian consumption of charcoal reached 4.6 million metric tons. The metallurgy sector, including pig iron, steel, iron alloys and silicon industries, consumed about 80% of charcoal. This sector produces charcoal in industrial facilities, mostly working with partial-combustion masonry kilns that do not recover byproduct gases and condensable compounds. Only charcoal is considered important in this process. Currently, mass and energy yields can be very low in such conventional kilns, about 30 and 50%, respectively, when byproducts are not recovered [2,3]. This means that millions of tons of chemicals are lost to the atmosphere as gases without any recycling.
One of the most important liquid products from wood pyrolysis is pyroligneous acid (PA). According to Souza et al. (2012) [4], pyrolysis liquids are referred to in the literature by terms such as pyroligneous tar, pyrolysis oil, bio-oil, bio-crude oil, bio-fuel oil, liquid wood, wood oil, liquid smoke, pyroligneous acid and wood distillates. However, a corrective remark must be inserted here since the expressions pyrolysis liquids and wood distillates by themselves are too comprehensive to be used as synonyms of PA. In turn, pyroligneous tar, pyrolysis oil, bio-oil, bio-fuel and bio-crude oil are commonly used to refer to the heavier oily and combustible portion of liquid products from flash pyrolysis of lignocellulosic raw materials, as pointed out by several authors [5,6,7] among many others. From a similar standpoint, the term liquid smoke also cannot be used as a synonym for PA since the so-called commercial liquid smoke is a result of fractionation, purification or concentration of carbonization liquid products, giving aqueous, oily or dry powder products [8]. This means that the use of the expression liquid smoke to characterize PA is not correct, while there are several types of LQ with properties quite distinct from each other. So, in order to standardize terms and to avoid ambiguity, it is better to consider both pyroligneous acid (PA) and wood vinegar as being the aqueous portion obtained from carbonization of wood and other lignocellulosic raw materials, as defined by several authors [9,10,11,12,13].
Charcoal making usually produces, as byproducts, non-condensable gases, tar and pyroligneous acid (hereafter referred to as PA). PA yields and chemical composition can widely vary depending on the type of the woody raw material and the pyrolysis process parameters, e.g., final temperature and heating rate [11,14]. Usually, PA is recovered from charcoal kilns by trapping the carbonization gases through a condensing unit or even simple metal pipes. After a certain interval (which can vary from days to weeks), wood tar, which is a heavier black oily fraction, decants at the bottom of the container and separates from PA. Agricultural uses of PA date back to the 1930s in Japan, when the product started to be applied as an antifungal and antibacterial agent on crops [12]. A large number of papers and technical reports show the undeniable efficiency of PA for soil conditioning [14,15,16] and for pest control in pure form or combined with conventional pesticides [4,12,17,18]. Additionally, PA has been successfully used to control fungal and bacterial diseases in plants [14,19] and as a herbicide [16]. Finally, PA is used as a plant growth enhancer, significantly stimulating the development of roots, stems, leaves, flowers, tubers and fruits, and also improving the sweet taste of fruits [14,20].
Despite being well-known worldwide and extensively used in agriculture, in Brazil the technical applications of PA are restricted mainly to small organic farming operations, leaving a much larger agribusiness market that can obtain benefits from the product. A significant element explaining the resistance to using PA is the ambiguous way of thinking, because many people strongly believe that PA can cause cancer and other diseases. This idea is related to the fact that PA can sometimes contain traces of wood tar, which is confused with coal tar, an undeniably toxic and carcinogenic compound [21]. Thus, disambiguation must be accomplished to enlighten people regarding the safety of using Eucalyptus PA. Besides this, there is a lack in the literature regarding the following points. (i) Research and technical works do not specify in a clear way what carbonization parameters were used to obtain the PA fraction, such as final temperature and heating rate; (ii) Scientific articles only rarely correlate the antibacterial and fungicidal properties of PA with the chemical compounds present in it; (iii) A significant number of Brazilian eucalyptus forests were established with clones of a hybrid from Eucalyptus urophylla × Eucalyptus grandis (cited in the national literature as Eucalyptus urograndis). Among these clones, GG100 stands out as one of the most used to form energy forests and for charcoal making in Brazil. However, despite such widespread application, there is no information about the chemical composition of the PA obtained from it. The present work aims to fill the gaps mentioned above, by clearly identifying the conditions applied to obtain PA from GG100 wood as raw material, as well as the standard method used for PA purification. Another specific goal is to identify the main components of PA by gas chromatography combined with mass spectrometry (GC-MS) and to present a brief review of PA’s compounds, including their main technical uses and possible biological effects.
2. Results and Discussion
Gravimetric yields (GY) of charcoal, total condensed liquids (crude PA) and non-condensable gases (NCG) from carbonization runs are shown in Table 1. These results obtained from charring runs agree with experimental data reported by Oliveira et al. (2006) [22], Pereira et al. (2013) [23] and Santos et al. (2013) [24], who all obtained similar yields and charcoal with acceptable quality in terms of industrial charcoal making.
Table 1.
Gravimetric yields from carbonization of Eucalyptus urograndis GG100 clone.
| Species | Gravimetric Yields (%) | ||
|---|---|---|---|
| Charcoal | Total Condensed Liquids | NCG | |
| Eucalyptus urograndis GG100 clone | 35.3 | 42.4 | 22.3 |
* NGC—non-condensable gases.
Table 2 shows the gravimetric yields of purified PA achieved by vacuum distillation of crude PA (total pyrolysis liquids) of Eucalyptus urograndis GG100 clone. The results attained by purification of crude PA were satisfactory for commercial purposes. Table 3 reports the properties of GG100 PA after distillation; they are in compliance with properties normally cited for PA in the literature cited here.
Table 2.
Gravimetric yields of purified PA from distillation.
| Species | Gravimetric Yields (%) | |
|---|---|---|
| Based on TPL * Mass | Based on Initial Dry Wood Mass | |
| Eucalyptus urograndis GG100 clone | 70.1 | 27.4 |
* Total pyrolysis liquids.
Table 3.
Properties of GG100 PA after distillation.
| Property | |
|---|---|
| Color | Yellow |
| pH | 2.85 |
| Titratable acidity | 0.03342 g NaOH/g sample |
| Density | 1.032 g cm−3 |
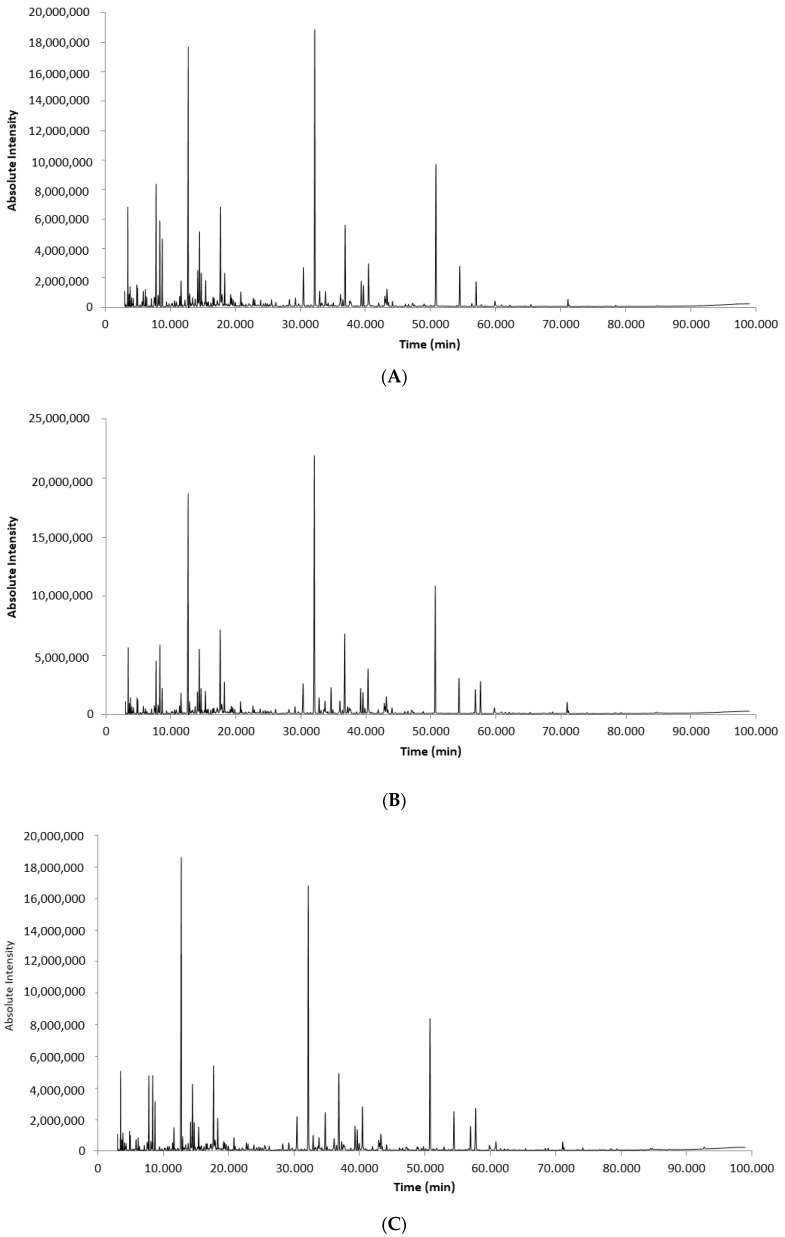
Figure 1 shows the total ion chromatograms of the Eucalyptus urograndis PA extracted with dichloromethane, diethyl ether and ethyl acetate (A, B and C, respectively). The profile of the chromatograms is similar, but the amount and type of compounds extracted by each solvent are different (as discussed shortly). Table 4 identifies the compounds extracted by each solvent. A total number of 93 main compounds were identified, divided into the following groups: phenolic compounds, aldehydes and ketones, furans and pyrans.
Figure 1.
Total ion chromatograms of extracts obtained by using dichloromethane (A), diethyl ether (B) and ethyl acetate (C).
Table 4.
Chemical compounds identified in the PA from Eucalyptus urograndis GG100 for dichloromethane, diethyl ether and ethyl acetate.
| Extraction Solvent | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dichloromethane | Diethyl Ether | Ethyl Acetate | |||||||||||
| Identified Peak # | Compound | Molecular Formula | Average Mass (Da) | Similarity (%) | OC | RT (min) | Area (%) | OC | RT (min) | Area (%) | OC | RT (min) | Area (%) |
| 1 | 2-methyl-2-pentanol | C6H14O | 102.175 | 87 | 3.178 | 0.05 | * | * | * | * | * | * | |
| 2 | Cyclopentanone | C5H8O | 84.116 | 98 | 3.483 | 2.39 | 3.475 | 1.83 | 3.467 | 1.64 | |||
| 3 | 2-methyl-cyclopentanone | C6H10O | 98.143 | 90 | 3.671 | 0.33 | 3.616 | 0.32 | 3.654 | 0.25 | |||
| 4 | Tetrahydro-2,2-dimethoxy-furan | C6H12O3 | 132.158 | 93 | 3.838 | 0.60 | 3.782 | 0.36 | 3.820 | 0.39 | |||
| 5 | 3-methyl-cyclopentanone | C6H10O | 98.143 | 89 | 4.051 | 0.31 | 3.991 | 0.29 | 4.032 | 0.19 | |||
| 6 | 2-methyl-pyridine | C6H7N | 93.127 | 96 | 4.166 | 0.07 | * | * | * | * | * | * | |
| 7 | 2-(methoxymethyl)-furan | C6H8O2 | 112.127 | 97 | 4.863 | 0.58 | 4.794 | 0.50 | 4.843 | 0.48 | |||
| 8 | Tetrahydro-2,5-dimethoxy-furan | C6H12O3 | 132.158 | 96 | 4.968 | 0.50 | 4.900 | 0.43 | 4.947 | 0.39 | |||
| 9 | 2-methyl-propanoic anhydride | C8H14O3 | 158.195 | 95 | 5.660 | 0.13 | * | * | * | * | * | * | |
| 10 | 1,4-dioxen | C4H6O2 | 86.089 | 88 | 5.855 | 0.73 | * | * | * | * | * | * | |
| 11 | N-nitrosodimethylamine | C2H6N2O | 74.082 | 94 | 6.192 | 0.60 | 6.085 | 0.19 | 6.161 | 0.41 | |||
| 12 | 5-methylhexahydro-4H-1,3-benzodioxin-4-one | C9H16O | 140.223 | 85 | * | * | * | 7.032 | 0.23 | * | * | * | |
| 13 | 2,4-hexadienal | C6H8O | 96.127 | 87 | 7.120 | 0.32 | * | * | * | * | * | * | |
| 14 | 3-pentanol | C5H12O | 88.148 | 93 | * | * | * | 7.556 | 0.14 | * | * | * | |
| 15 | 1-methoxy-2-butanol | C5H12O2 | 104.148 | 91 | * | * | * | * | * | * | 7.618 | 0.28 | |
| 16 | 4-hydroxy-3-hexanone | C6H12O2 | 116.158 | 88 | 7.646 | 0.38 | * | * | * | * | * | * | |
| 17 | 2-cyclopenten-1-one | C5H6O | 82.101 | 97 | 7.821 | 4.59 | 7.713 | 2.33 | 7.778 | 2.70 | |||
| 18 | 3,5-dimethyl-cyclohexanol | C8H16O | 128.212 | 85 | 8.137 | 0.56 | 8.040 | 0.34 | 8.134 | 0.38 | |||
| 19 | 2-methyl-2-cyclopenten-1-one | C6H8O | 96.127 | 97 | 8.404 | 3.38 | 8.307 | 3.14 | 8.368 | 2.90 | |||
| 20 | 1-hydroxy-2-butanone | C4H8O2 | 88.105 | 98 | 8.766 | 2.62 | 8.650 | 1.10 | 8.725 | 1.85 | |||
| 21 | 2-hydroxy-methyl ester-butanoic acid | C5H10O3 | 118.131 | 92 | 9.431 | 0.17 | * | * | * | 9.399 | 0.16 | ||
| 22 | 2-cyclohexen-1-one | C6H8O | 96.127 | 97 | 10.654 | 0.31 | * | * | * | * | * | * | |
| 23 | 3-furaldehyde | C5H4O2 | 96.084 | 94 | 11.442 | 0.38 | 11.324 | 0.29 | 11.405 | 0.30 | |||
| 24 | Butanoic acid, 2-ethylcyclohexyl ester | C12H22O2 | 198.302 | 85 | * | * | * | * | * | * | 11.617 | 1.08 | |
| 25 | 3-methyl-butanoic acid | C5H10O2 | 102.132 | 90 | 12.247 | 0.25 | * | * | * | * | * | * | |
| 26 | Furfural | C5H4O2 | 96.084 | 99 | 12.764 | 11.24 | 12.655 | 13.92 | 12.747 | 15.67 | |||
| 27 | 3,4-dimethyl-2-cyclopenten-1-one | C7H10O | 110.154 | 96 | 13.006 | 0.48 | * | * | * | 12.953 | 0.14 | ||
| 28 | 2,3,4-trimethyl-2-cyclopenten-1-one | C8H12O | 124.180 | 91 | 13.841 | 0.25 | 13.725 | 0.30 | 13.796 | 0.26 | |||
| 29 | 3-methyl-2-cyclopenten-1-one | C6H8O | 96.127 | 95 | 14.214 | 1.71 | 14.087 | 1.15 | 14.167 | 1.23 | |||
| 30 | 2-acetylfuran | C6H6O2 | 110.111 | 98 | 14.490 | 3.49 | 14.361 | 3.57 | 14.445 | 3.12 | |||
| 31 | Tetrahydro-2-furanmethanol | C5H10O2 | 102.132 | 93 | 14.759 | 1.59 | 14.637 | 1.40 | 14.714 | 1.36 | |||
| 32 | 1-isopropyl-1-cyclopentene | C8H14 | 110.197 | 90 | * | * | * | * | * | * | 15.232 | 0.08 | |
| 33 | 2,3-dimethyl-2-cyclopenten-1-one | C7H10O | 110.154 | 95 | 15.435 | 1.24 | 15.310 | 1.15 | 15.389 | 1.08 | |||
| 34 | 3,4,5-trimethyl-2-cyclopenten-1-one | C8H12O | 124.180 | 91 | 16.260 | 0.14 | * | * | * | 16.215 | 0.16 | ||
| 35 | 2-Butanone, 1-(acetyloxy)- | C6H10O3 | 130.142 | 93 | 16.741 | 0.33 | * | * | * | 16.693 | 0.27 | ||
| 36 | 1-acetylcyclohexene | C8H12O | 14.183 | 85 | * | * | * | * | * | * | 17.187 | 0.15 | |
| 37 | 3-methyl pyrrole | C5H7N | 81.116 | 89 | * | * | * | * | * | * | 17.320 | 0.18 | |
| 38 | 2,3-pentanedione | C5H8O2 | 110.116 | 88 | 16.570 | 0.42 | * | * | 16.528 | 0.30 | |||
| 39 | 3,4,4-trimethyl-2-cyclopenten-1-one | C8H12O | 124.180 | 85 | 17.236 | 0.18 | * | * | * | * | * | * | |
| 40 | 5-methyl-2-furancarboxaldehyde | C6H6O2 | 110.111 | 98 | 17.720 | 5.12 | 17.586 | 5.26 | 17.667 | 4.20 | |||
| 41 | Pentanoic acid, 4-oxo-, methyl ester | C6H10O3 | 130.142 | 88 | 17.937 | 1.01 | * | * | * | * | * | * | |
| 42 | Methyl-2-furoate | C6H6O3 | 126.110 | 98 | 18.362 | 1.65 | 18.223 | 1.91 | 18.316 | 1.59 | |||
| 43 | Butyrolactone | C4H6O2 | 86.089 | 95 | * | * | * | * | * | * | 19.218 | 0.39 | |
| 44 | 4-hydroxy-butanoic acid | C5H10O3 | 118.131 | 96 | 19.289 | 0.57 | * | * | * | * | * | * | |
| 45 | 3-ethyl-2-cyclopenten-1-one | C7H10O | 110.154 | 87 | 19.449 | 0.50 | * | * | * | * | * | * | |
| 46 | 2-acetyl-5-methylfuran | C7H8O2 | 124.137 | 92 | 19.665 | 0.24 | * | * | * | 19.617 | 0.30 | ||
| 47 | Methylbenzoate | C8H8O2 | 136.148 | 90 | 19.871 | 0.18 | * | * | * | 19.926 | 0.23 | ||
| 48 | 2,5-dihydro-3,5-dimethyl-2-furanone | C6H8O2 | 112.127 | 96 | 20.845 | 0.71 | 20.704 | 0.72 | 20.796 | 0.63 | |||
| 49 | Acetophenone | C8H8O | 120.148 | 88 | 21.018 | 0.15 | * | * | * | 20.972 | 0.17 | ||
| 50 | 5-methyl-2(5H)-Furanone | C5H6O2 | 98.100 | 90 | * | * | * | * | * | * | 22.043 | 0.09 | |
| 51 | 3-ethyl-2-hydroxy-2-ciclopenten-1-one | C7H10O2 | 126.153 | 92 | 22.757 | 0.38 | 22.615 | 0.46 | 22.707 | 0.39 | |||
| 52 | 2-furanmethanol (furfury alcohol) | C5H6O2 | 98.100 | 98 | 22.987 | 0.27 | 22.844 | 0.18 | 22.944 | 0.28 | |||
| 53 | 3-methyl-2(5H)-furanone | C5H6O2 | 98.100 | 92 | 23.984 | 0.24 | * | * | * | 23.839 | 0.28 | ||
| 54 | 4,5-dimethyl-4-hexen-3-one | C8H14O | 126.196 | 88 | * | * | * | 24.505 | 0.21 | 24.595 | 0.15 | ||
| 55 | 2(5H)-furanone | C4H4O2 | 84.073 | 92 | 25.561 | 0.25 | * | * | * | 25.502 | 0.22 | ||
| 56 | 2-propylcyclohexanone | C9H16O | 140.223 | 87 | * | * | * | * | * | * | 25.603 | 0.17 | |
| 57 | 3-methyl-4-hexen-2-one | C7H12O | 112.170 | 85 | * | * | * | * | * | * | 26.692 | 0.10 | |
| 58 | 1,2-dimethoxy-benzene (veratrol) | C8H10O2 | 138.164 | 92 | 26.231 | 0.14 | 26.089 | 0.28 | 26.184 | 0.20 | |||
| 59 | Methyl 4-hydroxybutanoate | C5H10O3 | 118.131 | 91 | 28.308 | 0.32 | * | * | * | 28.262 | 0.36 | ||
| 60 | 2,4-Dimethyl-1,3-cyclopentanedione | C7H10O2 | 126.153 | 89 | 29.232 | 0.44 | 29.089 | 0.51 | 29.180 | 0.51 | |||
| 61 | 3-methyl-1,2-cyclopentanedione | C6H8O2 | 112.127 | 97 | 30.487 | 2.37 | 30.331 | 2.42 | 30.430 | 2.25 | |||
| 62 | 2-methoxy-phenol (guaiacol) | C7H8O2 | 124.137 | 98 | 32.214 | 16.49 | 32.016 | 19.64 | 32.161 | 16.31 | |||
| 63 | 3-methyl-2-methoxy-phenol | C8H10O2 | 138.164 | 96 | 32.950 | 0.82 | 32.795 | 1.15 | 32.898 | 0.92 | |||
| 64 | Furan-2-carbaldehyde, (N’-nitroamidino)hydrazone | C6H7N5O3 | 197.054 | 85 | * | * | * | * | * | * | 34.748 | 2.29 | |
| 65 | 2,6-dimethyl-phenol | C8H10O | 122.164 | 97 | * | * | * | 34.921 | 0.21 | 35.027 | 0.19 | ||
| 66 | 2-methoxy-5-methyl-phenol | C8H10O2 | 138.164 | 97 | 36.158 | 0.63 | 35.998 | 0.80 | 36.107 | 0,63 | |||
| 67 | Maltol | C6H6O3 | 126.110 | 98 | 36.528 | 0.33 | 36.367 | 0.39 | 36.477 | 0.23 | |||
| 68 | 4-methyl-2-methoxy-phenol (creosol) | C8H10O2 | 138.164 | 97 | 36.880 | 4.73 | 36.722 | 5.91 | 36.830 | 4.87 | |||
| 69 | Phenol | C6H6O | 94.111 | 98 | 39.350 | 1.41 | 39.185 | 1.72 | 39.301 | 1.43 | |||
| 70 | 2-methyl-phenol (o-cresol) | C7H8O | 108.138 | 97 | 39.700 | 1.16 | 39.541 | 1.49 | 39.651 | 1.21 | |||
| 71 | 4-ethyl-2-methoxy-phenol | C9H12O2 | 152.190 | 98 | 40.487 | 2.38 | 40.327 | 3.11 | 40.434 | 2.61 | |||
| 72 | 4-methyl-phenol (p-cresol) | C7H8O | 108.138 | 97 | 42.984 | 0.54 | 42.818 | 0.77 | 42.931 | 0.67 | |||
| 73 | 2,6-dimethyl-phenol (2,6-xylenol) | C8H10O | 122.164 | 95 | 43.120 | 0.34 | 42.959 | 0.32 | 43.072 | 0.31 | |||
| 74 | 3-methyl-phenol (m-cresol) | C7H8O | 108.138 | 96 | 43.302 | 1.23 | 43.135 | 0.88 | 43.250 | 0.49 | |||
| 75 | 2,5-dimethy-phenol (2,5-xylenol) | C8H10O | 122.164 | 87 | * | * | * | * | * | * | 43.267 | 0.58 | |
| 76 | 3,4-dimethoxy-phenol | C8H10O3 | 154.163 | 91 | 43.531 | 0.21 | 43.369 | 0.21 | 43.480 | 0.17 | |||
| 77 | 4-propyl-2-methoxy-phenol | C10H14O2 | 166.217 | 92 | 44.172 | 0.24 | 44.013 | 0.45 | 44.121 | 0.37 | |||
| 78 | 2,4-dimethyl-phenol (2,4-xylenol) | C8H10O | 122.164 | 85 | * | * | * | 45.982 | 0.16 | 46.098 | 0.14 | ||
| 79 | 3-allyl-6-methoxy-phenol | C10H12O2 | 164.201 | 97 | * | * | * | 46.458 | 0.20 | 46.574 | 0.15 | ||
| 80 | 3,4-dimethyl-phenol (3,4-xylenol) | C8H10O | 122.164 | 93 | * | * | * | 47.043 | 0.34 | * | * | * | |
| 81 | 3-ethyl-phenol | C8H10O | 122.164 | 94 | * | * | * | 47.350 | 0.09 | * | * | * | |
| 82 | 3,5-dimethyl-phenol (3,5-xylenol) | C8H10O | 122.164 | 87 | * | * | * | 48.834 | 0.19 | * | * | * | |
| 83 | 4,5-dimethyl-imidazol | C5H8N2 | 96.130 | 98 | * | * | * | * | * | * | 49.749 | 0.21 | |
| 84 | 2,6-dimethoxy-phenol (syringol) | C8H10O3 | 154.163 | 97 | 50.829 | 8.78 | 50.665 | 9.90 | 50.770 | 8.48 | |||
| 85 | 4-methyl-2,6-dimethoxy-phenol | C9H12O3 | 168.190 | 80 | * | * | * | * | * | * | 54.427 | 2.52 | |
| 86 | 1,2,3-trimethoxy-benzene | C9H12O3 | 168.190 | 85 | 54.484 | 2.42 | 54.320 | 2.83 | * | * | * | ||
| 87 | 1,2,3-trimethoxy-5-methyl-benzene | C10H13O3 | 182.216 | 85 | 57.006 | 1.57 | 56.847 | 1.86 | 56.953 | 1.64 | |||
| 88 | 2,6-dimethoxy-4-allyl-phenol | C11H14O3 | 194.227 | 88 | * | * | * | 62.035 | 0.13 | 62.141 | 0.11 | ||
| 89 | Guaiacyl acetone | C10H12O3 | 180.201 | 90 | * | * | * | 65.253 | 0.11 | 65.365 | 0.10 | ||
| 90 | 2-methyl-5-amino-benzoxazole | C14H11NO | 209.243 | 88 | * | * | * | 68.719 | 0.13 | 68.834 | 0.12 | ||
| 91 | 2-acetyl-7-hydroxybenzofuran | C10H8O3 | 176.168 | 85 | * | * | * | * | * | * | 71.252 | 0.16 | |
| 92 | 2-amino-1-naphthol | C10H9NO | 159.185 | 89 | * | * | * | * | * | * | 74.131 | 0.17 | |
| 93 | Hydroquinone | C6H6O2 | 110.112 | 87 | * | * | * | 79.224 | 0.09 | 79.353 | 0.10 | ||
OC—Compound Occurrence in the respective extract: (# positive occurrence—marked in blue; negative occurrence—marked with an asterisk).
Regarding the sample extraction procedure, the addition of ammonium hydroxide to PA aqueous samples is an important step because it decreased the concentration of acetic acid extracted in the organic phase. It also increased the ionic strength of the solution, making the organic compounds less soluble in the aqueous phase and thus improving the efficiency of the liquid–liquid extraction, allowing detection of compounds even at low levels. As shown in Table 5, 65, 56 and 75 compounds were extracted by dichloromethane, diethyl ether and ethyl acetate, respectively, corresponding to 69.9%, 60.2% and 80.6% of the 93 compounds identified in GG100 clone PA. Therefore, in terms of number of extracted compounds, ethyl acetate was the most efficient solvent, followed by dichloromethane and diethyl ether. In terms of exclusive extraction, only four compounds were selectively extracted by diethyl ether (4.3% of the 93 compounds identified), against ten and 12 compounds exclusively extracted with dichloromethane and ethyl acetate. The results achieved are in accordance to those cited previously by Rungruang and Junyapoon (2010) [25], who found the same order of efficiency for these three solvents for the extraction of phenolic compounds from Eucalyptus wood.
Table 5.
Efficiency of solvents in the extraction of organic compounds from GG100 PA.
| Parameters | Extraction Solvent | ||
|---|---|---|---|
| Dichloromethane | Diethyl Ether | Ethyl Acetate | |
| Total number of compounds extracted by the solvent | 65 | 56 | 75 |
| % of the total extracted compounds | 69.9 | 60.2 | 80.6 |
| Number of compounds exclusively extracted by the solvent | 10 | 4 | 12 |
A significant number of the compounds identified here are the same cited in the literature as being components of liquid smoke used in the food industry [8,26,27,28]. Phenolic compounds are an important group identified both in PA and liquid smoke [8,26]. The same compounds are reported as being present in PA from different origins as well [4,13,29]. The high contents of guaiacol, phenol, cresols and furfural present in GG100 PA explain its antibacterial/antifungal activities, as pointed out by others authors [29], who stated that antibacterial and antifungal activity of PA from different sources cannot be attributed to a single compound, but instead to a combination of several ones.
A brief review of literature on some of the major components of GG100 PA is included from this point ahead, highlighting their properties, occurrence and biological effects, following the order of elution of the compounds shown in Table 4. 2-methyl-2-pentanol is flavoring agent used in food [30]. Cyclopentanone is a flavoring agent usually found in commercial liquid smoke [8,26]. 2-cyclopenten-1-one and its derivatives are food additives and are also found in commercial liquid smoke [8]. Furfural (2-furaldehyde) is a flavoring agent found in allspice and in commercial liquid smoke products [8,26]. 3-methyl-2-cyclopenten-1-one is a flavoring agent found in commercial liquid smoke [8,26]. 2-acetyl-furan is used in flavor compositions and contributes to aroma of many foods and beverages [30]. Tetrahydro-2-furanmethanol is a flavoring agent and food additive found in fermented soya hydrolysate (shoyu) and in liquid smoke [8,26,31]. 5-methyl-2-furancarboxaldehyde is a flavoring agent found in pepper, isolated from brown algae and other plant sources [31] and present in liquid smoke [8]. Methyl-2-furoate and 3-methyl-1,2-cyclopentanedione are flavoring agents [31]. Guaiacol is used medicinally as an expectorant, antiseptic, and local anesthetic [30]. Guaiacol and its derivatives have antioxidant properties and are present in commercial liquid smoke [8,26]. 4-methyl-2-methoxy-phenol (creosol) is a flavoring agent present in several foods and beverages [31] and in liquid smoke [8,26].
Phenol has some therapeutic value as a fungicide, antiseptic and disinfectant [31], with activity against a wide range of microorganisms including some viruses. Phenol is also one of the components of commercial liquid smoke products [8]. Cresols (o, m and p-cresol or 2,3 and 4-methy-phenol) are used as local antiseptics, parasiticides, disinfectants and as intestinal antiseptics [31], and are also present as components in liquid smoke products [8,26]. Other compounds present in PA, such as 4-ethyl-2-methoxy-phenol, maltol, 4-oxo-methyl-esther pentanoic acid (methyl levulinate), 2,6-dimethoxy-phenol (syringol) and its derivatives and xylenols, are used as flavoring agents [8,26,30].
Regarding the presence of mutagenic compounds, specifically polycyclic aromatic hydrocarbons (PAHs), several authors have pointed out that by removing wood tar from PAs, the PAHs are concomitantly removed. PAHs have a strong environmental impact because of their mutagenic and carcinogenic [32] character, which makes detection of these compounds important in processed foods, drinking water, air, etc. for health safety reasons. Liquid products from pyrolysis have been assessed to establish their acute toxicity and genotoxicity, but the results showed that crude pyrolysis liquids of eucalyptus wood had no mutagenic properties [33] because PAHs are strongly adsorbed to the pitch fraction and are not bioavailable. Furthermore, Pakdel and Roy (1988) [34] demonstrated that PAHs are thoroughly mixed with tar pitch, and only traces of them remained in the pure PA as oily tar fraction separated from aqueous PA. A feature that stands out in the chemical composition of PA is the presence of N-nitrosodimethylamine (11th compound in Table 4). This compound can be formed during the cooking of foods, especially cured meats and fish, which contain sodium nitrite as a preservative, but it is also found in several vegetables, cheeses, alcoholic beverages and fruits. It has been found to induce tumor formation in experimental animals, indicating it may also be a human carcinogen [35]. Likewise, phenol is reported as having suspected mutagenic properties [36]. The literature cited here also shows that for agriculture uses, PA is usually greatly diluted before applying the product, normally from 1:200 to 1:1.000 depending on the crop. Even though PA is used in diluted form, there is no dose level fixed for potentially carcinogenic substances that allows classifying them as not harmful. Thus, attention may be required regarding persistence times of N-nitrosodimethylamine and phenol in fruits and vegetables after PA is applied on crops.
Decantation is the most frequently used method for purification of PA and removing wood tar [11], but the method is a long-term strategy because it sometimes requires several months to achieve acceptable separation of PA from wood tar. Distillation is another method that can provide PAs free of PAHs in standardized quality with reliability and reproducible operational conditions [10]. There are still other methods currently used to eliminate PAHs from commercial liquid smoke flavorings [37] that most likely could be applied to refine PAs due to the close similarity in terms of chemical composition. On the other hand, phenolic compounds from Eucalyptus pyrolysis can be toxic to living organisms and cells, but they do not present any mutagenic character, as demonstrated by Pimenta et al. (2000) [33]. In general, phenolic compounds are widely used in clinical dentistry as sedatives, disinfectants and medication because despite being cytotoxic agents, they have no mutagenic effect [38].
Based on these observations about GG100 PA’s chemical composition, it is not evident that since the product likely is free of PAHs after purification, it can be applied without any concern in agriculture or as additive in animal feed, because of the presence of phenols and N-nitrosodimethylamine in its chemical composition. Further research should be carried out to isolate and specifically test the mutagenic character of crude GG100 PA and its phenolic fraction. Effective methods to isolate the phenolic fraction from PA are reported in the literature [29,39].
3. Materials and Methods
3.1. Production and Purification of PAs
The wood for the experiment was obtained from a planted forest tended by the Agricultural Sciences Unit of Federal University of Rio Grande do Norte, located in the city of Macaíba, Rio Grande do Norte. The procedures for log collection and wood sampling followed the method described by Santos et al. (2013) [24]. Wood samples consisted of 2-cm-thick disks, each divided into four wedges. Wood wedges were oven dried for 48 h at 103 ± 1 °C until 0% moisture content. Then they were placed in a metal container in batches of about 500 g and were carbonized using a laboratory furnace. The furnace was equipped with a device designed to trap and collect the condensable portion of pyrolysis gases, and during all carbonization runs, the condenser was water cooled and maintained at 25 °C. Ten charring runs were carried out at a heating rate of 1.25 °C/min until final temperature of 450 °C, which was held for 30 min. After the charring runs, the total condensed liquids were immediately stored in a refrigerator at 2 °C until use. The liquids from 10 pyrolysis runs were combined to form a composite sample. From the experimental data, the yields of charcoal, total condensed liquids and non-condensable gases (NCG) were determined. Yields of NGC were obtained by difference. Then the composite sample of condensed liquids was bi-distilled under a 1.0 mm HG vacuum at 100 °C to obtain the purified PAs, with the distillation process being interrupted as soon as the temperature reached 102–105 °C. Wood tar and heavy oils from each distillation were discarded. After PA purification, the following properties were determined: pH; titratable acidity by using a Metrhom Titrando device (Herisau, Switzerland) 0.1 mol L−1 NaOH titration solution; density (Koehler K86201 automatic density meter, Bohemia, NY, USA); and color.
3.2. GC-MS Analysis of PA
First, 1.5 mL of concentrated ammonium hydroxide solution (Ammonia Solution UN 2672, Caledon, ON, Canada) was added to 5 mL aliquots of the aqueous samples of PA to increase the pH to around 5. Then extractions were carried out by adding 3 mL of the different solvents separately: dichloromethane (Tedia, Aparecida de Goiânia, Brazil), diethyl ether and ethyl acetate (Merck, Kenilworth, NJ, USA). All the solvents were HPLC grade. Three extracts were produced, respectively. After liquid-liquid extraction, 1 mL of the organic fraction obtained by each solvent was transferred to a GC vial and was promptly analyzed.
The GC-MS analyses of the samples were carried out with a Shimadzu QP 2010 system. The separation was performed in a CP-Wax column (Restek 52 DB, 30 m length, 0.25 mm diameter, 0.25 μm film thickness), keeping the injector temperature at 250 °C. The chromatographic runs were carried out in order to achieve the best separation of compounds, until the following routine of analysis could be defined. The samples (1 µL) were injected in a split ratio of 1:10, and the oven temperature program was 50 °C for 2 min, 2 °C min−1 from 50 to 240 °C, maintained for 2 min. Helium was used as a carrier gas at a constant flow rate of 1 mL min-1. Major (>20% area) and minor compounds (~0.02%) were detected and identified based on their characteristic mass spectra by comparison with the NIST library. All of the chemical compounds reported here had mass spectrum similarity above 85%.
After chemical identification of the compounds present in each extract sample, we searched the literature to determine the main uses and their biological effects. The goal here was not to perform an exhaustive review, but only a brief appraisal to highlight possible effects of PA components on human and animal health.
4. Conclusions
This work describes the efficiency of the liquid–liquid extraction of PA from Eucalyptus GG100 clone with dichloromethane, diethyl ether and ethyl acetate, followed by analysis of the extracts by GC-MS to identify the main compounds. The GG100 clone is planted in large scale in Brazil and the main components of its PA have antibacterial, antifungal and antioxidant properties, of interest for agricultural uses. Higher contents of guaiacol, phenol, cresols and furfural seem to explain the antibacterial and antifungal activity shown by PA, as reported previously in the literature. Besides that, we found that GG100 PA consists of a mixture of chemicals very similar to those in liquid smoke additives regularly used in the food industry. However, the presence of N-nitrosodimethylamine and phenol in PA composition may require attention regarding persistence times of these compounds in plants and the environment in case of applying PA on edible crops, since those two compounds have been found to be carcinogenic and are suspected of being carcinogenic, respectively.
Acknowledgments
This work was funded by the Graduate Program in Forest Science of the Department of Forest Engineering of Federal University of Rio Grande do Norte (Brazil). K.M.G. Lima acknowledges the CNPq Grant (305962/2014-0).
Author Contributions
A.S.P. designed the experiments; M.F. performed the GC-MS analysis of PA; T.C.V.M. performed the production and purification of PAs. K.M.G.L. analyzed the data. All authors participated in writing the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Zulu L.C. The forbidden fuel: Charcoal, urban woodfuel demand and supply dynamics, community forest management and woodfuel policy in Malawi. Energy Policy. 2010;38:3717–3730. doi: 10.1016/j.enpol.2010.02.050. [DOI] [Google Scholar]
- 2.Arruda T.P.M.D., Pimenta A.S., Vital B.R., Lucia R.M.D., Acosta F.C. Evalution of two carbonization routines in rectangular kilns. Rev. Árvore. 2011;35:949–955. doi: 10.1590/S0100-67622011000500020. [DOI] [Google Scholar]
- 3.Jesus M.S. Master’s Thesis. Universidade Federal de Lavras; Lavras, Brazil: 2016. Mass and Energy Balance on Pyrolysis of Eucalyptus Wood in Industrial Scale; p. 89. [Google Scholar]
- 4.Souza J.B.G., Ré-Poppi N., Raposo J.L. Characterization of pyroligneous acid used in agriculture by gas chromatography-mass spectrometry. J. Braz. Chem. Soc. 2012;23:610–617. doi: 10.1590/S0103-50532012000400005. [DOI] [Google Scholar]
- 5.Zhu X.F., Lu Q. Production of Chemicals from Selective Fast Pyrolysis of Biomass. In: Momba M.N.B., editor. Biomass. InTech; London, UK: 2010. p. 19. [Google Scholar]
- 6.Radlein D., Quignard A. A Short Historical Review of Fast Pyrolysis of Biomass. Oil Gas Sci. Technol. 2013;68:765–783. doi: 10.2516/ogst/2013162. [DOI] [Google Scholar]
- 7.Alsbou E.I. Ph.D. Thesis. Department of Chemistry, Memorial University of Newfoundland; St. John’s, NL, Canada: 2014. Pyrolysis Bio-Oil as a Renewable Fuel and Source of Chemicals: Its Production, Characterization and Stability; p. 174. [Google Scholar]
- 8.Montazeri N., Oliveira A.C.M., Himelbloom B.H., Leigh M.B., Crapo C.A. Chemical characterization of commercial liquid smoke products. Food Sci. Nutr. 2013;1:102–115. doi: 10.1002/fsn3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto T. Present status of wood vinegar studies in Japan for agricultural usage; Proceedings of the 7th International Congress of the Society for the Advancement of Breeding Researches in Asia and Oceania (SABRAO); Taichung, Taiwan. 16–20 November 1994; pp. 811–820. [Google Scholar]
- 10.Higashino T., Shibata A., Yatagai M. Basic Study for Establishing Specifications for Wood Vinegar by distillation I. Mokuzai Gakkaishi. 2005;51:180–188. doi: 10.2488/jwrs.51.180. [DOI] [Google Scholar]
- 11.Campo A.D. Técnicas para produção de extrato pirolenhoso para uso agrícola. Embrapa. 2007;65:1–8. [Google Scholar]
- 12.Tiilikkala K., Fagernäs L., Tiilikkala J. History and Use of Wood Pyrolysis Liquids as Biocide and Plant Protection Product. Open Agric. J. 2010;4:111–118. doi: 10.2174/1874331501004010111. [DOI] [Google Scholar]
- 13.Zhai M., Shi G., Wang Y., Mao G., Wang D., Wang Z. Chemical Compositions and Biological Activities of Pyroligneous Acids from Walnut Shell. Bioresource. 2015;10:1715–1729. doi: 10.15376/biores.10.1.1715-1729. [DOI] [Google Scholar]
- 14.Yoshimoto T. Toward enhanced and sustainable agricultural productivity in the 2000’s: Breeding research and biotechnology; Proceedings of the 7th lnternational Congress of the Society for the Advancement of Breeding Researches in Asia and Oceanla (SABRAO) and lnternatiollal Symposium of WVorld Sustainable Agriculture Association (H/SAA); Taipei, Taiwan. 16–20 November 1993; pp. 811–820. [Google Scholar]
- 15.Steiner C. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia. 2008;51:359–366. doi: 10.1016/j.pedobi.2007.08.002. [DOI] [Google Scholar]
- 16.Togoro A., Silva J.A.S., Cazetta J.O. Chemical changes in oxisol treated with pyroligneous acid. Ciênc. Agrotecnol. 2014;38:113–121. doi: 10.1590/S1413-70542014000200002. [DOI] [Google Scholar]
- 17.Thuler R.T., Bortoli S.A., Barbosa J.C. Effectiveness of chemical insecticides and plant products for the control of Plutella xylostella. Científica. 2007;35:166–174. [Google Scholar]
- 18.Kim D.U., Seo H.E., Lee S., Lee K. Effects of wood vinegar mixed with inseticides on the mortalities of Nilaparvata lugens and Laodeophax striatellus (Homoptera: Delphacidae) Anim. Cells Syst. 2008;12:47–52. doi: 10.1080/19768354.2008.9647153. [DOI] [Google Scholar]
- 19.Payamara J. Usage of wood vinegar as new organic substance. Int. J. ChemTech Res. 2011;3:1658–1662. [Google Scholar]
- 20.Zulkarami B., Ashrafuzzman M., Husni M., Mohamed R.I. Effect of pyroligneous acid on growth, yield and quality improvement of rockmellon in soiless culture. Aust. J. Crop Sci. 2011;5:1508–1514. [Google Scholar]
- 21.14th Report on Carcinogens. [(accessed on 16 October 2017)]; Available online: https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html.
- 22.Oliveira E., Vital B.R., Pimenta A.S., Lucia R.M.D., Ladeira A.M.M., Carneiro A.C.O. Anatomical structure and charcoal quality of Mimosa tenuiflora (Willd.) Poir. wood. Rev. Árvore. 2006;30:311–318. doi: 10.1590/S0100-67622006000200018. [DOI] [Google Scholar]
- 23.Pereira B.L.C., Carneiro A.D.C.O., Carvalho A.M.M.L., Colodette J.L., Oliveira A.C., Fontes M.P.F. Influence of Chemical Composition of Eucalyptus Wood on Gravimetric Yield and Charcoal Properties. BioResources. 2013;8:4574–4592. doi: 10.15376/biores.8.3.4574-4592. [DOI] [Google Scholar]
- 24.Santos R.C., Carneiro A.C.O., Pimenta A.P., Castro R.V., Marinho I.V., Trugilho P.F., Alves I.C.N., Castro A.F.N.M. Energy potential of species from forest management plan for the Rio Grande do Norte State. Ciênc. Florest. 2003;23:493–504. doi: 10.5902/198050989293. [DOI] [Google Scholar]
- 25.Rungruang P., Suwannee J. Antioxidative activity of phenolic compounds in pyroligneous acid produced from Eucalyptus wood; Proceedings of the 8th International Symposium on Biocontrol and Biotechnology; Pattaya, Thailand. 4–6 October 2010; Khon Kaen, Thailand: Institute of Technology Ladkrabang and Khon Kaen University; 2010. pp. 102–106. [Google Scholar]
- 26.Nollet L.M., Boylston T., Chen F., Coggins P.C., Gloria M.B. In: Handbook of Meat, Poultry & Seafood Quality. Nollet L.M.L., editor. Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2007. [Google Scholar]
- 27.Achmadi S.S., Mubarik N.R., Nursyamsi R., Septiaji P. Characterization of redistilled liquid smoke of Oil-palm shells and its application as fish preservatives. J. Appl. Sci. 2013;13:401–408. doi: 10.3923/jas.2013.401.408. [DOI] [Google Scholar]
- 28.Budaraga I.K., Arnim Y.M., Bulanin U. Analysis of liquid smoke chemical components with GC MS from different raw materials variation production and pyrolysis temperaturelevel. Int. J. ChemTech Res. 2016;9:694–708. [Google Scholar]
- 29.Yang J.-F., Yang C.-H., Liang M.-T., Gao Z.-J., Wu Y.-W., Chuang L.-Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules. 2016;21:1150. doi: 10.3390/molecules21091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Human Metabolome Database (HMDB) [(accessed on 16 October 2017)]; Available online: http://www.hmdb.ca/
- 31.O’Neil M.J., Chemistry R.S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Royal Society of Chemistry; Cambridge, UK: 2013. [Google Scholar]
- 32.Samanta S.K., Singh O.V., Jain R.K. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends Biotechnol. 2002;20:243–248. doi: 10.1016/S0167-7799(02)01943-1. [DOI] [PubMed] [Google Scholar]
- 33.Pimenta A.S., Bayona J.M., García M.T., Solanas A.M. Evaluation of acute toxicity and genotoxicity of liquid products from pyrolysis of Eucalyptus grandis wood. Arch. Environ. Contam. Toxicol. 2000;38:169–175. doi: 10.1007/s002449910022. [DOI] [PubMed] [Google Scholar]
- 34.Pakdel H., Roy C. American Chemical Society Symposium Series. ACS eBooks; Washington, DC, USA: 1988. Chemical characterization of wood pyrolysis oils obtained in a vacuum pyrolysis multiple-hearth reactor; pp. 203–219. [Google Scholar]
- 35.Substance Evaluation—CoRAP. [(accessed on 29 November 2017)]; Available online: https://echa.europa.eu/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table/-/dislist/details/0b0236e1807e9220.
- 36.N-Nitrosodimethylamine (Code C44417) [(accessed on 16 October 2017)]; Available online: https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C44417.
- 37.Simko P. Factors affecting elimination of polycyclic aromatic hydrocarbons from smoked meat foods and liquid smoke flavorings. Mol. Nutr. Food Res. 2005;49:637–647. doi: 10.1002/mnfr.200400091. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y.C., Tai K.W., Huang F.M., Huang M.F. Cytotoxic and nongenotoxic effects of phenolic compounds in human pulp cell cultures. J. Endod. 2000;26:440–443. doi: 10.1097/00004770-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Xavier L., Freire M.S., Vidal-Tato I., González-Álvarez J. Recovery of phenolic compounds from Eucalyptus wood wastes using ethanol-salt-based. Maderas Cienc. Tecnol. 2017 doi: 10.4067/S0718-221X2017005000001. [DOI] [Google Scholar]