Abstract
The objectives of the present study were to characterize the chemical composition, antioxidant activity and hepatoprotective effect of the polysaccharides from Taishan Pinus massoniana pollen (TPPPS). HPLC analysis showed that TPPPS was an acidic heteropolysaccharide with glucose and arabinose as the main component monosaccharides (79.6%, molar percentage). Fourier transform-infrared spectroscopy (FT-IR) analysis indicated that the spectra of TPPPS displayed infrared absorption peaks characteristic of polysaccharides. In in vitro assays TPPPS exhibited different degrees of dose-dependent antioxidant activities , and this was further verified by suppression of CCl4-induced oxidative stress in the liver with three tested doses of TPPPS (100, 200, and 400 mg/kg bw) in rats. Pretreatment with TPPPS significantly decreased the levels of alanine aminotransferase (AST), aspartate aminotransferase (ALT), alkaline phosphatase (ALP), lactic dehydrogenase (LDH) and malondialdehyde (MDA) against CCl4 injuries, and elevated the activities of superoxide dismutase (SOD) as well as glutathione peroxidase (GSH-Px). Histopathological observation further confirmed that TPPPS could protect the liver tissues from CCl4-induced histological alternation. These results suggest that TPPPS has strong antioxidant activities and significant protective effect against acute hepatotoxicity induced by CCl4. The hepatoprotective effect may partly be related to its free radical scavenging effect, increasing antioxidant activity and inhibiting lipid peroxidation.
Keywords: Taishan Pinus massoniana pollen, polysaccharides, characterization, antioxidant activity, hepatoprotective effect
1. Introduction
Reactive oxygen species (ROS) generated in aerobic organisms during the respiration process, including free radicals such as superoxide anion (O2•−), hydroxyl radical (OH•) and hydrogen peroxide (H2O2), play important roles in degenerative and pathological processes such as cancer, aging, inflammation and fibrosis [1,2,3]. Mammalian cells constantly exposed to excessive ROS may suffer detrimental effects such as lipid peroxidation of cellular membranes, enzyme inactivation, DNA breakage, alteration of lipid–protein interaction and eventually the promotion of mutations that initiate tumor progression [4]. It has also been found that ROS are involved in various acute and chronic liver diseases [5]. Although the synthetic antioxidants such as propyl gallate and butylated hydroxytoluene could effectively inhibit the oxidative damage, their potential toxicity in human body has been reported and is of concern [6]. In recent years, many natural polysaccharides, which are widely found in animals, plants and microorganisms, have been demonstrated to play an important role as free radical scavengers in the prevention of oxidative damage in living organism, and can be exploited as novel potential antioxidants to prevent the damage of ROS [7,8,9]. Therefore, discovery and assessment of natural polysaccharides as new functional antioxidant medicines have become a hot research field.
Pinus massoniana pollen, a traditional food supplement and medicine in China, is continually reported to have a wide range of health benefits including alleviating fatigue, delaying apolexis, and treating disease [10]. These beneficial effects are attributed to its variety of chemical ingredients including proteins, vitamins, enzymes and coenzymes, fats, flavonoids, nucleic acids, monosaccharides, polysaccharides, phospholipids, and other nutrients [11]. Recent studies have demonstrated that Masson pine pollen had a hepatoprotective effect which was related to its antioxidant properties [12]. Taishan Pinus massoniana pollen polysaccharide (TPPPS), is extracted from Pinus massoniana pollen collected from Mount Tai (Chinese name: Taishan). Our previous studies have confirmed that TPPPS could enhance immunological functions in mice, rabbits and chickens, and significantly improve the immune response of the subunit vaccine [13,14,15,16]. However, there is relatively little information pertaining to the antioxidant and hepatoprotective activities of TPPPS. Therefore, the aim of the present study was firstly to characterize the chemical composition of TPPPS and its antioxidant activity in vitro and secondly, to explore the in vivo protective effect against carbon tetrachloride (CCl4)-induced hepatic damage in rat.
2. Results
2.1. Physicochemical Properties of TPPPS
The crude TPPPS were obtained from dry Taishan Pinus massoniana pollen using hot water extraction and ethanol precipitation. After deproteinization, dialysis and purification by gel-filtration chromatography, the yield of TPPPS was 4.28% of the starting material weight. The total carbohydrate content in TPPPS was 94.7%, as determined by the phenol–sulfuric acid method. As determined by the sulfuric acid-carbazole method, the content of uronic acid in TPPPS was 12.49%. Purified TPPPS appeared as a white powder, with no absorption at 260 nm in the UV spectrum, indicating the absence of nucleic acids. In addition, the Coomassie Brilliant Blue method showed that the protein content in TPPPS was 1.92%, suggesting that TPPPS might be a protein-bound polysaccharide.
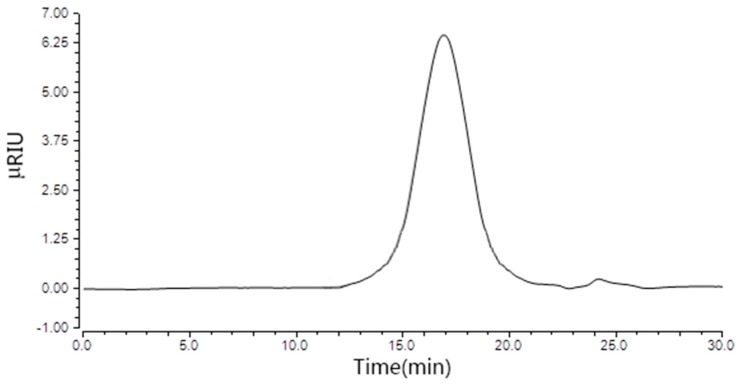
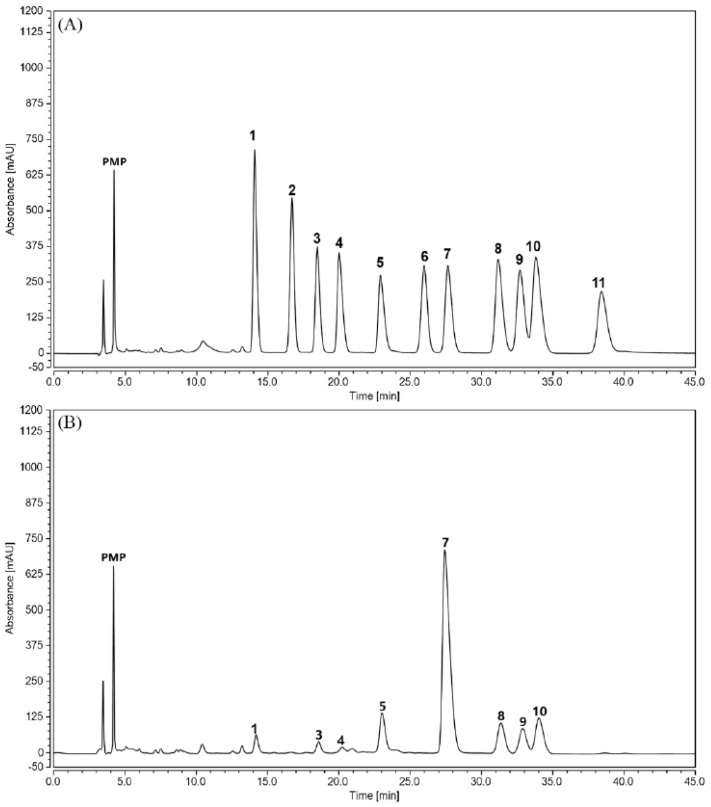
As shown in Figure 1, TPPPS was eluted as a single symmetrical peak in the high performance gel permeation chromatography (HPGPC) chromatogram, indicating that it was a homogeneous polysaccharide. The monosaccharide composition and content in TPPPS were determined using a high-performance liquid chromatography (HPLC) method (Figure 2). Following derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP) eleven kinds of standard monosaccharides could be separated well through the column within 45 min. As shown in Figure 2B, TPPPS was composed of mannose, rhamnose, gluconic acid, galacturonic acid, glucose, galactose, xylose and arabinose, and their corresponding mole percentages were 1.76%, 1.67%, 0.78%, 5.39%, 69.17%, 5.18%, 5.63% and 10.43% of all the total monosaccharides, respectively.
Figure 1.
HPGPC profile of TPPPS.
Figure 2.
The HPLC chromatograms of PMP derivatives of component monosaccharides released from 11 standard monosaccharides (A) and TPPPS (B). Peaks: (1) mannose, (2) glucosamine, (3) rhamnose, (4) gluconic acid, (5) galacturonic acid, (6) galactosamine, (7) glucose, (8) galactose, (9) xylose, (10) arabinose, (11) fucose (internal standard).
2.2. FT-IR Spectrum of TPPPS
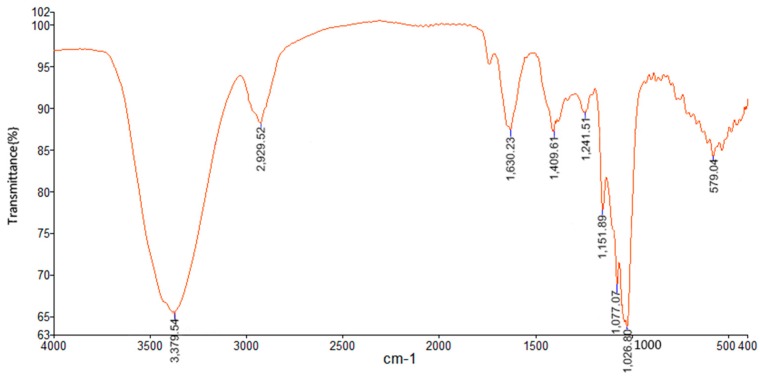
To characterize the TPPPS composition more precisely, the characteristic FT-IR spectrum was recorded in the wavelength range of 4000–400 cm−1 (Figure 3). Specifically, the strong and broad absorption peak at 3379 cm−1 is a characteristic stretching vibration of O-H, and the weak peak at 2929 cm−1 was caused by the stretching vibration of the C–H bonds. As reported, the characteristic absorption peak at 1026 and 1077 cm−1 was ascribed to the bending vibration of C–OH side groups and C–O–C glycosidic bond, indicating that there existed pyranose unit in TPPPS [17,18]. In addition, the stretching absorption peak at 1630 cm−1 along with the peak at 1409 cm−1 indicated the presence of carboxylic group (COO−), which conforms to the presence of uronic acids detected by the HPLC method [19,20].
Figure 3.
FT-IR spectrum of TPPPS in the frequency range of 4000–400 cm−1.
2.3. In Vitro Antioxidant Activity of TPPPS
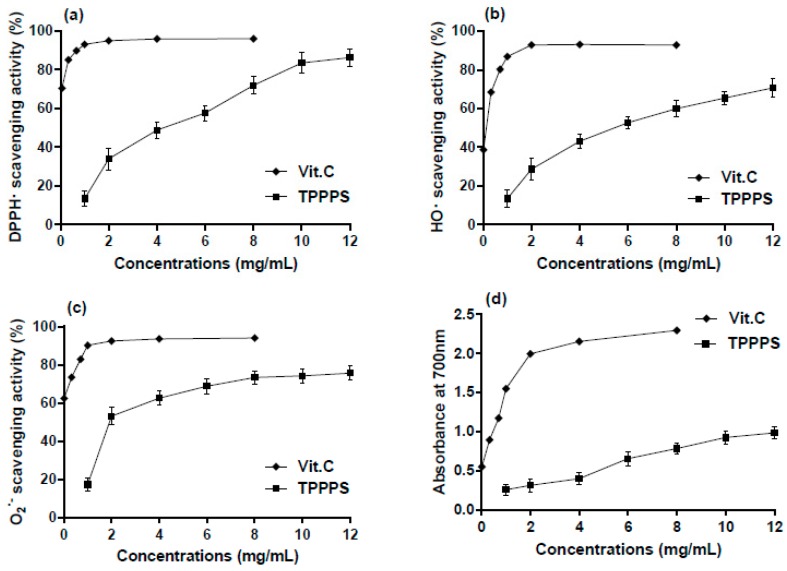
In this study, the in vitro antioxidant capacity of TPPPS was estimated with DPPH•, O2•− and HO• and reducing power systems (Figure 4).
Figure 4.
In vitro antioxidant activities of TPPPS determined using DPPH• scavenging assay (a); HO• scavenging assay (b); O2•− scavenging assay (c) and ferric reducing powder assay (d). Vit. C was used as positive control.
As shown in Figure 4a, the DPPH•-scavenging activities of TPPPS were increased along with the increase of concentration. At the concentration of 12 mg/mL the DPPH•-scavenging activity of TPPPS (86.31%) was close to that of the reference substance vitamin C (Vit. C, 94.96%). Similarly, TPPPS also exhibited obvious scavenging activity against HO• in the range of 13.43–70.71% at the concentration of 1–12 mg/mL (Figure 4b). In addition, TPPPS was also observed to possess the high ability to scavenge O2•− (Figure 4c). The scavenging ratio was 14.47% at 1 mg/mL of TPPPS, and reached 75.74% when the concentration increased to 12 mg/mL. Furthermore, the antioxidant capacity of TPPPS was assessed by means of reducing power. As expected, in the broad range of 1–12 mg/mL, the phenomenon of concentration-dependence was still obvious (Figure 4d), and the reducing power (increased absorbance at 700 nm) of TPPPS ranged from 0.20 to 0.68 in a concentration-dependent manner.
2.4. The Toxicity of TPPPS
The oral toxicity of TPPPS was determined in male Wistar rats. There was no mortality observed in all the groups. In addition, all treatments caused no significant effects on food and water intake, general signs, pathological changes of organs, hematological and biochemical parameters (data not shown). Thus, the present study confirmed that TPPPS does not cause any apparent toxicity in a rat model.
2.5. Effects of TPPPS on Body Weight, Liver Weight and Hepatosomatic Index (HI)
The effects of TPPPS on the body weight, liver weight and HI for experimental rats are summarized in Table 1. For the CCl4 (model control) group, significant increases in body weight, liver weight and HI , respectively, were observed compared to the normal control group (p < 0.05, p < 0.01). However, the CCl4-induced increases in the liver weight and HI could be decreased by the pretreatment with TPPPS at the higher doses of 200 and 400 mg/kg bw relative to the CCl4 group (p < 0.05, p < 0.01, respectively). A similar decreasing effects were also observed on both the indexes with the pretreatment of the positive control Bifendate Pills (BP) at 100 mg/kg bw. The results indicated that the administration of TPPPS to rats may generate a comparable preventive effect against CCl4-induced liver damage as BP.
Table 1.
Effects of TPPPS on body weight, liver weight and HI of CCl4-intoxicated rats.
| Treatments | Doses | Body Weight (g) | Liver Weight (g) | HI (%) |
|---|---|---|---|---|
| Normal | - | 285 ± 17 | 13.2 ± 2.7 | 4.62 ± 0.48 |
| CCl4 | - | 317 ± 15 * | 18.3 ± 2.4 ** | 5.77 ± 0.23 ** |
| CCl4 + TPPPS | 100 mg/kg | 305 ± 18 | 16.4 ± 1.9 | 5.36 ± 0.29 # |
| CCl4 + TPPPS | 200 mg/kg | 299 ± 17 | 14.8 ± 2.4 # | 4.95 ± 0.36 ## |
| CCl4 + TPPPS | 400 mg/kg | 300 ± 16 | 14.8 ± 2.6 # | 4.92 ± 0.22 ## |
| CCl4 + BP | 100 mg/kg | 299 ± 16 | 14.8 ± 1.9 # | 4.94 ± 0.56 ## |
Values are means ± SD for six rats in each group. * p < 0.05, ** p < 0.01, compared to the normal control group. # p < 0.05, ## p < 0.01, compared to the model control group.
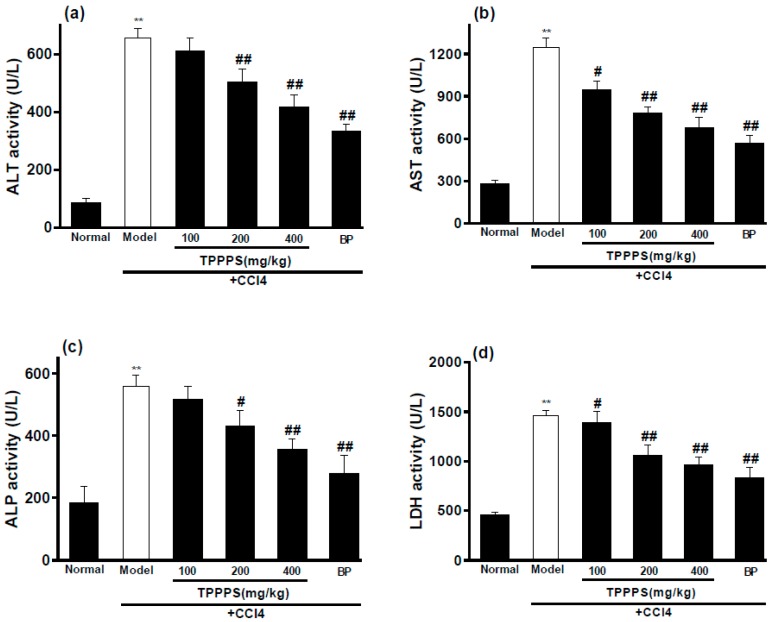
2.6. Effects of TPPPS on Activities of ALT, AST, ALP and LDH in Serum
The protective effects of TPPPS on serum ALT, AST, ALP and LDH activities are presented in Figure 5. The model control group exhibited significantly higher levels of ALT, AST, ALP and LDH in serum compared with the normal control group (p < 0.01), reflecting the tissue damages in the liver. As expected, the administration of TPPPS prior to the CCl4 intoxication effectively decreased the CCl4-induced elevations of serum enzymes (ALT, AST, ALP and LDH) in a dose-dependent manner. Specifically, at dosages of 200 and 400 mg/kg bw of TPPPS, the activities of ALT, AST, ALP and LDH were significantly decreased relative to the model control group (p < 0.05 or p < 0.01), which were close to that of the positive control BP at 100 mg/kg bw.
Figure 5.
Effects of TPPPS on the enzymic activities of ALT (a); AST (b); ALP(c) and LDH (d) in serum after treatment with CCl4 in rats. Values are expressed as means ± SD for 6 rats in each group. ** p < 0.01, compared to the normal control group. # p < 0.05, ## p < 0.01, compared to the model control group. Bifendate Pills (BP) was used as positive control.
2.7. Effects of TPPPS on the Levels of MDA, SOD and GSH-Px in Hepatic Tissue
As shown in Table 2, there was a significant increase in MDA level and decrease in the activities of SOD as well as GSH-Px in model control group compared with the normal control group (p < 0.01), suggesting stronger oxidative stress and lipid peroxidation in liver tissue. However, the administration of TPPPS decreased the level of MDA, whereas enhanced the activities of SOD and GSH-Px in a dosage-dependent manner. Especially, at the dosage of 200 and 400 mg/kg bw, the MDA level in hepatic tissue was significantly reduced, whereas the SOD and GSH-Px activities were significantly elevated in comparison with the model control group (p < 0.05 or p < 0.01). The protective effects of TPPPS were nearly as good as BP at 100 mg/kg bw.
Table 2.
Effects of TPPPS on the levels of MDA, SOD and GSH-Px in hepatic tissue of CCL4-induced liver injury in rats.
| Treatments | Dose | MDA (nmol/mg Protein) | SOD (U/mg Protein) | GSH-Px (U/mg Protein) |
|---|---|---|---|---|
| Normal | - | 2.23 ± 0.31 | 117.88 ± 16.20 | 67.20 ± 4.35 |
| CCl4 | - | 7.63 ± 1.17 ** | 45.58 ± 9.89 ** | 34.96 ± 3.76 ** |
| CCl4 + TPPPS | 100 mg/kg | 5.99 ± 1.31 # | 55.95 ± 8.64 # | 39.51 ± 4.62 |
| CCl4 + TPPPS | 200 mg/kg | 4.71 ± 0.92 # | 77.10 ± 5.34 ## | 45.56 ± 5.14 # |
| CCl4 + TPPPS | 400 mg/kg | 3.71 ± 0.48 ## | 94.74 ± 7.10 ## | 56.79 ± 8.36 ## |
| CCl4 + BP | 100 mg/kg | 4.40 ± 0.38 # | 79.27 ± 6.31 ## | 50.88 ± 3.47 ## |
Values are means ± SD for six rats in each group. ** p < 0.01, compared to the normal control group. # p < 0.05, ## p < 0.01, compared to the model control group.
2.8. Histopathological Examination of Liver Tissues
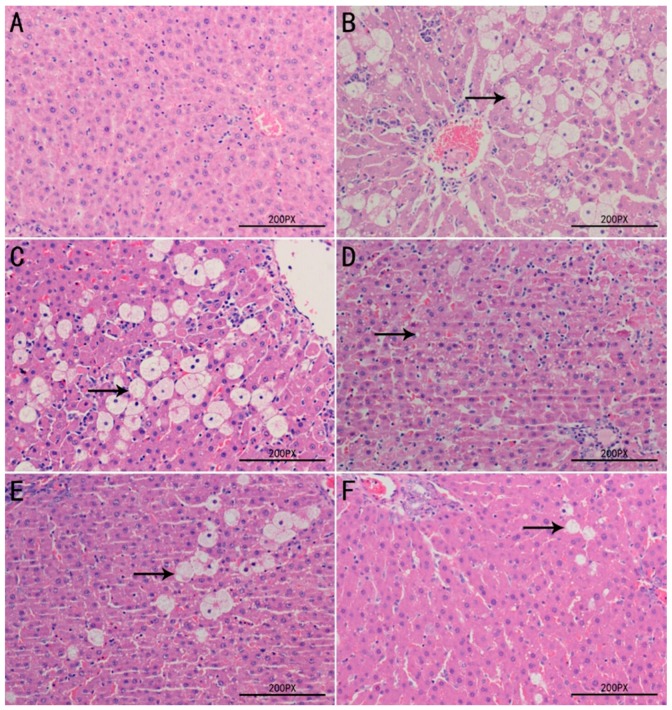
The protective effects exerted by TPPPS against CCl4–induced hepatotoxicity were further confirmed by histological assessment (Figure 6). The histological feature of the liver sections of the normal control group showed that hepatocytes were well-preserved and uniform cytoplasm, and prominent nuclei and central veins were obvious visible (Figure 6A). Compared with the normal control group, the sections of model control group revealed serious pathological changes characterized by hepatocellular degeneration and necrosis around the central vein, cytoplasmic vacuolation, inflammatory cell infiltration and loss of cellular boundaries (Figure 6B). However, as demonstrated in Figure 6C–F, the pre-administration with TPPPS remarkably ameliorated the hepatic histopathological lesions induced by CCl4 in a dose-dependent manner, and this was in good agreement with the results of biochemical assays.
Figure 6.
Effects of TPPPS on liver histopathological changes of rats using H & E staining (×200). Liver tissues of normal control group (A); Vehicle + CCl4 group (B); 100 mg/kg bw TPPPS + CCl4 group (C); 200 mg/kg bw TPPPS + CCl4 group (D); 400 mg/kg bw TPPPS + CCl4 group (E); 100 mg/kg bw BP + CCl4 group (F). Arrow indicate hepatocyte ballooning.
3. Discussion
In recent years, the potential of using antioxidants, especially the natural antioxidants, in preventing and curing diseases has attracted enormous interest. Antioxidants may have a protective effect in preventing cancer, aging, heart and liver disease or lessening their severity [21]. This protective effect is attributed to their ability to scavenge ROS, which are generated during the oxidative stress. Thus, the usefulness of antioxidants in protecting cellular components against oxidative damage is well established [22]. In the present study, it was reported for the first time the antioxidant and hepatoprotective activities of TPPPS.
In our study, the TPPPS have been successfully extracted from pulverized Pinus massoniana pollen collected from the Taishan region. Furthermore, TPPPS was characterized as an acidic heteropolysaccharide, rich in glucose (69.17%) and arabinose (10.43%) among all the monosaccharides. The infrared spectrum of TPPPS showed characteristic absorption peaks of polysaccharides. In addition, the in vitro assays to measure scavenging of DPPH•, O2•− and HO• and ferric-reducing power showed that TPPPS had a concentration-dependent antioxidant effect. The data presented here indicate that TPPPS has good antioxidant capacity and the potential to be explored as a strong antioxidant.
Many studies have showed that some natural products with antioxidant activity also have hepatoprotective activity [23]. The hepatic injury model is commonly used to detect the hepatoprotective activity of drugs in vivo [24]. CCl4-induced liver damage is the best characterized system of xenobiotic-induced hepatotoxicity and a commonly used model for the hepatoprotective drugs screening [25,26]. The mechanism of liver injury induced by CCl4 may be associated with the severity of lipid peroxidation and the depletion of antioxidant status which is caused by damage of the cell membrane and the organelles of the hepatocyte [27,28,29]. It has been reported that administration of CCl4 in rats caused the increases in ALT, AST as well as ALP activities, lipid peroxidation products, and hepatocellular necrosis, and a decrease in antioxidative enzymes [30,31]. In the present study, administering intraperitoneally (ip) with 2.0 mL/kg bw of 50% CC14 caused dramatic elevations of ALT, AST, ALP and LDH activities in serum and serious histopathological changes in liver tissue, indicating hepatotoxicity induced by administration of CC14 in rats. However, the pretreatment of TPPPS, especially at the higher dosages of 200 and 400 mg/kg bw, significantly decreased the activities of ALT, AST, ALP and LDH, suggesting that TPPPS may effectively protect hepatocytes against the toxic effects of CCl4. These results indicated that TPPPS possess the potent hepatoprotective activity in vivo.
In order to more clarify the mechanisms of hepatoprotective activity of TPPPS, the effect on hepatic antioxidant defense system was explored. It is well known that SOD and GSH-Px are two major enzymes in antioxidant-defense system of organism, and MDA is the main product of lipid peroxidation and used as an indicator of reflecting oxidative damage and antioxidation [32,33]. The results of the present study showed that administration of TPPPS significantly elevated the enzymatic activities SOD and GSH-Px, and inhibited the formation of MDA in liver tissues compared to the model control group, especially at the dosages of 200 and 400 mg/kg bw, suggesting that TPPPS possesses in vivo antioxidative activities. Histological changes in the liver can directly reflect the degree of liver injury and repair. In the present study, in agreement with the results of the biochemical parameters assay in serum and liver tissues, administration of TPPPS reduced the histopathological alteration induced by CCl4. This may be due to preventing the toxic chemical reactions from forming highly reactive radicals induced by CCl4.
4. Materials and Methods
4.1. Materials and Reagents
The Taishan Pinus massoniana pollen was collected from the Taishan Region in Shandong Province, China. The voucher specimens were deposited in the College of Veterinary Medicine & Animal Science of Shandong Agricultural University. Monosaccharide standards, 1-phenyl-3-methyl-5-pyrazolonde (PMP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid (Vit. C) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). CCl4 was purchased from Tianjin Fengchuan Chemical Reagent Science and Technology Co., Ltd. (Tianjin, China). Bifendate Pills (BP) was purchased from Beijing Union Pharmaceutical Factory (Beijing, China). Assay kits for malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals and reagents were of analytical grade.
4.2. Animals
Male Wistar rats weighing 180–200 g were purchased from the Experiment Animal Center of Academy of Military Medical Sciences (Beijing, China). The rats were housed under ideal laboratory conditions (12 h light/12 h darkness cycle, 45–55% relative humidity and temperature 23–25 °C), maintained on standard pellet diet and water ad libitum throughout the experimental period. All procedures involving animals were performed in accordance with the guidelines for the care and use of laboratory animals, as adopted and promulgated by Shandong Agricultural University Animal Care and Use Committee (Permit number: SDAUA-2014-010).
4.3. Preparation of TPPPS
Dry Taishan Pinus massoniana pollen collected from the Taishan Region in China, was passed through a 260 mesh sieve, and pulverized by an Ultra-Micro Pulverizer (Zhengxin Science and Technology Co., Ltd., Taian, China). TPPPS was extracted in our laboratory according to the method described by Wei et al. [13] with some modifications. Briefly, crude TPPPS were extracted by water decoction and one-step ethanol precipitation, in which ethanol was added to the decoction to obtain ethanol concentration of 80% (v/v). The crude TPPPS was again purified using Sevag’s method to eliminate protein [34], active carbon absorption to remove the pigment and through a Sephadex G-75 column eluted with 0.15 M NaCl solution. Then the fraction with the highest peak on the distribution curve was dialyzed, concentrated, and freeze-dried to obtain the purified TPPPS. The yield of TPPPS were calculated.
4.4. Preliminary Characterization of TPPPS
The total carbohydrate content of TPPPS was determined by a modified phenol-sulfuric acid colorimetric method with glucose as a standard [35]. Uronic acid contents were estimated using a modified sulfuric acid-carbazole method with gluconic acid as the standard [36]. In addition, proteins in the polysaccharides were determined by the method using Coomassie Brilliant Blue G-250 with bovine serum albumin as the standard [37]. The homogeneity of TPPPS were determined by the HPGPC method described by Yan et al. [38]. The monosaccharide components of the TPPPS were analyzed by reverse-phase HPLC using the PMP derivatization procedure with some modification [20]. FT-IR spectroscopy was employed to detect the main functional groups in TPPPS. The FT-IR spectrum was recorded on a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific Inc., Madison, MA, USA) in the wavenumber region of 4000–400 cm−1. The TPPPS sample was mixed and grounded with potassium bromide. The spectrum was obtained from 120 scans at 4 cm−1.
4.5. Determination of Antioxidant Activity In Vitro
The antioxidant activities of TPPPS were demonstrated by ferric reducing powder and three radicals scavenging methods reported previously, including DPPH•, O2•− and HO•. Each test was performed in triplicates.
4.5.1. Scavenging Activity on DPPH•
The assay was performed as described by Wang et al. [39] with slight modification. In brief, 1.0 mL of TPPPS solution at various concentrations (1–12 mg/mL) was mixed with 3.0 mL of 0.1 mM DPPH in aqueous methanol. The mixed solutions were measured at absorbance of 517 nm after incubation for 30 min in the dark at room temperature. The DPPH• scavenging activity (%) was calculated with the following formula: Scavenging activity (%) = [1 − (As − Ab)/A0] × 100, where A0 was the absorbance without sample, As was the absorbance with sample, and Ab was the absorbance of ground color. Vit. C was used as positive control.
4.5.2. Scavenging Activity on HO•
The scavenging activity of TPPPS on HO• was measured according to the method described by Huang et al. [40]. In brief, 1.0 mL TPPPS solution at various concentrations (1–12 mg/mL) were incubated with FeSO4 (1 mL, 4 mM), salicylic acid-ethanol (1 mL, 6 mM), and H2O2 (1 mL, 2 mM) at 37 °C for 60 min, and then the absorbance at 510 nm was measured. The scavenging activity was calculated by the following equation: Scavenging activity (%) = [1 − (A1 − A2)/A0] × 100, where A0 was the absorbance of the control, A1 was the absorbance of the sample and A2 was the absorbance without H2O2. Vit. C was used as positive control.
4.5.3. Scavenging Activity on O2•−
The activities of TPPPS to scavenge O2•− were determined using the method previously reported [41]. 1.0 mL nitroblue tetrazolium (NBT), 1.0 mL reduced nicotinamide adenine dinucleotide (NADH), 1.0 mL TPPPS solution at various concentrations (1–12 mg/mL) and 0.4 mL phenazine methosulfate (PMS) were successively added into test tube, then the reaction mixture was incubated at room temperature for 5 min and the absorbance of the mixture solution was determined at 560 nm against blank (mixture without PMS was used as blank). The scavenging capability of TPPPS was calculated according to the following formula: Scavenging activity (%) = [1 − (Abs. of sample − Abs. of blank)/Abs. of control] × 100. Vit. C was used as positive control.
4.5.4. Measurement of Reducing Power
The reducing power of TPPPS was measured according to the method of Tian et al. [19]. Briefly, 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% (w/v) K3Fe(CN)6 solution were added to 1.0 mL of TPPPS solution at various concentrations (1–12 mg/mL). The mixture was incubated at 50 °C for 20 min, and then 2.5 mL 10% (w/v) trichloroacetic acid (TCA) solution was added, and the mixture was further centrifuged at 3000 r/min for 10 min. The absorbance at 700 nm was measured immediately. An enhanced absorbance of the reaction mixture indicated a high reducing power. Vit. C was used as a positive control.
4.6. Determination of the Toxicity of TPPPS
Male Wistar rats weighing 180–200 g was randomly divided into seven groups of five animals each. The normal control group received saline (10 mL/kg, oral) and other groups received 50, 100, 200, 400, 800 and 1600 mg/kg of TPPPS dispersion in saline. Food and water intake, general signs, and mortality were observed continuously for 14 days. Blood samples and pathology of organs were evaluated at day 14.
4.7. Determination of Hepatoprotective Activity In Vivo
4.7.1. CCl4-induced Hepatotoxicity Experiment
After a 7-day acclimatization period, a total of 36 rats were randomly divided into six groups of six rats each. For the normal and model control groups, rats were given a single dose of physiological saline (2.0 mL) once daily intragastrically (ig). For the BP (positive) group, rats received 100 mg/kg bw of reference drug BP (2.0 mL, ig) once daily. For the TPPPS-treated groups, rats were given 100, 200, or 400 mg/kg bw of TPPPS (2.0 mL, ig) once daily, respectively. All the administrations were conducted at between sixteen and seventeen o’clock for 14 consecutive days. On the 15th day, all the rats except the normal control group were given a 50% CCl4/peanut oil mixture (v/v, 2.0 mL/kg bw) intraperitoneally (ip) to induce hepatic injury, while the normal control group received peanut oil alone. After 2 h, all the rats were fasted with only water provided for 24 h. And then all of the rats were weighed and anesthetized by pentobarbital sodium (40 mg/kg bw, ip). Blood samples were immediately collected from the heart, centrifuged to obtain sera, and stored at −70 °C for further biochemical analysis. Livers were immediately taken out and weighted after rinsing by ice-cold physiological saline and then stored at −80 °C before use. Hepatosomatic index (HI) was calculated according to the following formula: HI = liver weight/body weight × 100%.
4.7.2. Serum Biochemical Indicators Assay
The activities of ALT, AST, ALP and LDH were determined with an Automatic Biochemical Analyzer (AU2700, Olympus, Kanagawa, Japan) using diagnostic reagent kits. All the experiments were conducted in triplicates, and the average counts were obtained from each individual sample.
4.7.3. Antioxidative Indicators Assay of Liver Homogenate
The liver samples were homogenized in 9-fold volume cold physiological saline, and then the homogenates were centrifuged at 1500× g for 10 min. The clear supernatant fraction was used for SOD, GSH-Px and MDA analysis with the kits according to the instructions. The protein concentration in homogenates was measured by the method of Coomassie Brilliant Blue with bovine serum albumin as a standard [37].
4.7.4. Histopathological Examination of Liver
Some parts of liver tissues were fixed with a 4% paraformaldehyde solution at 4 °C for 24 h. Fixed liver tissues were embedded in paraffin wax, cut into sections (~5 μm) and stained with hematoxylin-eosin (H & E) solution. The slides were observed under an Olympus® light microscope (Olympus Corporation, Tokyo, Japan) and photographed.
4.8. Statistical Analysis
The data were expressed as means ± standard deviation (SD) and evaluated by one-way analysis of variance followed by the LSD-test using the SPSS 18.0 system (SPSS Inc., Chicago, IL, USA). The significant difference was considered at p < 0.05, and extremely significant difference was considered at p < 0.01.
5. Conclusions
In this study, TPPPS displayed overall antioxidant activity against in vitro oxidative stress as well as in vivo CCl4-induced oxidative hepatotoxicity in rat. The hepatoprotective activity of TPPPS may, at least partly, be due to its free radical-scavenging activity, enhancing the antioxidant capacity of the liver. Our findings provide a base for further exploitation of TPPPS as a novel hepatoprotective pharmaceutical ingredient. Further work on the structure, function and mechanisms of action is in progress.
Acknowledgments
The project was supported by the Shandong Natural Science Foundation of China (ZR2016CM26) and National Natural Science Foundation of China (3177130834) and Funds of Shandong “Double Tops” Program.
Author Contributions
Zhengui Yan and Ruiliang Zhu conceived and designed the experiments; Changming Zhou and Shaojie Yin performed the experiments and analyzed the data; Zhongfang Yu, Yuxiang Feng, Kai Wei, Weiming Ma and Lijiang Ge contributed reagents/materials/analysis tools; Changming Zhou and Zhengui Yan wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Valle L.G. Oxidative stress in aging: The oretical outcomes and clinical evidences in humans. Biomed. Aging Pathol. 2011;1:1–7. doi: 10.1016/j.biomag.2011.03.001. [DOI] [Google Scholar]
- 3.Sánchez-Valle V., Chavez-Tapia N.C., Uribe M., Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 4.Marczak A., Bukowska B. ROS production and their influence on the cellular antioxidative system in human erythrocytes incubated with daunorubicin and glutaraldehyde. Environ. Toxicol. Pharmacol. 2013;36:171–181. doi: 10.1016/j.etap.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Bruck R., Aeed H., Avni Y., Shirin H., Matas Z., Shahmurov M., Avinoach I., Zozulya G., Weizman N., Hochman A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J. Hepatol. 2004;40:86–93. doi: 10.1016/S0168-8278(03)00504-X. [DOI] [PubMed] [Google Scholar]
- 6.Carocho M., Ferreira I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Kardošová A., Machova E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006;77:367–373. doi: 10.1016/j.fitote.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Fan J., Wu Z., Zhao T., Sun Y., Ye H., Xu R., Zeng X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr. Polym. 2014;101:990–997. doi: 10.1016/j.carbpol.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Chen H., Ju Y., Li J., Yu M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012;50:214–218. doi: 10.1016/j.ijbiomac.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Yang S., Wei K., Jia F., Zhao X., Cui G., Guo F., Zhu R. Characterization and Biological Activity of Taishan Pinus massoniana Pollen Polysaccharide In Vitro. PLoS ONE. 2015;10:e0115638. doi: 10.1371/journal.pone.0115638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X., Sun X., Yu Z. Effective Components and Pharmacological Function of Pine Pollen. J. Northeast For. Univ. 2007;35:78–80. [Google Scholar]
- 12.Lee K.H., Kim A.J., Choi E.M. Antioxidant and antiinflammatory activity of pine pollen extract In Vitro. Phytother. Res. 2009;23:41–48. doi: 10.1002/ptr.2525. [DOI] [PubMed] [Google Scholar]
- 13.Wei K., Sun Z., Yan Z., Tan Y., Zhu X., Wang X., Sheng P., Zhu R. Effects of Taishan Pinus massoniana pollen polysaccharide on immune response of rabbit haemorrhagic disease tissue inactivated vaccine and on production performance of Rex rabbits. Vaccine. 2011;29:2530–2536. doi: 10.1016/j.vaccine.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Cui G., Zhong S., Yang S., Zuo X., Liang M., Sun J., Liu J., Zhu R. Effects of Taishan Pinus massoniana pollen polysaccharide on the subunit vaccine of Proteus mirabilis in birds. Int. J. Biol. Macromol. 2013;56:94–98. doi: 10.1016/j.ijbiomac.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X., Liang M., Yang P., Guo F., Pan D., Huang X., Li Y., Wu C., Qu T., Zhu R. Taishan Pinus massoniana pollen polysaccharides promote immune responses of recombinant Bordetella avium ompA in BALB/c mice. Int. Immunopharmacol. 2013;17:793–798. doi: 10.1016/j.intimp.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Guo F., Xue C., Wu C., Zhao X., Qu T., He X., Guo Z., Zhu R. Immunoregulatory effects of Taishan Pinus massoniana pollen polysaccharide on chicks co-infected with avian leukosis virus and Bordetella avium early in ovo. Res. Vet. Sci. 2014;96:260–266. doi: 10.1016/j.rvsc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M., Yang N., Yang B., Jiang Y., Zhang G. Structural characterization of water-soluble polysaccharides from Opuntia monacantha cladodes in relation to their anti-glycated activities. Food Chem. 2007;105:1480–1486. doi: 10.1016/j.foodchem.2007.05.031. [DOI] [Google Scholar]
- 18.Maciel J.S., Chaves L.S., Souza B.W., Teixeira D.I., Freitas A.L., Feitosa J.P., de Paula R.C. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydr. Polym. 2008;71:559–565. doi: 10.1016/j.carbpol.2007.06.026. [DOI] [Google Scholar]
- 19.Tian L., Zhao Y., Guo C., Yang X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011;83:537–544. doi: 10.1016/j.carbpol.2010.08.023. [DOI] [Google Scholar]
- 20.Wu M., Xu L., Zhao L., Xiao C., Gao N., Luo L., Yang L., Li Z., Chen L., Zhao J. Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs. 2015;13:2063–2084. doi: 10.3390/md13042063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L.-C., Ho J.-A., Shieh M.-C., Lu I.-W. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J. Agric. Food. Chem. 2005;53:4207–4212. doi: 10.1021/jf0479517. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Wahhab M.A., Abdel-Galil M.M., El-Lithey M. Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J. Pineal Res. 2005;38:130–135. doi: 10.1111/j.1600-079X.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Hiraganahalli B.D., Chinampudur V.C., Dethe S., Mundkinajeddu D., Pandre M.K., Balachandran J., Agarwal A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 2012;8:116–123. doi: 10.4103/0973-1296.96553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian L.-H., Wu Y.-L., Song S.-Z., Wan Y., Xie W.-X., Li X., Bai T., Ouyang B.-Q., Nan J.-X. Gentiana manshurica Kitagawa reverses acute alcohol-induced liver steatosis through blocking sterol regulatory element-binding protein-1 maturation. J. Agric. Food. Chem. 2010;58:13013–13019. doi: 10.1021/jf103976y. [DOI] [PubMed] [Google Scholar]
- 25.Hassan H.H., El-Banna S.G., Elhusseiny A.F., Mansour E.-S.M. Antioxidant Activity of New Aramide Nanoparticles Containing Redox-Active N-phthaloyl Valine Moieties in the Hepatic Cytochrome P450 System in Male Rats. Molecules. 2012;17:8255–8275. doi: 10.3390/molecules17078255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston D.E., Kroening C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Basic Clin. Pharmacol. Toxicol. 1998;83:231–239. doi: 10.1111/j.1600-0773.1998.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 27.Singh B., Saxena A., Chandan B., Anand K., Suri O., Suri K., Satti N. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia. 1998;69:135–140. [Google Scholar]
- 28.Cheng D., Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16:2542–2550. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karakus E., Karadeniz A., Simsek N., Can I., Kara A., Yildirim S., Kalkan Y., Kisa F. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in liver of rats treated with carbon tetrachloride (CCl4) J. Hazard. Mater. 2011;195:208–213. doi: 10.1016/j.jhazmat.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Yan F., Zhang Q.Y., Jiao L., Han T., Zhang H., Qin L.P., Khalid R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomed. Int. J. Phytother. Phytopharmacol. 2009;16:805–813. doi: 10.1016/j.phymed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Charles A.L., Huang T.C. Sweet cassava polysaccharide extracts protects against CCl4 liver injury in Wistar rats. Food Hydrocoll. 2009;23:1494–1500. doi: 10.1016/j.foodhyd.2008.08.011. [DOI] [Google Scholar]
- 32.Maheshwari D., Kumar M.Y., Verma S.K., Singh V.K., Singh S.N. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2011;49:2422–2428. doi: 10.1016/j.fct.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 33.Sigala F., Kostopanagiotou G., Andreadou I., Kavatzas N., Felekouras E., Sigalas P., Bastounis E., Papalambros E. Histological and lipid peroxidation changes after administration of 2-acetylaminofluorene in a rat liver injury model following selective periportal and pericentral damage. Toxicology. 2004;196:155–163. doi: 10.1016/j.tox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Zha X.-Q., Xiao J.-J., Zhang H.-N., Wang J.-H., Pan L.-H., Yang X.-F., Luo J.-P. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012;134:244–252. doi: 10.1016/j.foodchem.2012.02.129. [DOI] [Google Scholar]
- 35.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 36.Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 37.Lott J.A., Stephan V.A., Pritchard K.A., Jr. Evaluation of the Coomassie Brilliant blue G-250 method for urinary protein. Clin. Chem. 1983;29:1946–1950. [PubMed] [Google Scholar]
- 38.Yan Z.H., Fan R.F., Yin S.J., Zhao X.N., Liu J.Z., Li L.H., Zhang W.Q., Ge L.J. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015;80:573–580. doi: 10.1016/j.ijbiomac.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 39.Wang D.Y., Zhao Y., Jiao Y.D., Yu L.H., Yang S., Yang X.B. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 2012;88:1453–1459. doi: 10.1016/j.carbpol.2012.02.046. [DOI] [Google Scholar]
- 40.Huang X.Q., Tu Z.C., Jiang Y., Xiao H., Zhang Q.T., Wang H. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of lentinan. Int. J. Biol. Macromol. 2012;51:926–932. doi: 10.1016/j.ijbiomac.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Chen S.K., Tsai M.L., Huang J.R., Chen R.H. In vitro antioxidant activities of low-molecular-weight polysaccharides with various functional groups. J. Agric. Food Chem. 2009;57:2699–2704. doi: 10.1021/jf804010w. [DOI] [PubMed] [Google Scholar]