Abstract
Traditional Chinese medicine has been practiced for centuries in East Asia. Herbs are used to maintain health and cure disease. Certain Chinese herbs are known to protect and improve the brain, memory, and nervous system. To apply ancient knowledge to modern science, some major natural therapeutic compounds in herbs were extracted and evaluated in recent decades. Emerging studies have shown that herbal compounds have neuroprotective effects or can ameliorate neurodegenerative diseases. To understand the mechanisms of herbal compounds that protect against neurodegenerative diseases, we summarize studies that discovered neuroprotection by herbal compounds and compound-related mechanisms in neurodegenerative disease models. Those compounds discussed herein show neuroprotection through different mechanisms, such as cytokine regulation, autophagy, endoplasmic reticulum (ER) stress, glucose metabolism, and synaptic function. The interleukin (IL)-1β and tumor necrosis factor (TNF)-α signaling pathways are inhibited by some compounds, thus attenuating the inflammatory response and protecting neurons from cell death. As to autophagy regulation, herbal compounds show opposite regulatory effects in different neurodegenerative models. Herbal compounds that inhibit ER stress prevent neuronal death in neurodegenerative diseases. Moreover, there are compounds that protect against neuronal death by affecting glucose metabolism and synaptic function. Since the progression of neurodegenerative diseases is complicated, and compound-related mechanisms for neuroprotection differ, therapeutic strategies may need to involve multiple compounds and consider the type and stage of neurodegenerative diseases.
Keywords: traditional Chinese medicine, herbal compounds, neuroprotection agent, neurodegenerative disease, cytokine regulation, autophagy, ER stress, glucose metabolism, synaptic function
1. Introduction
Neurodegenerative diseases result in the progressive degeneration or death of neurons, which cannot regenerate. The loss of neurons can cause behavioral or cognitive deficits, or both. Neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and spinocerebellar atrophy (SCA), are closely related to protein aggregations [1]. There are two major types of protein aggregates in AD: the β-amyloid (Aβ) peptide derived from the amyloid precursor protein (APP), and tau protein aggregates, both of which can lead to death of neurons [2]. PD is characterized by misfolded protein aggregates. The abnormal α-synuclein protein that forms Lewy body aggregates is broadly distributed in brain regions [3,4]. ALS is caused by motor neuron degeneration in the spinal cord and cortex. Some evidence has shown that ubiquitinated proteins can accumulate in motor neurons leading to cell death [5]. SCA is a kind of polyglutamine (polyQ) disorder disease, and polyQ aggregates can cause transcriptional dysregulation leading to cellular toxicity [6].
Traditional Chinese medicine (TCM) has been practiced for centuries in East Asia. Herbs and herbal preparations are used to maintain health and cure disease. In the past few years, Chinese herbal medicines have been studied in different fields. Some Chinese herbs are known to protect and improve the brain, memory, and nervous system. However, the ingredients in various herbs are complex, and the active components in herbs must be identified to apply ancient knowledge to modern science. The extraction and analysis of components in herbs have increased in recent years [7], and many major natural therapeutic compounds in herbs have been discovered, extracted, and analyzed in recent decades [8]. Several Chinese herbs have been used to attenuate the progression of neurodegenerative diseases, and some of their active components have been identified [9,10]. After discovering an active herbal compound, the actual mechanism of the active components must be investigated.
To understand how herbal compounds attenuate neurodegenerative diseases, we summarized studies that investigated the role of herbal compounds in various neurodegenerative disease models. We categorized the compound-related mechanisms into various categories, including regulation of cytokines, autophagy, endoplasmic reticulum (ER) stress, glucose metabolism, and synaptic function.
2. Chinese Herbal Drugs for Neuroprotection via Cytokine Regulation
2.1. Neuroinflammation in the Nervous System
Neuroinflammation is usually induced by cytokines and can lead to neuronal death. In the central nervous system (CNS), microglial cells are the source of inflammatory cytokines. Proinflammatory cytokines, including interleukins (ILs), transforming growth factor (TGF)-β1, and tumor necrosis factor (TNF)-α usually make neurodegenerative disease worse. IL-1β induce neuronal injury not only by producing reactive oxygen species (ROS), but also by activating the microglial cell. It can also induce increased glycogen consumption in astrocytes, leading to elevated levels of toxic substances, thereby affecting cellular metabolism [11,12]. In addition, IL-1β and TNF-α induce neurotoxicity through glutamate production [13].
2.2. Cytokines and Neuronal Diseases
Neurodegenerative diseases, including AD and PD, are associated with chronic inflammatory responses [14]. Analysis of cerebrospinal fluid from patients with AD suggested that the IL-12 and IL-23 signaling pathways are activated [15]. A recent study showed that the gut microbiota regulates motor deficits and neuroinflammation in a PD model, and expressions of two proinflammatory cytokines increased in the presence of host microbiota [16]. A meta-analysis also showed higher peripheral levels of ILs in PD patients [17]. In animal study, NLRP3 (an inflammasome sensor)-deficient APP/PS1 AD mice showed improved Aβ clearance by microglia, lower Aβ-induced IL-1β formation in the brain, and decreased the Aβ-induced suppression of synaptic plasticity [18]. Furthermore, ILs can modulate the size of ischemic damage in a rodent stroke model as also found in neurodegenerative diseases [19]. IL-1, IL-6, TNF-α, and C-reactive protein (CRP) are potential targets of stroke therapy. Studies have reported that these cytokines are associated with depression [20,21,22]. According to these evidences, regulating inflammatory cytokines could be a strategy to treat neurodegenerative diseases, stroke, and mental illness. Therefore, we list some herbs that attenuate the progression of neurological disease progression through cytokine regulation.
2.3. Chinese Herbaldrugs that Benefit Neuronal Diseases via Cytokine Regulation
Isobavachalcone and paeonol are the main components of the herbs Psoralea corylifolia and Cortex Moutan, which were applied for PD therapy. In a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model, both isobavachalcone and paeonol inhibited the overactivation of microglia, and decreased expressions of ILs and also prolonged the residence time of mice on a Rota-rod and alleviated neuronal necrosis [23,24].
Icariin was an anti-inflammation compound extracted from Chinese herb Epimedium segittatum and worked on several neuronal diseases involved in inflammatory response. In an AD animal model, icariin significantly attenuated Aβ deposition, microglial activation, and TGF-β1 immunoreactivity at amyloid plaques and restored impaired nesting ability [25]. Icariside (ICS) II, a novel phosphodiesterase 5 inhibitor derived from the herb Epimedium brevicornum, and tetrandrine, a bisbenzylisoquinoline alkaloid isolated from the herbal radix Stephania tetrandra, both inhibit inflammatory factors and improve cognitive deficits in AD animal models [26,27]. ICS II decreased levels of Aβ1-40 and Aβ1-42 and inhibited inflammatory factors, including IL-1β, TNF-α, cyclooxygenase (COX)-2, and TGF-β1, in AD. Tetrandrine treatment inhibit nuclear factor kappaB (NF-κB) activity and downregulate IL-1β and TNF-α expression.
Stroke is highly correlated with post infarction inflammation. For anti-inflammation, icariin was not only applied to AD but also stroke therapy. In an animal stroke model, icariin pretreatment reduced cytokine levels (IL-1β and TGF-β1), decreased a neurological deficit score, and reduced the infarct volume [28]. Ampelopsin (AMP), extracted from Ampelopsis grossedentata, attenuated neurological deficits and reduced infarct volumes, brain edema, immunoglobulin G (IgG) exudation, and neuron degeneration by inhibition of the middle cerebral arterial occlusion-induced IL-1β and TNF-α release in animal model [29]. Eriodictyol extracted from the herb Dracocephalum rupestre reduced TNF-α, inducible nitric oxide (NO) synthase (iNOS), and glial fibrillary acidic protein (GFAP) expressions, as well as prevented neuronal death, reduced the infarct area, and ameliorated neurological and memory deficits [30]. Ruscogenin isolated from the herb Ophiopogon japonicas decreased the infarct size and improved neurological deficits by NF-κB inhibition that suppresses iNOS, COX-2, TNF-α, and IL-1β [31]. The 6-hydroxycleroda-3,13-dien-15,16-olide (PL3) has been extracted from Polyalthia longifolia var. pendula and was reported to inhibits microglia-mediated inflammation and inflammation-related neuronal cell death by inhibition of the NF-κB signaling pathway [32].
For mental illness, berberine, a major constituent alkaloid isolated from Coptis chinensis, prevents depressive-like behaviors in mice by suppressing IL-1β, IL-6, and TNF-α expression levels, as well as microglial activation [33]. Icariin also exerts antidepressant-like effects in rats with mild chronic stress, which may be mediated by an enhanced anti-inflammatory effects by inhibition of NF-κB signaling and the NLRP3-inflammasome/caspase-1/IL-1β axis [34].
2.4. Brief Summary of Chinese Herbal Compounds on Neuroinflammation
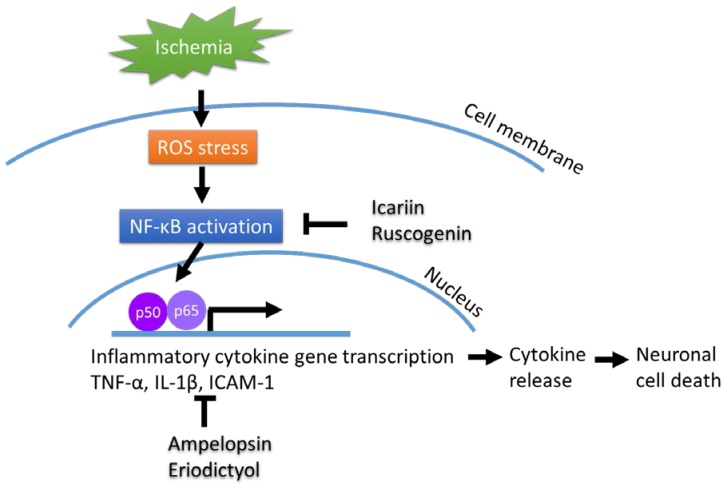
In summary, Chinese herbs that regulate the NF-κB signaling pathway and its downstream targets, such as IL-1β and TNF-α, usually attenuate inflammatory responses and protect against neuronal death (Figure 1), suggesting that treatment with these herbs may provide potential therapies for neurodegenerative diseases.
Figure 1.
Chinese herbal compound decrease NF-κB activation and cytokines release after ischemia injury.
3. Chinese Herbal Drugs for Neuroprotection via Autophagy
3.1. The Importance of Regulating Autophagy in the Nervous System
Autophagy, which has garnered much attention in the past few years, is an intracellular recycling and metabolic process that is critical in different areas of cell physiology. Cytosolic components can be engulfed by the cell membrane and sequestered. After formation of autophagosome, the sequestered organelles and cell debris are delivered to a lysosome; then, the outer membrane of the autophagosome fuses with the lysosome to maintain homeostasis by lysosomal hydrolase [35]. This ensures a dynamic balance between the production and degradation of molecules and cell flow, and any damage to this important intracellular route results in a variety of different diseases such as neurodegenerative diseases [36,37,38], metabolic-related disorders [39], cancer [40], and cardiovascular and pulmonary diseases [41,42].
3.2. Autophagy Dysregulation and Neurodegenerative Diseases
It is well-known that many neurodegenerative diseases involve protein aggregation and deposition, such as AD, PD, ALS, and polyQ diseases [43]. Clearance of these abnormal proteins may be the key point to slow down disease progress. Therefore, regulation of autophagy can be a strategy to improve the pathology of neurodegenerative diseases by intracellular cleaning and inhibiting the accumulation of misfolded proteins. Although autophagy plays an important role in neurodegenerative diseases, the detailed mechanism remains unclear.
3.3. Chinese Herbal Compounds Promote the Regulation of Autophagy and Improve Neurodegenerative Diseases
Wang et al. summarized autophagy modulators from Chinese herbs for neurodegenerative diseases in the Journal of Ethnopharmacology [44]. The study’s authors collected literature from scholarly databases published during a nine-year period (2007–2015) and investigated the effects of Chinese herbal compounds or their natural extracts on regulation of autophagy. We summarize some autophagy regulators from Chinese herbs in terms of the therapeutic effects on neurodegenerative disease, and it can provide new pharmacological applications for drug development.
Overall, a number of articles have been published on modulation of autophagy by Chinese herbal medicine in the past five years. The dual roles of up- or downregulation of autophagy by natural compounds for neuroprotection have been revealed. The impairment of autophagolysosome formation and maturation may contribute to the gradual accumulation of Aβ and phosphorylated tau proteins in AD. It has been reported that resveratrol, luteolin, berberine, Thamnolia vermicularis extract, carnosic acid, and ginsenoside compound K derived from different Chinese herbs promote clearance of Aβ accumulations [45,46,47,48,49,50], inhibit the production of hyperphosphorylated tau, and improve the behavioral performance via the adenosine monophosphate-activated protein kinase (AMPK)-dependent, phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), and AMPK/raptor/mTOR signaling pathways. In contrast, autophagy has been shown to be necessary for apoptotic cell death, placing it upstream of apoptosis, which means that autophagy can induce apoptosis, leading to cell death. In this way, it was reported that triptolide significantly reduces cytotoxicity and apoptosis by inhibiting the autophagic pathway in PC12 cells [51].
Studies have demonstrated that autophagy is also involved in the pathogenesis of PD. Baicalein, hederagenin, carnosic acid, sulforaphane, piperine, triptolide, conophylline, and resveratrol [52,53,54,55,56,57,58,59,60] significantly upregulate autophagy to prevent behavioral deficits, attenuate dopaminergic neuronal loss, neuronal apoptosis, and mitochondrial dysfunction, suppress oligomerized α-synuclein, and restore the interactions of parkin and beclin-1 via the AMPK-mTOR signaling cascade and heme oxygenase-1, LC3II, and p62 signaling. In contrast, oleuropein, baicalein, and cucurbitacin E decreased neuronal death and autophagic flux by inhibiting autophagic initiation in 6-OHDA or MPP+-infused substantia nigra (SN) PD models [53,61,62].
In ALS, Berberine upregulated the deregulated mTOR/p70S6K signal and activated an autophagic degradation pathway to promote clearance of TDP-43 [63]. On the other hand, n-butylidenephthalide (Bdph) downregulated autophagy by enhancing p-mTOR, decreasing LC3II to prolong the lifespan, and attenuating motor neuron loss in ALS mice [64,65].
The roles of Chinese herbal medicines in other neurodegenerative diseases were published in the past three years. Sulforaphane, neferine, conophylline, and resveratrol eliminated mutant Huntingtin (Htt) aggregates [58,66,67,68], and cleared Htt protein and inclusions against mutant Htt or CAG repeats by activating AMPK-mTOR signaling. Both breviscapine and ginsenoside Rb1 extracted from Chinese herbs played neuroprotective roles by downregulating autophagy. Breviscapine reduced infarct volumes and neuro-functional deficiencies in a cerebral ischemic rat model [69], and ginsenoside Rb1 decreased the loss of motor neurons and promoted functional recovery in a spinal cord injury model [70]. AMP inhibited D-gal-induced apoptosis and rescued impaired autophagy of neurons by upregulating SIRT1 and downregulating the mTOR signal pathways [71].
3.4. Brief Summary of Chinese Herbal Compounds Involved in Regulating Autophagy
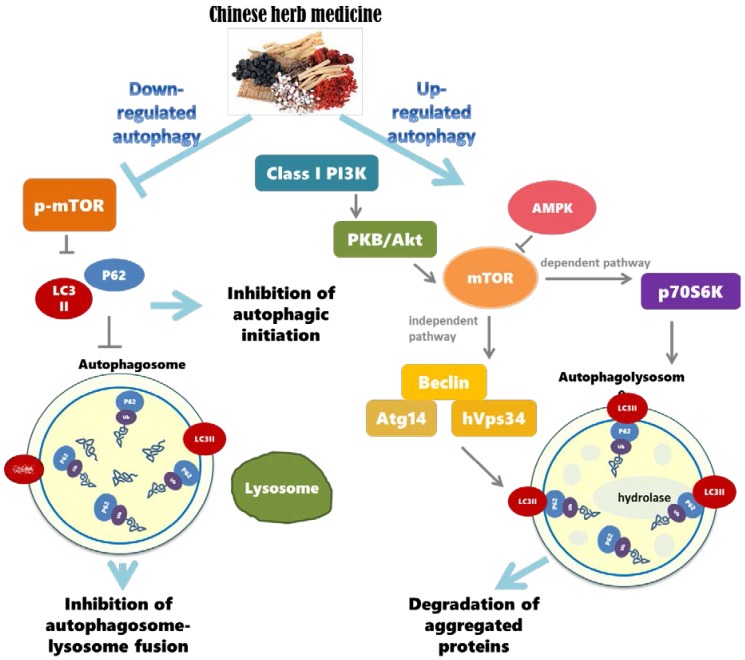
Many studies have investigated the regulation of autophagy by extracts of Chinese herbal medicines. Results suggest that herbal compounds derived from Chinese herbal medicines are able to protect the functions of neurons against mutant proteins and protein aggregates. These extracts may regulate autophagy via various pathways, especially the PI3K/AKT/mTOR pathway. The mTOR is an important key for autophagy, and most studies reported that those extracts from Chinese herbal medicines may regulate autophagy via mTOR signaling (Figure 2). Results indicated that herbal compounds extracted from plants hold great potential for treating neurodegenerative diseases, and targeting the modulation of autophagy for therapeutic effects is a great strategy for neuroprotection.
Figure 2.
Autophagy regulation by Chinese herb medicine.
4. Chinese Herbal Drugs for Neuroprotection via Reduced ER Stress
4.1. ER Function and Maintenance of Neurons
The ER is a multifunctional organelle, involved in lipid and sterol synthesis, protein post-translational modifications, and protein transport [72]. The neuronal ER also mediates the Ca2+ intracellular signal, producing local or global cytosolic calcium in neurons [73]. Neurons are highly bipolar cells with very long dendrites and axons. The shape of the ER in neurons is between those of dendrites and axons. ER tubules extend along microtubules to build up the transport network [74]. The neuronal ER network is dynamically remodeled during neuronal development. During dendrite and axon developmental processes, the ER rapidly extends bidirectionally and retains protein trafficking from the soma [75]. The neuronal ER in soma and dendrites has an abundant ribosomal distribution [76]. The dendritic ER structure is modulated by external cellular signals, when type I metabotropic glutamate receptors are activated [77], and the axonal ER is rich in Ca2+ ATPase and IP3R [78]. Thus, the ER is a very important organelle during neuron development.
4.2. The Role of ER Stress in Neuronal Diseases
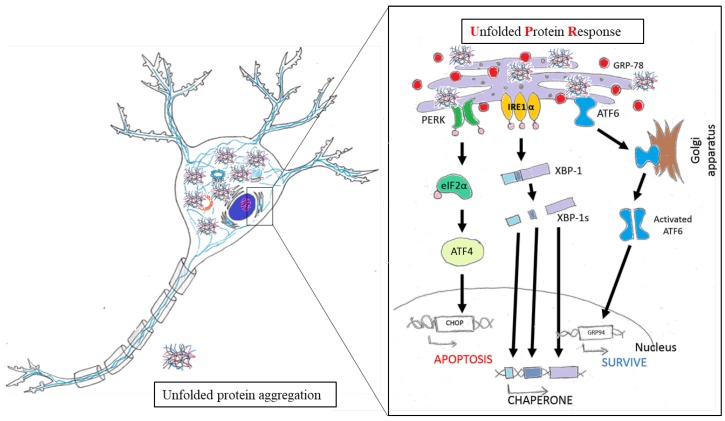
Most neurodegenerative diseases are caused by misfolded protein accumulation. Proteins that accumulate abnormally in the ER generate stressful conditions termed ER stress. When a cell faces this situation, it activates the unfolded protein response (UPR) [79]. In the initial stage, the UPR is regulated by three ER stress sensors: inositol-requiring protein-1 (IRE1), activating transcription factor-6 (ATF6), and protein kinase RNA (PKR)-like ER kinase (PERK). When the UPR is activated, neurons downregulate protein translation, enhance translation of chaperone protein, and begin to produce chaperones and other components for the accurate folding of proteins [80,81]. However, when facing prolonged ER stress, the cell activates the UPR-apoptosis process. C/EBP homologous protein (CHOP) and caspase-12 are induced by ATF4 and X box-binding protein-1 (XBP-1) [82]. In AD, overexpressed Aβ forms oligomers, which accumulate in the ER lumen and activate the proapoptotic ER stress response. Brains of AD show high expressions of UPR markers, such as GRP78 and p-PERK, in the cortex and hippocampal region [83,84,85,86]. Similarly, α-synuclein accumulates in the ER then disrupts protein transport from ER to Golgi in PD [87,88]. In ALS, mutant SOD1 was observed in the ER and was found to be associated with motor neuron death [89,90,91,92]. According to those studies, UPR signaling plays an important role in neuroprotection (Figure 3). Thus, discovering new compounds to reduce ER stress and restore ER function is very important.
Figure 3.
Schematic representation of the unfolded protein response pathway.
4.3. Chinese Herbal Drugs Reduce ER Stress and Benefit Nervous System
Astragaloside IV has been extracted from the roots of Astragalus membranaceus, and was found to inhibit the ER stress apoptosis pathway [93]. Astragaloside IV also promoted neurite outgrowth and increased survival of dopaminergic neurons in a PD model [94]. Baicalein, which is isolated from roots of Scutellaria baicalensis and S. lateriflora, were previously introduced in the autophagy section, also have potential to apply for the treatment of PD via ER stress regulation. It inhibits CHOP expression in the HT-22 cell line and rotenone-induced apoptosis in dopaminergic SH-SY5Y cells by inhibiting brefeldin A (BFA)-induced CHOP, GRP78, ATF6, and p-eIFα expressions, and reducing apoptotic caspase-3 and caspase-12 splicing [95,96]. Crocin, isolated from saffron, has been used in TCM for decades. Crocin attenuates MPP(+)-induced cell apoptosis by inhibiting CHOP expression and the Wnt signaling pathway in PC12 PD cell model [97]. Crocin also inhibited the effect of tau protein aggregation in AD in an in vitro model [98]. Interestingly, heroin-addicted rats showed significant downregulation of PERK, eIF2a, and CHOP expressions and a reduction in neuron apoptosis [99]. Taken together, ER stress in neural diseases is well connected with herbal compound treatment.
4.4. Brief Summary of Chinese Herbal Drugs Involved in ER Stress
Unfolded protein response pathway is highly involved in the first step of neurodegenerative diseases, especially in amyloid triggered diseases including AD, PD, ALS, and Huntington disease (HD). According to these evidences, there are two candidate pathways for treating neurodegenerative diseases. One is to inhibit the ER stress-activated apoptotic pathway, and the other one is to enhance chaperone protein translation. However, there are still few Chinese herbs derived pure compounds were identified to improve ER function in neurodegenerative models. Only astragaloside IV, baicalein, and crocin were demonstrated to reduce ER stress or ER-stress-induced apoptosis directly and benefit neuron survival. Therefore, there is still great potential to study the herb-derived compound on ER stress regulation.
5. Chinese Herbal Drugs for Neuroprotection via Glucose Metabolism
5.1. Effects of Glucose Metabolism on Neurophysiology
The energy requirements of the brain are relatively higher than other parts of the body, particularly in humans. About 20% of the body’s energy consumption occurs in less than 2% of a person’s weight [100]. The brain has specialized glial cells, astrocytes, which are responsible for the delivery, production, storage of brain energy, and metabolism of neurons [101]. That is, neurons rely on glia cells and astrocytes to supply their extreme energy costs and maintain functions. The glucose metabolism pathway and the glucose level in the brain have potential to alter neuron function and survival. Moreover, glucose metabolism is also interconnected with the production of major neurotransmitters as discussed below. Maintaining the balances of glucose uptake maybe one key factor to keep brain neurons healthy.
5.2. Dysregulation of Glucose Metabolism in Neurodegenerative Diseases
The correlation of diabetes (one major glucose metabolism disease) and neurodegenerative disease, especially dementia and AD, were reported recently. People with diabetes or metabolism diseases have higher risk of neurodegenerative diseases [102,103]. To emphasize the importance of metabolism dysregulation on neurodegenerative diseases, AD was also suggested as “Type 3 diabetes” [104]. Looking into the mechanisms, the dysregulation of systemic glucose metabolism, such as insulin deficiency or insulin resistance, may also influence the uptake of glucose in human brain. Many studies showed a reduction in insulin production and glucose uptake in the aged, dementia brains [105], and even in patients with diabetes [106]. Dysregulation of glucose metabolism can lead to reduce the energy source, increase oxidative stress and inflammatory response, then damage the brain neurons in AD [107]. In addition, use of fluorodeoxyglucose uptake by PET scans exhibited a reduction of glucose in Aβ damaged brains [108]. These results reveal correlations between glucose metabolism and neurodegenerative diseases.
5.3. Potential of Chinese Herbal Drugs to Improve Neural Metabolism
A triterpenoid compound, maslinic acid (2-α, 3-β-dihydroxyolean-12-en-28-oic acid), isolated from the wax skin of Olea europaea, can increase glycogen synthesis and prevent norepinephrine-induced glycogenolysis in cortical astrocytes [109]. Qian et al. demonstrated that maslinic acid ameliorates neuron injury and apoptosis which accompanied the inhibition of oxygen-glucose deprivation-induced NO production and iNOS expression [110]. The effect of baicalein on preventing neuronal death induced by glutamate and glucose deprivation was also examined [111]. These compounds may have potential for neuronal diseases treatment. Resveratrol has been studied for decades. Its neuroprotective mechanism varies, such as its effects not only on oxidative damage and chronic inflammation, but especially on activating expression of sirtuins, a protein involved in glucose metabolism [112]. Supplementation with resveratrol for 26 weeks (at 200 mg/day) enhanced memory performance in healthy older adults [113]. Moreover, the above double-blind study showed that resveratrol intake significantly reduced serum glycated hemoglobin (HbA1c) compared to a placebo group, and the decreased HbA1c was significantly correlated with an individual’s functional connectivity. There indeed is an effect of glucose metabolism on neural function. This is a great potential to benefit neurodegenerative diseases via metabolism regulation with Chinese herb drugs. Although there are still few herb drugs targeting on systemic and nerve system metabolism, a more-detailed assessment is forthcoming to clarify this issue.
6. Chinese Herbal Drugs for Neuroprotection via Neurotransmitters and Synaptic Function
6.1. Neurotransmitters on Synapse and Neural Function
Synapse is the basic component for neural communication and neural information flow in nervous system. Stimulations of neurons benefit the maintenance of neuronal function and survival. Neurotransmitters play the major role on synaptic function. The life cycle of neurotransmitter including the production, packaging, release, recycling, and postsynaptic uptake [114]. Glutamate, acetylcholine (ACh), and γ-aminobutyric acid (GABA) are common neurotransmitters in CNS. Glutamate and ACh are excitatory neurotransmitters [115], and GABA is inhibitory neurotransmitter [116]. The tricarboxylic acid (TCA) cycle is further extended to produce glutamate and GABA in the CNS, called “the GABA shunt” [117]. Pyruvate is oxidized to acetyl-CoA in mitochondria, and acetyl-CoA and choline are the material of ACh that synthesized by choline acetyltransferase [118]. Released neurotransmitters can be recycled or re-uptake by nearby astrocytes or pre-/post-synaptic terminals. The released glutamates from presynaptic neurons were recycled by astrocytes via the glutamate–glutamine cycle. Glutamate is converted into glutamine by glutamine synthetase in astrocytes then shuttled to neurons [101]. Released GABA was uptake by GABA transporter in presynaptic terminal. In the synapse cleft, released ACh is broken down into choline and acetate by acetylcholinesterase, and choline is transported into the axon terminal as the recycled material of ACh. Overall, the pre-synapse axons, post-synapse dendrites, and astrocytes can re-uptake released neurotransmitter with re-uptake pump to recycle the neurotransmitter around the synapse as materials to synthesis new neurotransmitter.
6.2. Neurotransmitters and Synaptic Function in Neurodegenerative Diseases
Many reports demonstrated the misfolded amyloids would accumulate around the synapses and block the releasing and recycling of neurotransmitter [119,120,121]. The dysregulation of neurotransmitter also causes the abnormality of post-synapse stimulation, including hyper-activation and hypo-activation, and causes neuron disfunction or death [122,123]. It was revealed decades ago that inhibiting NMDA receptors prolongs survival in patients with ALS [124]. A previous study also showed that Aβ is correlated with extracellular GABA levels [125], which raised the theory of the involvement of GABA in the physiopathology of AD [126]. Pimlott et al. demonstrated the reduction of nicotinic ACh receptor (nAchR) in AD, PD, and dementia with Lewy bodies [127].
6.3. Chinese Herbal Drugs Improve Neurotransmitter Production and Synaptic Function
Our recent data revealed that a small molecule from the root of Angelica sinensis has therapeutic potential in SCA3. This small molecule, Bdph, regulates tryptophan metabolism and decreases the downstream neurotoxic product, quinolinic acid (QA, a NMDA receptor agonist) [128]. Moreover, Bdph reduces Aβ40 secretion and attenuates AD-like cytopathy in AD models [129]. Huperzine A has been extracted from the firmoss Huperzia serrata and competes with the NMDA receptor on polyamine-binding sites [130]. Huperzine A has treatment potential for AD and vascular dementia [131]. The type A GABA receptor is a postulated target in age- and AD-related neuronal degeneration [125]. The neuroprotective effect of kava, a modulator of the GABA receptor, has been reviewed for the detailed mechanism of its antianxiety and sleep-inducing abilities [132]. Some other herb extracts were reported to improve neurotransmitter and synapse function. However, the major functional compounds are still unclear and need further identification [133]. Moreover, the Chinese herbal drugs that benefit neurons mentioned in the above sections may also have potential to work on synaptic plasticity. Further studies of herbal drugs on synapse and neurotransmitter would provide new direction of herbal drugs in neurodegenerative diseases.
7. Discussion
The evidence suggests that Chinese herbal drugs have neuroprotective abilities in various neurodegenerative disease models in different ways (Table 1). One is to attenuate the inflammatory response through cytokine regulation, and several previous experiments showed that the IL-1β and TNF-α signaling pathways could be inhibited to benefit neurons in neurodegenerative diseases. Regulating the mTOR signaling pathway in autophagy is also a neuroprotective strategy, and herbal compounds from Chinese herbs have different effects on this signaling pathway. Due to opposite roles of the mTOR pathway in neurons being still largely unknown, the detailed mechanisms of autophagy on neurodegenerative diseases are still in need of further study. Several neurodegenerative diseases have unusual protein misfolding and accumulation in the ER, and herbal compounds inhibit UPR-related apoptosis, which prevents neurons undergoing ER stress from dying. Due to the correlation between metabolic abnormalities and neurodegenerative diseases, such as AD, revealed recently, there is a large potential to apply herb compounds to maintain metabolism balance to combat neurodegenerative diseases. Besides the glucose metabolism, there are also some mechanisms and metabolic pathways related to neuroprotection not mentioned above. As neurons can die due to aberrant protein accumulation, the efficiency of waste clearance and improving brain blood flow are important. Furthermore, nutrient support from glial cells to neurons is also important. Although herbal compounds have neuroprotective abilities, the off-target effect of herbal compounds remains a concern. Some of the herbal compounds involved more than one mechanism, such as AMP, baicalein, berberine, Bdph, and resveratrol, and the neuroprotective specificity by these compounds still need to be confirmed. Moreover, herbal compounds show opposite impact on the mTOR pathway for neuroprotection in neurodegenerative disease models. This indicates that autophagy is complex in neurodegenerative diseases and the regulation of autophagy for neuroprotection may vary with the disease type and stage. Inhibiting UPR-related apoptosis prevents neurons undergoing ER stress from dying, but the ER stress is still high in neurons. The way to inhibit ER-stress-induced cell death may be insufficient to cure neurodegenerative diseases, and releasing ER stress may be needed. As the progress of neurodegenerative diseases is complicated and the herbal compounds have different impacts on the nervous system, combinations of compounds may be needed. In addition, therapeutic strategies may vary with the type and progression of disease.
Table 1.
Chinese herbal compounds have neuroprotection ability to various neurological disorders through different mechanisms and metabolisms.
| Herbal Drugs | Plant Sources | Structure | Involved Mechanism (s) | Treated Model | Main Citations |
|---|---|---|---|---|---|
| 6-hydroxycleroda-3,13-dien-15,16-olide (PL3) | Polyalthia longifolia var. pendula |  |
cytokine regulation | LPS-induced model | [32] |
| Ampelopsin | Ampelopsis grossedentata | 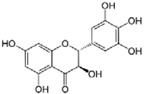 |
cytokine regulation, autophagy regulation | Stroke, brain aging | [29,71] |
| Astragaloside IV | Astragalus membranaceus | 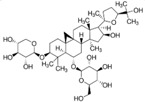 |
ER stress | PD | [94] |
| Baicalein | Scutellaria baicalensis and S. lateriflora | 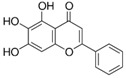 |
autophagy regulation, ER stress | PD | [53,54,96] |
| Berberine | Coptis chinensis | 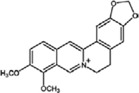 |
cytokine regulation, autophagy regulation | depression, AD, and ALS | [33,47,63] |
| Breviscapine | Erigerin breviscapus |  |
autophagy regulation | cerebral ischemic | [69] |
| n-butylidenephthalide | Angelica sinensis |  |
autophagy regulation, neurotransmitters and synaptic function | ALS, SCA3, and down syndrome model | [64,65,128,129] |
| Carnosic acid | Rosmarinus officinalis |  |
autophagy regulation | AD and PD | [50,55] |
| Conophylline | Ervatamia microphylla |  |
autophagy regulation | PD and HD | [58] |
| Crocin | Crocus sativus | 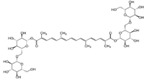 |
ER stress | PD and AD | [97,98] |
| Cucurbitacin E | Wilbrandia ebracteata | 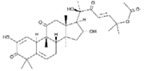 |
autophagy regulation | PD | [62] |
| Eriodictyol | Dracocephalum rupestre | 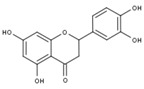 |
cytokine regulation | stroke | [30] |
| Ginsenoside compound K | Panax ginseng | 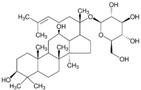 |
autophagy regulation | AD | [46] |
| Ginsenoside Rb1 | Panax ginseng |  |
autophagy regulation | spinal cord injury | [70] |
| Hederagenin | Hedera helix |  |
autophagy regulation | PD | [59] |
| Huperzine A | Huperzia serrata |  |
neurotransmitters and synaptic function | AD | [130,131] |
| Icariin | Epimedium segittatum |  |
cytokine regulation | AD, stroke, depression | [25,28,34] |
| Icariside II | Epimedium brevicornum | 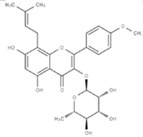 |
cytokine regulation | AD | [26] |
| Isobavachalcone | Psoralea corylifolia | 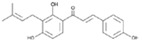 |
cytokine regulation | PD | [24] |
| Luteolin | Lonicera japonica | 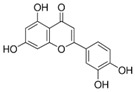 |
autophagy regulation | AD | [48] |
| Maslinic acid | Olea europaea | 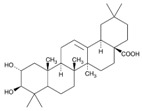 |
glucose metabolism | oxygen-glucose deprivation-induced injury model | [110] |
| Neferine | Nelumbo nucifera | 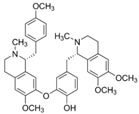 |
autophagy regulation | HD | [68] |
| Oleuropein | Olea europaea | 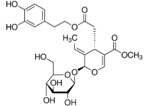 |
autophagy regulation | PD | [61] |
| Paeonol | Cortex Moutan | 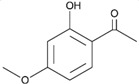 |
cytokine regulation | PD | [23] |
| Piperine | Piper longum L. | 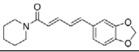 |
autophagy regulation | PD | [57] |
| Resveratrol | red wine |  |
autophagy regulation, glucose metabolism | AD, PD, HD, and healthy older adults | [45,56,67,113] |
| Ruscogenin | Ophiopogon japonicas |  |
cytokine regulation | Ischemic stroke | [31] |
| Sulforaphane | cruciferous vegetables |  |
autophagy regulation | PD, HD | [60,66] |
| Tetrandrine | Stephania tetrandra |  |
cytokine regulation | AD | [27] |
| Thamnolia vermicularis extract | Thamnolia vermicularis | 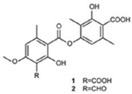 |
autophagy regulation | AD | [49] |
| Triptolide | Tripterygium wilfordii | 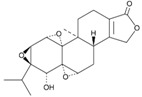 |
autophagy regulation | AD, PD | [51,52] |
ER, endoplasmic reticulum; LPS, lipopolysaccharide; PD, Parkinson’s disease; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; SCA3, spinocerebellar ataxia type 3; HD, Huntington disease.
Acknowledgments
This work was supported by Bio-innovation Center and financially supported by the Ministry of Science and Technology, Taiwan (MOST 106-2320-B-303-001-MY3 and MOST 106-2320-B-303-002-MY3), Buddhist Tzu Chi Medical Foundation and Buddhist Tzu Chi General Hospital, Hualien, Taiwan.
Author Contributions
S.-Z.L. and H.-J.H. initiated this project. C.-Y.C. and H.-C.T. edited, organized, and wrote the article. K.-Y.L., H.-M.C., S.-F.T., M.-H.H., and C.-A.L. wrote the paragraphs and drew the figures. All authors reviewed this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 2.Bloom G.S. Amyloid-β and tau: The trigger and bullet in alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 3.Gaugler M.N., Genc O., Bobela W., Mohanna S., Ardah M.T., El-Agnaf O.M., Cantoni M., Bensadoun J.-C., Schneggenburger R., Knott G.W. Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123:653–669. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- 4.Braak H., Tredici K.D. Neuroanatomy and Pathology of Sporadic Parkinson's Disease. Springer; Berlin/Heidelberg, Germany: 2009. [PubMed] [Google Scholar]
- 5.Blokhuis A.M., Groen E.J., Koppers M., van den Berg L.H., Pasterkamp R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakahira H., Breuer P., Hayer-Hartl M.K., Hartl F.U. Molecular chaperones as modulators of polyglutamine protein aggregation and toxicity. Proc. Natl. Acad. Sci. USA. 2002;99:16412–16418. doi: 10.1073/pnas.182426899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huie C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002;373:23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang J.F., Wei D.Q., Chou K.C. Drug candidates from traditional chinese medicines. Curr. Top. Med. Chem. 2008;8:1656–1665. doi: 10.2174/156802608786786633. [DOI] [PubMed] [Google Scholar]
- 9.Chen L.W., Wang Y.Q., Wei L.C., Shi M., Chan Y.S. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of parkinson’s disease. CNS Neurol. Disord. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z.K., Yang H.Q., Chen S.D. Traditional chinese medicine: A promising candidate for the treatment of alzheimer’s disease. Transl. Neurodegener. 2013;2:6. doi: 10.1186/2047-9158-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J.S., John G.R., Sikora A., Lee S.C., Brosnan C.F. Modulation of interleukin-1beta and tumor necrosis factor alpha signaling by p2 purinergic receptors in human fetal astrocytes. J. Neurosci. 2000;20:5292–5299. doi: 10.1523/JNEUROSCI.20-14-05292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavillet M., Allaman I., Magistretti P.J. Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia. 2008;56:975–989. doi: 10.1002/glia.20671. [DOI] [PubMed] [Google Scholar]
- 13.Ye L., Huang Y., Zhao L., Li Y., Sun L., Zhou Y., Qian G., Zheng J.C. Il-1beta and tnf-alpha induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 2013;125:897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borsini A., Zunszain P.A., Thuret S., Pariante C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Vom Berg J., Prokop S., Miller K.R., Obst J., Kalin R.E., Lopategui-Cabezas I., Wegner A., Mair F., Schipke C.G., Peters O., et al. Inhibition of il-12/il-23 signaling reduces alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 16.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin X.Y., Zhang S.P., Cao C., Loh Y.P., Cheng Y. Aberrations in peripheral inflammatory cytokine levels in parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 18.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in alzheimer’s disease. Nat. Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 19.Lambertsen K.L., Biber K., Finsen B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 22.Martinez J.M., Garakani A., Yehuda R., Gorman J.M. Proinflammatory and “resiliency” proteins in the csf of patients with major depression. Depression Anxiety. 2012;29:32–38. doi: 10.1002/da.20876. [DOI] [PubMed] [Google Scholar]
- 23.Shi X., Chen Y.H., Liu H., Qu H.D. Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced parkinson’s disease in mice. Mol. Med. Rep. 2016;14:2397–2404. doi: 10.3892/mmr.2016.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing H., Wang S., Wang M., Fu W., Zhang C., Xu D. Isobavachalcone attenuates MPTP-induced parkinson’s disease in mice by inhibition of microglial activation through NF-kappab pathway. PLoS ONE. 2017;12:e0169560. doi: 10.1371/journal.pone.0169560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z.Y., Li C., Zug C., Schluesener H.J. Icariin ameliorates neuropathological changes, tgf-beta1 accumulation and behavioral deficits in a mouse model of cerebral amyloidosis. PLoS ONE. 2014;9:e104616. doi: 10.1371/journal.pone.0104616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He F.Q., Qiu B.Y., Zhang X.H., Li T.K., Xie Q., Cui D.J., Huang X.L., Gan H.T. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-kappab activation in a rat model of alzheimer’s disease induced by amyloid-beta(1-42) Brain Res. 2011;1384:89–96. doi: 10.1016/j.brainres.2011.01.103. [DOI] [PubMed] [Google Scholar]
- 27.Yin C., Deng Y., Gao J., Li X., Liu Y., Gong Q. Icariside ii, a novel phosphodiesterase-5 inhibitor, attenuates streptozotocin-induced cognitive deficits in rats. Neuroscience. 2016;328:69–79. doi: 10.1016/j.neuroscience.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Xiong D., Deng Y., Huang B., Yin C., Liu B., Shi J., Gong Q. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by nf-kappab, pparalpha and ppargamma in rats. Int. Immunopharmacol. 2016;30:157–162. doi: 10.1016/j.intimp.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Ye X.L., Lu L.Q., Li W., Lou Q., Guo H.G., Shi Q.J. Oral administration of ampelopsin protects against acute brain injury in rats following focal cerebral ischemia. Exp. Ther. Med. 2017;13:1725–1734. doi: 10.3892/etm.2017.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira Ede O., Fernandes M.Y., Lima N.M., Neves K.R., Carmo M.R., Lima F.A., Fonteles A.A., Menezes A.P., Andrade G.M. Neuroinflammatory response to experimental stroke is inhibited by eriodictyol. Behav. Brain Res. 2016;312:321–332. doi: 10.1016/j.bbr.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Guan T., Liu Q., Qian Y., Yang H., Kong J., Kou J., Yu B. Ruscogenin reduces cerebral ischemic injury via nf-kappab-mediated inflammatory pathway in the mouse model of experimental stroke. Eur. J. Pharmacol. 2013;714:303–311. doi: 10.1016/j.ejphar.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Shih Y.T., Hsu Y.Y., Chang F.R., Wu Y.C., Lo Y.C. 6-hydroxycleroda-3,13-dien-15,16-olide protects neuronal cells from lipopolysaccharide-induced neurotoxicity through the inhibition of microglia-mediated inflammation. Planta Medica. 2010;76:120–127. doi: 10.1055/s-0029-1186005. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.M., Niu L., Wang L.L., Bai L., Fang X.Y., Li Y.C., Yi L.T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017;134:220–227. doi: 10.1016/j.brainresbull.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Liu B., Xu C., Wu X., Liu F., Du Y., Sun J., Tao J., Dong J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience. 2015;294:193–205. doi: 10.1016/j.neuroscience.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 35.Burman C., Ktistakis N.T. Autophagosome formation in mammalian cells. Semin. Immunopathol. 2010;32:397–413. doi: 10.1007/s00281-010-0222-z. [DOI] [PubMed] [Google Scholar]
- 36.Cherra S.J., 3rd., Chu C.T. Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marino G., Madeo F., Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr. Opin. Cell Biol. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Nah J., Yuan J., Jung Y.K. Autophagy in neurodegenerative diseases: From mechanism to therapeutic approach. Mol. Cells. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satriano J., Sharma K. Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol. Dial. Transplant. 2013;28(Suppl. 4):iv29–iv36. doi: 10.1093/ndt/gft229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang J.L., Zhang H.H., Lai M.D. Role of autophagy and apoptosis in tumor. Zhonghua Bing Li Xue Za Zhi. 2012;41:573–576. doi: 10.3760/cma.j.issn.0529-5807.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Ren S.Y., Xu X. Role of autophagy in metabolic syndrome-associated heart disease. Biochim. Biophys. Acta. 2015;1852:225–231. doi: 10.1016/j.bbadis.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryter S.W., Nakahira K., Haspel J.A., Choi A.M. Autophagy in pulmonary diseases. Annu. Rev. Physiol. 2012;74:377–401. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 43.Shastry B.S. Neurodegenerative disorders of protein aggregation. Neurochem. Int. 2003;43:1–7. doi: 10.1016/S0197-0186(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 44.Wang S.F., Wu M.Y., Cai C.Z., Li M., Lu J.H. Autophagy modulators from traditional chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 2016;194:861–876. doi: 10.1016/j.jep.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed T., Javed S., Javed S., Tariq A., Samec D., Tejada S., Nabavi S.F., Braidy N., Nabavi S.M. Resveratrol and alzheimer’s disease: Mechanistic insights. Mol. Neurobiol. 2017;54:2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 46.Guo J., Chang L., Zhang X., Pei S., Yu M., Gao J. Ginsenoside compound k promotes beta-amyloid peptide clearance in primary astrocytes via autophagy enhancement. Exp. Ther. Med. 2014;8:1271–1274. doi: 10.3892/etm.2014.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang M., Jiang X., Liang Y., Liu Q., Chen S., Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in app/tau/ps1 mouse model of alzheimer’s disease. Exp. Gerontol. 2017;91:25–33. doi: 10.1016/j.exger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Kwon K., Kwon Y.S., Kim S.W., Yu K., Lee K.H., Kwon O.Y. Luteolin-induced apoptosis through activation of endoplasmic reticulum stress sensors in pheochromocytoma cells. Mol. Med. Rep. 2017;16:380–386. doi: 10.3892/mmr.2017.6582. [DOI] [PubMed] [Google Scholar]
- 49.Li C., Guo X.D., Lei M., Wu J.Y., Jin J.Z., Shi X.F., Zhu Z.Y., Rukachaisirikul V., Hu L.H., Wen T.Q., et al. Thamnolia vermicularis extract improves learning ability in app/ps1 transgenic mice by ameliorating both abeta and tau pathologies. Acta Pharmacol. Sin. 2017;38:9–28. doi: 10.1038/aps.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J., Su H., Qu Q.M. Carnosic acid prevents beta-amyloid-induced injury in human neuroblastoma sh-sy5y cells via the induction of autophagy. Neurochem. Res. 2016;41:2311–2323. doi: 10.1007/s11064-016-1945-6. [DOI] [PubMed] [Google Scholar]
- 51.Xu P., Li Z., Wang H., Zhang X., Yang Z. Triptolide inhibited cytotoxicity of differentiated pc12 cells induced by amyloid-beta(2)(5)(-)(3)(5) via the autophagy pathway. PLoS ONE. 2015;10:e0142719. doi: 10.1371/journal.pone.0142719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu G., Gong X., Wang L., Liu M., Liu Y., Fu X., Wang W., Zhang T., Wang X. Triptolide promotes the clearance of alpha-synuclein by enhancing autophagy in neuronal cells. Mol. Neurobiol. 2017;54:2361–2372. doi: 10.1007/s12035-016-9808-3. [DOI] [PubMed] [Google Scholar]
- 53.Hung K.C., Huang H.J., Wang Y.T., Lin A.M. Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the mpp(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016;194:522–529. doi: 10.1016/j.jep.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 54.Kuang L., Cao X., Lu Z. Baicalein protects against rotenone-induced neurotoxicity through induction of autophagy. Biol. Pharm. Bull. 2017;40:1537–1543. doi: 10.1248/bpb.b17-00392. [DOI] [PubMed] [Google Scholar]
- 55.Lin C.Y., Tsai C.W. Carnosic acid attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells by inducing autophagy through an enhanced interaction of parkin and beclin1. Mol. Neurobiol. 2017;54:2813–2822. doi: 10.1007/s12035-016-9873-7. [DOI] [PubMed] [Google Scholar]
- 56.Lin T.K., Chen S.D., Chuang Y.C., Lin H.Y., Huang C.R., Chuang J.H., Wang P.W., Huang S.T., Tiao M.M., Chen J.B., et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014;15:1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J., Chen M., Wang X., Wang Y., Duan C., Gao G., Lu L., Wu X., Wang X., Yang H. Piperine induces autophagy by enhancing protein phosphotase 2a activity in a rotenone-induced parkinson’s disease model. Oncotarget. 2016;7:60823–60843. doi: 10.18632/oncotarget.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasazawa Y., Sato N., Umezawa K., Simizu S. Conophylline protects cells in cellular models of neurodegenerative diseases by inducing mammalian target of rapamycin (mTOR)-independent autophagy. J. Biol. Chem. 2015;290:6168–6178. doi: 10.1074/jbc.M114.606293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu A.G., Zeng W., Wong V.K., Zhu Y.Z., Lo A.C., Liu L., Law B.Y. Hederagenin and alpha-hederin promote degradation of proteins in neurodegenerative diseases and improve motor deficits in MPTP-mice. Pharmacol. Res. 2017;115:25–44. doi: 10.1016/j.phrs.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Q., Chen B., Wang X., Wu L., Yang Y., Cheng X., Hu Z., Cai X., Yang J., Sun X., et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, NRF2, and autophagy pathways. Sci. Rep. 2016;6:32206. doi: 10.1038/srep32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achour I., Arel-Dubeau A.M., Renaud J., Legrand M., Attard E., Germain M., Martinoli M.G. Oleuropein prevents neuronal death, mitigates mitochondrial superoxide production and modulates autophagy in a dopaminergic cellular model. Int. J. Mol. Sci. 2016;17:e1293. doi: 10.3390/ijms17081293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arel-Dubeau A.M., Longpre F., Bournival J., Tremblay C., Demers-Lamarche J., Haskova P., Attard E., Germain M., Martinoli M.G. Cucurbitacin e has neuroprotective properties and autophagic modulating activities on dopaminergic neurons. Oxid. Med. Cell. Longev. 2014;2014:425496. doi: 10.1155/2014/425496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang C.F., Lee Y.C., Lee K.H., Lin H.C., Chen C.L., Shen C.J., Huang C.C. Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS. J. Biomed. Sci. 2016;23:72. doi: 10.1186/s12929-016-0290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsueh K.W., Chiou T.W., Chiang S.F., Yamashita T., Abe K., Borlongan C.V., Sanberg P.R., Huang A.Y., Lin S.Z., Harn H.J. Autophagic down-regulation in motor neurons remarkably prolongs the survival of ALS mice. Neuropharmacology. 2016;108:152–160. doi: 10.1016/j.neuropharm.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Q.M., Zhang J.J., Li S., Chen S., Le W.D. N-butylidenephthalide treatment prolongs life span and attenuates motor neuron loss in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. CNS Neurosci. Ther. 2017;23:375–385. doi: 10.1111/cns.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Hettinger C.L., Zhang D., Rezvani K., Wang X., Wang H. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for huntington’s disease. J. Neurochem. 2014;129:539–547. doi: 10.1111/jnc.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vidoni C., Secomandi E., Castiglioni A., Melone M.A.B., Isidoro C. Resveratrol protects neuronal-like cells expressing mutant huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2017 doi: 10.1016/j.neuint.2017.05.013. S0197-0186(17)30243-7. [DOI] [PubMed] [Google Scholar]
- 68.Wong V.K., Wu A.G., Wang J.R., Liu L., Law B.Y. Neferine attenuates the protein level and toxicity of mutant huntingtin in PC-12 cells via induction of autophagy. Molecules. 2015;20:3496–3514. doi: 10.3390/molecules20033496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pengyue Z., Tao G., Hongyun H., Liqiang Y., Yihao D. Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed. Pharmacother. 2017;90:69–76. doi: 10.1016/j.biopha.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 70.Wang P., Lin C., Wu S., Huang K., Wang Y., Bao X., Zhang F., Huang Z., Teng H. Inhibition of autophagy is involved in the protective effects of ginsenoside RB1 on spinal cord injury. Cell. Mol. Neurobiol. 2017 doi: 10.1007/s10571-017-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kou X., Liu X., Chen X., Li J., Yang X., Fan J., Yang Y., Chen N. Ampelopsin attenuates brain aging of D-gal-induced rats through miR-34a-mediated SIRT1/mTOR signal pathway. Oncotarget. 2016;7:74484–74495. doi: 10.18632/oncotarget.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baumann O., Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 73.Verkhratsky A. The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium. 2002;32:393–404. doi: 10.1016/S0143416002001896. [DOI] [PubMed] [Google Scholar]
- 74.Bola B., Allan V. How and why does the endoplasmic reticulum Move? Biochem. Soc. Trans. 2009;37:961–965. doi: 10.1042/BST0370961. [DOI] [PubMed] [Google Scholar]
- 75.Bannai H., Inoue T., Nakayama T., Hattori M., Mikoshiba K. Kinesin dependent, rapid, bi-directional transport of er sub-compartment in dendrites of hippocampal neurons. J. Cell Sci. 2004;117:163–175. doi: 10.1242/jcs.00854. [DOI] [PubMed] [Google Scholar]
- 76.Broadwell R., Cataldo A. The neuronal endoplasmic reticulum: Its cytochemistry and contribution to the endomembrane system. I. Cell bodies and dendrites. J. Histochem. Cytochem. 1983;31:1077–1088. doi: 10.1177/31.9.6309951. [DOI] [PubMed] [Google Scholar]
- 77.Banno T., Kohno K. Conformational changes of the smooth endoplasmic reticulum are facilitated by l-glutamate and its receptors in rat purkinje cells. J. Comp. Neurol. 1998;402:252–263. doi: 10.1002/(SICI)1096-9861(19981214)402:2<252::AID-CNE9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 78.Aihara Y., Inoue T., Tashiro T., Okamoto K., Komiya Y., Mikoshiba K. Movement of endoplasmic reticulum in the living axon is distinct from other membranous vesicles in its rate, form, and sensitivity to microtubule inhibitors. J. Neurosci. Res. 2001;65:236–246. doi: 10.1002/jnr.1147. [DOI] [PubMed] [Google Scholar]
- 79.Xu C., Bailly-Maitre B., Reed J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001;26:504–510. doi: 10.1016/S0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 81.Ledoux S., Yang R., Friedlander G., Laouari D. Glucose depletion enhances p-glycoprotein expression in hepatoma cells. Cancer Res. 2003;63:7284–7290. [PubMed] [Google Scholar]
- 82.Remondelli P., Renna M. The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front. Mol. Neurosci. 2017;10:187. doi: 10.3389/fnmol.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imaizumi K., Miyoshi K., Katayama T., Yoneda T., Taniguchi M., Kudo T., Tohyama M. The unfolded protein response and alzheimer’s disease. Biochim. Biophys. Acta. 2001;1536:85–96. doi: 10.1016/S0925-4439(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 84.Gouras G.K., Tsai J., Naslund J., Vincent B., Edgar M., Checler F., Greenfield J.P., Haroutunian V., Buxbaum J.D., Xu H. Intraneuronal aβ42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/S0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandez-Vizarra P., Fernandez A., Castro-Blanco S., Serrano J., Bentura M., Martinez-Murillo R., Martinez A., Rodrigo J. Intra-and extracellular aß and phf in clinically evaluated cases of alzheimer’s disease. Histol. Histopathol. 2004;19:823–844. doi: 10.14670/HH-19.823. [DOI] [PubMed] [Google Scholar]
- 86.Ho Y.-S., Yang X., Lau J.C.-F., Hung C.H.-L., Wuwongse S., Zhang Q., Wang J., Baum L., So K.-F., Chang R.C.-C. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: Implication in alzheimer’s disease pathogenesis. J. Alzheimers Dis. 2012;28:839–854. doi: 10.3233/JAD-2011-111037. [DOI] [PubMed] [Google Scholar]
- 87.Bellucci A., Navarria L., Zaltieri M., Falarti E., Bodei S., Sigala S., Battistin L., Spillantini M., Missale C., Spano P. Induction of the unfolded protein response by α-synuclein in experimental models of parkinson’s disease. J. Neurochem. 2011;116:588–605. doi: 10.1111/j.1471-4159.2010.07143.x. [DOI] [PubMed] [Google Scholar]
- 88.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F. Α-synuclein blocks er-golgi traffic and rab1 rescues neuron loss in parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kikuchi H., Almer G., Yamashita S., Guégan C., Nagai M., Xu Z., Sosunov A.A., McKhann G.M., Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an als model. Proc. Natl. Acad. Sci. USA. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishitoh H., Kadowaki H., Nagai A., Maruyama T., Yokota T., Fukutomi H., Noguchi T., Matsuzawa A., Takeda K., Ichijo H. Als-linked mutant sod1 induces er stress-and ask1-dependent motor neuron death by targeting derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urushitani M., Ezzi S.A., Matsuo A., Tooyama I., Julien J.-P. The endoplasmic reticulum-golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to als. FASEB J. 2008;22:2476–2487. doi: 10.1096/fj.07-092783. [DOI] [PubMed] [Google Scholar]
- 92.Atkin J.D., Farg M.A., Turner B.J., Tomas D., Lysaght J.A., Nunan J., Rembach A., Nagley P., Beart P.M., Cheema S.S. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y., Gui D., Chen J., He D., Luo Y., Wang N. Down-regulation of PERK-ATF4-chop pathway by astragaloside iv is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell. Physiol. Biochem. 2014;33:1975–1987. doi: 10.1159/000362974. [DOI] [PubMed] [Google Scholar]
- 94.Chan W.-S., Durairajan S.S.K., Lu J.-H., Wang Y., Xie L.-X., Kum W.-F., Koo I., Yung K.K.L., Li M. Neuroprotective effects of astragaloside iv in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem. Int. 2009;55:414–422. doi: 10.1016/j.neuint.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 95.Choi J.H., Choi A.Y., Yoon H., Choe W., Yoon K.-S., Ha J., Yeo E.-J., Kang I. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and chop induction. Exp. Mol. Med. 2010;42:811–822. doi: 10.3858/emm.2010.42.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song J.-X., Choi M.Y.-M., Wong K.C.-K., Chung W.W.-Y., Sze S.C.-W., Ng T.-B., Zhang K.Y.-B. Baicalein antagonizes rotenone-induced apoptosis in dopaminergic SH-SY5Y cells related to parkinsonism. Chin. Med. 2012;7:1. doi: 10.1186/1749-8546-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G.F., Zhang Y., Zhao G. Crocin protects PC12 cells against MPP(+)-induced injury through inhibition of mitochondrial dysfunction and er stress. Neurochem. Int. 2015;89:101–110. doi: 10.1016/j.neuint.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Karakani A.M., Riazi G., Mahmood Ghaffari S., Ahmadian S., Mokhtari F., Jalili Firuzi M., Zahra Bathaie S. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med. Sci. 2015;18:485–492. [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Y.L., Zhang Y., Cao J.P., Wu S.B., Cai X.H., Zhang Y.C., Zhang R.J., Song X.G., Zhang L.D. Regulation of the endoplasmic reticulum stress response and neuroprotective effects of acupuncture on brain injury caused by heroin addiction. Acupunct. Med. 2017;35:366–373. doi: 10.1136/acupmed-2016-011220. [DOI] [PubMed] [Google Scholar]
- 100.Drubach D. The Brain Explained. Pearson Prentice Hall; Upper Saddle River, NJ, USA: 2000. [Google Scholar]
- 101.Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 102.Ott A., Stolk R.P., van Harskamp F., Pols H.A., Hofman A., Breteler M.M. Diabetes mellitus and the risk of dementia: The rotterdam study. Neurology. 1999;53:1937–1942. doi: 10.1212/WNL.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 103.Akter K., Lanza E.A., Martin S.A., Myronyuk N., Rua M., Raffa R.B. Diabetes mellitus and alzheimer's disease: Shared pathology and treatment? Br. J. Clin. Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De la Monte S.M., Wands J.R. Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mosconi L. Glucose metabolism in normal aging and alzheimer’s disease: Methodological and physiological considerations for pet studies. Clin. Transl. Imaging. 2013;1 doi: 10.1007/s40336-013-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts R.O., Knopman D.S., Cha R.H., Mielke M.M., Pankratz V.S., Boeve B.F., Kantarci K., Geda Y.E., Jack C.R., Jr., Petersen R.C., et al. Diabetes and elevated hemoglobin a1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 2014;55:759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soucek T., Cumming R., Dargusch R., Maher P., Schubert D. The regulation of glucose metabolism by hif-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39:43–56. doi: 10.1016/S0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- 108.Horwood N., Davies D.C. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in alzheimer’s disease. Virchows Arch. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 109.Guan T., Li Y., Sun H., Tang X., Qian Y. Effects of maslinic acid, a natural triterpene, on glycogen metabolism in cultured cortical astrocytes. Planta Med. 2009;75:1141–1143. doi: 10.1055/s-0029-1185481. [DOI] [PubMed] [Google Scholar]
- 110.Qian Y., Guan T., Tang X., Huang L., Huang M., Li Y., Sun H. Maslinic acid, a natural triterpenoid compound from olea europaea, protects cortical neurons against oxygen-glucose deprivation-induced injury. Eur. J. Pharmacol. 2011;670:148–153. doi: 10.1016/j.ejphar.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 111.Lee H.H., Yang L.L., Wang C.C., Hu S.Y., Chang S.F., Lee Y.H. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res. 2003;986:103–113. doi: 10.1016/S0006-8993(03)03197-4. [DOI] [PubMed] [Google Scholar]
- 112.Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2011. Botanical phenolics and neurodegeneration. [Google Scholar]
- 113.Witte A.V., Kerti L., Margulies D.S., Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards R.H. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Meldrum B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 116.Bowery N.G., Smart T.G. Gaba and glycine as neurotransmitters: A brief history. Br. J. Pharmacol. 2006;147(Suppl. 1):S109–S119. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bak L.K., Schousboe A., Waagepetersen H.S. The glutamate/gaba-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 118.Prado M.A., Reis R.A., Prado V.F., de Mello M.C., Gomez M.V., de Mello F.G. Regulation of acetylcholine synthesis and storage. Neurochem. Int. 2002;41:291–299. doi: 10.1016/S0197-0186(02)00044-X. [DOI] [PubMed] [Google Scholar]
- 119.Barone P. Neurotransmission in parkinson’s disease: Beyond dopamine. Eur. J. Neurol. 2010;17:364–376. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 120.Foerster B.R., Pomper M.G., Callaghan B.C., Petrou M., Edden R.A., Mohamed M.A., Welsh R.C., Carlos R.C., Barker P.B., Feldman E.L. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-t proton magnetic resonance spectroscopy. JAMA Neurol. 2013;70:1009–1016. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Newington J.T., Harris R.A., Cumming R.C. Reevaluating metabolism in alzheimer’s disease from the perspective of the astrocyte-neuron lactate shuttle model. J. Neurodegener. Dis. 2013;2013:234572. doi: 10.1155/2013/234572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mucke L., Selkoe D.J. Neurotoxicity of amyloid beta-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., Bien-Ly N., Yoo J., Ho K.O., Yu G.Q., Kreitzer A., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shaw P.J., Ince P.G. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J. Neurol. 1997;244(Suppl. 2):S3–S14. doi: 10.1007/BF03160574. [DOI] [PubMed] [Google Scholar]
- 125.Marczynski T.J. Gabaergic deafferentation hypothesis of brain aging and alzheimer’s disease revisited. Brain Res. Bull. 1998;45:341–379. doi: 10.1016/S0361-9230(97)00347-X. [DOI] [PubMed] [Google Scholar]
- 126.Nava-Mesa M.O., Jimenez-Diaz L., Yajeya J., Navarro-Lopez J.D. Gabaergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of alzheimer’s disease. Front. Cell. Neurosci. 2014;8:167. doi: 10.3389/fncel.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pimlott S.L., Piggott M., Owens J., Greally E., Court J.A., Jaros E., Perry R.H., Perry E.K., Wyper D. Nicotinic acetylcholine receptor distribution in alzheimer’s disease, dementia with lewy bodies, parkinson’s disease, and vascular dementia: In vitro binding study using 5-[(125)i]-a-85380. Neuropsychopharmacology. 2004;29:108–116. doi: 10.1038/sj.npp.1300302. [DOI] [PubMed] [Google Scholar]
- 128.Rajamani K., Liu J.W., Wu C.H., Chiang I.T., You D.H., Lin S.Y., Hsieh D.K., Lin S.Z., Harn H.J., Chiou T.W. N-butylidenephthalide exhibits protection against neurotoxicity through regulation of tryptophan 2, 3 dioxygenase in spinocerebellar ataxia type 3. Neuropharmacology. 2017;117:434–446. doi: 10.1016/j.neuropharm.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 129.Chang C.Y., Chen S.M., Lu H.E., Lai S.M., Lai P.S., Shen P.W., Chen P.Y., Shen C.I., Harn H.J., Lin S.Z., et al. N-butylidenephthalide attenuates alzheimer’s disease-like cytopathy in down syndrome induced pluripotent stem cell-derived neurons. Sci. Rep. 2015;5:8744. doi: 10.1038/srep08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang J.M., Hu G.Y. Huperzine a, a nootropic alkaloid, inhibits n-methyl-d-aspartate-induced current in rat dissociated hippocampal neurons. Neuroscience. 2001;105:663–669. doi: 10.1016/S0306-4522(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 131.Yang G., Wang Y., Tian J., Liu J.P. Huperzine a for alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE. 2013;8:e74916. doi: 10.1371/journal.pone.0074916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tzeng Y.M., Lee M.J. Neuroprotective properties of kavalactones. Neural Regen. Res. 2015;10:875–877. doi: 10.4103/1673-5374.158335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Breyer A., Elstner M., Gillessen T., Weiser D., Elstner E. Glutamate-induced cell death in neuronal HT22 cells is attenuated by extracts from st. John’s wort (hypericum perforatum l.) Phytomedicine. 2007;14:250–255. doi: 10.1016/j.phymed.2007.02.001. [DOI] [PubMed] [Google Scholar]