Abstract
The formation of inclusion complexes of the water-soluble p-sulfonatocalix[n]arenes, where n = 4 or 6, with the Chemical Warfare Agent (CWA) GD, or Soman, and commonly used dialkyl methylphosphonate simulants has been studied by experimental solution NMR methods and by Molecular Mechanics (MMFF) and semi-empirical (PM6) calculations. Complex formation in non-buffered and buffered solutions is driven by the hydrophobic effect, and complex stoichiometry determined as 1:1 for all host:guest pairs. Low affinity complexes (Kassoc < 100 M−1) are observed for all guests, attributed to poor host–guest complementarity and the role of buffer cation species accounts for the low affinity of the complexes. Comparison of CWA and simulant behavior adds to understanding of CWA–simulant correlations and the challenges of simulant selection.
Keywords: supramolecular, hydrophobic effect, chemical warfare agent, nerve agent, complexation, inclusion complex, computational
1. Introduction
Organophosphorus (OP) nerve agents are a class of Chemical Warfare Agent (CWA) that function by inhibiting the breakdown of the neurotransmitter acetylcholine (ACh) by acetylcholinesterase (AChE). The affinity of OP nerve agents for AChE is in part based on their structural similarities to ACh, and exposure to nerve agents such as GB (Sarin), GD (Soman) and VX results in miosis, muscle tremors, salivation, lachrymation and eventually heart and respiratory failure leading to death [1,2]. First developed in the 1940s and 1950s, proliferation of such compounds through the second half of the twentieth century led to the development of the Chemical Weapons Convention and the establishment of the Organization for the Prohibition of Chemical Weapons (OPCW) in the early 1990s [3,4]. However, events in the 1980s in Iraq [5], 1990s in Tokyo [6] and more recently in Syria [7] indicate the threat still posed by these chemicals.
Whilst there are established detection and decontamination technologies readily available, recent efforts in the application of supramolecular chemistry to CWA hazard mitigation have gained interest and shown significant promise [8]. Examples include the catalytic breakdown of OP materials by metallo-organic cages [9], the development of hydrogen bond-donating organocatalysts [10] and the use of supramolecular gels as CWA-responsive materials [11].
All of the G- and V-series agents contain aliphatic side-chains, potentially suitable for inclusion into the hydrophobic cavities of appropriate cavitands through the hydrophobic effect. This has been demonstrated widely for the cyclodextrins (CDs), with GB shown to bind to α-CD and GD to β-CD [12]. A recent review of the complexation of toxic OP materials (including related pesticides) has recently been published by Estour [13], and Worek and co-workers have demonstrated the use of functionalized β-CDs as potential medical countermeasures for OP poisoning [14].
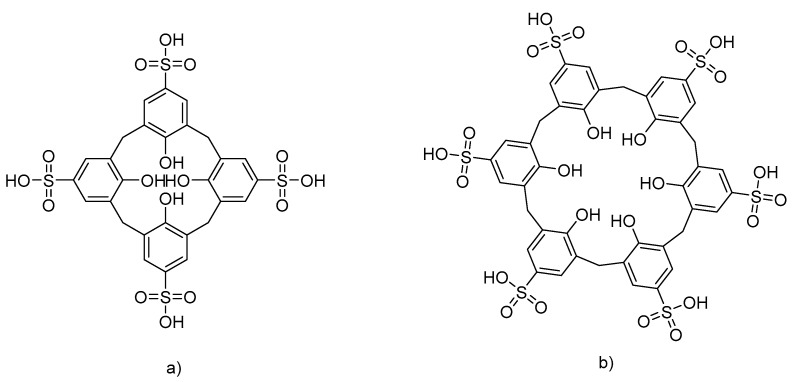
Water-soluble sulfonato-calixarenes (SCX; Figure 1) are increasingly well-known host systems that can form strong inclusion complexes driven by the hydrophobic effect [15]. Formation of SCX inclusion complexes with small organic molecules is well-known and examples of guests include small, neutral organic molecules (e.g., alcohols, ketones, nitriles) [16], neutral aromatic guests [17] and ACh [18].
Figure 1.
Water-soluble calixarenes (a) p-sulfonatocalix[4]arene (SCX4) and (b) p-sulfonatocalix[6]arene (SCX6).
Recently, Worek and co-workers have reported on the development of SCXs modified with hydroxamic acid, which is known to detoxify OP nerve agents [19]. The functionalized calixarenes show a clear enhancement in the half-life of the detoxification reactions of V-series agents and GD compared to the reactive components in the absence of the calixarene host.
The development of new responsive materials, such as sensors and catalysts, is generally conducted with simulants, also commonly referred to as analogues or surrogates [20], prior to the studies using actual CWAs. Due to the inherent toxicity and the control of the actual CWAs, a range of simulant materials have been proposed that possesses similar properties, such as chemical structure and physico-chemical behavior, but with considerably lower toxicity [21]. In some cases, agent–simulant correlation is understood sufficiently to suggest simulant selection [22], but this may not be the case with regard to supramolecular complex formation.
In a recent study, we reported comparative agent–simulant data in the hydrophobically-driven inclusion complexes of β-CD with GD, common trialkylphosphates and dialkyl methylphosphonates, using both experimental and computational DFT approaches; considerable differences in complex affinity between GD and simulants were observed. Furthermore, the use of computational methods allowed for deconvolution of association constants to calculate diastereoisomer-specific complex affinities of the four diastereoisomers of GD [P(+)C(+), P(+)C(−), P(−)C(−), P(−)C(+)] [23]. A further theoretical study has considered the formation of inclusion complexes of the CWA GB and a range of simulants by α-cyclodextrin, alongside spectroscopic calculations for the V-series agents VX and VG and the G-series agents GA, GB, GD and GF [24].
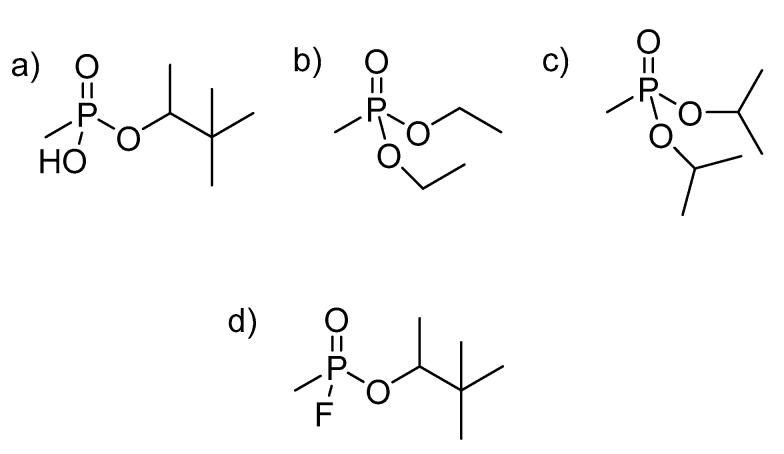
Herein we report the complexation behavior of the simulants pinacolyl methyl phosphonate (PMP), diisopropyl methylphosphonate (DIMP) and diethyl methyl phosphonate (DEMP; Figure 2) with sulfonatocalix[4]arene (SCX4) and sulfonatocalix[6]arene (SCX6), which have been compared directly to the complexation behavior with GD (Figure 2). Molecular mechanics (MMFF) calculations followed by geometry refinement by semiempirical methods (PM6) on GD, simulants, calixarenes and their complexes were used to further investigate complex formation and to suggest how GD and the simulants interacted with the calixarenes.
Figure 2.
Structures of OP compounds commonly used as nerve agent simulants: (a) pinacolyl methylphosphonate (PMP); (b) diethyl methylphosphonate (DEMP); (c) diisopropylmethylphosphonate (DIMP) and (d) the nerve agent GD (Soman).
2. Materials and Methods
2.1. Materials
The chemicals p-sulfonatocalix[4]arene (SCX4), p-sulfonatocalix[6]arene (SCX6), diethylmethyl phosphonate (DEMP), and pinacolyl methylphosphonate (PMP) were purchased from Sigma-Aldrich and used as supplied. Diisoproyl methylphosphonate (DIMP) was purchased from Alpha Aesar and used as supplied. Soman (GD) was produced at Dstl and its purity was confirmed by in-house methods.
2.2. Experimental Methods
All NMR experiments were recorded at 9.4 T using a Bruker AVHD 400 MHz spectrometer equipped with a 5 mm BBFO+ probe head at a temperature of 293 K. 1H-NMR was recorded at 400.16 MHz using an excitation pulse of (π/6) of 3.4 µs and a recycle delay of 2 s. A total of 16 scans were acquired. 31P{1H}-NMR was recorded at 161.92 MHz using an excitation pulse of (π/6) of 3.6 µs and a recycle delay of 2 s using WALTZ-16 1H decoupling during acquisition. A total of 128 scans were acquired. Solvent environments were D2O or buffered D2O. Where buffer was used, this was a 0.25 M, pH 7 phosphate buffer comprised of Na2HPO4 and NaH2PO4, and verified using a Metler Toledo SevenCompact pH/ion InLab® Pro ISM probe.
In typical Job plot experiments, solutions of p-sulfonatocalix[n]arene (where n = 4 or 6) were prepared in D2O or phosphate buffered D2O, pH 7, 0.1 M, to give a total volume of 6 ml of 0.1 M SCX4 or SCX6 as were solutions of the same volume and concentration of the appropriate OP compound (DEMP, DIMP, PMP or GD). These solutions were then divided up into 11 × 1 mL mixtures where the sum concentration of the mixtures remained 0.1 M but the ratio of host to guest varied through the range 0:10, 1:9, 2:8 … 8:2, 9:1, 10:0. 1H-NMR spectra were recorded for each sample, analysed using ACD Spectrus Processor and used to construct Job plots.
In typical NMR titration experiments, a solution of the appropriate OP compound (DEMP, DIMP, PMP or GD) in D2O or phosphate buffered D2O, pH 7, 0.1 M, was prepared, to give a total volume of 17 mL of 1 mM solution, and subsequently divided into 17 equal volume samples (1 mL). To each vial was added an aliquot of a solution of the p-sulfonatocalix[n]arene (0.1 M) in an identical solvent to achieve molar equivalency of 0, 0.2, 0.4 … 2.0, 2.5, 3.0, 4.0, 5.0, 7.0, 10.0. 1H- and/or 31P-NMR were recorded of each sample and titration curves constructed following analysis by ACD Spectrus Processor. Data fitting was conducted using WinEQNMR [25].
2.3. Computational Details
Calculations were undertaken using the Spartan’16 Parallel Suite [26] running on a Mac Pro with 3.5 GHz 6-Core Intel Xenon E5 processors and two threads per core. Calixarenes, GD and simulants were constructed using the Build option. A full conformational search of calixarene hosts, in the acid form, using molecular mechanics (MMFF) was followed by geometry refinement by semi-empirical methods (PM6). The same process was adopted for GD and the simulants which were then introduced into the central cavities of the calixarenes and the geometries of the complexes optimized (MMFF/PM6). Acidic protons were removed from both calixarenes, the sulfonyl groups given partial double bond character and sodium counter ions added to generate their sodium sulfonate forms. GD and simulant complex geometries were determined as for the acid forms. Simulations were attempted using explicitly solvated systems (4 to 18 water molecules) and a continuum model, but these failed to refine successfully.
3. Results and Discussion
3.1. Experimental Investigation of Host–Guest Behaviour
Preliminary 1H-NMR data of D2O and buffered D2O (phosphate, pH 7, 0.25 M) solutions of SCX host compounds and organophosphonate guests revealed upfield chemical shift perturbations in aliphatic guest proton environments and (up/down)field perturbations in the calixarenes’ aromatic and aliphatic environments, indicative of inclusion complex formation. Typically, perturbations in the guest molecule proton environments were greater in magnitude than those experienced by the calixarene host protons. For example, in DIMP-SCX4 unbuffered D2O solutions at a molar ratio of 1:2 the iPr CH3 protons were perturbed upfield by ca. −0.063 ppm, and under analogous conditions the terminal CH3 protons of DEMP were perturbed by −0.09 ppm (Figure 3 and Supplementary Materials). In buffered solutions, slightly smaller magnitude perturbations of −0.035 and −0.05 ppm were also observed.
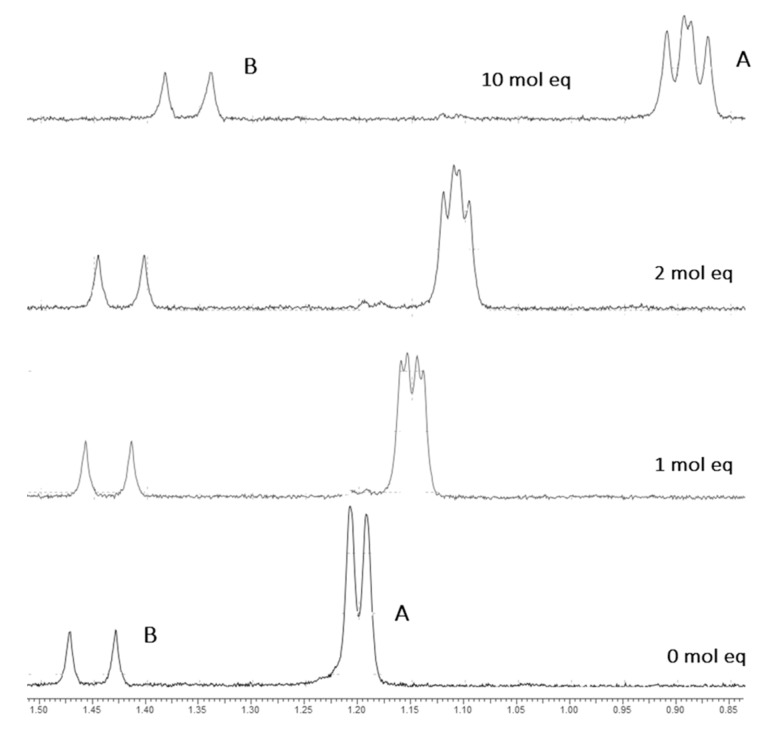
Figure 3.
A stack plot of DIMP 1H environments in aqueous solution with increasing SCX4 concentration (stated as molar equivalents). Peak A corresponds to the iPr CH3 proton environment and undergoes shift perturbations of −0.32 ppm by the addition of 10 molar equivalents of SCX4. Peak B corresponds to the P-CH3 group, and also undergoes upfield shift perturbations (D2O, 298 K).
Analysis of 31P-NMR spectra also indicated formation of inclusion complexes for all simulants studied as evidenced by a downfield shift perturbation in 31P resonances upon addition of the calixarene species. For example, in unbuffered solutions 31P-NMR data revealed changes in chemical shift upon addition of two molar equivalent of SCX4 for DEMP (δ = 35.45 ppm) of Δδ = −0.053 and for DIMP (δ = 32.90 ppm) of Δδ = −0.065 ppm. In buffered solutions, similar upfield shifts were also observed, with Δδ = −0.027 and −0.030 at two molar equivalents of SCX4 for DEMP and DIMP, respectively. Smaller chemical shift perturbations were observed for both compounds in the presence of SCX6 with Δδ = −0.004 ppm for DEMP in both unbuffered and buffered solutions, and Δδ = −0.038 and −0.008 ppm for DIMP in unbuffered and buffered solutions, respectively. It should be noted that the addition of either SCX species considerably acidifies the solution, and by the addition of two molar equivalents of SCX4 to solutions of DEMP and DIMP the pH had dropped from approximately seven to around two, and for PMP from 2.9 to 2 (see Supplementary Materials). In order to investigate the possible effects of pH on the system the acyclic compound 4-hydroxybenzene sulfonic acid was titrated into non-buffered D2O solutions of DEMP, DIMP and PMP and 31P-NMR examined. In the case of DEMP and DIMP the 31P resonances were invariant to the addition of 4-hydroxybenzene sulfonic acid, indicating both a lack of pH effects and confirming that complexation is driven by the hydrophobic cavity of the cyclic hosts. In the case of PMP, however, considerable perturbation of the P-centre was observed, indicating the formation of different solution species. Therefore, as this could not be separated from the complexation process, PMP data is reported in buffered solutions using only 1H-NMR for data fitting analyses.
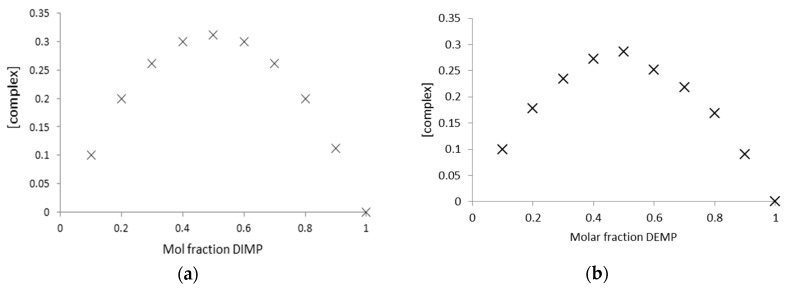
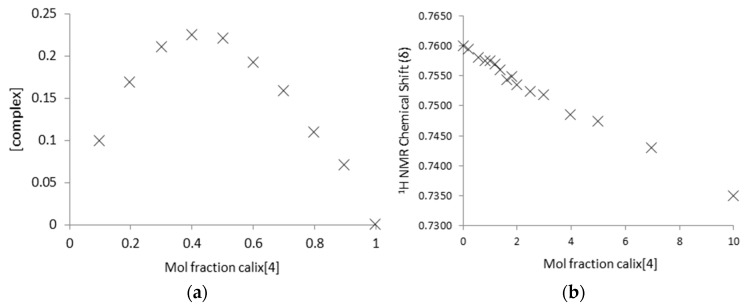
Job plot analysis using 1H-NMR data was conducted on DEMP and DIMP-calixarene pairs and used to determine host–guest complex stoichiometry in unbuffered D2O. For DEMP and DIMP a clear 1:1 complex stoichiometry was indicated by plot maxima at ≈0.5 with both sizes of calixarenes and by analysis of both calixarene and OP proton environments (Figure 4).
Figure 4.
Example Job Plot analyses of (a) SCX4 and DIMP and (b) SCX6 and DEMP (D2O, 298 K, [total] = 0.01 M).
For PMP monitoring, the proton resonances of the tBu group of the molecule indicated a 1:1 binding interaction, however, the P-CH3 environments in particular gave rise to skewed plots in D2O and did not furnish expected 1:1 curves in buffered solutions. This suggests that the tBu moiety is favorably included in the calixarene cavity and the P-CH3 group is closer to the polar water environment due to its proximity to the polar P=O and O-H functionalities. It may also indicate the presence of multiple binding modes or conformations in the PMP–host complex.
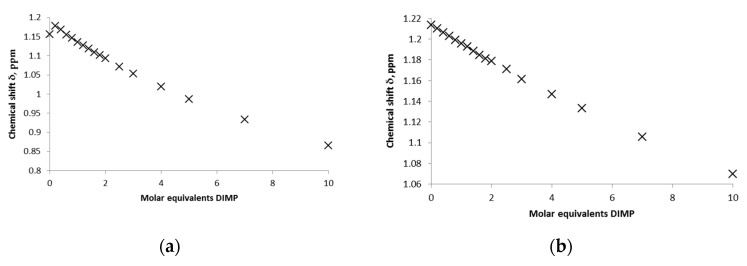
Quantitative 1H-NMR titration experiments were then conducted in both D2O and buffered D2O for DEMP, DIMP and PMP (buffered only) with both calixarenes in order to determine complex affinity. Examples of the resulting titration curves for DIMP and PMP with SCX4 are shown in Figure 5.
Figure 5.
NMR titration plots in D2O following the alkyl termini of DIMP (iPr) in (a) unbuffered D2O and (b) phosphate buffered (0.25 M, pH 7) solutions (298 K, [initial] = 0.01 M).
Data fitting was successful for all simulant–calixarene host–guest pairs with the exception of the DEMP:SCX6 pair. The calculated association constants, K11, are given in Table 1. For the SCX4 host the low complex affinity is nearly identical in D2O solutions with 64 and 53 M−1 for DEMP and DIMP, respectively. The presence of buffer appears to lower the complex affinity, as has been reported by others for phosphate buffers. In the case of the larger SCX6 host, there is an indication of complex formation with DEMP but the data could not be fitted satisfactorily to allow association constants to be determined. For the DIMP:SCX6 pair there is a slight decrease in complex affinity in D2O from 65 to 20 M−1; again a lower affinity (16 M−1) is observed in the buffered solutions. In the case of PMP, low affinity complexes were observed for both SCX host species in buffered solutions, and determination of complex affinity in non-buffered solutions was not feasible, as discussed earlier.
Table 1.
Association constants (Kassoc/M−1) for host and guest complexes in 0.25 M phosphate-buffered D2O and non-buffered D2O calculated with WinEQNMR software [25]. Errors stated are those obtained from the data fitting process and fit plots are presented in the Supplementary Materials.
| p-Sulfonatocalix[n]arene | Kassoc, n = 4 | Kassoc, n = 6 | ||
|---|---|---|---|---|
| No Buffer | Buffer | No Buffer | Buffer | |
| DIMP | 64 ± 2 | 20 ± 1 | 20 ± 8 | 16 ± 2 |
| DEMP | 53 ± 2 | 21 ± 2 | a | a |
| PMP | n/a | 20 ± 1 | b | 95 ± 24 |
| GD | n/a | 75 ± 2 | b | <10 |
a Unable to fit; b Experiment not conducted.
Phosphate buffer was employed by Douteau-Guével et al. who note that other biological buffers interact strongly with p-sulfonatocalix[n]arenes but that phosphate buffer had no influence on complexation and could be used from pH 5 to 8 [27]. Consequently, buffered solutions (phosphate, pH 7, 0.25 M) were used in the GD-calix titration experiments, in order to minimize the hydrolysis of GD during the titration experiments. Job plots constructed by monitoring the tBu environment indicated the formation of 1:1 stoichiometry complexes of GD with both calixarene hosts (Figure 6), however, analysis of the P-CH3 group provided little indication of complex formation. This is suggestive of a host–guest complex conformation in which the pinacolyl side-chain is included in the cavity and the P-center is closer to the solvent environment. In the case of SCX6 Job plots were less clear, indicative of weaker interaction between the host and guest. Quantitative 1H-NMR titration experiments were again conducted and association constants determined by EQNMR analysis as K11 = 75 and <10 M−1 for the SCX4 and SCX6 hosts, respectively. It should be noted that, unlike with β-cyclodextrin [23], there was no observation of chiral discrimination as a result of complex formation. This is expected, given that the SCX hosts are achiral and chiral recognition using calixarenes is usually achieved through either the incorporation of chiral functionality or of asymmetry [28,29].
Figure 6.
Analysis of GD and SCX4 association under buffered conditions from (a) a Job plot and (b) NMR titration experiments following tBu proton environment.
Affinities between GD and SCX6 were lower than for the SCX4 host, which may be attributed to the larger cavity size of SCX6 host and the poor fit with the size of the guest molecules. It is interesting that PMP exhibited a stronger association with SCX6 than SCX4 despite its similarity in size and structure to GD.
3.2. Computational Studies of Host–Guest Complexation
Calculations were undertaken in the gas phase and solvated state with explicit water molecules introduced into the simulations. The latter calculations encountered problems upon refinement at semi-empirical level so discussion has been restricted to the gas phase results. We have previously shown that, at this level of theory, solution phase host–guest complexes are quite adequately represented by gas phase models [30,31].
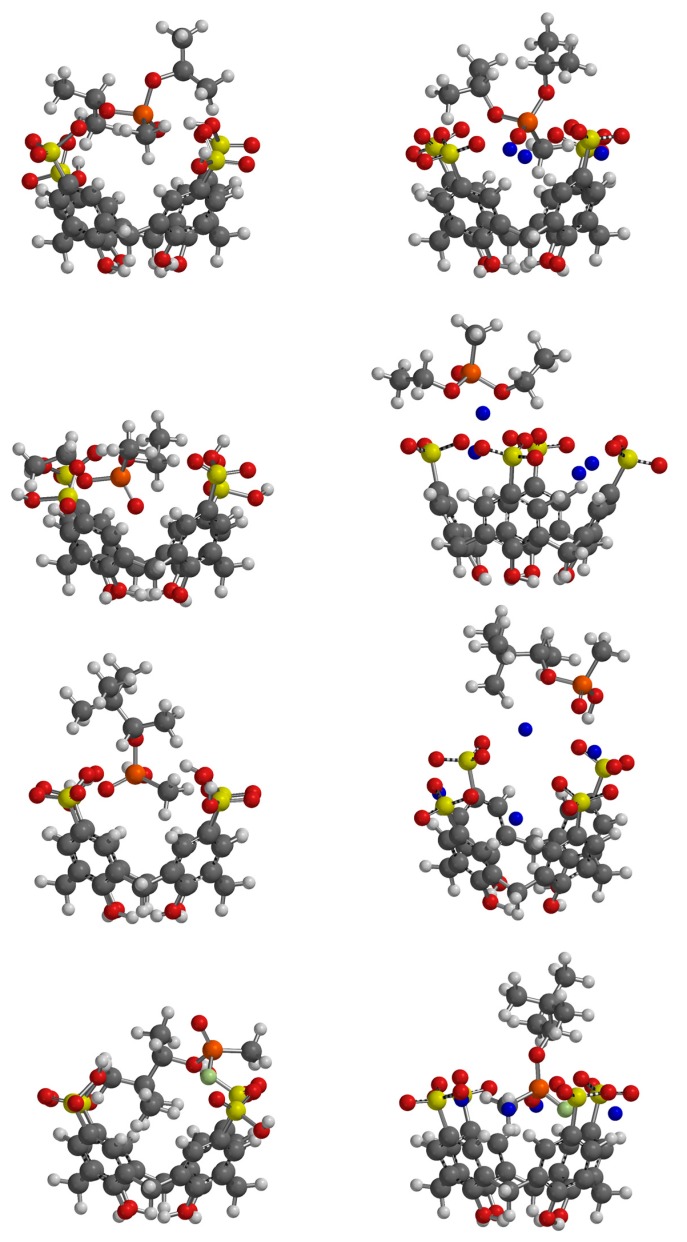
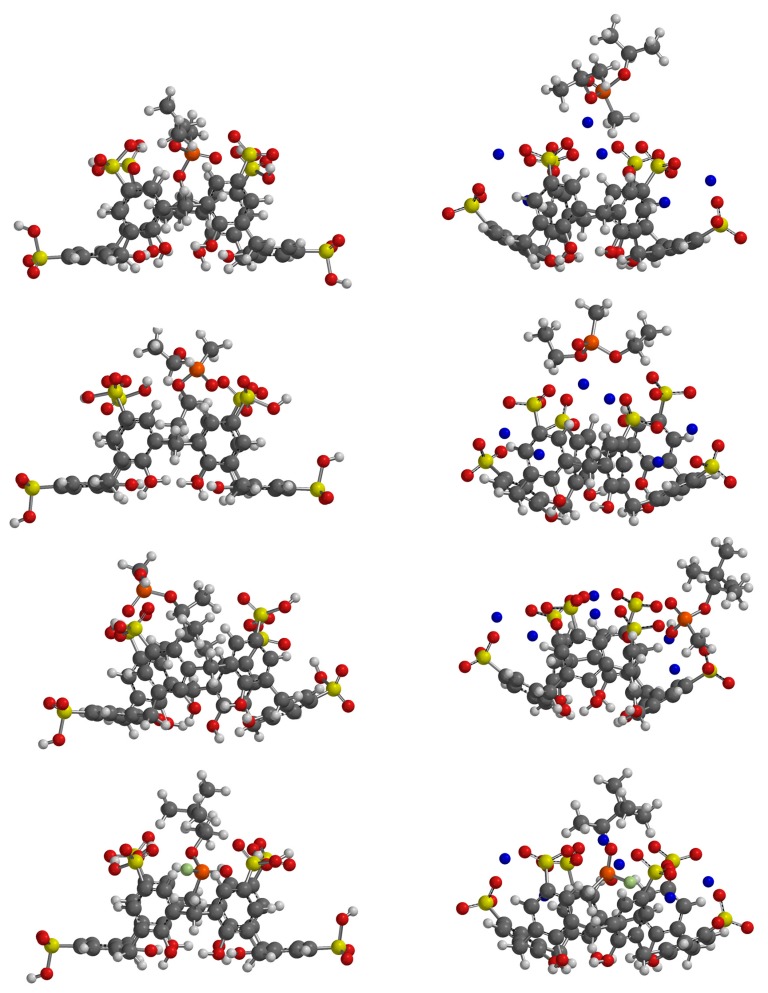
While binding affinities were not reproduced well, the resulting geometries of the complexes give some insights into the potential binding of GD and simulants by the two calixarenes (Figure 7 and Figure 8). It is clear that where differences in binding affinities occur upon buffering, it is the formation of sodium salts of the calixarenes which reduces the access to the macrocyclic cavity. In the case of SCX6, this is accompanied by large conformational changes in which the sodium cations bridge adjacent sulfonate groups (Figure 8). Inspection of the different complexes shows that hydrogen bonding between the interior of the macrocyclic hosts and their guests is largely restricted to their sulfonic acid forms (Table 2 and Table 3). Only PMP, of all the species tested, can act as a hydrogen bond donor which leads to synergistic interactions in the case of both SCX4 and SCX6 and could potentially lead to higher stability complexes in the unbuffered system. In the case of the PMP:SCX6 complex the guest is held by two hydrogen bonds to the phosphate oxygen atom. One alkyl substituent is buried within the calixarene cavity suggesting further stabilization through hydrophobic interactions. Under buffered conditions the sulfonate salts of the macrocycles are much less amenable to binding PMP. The geometry of the PMP:SCX6 complex suggests that PMP only weakly associates with the exterior of buffered calix[6]sulfonate. While the calixarenes studied are also likely to adopt numerous conformers in solution, the models chosen here represent the most stable based upon conformational analysis.
Figure 7.
SCX4 acid (left) and sodium sulfonate (right) complexes with (from top) DIMP, DEMP, PMP and GD.
Figure 8.
SCX6 acid (left) and sodium sulfonate (right) complexes with (from top) DIMP, DEMP, PMP and GD.
Table 2.
Hydrogen bonding (Å) between SCX4 host and guests.
| Guest | No Buffer | Buffer | ||
|---|---|---|---|---|
| O…HOS a | P=O…HOS | OH…O3S | OH…O3S | |
| DIMP | 1.859 | 1.642 | ||
| DEMP | 1.899 | |||
| PMP | 1.993 | 1.676 | 1.711 | 1.429 |
| GD | 2.143 | |||
a bridging to the P-O-R oxygen except for PMP where it is P-O-H.
Table 3.
Hydrogen bonding (Å) between SCX6 host and guests.
| Guest | No Buffer | Buffer | ||
|---|---|---|---|---|
| O…HOS a | P=O…HOS | OH…O3S | OH…O3S | |
| DIMP | 1.183 | 1.750 | ||
| DEMP | 2.123 | |||
| PMP | 1.625, 1.670 | 1.768 | 1.573 | |
| GD | 2.441 | |||
a bridging to the P-O-R oxygen except for PMP where it is P-O-H.
The effects of C–H…π interactions were analysed from the models generated by measuring the interactions between methyl hydrogens and the centroids of the closest aromatic groups, for both the SCX4 (Table 4) and SCX6 (Table 5) hosts. A majority were below three angstrom, consistent with literature data. [32] The angles between aromatic centroid, hydrogen atom and closest aromatic carbon atom were determined and found to range between 27° and 32°, also in agreement with crystallographic data for C–H…π interactions. Most noticeable is the lack of C–H…π interactions in the buffered state for both calixarenes which reflects the lack of inclusion seen in most cases.
Table 4.
C–H…π interactions (Å and °) between SCX4 host and guests.
| Guest | No Buffer | Buffer | ||
|---|---|---|---|---|
| C–H…π | α a | C–H…π | α a | |
| DIMP | 3.066 | 27.2 | 2.968 | 24.4 |
| DEMP | 3.402 | 23.5 | ||
| PMP | 3.051 | 25.8 | ||
| GD | 2.496 | 29.6 | 2.803 | 29.0 |
| 2.532 | 28.4 | |||
a angle between aromatic centroid, hydrogen atom and closest aromatic carbon atom.
Table 5.
C–H…π interactions (Å and °) between SCX6 host and guests.
| Guest | No Buffer | Buffer | ||
|---|---|---|---|---|
| C–H…π | α a | C–H…π | α a | |
| DIMP | 2.313 | 31.6 | ||
| 2.398 | 31.1 | |||
| 2.741 | 29.4 | |||
| 2.807 | 29.0 | |||
| DEMP | 2.919 | 29.3 | ||
| PMP | 3.016 | 29.9 | ||
| GD | 2.931 | 29.0 | 3.266 | 25.4 |
a angle between aromatic centroid, hydrogen atom and closest aromatic carbon atom.
3.3. Discussion of Host–Guest Behavior and Agent–Simulant Comparisons
While there is considerable literature on the inclusion properties of SCX4 and SCX6, many of the papers focus on complexes with a potential biological importance [27,33,34,35,36]. Calixarene binding to the OP VX has been reported [19] and, as we have demonstrated, the formation of host–guest inclusion complexes of the OP nerve agent GD and commonly used simulants with SCX4 and SCX6 is clearly feasible. In agreement with the literature, this appears to be driven by the hydrophobic inclusion of aliphatic groups [16,37]. In contrast to reports by ourselves and others on the inclusion of OP CWAs and related compounds with cyclodextrins [13,23], the affinity of the complexes is in most cases fairly weak. This may be a result of non-optimized size match between the aliphatic side-chains of the guest species and the internal hydrophobic cavity, or the relative hydrophobic nature of the cyclodextrin and SCX cavities. For DIMP, DEMP and GD the affinity of the SCX6 complexes was lower than SCX4, indicating a lower stability complex and poorer match between host and guest.
There is very little difference in the affinity of the cavity for DIMP, DEMP and PMP versus GD. Notably, PMP is the only guest capable of synergistic hydrogen bonding with SCX4 and SCX6 particularly when the hosts are in the acidic form. The computational simulations indicate that binding to all guests except PMP is less likely when the both hosts are in the sodium (buffered) form. Under these conditions, the sodium cations form networks with the sulfonate groups which block the approach of hosts. Only PMP, by virtue of its phosphate hydroxyl group, can interact with the sodium sulfonate forms of SCX4 and SCX6. Even so, fewer hydrogen bonding interactions are predicted with the calixarene sodium salts when moving from neutral to buffered solutions of SCX4 and SCX6.
4. Conclusions
The hydrophobically-driven inclusion of the nerve agent GD and the organophosphonate species DEMP, DIMP and PMP has been demonstrated using experimental NMR techniques and the stoichiometry and affinity of the resulting complexes determined. Computational modeling approaches have been used to further elucidate the mechanism of binding, and to explain differences in observed complexation behavior between agents and simulants. It appears that in buffered solutions the effects of the cations significantly reduce the abilities of guest molecules to bind to the calixarene hosts. This, and poor host–guest complementarity, contributes to the weak binding observed for buffered SCX6 complexes. This work adds to our recent efforts to more fully understand the supramolecular behavior of CWAs and to furnish agent–simulant correlations to aid in simulant selection and extrapolation of simulant-only data.
Acknowledgments
MRS and JE thank Dstl for funding this work. MRS and JE also thank James Jones (CBR Division, Dstl) for NMR service support. PJC thanks the US Army Research Office, contract W911NF-15-1-0624, for hardware and software.
Supplementary Materials
The following are available online: pH data (Table S1), Job plot and NMR titration data (Figures S1–S38) and SCX stack plot (Figure S39).
Author Contributions
M.R.S. conceived and designed the experiments, M.R.S. and J.A.E. carried out the experiments and analyzed the data. P.J.C. contributed computational data and associated analysis. All authors contributed to the writing of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Timperley C.M., editor. Best Synthetic Methods: Organophosphorus (V) Chemistry. Elsevier; Oxford, UK: 2015. General Overview; pp. 1–90. [Google Scholar]
- 2.Thierman H., Aurbek N., Worek F. Treatment of Nerve Agent Poisoning. In: Worek F., Jenner J., Thiermann H., editors. Chemical Warfare Toxicology: Volume 2: Management of Poisoning. Royal Society of Chemistry; Cambridge, UK: 2016. pp. 1–42. [Google Scholar]
- 3.Kim K., Tsay O.G., Atwood D.A., Churchill D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2011;111:5345–5403. doi: 10.1021/cr100193y. [DOI] [PubMed] [Google Scholar]
- 4.Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction, Technical Secretariat of the Organisation for the Prohibition of Chemical Weapons, 2005. [(accessed on 29 September 2017)]; Available online: https:www.opcw.org/fileadmin/OPCW/CWC/CWC_en.pdf.
- 5.Black R.M., Read R.W. Environmental and biomedical sample analysis in support of allegations of use of chemical warfare agents. Toxin Rev. 2007;26:275–298. doi: 10.1080/15569540701474328. [DOI] [Google Scholar]
- 6.Yokoyama K., Araki S., Murata K., Nishikitani M., Okumura T., Ishimatsu S., Takasu N. Neurobehavioral and Central and Autonomic Nervous System Effects of Tokyo Subway Sarin Poisoning. J. Physiol. (Paris) 1998;92:317–323. doi: 10.1016/S0928-4257(98)80040-5. [DOI] [PubMed] [Google Scholar]
- 7.Rice H., Dalton C.H., Price M.E., Graham S.J., Green A.C., Jenner J., Groombridge H.J., Timperley C.M. Toxicity and medical countermeasure studies on the organophosphorus nerve agents VM and VX. Proc. R. Soc. A. 2015;471:20140891. doi: 10.1098/rspa.2014.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambrook M.R., Notman S. Supramolecular chemistry and chemical warfare agents: From fundamentals of recognition to catalysis and sensing. Chem. Soc. Rev. 2013;42:9251–9267. doi: 10.1039/c3cs60230c. [DOI] [PubMed] [Google Scholar]
- 9.Bolliger J.L., Belengeur A.M., Nitschke J.R. Enantiopure Water-Soluble [Fe4L6] Cages: Host-Guest Chemistry and Catalytic Activity. Angew. Chem. Int. Ed. 2013;52:7958–7962. doi: 10.1002/anie.201302136. [DOI] [PubMed] [Google Scholar]
- 10.Hiscock J.R., Sambrook M.R., Cranwell P.B., Watts P., Vincent J.C., Xuereb N.J., Wells N.J., Raja R., Gale P.A. Tripodal molecules for the promotion of phosphoester hydrolysis. Chem. Commun. 2014;50:6217–6220. doi: 10.1039/C4CC00333K. [DOI] [PubMed] [Google Scholar]
- 11.Hiscock J.R., Sambrook M.R., Ede J.A., Wells N.J., Gale P.A. Disruption of a binary organogel by the chemical warfare agent soman (GD) and common organophosphorus simulants. J. Mater. Chem. A. 2015;3:1230. doi: 10.1039/C4TA04834B. [DOI] [Google Scholar]
- 12.Désiré B., Saint-André S. Inactivation of sarin and soman by cyclodextrins in vitro. Experientia. 1987;43:395–397. doi: 10.1007/BF01940424. [DOI] [PubMed] [Google Scholar]
- 13.Letort S., Balieu S., Erb W., Géraldine G., Estour F. Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds. Beilstein J. Org. Chem. 2016;12:204–228. doi: 10.3762/bjoc.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worek F., Seeger T., Zengerle M., Kubik S., Thiermann H., Wille T. Effectiveness of a substituted β-cyclodextrin to prevent cyclosarin toxicity in vivo. Toxicol. Lett. 2014;226:222–227. doi: 10.1016/j.toxlet.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Guo D.-S., Liu Y. Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc. Chem. Res. 2014;47:1925–1934. doi: 10.1021/ar500009g. [DOI] [PubMed] [Google Scholar]
- 16.Arena G., Contino A., Gulino F.G., Magrí A., Sciotto D., Ungaro R. Complexation of small neutral organic molecules by water soluble calix[4]arenes. Tetrahedron Lett. 2000;41:9327–9330. doi: 10.1016/S0040-4039(00)01687-7. [DOI] [Google Scholar]
- 17.Rehm M., Frank M., Schatz J. Water-soluble calixarenes—Self-aggregation and complexation of noncharged aromatic guests in buffered aqueous solution. Tetrahedron Letts. 2009;50:93–96. doi: 10.1016/j.tetlet.2008.10.089. [DOI] [Google Scholar]
- 18.Lehn J.M., Meric R., Vigneron J.-P., Cesario M., Guilhem J., Pascard C., Asfari Z., Vicens J. Binding of acetylcholine and other quaternary ammonium cations by sulfonated calixarenes. Crystal structure of a [choline-tetrasulfonated calix[4]arene] complex. Supramol. Chem. 1995;5:97–103. doi: 10.1080/10610279508029480. [DOI] [Google Scholar]
- 19.Schneider C., Bierwisch A., Koller M., Worek F., Kubik S. Detoxification of VX and Other V-Type Nerve Agents in Water at 37 °C and pH 7.4 by Substituted Sulfonatocalix[4]arenes. Angew. Chem. Int. Ed. 2016;55:12668–12672. doi: 10.1002/anie.201606881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavoie J., Srinivasan S., Nagarajan R. Using cheminformatics to find simulants for chemical warfare agents. J. Hazard. Mater. 2011;194:85–91. doi: 10.1016/j.jhazmat.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 21.Bartelt-Hunt S.L., Knappe D.R.U., Barlaz M.A. A review of chemical warfare agent simulants for the study of environmental behaviour. Crit. Rev. Environ. Sci. Technol. 2008;38:112–136. doi: 10.1080/10643380701643650. [DOI] [Google Scholar]
- 22.Willis M.P., Varady M.J., Pearl T.P., Fouse J.C., Riley P.C., Mantooth B.A., Lalain T. Physics-based agent to simulant correlations for vapor phase mass transport. J. Hazard. Mater. 2013;263:479–485. doi: 10.1016/j.jhazmat.2013.09.064. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook M.R., Vincent J.C., Ede J.A., Gass I.A., Cragg P.J. Experimental and computational study of the inclusion complexes of β-cyclodextrin with the chemical warfare agents soman (GD) and commonly used simulants. RSC Adv. 2017;7:38069–38076. doi: 10.1039/C7RA03328A. [DOI] [Google Scholar]
- 24.Sambrook M.R., Gass I.A., Cragg P.J. Spectroscopic and inclusion properties of G-series chemical warfare agents and their simulants: A DFT study. Supramol. Chem. 2017 doi: 10.1080/10610278.2017.1401074. [DOI] [Google Scholar]
- 25.Hynes M.J. EQNMR: A computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans. 1993;2:311–312. doi: 10.1039/dt9930000311. [DOI] [Google Scholar]
- 26.Spartan’16. Wavefunction Inc.; Irvine, CA, USA: 2016. [Google Scholar]
- 27.Douteau-Guével N., Coleman A.W., Morel J.-P., Morel-Desrosiers N. Complexation of the basic amino acids lysine and arginine by three sulfonatocalix[n]arenes (n = 5 4, 6 and 8) in water: Microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation. J. Chem. Soc. Perkin Trans. 1999;2:629–633. doi: 10.1039/a806855k. [DOI] [Google Scholar]
- 28.Zheng Y.-S., Zhang C. Exceptional chiral recognition of racemic carboxylic acids by calix[4]arenes bearing optically pure α,β-amino alcohol groups. Org. Lett. 2004;6:1189–1192. doi: 10.1021/ol036122o. [DOI] [PubMed] [Google Scholar]
- 29.Li S.-Y., Xu Y.-W., Liu J.-M., Su C.-Y. Inherently chiral calixarenes: Synthesis, optical resolution, chiral recognition and asymmetric catalysis. Int. J. Mol. Sci. 2011;12:429–455. doi: 10.3390/ijms12010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan R., Cragg P.J. Supramolecular chemistry in silico. Supramol. Chem. 2008;20:443–451. doi: 10.1080/10610270701302465. [DOI] [Google Scholar]
- 31.Miah M., Pavey K.D., Gun’ko V.M., Sheehan R., Cragg P.J. Observation of transient metal inclusion in oxacalix[3]arenes. Supramol. Chem. 2004;16:185–192. doi: 10.1080/10610270310001644473. [DOI] [Google Scholar]
- 32.Suezawa H., Ishihara S., Umezawa Y., Tsuboyama S., Nishio M. The aromatic CH/π hydrogen bond as an important factor in determining the relative stability of diastereomeric salts relevant to enantiomeric resolution: A crystallographic database study. Eur. J. Org. Chem. 2004:4816–4822. doi: 10.1002/ejoc.200400373. [DOI] [Google Scholar]
- 33.Douteau-Guével N., Coleman A.W., Morel J.-P., Morel N. Complexation of basic amino acids by water-soluble calixarene sulphonates as a study of the possible mechanisms of recognition of calixarene sulphonates by proteins. J. Phys. Org. Chem. 1998;11:693–696. doi: 10.1002/(SICI)1099-1395(1998100)11:10<693::AID-POC18>3.0.CO;2-8. [DOI] [Google Scholar]
- 34.Kalchenko O.I., Perret F., Morel-Desrosiers N., Coleman A.W. A comparative study of the determination of the stability constants of inclusion complexes of p-sulfonatocalix[4]arene with amino acids by RP-HPLC and 1H NMR. J. Chem. Soc. Perkin Trans. 2001;2:258–263. doi: 10.1039/b005497f. [DOI] [Google Scholar]
- 35.Douteau-Guével N., Perret F., Coleman A.W., Morel J.-P., Morel-Desrosiers N. Binding of dipeptides and tripeptides containing lysine or arginine by p-sulfonatocalixarenes in water: NMR and microcalorimetric studies. J. Chem. Soc. Perkin Trans. 2002;2:524–532. doi: 10.1039/b109553f. [DOI] [Google Scholar]
- 36.Lazar A., Da Silva E., Navaza A., Barbey C., Coleman A.W. A new packing motif for para-sulfonatocalix[4]arene: The solid state structure of the para-sulfonatocalix[4]arene D-arginine complex. Chem. Commun. 2004:2162–2163. doi: 10.1039/b408863h. [DOI] [PubMed] [Google Scholar]
- 37.Li H., Song J.-P., Chao J.-B., Shuang S.-M., Dong C. Study on the inclusion behavior of p-sulfonatocalix[6]arene with propranolol by spectrofluorimetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;97:155–160. doi: 10.1016/j.saa.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.