Abstract
Sakuranetin (1) is a rice flavanone-type phytoalexin. We have already reported that the metabolites from the detoxification of 1 by Pyricularia oryzae are naringenin (2) and sternbin. In this study, we investigated whether the rice sheath blight fungus Rhizoctonia solani, another major rice pathogen, can detoxify 1. The extract of R. solani suspension culture containing 1 was analyzed by LC-MS to identify the metabolites of 1. Three putative metabolites of 1 were detected in the extract from the R. solani suspension culture 12 h after the addition of 1, and they were identified as 2, sakuranetin-4′-O-β-d-xylopyranoside (3), and naringenin-7-O-β-d-xylopyranoside (4) by NMR, LC-MS/MS, and GC-MS analyses. The accumulation of 2, 3, and 4 reached their maximum levels 9–12 h after the addition of 1, whereas the content of 1 decreased to almost zero within 9 h. The antifungal activities of 3 and 4 against R. solani were negligible, and 2 showed weaker antifungal activity than 1. We concluded that 2, 3, and 4 are metabolites from the detoxification of 1 by R. solani. Xylosylation is a rare and efficient detoxification method for phytoalexins.
Keywords: phytoalexin, flavonoid, rice, Rhizoctonia solani, xylosylation
1. Introduction
Phytoalexins are antimicrobial secondary metabolites that are produced in plants de novo after pathogen attack [1]. In rice plants, 19 phytoalexins have been reported, including 14 labdane-related diterpenes (momilactones, oryzalexins, and phytocassanes), one casbene-type diterpene (ent-10-oxodepressin), one flavanone (sakuranetin), and two amides (N-benzoyltryptamine and N-cinnamoyltryptamine) [2]. We demonstrated that the rice phytoalexins play an important role in blast disease resistance in rice plants [3,4,5].
Phytopathogenic microorganisms can detoxify phytoalexins to prevent their antimicrobial activities [6,7,8,9]. We have previously reported that the rice diterpenoid phytoalexin momilacotone A and the flavonoid phytoalexin sakuranetin (1) can be metabolized and detoxified by the rice blast fungus Pyricularia oryzae [3,5,10,11]. The detoxified metabolites of 1 were identified as naringenin (2) and sternbin, which are derived from 1 via 7-O-demethylation and 3′-hydroxylation, respectively [10].
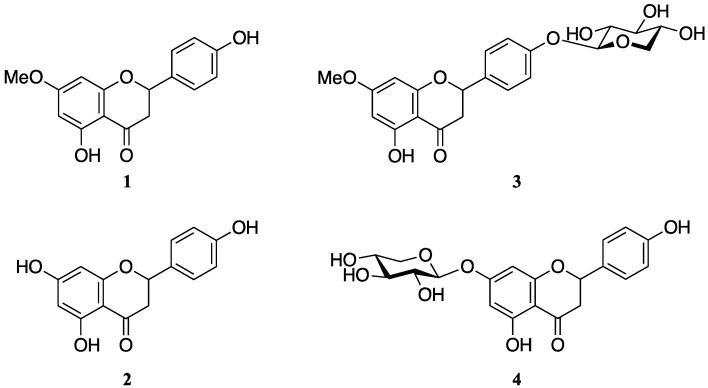
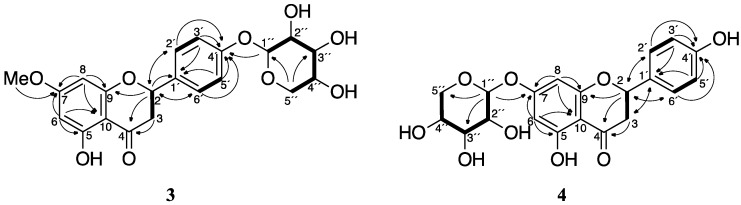
Rice sheath blight fungus, Rhizoctonia solani, is another major fungal pathogen in rice [12], and 1 has been reported to show antifungal activity against R. solani [13]. We are interested in whether R. solani can also detoxify 1 and whether the detoxified metabolites are different from those of P. oryzae. In this study, the detoxified metabolites of 1 were identified from the R. solani suspension culture. We identified two xylosylated flavanones specific to R. solani, sakuranetin-4′-O-β-d-xylopyranoside (3) and naringenin-7-O-β-d-xylopyranoside (4), as well as 2, which is also a metabolite of P. oryzae, as the detoxified metabolites of 1 (Figure 1).
Figure 1.
Structures of sakuranetin (1), naringenin (2), sakuranetin-4′-O-β-d-xylopyranoside (3), and naringenin-7-O-β-d-xylopyranoside (4).
2. Results and Discussion
2.1. Sakuranetin (1) is Metabolized by Rhizoctonia solani
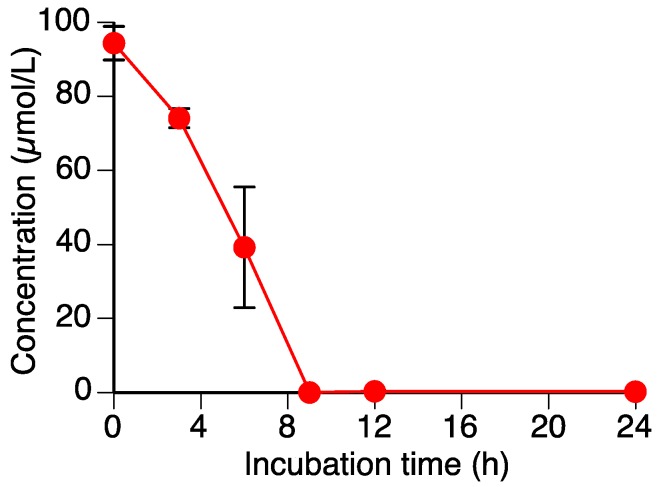
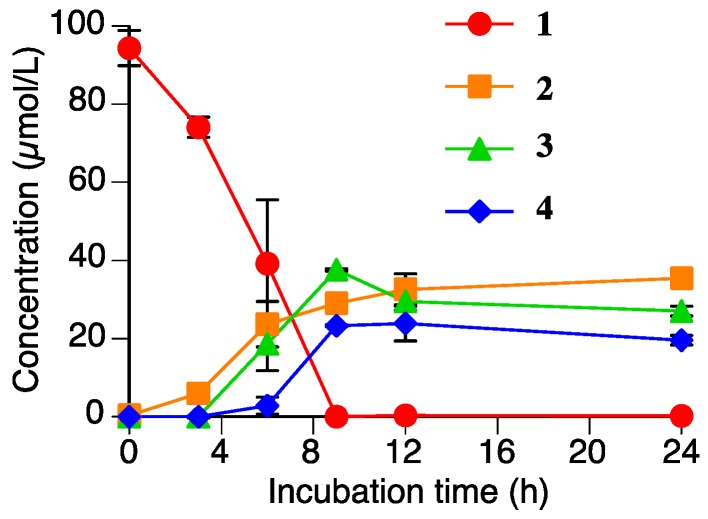
As shown in Figure 2, the level of sakuranetin (1) decreased to almost zero within 9 h in the R. solani suspension culture. We have already shown that another rice pathogenic fungus, P. oryzae, can metabolize 1, which suggested that R. solani could also metabolize 1.
Figure 2.
Time-dependent decrease in sakuranetin (1) content in the Rhizoctonia solani suspension culture. Values are reported as the mean ± SD (n = 3).
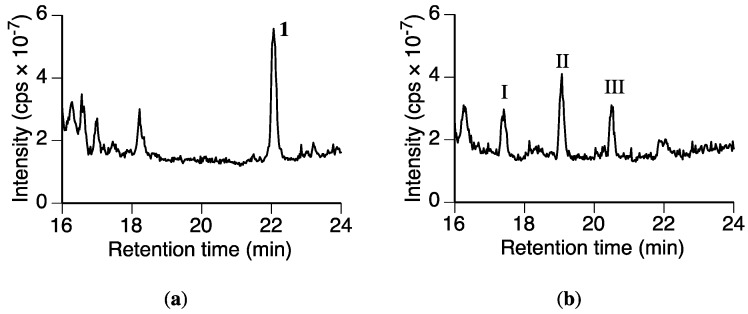
We then screened the fungal culture for putative metabolites of 1. The MeOH extracts from the fungal culture containing 1 after incubation for 0 and 12 h were analyzed by LC-MS. The total ion current chromatograms of each extract were compared (Figure 3). Compound 1 was detected in the 0 h extract at tR 22.1 min, whereas only a trace amount of 1 was present in the 12 h extract. Three new peaks were detected at tR 17.4 (I), 19.1 (II), and 20.5 min (III) in the 12 h extract. These peaks were not detected in a culture without 1 or in the medium containing 1 without the fungus (Figure S1). Therefore, we were certain these peaks were from the possible metabolites of 1.
Figure 3.
Total ion current chromatograms obtained from the Rhizoctonia solani suspension cultures containing sakuranetin (1) using LC-MS. (a) Chromatogram after 0 h of incubation following the addition of 1; (b) 12 h of incubation after the addition of 1.
2.2. Identification of Naringenin (2) in the Rhizoctonia solani Suspension Culture Containing Sakuranetin (1)
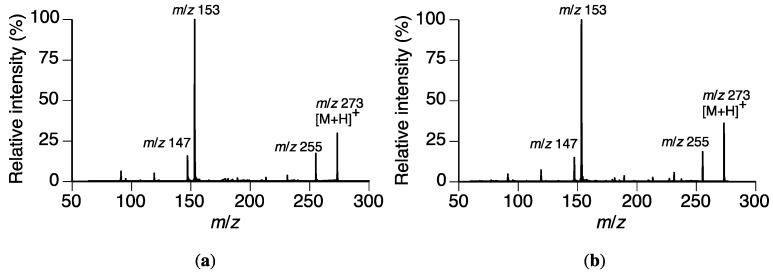
The mass spectrum of peak II showed a plausible [M + H]+ ion at m/z 273, which was consistent with the [M + H]+ of naringenin (2). The tR of II (19.1 min) coincided that of 2, which had been identified as a metabolite of 1 by P. oryzae in our previous study [10]. The tR and collision-induced dissociation-mass spectrum (CID-MS) of peak II were compared with those of 2 in LC-MS/MS analysis. The tR and CID-MS were consistent as shown in Figure 4. Therefore, we concluded that peak II was from 2.
Figure 4.
ESI-CID-MS results of a potential metabolite (peak II) in the Rhizoctonia solani suspension culture containing sakuranetin (1) and naringenin (2). (a) Peak II; (b) naringenin (2).
2.3. Purification and Identification of Sakuranetin-4′-O-β-d-xylopyranoside (3) and Naringenin-7-O-β-d-xylopyranoside (4) in Rhizoctonia solani Suspension Culture Containing Sakuranetin (1)
Peaks I and III showed significant ions at m/z 273 and 287, respectively, in their electrospray ionization-mass spectra (ESI-MS) (Figure S2). The ions are identical to the protonated molecules of 2 and 1, respectively; however, their tRs are different from those of 2 and 1. Therefore, we speculated that peaks I and III are derived from compounds that include naringenin and sakuranetin moieties, respectively.
We then tried to purify compounds 3 (peak III) and 4 (peak II) from the R. solani suspension culture containing 1 based on LC-MS analysis. Compounds 3 (1.1 mg, a white solid) and 4 (2.4 mg, a yellow oil) were successfully purified from 300 mL of the R. solani suspension culture containing 10 mg of 1. Finally, 4.4 mg of 3 and 9.4 mg of 4 were obtained from 50 mg of 1 after several purification steps.
The ESI-MS of 3 showed plausible [M + H]+ and [M + Na]+ ions at m/z 419 and 441, respectively, suggesting a molecular weight of 418 (Figure S2). High-resolution mass spectrometry (HRMS) analysis using fast atom bombardment (FAB) ionization of 3 suggested a molecular formula of C21H22O9, which has eleven degrees of unsaturation. The 13C NMR and DEPT spectra revealed that 3 has 19 inequivalent carbons; namely, one methyl, two methylenes, nine methines, and seven quaternary carbons (Table 1). The 1H, 13C, HSQC, COSY, and HMBC spectra suggested the structure of sakuranetin is conserved in 3 (Table 1 and Figure 5). The TOCSY and COSY correlations of the remaining signals and their chemical shifts indicated that a pentose moiety should be present in 3. The HMBC data suggested that the pentosyl group forms a pentopyranosyl structure, and that the pentopyranosyl group is connected to the oxygen at the 4′-position in sakuranetin.
Table 1.
13C and 1H NMR data for compounds 3 (methanol-d4) and 4 (acetone-d6).
| Position | Compound 3 | Compound 4 | ||
|---|---|---|---|---|
| δC | δH
(Multiplicity, J in Hz) |
δC | δH
(Multiplicity, J in Hz) |
|
| 2 | 80.2 | 5.45 (dd, 12.7, 3.1) | 80.15/80.17 1 | 5.50 (dd, 13.0, 3.2) |
| 3 | 44.1 | 2.79 (dd, 17.2, 3.1) | 43.58/43.60 1 | 2.78 (m) 2 |
| 3.14 (dd, 17.2, 12.7) | 3.24/3.25 1 (dd, 17.0, 13.0) | |||
| 4 | 197.9 | 197.97/197.99 1 | ||
| 5 | 165.3 | 164.72/164.76 1 | ||
| 6 | 95.8 | 6.05 (d, 2.3) | 97.6 | 6.11/6.12 1 (d, 2.2) |
| 7 | 169.6 | 166.46/166.53 1 | ||
| 8 | 95.0 | 6.08 (d, 2.3) | 96.4 | 6.15 (d, 2.2) |
| 9 | 164.6 | 164.2 | ||
| 10 | 104.1 | 104.5 | ||
| 7-O-Me | 56.3 | 3.81 (s) | – | – |
| 5-OH | – | – | 12.07 | |
| 1′ | 134.2 | 130.63/130.67 1 | ||
| 2′, 6′ | 128.8 | 7.44 (d, 8.7) | 129.10/129.14 1 | 7.41 (d, 8.7) |
| 3′, 5′ | 117.9 | 7.11 (d, 8.7) | 116.3 | 6.91 (d, 8.7) |
| 4′ | 159.2 | 158.84/158.85 1 | ||
| 1″ | 102.8 | 4.90 (d, 7.2) | 101.39/101.46 1 | 5.06/5.07 1 (d, 7.0 or 5.8) 3 |
| 2″ | 74.7 | 3.44 (m)2 | 74.1 | 3.48 (m) 2 |
| 3″ | 77.7 | 3.44 (m)2 | 77.4 | 3.48 (m) 2 |
| 4″ | 71.0 | 3.57 (m) | 70.6 | 3.60 (m) |
| 5″ | 66.9 | 3.37 (dd, 11.4, 10.2) | 66.6 | 3.50 (m) 2 |
| 3.92 (dd, 11.4, 5.3) | 3.91 (dd, 11.2, 4.8) | |||
1 Each diastereomer showed different chemical shifts. 2 The chemical shifts were estimated by an HSQC experiment. 3 Two possibilities are listed for the J values; the true values could not be confidently assigned due to the overlapping signals of the two diastereomers.
Figure 5.
Key 2D NMR correlations for compounds 3 and 4. The COSY and TOCSY correlations are represented by bold lines, and the HMBC are represented by arrows from H to C.
The pentopyranosyl group in 3 was determined by GC-MS analysis of the hydrolysate of 3. The hydrolysate of 3 was trimethylsilylated and then subjected to GC-MS. d-Xylose, d-ribose, and l-arabinose, which are common pentoses in natural products, were also trimethylsilylated and analyzed by GC-MS. The chromatograms of the trimethylsilylated (TMS) derivatives of the pentoses showed four peaks, which were indicative of the α-pyranose, β-pyranose, α-furanose, and β-furanose forms of the compounds [14]. The TMS derivative of the hydrolysate of 3 showed four peaks with tRs that coincided with those of xylose. The mass spectra of the four peaks derived from the hydrolysate of 3 also coincided with those of the xylose derivatives (Figure S3). Although the absolute stereochemistry of xylose was not determined experimentally, the d-form is a reasonable assignment because the l-form is not known as a natural product. The anomeric position was determined to be in the β-configuration based on the J value (7.2 Hz) of H-1″. We therefore concluded that 3 is sakuranetin-4′-O-β-d-xylopyranoside (Figure 1). The stereochemistry of C-2 was not determined in this study.
The ESI-MS of 4 showed a plausible [M + Na]+ ion at m/z 427, suggesting a molecular weight of 404 (Figure S2). HRMS analysis using FAB ionization of 4 was indicative of a molecular formula of C20H20O9, which includes eleven degrees of unsaturation. Some of the 13C NMR signals of 4 were observed as double signals that had very similar, but not identical, chemical shifts (Table 1). This suggested that purified 4 was a mixture of two diastereomers. However, the diastereoisomers were not chromatographically separable. We determined 4 was a diastereomeric mixture by a similar manner as we used to determine the structure of 3. The NMR spectra of 4 revealed that a pentopyranose was connected to the 7-oxygen in naringenin (Table 1 and Figure 5). GC-MS analysis revealed that the pentose is a xylose moiety (Figure S4). We thus concluded that 4 is naringenin-7-O-β-d-xylopyranoside (Figure 1). The difference in the diastereomers must be the stereochemistry of C-2. However, the NMR spectra of 3 indicated that purified 3 seemed to be the single diastereomer. This suggested that R. solani may preferentially xylosylate a specific enantiomer of 1.
Naringenin-7-O-xyloside was reported as a biotransformation product of naringenin by genetically engineered Escherichia coli that could express a glycosyltransferase from Arabidopsis thaliana and some other related enzymes [15]. The structure of naringenin-7-O-xyloside was confirmed only by LC-MS/MS analysis in that report.
2.4. Accumulation of Naringenin (2), Sakuranetin-4′-O-β-d-xylopyranoside (3), and Naringenin-7-O-β-d-xylopyranoside (4) in the Rhizoctonia solani Suspension Culture Containing Sakuranetin (1)
Figure 6 shows the time-dependent accumulation of 2, 3, and 4 in the R. solani suspension culture containing 1 (100 µmol/L). The level of 1 decreased to nearly zero in the 9 h after the addition of 1. The levels of 2, 3, and 4 gradually increased in the first 12 h, and the levels were relatively constant until 24 h. This result strongly supported that 2, 3, and 4 are products of the metabolism of 1 by R. solani. The total accumulation of 2, 3, and 4 in the suspension culture after 9 h of incubation was 90 µmol/L. Therefore, these three compounds must be the major metabolites of 1 in the R. solani suspension culture.
Figure 6.
Time-dependent accumulations of naringenin (2), sakuranetin-4′-O-β-d-xylopyranoside (3), and naringenin-7-O-β-d-xylopyranoside (4), and the decreasing sakuranetin (1) content in the Rhizoctonia solani suspension culture. Values are presented as the mean ± SD (n = 3).
We have already reported that 1 can be metabolized by P. oryzae, and that the metabolites were identified as 2 and sternbin [10]. Compound 2 is a common metabolite to both P. oryzae and R. solani, but the xylosylated flavanones, 3 and 4, are specific to R. solani. Our previous study demonstrated that the accumulation levels of the metabolites of P. oryzae, 2 and sternbin, reached their maximum levels after 6–8 h of incubation and then decreased to the almost zero as the incubation time increase to 24 h [10]. However, the levels of the metabolites from R. solani, 2, 3, and 4 did not decrease significantly in 24 h of incubation.
2.5. Antifungal Activities of Naringenin (2), Sakuranetin-4′-O-β-d-xylopyranoside (3), and Naringenin-7-O-β-d-xylopyranoside (4)
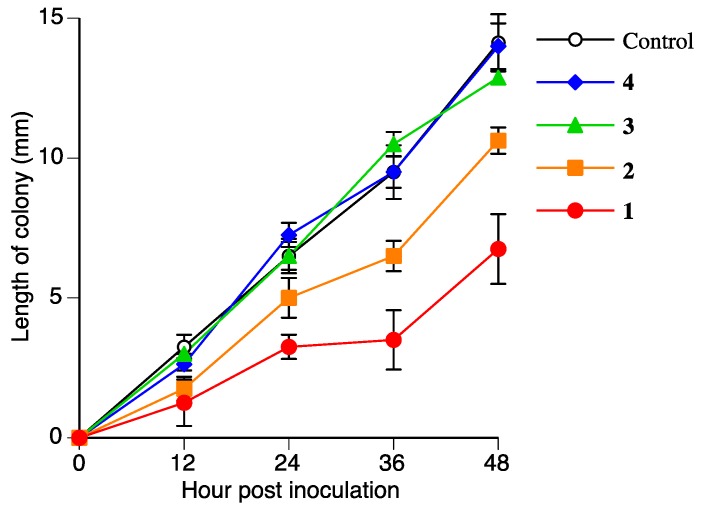
The antifungal activities of 2, 3, and 4 were measured to determine if 2, 3, and 4 are detoxified metabolites of 1. Figure 7 and Table 2 show the antifungal activities of 2, 3, and 4 against R. solani. The antifungal activity of 2 was lower than that of 1. The antifungal activities of 3 and 4 were almost negligible. This result suggested that R. solani could detoxify 1 in the suspension culture.
Figure 7.
Antifungal activities of 1, 2, 3, and 4 (300 µmol/L) against Rhizoctonia solani mycelium growth. Values are presented as the mean ± SD (n = 4).
Table 2.
Inhibitory activity of 1, 2, 3, and 4 on Rhizoctonia solani mycelium growth after 48 h of incubation.
| Compound | Concentration (µmol/L) | ||
|---|---|---|---|
| 75 | 150 | 300 | |
| Length of Colony [mm] (Inhibition [%]) | |||
| 1 | 9.8 ± 0.6 (31) | 8.1 ± 0.7 (42) | 6.8 ± 1.2 (52) |
| 2 | 14.5 ± 0.6 (−3) | 11.3 ± 0.2 (20) | 10.6 ± 0.5 (25) |
| 3 | 12.8 ± 0.2 (10) | 13.1 ± 0.9 (7) | 12.9 ± 0.2 (9) |
| 4 | 14.4 ± 0.2 (−2) | 14.0 ± 0.2 (1) | 14.0 ± 0.8 (1) |
Values are presented as the mean ± SD (n = 4). Inhibition [%] = (1-colony length of sample/colony length of control) × 100. Colony length of the control was 14.1 ± 1.0 mm.
Glucosylation is known to be a common detoxification method for phytoalexins [6,9]. However, to the best of our knowledge, xylosylation detoxification of a phytoalexin has not been reported. We therefore concluded that xylosylation is a rare and efficient mode of detoxification of phytoalexins.
3. Materials and Methods
3.1. General Analytical Methods
1H NMR (400 MHz), 13C NMR (100 MHz), and 2D-NMR spectra were acquired on an AVANCE III FT-NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany) equipped with a 5 mm BBFO probe. The chemical shifts were referenced to residual 1H or 13C signals of the solvents: acetone-d6 (δH 2.05; δC 29.84) or methanol-d4 (δH 3.31; δC 49.00). The UV spectra were acquired using a V-550 spectrometer (Jasco, Tokyo, Japan); the samples for UV spectroscopy were dissolved in MeOH. LC-MS and LC-MS/MS were performed with a 3200 QTRAP LC/MS/MS system (SCIEX, Framingham, MA, USA) coupled with a Prominence UFLC system (Shimadzu Co., Kyoto, Japan). The FAB-MS were recorded with a JMS-BU25 mass spectrometer (Jeol, Tokyo, Japan) in the negative ion mode; glycerol was used as the matrix and argon was used as the FAB gas. Polyethylene glycol was used as an internal standard for HRMS analysis. GC-MS was performed with a JMS-BU25 mass spectrometer coupled with an HP6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA). Preparative HPLC was carried out with PU-980 HPLC pumps and an MD-910 photodiode array detector (Jasco).
3.2. Chemicals
Sakuranetin (1) was chemically synthesized from naringenin (2; Sigma-Aldrich, St. Louis, MO, USA) according to a previously reported method [16]. The sakuranetin and naringenin used in this study were racemic mixtures. d-Xylose was purchased from Tokyo Chemical Industry (Tokyo, Japan). d-Ribose was purchased from Wako Pure Chemical Industries (Osaka, Japan). l-Arabinose was purchased from Nacalai Tesque (Kyoto, Japan). A Sylon BFT kit (BSTFA + TMCS, 99:1; Supelco, Bellefonte, PA, USA) was used for trimethylsilylation.
3.3. Fungal Material
The rice sheath blight fungus (Rhizoctonia solani MAFF305003) was obtained from NARO Genebank Project (Tsukuba, Japan) and was maintained on a potato dextrose agar (PDA) medium (Nissui Pharmaceutical, Tokyo, Japan) as a stock culture. A small portion of this stock culture was inoculated and grown on PDA in a Petri dish (9 mm in diameter) at 26 °C in the dark prior to use in the following experiments.
3.4. Incubation of the Rhizoctonia solani Suspension Cultures with Sakuranetin (1)
A portion of approximately 1 × 1 cm of the fungal layer was excised from the 5 dpi PDA medium. The fungal layer was homogenized using a spatula and suspended in 50 mL of potato dextrose broth (PDB; Sigma-Aldrich). The fungal culture was incubated for 3 days in the dark at 27 °C with rotary shaking at 150 rpm. A spherical mycelial cluster (approximately 5 mm in diameter) that was formed was transferred to fresh PDB (1 mL) to which 7.0 mmol/L 1 in MeOH (15 µL) had been added. The medium was incubated for 3–24 h at 27 °C with reciprocal shaking at 200 strokes/min.
3.5. Screening of the Sakuranetin (1) Metabolites from the Rhizoctonia solani Suspension Culture
Following the addition of 1, the medium (1 mL) was incubated for 0 or 12 h and then diluted with MeOH (8 mL). The extract was filtered through a cotton-plugged Pasteur pipette. The filtrate was evaporated to dryness in vacuo, the residue was dissolved in 800 µL of MeOH, and the resulting solution was filtered through a 0.22-µm membrane filter. A 10-µL aliquot of the solution was subjected to LC-MS analysis to detect the putative metabolites of 1. The Turbo V ion source was operated in the positive electrospray ionization (ESI) mode. LC separation of the analytes was achieved on a Unison UK-18 column (150 × 2.0 mm i.d., 3.0 µm particle size; Imtact Co., Kyoto, Japan) with a binary gradient of 0.1% (v/v) aqueous HCOOH (solvent A) and MeOH containing 0.1% HCOOH (solvent B) at a flow rate of 0.2 mL/min at 40 °C. The solvent gradient elution was performed with the following program: (i) initial, 20% B; (ii) 0–5 min, isocratic elution with 20% B; (iii) 5–25 min, a linear gradient from 20% B to 100% B; and (iv) 25–30 min, isocratic elution with 100% B. The following parameters were used for the ion source and MS: (i) curtain gas (CUR), 20 psi; (ii) temperature (TEM), 450 °C; (iii) nebulizer gas (GS1), 50 psi; (iv) GS2, 50 psi; (v) ion spray voltage (IS), 5200 V; (vi) declustering potential (DP), 51.0 V; and (vii) entrance potential (EP), 7.5 V. The scan range for the MS anaysis was set to m/z 100–700 (enhanced MS scan).
3.6. LC-MS/MS Analysis to Identify Naringenin (2) as a Metabolite
LC-ESI-CID-MS analysis was performed according to a previously described method [10].
3.7. Purification of Sakuranetin-4′-O-β-d-xylopyranoside (3) and Naringenin-7-O-β-d-xylopyranoside (4) from the Rhizoctonia solani Suspension Culture
A portion of approximately 1 × 1 cm of the fungal layer was excised from the 5 dpi PDA medium. The fungal layer was homogenized using a spatula and suspended in PDB (300 mL). The fungal culture was incubated for 5 days in the dark at 27 °C with rotary shaking at 150 rpm. After the addition of 35 mmol/L 1 in MeOH (1 mL), the culture was incubated for 18 h under the same conditions. MeOH (200 mL) was added to the culture, and the mixture was homogenized with a Physcotron homogenizer (Microtec, Funabashi, Japan). The homogenate was filtered through filter paper, and the filtrate was concentrated in vacuo. The concentrate (250 mL) was extracted with EtOAc (250 mL × 3) and the organic layer was concentrated to dryness in vacuo. The residue of the EtOAc extract was dissolved in MeOH to a concentration of 4% (v/v) and separated on a Luna C18(2) column (25 × 1 cm i.d., 5 µm particle size, Phenomenex, Torrance, CA, USA) with a binary gradient of H2O (solvent A) and MeOH (solvent B) at a flow rate of 2 mL/min at 40 °C. The gradient elution was performed with the following program: (i) initial, 40% B; (ii) 0–50 min, isocratic elution with 40% B; (iii) 50–80 min, a linear gradient from 40% B to 70% B; and (iv) 80–100 min, isocratic elution with 40% B. The injection volume in each run was 50 µL, and the sample was separated repeatedly under the same conditions. The detection wavelength was 280 nm. The presence of 3 and 4 was confirmed using LC/MS under the same conditions as were used in the screening of the metabolites. The peak containing 3 (tR 77–78 min) was collected and evaporated to dryness in vacuo to afford 1.1 mg of 3. The peak containing 4 (tR 43–46 min) was collected and evaporated to dryness in vacuo to afford 2.4 mg of 4.
Sakuranetin-4′-O-β-d-xylopyranoside (3). HRMS (FAB): m/z 417.1172 ([M − H]−); calcd. for C21H21O9, 417.1185. 1H and 13C NMR (methanol-d4): see Table 1; UV (MeOH) λmax (log ε): 288 (4.0).
Naringenin-7-O-β-d-xylopyranoside (4). HRMS (FAB): m/z 403.1042 ([M − H]−); calcd. for C20H19O9, 403.1029. 1H and 13C NMR (acetone-d6): see Table 1. UV (MeOH) λmax (log ε): 283 (4.3).
3.8. GC-MS Analysis of the Hydrolysates of Sakuranetin-4′-O-β-d-xylopyranoside (3) and Naringenin-7-O-β-d-xylopyranoside (4)
The hydrolysis reaction was performed according to a previous study [17]. Compound 3 or 4 (1 µg) was hydrolyzed with 40 µL of 2 mol/L TFA at 100 °C for 2 h. The hydrolysate mixture was concentrated to dryness in vacuo. The residue was trimethylsilylated with 50 µL of BSTFA + TMCS (99:1) at 70 °C for 3 h. The trimethylsilylated sample (2 µL) was subjected to GC-MS analysis. GC separation was carried out on a Zebron ZB-5MS column (30 m × 0.25 mm i.d., 0.25 µm film thickness, Phenomenex) under the following conditions: injector temperature, 280 °C; carrier gas, helium; and flow rate, 1.0 mL/min. The temperature program of the column oven was set to hold at 70 °C for 1 min, then increase at 10 °C/min to 300 °C, and finally hold at 300 °C for 3 min. The conditions used for the mass spectrometer were as follows: ionization mode, EI (70 eV); ion source temperature, 200 °C; scan range, m/z 61–760; and scan rate, 1 s/scan.
3.9. Quantitation of Sakuranetin (1) and Its Metabolites in the Rhizoctonia solani Suspension Culture
After incubation, MeOH (8 mL) was added to the medium (1 mL), and the resulting suspension was shaken for 1 h. The extract was filtered through a cotton-plugged Pasteur pipette and a membrane filter (0.22 µm). A portion of the filtrate (0.1 mL) was diluted with MeOH (0.9 mL), and 2 µL of the dilution was subjected to LC-MS/MS analysis. The Turbo V ion source was operated in the negative ESI mode. LC separation of the analytes was achieved on a TSK-gel ODS-100V column (50 × 2.0 mm i.d., 3.0 µm particle size; Tosoh Corp., Tokyo, Japan) with a binary gradient of 0.1% (v/v) aqueous HCOOH (solvent A) and MeOH containing 0.1% HCOOH (solvent B) at a flow rate of 0.2 mL/min at 40 °C. The solvent gradient elution was performed according to the following program: (i) initial, 20% B; (ii) 0–1 min, isocratic elution with 20% B; (iii) 1–6 min, a linear gradient from 20% B to 100% B; and (iv) 6–9 min, isocratic elution with 100% B. The following parameters were used for the ion source: (i) CUR, 10 psi; (ii) TEM, 300 °C; (iii) GS1, 30 psi; (iv) GS2, 80 psi; and (v) IS, −4500 V. The selective reaction monitoring (SRM) transitions and MS parameters were optimized to detect 1–4 using Analyst 1.6.2 software (SCIEX). The following SRM transitions (tR) were monitored: (1) m/z 285 → 119 (7.3 min), (2) m/z 271 → 151 (6.6 min), (3) m/z 417 → 285 (6.9 min), and (4) m/z 403 → 271 (6.1 min). The optimized MS parameters for each compound were as follows: (1) −45 V DP, −4.5 V EP, and −24 V CE; (2) −45 V DP, −6.5 V EP, and −22 V CE; (3) −60 V DP, −3 V EP, and –18 V CE; and (4) −40 V DP, −3.5 EP, and −22 CE. Calibration curves were prepared using the SRM peak areas of standards for 1 and 2–4 in concentration ranges of 20–2000 ng/mL and 10–1000 ng/mL, respectively.
3.10. Assay of the Antifungal Activity Against Rhizoctonia solani
The Fungal colony growth inhibition assay was performed according to a previously described method, which used Pyricularia oryzae as the test fungus [18]. In this study, R. solani was used instead of P. oryzae.
4. Conclusions
Xylosylated flavanones sakuranetin-4′-O-β-d-xylopyranoside (3) and naringenin-7-O-β-d-xylopyranoside (4) as well as naringenin (2) were identified as detoxified metabolites of sakuranetin (1) by the rice sheath blight fungus Rhizoctonia solani. Compound 2 is a common detoxified metabolite of 1 by both R. solani and Pyricularia oryzae. However, xylosylation by R. solani is a rare and efficient detoxification path of phytoalexins.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
S.K. and M.H. conceived and designed the experiments; S.K. performed the experiments; S.K., H.T., and M.H. analyzed the data; S.K. and M.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 3 and 4 are available from the authors.
References
- 1.Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Horie K., Sakai K., Okugi M., Toshima H., Hasegawa M. Ultraviolet-induced amides and casbene diterpenoids from rice leaves. Phytochem. Lett. 2016;15:57–62. doi: 10.1016/j.phytol.2015.11.009. [DOI] [Google Scholar]
- 3.Hasegawa M., Mitsuhara I., Seo S., Okada K., Yamane H., Iwai T., Ohashi Y. Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules. 2014;19:11404–11418. doi: 10.3390/molecules190811404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyomasu T., Usui M., Sugawara C., Otomo K., Hirose Y., Miyao A., Hirochika H., Okada K., Shimizu T., Koga J., et al. Reverse genetic approach to verify physiological roles of rice phytoalexins: Characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol. Plant. 2014;150:55–62. doi: 10.1111/ppl.12066. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa M., Mitsuhara I., Seo S., Imai T., Koga J., Okada K., Yamane H., Ohashi Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant Microbe Interact. 2010;23:1000–1011. doi: 10.1094/MPMI-23-8-1000. [DOI] [PubMed] [Google Scholar]
- 6.Pedras M.S.C., Abdoli A. Pathogen inactivation of cruciferous phytoalexins: Detoxification reactions, enzymes and inhibitors. RSC Adv. 2017;7:23633–23646. doi: 10.1039/C7RA01574G. [DOI] [Google Scholar]
- 7.VanEtten H.D., Matthews D.E., Matthews P.S. Phytoalexin detoxification: Importance for pathogenicity and practical implications. Annu. Rev. Phytopathol. 1989;27:143–164. doi: 10.1146/annurev.py.27.090189.001043. [DOI] [PubMed] [Google Scholar]
- 8.VanEtten H., Temporini E., Wasmann C. Phytoalexin (and phytoanticipin) tolerance as a virulence trait: Why is it not required by all pathogens? Physiol. Mol. Plant Pathol. 2001;59:83–93. doi: 10.1006/pmpp.2001.0350. [DOI] [Google Scholar]
- 9.Pedras M.S.C., Ahiahonu P.W.K. Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry. 2005;66:391–411. doi: 10.1016/j.phytochem.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Katsumata S., Hamana K., Horie K., Toshima H., Hasegawa M. Identification of sternbin and naringenin as detoxified metabolites from the rice flavanone phytoalexin sakuranetin by Pyricularia oryzae. Chem. Biodiver. 2017;14:e1600240. doi: 10.1002/cbdv.201600240. [DOI] [PubMed] [Google Scholar]
- 11.Imai T., Ohashi Y., Mitsuhara I., Seo S., Toshima H., Hasegawa M. Identification of a degradation intermediate of the momilactone A rice phytoalexin by the rice blast fungus. Biosci. Biotechnol. Biochem. 2012;76:414–416. doi: 10.1271/bbb.110756. [DOI] [PubMed] [Google Scholar]
- 12.Ogoshi A. Ecology and pathogenicity of anastomosis and intraspecific groups of Rhizoctonia solani Kühn. Annu. Rev. Phytopathol. 1987;25:125–143. doi: 10.1146/annurev.py.25.090187.001013. [DOI] [Google Scholar]
- 13.Park H.L., Yoo Y., Hahn T.-R., Bhoo S.H., Lee S.-W., Cho M.-H. Antimicrobial activity of UV-induced phenylamides from rice leaves. Molecules. 2014;19:18139–18151. doi: 10.3390/molecules191118139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paez M., Martínez-Castro I., Sanz J., Olano A., Garcia-Raso A., Saura-Calixto F. Identification of the components of aldoses in a tautomeric equilibrium mixture as their trimethylsilyl ethers by capillary gas chromatography. Chromatographia. 1987;23:43–46. doi: 10.1007/BF02310417. [DOI] [Google Scholar]
- 15.Simkhada D., Kim E., Lee H.C., Sohng J.K. Metabolic engineering of Escherichia coli for the biological synthesis of 7-O-xylosyl naringenin. Mol. Cells. 2009;28:397–401. doi: 10.1007/s10059-009-0135-7. [DOI] [PubMed] [Google Scholar]
- 16.Aida Y., Tamogami S., Kodama O., Tsukiboshi T. Synthesis of 7-methoxyapigeninidin and its fungicidal activity against Gloeocercospora sorghi. Biosci. Biotechnol. Biochem. 1996;60:1495–1496. doi: 10.1271/bbb.60.1495. [DOI] [PubMed] [Google Scholar]
- 17.Füzfai Z., Molnár-Perl I. Gas chromatographic–mass spectrometric fragmentation study of flavonoids as their trimethylsilyl derivatives: Analysis of flavonoids, sugars, carboxylic and amino acids in model systems and in citrus fruits. J. Chromatogr. A. 2007;1149:88–101. doi: 10.1016/j.chroma.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Horie K., Inoue Y., Sakai M., Yao Q., Tanimoto Y., Koga J., Toshima H., Hasegawa M. Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015;63:4050–4059. doi: 10.1021/acs.jafc.5b00785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.