Abstract
Glycogen synthase kinase-3β (GSK-3β) is a multifunctional serine/threonine protein kinase that was originally identified as an enzyme involved in the control of glycogen metabolism. It plays a key role in diverse physiological processes including metabolism, the cell cycle, and gene expression by regulating a wide variety of well-known substances like glycogen synthase, tau-protein, and β-catenin. Recent studies have identified GSK-3β as a potential therapeutic target in Alzheimer´s disease, bipolar disorder, stroke, more than 15 types of cancer, and diabetes. GSK-3β is one of the most attractive targets for medicinal chemists in the discovery, design, and synthesis of new selective potent inhibitors. In the current study, twenty-eight Amaryllidaceae alkaloids of various structural types were studied for their potency to inhibit GSK-3β. Promising results have been demonstrated by alkaloids of the homolycorine-{9-O-demethylhomolycorine (IC50 = 30.00 ± 0.71 µM), masonine (IC50 = 27.81 ± 0.01 μM)}, and lycorine-types {caranine (IC50 = 30.75 ± 0.04 μM)}.
Keywords: Amaryllidaceae alkaloids, Alzheimer’s disease, glycogen synthase kinase-3β, masonine, caranine, 9-O-demethylhomolycorine
1. Introduction
Glycogen synthase kinase-3β (GSK-3β) is a ubiquitous pleiotropic serine/threonine kinase that plays crucial roles in cellular functions, including cell-cycle regulation, differentiation, and proliferation, and gene expression by regulating a wide variety of known targets such as glycogen synthase, τ-protein, and β-catenin [1]. GSK-3 is involved in cellular signaling, including Wnt and Hedgehog pathways, and in neuronal development, insulin pathways, transcription, cell division, cell survival, and cell death [1,2,3]. Due to its multifarious roles, aberrant activity of GSK-3 underlines a variety of disorders including Alzheimer´s disease (AD) [4], cancer [5], diabetes [6], cardiovascular disorders [7], and psychiatric disorders [8].
One of the neuropathological characteristics of AD is the presence of neurofibrillary tangles (NFTs) consisting of paired helical filaments, with the main component being hyperphosphorylated τ-protein. Phosphorylation of τ-proteins is primarily dependent on GSK-3β and cyclin-dependent kinase 5 (CDK5) [9]. Genetic and epidemiological studies indicate that GSK-3β is deregulated in AD through alterations in upstream Wnt and insulin signaling pathway intermediates. This may be the reason behind tau hyperphosphorylation and, later on, the formation of NFTs. GSK-3β may also induce the formation of amyloid β-protein (Aβ), a further neuropathological marker for AD. Aβ is aggregated and deposited in the AD brain and causes dysfunction of neurons, inflammation, and oxidative stress [10]. Aβ production is facilitated by overexpression of β-site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1) and of presenilin 1 (PS1) [11]. Increased GSK-3β activity in the brains of patients with AD, and its pathological activation facilitates Aβ production [12]. Therapeutic concentrations of lithium, a GSK-3 inhibitor, block the production of Aβ peptides and the accumulation of Aβ peptides in the brains of mice that overproduce APP [13,14]. Clinical studies have evaluated the safety and efficacy of the irreversible GSK-3β inhibitor tideglusib in the treatment of patients with AD [15,16]. Tideglusib is a thiadiazolidinone that reduces tau phosphorylation in murine primary neurons. In a pilot, double-blind, placebo-controlled, randomized, escalating dose trial, 30 patients with mild to moderate Alzheimer´s disease were enrolled and received either tideglusib or placebo (orally) at escalating doses for a total of 20 weeks. The objective of this pilot study was to evaluate safety and tolerability of tideglusib with strict criteria for drug escalation or withdrawal. Tideglusib was well tolerated by 65% of the patients [16].
GSK-3β has been implicated in playing a role in cancers which are resistant to chemo-, radio-, and targeted therapy [17]. It has been shown to be a potential mediator in contributing to neoplastic transformation, in part because it belongs to both the canonical Wnt/β-catenin and the PI3K/Akt signaling systems, the two major pathways often dysregulated in cancer [18]. GSK-3 inhibitors may eventually be used in the treatment of certain cancers. GSK-3 is believed to exert pro-proliferative effects in solid cancers including: colorectal cancer, glioblastoma, pancreatic cancer, ovarian cancer, and blood cancers [19].
A number of publications have emerged describing diverse molecules that inhibit GSK-3β, such as manzamine alkaloids [20], pyrazolopyrimidines [21], pyridyloxadiazoles [22], thiadiazolidindiones [23], maleimides [24], and paullones (a group of benzazepinones) [25]. Current advances in the search for GSK-3 inhibitors have been recently reviewed [1,13,26].
Amaryllidaceae alkaloids, consisting of a nitrogen-containing polycyclic structure, are produced exclusively by plants of the Amaryllidaceae family. These compounds have attracted considerable attention, most prominently because of their inhibition of acetylcholinesterase (AChE) and activity against drug-resistant cancers with dismal prognoses [27,28,29,30]. The best known Amaryllidaceae alkaloid, galanthamine, is used in the treatment of Alzheimer’s disease, as a long acting, selective, reversible, and competitive AChE inhibitor [28]. Further Amaryllidaceae alkaloids, such as pancratistatine, narciclasine, lycorine, haemanthamine, distichamine, and their derivatives, are known for their potent cell line specific anticancer properties, and some of them are involved at various stages of development, with a clinical candidate earmarked for commercialization within the next decade [31,32].
In our search for active natural products against neurological and cancer disorders, we have discovered the potency of Amaryllidaceae alkaloids to inhibit GSK-3β.
2. Results and Discussion
2.1. Amaryllidaceae Alkaloids
In the current study, 28 Amaryllidaceae alkaloids (Figure 1) of seven structural types: belladine (1), haemanthamine (2–6), crinine (7–10), galanthamine (11–13), lycorine (14–19), tazettine (20), and homolycorine (21–28), were studied for their ability to inhibit GSK-3β. All compounds have been previously isolated in our laboratory from different Amaryllidaceae plants.
Figure 1.
Structures of the studied Amaryllidaceae alkaloids.
2.2. Potency of Amaryllidaceae Alkaloids to Inhibit GSK-3β
The inhibitory activity of the compounds was first screened at a concentration of 50 µM (Table 1); a synthetic arylindolemaleimide derivative, SB-415286, was used as a positive standard. This compound is a highly selective GSK-3 inhibitor developed by GlaxoSmithKline that inhibits GSK-3 as well as other organic inhibitors of synthetic origin (e.g., thiadiazolidinones, oxadiazole analogues), within the low nanomolar concentration range [23,24,33].
Table 1.
Screening of Amaryllidaceae alkaloids for their potency to inhibit GSK-3β (conc. 50 µM).
| Structural Type | Alkaloid | % of Inhibition |
|---|---|---|
| Belladine | Beladine (1) | 34.4 ± 2.7 |
| Haemanthamine | Epimaritidine (2) | 45.2 ± 1.1 |
| Haemanthamine (3) | 52.4 ± 0.1 | |
| Haemanthidine (4) | 33.0 ± 2.2 | |
| Hamayne (5) | 33.9 ± 0.1 | |
| Seco-isopowellaminone (6) | 38.5 ± 0.8 | |
| Crinine | Ambelline (7) | 38.0 ± 0.8 |
| Crinine (8) | 39.6 ± 5.4 | |
| Undulatine (9) | 43.3 ± 4.0 | |
| Crinamidine (10) | 32.1 ± 7.9 | |
| Galanthamine | Chlidanthine (11) | 37.9 ± 9.5 |
| Narwedine (12) | 37.7 ± 0.3 | |
| Lycoraminone (13) | 38.9 ± 1.0 | |
| Lycorine | Caranine (14) | 61.8 ± 9.2 |
| Lycorine (15) | 32.9 ± 0.2 | |
| 1-O-Acetyllycorine (16) | 49.9 ± 1.9 | |
| Galanthine (17) | 26.4 ± 7.7 | |
| 9-O-Demethylgalanthine (18) | 50.9 ± 8.9 | |
| Norpluviine (19) | 45.0 ± 4.3 | |
| Tazettine | Tazettine (20) | 49.2 ± 0.3 |
| Homolycorine | Hippeastrine (21) | 10.7 ± 2.5 |
| Homolycorine (22) | 54.4 ± 0.6 | |
| 9-O-Demethylhomolycorine (23) | 63.6 ± 1.3 | |
| Masonine (24) | 66.0 ± 4.0 | |
| Lycorenine (25) | 47.6 ± 3.5 | |
| O-Ethyllycorenine (26) | 57.7 ± 3.5 | |
| Oduline (27) | 57.7 ± 4.4 | |
| Tetrahydromasonine (28) | 22.4 ± 0.2 |
The best results in preliminary screening were demonstrated by alkaloids of the homolycorin-type (21–28). Most of the substances tested in this group showed an activity at 50 μM of more than 50%. After preliminary screening, the three most active compounds: caranine (14), 9-O-demethylhomolycorine (23), and masonine (24), were selected for IC50 determination.
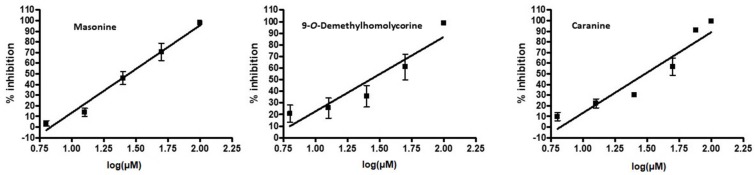
The measurements were performed in triplicate and the values given are the average obtained after at least two measurements. The IC50 values of the selected alkaloids are in the micromolar range (about 30 µM) and were obtained for three of the selected compounds (Table 2). The highest GSK-3β inhibition potency has been demonstrated by two homolycorine-type Amaryllidaceae alkaloids, masonine (24, IC50 = 27.81 ± 0.01 µM; Figure 2) and 9-O-demethylhomolycorine (23, IC50 = 30.00 ± 0.71 µM; Figure 2), and one lycorine-type alkaloid caranine (14, IC50 = 30.75 ± 0.04 µM; Figure 2). The low number of available homolycorine-type alkaloids precluded a detailed structure-activity relationship (SAR) study, but their general features can still be described. It seems that the presence of hydroxyl substitution at position 2, as in hippeastrine (21; see Figure 1), is connected with a distinct reduction of GSK-3β inhibitory activity (10.65% of GSK-3β inhibition at 50 µM) compared with masonine (66.0% of GSK-3β inhibition at 50 µM), 9-O-demethylhomolycorine (63.6% of GSK-3β inhibition at 50 µM), oduline (57.7% of GSK-3β inhibition at 50 µM), and O-ethyllycorenine (57.7% of GSK-3β inhibition at 50 µM), where no substituent (e.g., hydroxy or methoxy group, etc.) in position C-2 is present. The opening of the tetrahydropyrane ring in tetrahydromasonine (28, see Figure 1) also reduces the GSK-3β inhibitory potency of homolycorine-type alkaloids (Table 1). For a detailed SAR study of homolycorine-type of Amaryllidaceae alkaloids, it is necessary to study a wider range of natural or semi-synthetic analogues of active alkaloids.
Table 2.
The potency to inhibit GSK-3β (IC50) of selected Amaryllidaceae alkaloids.
| Alkaloid | IC50 (µM) * |
|---|---|
| Caranine (14) | 30.75 ± 0.04 |
| 9-O-Demethylhomolycorine (23) | 30.00 ± 0.71 |
| Masonine (24) | 27.81 ± 0.05 |
| SB-415286 ** | 70.00 nM |
* Data are the means ± Standard Deviation (SD) of three independent replications, ** SB-415286, a compound used as a standard.
Figure 2.
Linear graph of IC50 assay of GSK-3β treated with selected Amaryllidaceae alkaloids. Concentrations of alkaloids were 6.25; 12.5; 25; 50 and 100 μM. Activity is presented as % inhibition.
The most interesting GSK-3β inhibition potency of natural products have been demonstrated by the alkaloid manzamine A (IC50 = 10.2 µM), isolated from a common Indonesian sponge Acanthostrongylophora and its semisynthetic analogue 1 [20], by indole alkaloid hymenialdisine (HD, IC50 = 10 nM) [34], isolated from marine sponges from the Agelasidae, Axinellidae, and Halichondriidae families [35,36], as well as meridianin E (IC50 = 2.5 µM) [37] isolated from ascidian Aplidium meridianum. The mechanism of action has been studied in case of HD. The kinetic experiments were performed by varying both ATP levels and HD concentrations. The results of double-reciprocal plotting indicated that HD is a competitive inhibitor for ATP [34]. Compounds isolated from endophytic fungus Cosmospora vilior have also been studied for their potency to inhibit GSK-3β [38]. Cosmochlorin A and cosmochlorine B showed GSK-3β inhibition activity at IC50 values of 62.5 and 60.6 µM, respectively [38].
3. Experimental
3.1. Amaryllidaceae Alkaloids
All Amaryllidaceae alkaloids tested have been previously isolated at the Department of Pharmaceutical Botany, Faculty of Pharmacy in Hradec Králové from various Amaryllidaceae plant species (Zephyranthes robusta [39,40], Chlidanthus fragrans [27,41], Nerine bowdenii [42], Narcissus poeticus cv. Pink Parasol [43], and N. poeticus cv. Brackenhurst [44]). The purity of all compounds (≥ 98%) was determined by 1H and 13C NMR spectroscopy.
3.2. GSK-3β Assay
Kinase-Glo Kit was obtained from Promega (Promega Biotech Iberica, S.L., Madrid, Spain), and human recombinant GSK-3β and GSM substrate mimicking Glycogen Muscle Synthase from Merck Millipore (Darmstadt, Germany). Adenosine 5-triphosphate (ATP) disodium salt hydrate, ammonium acetate, ammonium hydroxide, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), ethylene glycol-bis(-aminoethylether)-N,N,N,N-tetraacetic acid tetrasodium salt (EGTA), ethylenediaminetetraacetic acid (EDTA), dimethyl sulfoxide (DMSO), magnesium acetate tetrahydrate, formic acid, and 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione were purchased from Sigma-Aldrich (St. Louis, MO, USA). The GSK-3β selective inhibitor SB-415286 ([3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione]) was purchased from Selleck Chemicals (Houston, TX, USA). Ultrapure water was obtained using a Purite LTD water purification system (Thame, UK). The experiments were carried out using a Victor X3 multimode plate reader (Perkin Elmer, MA, USA).
GSK-3β activity and inhibition were studied according to the luminescent method of Baki et al. using a Kinase-Glo reagent kit [45]. The reaction was performed in 96-well white plates. Each well contained 10 µL of test compound (dissolved in DMSO) at 1 mM concentration and diluted in advance in an assay buffer (pH 7.5) containing 50 mM HEPES, 1 mM EDTA, 1 mM EGTA, and 15 mM magnesium acetate, to the desired concentration, 10 µL of ATP (1 µM final concentration), 10 µL of 100 µM GSM and 10 µL of GSK-3β (20 ng). Ten microliters of either buffer or SB-415286 solution (5 µM final concentration) was added instead of test compound solution in order to obtain the positive (maximum activity) and negative control (total inhibition), respectively. The final DMSO concentration in the reaction mixture did not exceed 5%. The mix was left to react at 37 °C for 30 min. Then the enzymatic reactions were stopped with 40 µL of Kinase-Glo reagent. Glow-type luminescence was recorded after 10 min. The activity is proportional to the difference of the total and consumed ATP. The inhibition activities were calculated on the basis of maximal activity, measured in the absence of inhibitor, and the maximal inhibition was measured in the presence of the reference compound. The IC50 values were calculated using the GraphPad Prism 4.0 program (GraphPad Software Inc., CA, USA).
4. Conclusions
In conclusion, GSK-3β is an enzyme with a very large number of different actions in intracellular signaling systems. Many of the pathways that use GSK-3β as a regulator have links to human diseases and, thus, have great potential as a target for therapeutic prevention. Currently, GSK-3β inhibitors have great promise as drugs for the pharmacotherapy of severe pathologies such as cancer, AD, mood disorders, diabetes, stroke, and many others. Since the introduction of galanthamine into the treatment of AD, Amaryllidaceae alkaloids have been an important source for the discovery of potential therapeutic agents.
In the present study, the potency of Amaryllidaceae alkaloids to inhibit GSK-3β has been studied. The results obtained suggest Amaryllidaceae alkaloids constitute an interesting scaffold. Since Amaryllidaceae alkaloids can easily be isolated from natural sources in amounts which allow for the preparation of their derivatives, thus the active GSK-3β inhibitors will be used in the design of more potent semisynthetic compounds. The type of GSK-3β inhibition of active alkaloids, and their semisynthetic derivatives, will be studied in future experiments.
Acknowledgments
This project has been supported by a grant from Charles University Nr. SVV 260412.
Author Contributions
D.H., A.D.S., L.D., and V.A. contributed to the measurement of GSK-3β inhibition activity of Amaryllidaceae alkaloids. K.B., T.S., K.K., A.H., M.Š., and L.C. contributed in the isolation of Amaryllidaceae alkaloids and their unambiguous identification (MS, NMR, IR etc.). L.C. and V.A. designed the study, supervised the laboratory work, and contributed to critical reading of the manuscript. All of the authors read the final manuscript and approved the submission.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds, except of 6, 10, 12, 13, 16, and 26, are available from the authors.
References
- 1.Saraswati A.P., Ali Hussaini S.M., Krishna N.H., Babu B.N., Kamal A. Glykogen synthase kinase-3 and its inhibitors: Potential target for various therapeutics conditions. Eur. J. Med. Chem. 2018;144:843–858. doi: 10.1016/j.ejmech.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P., Frame S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 3.Phukan S., Babu V.S., Kannoji A., Hariharan R., Balaji V.N. GSK3β: Role in therapeutic landscape and development of modulators. Br. J. Pharmacol. 2010;160:1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maqbool M., Mobashir M., Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016;107:63–81. doi: 10.1016/j.ejmech.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Luo J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriksen E.J., Dokken B.B. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr. Drug Targets. 2006;7:1435–1441. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- 7.Lal H., Ahmad F., Woodgett J., Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ. Res. 2015;116:138–149. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jope R.S., Roh M.S. Glykogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plattner F., Angelo M., Giese K.P. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- 10.Palop J.J., Mucke L. Amyloid-β induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petanceska S.S., Seeger M., Checler F. Mutant presenilin 1 increases the levels of Alzheimer amyloid β-peptide Aβ42 in late compartments of the constitutive secretory pathway. J. Neurochem. 2000;74:1878–1884. doi: 10.1046/j.1471-4159.2000.0741878.x. [DOI] [PubMed] [Google Scholar]
- 12.Rockenstein E., Torrance M., Adame A., Mante M., Baron P., Rose J.B., Crews L., Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez A. Preclinical efficacy on GSK-3 inhibitors: Towards a future generation of powerful drugs. Med. Res. Rev. 2008;28:773–796. doi: 10.1002/med.20119. [DOI] [PubMed] [Google Scholar]
- 14.Phiel C.J., Wilson C.A., Lee V.M., Klein P.S. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez J.M., Fuertes A., Orozco L., del Monte-Millan M., Deldago E., Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J. Biol. Chem. 2012;287:893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Ser T., Steinwachs K.C., Gertz H.J., Andress M.V., Gomez-Carrillo B., Medina M., Vericat J.A., Redondo P., Fleet D., Leon T. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: A pilot study. J. Alzheimer Dis. 2013;33:205–215. doi: 10.3233/JAD-2012-120805. [DOI] [PubMed] [Google Scholar]
- 17.Shimura T. Acquired radioresistance of cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J. Radiat. Res. 2011;52:539–544. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- 18.Jope R.S., Yuskaitis C.J., Beurel E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCubrey J.A., Steelman L.S., Bertrand F.E., Davis N.M., Sokolosky M., Abrams S.L., Montalto G., D’Assoro A.B., Libra M., Nicoletti F., et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamann M., Alonso D., Martín-Aparicio E., Fuertes A., Pérez-Puerto M.J., Castro A., Morales S., Navarro M.L., Del Monte-Millán M., Medina M., et al. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J. Nat. Prod. 2007;70:1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]
- 21.Witherington J., Bordas V., Garland S.L., Hickey D.M.B., Ife R.J., Liddle J., Saunders M., Smith D.G., Ward R.W. 5-Aryl-pyrazolo[3,4-b]pyridines: Potent inhibitors of glycogen synthase kinase-3 (GSK-3) Bioorg. Med. Chem. 2003;13:1577–1580. doi: 10.1016/S0960-894X(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 22.Naerum L., Norskov-Lauritsen L., Olesen P.H. Scaffold hopping and optimization towards libraries of glycogen synthase kinase-3 inhibitors. Bioorg. Med. Chem. Lett. 2002;12:1525–1528. doi: 10.1016/S0960-894X(02)00169-5. [DOI] [PubMed] [Google Scholar]
- 23.Martinez A., Alonso M., Castro A., Perez C., Moreno F.J. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: Thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 24.Coghlan M.P., Culbert A.A., Cross D.A.E., Corcoran S.L., Yates J.D., Pearce N.J., Rausch O.L., Murphy G.J., Carter P.S., Cox L.R., et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000;7:793–803. doi: 10.1016/S1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 25.Leost M., Schultz C., Link A., Wu Y.Z., Biernat J., Man-Delkow E.M., Bibb J.A., Snyder G.L., Greengard P., Zaharevitz D.W., et al. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandey M.K., DeGrado T.R. Glycogen synthase kinase-3 (GSK-3)-targeted therapy and imaging. Theranostics. 2016;6:571–593. doi: 10.7150/thno.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doskočil I., Hošťálková A., Šafratová M., Benešová N., Havlík J., Havelek R., Kuneš J., Královec K., Chlebek J., Cahlíková L. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem. Lett. 2015;13:394–398. doi: 10.1016/j.phytol.2015.08.004. [DOI] [Google Scholar]
- 28.Ago Y., Koda K., Takuma K., Matsuda T. Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J. Pharm. Sci. 2011;116:6–17. doi: 10.1254/jphs.11R01CR. [DOI] [PubMed] [Google Scholar]
- 29.Cahlíková L., Pérez D.I., Štěpánková Š., Chlebek J., Šafratová M., Hošťálková A., Opletal L. In vitro inhibitory effects of 8-O-demethylmaritidine and undulatine on acetylcholinesterase and their predicted penetration across the blood-brain barrier. J. Nat. Prod. 2015;78:1189–1192. doi: 10.1021/acs.jnatprod.5b00191. [DOI] [PubMed] [Google Scholar]
- 30.Cedrón J.C., Ravelo A.G., León L.G., Padrón J.M., Estévez-Braun A. Antiproliferative and structure activity relationships of Amaryllidaceae alkaloids. Molecules. 2015;20:13854–13863. doi: 10.3390/molecules200813854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Goietsenoven G., Hutton J., Becker J.P., Lallemand B., Robert F., Lefranc F., Pirker C., Vandenbussche G., Van Antwerpen P., Evidente A., et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010;24:4575–4584. doi: 10.1096/fj.10-162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma D., Pignanelli C., Tarade D., Gilbert T., Noel M., Mansour F., Adams S., Dowhayko K., Vshyvenko S., Hudlicky T., et al. Cancer cell mitochondria targeting by pancratistatin analogs is dependent on functional complex II and III. Sci. Rep. 2017;7:42957. doi: 10.1038/srep42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoh M., Kunitomo J., Kimura E., Hayase Y., Kobayashi H., Uchiyama N., Kawamoto T., Tanaka T., Mol C., Dougan D.R. Design, synthesis and structure-activity relationships of 1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3β. Bioorg. Med. Chem. 2009;17:2017–2029. doi: 10.1016/j.bmc.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Meijer L., Thunnissen A.-M.W.H., White A.W., Garnier M., Nikolic M., Tsai L.-H., Walter J., Cleverley K.E., Salinas P.C., Wu Y.-Z., et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000;7:51–63. doi: 10.1016/S1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa I., Kobayashi M., Kitanaka K., Kido M., Kyogoku Y. Marine natural products XII. On the chemical constituents of the Okinawan marine sponge Hymeniacidon aldis. Chem. Pharm. Bull. 1983;31:2321–2328. doi: 10.1248/cpb.31.2321. [DOI] [Google Scholar]
- 36.Cimino G., de Rosa S., de Stefano S., Mazzarella L., Puliti R., Sodano G. Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett. 1982;23:767–768. doi: 10.1016/S0040-4039(00)86943-9. [DOI] [Google Scholar]
- 37.Gompel M., Leost M., Bal De Kier J.E., Puricelli L., Hernandez F.L., Palermo J., Meijer L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004;14:1703–1707. doi: 10.1016/j.bmcl.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Shiono Y., Miyazaki N., Murayyma T., Harizon T.K., Katja D.G., Supratman U., Nakata J., Kakihara Y., Saeki M., Yoshida J., et al. GSK-3β inhibitory activities of novel dichlororesorcinol derivatives from Cosmopora vilior isolated from mangrove plant. Phytochem. Lett. 2016;18:122–127. doi: 10.1016/j.phytol.2016.09.007. [DOI] [Google Scholar]
- 39.Kulhánková A., Cahlíková L., Novák Z., Macáková K., Kuneš J., Opletal L. Alkaloids from Zephyranthes robusta Baker and their acetylcholinesterase and butyrylcholinesterase-inhibitory activity. Chem. Biodivers. 2013;10:1120–1127. doi: 10.1002/cbdv.201200144. [DOI] [PubMed] [Google Scholar]
- 40.Šafratová M., Novák Z., Kulhánková A., Kuneš J., Hrabinová M., Jun D., Macáková K., Opletal L., Cahlíková L. Revised NMR data for 9-O-demethylgalanthine: An alkaloid from Zephyranthes robusta (Amaryllidaceae) and its biological activity. Nat. Prod. Commun. 2014;9:787–788. [PubMed] [Google Scholar]
- 41.Cahlíková L., Hrabinová M., Kulhánková A., Benešová N., Chlebek J., Jun D., Novák Z., Kuča K., Macáková K., Opletal L. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013;8:1541–1544. [PubMed] [Google Scholar]
- 42.Vaněčková N., Hošťálková A., Šafratová M., Kuneš J., Hulcová D., Hrabinová M., Doskočil I., Štěpánková Š., Opletal L., Nováková L., et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016;6:80114–80120. doi: 10.1039/C6RA20205E. [DOI] [Google Scholar]
- 43.Šafratová M., Hošťálková A., Hulcová D., Breiterová K., Hrabcová V., Machado M., Fontinha D., Prudêncio M., Kuneš J., Chlebek J., et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2017;41:208–218. doi: 10.1007/s12272-017-1000-4. [DOI] [PubMed] [Google Scholar]
- 44.Havlasová J., Šafratová M., Siatka T., Štěpánková Š., Ločárek M., Opletal L., Hrabinová M., Jun D., Benešová N., Novák Z., et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat. Prod. Commun. 2014;9:1151–1155. [PubMed] [Google Scholar]
- 45.Baki A., Bielik A., Molnár L., Szendrei G., Keserü G.M. A high throughput luminescent assay for glycogen synthase kinase-3β inhibitors. ASSAY Drug. Dev. Technol. 2007;5:75–83. doi: 10.1089/adt.2006.029. [DOI] [PubMed] [Google Scholar]