Abstract
Two major obstacles for successful cancer treatment are the toxicity of cytostatics and the development of drug resistance in cancer cells during chemotherapy. Acquired or intrinsic drug resistance is responsible for almost 90% of treatment failure. For this reason, there is an urgent need for new anticancer drugs with improved efficacy against cancer cells, and with less toxicity on normal cells. There are impressive examples demonstrating the success of natural plant compounds to fight cancer, such as Vinca alkaloids, taxanes, and anthracyclines. Artesunic acid (ARTA), a drug for malaria treatment, also exerts cytotoxic activity towards cancer cells. Multidrug resistance often results from drug efflux pumps (ABC-transporters) that reduce intracellular drug levels. Hence, it would be interesting to know, whether ARTA could overcome drug resistance of tumor cells, and in what way ABC-transporters are involved. Different derivatives showing improved features concerning cytotoxicity and pharmacokinetic behavior have been developed. Considering both drug sensitivity and resistance, we chose a sensitive and a doxorubicin-resistant leukemia cell line and determined the killing effect of ARTA on these cells. Molecular docking and doxorubicin efflux assays were performed to investigate the interaction of the derivatives with P-glycoprotein. Using single-cell gel electrophoresis (alkaline comet assay), we showed that the derivatives of ARTA induce DNA breakage and accordingly programmed cell death, which represents a promising strategy in cancer treatment. ARTA activated apoptosis in cancer cells by the iron-mediated generation of reactive oxygen species (ROS). In conclusion, ARTA derivatives may bear the potential to be further developed as anticancer drugs.
Keywords: chemotherapy; multi-drug resistance; artemisinin, egonol, thymoquinone, hybrids
1. Introduction
The active compound artemisinin is derived from Artemisia annua L. (qinhao, sweet wormwood), a medical plant used in traditional Chinese medicine, and its semisynthetic derivatives artesunate and dihydroartemisinin exert not only antimalarial activity, even to otherwise drug-resistant Plasmodia [1], but also display inhibitory activity towards other diseases, including cancer in vitro [2,3,4,5,6,7,8] and in vivo [9,10]. Artemisinin and its derivatives also revealed anticancer activity in clinical pilot trials with human and veterinarian cancer patients [11,12,13,14,15,16,17,18]. More recently, it turned out that the bioactivity spectrum is much broader, and that artemisinin and its derivatives may also be valuable to treat other diseases, e.g., viral infections, schistosomiasis, trypanosomiasis, atherosclerosis, or diabetes [19,20,21,22,23]. Interesting features of artemisinin include activity against multidrug-resistant cancer cells [24], and good tolerance [25]. Because artemisinin has saved the lives of millions of patients, the Chinese scientist Youyou Tu, who discovered the antimalarial activity of this compound found in A. annua, was honored with the Nobel Prize for Medicine or Physiology in 2015 [26]. An endoperoxide bridge constitutes the active moiety, because its cleavage leads to the formation of reactive oxygen species (ROS) [27] and carbon-centered radicals [28]. In the malaria parasites, artesunic acid (ARTA) causes the iron(II)-mediated alkylation of heme and several other proteins, such as the translationally controlled tumor protein (TCTP), histidine-rich protein, and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) [29,30]. Tumor cells contain less iron than erythrocytes, but more than other normal tissues [31]. Iron may also be critical for the action of artemisinin-type drugs towards tumor cells, because it is correlated with the expression of the transferrin receptor (CD71), which is responsible for cellular iron uptake [32,33,34,35,36,37].
In malaria treatment, there is no cross-resistance of artemisinin and its derivatives to other antimalarial drugs. Therefore, it is reasonable to ask, whether or not ARTA is involved in the multidrug-resistance (MDR) phenotype in tumor cells.
ABC (ATP-binding cassette) transporters have a key function in MDR, where tumor cells develop resistance to a relatively wide range of drugs that have no structural or pharmacological similarities [38]. P-glycoprotein is a well-known member of this family and has a diverse spectrum of substrates, varying in size from 200 to 1900 Da [39]. It is responsible for the efflux of many xenobiotics, including numerous anticancer agents. In 1981, Tsuruo et al. first described verapamil, a calcium channel blocker, as an inhibitor of P-glycoprotein-mediated drug efflux [40]. Since then, there is still an ongoing search for new modulators of P-glycoprotein [41,42,43,44,45,46,47,48,49,50,51,52,53]. First-generation modulators, originally not used in cancer therapy, were highly toxic at suitable doses. Their derivatives, the second-generation modulators, had better efficacies, but had alarming pharmacokinetic interactions with anticancer drugs. Hence, new third-generation drugs have been developed with the hope that they would have a higher specificity for P-glycoprotein and fewer toxic effects. Three major binding sites were described for P-glycoprotein [54]: the H-site favors Hoechst 33,342, the R-site interacts with rhodamine 123, and a third site named the M-(modulatory) site.
To investigate the interaction with P-glycoprotein, we screened new ARTA derivatives, the design and development of which was inspired by the powerful concepts of natural product hybridization [55,56] and dimerization [57]. In addition, the possible mode of binding of these compounds was studied using molecular docking. Furthermore, we chose sensitive and doxorubicin-resistant leukemia cell lines to determine the cytotoxicity of a panel of ARTA derivatives on these cells. ARTA induced DNA damage and triggered apoptotic cell death in various tumor cell lines [24,58,59,60,61]. Therefore, we quantified the DNA damage of this series of ARTA derivatives by the alkaline comet assay.
2. Materials and Methods
2.1. Compounds
The syntheses of compounds REI230, REI235, TF27, and TF29 have been previously reported, [62] and are shown in Scheme 1. The chemical structures and physical–chemical characterization of compounds TF19, TF26, REI213, REI220+226, REI234, REI259, VK3, and HH3 are presented in the Supplementary Materials.
Scheme 1.
Chemical structures of artemisinin-, egonol-, and thymoquinone-based hybrids, dimers, and trimers studied in this work.
2.2. Cell Culture
Human leukemic CCRF-CEM and P-glycoprotein-expressing CEM/ADR5000 cells were obtained from the University of Jena (Department for Pediatrics, University of Jena, Jena, Germany). Cells were cultured in RPMI 1640 medium (Life Technologies, Schwerte, Germany), supplemented with 10% FBS (Life Technologies) and 1% penicillin (1000 U/mL)/streptomycin (100 µg/mL) (Life Technologies). They were maintained in a humidified, 5% CO2 supplied atmosphere in an incubator at 37 °C. CEM/ADR5000 cells were treated with 5000 ng/mL doxorubicin (Medical Center, Johannes Gutenberg University, Mainz, Germany) once per week to retain their resistance phenotype. The MDR profile has been previously reported [63,64,65]. Cells were passaged twice a week and then used for experiments in the logarithmic phase.
2.3. Resazurin Reduction Assay
The resazurin reduction assay was used to detect the cytotoxicity of ARTA derivatives towards sensitive and multidrug-resistant cells. Viable cells are able to reduce resazurin to the highly fluorescent resorufin, and this fluorescence can be measured with the Tecan Reader Infinite m200 Pro (Crailsheim, Germany). The method was previously described [66,67]. Briefly, CCRF-CEM and CEM/ADR5000 cells were seeded in the appropriate density (10,000 cells/well) in 96-well cell plates, with a total volume of 200 µL per well. Compounds were added in varying concentrations (0.001, 0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, and 100 µM). Each concentration was tested six times per experiment, and each experiment was repeated three times. Additionally, we tested the CEM/ADR5000 cells with doxorubicin alone and in combination with three different ARTA derivatives (10 µM) or verapamil (0.1, 0.3, 1, 3, 10, and 100 µM). After 72 h at 37 °C and 5% CO2, 20 µL resazurin (Sigma-Aldrich, Taufkirchen, Germany) 0.01% w/v in ddH2O was added to each well and further incubated for 4 h. The plates were measured using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. The test compound concentrations required to inhibit 50% of cell proliferation were represented by IC50 values calculated from dose–response curves.
2.4. Molecular Docking
Two-dimensional structures of ARTA and its derivatives were drawn and converted to 3D structures using the Corina Online Demo, and were saved in PDB format. Using the X-ray crystallography-based structure of a mouse P-glycoprotein as a template (PDB code: 5KOY), the homology structure of human P-glycoprotein was modeled as described [68]. The PDB file was converted to the PDBQT format using AutodockTools-1.5.6rc3. A grid box (coordinates of three dimensions: [grid center]: X: 21.092, Y: 92.594 and Z: 24.0; number of grid points in the three dimensions [npts]: X: 120, Y: 98 and Z: 100; spacing: 0.375) was constructed to define the transmembrane docking spaces of every type of atom in the ligand energies, which are used to predict the binding energies of the ligand; transmembrane docking spaces were calculated with the Autogrid 4.2 (The Scripps research Institute, Molecular Graphics Laboratory, La Jolla, CA, USA). Docking parameters were set to 250 runs and a 2,500,000 energy evaluation was set for each cycle. Using the Autodock 4.2 (Molecular Graphics Laboratory), we docked every ligand via the Lamarckian algorithm. The binding energies and interacting amino acids were received from DLG files, and the images were obtained using Visual Molecular Dynamics VMD (University of Illinois at Urbana Champaign, Champaign, IL, USA).
2.5. Flow Cytometry
CCRF-CEM and CEM/ADR5000 cells were exposed to doxorubicin (10 µM) (in the presence and absence of verapamil) and ARTA and its derivatives (10 µM). After incubation for 24 h, cells were harvested by centrifugation at 1500× g for 5 min. The supernatant was removed and the cells were suspended in a RPMI colorless medium. The fluorescence intensity of the intracellular doxorubicin was determined using a flow cytometer FACScalibur (Becton-Dickinson, Heidelberg, Germany), equipped with an ultraviolet argon laser (excitation at 488 nm, emission at 530/30 and 570/30 nm band-pass filters). The experiment was repeated thrice. Viable cells were gated, and we obtained the log fluorescence of single cells in forward and side light-scatter, based on the acquisition of data from 20,000 cells.
2.6. Single Cell Gel Electrophoresis (Alkaline Comet Assay)
DNA single-strand breaks were determined and calculated by single-cell gel electrophoresis. We used the OxiSelect™ Comet Assay Kit (Cell Biolabs-BIOCAT, Heidelberg, Germany). The alkaline comet assay detects both single and double DNA strand breaks. Radical molecules formed by ARTA derivatives generate DNA lesions and strand breaks. The DNA fragments migrate in the electrophoretic gel and form comets. With increasing DNA damage, the tail intensity increases rather than its length; tail length is determined primarily by the length of the loops. Here, we used a recently described protocol [69]. Exponentially growing cells were exposed to ARTA derivatives (10 µM) for 24 h, and then processed according to the manufacturer’s instructions. We varied both lysis times and electrophoresis times by 30 min. Fifteen cells per sample were analyzed with casplab 1.1.3b.2 software (http://casplab.com), (version 1.1.3 beta 2, CASPLAB, Wroclaw, Poland).
3. Results
To analyze the cytotoxic effects of ARTA derivatives, CCRF-CEM and P-glycoprotein-overexpressing CEM/ADR5000 cells were treated with the compounds for 72 h, and then the cell viability was evaluated using the resazurin reduction assay. Since drug resistance is a major problem in cancer chemotherapy, we assessed whether or not the derivatives bypassed MDR. The results are shown in Table 1. Most of the derivatives inhibited proliferation by 50% at concentrations below 10 µM. With the exception of TF26 and TF29, CEM/ADR5000 cells showed no or negligible cross-resistance towards the derivatives. Except for TF26 and TF29, the degree of resistance of the CEM/ADR5000 cells was much lower when compared to doxorubicin.
Table 1.
The cytotoxicity of artesunic acid (ARTA) derivatives and doxorubicin. CCRF-CEM and P-glycoprotein-expressing CEM/ADR5000 cells were incubated for 72 h with artemisinin-, egonol-, and thymoquinone-based hybrids. Resazurin assays were performed to determine dose–response curves and IC50 measurements were calculated (mean ± SD). Experiments were repeated at least twice. Resistance indices were obtained by dividing the IC50 values on the resistant cell line through that of the sensitive cell line.
| Compound | IC50 (µM) ± SD | Degree of Resistance | |
|---|---|---|---|
| CCRF-CEM | CEM/ADR5000 | ||
| Doxorubicin | 0.0033 ± 0.00065 | 1.613 ± 0.166 | 488.79 |
| Artesunic acid | 0.069 ± 0.03 | 0.189 ± 0.003 | 2.739 |
| DHA | 0.085 ± 0.003 | 0.265 ± 0.008 | 3.118 |
| REI213 | 0.568 ± 0.215 | 0.582 ±0.224 | 1.025 |
| REI220+26 | 43.685 ± 4.385 | 17.450 ± 1.010 | 0.399 |
| REI230 | 2.748 ± 0.021 | 2.789 ± 0.018 | 1.015 |
| REI234 | 0.0018 ± 0.0001 | 0.0068 ± 0.0006 | 3.778 |
| REI235 | 0.092 ± 0.006 | 0.199 ± 0.023 | 2.163 |
| REI259 | 0.876 ± 0.192 | 3.852 ± 1.021 | 4.397 |
| TF19 | 6.071 ± 0.247 | 5.663 ± 0.190 | 0.933 |
| TF26 | 0.0027 ± 0.001 | 7.872 ± 0.594 | 2915.556 |
| TF27 | 0.0024 ± 0.0001 | 0.196 ± 0.008 | 81.667 |
| TF29 | 0.0021 ± 0.0003 | 0.485 ± 0.210 | 230.952 |
| HH3 | 7.343 ± 0.911 | 7.071 ± 1.408 | 0.963 |
| VK3 | 0.134 ± 0.140 | 5.210 ± 0.153 | 38.881 |
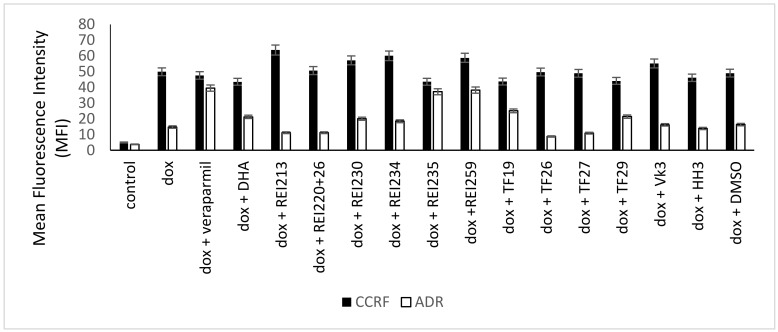
To measure the P-glycoprotein inhibitory activity of the ARTA derivatives, doxorubicin efflux assays were performed; relative intracellular doxorubicin concentrations were measured by flow cytometry. For comparison, the doxorubicin efflux assays were executed in the presence of a known P-glycoprotein modulator, verapamil. Figure 1 shows the results of the flow cytometric measurements. We measured low fluorescence intensities (MFIs). The MFIs of doxorubicin-treated cells were the lowest because of the efflux transport by P-glycoprotein. Concurrent treatment with verapamil inhibited P-glycoprotein, hence, these MFI values were the highest. Treatment with doxorubicin and the ARTA derivatives was found to be between both standards. Some of the derivatives did not interact with P-glycoprotein, and some inhibited the ABC-transporter (e.g., REI235, REI259, and TF19).
Figure 1.
Flow cytometric quantification of intracellular doxorubicin fluorescence in CCRF-CEM and CEM/ADR5000 cells treated with doxorubicin alone, doxorubicin and artemisinin-, egonol-, and thymoquinone-based hybrids, or doxorubicin and the P-glycoprotein inhibitor verapamil, as a positive control after incubation for 24 h.
To confirm the flow cytometry results, we carried out resazurin reduction assays by combining doxorubicin with the P-glycoprotein-modulating ARTA derivatives. We tested doxorubicin alone, in combination with verapamil (a known modulator of P-glycoprotein), and each of the three ARTA derivatives. Table 2 summarizes the IC50 values and the degrees of resistance reversal, which were calculated by dividing the IC50 values of doxorubicin alone by the IC50 values of doxorubicin in combination with the modulators.
Table 2.
IC50 values of CEM/ADR5000 upon treatment of doxorubicin combined with verapamil, or three hybrids, as measured by resazurin assays and the degrees of resistance reversal. Verapamil (20 µM) or derivatives (10 µM) were used in combination with doxorubicin (0.1–100 µM). Experiments were repeated at least twice, and for each concentration at least in triplicate.
| Modulator | IC50 (µM) in Combination with Doxorubicin ± SD | Fold Change in IC50 (Degree of Resistance Reversal) |
|---|---|---|
| - | 2.19 ± 0.041 | - |
| Verapamil | 0.69 ± 0.170 | 3.17 |
| REI259 | 0.84 ± 0.020 | 2.61 |
| REI235 | 0.85 ± 0.077 | 2.58 |
| TF19 | 1.43 ± 0.034 | 1.53 |
To consider the mode of binding of the ARTA derivatives, we performed molecular docking on human P-glycoprotein at the transmembrane domain (TDM). Table 3 summarizes the results for each compound, providing the lowest binding energies. The total number of interacting amino acids and the amino acids involved in hydrogen bonds are also displayed in Table 3.
Table 3.
Molecular docking of artemisinin-, egonol-, and thymoquinone-based hybrids on homology-modelled human P-glycoprotein in the transmembrane domain.
| Compound | Lowest Binding Energy (kcal/mol) | Mean Binding Energy (Kcal/mol) | Number of Interacting AA | AA Involved in H-Bond | pKi |
|---|---|---|---|---|---|
| REI213 | −12.945 (± 0.015) | −11.83 (± 0.11) | 13 | Gln725, Tyr953 | 9.4 |
| REI20+26 | −11.055 (± 0.105) | −10.31 (± 0.0) | 13 | - | 7.8 |
| REI230 | −12–78 (± 0.01) | 12.34 (± 0.12) | 11 | - | 9.3 |
| REI234 | −12.84 (± 0.035) | 12.185 (± 0.095) | 13 | Tyr953 | 9.5 |
| REI235 | −13.01 (± 0.01) | 12.24 (± 0.0) | 13 | - | 9.5 |
| TF19 | 11.425 (± 0.755) | 11.015 (± 0.345) | 17 | - | 8.9 |
| TF26 | −13.68 (± 0.425) | 13.3 (± 0.81) | 15 | Gln195 | 9.7 |
| TF27 | −14.815 (± 0.205) | −12.75 (± 0.37) | 15 | Gln990 | 11.0 |
| TF29 | −15.545 (± 0.049) | −13.845 (± 0.665) | 10 | Gln990 | 11.75 |
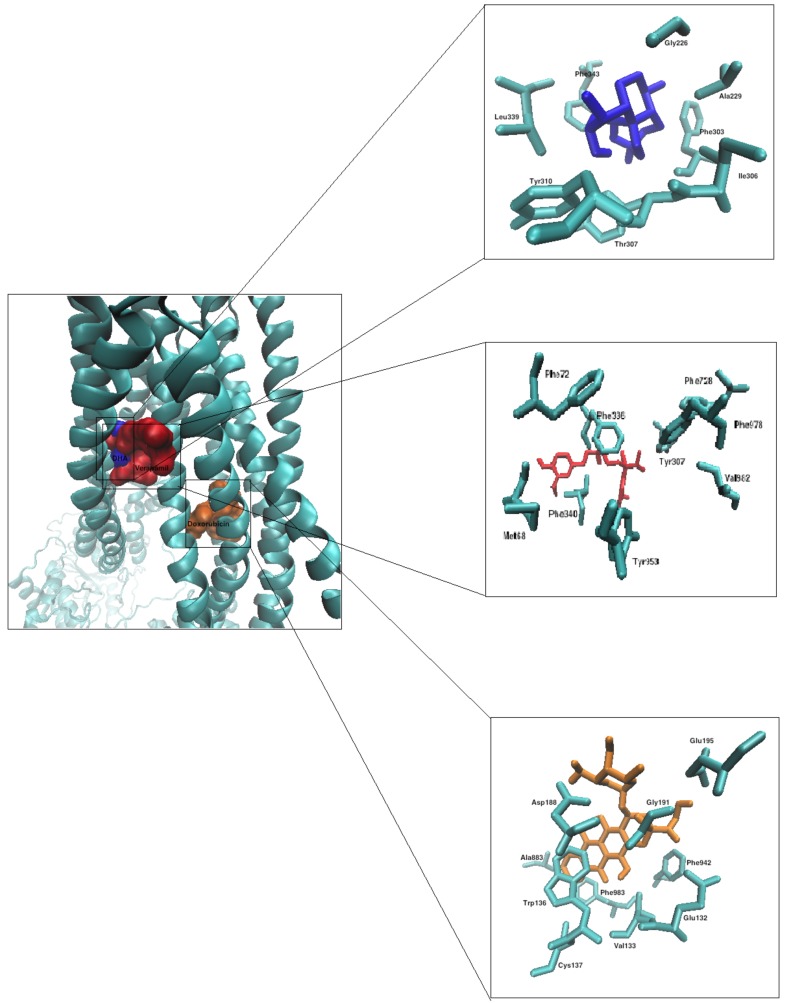
Figure 2 demonstrates the binding modes of dihydroartemisinin, verapamil, and doxorubicin with the respective interacting amino acids.
Figure 2.
Molecular Docking of dihydroartemisinin (blue), verapamil (red), and doxorubicin (orange) to P-glycoprotein (represented in a new cartoon style shown as cyan), corresponding interacting amino acids are shown for each compound.
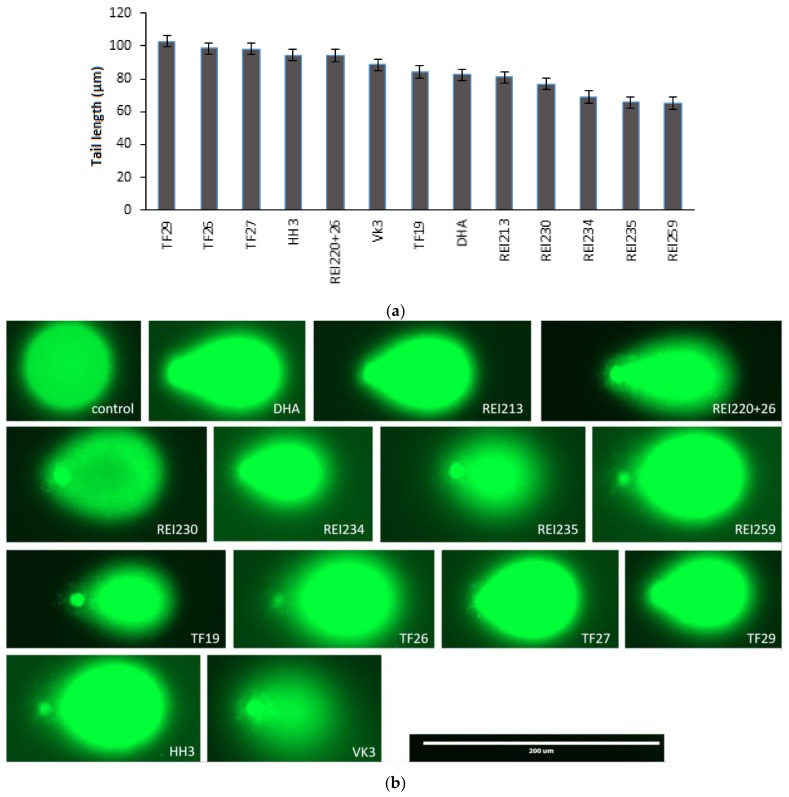
Furthermore, we examined the induction of DNA damage triggered by the artemisinin-, egonol-, and thymoquinone-based hybrids. For this reason, we performed the alkaline comet assay. CCRF-CEM cells were incubated with the derivatives for 24 h. As shown in Figure 3a, the tail lengths varied between 102.91 (± 0.53) and 65.15 (± 0.20) µm. Representative images of CCRF-CEM cells subjected to the alkaline comet assay upon treatment with ARTA derivatives are shown in Figure 3b.
Figure 3.
DNA damage induced by artemisinin-, egonol-, and thymoquinone-based hybrids. CCRF-CEM cells were each treated with10 µM of the above compounds and harvested for 24 h. The cells were subjected to alkaline single-cell gel electrophoresis as described. (a) Quantified tail lengths are the mean values of at least three independent experiments ± SD; (b) Representative images of cells treated with ARTA derivatives.
4. Discussion
In the present investigation, we demonstrated the cytotoxic activity of 12 novel ARTA-based derivatives on sensitive CCRF-CEM and multidrug-resistant CEM/ADR5000 cells. The IC50 values ranged from 0.0018 to 43.685 µM, and for most of the compounds we achieved low resistance indices. It is known for ARTA and its derivatives that their cytotoxicity is attributed to the cleavage of the endoperoxide bridge and subsequent ROS generation. One of the compounds, REI220+26, displayed higher cytotoxicity in CEM/ADR5000 than in CCRF-CEM cells. This ability of compounds to kill multidrug-resistant cells with greater efficacy than their parenteral drug-sensitive cells is termed collateral sensitivity [70,71]. The mechanism is still not completely understood, but it has been proposed that substances discharged by the ABC transporter deplete ATP [72], subsequent replenishment of ATP generates ROS by oxidative phosphorylation [73], and many collateral sensitive substances that are lipophilic re-enter the cell. Hence, a futile cycling [74] may develop, leading to ATP depletion and preferential killing of multidrug-resistant cells. Previously, it has been shown that twice the amount of ATP is consumed in multidrug-resistant cell lines in comparison to sensitive cells [74]. CEM/ADR5000 cells overexpress P-glycoprotein [63,65,75] and exhibit MDR with cross-resistance between anthracyclines, Vinca alkaloids, taxanes, and other drugs [64]. However, treatment of the CEM/ADR5000 cells with ARTA derivatives resulted in considerably enhanced intracellular retention of doxorubicin through inhibition of P-glycoprotein activity. The results obtained by flow cytometry were confirmed by resazurin assays that assessed the effect of the three most potent compounds REI235, REI259, and TF19 in combination with doxorubicin.
Furthermore, we used in silico molecular docking to investigate the possible interactions of these compounds with P-glycoprotein. With the exception of TF19, all derivatives revealed a similar binding site at the transmembrane region of P-glycoprotein. This binding site was close to the one of verapamil, and similar degrees of resistance reversal were observed. For this reason, we conclude that both the ARTA derivatives and verapamil may share a similar mechanism of inhibiting P-glycoprotein. Verapamil inhibits P-glycoprotein-mediated drug efflux in a competitive manner.
Most anti-cancer drugs kill tumor cells by the induction of programmed cell death, and it has been first shown by Efferth et al. that ARTA induces apoptosis in leukemia cells [4]. This result was subsequently corroborated by several other groups (for review see [6]). ARTA induces apoptosis in a different manner than doxorubicin. This may be the reason why ARTA induced apoptosis in doxorubicin-resistant cells [76]. In this study, we demonstrated by comet assays that the ARTA derivatives caused DNA damage. Recently, it was shown that ARTA induced DNA damage, presumably by ROS [59]. Furthermore, protein alkylation is a reason for ARTA-induced cytotoxicity [77,78]. The cleavage of the endoperoxide moiety of ARTA in the presence of ferrous iron by a Fenton-type reaction leads to the formation of ROS, as well as carbon-centered radical molecules. In conclusion, we described a panel of novel ARTA derivatives, most of which were not cross-resistant or collateral sensitive in multidrug-resistant cells. Some of the derivatives inhibited doxorubicin efflux by P-glycoprotein, leading to the reversal of doxorubicin resistance. Our data might inspire further investigations to clarify the exact signaling pathways of ARTA in cancer cells. ARTA derivatives may be useful drugs to fight MDR in cancer cells and improve the treatment of refractory tumors in clinics.
Acknowledgments
We would like to thank the kind staff of the flow cytometry core facility of the Institute of Molecular Biology (IMB, Mainz, Germany).
Abbreviations
AA, amino acid; ABC, ATP-binding cassette; ARTA, artesunic acid; DOX, doxorubicin; MDR, multidrug resistance; ROS, reactive oxygen species; SERCA, sarco/endoplasmic reticulum Ca2+ATPase; TCTP, translationally controlled tumor protein.
Supplementary Materials
The following are available online. Physical-chemical characterization of selected compounds and representative flow cytometric histograms of doxorubicin uptake assay in CEM/ADR5000 cells.
Author Contributions
Svetlana B. Tsogoeva and Thomas Efferth conceived and designed the experiments; Lisa Gruber, Sara Abdelfatah, Tony Fröhlich, Christoph Reiter and Volker Klein performed the experiments and analyzed the data; Lisa Gruber and Thomas Efferth wrote and revised the paper
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Daddy N.B., Kalisya L.M., Bagire P.G., Watt R.L., Towler M.J., Weathers P.J. Artemisia annua dried leaf tablets treated malaria resistant to ACT and i.v. artesunate: Case reports. Phytomedicine. 2017;32:37–40. doi: 10.1016/j.phymed.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woerdenbag H.J., Moskal T.A., Pras N., Malingre T.M., el-Feraly F.S., Kampinga H.H., Konings A.W. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod. 1993;56:849–856. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]
- 3.Lai H., Singh N.P. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91:41–46. doi: 10.1016/0304-3835(94)03716-V. [DOI] [PubMed] [Google Scholar]
- 4.Efferth T., Rücker G., Falkenberg M., Manns D., Olbrich A., Fabry U., Osieka R. Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs. Arzneimittelforschung. 1996;46:196–200. [PubMed] [Google Scholar]
- 5.Efferth T. Artemisinin—Second career as anticancer drug? World J. Tradit. Chin. Med. 2015;1:2–25. doi: 10.15806/j.issn.2311-8571.2015.0036. [DOI] [Google Scholar]
- 6.Konstat-Korzenny E., Ascencio-Aragón J., Niezen-Lugo S., Vázquez-López R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med. Sci. 2018;6:19. doi: 10.3390/medsci6010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Efferth T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017;139:56–70. doi: 10.1016/j.bcp.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Moore J.C., Lai H., Li J.R., Ren R.L., McDougall J.A., Singh N.P., Chou C.K. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995;98:83–87. doi: 10.1016/S0304-3835(06)80014-5. [DOI] [PubMed] [Google Scholar]
- 10.Dell’Eva R., Pfeffer U., Vene R., Anfosso L., Forlani A., Albini A., Efferth T. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem. Pharmacol. 2004;68:2359–2366. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Berger T.G., Dieckmann D., Efferth T., Schultz E.S., Funk J.O., Baur A., Schuler G. Artesunate in the treatment of metastatic uveal melanoma—First experiences. Oncol. Rep. 2005;14:1599–1603. doi: 10.3892/or.14.6.1599. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z.Y., Yu S.Q., Miao L.Y., Huang X.Y., Zhang X.P., Zhu Y.P., Xia X.H., Li D.Q. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6:134–138. doi: 10.3736/jcim20080206. [DOI] [PubMed] [Google Scholar]
- 13.Jansen F.H., Adoubi I., Kouassi J.C., DE C., Jansen N., Tschulakow A., Efferth T. First study of oral Artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor markers. Anticancer Res. 2011;31:4417–4422. [PubMed] [Google Scholar]
- 14.Rutteman G.R., Erich S.A., Mol J.A., Spee B., Grinwis G.C., Fleckenstein L., London C.A., Efferth T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer Res. 2013;33:1819–1827. [PubMed] [Google Scholar]
- 15.Breuer E., Efferth T. Treatment of iron-loaded veterinary sarcoma by Artemisia annua. Nat. Prod. Bioprospect. 2014;4:113–118. doi: 10.1007/s13659-014-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efferth T. Cancer combination therapy of the sesquiterpenoid artesunate and the selective EGFR-tyrosine kinase inhibitor erlotinib. Phytomedicine. 2017;37:58–61. doi: 10.1016/j.phymed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Krishna S., Ganapathi S., Ster I.C., Saeed M.E., Cowan M., Finlayson C., Kovacsevics H., Jansen H., Kremsner P.G., Efferth T., et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2015;2:82–90. doi: 10.1016/j.ebiom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Hagens C., Walter-Sack I., Goeckenjan M., Osburg J., Storch-Hagenlocher B., Sertel S., Elsässer M., Remppis B.A., Edler L., Munzinger J., et al. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2) Breast Cancer Res. Treat. 2017;164:359–369. doi: 10.1007/s10549-017-4261-1. [DOI] [PubMed] [Google Scholar]
- 19.Efferth T., Romero M.R., Wolf D.G., Stamminger T., Marin J.J., Marschall M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008;47:804–811. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 20.Saeed M.E., Krishna S., Greten H.J., Kremsner P.G., Efferth T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 2016;110:216–226. doi: 10.1016/j.phrs.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W., Cen Y., Song Y., Li P., Qin R., Liu C., Zhao Y., Zheng J., Zhou H. Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine. 2016;23:1259–1266. doi: 10.1016/j.phymed.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Efferth T.R.M., Bilia A.R., Osman A.G., Elsohly M., Wink M., Bauer R., Khan I., Bergonzi M.C., Marin J.J.G. Expanding the therapeutic spectrum of artemisinin: Activity against infectious diseases beyond malaria and novel pharmaceutical developments. World J. Tradit. Chin. Med. 2016;2:1–23. doi: 10.15806/j.issn.2311-8571.2016.0002. [DOI] [Google Scholar]
- 23.Li J., Casteels T., Frogne T., Ingvorsen C., Honore C., Courtney M., Huber K.V., Schmitner N., Kimmel R.A., Romanov R.A., et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell. 2017;168:86–100. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efferth T., Sauerbrey A., Olbrich A., Gebhart E., Rauch P., Weber H.O., Hengstler J.G., Halatsch M.E., Volm M., Tew K.D., et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro I.R., Olliaro P. Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med. Trop. 1998;58(Suppl. 3):50–53. [PubMed] [Google Scholar]
- 26.Tu Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2016;55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 27.Berman P.A., Adams P.A. Artemisinin enhances heme-catalysed oxidation of lipid membranes. Free Radic. Biol. Med. 1997;22:1283–1288. doi: 10.1016/S0891-5849(96)00508-4. [DOI] [PubMed] [Google Scholar]
- 28.Meshnick S.R., Yang Y.Z., Lima V., Kuypers F., Kamchonwongpaisan S., Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu) Antimicrob. Agents Chemother. 1993;37:1108–1114. doi: 10.1128/AAC.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asawamahasakda W., Ittarat I., Pu Y.M., Ziffer H., Meshnick S.R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 1994;38:1854–1858. doi: 10.1128/AAC.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckstein-Ludwig U., Webb R.J., Van Goethem I.D., East J.M., Lee A.G., Kimura M., O’Neill P.M., Bray P.G., Ward S.A., Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 31.Shterman N., Kupfer B., Moroz C. Comparison of transferrin receptors, iron content and isoferritin profile in normal and malignant human breast cell lines. Pathobiology. 1991;59:19–25. doi: 10.1159/000163611. [DOI] [PubMed] [Google Scholar]
- 32.Judd W., Poodry C.A., Strominger J.L. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J. Exp. Med. 1980;152:1430–1435. doi: 10.1084/jem.152.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc. Natl. Acad. Sci. USA. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatter K.C., Brown G., Trowbridge I.S., Woolston R.E., Mason D.Y. Transferrin receptors in human tissues: Their distribution and possible clinical relevance. J. Clin. Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efferth T., Benakis A., Romero M.R., Tomicic M., Rauh R., Steinbach D., Hafer R., Stamminger T., Oesch F., Kaina B., et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic. Biol. Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Kelter G., Steinbach D., Konkimalla V.B., Tahara T., Taketani S., Fiebig H.H., Efferth T. Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS ONE. 2007;2:e798. doi: 10.1371/journal.pone.0000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooko E., Saeed M.E., Kadioglu O., Sarvi S., Colak M., Elmasaoudi K., Janah R., Greten H.J., Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Andreoli T.E. Cecil Essentials of Medicine. Saunders; Philadelphia, PA, USA: 1997. [Google Scholar]
- 39.Schinkel A.H., Jonker J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003;55:3–29. doi: 10.1016/S0169-409X(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 40.Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Enhancement of vinblastine-induced cytotoxicity by lysolecithin and phosphatidylinositol. Cancer Lett. 1981;13:133–137. doi: 10.1016/0304-3835(81)90139-7. [DOI] [PubMed] [Google Scholar]
- 41.Ford J.M., Yang J.M., Hait W.N. P-glycoprotein-mediated multidrug resistance: Experimental and clinical strategies for its reversal. Cancer Treat. Res. 1996;87:3–38. doi: 10.1007/978-1-4613-1267-3_1. [DOI] [PubMed] [Google Scholar]
- 42.Fojo T., Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22:7512–7523. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- 43.Barthomeuf C., Bourguet-Kondracki M.L., Kornprobst J.M. Marine metabolites overcoming or circumventing multidrug resistance mediated by ATP-dependent transporters: A new hope for patient with tumors resistant to conventional chemotherapy. Anticancer Agents Med. Chem. 2008;8:886–903. doi: 10.2174/187152008786847729. [DOI] [PubMed] [Google Scholar]
- 44.Molnar J., Engi H., Hohmann J., Molnar P., Deli J., Wesolowska O., Michalak K., Wang Q. Reversal of multidrug resitance by natural substances from plants. Curr. Top. Med. Chem. 2010;10:1757–1768. doi: 10.2174/156802610792928103. [DOI] [PubMed] [Google Scholar]
- 45.Eichhorn T., Efferth T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J. Ethnopharmacol. 2012;141:557–570. doi: 10.1016/j.jep.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 46.Amiri-Kordestani L., Basseville A., Kurdziel K., Fojo A.T., Bates S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updates. 2012;15:50–61. doi: 10.1016/j.drup.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeino M., Saeed M.E., Kadioglu O., Efferth T. The ability of molecular docking to unravel the controversy and challenges related to P-glycoprotein–A well-known, yet poorly understood drug transporter. Investig. New Drugs. 2014;32:618–625. doi: 10.1007/s10637-014-0098-1. [DOI] [PubMed] [Google Scholar]
- 48.Abdelfatah S.A., Efferth T. Cytotoxicity of the indole alkaloid reserpine from Rauwolfia serpentina against drug-resistant tumor cells. Phytomedicine. 2015;22:308–318. doi: 10.1016/j.phymed.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y., Ding Y., Zhang T., An H. Sinapine reverses multi-drug resistance in MCF-7/dox cancer cells by downregulating FGFR4/FRS2α-ERK1/2 pathway-mediated NF-κB activation. Phytomedicine. 2016;23:267–273. doi: 10.1016/j.phymed.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Reis M.A., Ahmed O.B., Spengler G., Molnár J., Lage H., Ferreira M.J. Jatrophane diterpenes and cancer multidrug resistance—ABCB1 efflux modulation and selective cell death induction. Phytomedicine. 2016;23:968–978. doi: 10.1016/j.phymed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Teng Y.N., Sheu M.J., Hsieh Y.W., Wang R.Y., Chiang Y.C., Hung C.C. β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function. Phytomedicine. 2016;23:316–323. doi: 10.1016/j.phymed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Lu J., Zhang Y., Sun M., Liu M., Wang X. Comprehensive assessment of Cucurbitacin E related hepatotoxicity and drug-drug interactions involving CYP3A and P-glycoprotein. Phytomedicine. 2017;26:1–10. doi: 10.1016/j.phymed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Umsumarng S., Pitchakarn P., Yodkeeree S., Punfa W., Mapoung S., Ramli R.A., Pyne S.G., Limtrakul P. Modulation of P-glycoprotein by Stemona alkaloids in human multidrug resistance leukemic cells and structural relationships. Phytomedicine. 2017;34:182–190. doi: 10.1016/j.phymed.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Ferreira R.J., Ferreira M.J., dos Santos D.J. Molecular docking characterizes substrate-binding sites and efflux modulation mechanisms within P-glycoprotein. J. Chem. Inf. Model. 2013;53:1747–1760. doi: 10.1021/ci400195v. [DOI] [PubMed] [Google Scholar]
- 55.Tietze L.F., Bell H.P., Chandrasekhar S. Natural product hybrids as new leads for drug discovery. Angew. Chem. Int. Ed. 2003;42:3996–4028. doi: 10.1002/anie.200200553. [DOI] [PubMed] [Google Scholar]
- 56.Tsogoeva S.B. Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini-Rev. Med. Chem. 2010;10:773–793. doi: 10.2174/138955710791608280. [DOI] [PubMed] [Google Scholar]
- 57.Fröhlich T., Capci Karagoz A., Reiter C., Tsogoeva S.B. Artemisinin-derived dimers: Potent antimalarial and anticancer agents. J. Med. Chem. 2016;59:7360–7388. doi: 10.1021/acs.jmedchem.5b01380. [DOI] [PubMed] [Google Scholar]
- 58.Efferth T., Olbrich A., Bauer R. mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem. Pharmacol. 2002;64:617–623. doi: 10.1016/S0006-2952(02)01221-2. [DOI] [PubMed] [Google Scholar]
- 59.Li P.C., Lam E., Roos W.P., Zdzienicka M.Z., Kaina B., Efferth T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008;68:4347–4351. doi: 10.1158/0008-5472.CAN-07-2970. [DOI] [PubMed] [Google Scholar]
- 60.Berdelle N., Nikolova T., Quiros S., Efferth T., Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 61.Reichert S., Reinboldt V., Hehlgans S., Efferth T., Rodel C., Rodel F. A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother. Oncol. 2012;103:394–401. doi: 10.1016/j.radonc.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Reiter C., Fröhlich T., Gruber L., Hutterer C., Marschall M., Voigtländer C., Friedrich O., Kappes B., Efferth T., Tsogoeva S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015;23:5452–5458. doi: 10.1016/j.bmc.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 63.Gillet J.P., Efferth T., Steinbach D., Hamels J., de Longueville F., Bertholet V., Remacle J. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64:8987–8993. doi: 10.1158/0008-5472.CAN-04-1978. [DOI] [PubMed] [Google Scholar]
- 64.Efferth T., Konkimalla V.B., Wang Y.F., Sauerbrey A., Meinhardt S., Zintl F., Mattern J., Volm M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008;14:2405–2412. doi: 10.1158/1078-0432.CCR-07-4525. [DOI] [PubMed] [Google Scholar]
- 65.Kadioglu O., Cao J., Kosyakova N., Mrasek K., Liehr T., Efferth T. Genomic and transcriptomic profiling of resistant CEM/ADR-5000 and sensitive CCRF-CEM leukaemia cells for unravelling the full complexity of multi-factorial multidrug resistance. Sci. Rep. 2016;6:36754. doi: 10.1038/srep36754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuete V., Mbaveng A.T., Nono E.C., Simo C.C., Zeino M., Nkengfack A.E., Efferth T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2016;23:856–863. doi: 10.1016/j.phymed.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Kuete V., Mbaveng A.T., Sandjo L.P., Zeino M., Efferth T. Cytotoxicity and mode of action of a naturally occurring naphthoquinone, 2-acetyl-7-methoxynaphtho[2,3-b]furan-4,9-quinone towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2017;33:62–68. doi: 10.1016/j.phymed.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Tajima Y., Nakagawa H., Tamura A., Kadioglu O., Satake K., Mitani Y., Murase H., Regasini L.O., Bolzani Vda S., Ishikawa T., et al. Nitensidine A, a guanidine alkaloid from Pterogyne nitens, is a novel substrate for human ABC transporter ABCB1. Phytomedicine. 2014;21:323–332. doi: 10.1016/j.phymed.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 69.Kadioglu O., Chan A., Cong Ling Qiu A., Wong V.K.W., Colligs V., Wecklein S., Freund-Henni Rached H., Efferth T., Hsiao W.W. Artemisinin derivatives target topoisomerase 1 and cause DNA damage in silico and in vitro. Front. Pharmacol. 2017;8:711. doi: 10.3389/fphar.2017.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall M.D., Handley M.D., Gottesman M.M. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009;30:546–556. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pluchino K.M., Hall M.D., Goldsborough A.S., Callaghan R., Gottesman M.M. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist. Updates. 2012;15:98–105. doi: 10.1016/j.drup.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottesman M.M., Ambudkar S.V., Xia D. Structure of a multidrug transporter. Nat. Biotechnol. 2009;27:546–547. doi: 10.1038/nbt0609-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karwatsky J., Lincoln M.C., Georges E. A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity. Biochemistry. 2003;42:12163–12173. doi: 10.1021/bi034149+. [DOI] [PubMed] [Google Scholar]
- 74.Broxterman H.J., Pinedo H.M., Kuiper C.M., Kaptein L.C., Schuurhuis G.J., Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J. 1988;2:2278–2282. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- 75.Kimmig A., Gekeler V., Neumann M., Frese G., Handgretinger R., Kardos G., Diddens H., Niethammer D. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990;50:6793–6799. [PubMed] [Google Scholar]
- 76.Efferth T., Giaisi M., Merling A., Krammer P.H., Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE. 2007;2:e693. doi: 10.1371/journal.pone.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ismail H.M., Barton V.E., Panchana M., Charoensutthivarakul S., Biagini G.A., Ward S.A., O’Neill P.M. A Click Chemistry-Based Proteomic Approach Reveals that 1,2,4-Trioxolane and Artemisinin Antimalarials Share a Common Protein Alkylation Profile. Angew. Chem. Int. Ed. Engl. 2016;55:6401–6405. doi: 10.1002/anie.201512062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Y., Li W., Xiao Y. Profiling of multiple targets of artemisinin activated by hemin in cancer cell proteome. ACS Chem. Biol. 2016;11:882–888. doi: 10.1021/acschembio.5b01043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.