Abstract
Tinospora sinensis, a kind of Chinese folk medicine, has functions of harmonizing qi and blood, dredging the channels and collaterals, calming and soothing the nerves. In the present study, a method based on high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometry (HPLC-LTQ-Orbitrap) was developed for the systematical characterization of the non-diterpenoid constituents which possessed remarkable biological activities in T. sinensis, like anti-tumor, anti-inflammatory, hypoglycemic activity and immunomodulatory activity. Based on the accurate mass measurement (<5 ppm), retention times and MS fragmentation ions, 60 non-diterpenoid constituents were unambiguously or tentatively characterized from T. sinensis extract, including 27 alkaloids, 23 phenylpropanoids, seven sesquiterpenoids and three other constituents. Among them, 13 compounds were tentatively identified as new compounds. Finally, three of the non-diterpenoid constituents were purified and identified, which further confirmed the validity of the results. This study demonstrated that the HPLC-LTQ-Orbitrap MSn platform was a useful and efficient analytical tool to screen and identify constituents in natural medicine.
Keywords: HPLC-LTQ-Orbitrap, non-diterpenoids, fragmentation pathway, Tinospora sinensis
1. Introduction
Tinospora sinensis is derived from the dried stems of Tinospora sinensis (Lour.) Merr. (family Menispermaceae), which was officially documented in the Chinese Pharmacopoeia (2015) with the name Kuanjinteng [1]. As a kind of folk medicine which is mainly distributed in China, India and Southeast Asia, T. sinensis is commonly employed to treat various diseases. For instance, Tibetan medicine thought that T. sinensis could be used to treat rheumatoid arthritis, while Indian Ayurveda usually employed this ethnic drug in treatment for diabetes [2]. Pharmacological studies and clinical practice have demonstrated that the extracts of T. sinensis possessed various biological activities, including anti-inflammatory, anti-oxidative, anti-radiant, insecticidal and immunosuppressive effects [3,4,5,6].
T. sinensis has complicated chemical composition, including diterpenoids, alkaloids, phenylpropanoids, sesquiterpenes, triterpenoids, sterols, amino acids, and so on. Among them, diterpenoids are considered as the most abundant constituents. In previous work, we have systematic reported of diterpenoids in T. sinensis. A total of 63 diterpenoids were preliminarily identified, including 10 diterpenoid aglycones and 53 diterpenoid glycosides [7]. However, some non-diterpenoid constituents show good pharmacological activities which are probably closely related to its traditional efficacy. For example, two carboxylic acid esters isolated from T. sinensis showed significant PTP1B inhibitory activity in vitro, which represented a novel strategy for the treatment of type II diabetes [8]. Diosgenin isolated from T. sinensis showed good anti-inflammatory activity in carrageenan induced inflammation (paw edema) rodent model [9]. Three isoquinoline alkaloids isolated from T. cordifolia stem, named jatrorrhizine, palmatine and magnoflorine, were proved to possess the potential inhibitory effect on α-glucosidase in vitro and in vivo [10]. Tans-syringin, a typical phenylpropanoid glycoside which was abundant in T. sinensis, showed various activities of anti-tumor, anti-inflammatory, and hypoglycemic activities [11,12,13]. In addition, two sesquiterpenes of tinocordiside and 11-hydroxymustakone isolated from Tinospora cordifolia showed significant immunomodulatory activity [14]. Therefore, in order to comprehensively expound the material foundation of efficacy of T. sinensis, we propose a strategy to screen and identify the non-diterpenoid constituents in this herb.
As a powerful tool with the high resolution and excellent sensitivity to analyze multi-constituents in complex matrices, HPLC-ESI-MSn has been employed for characterization of phytochemical compounds in many areas of food and biological analyses [15,16]. The hybrid linear ion trap-Orbitrap mass spectrometer (LTQ-Orbitrap) was characterized by the higher mass resolution and mass accuracy (within 5 ppm) of orbitrap, MSn scanning function, and high trapping capacity of the linear ion trap [17,18]. Orbitrap allows the potent detection of a great deal of chemical constituents of similar accurate mass with high confidence of compounds identification, especially combined with retention times and the use of mass spectral libraries constructed with authentic standards [19]. These advantages facilitate to rapidly identify and characterize of multiple constituents in TCMs. In general, compounds with the same carbon skeleton usually have similar fragmentation pathway and characteristic product ions in collision-induced dissociation (CID) mode, so that this method also could be employed to identify novel constituents in TCMs [20]. In this study, a method with HPLC-LTQ-Orbitrap was established to comprehensively analyze the non-diterpenoid constituents in T. sinensis.
2. Results and Discussion
2.1. Identification of the Constituents by HPLC-LTQ-Orbitrap MSn
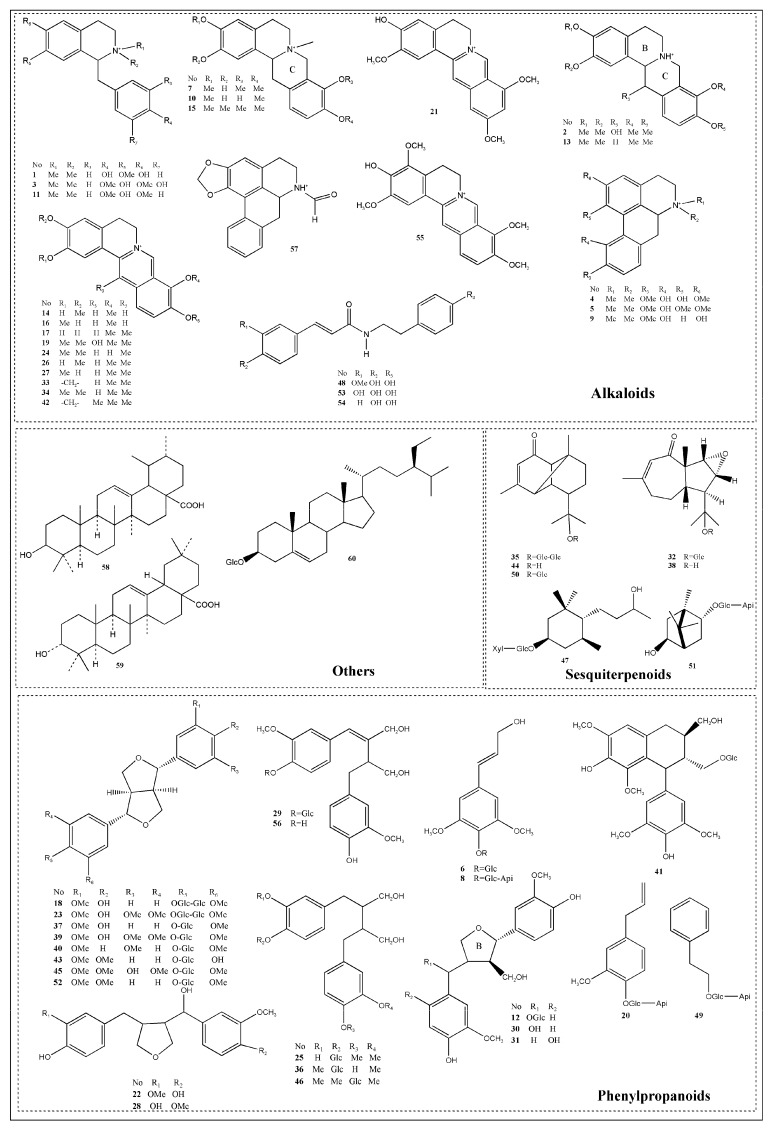
In order to get adequate structural information of the chemical constituents in T. Sinensis and reveal as many chemical compounds as possible, both positive and negative modes were employed for the comprehensive analysis. For the available standard compounds, these compounds were identified by comparing retention time (tR) and/or accurate mass. For the standard unavailable compounds, the molecular formula of which were confirmed by compared with the HRMS molecular formula database built in-home, the high-accuracy protonated precursors with an error less than 5 ppm and related literatures. In the present study, a total of 60 compounds (Table 1, Figure 1) were identified or tentatively identified from T. Sinensis extract, including 27 alkaloids, 23 phenylpropanoids, seven sesquiterpenoids and three others. A typical total ion chromatogram (TIC) of T. sinensis in positive and negative ion mode is presented in Figure 2.
Table 1.
Identification of chemical constituents of T. sinensis by HPLC-LTQ-Orbitrap.
| NO. | tR/min | Identification | Experical Formula | Proposal Ions | Theoretical Mass m/z | Experimental Mass m/z |
Mass Error (ppm) |
MS2 Data (Measured) |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.05 | Lotusine b | C19H24NO3 | [M]+ | 314.17507 | 314.17465 | −1.337 | 269 (100), 237 (8), 107 (12.85), 175 (15), 282 (4.7), 299(25) |
| 2 | 8.02 | 13-Hydroxy-2,3,9,10-tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquino[3,2-α]-isoquinolinium *,b | C21H26NO5 | [M]+ | 372.18054 | 372.17999 | −1.503 | 192 (100), 177 (3), 176 (0.1), 208 (29), 165 (0.15), 356 (0.61), 354 (1.79) |
| 3 | 8.96 | Tembetarine | C20H26NO4 | [M]+ | 344.18563 | 344.18536 | −0.798 | 299 (100), 175 (44), 137 (17), 267 (12), 312 (11), 206 (2), 329 (3) |
| 4 | 9.41 | Magnoflorine | C20H24NO4 | [M]+ | 342.16998 | 342.16946 | −1.534 | 297 (100), 265 (23), 311 (16), 310 (1.5), 282 (2.6), 237 (1) |
| 5 | 11.02 | 2,11-dihydroxy-10-methoxy-6,6-dimethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-6-ium *,b | C22H24NO7 | [M − H + 2HCOOH]− | 414.15472 | 414.15561 | 2.128 | 354 (100), 368 (42), 353 (30), 386 (12), 369 (0.3) |
| 6 | 11.24 | Trans-syringin a | C18H25O11 | [M − H + HCOOH]− | 417.13913 | 417.13998 | 2.019 | 371 (3.3), 209 (100), 191 (10.62), 179 (12.32), 387 (4.76), 373 (10.54), 399 (13.63), 381 (7.27) |
| 7 | 11.40 | S-trans-N-methyltetra-hydrocolumbamine | C21H26NO4 | [M]+ | 356.18563 | 356.18527 | −1.024 | 192 (100), 177 (1.2), 190 (1), 325 (0.1) |
| 8 | 13.40 | Tinosinen | C22H32O13Na | [M + Na]+ | 527.17351 | 527.17303 | −0.914 | 317 (100), 496 (7), 395 (3.8), 233 (3) |
| 9 | 14.23 | Menisperine | C21H26NO4 | [M]+ | 356.18563 | 356.18555 | −0.238 | 311 (100), 279 (74), 251 (1.8), 325 (6.6), 296 (7.1) |
| 10 | 14.59 | Cyclanoline | C20H24NO4 | [M]+ | 342.16998 | 342.17026 | 0.805 | 192 (100), 190 (4), 177 (3.8), 311 (1.4) |
| 11 | 15.63 | Colletine b | C20H26NO3 | [M]+ | 328.19072 | 328.19031 | −1.250 | 283 (100), 251 (8), 175 (19), 143 (4), 144 (1), 296 (5.6), 313 (1.7) |
| 12 | 16.16 | Tanegoside | C27H35O14 | [M − H + HCOOH]− | 583.20213 | 583.20276 | 1.077 | 375 (100), 537 (17.89), 357 (0.32), 327 (18.28), 568 (0.59), 565 (0.34), 522 (0.25) |
| 13 | 18.02 | Tetrahydropamatine | C21H26NO4 | [M]+ | 356.18563 | 356.18588 | 0.688 | 192 (100), 177 (2.4), 190 (2), 165 (0.2), 340 (0.5) |
| 14 | 18.97 | Stepharanine | C19H18NO4 | [M]+ | 324.12303 | 324.12292 | −0.115 | 309 (100), 280 (2.2), 294 (0.34), 292 (0.16), 307 (3.2) |
| 15 | 19.39 | 2,3,9,10-Tetramethoxy-7-methyl-5,8,13,13a-tetrahydro-6H-isoquino[3,2-α]-isoquinolinium *,b | C22H28NO4 | [M]+ | 370.20128 | 370.20117 | −0.310 | 206 (100), 204 (2.2), 191 (1.5), 190 (1.47), 165 (0.24), 355 (0.38) |
| 16 | 19.95 | Dehydrodiscretamine | C19H18NO4 | [M]+ | 324.12303 | 324.12311 | 0.075 | 309 (100), 280 (1.2), 306 (0.17), 294 (0.08), 292 (0.05) |
| 17 | 20.36 | Demethyleneberberine | C19H18NO4 | [M]+ | 324.12303 | 324.12277 | −0.137 | 309 (100), 280 (11), 308 (9), 294 (0.8), 292 (0.56), 306 (0.22) |
| 18 | 21.15 | Pinoresinol-di-O-β-d-glucopyranoside b | C32H42O16Na | [M + Na]+ | 705.23650 | 705.23627 | −0.335 | 543 (100), 687 (1), 528 (0.58), 381 (0.16), 661 (0.16) |
| 19 | 22.62 | 13-Hydroxypalmatine | C21H22NO5 | [M]+ | 368.14924 | 368.14908 | −0.169 | 353 (100), 352 (99), 350 (20), 324 (21), 334 (0.6), 338 (1) |
| 20 | 24.27 | Icariside D1 | C19H28O10Na | [M + Na]+ | 439.15746 | 439.15729 | −0.406 | 307 (100), 421 (2.6), 275 (1.6), 403 (0.16), 407 (0.24) |
| 21 | 25.24 | 3-Hydroxy-2,9,11-trimethoxy-5,6-dihydro isoquino[3,2-α]-isoquinolinylium | C20H20NO4 | [M]+ | 338.13868 | 338.13861 | −0.075 | 323 (100), 295 (0.6), 308 (0.2), 294 (2), 320 (0.05) |
| 22 | 26.15 | 3(a,4-dihydroxy-3-methoxybenzyl)-4-(4-hydroxy-3-methoxybenzyl) tetrahydrofuran | C20H23O6 | [M − H]− | 359.14891 | 359.14923 | 0.315 | 341 (100), 344 (0.11), 329 (51), 311 (0.32), 205 (1.19) |
| 23 | 26.55 | Syringaresinol-di-O-β-d-glucoside b | C35H47O20 | [M − H + HCOOH]− | 787.26552 | 787.26581 | 0.368 | 579 (100), 417 (14), 769( 12), 741 (14), 723 (4) |
| 24 | 26.73 | Palmaturbine | C20H20NO4 | [M]+ | 338.13868 | 338.13837 | −0.315 | 323 (100), 295 (0.5), 308 (0.1), 294(2) |
| 25 | 27.41 | 2-{4-[4-(3,4-Dimethoxy-phenyl)-2,3-bis-hydroxymethylbutyl]-2-hydroxyphenoxy}-6-hydroxymethyl-tetrahydropyran-3,4,5-triol *,b | C26H36O11Na | [M + Na]+ | 547.21498 | 547.21509 | 0.107 | 532 (9.45), 385 (100), 367 (0.31), 349 (0.04), 514 (0.32), 517 (0.84), 529 (2.46), 531(1.5), 395(0.32) |
| 26 | 27.98 | Jatrorrhizine | C20H20NO4 | [M]+ | 338.13868 | 338.13834 | −0.345 | 323 (100), 322 (6), 295 (1), 308 (0.4), 294 (5), 320 (0.02) |
| 27 | 29.04 | Columbamine | C20H20NO4 | [M]+ | 338.13868 | 338.13843 | −0.255 | 323 (100), 322 (14), 308 (1.5), 295 (1), 294 (16) |
| 28 | 31.17 | 4-{4-[(3,4-Dimethoxy-phenyl)hydroxymethyl]-tetrahydrofuran-3-ylmethyl}-benzene-1,2-diol *,b | C20H23O6 | [M − H]− | 359.14891 | 359.14935 | 0.435 | 341 (100), 344 (0.28), 329 (48), 311 (1.41), 191 (0.22), 343 (0.06) |
| 29 | 31.50 | Sagitiside A | C26H34O11Na | [M + Na]+ | 545.19933 | 545.19855 | −1.436 | 530 (100), 383 (34), 527 (14.88), 515 (11.30), 514 (3.30), 245 (1.32) |
| 30 | 31.97 | 8′-Epitanegool | C20H24O7Na | [M + Na]+ | 399.14142 | 399.14093 | −1.238 | 202 (100), 351 (37), 381 (33), 384 (22), 368 (8), 369 (9), 219 (7.5) |
| 31 | 33.68 | 4-[5-(4-Hydroxy-3-methoxyphenyl)-4-hydroxymethyl-tetrahydrofuran-3-ylmethyl]-6-methoxy-benzene-1,3-diol *,b | C20H24O7Na | [M + Na]+ | 399.14142 | 399.14133 | −0.236 | 202 (100), 351 (27), 381 (19.7), 384 (24), 369 (12), 368 (4), 219 (8.3) |
| 32 | 33.78 | Tinocordifolioside | C21H32O8Na | [M + Na]+ | 435.19893 | 435.19821 | −1.675 | 417 (20.57), 402 (0.13), 399 (0.78), 407 (8.28), 389 (1.93), 420 (0.53), 405 (0.77), 273 (100), 255 (2.15), 245 (0.15) |
| 33 | 33.97 | Berberine a | C20H18NO4 | [M]+ | 336.12303 | 336.12317 | 0.135 | 321 (100), 320 (25), 292 (21), 306 (1), 334 (0.66) |
| 34 | 35.15 | Palmatine | C21H22NO4 | [M]+ | 352.15433 | 352.1539 | −0.435 | 337 (69), 336 (100), 322 (0.5), 308 (20) |
| 35 | 37.33 | 6-{1-[3,4-Dihydroxy-6-hydroxymethyl-5-(3,4,5-trihydroxy-6-hydroxy-methyl-tetrahydropyran-2-yloxy)-tetrahydro-pyran-2-yloxy]-1-methylethyl}-2,12-dimethyltricyclo-[6.4.0.02,9]-dodec-11-en-10-one *,b | C27H42O12Na | [M + Na]+ | 581.25684 | 581.25641 | −0.753 | 365 (100), 347 (1.22), 563 (0.7), 551 (0.17), 533 (0.10), 419 (1.26), 401 (0.12), 257 (0.11) |
| 36 | 37.77 | 2-{4-[4-(4-Hydroxy-3-methoxyphenyl)-2,3-bis-hydroxymethylbutyl]-2-methoxyphenoxy}-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol *,b | C26H36O11Na | [M + Na]+ | 547.21498 | 547.21448 | −0.919 | 532 (100), 367 (42), 385 (35), 349 (8.6), 529 (13), 514 (5.8), 517 (2.5) |
| 37 | 38.60 | Pinoresinol-O-β-d-glucopyranoside | C26H31O11 | [M−H]− | 519.18608 | 519.1864 | 0.601 | 357 (100), 151 (0.21), 501 (0.04), 342 (0.08), 339 (0.02) |
| 38 | 38.69 | Tinocordifolin | C15H22O3Na | [M + Na]+ | 273.14611 | 273.14627 | 0.565 | 258 (11.24), 243 (10.32), 245 (27.57), 230 (4.6), 255 (100), 227 (27.93) |
| 39 | 39.57 | Syringaresinol-O-β-d-glucopyranoside | C28H36O13Na | [M + Na]+ | 603.20481 | 603.20416 | −1.081 | 441 (100), 573 (8.88), 588 (6.6), 585 (1.8), 426 (0.49), 423 (0.28) |
| 40 | 39.81 | 2-{4-[4-(3,5-Dimethoxy-phenyl)tetrahydro-furo[3,4-c]furan-1-yl]-2-methoxyphenoxy}-6-hydroxymethyl-tetrahydropyran-3,4,5-triol *,b | C28H35O13 | [M − H + HCOOH]− | 579.20721 | 579.20740 | 0.315 | 417 (100), 165 (0.08), 402 (0.08), 564 (0.04), 547 (0.02) |
| 41 | 40.47 | Lyoniresinol-2α-O-β-d-glucopyranoside | C28H38O13Na | [M + Na]+ | 605.22046 | 605.22021 | −0.252 | 443 (100), 425 (2), 413 (10), 395 (40.88), 412 (2.62), 590 (1.71), 576 (1.14), 574 (15.34), 587 (8.55) |
| 42 | 40.47 | 13-methylberberine b | C21H20NO4 | [M]+ | 350.13868 | 350.13852 | −0.47 | 335 (100), 334 (99), 306 (39), 332 (0.85), 320 (0.56) |
| 43 | 41.22 | 2-{4-[4-(3,4-Dimethoxy-phenyl)tetrahydro-furo[3,4-c]furan-1-yl]-2-hydroxyphenoxy}-6-hydroxymethyl-tetrahydropyran-3,4,5-triol *,b | C26H32O11Na | [M + Na]+ | 543.18368 | 543.18329 | −0.723 | 309 (100), 381 (38), 527 (25), 528 (24), 525 (17), 363 (7.8), 513 (8), 512 (6.49) |
| 44 | 41.23 | Tinosinenside | C26H40O11Na | [M + Na]+ | 551.24628 | 551.24579 | −0.895 | 335 (100), 503 (0.2), 521 (0.25), 533 (0.9), 419 (1.57), 387 (0.19), 257 (0.31), 239 (0.59), 203 (0.41) |
| 45 | 41.43 | 2-{4-[4-(3-Hydroxy-4,5-dimethoxyphenyl)-tetrahydrofuro[3,4-c]-furan-1-yl]-2,6-dimethoxy-phenoxy}-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol *,b | C28H36O13Na | [M + Na]+ | 603.20481 | 603.20441 | –0.667 | 441 (100), 573 (9.72), 588 (6.62), 585 (4.68), 426 (0.88), 423 (5.25), 587 (1) |
| 46 | 41.91 | Tinosposide A a | C27H35O11 | [M − H]− | 535.21738 | 535.21783 | 0.825 | 373 (100), 520 (1.14), 358 (4.6), 517 (1.05), 505 (0.39), 357 (0.33), 358 (3.92) |
| 47 | 41.97 | 3,9-Dihydroxy-megastigmane-3-O-β-d-glucopyranosyl(6→1)-β-d-xylpyranoside | C25H35O13 | [M − H + HCOOH]− | 543.20721 | 543.20740 | 0.336 | 528 (0.34), 497 (100), 525 (0.66), 507 (0.51), 411 (0.32) |
| 48 | 41.99 | N-trans-caffeoyltyramine a, b | C17H18NO4 | [M + H]+ | 300.12303 | 300.12320 | 0.551 | 163 (100), 138 (8.48), 135 (0.66), 121 (9.2) |
| 49 | 42.29 | 4-allyl-2-methoxyphenyl-6-O-β-d-glucopyranosyl-(6→1)-β-d-apiofuranoside | C21H30O11Na | [M + Na]+ | 481.16803 | 481.16748 | –1.149 | 349 (100), 317 (86), 440 (23), 291 (11), 466 (8) |
| 50 | 43.42 | Tinocordiside a | C21H32O7Na | [M + Na]+ | 419.20402 | 419.20309 | –2.229 | 401 (1.1), 391 (0.15), 375 (0.2), 257 (32.05), 239 (59.3), 203 (100), 185 (4.15) |
| 51 | 43.80 | Angelicoidenol-2-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | C21H36O11Na | [M + Na]+ | 487.21498 | 487.21439 | –1.217 | 469 (5.76), 472 (0.37), 457 (1.55), 439 (1.50), 355 (100), 337 (0.12), 193 (0.12) |
| 52 | 49.24 | Pinoresinol monomethyl ether-O-β-d-gluco-pyranoside | C28H35O13 | [M − H + HCOOH]− | 579.20721 | 579.20703 | –0.324 | 563 (0.5), 371 (100), 533 (15.8), 417 (5.4), 561 (0.36), 399 (0.56) |
| 53 | 49.78 | N-p-Coumaroyltyramine b | C17H18NO3 | [M + H]+ | 284.12811 | 284.12802 | –0.352 | 147 (100), 138 (0.18), 121 (0.94), 119 (1.87) |
| 54 | 51.86 | N-trans-feruloyltyramine a | C18H20NO4 | [M + H]+ | 314.13868 | 314.13855 | –0.428 | 177 (100), 145 (7.8), 117 (0.89), 149 (0.14), 138 (0.02) |
| 55 | 70.84 | 3-Hydroxy-2,4,9,10-tetramethoxy-5,6-dihydro-isoquino[3,2-α]-isoquinolinylium *,b | C21H22NO5 | [M]+ | 368.14924 | 368.14905 | –0.199 | 352 (100), 353 (85), 337 (62), 322 (27), 321 (2.4), 350 (1.5), 338 (12), 324 (17) |
| 56 | 78.44 | 2-(4-Hydroxy-3-methoxy-benzyl)-3-(4-hydroxy-3-methoxy-benzylidene)-butane-1,4-diol *,b | C20H24O6Na | [M + Na]+ | 383.14650 | 383.14645 | –0.156 | 365 (100), 368 (43), 351 (12), 347 (10), 245 (1) |
| 57 | 80.17 | N-Formylannonain | C18H16NO3 | [M + H]+ | 294.11246 | 294.11221 | –0.884 | 249 (100), 219 (3.2), 264 (7), 266 (0.85), 236 (0.8) |
| 58 | 116.95 | Ursolic acid | C30H47O3 | [M − H]− | 455.35197 | 455.35257 | 1.314 | 437 (21.06), 419 (4.9), 411 (45.93), 397 (13.61), 410 (14.24), 407 (19.45) |
| 59 | 118.78 | Oleanic acid | C30H47O3 | [M − H]− | 455.35197 | 455.35291 | 2.060 | 437 (1.65), 419 (1.63), 411 (4.66), 397 (5.75), 410 (2.13), 407 (100) |
| 60 | 125.78 | β-Sitosterol glycoside | C36H61O8 | [M − H + HCOOH]− | 621.43609 | 621.43665 | 0.893 | 575 (100), 560 (21), 603 (26.74), 585 (15), 606 (8.33) |
a Comparison with standards; b Firstly characterized in the genus Tinospora; * Tentatively identified as new compound.
Figure 1.
The structures of compounds identified in T. sinensis.
Figure 2.
TIC chromatogram of T. sinensis: (A) positive ion mode; (B) negative ion mode.
2.1.1. Structural Characterization and Identification of Alkaloids
Compound 33 produced a [M]+ ion at m/z 336.12317 (C20H18NO4, mass error = 0.135 ppm). It yielded a series of ions at m/z 321 [M − CH3]+, m/z 320 [M − CH3 − H]+ and 306 [M − CH3 − CH3]+, suggesting the presence of adjacent methoxyl groups. The product ion at m/z 320 also generated the predominant produce ions at m/z 292 [M − CH3 − H − CO]+. In addition, the [M − 2H]+ ion at m/z 334 suggested the presence of C5–C6 carbon-carbon single bonds, which might due to a more stable π-conjugated system was formed by the losses of two hydrogen. By comparison with reference standard, compound 33 was predicatively deduced as berberine.
Compounds 21, 24, 26 and 27 generated their [M]+ ions at m/z 338.13861 (mass error = −0.075 ppm), m/z 338.13837 (mass error = −0.315 ppm), m/z 338.13834 (mass error = −0.345 ppm) and m/z 338.13843 (mass error = −0.255 ppm) (C20H20NO4), respectively. After the CID cleavage, all of them produced [M − CH3]+, [M − CH3 − CH3]+, [M − CH3 − CO]+ and [M − CH4 − CO]+ ions at m/z 323, m/z 308, m/z 295 and m/z 294, respectively. The m/z 320 [M − H2O]+ ions of compounds 21 and 26 suggested the presence of C-3 hydroxyl groups. Moreover, the [M − CH3 − H]+ ions of compounds 26 and 27 at m/z 322 suggested the presence of C9–C10 methoxyl groups. Therefore, combined with bibliography data and fragmentation pathways, these four compounds were tentatively identified as 3-hydroxy-2,9,11-tri-methoxy-5,6-dihydroisoquino[3,2-α]isoquinolinylium, palmaturbine, jatrorrhizine and columbamine, respectively [5,21].
Compound 34 showed the [M]+ ion at m/z 352.15390 (C21H22NO4, mass error = −0.435 ppm). In the MS2 spectrum, m/z 336 [M − CH3 − H]+ was identified as the base peak. Other product ions like m/z 337 [M − CH3]+, m/z 322 [M − 2CH3]+ and m/z 308 [M − CH3 − H − CO]+ were also observed. By comparison with the literature data, compound 34 was tentatively identified as palmatine [22].
Compounds 14, 16 and 17 gave their [M]+ ions at m/z 324.12292 (mass error = −0.115 ppm), m/z 324.12311 (mass error = 0.075 ppm) and m/z 324.12277 (mass error = −0.137 ppm) (C19H18NO4), respectively. They all produced the [M − CH3]+, [M − 2CH3]+, [M − 2CH3 − 2H]+ and [M − CH3 − H − CO]+ ions at m/z 309, m/z 294, m/z 292 and m/z 280, respectively. Besides, the [M − H2O]+ ions at m/z 306 of compounds 16 and 17 suggested that the presence of C-3 hydroxyl group. In addition, compound 17 could generate [M − CH3 − H]+ at m/z 308, suggesting that there were C9–C10 methoxyl groups at the skeleton [23]. The proposed fragmentation pathway of compound 14, 16 and 17 are shown in Scheme 1. Therefore, compounds 14, 16 and 17 were tentatively ascertained as stepharanine, dehydrodiscretamine and demethyleneberberine.
Scheme 1.
The proposed fragmentation pathway of stepharanine, dehydrodiscretamine and demethyleneberberine.
Compounds 19 and 55 yielded their quasi-molecular ions [M]+ at m/z 368.14908 (mass error = −0.169 ppm) and m/z 368.14905 (mass error = −0.199 ppm) (C21H22NO5), respectively. Both of them generated the same ESI-MS2 ions at m/z 353 [M − CH3]+, m/z 352 [M − CH3 − H]+, m/z 338 [M − 2CH3]+ and 324 [M − CH3 − H − CO]+. In addition, the ion at m/z 350 [M − H2O]+ suggested the presence of hydroxyl group at C-3 or C-13. Meanwhile, compound 55 displayed the ion [M − OCH3]+ at m/z 337, which indicated there were adjacent methoxyl and phenolic hydroxyl groups at the skeleton [23]. Therefore, the phenolic hydroxyl group of compound 19 and compound 55 existed at C-13 and C-3, respectively. Compound 19 and 55 were presumed to be 13-hydroxypalmatine and 3-hydroxy-2,4,9,10-tetramethoxy-5,6-dihydro-isoquino[3,2-α]isoquinolinylium.
Compound 42 showed the [M]+ ion at m/z 350.13852 (C21H20NO4, mass error = −0.470 ppm). It generated a serial of ions at m/z 335 [M − CH3]+, m/z 334 [M − CH3 − H]+, m/z 332 [M − H2O]+, m/z 320 [M − 2CH3]+ and m/z 306 [M − CH3 − H − CO]+. Thus, compound 42 was tentatively determined as 13-methylberberine.
Compound 9 and 13 generated [M]+ ions at m/z 356.18555 (mass error = −0.238 ppm) and m/z 356.18588 (mass error = 0.688 ppm) (C21H26NO4), respectively. The MS2 spectrum of compound 9 could produce the ion at m/z 311 [M − (CH3)2NH]+, which was the characteristic fragment ion of aporphine-type alkaloids [23]. The ion of compound 13 at m/z 340 was generated by loss a CH4 from the quasi-molecular ion. The ions at m/z 192 and m/z 165 were produced by Retro-Diels-Alder (RDA) cleavage fragmentation at 8, 13-position of the C-ring. Moreover, the product ion at m/z 192 generated the minor ion at m/z 190 and m/z 177 by the loss of two hydrogen ions and a methyl group, respectively. Therefore, according to the literature data, compounds 9 and 13 were tentatively deduced as menisperine and tetrahydropamatine [24].
Compound 2 gave a [M]+ ion at m/z 372.17999 (C21H26NO5, mass error = −1.503 ppm). The ion at m/z 354 [M − H2O]+ suggested the presence of C-3 or C-13 hydroxyl group. Meanwhile, the ion at m/z 356 [M − CH3 − H]+ indicated the presence of C9–C10 methoxyl groups [23]. The ion at m/z 192 [M + H − 181]+ was produced by RDA cleavage fragmentation at 8,13-position of the C-ring. The product ion at m/z 192 generated the minor ion at m/z 177 by the loss of a methyl group. Moreover, the ions at m/z 208 and m/z 165 were produced by α-cleavage fragmentation at B-ring. Therefore, compound 2 was tentatively presumed to be 13-hydroxy-2,3,9,10-tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquino[3,2-α]isoquinolinium.
Compound 7 generated its [M]+ ion at m/z 356.18527 (C21H26NO4, mass error = −1.024 ppm). The ion at m/z 192 [M + H − 185]+ was yielded by RDA cleavage fragmentation at 8,13-position of the C-ring. Moreover, the product ion at m/z 192 generated the minor ions at m/z 190 and m/z 177 by the loss of two hydrogen ions and a methyl group, respectively. Moreover, the [M − OCH3]+ ions at m/z 325 indicated there were adjacent methoxyl and phenolic hydroxyl groups at the skeleton [23]. Therefore, compound 7 was tentatively deduced to be S-trans-N-methyltetrahydrocolumbamine.
Compound 15 generated its [M]+ ion at m/z 370.20117 (C22H28NO4, mass error = −0.310 ppm). It produced the ion at m/z 355, which involved the loss of a methyl group. The reaction of RDA cleavage took place in the course of which the characteristic ions at m/z 206 and m/z 165 are generated. In addition, the product ion at m/z 206 generated a serial of ions at m/z 204, m/z 191 and m/z 190 by the loss of two hydrogen ions, a methyl group and a molecule of methane, respectively. Therefore, compound 15 was tentatively determined as 2,3,9,10-tetramethoxy-7-methyl-5,8,13,13a-tetrahydro-6H-isoquino[3,2-α]isoquinolinium.
Compounds 4 and 10 produced their [M]+ ions at m/z 342.16946 (mass error = −1.534 ppm) and m/z 342.17026 (mass error = 0.805 ppm) (C20H24NO4), respectively. Both of them generated the same ions at m/z 311 [M − OCH3]+. In the MS2 spectrum, the ion at m/z 192 which was generated by compound 10 suggested that the reaction of RDA cleavage took place. The further ions at m/z 190 [M – 150 − 2H]+ and 177 [M – 150 − CH3]+ indicated that compound 10 belonged to N-methyltetrahydroprotoberberine-type alkaloids [23]. However, compound 4 could generated the ESI-MS2 base peak ion at m/z 297, which involved the loss of a molecule of (CH3)2NH, a characteristic fragment ion of aporphine-type alkaloids. The proposed fragmentation pathway of compound 4 is shown in Scheme 2. Combined with bibliography data and fragmentation pathways, these two compounds were tentatively ascertained as magnoflorine and cyclanoline [25].
Scheme 2.
The proposed fragmentation pathway of magnoflorine.
Compound 5 generated an [M − H + 2HCOOH]− ion at m/z 414.15561 (C22H24NO7, mass error = 2.128 ppm). The ion at m/z 369 was produced by the loss of (CH3)2NH from the quasi-molecular ion, which suggested the compound 5 might be a kind of aporphine-type alkaloid [23].Moreover, it also generated fragments at m/z 386 [M − H + 2HCOOH − CO]− and m/z 354 [M − H + 2HCOOH − CO − CH3OH]−. Therefore, compound 5 was tentatively determined as 2,11-dihydroxy-10-methoxy-6,6-dimethyl-5,6,6a,7-tetra-hydro-4H-dibenzo[de,g]quinolin-6-ium.
Compounds 1, 3 and 11 produced their [M]+ ions at m/z 314.17465 (C19H24NO3, mass error = −1.337 ppm), 344.18536 (C20H26NO4, mass error = −0.798 ppm) and 328.19031 (C20H26NO3, mass error = −1.250 ppm), respectively. All of them generated [M − CH3]+, [M − CH3OH]+, [M − (CH3)2NH]+ and [M − (CH3)2NH − CH3OH]+ ion at m/z 299, m/z 282, m/z 269, m/z 237, and m/z 329, m/z 312, m/z 299, m/z 267, and m/z 313, m/z 296, m/z 283, m/z 251, respectively. The MS2 ions at m/z 175 (C11H11O2) were generated by α-cleavage fragmentation from 9,10-position of quasi-molecular ions. Compound 3 also yielded the ions at m/z 206 and m/z 137 through α-cleavage fragmentation at 8,9-position of [M]+ ion. Moreover, the product ion of compound 11 at m/z 175 generated the major ions at m/z 144 and m/z 143 by the loss of a methoxyl group and a molecule of methanol, respectively. Therefore, compound 1, 3 and 11 were tentatively deduced as lotusine, tembetarine and colletine, respectively.
Compound 57 generated the [M + H]+ ion at m/z 294.11221 (C18H16NO3, mass error = −0.884 ppm). It generated a serial of ions at m/z 249 [M + H − CONH3]+, m/z 219 [M + H − CONH3 − CH2O]+, m/z 264 [M + H − CH2O]+, m/z 266 [M + H − CO]+ and m/z 236 [M + H − CO − CH2O]+. According to the fragmentation patterns, compound 57 was tentatively ascertained as N-formylannonain.
Compounds 48, 53 and 54 generated their [M + H]+ ions at m/z 300.12320 (C17H18NO4, mass error = 0.551 ppm), m/z 284.12802 (C17H18NO3, mass error = −0.352 ppm) and m/z 314.13855 (C18H20NO4, mass error = −0.428 ppm), respectively. The ions at m/z 138 (C8H12NO) were generated by α-cleavage fragmentation at 9,10-position of [M + H]+ ion. The ESI-MS2 base peak ion of compound 54 at m/z 177 [M + 2H − C8H12NO]+ further generated [M + 2H − C8H12NO − CO]+, [M + 2H − C8H12NO − CH3OH]+ and [M + 2H − C8H12NO − CO − CH3OH]+ at m/z 149, m/z 145 and m/z 117, respectively. Similarly, the ESI-MS2 base peak ion of compound 48 at m/z 163 [M + 2H − C8H12NO]+ further generated [M + 2H − C8H12NO − CO]+ and [M + 2H − C8H12NO − C2H2O]+ at m/z 135 and 121, respectively. The ESI-MS2 base peak ion of compound 53 at m/z 147 [M + 2H − C8H12NO]+ further generated [M + 2H − C8H12NO − CO]+ at m/z 119. In addition, the ion of compound 53 at m/z 138 could generate ion at m/z 121 by the loss of NH3. By comparison with reference standards, compound 48 and 54 were predicatively deduced as N-trans-caffeoyltyramine and N-trans-feruloyltyramine. Compound 53 was tentatively identified as N-p-coumaroyltyramine.
2.1.2. Structural Characterization and Identification of Phenylpropanoids
Compound 6 produced the [M − H + HCOOH]− ions at m/z 417.13998 (C18H25O11, mass error = 2.019 ppm). Its MS2 spectrum produced ions at m/z 399 and m/z 381, which involved the loss of one and two molecules of H2O, respectively. Moreover, the deprotonated molecular ions yield [M − H + HCOOH − CH2O]− at m/z 387, [M − H + HCOOH − CO2]− at m/z 373. In addition, compound 6 also produced a serial of ions at m/z 371 [M − H]−, m/z 209 [M − H − Glc]−, m/z 191 [M − H − Glc − H2O]−. The proposed fragmentation pathway of compound 6 is shown in Scheme 3. Compared with the tR values and mass spectra with the reference standard, compound 6 was predicatively characterized as trans-syringin.
Scheme 3.
The proposed fragmentation pathway of trans-syringin.
Compound 8 generated [M + Na]+ ion at m/z 527.17303 (C22H32O13Na, mass error = −0.914 ppm). The [M + Na]+ ion produced the ions at m/z 395 and m/z 233 in the MS2 spectrum, which originated from the neutral loss of an apiose moiety and a disaccharide moiety, which was composed of one molecule of glucose and one molecule of apiose. In addition, the molecular ion also produced the minor ion at m/z 496 [M + Na − OCH3]+. Thus, compound 8 was tentatively determined as tinosinen.
Compounds 20 and 49 yielded their [M + Na]+ ions at m/z 439.15729 (C19H28O10Na, mass error = −0.406 ppm) and m/z 481.16748 (C21H30O11Na, mass error = −1.149 ppm), respectively. Both of them generated [M + Na − Api]+ ions at m/z 307 and m/z 349, respectively. Compound 20 could generate a series of ions at m/z 421 [M + Na − H2O]+, m/z 403 [M + Na − 2H2O]+, m/z 407 [M + Na − CH3OH]+ and m/z 275 [M + Na − Api − H2O]+. Compound 49 displayed fragment ions at m/z 466, m/z 440 and m/z 317 corresponding to [M + Na − CH3]+, [M + Na − C3H5]+ and [M + Na − Api − CH3OH]+, respectively. By comparing with the literature data, compound 49 and 20 were tentatively deduced as 4-allyl-2-methoxyphenyl-6-O-β-d-glucopyranosyl(6→1)-β-d-apiofuranoside and icariside D1 [26].
Compound 18 generated [M + Na]+ ion at m/z 705.23627 (C32H42O16Na, mass error = −0.335 ppm). The ions at m/z 687 and m/z 661 were yielded by neutral loss of H2O and CO2, respectively. Moreover, the molecular ion generated the major ions at m/z 543 [M + Na − Glc]+, m/z 381 [M + Na − 2Glc]+ and m/z 528 [M + Na − Glc − CH3]+. According to the literature data, compound 18 was ascertained as pinoresinol-di-O-β-d-glucopyranoside [27].
Compounds 39 and 45 produced their [M + Na]+ ion at m/z 603.20416 (mass error = −1.081 ppm) and m/z 603.20441 (mass error = −0.667 ppm) (C28H36O13Na). Both of the molecular ions generated [M + Na − H2O]+, [M + Na − CH3]+, [M + Na − 2CH3]+, [M + Na − Glc]+, [M + Na − Glc − CH3]+ and [M + Na − Glc − H2O]+ at m/z 585, m/z 588, m/z 573, m/z 441, m/z 426 and m/z 423, respectively. In addition, compound 45 also yielded the [M + Na − CH3 − H]+ at m/z 587, which suggested the presence of adjacent methoxyl groups. Therefore, combined with literature data and fragmentation pathways, these two compounds were tentatively presumed to be syringaresinol-O-β-d-glucopyranoside and 2-{4-[4-(3-hydroxy-4,5-dimethoxyphenyl)-tetrahydrofuro[3,4-c]furan-1-yl]-2,6-dimethoxyphenoxy}-6-hydroxymethyltetra-hydropyran-3,4,5-triol, respectively [27].
Compound 43 produced [M + Na]+ ion at m/z 543.18329 (C26H32O11Na, mass error = −0.723 ppm). The ESI-MS2 base peak ion was [C13H18O7 + Na]+ at m/z 309. Moreover, it also generated ions at m/z 525 [M + Na − H2O]+, m/z 528 [M + Na − CH3]+, m/z 513 [M + Na − 2CH3]+, m/z 512 [M + Na − OCH3]+, m/z 381 [M + Na − Glc]+ and m/z 363 [M + Na − Glc − H2O]+. In addition, the distinctive ion at m/z 527 [M + Na − CH3 − H]+ indicated the presence of adjacent methoxyl groups. Thus, compound 43 was tentatively determined as 2-{4-[4-(3,4-dimethoxyphenyl)-tetrahydrofuro[3,4-c]furan-1-yl]-2-hydroxyphenoxy}-6-hydroxy-methyltetrahydropyran-3,4,5-triol.
Compound 23 generated [M − H + HCOOH]− ion at m/z 787.26581 (C35H47O20, mass error = 0.368 ppm). It produced a serial of ions at m/z 769 [M − H + HCOOH − H2O]−, m/z 741 [M − H]−, m/z 23 [M − H − H2O]−, m/z 579 [M − H + HCOOH − Glc]− and m/z 417 [M − H + HCOOH − 2Glc]−. According to the literature data, compound 34 was deduced as syringaresinol-di-O-β-d-glucopyranoside [27].
Compound 37 showed its [M − H]− ion at m/z 519.18640 (C26H31O11, mass error = 0.601 ppm). The base peak ion at m/z 357 was originated from the neutral loss of a glucose moiety. It further generated the fragment ions at m/z 342 and m/z 339 by the loss of a molecule of methyl and H2O, respectively. In addition, it also could produce [M − H − H2O]− at m/z 501. According to the literature data, it was tentatively ascertained as pinoresinol-O-β-d-glucopyranoside [27].
Compounds 40 and 52 produced their [M − H + HCOOH]− ions at m/z 579.20740 (mass error = 0.315 ppm) and m/z 579.20703 (mass error = −0.324 ppm) (C28H35O13). Both of them produced [M − H + HCOOH − Glc]− at m/z 417. Compound 52 generated a serial of ions at m/z 564 [M − H + HCOOH − CH3]− and m/z 402 [M − H + HCOOH − Glc − CH3]−. Compound 40 could produce [M − H + HCOOH − H2O]−, [M − H + HCOOH − Glc − H2O]−, [M − H]− and [M − H − Glc]− at m/z 561, m/z 399, m/z 533 and m/z 371, respectively. In addition, compound 40 also generated the ion at m/z 563 [M − H + HCOOH − CH4]−, which suggested the presence of adjacent methoxyl groups. Therefore, according to the fragmentation pathways and literature data, compound 40 and 52 was tentatively presumed to be 2-{4-[4-(3,5-dimethoxyphenyl)-tetrahydrofuro[3,4-c]furan-1-yl]-2-methoxyphenoxy}-6-hydroxymethyltetrahydropyran-3,4,5-triol and pinoresinol monomethyl ether-O-β-d-glucopyranoside [27].
Compounds 29 and 56 generated their [M + Na]+ ions at m/z 545.19855 (C26H34O11Na, mass error = −1.436 ppm) and m/z 383.14645 (C20H24O6Na, mass error = −0.156 ppm), respectively. The [M + Na]+ ion of compound 29 produced the aglycone ion at m/z 383 in the MS2 spectrum, which originated from the neutral loss of an glucose moiety (162 Da). Both of them could generate the same MS2 ions at m/z 245, which produced by a loss of neutral fragment (C8H10O2) from the ion at m/z 383. In addition, compound 29 generated a serial of ions at m/z 530 [M + Na − CH3]+, m/z 527 [M + Na − H2O]+, m/z 515 [M + Na − 2CH3]+, m/z 514 [M + Na − OCH3]+. Compound 56 generated [M + Na − CH3]+, [M + Na − H2O]+, [M + Na − CH3OH]+ and [M + Na − 2H2O]+ at m/z 368, m/z 365, m/z 351 and m/z 347, respectively. The proposed fragmentation pathway of compound 29 is shown in Scheme 4. Comparing with the literature data and respective fragmentation pathways, compound 29 and 56 was plausibly described as sagitiside A and 2-(4-hydroxy-3-methoxybenzyl)-3-(4-hydroxy-3-methoxybenzylidene)butane-1,4-diol.
Scheme 4.
The proposed fragmentation pathway of sagitiside A.
Compound 46 produced its [M − H]− ion at m/z 535.21783 (C27H35O11, mass error = 0.825 ppm). Its ESI-MS2 base peak ion at m/z 373 was generated by losing dehydrated glucose, and the major product ions at m/z 520 and m/z 505 were produced by the loss of one and two molecules of methyl from the quasi-molecular ion, respectively. In addition, the minor ion at m/z 357 [M − H − Glc − CH4]− generated from the ion at m/z 373 suggested the presence of adjacent methoxyl groups. By comparison with reference standard and literature data, compound 46 was proposed to be tinosposide A [28].
Compound 25 and 36 showed their [M + Na]+ ions at m/z 547.21509 (mass error = 0.107 ppm) and m/z 547.21448 (mass error = −0.919 ppm) (C26H36O11Na). Both of their molecular ions generated a series of ions at m/z 532 [M + Na − CH3]+, m/z 529 [M + Na − H2O]+, m/z 517 [M + Na − CH3]+, m/z 514 [M + Na − CH3 − H2O]+, m/z 385 [M + Na − Glc]+, m/z 367 [M + Na − Glc − H2O]+ and m/z 349 [M + Na − Glc − 2H2O]+. Moreover, compound 25 could also produce ions at m/z 531 and m/z 383 by the loss of CH4 and neutral fragment (C9H12O2), respectively. It suggested that the presence of adjacent methoxyl groups. Therefore, according to the fragmentation pathways, compound 25 and 36 were deduced as 2-{4-[4-(3,4-dimethoxyphenyl)-2,3-bishydroxymethylbutyl]-2-hydroxyphenoxy}-6-hydroxymethyltetrahydro-pyran-3,4,5-triol and 2-{4-[4-(4-hydroxy-3-methoxyphenyl)-2,3-bishydroxymethylbutyl]-2-methoxy-phenoxy}-6-hydroxymethyl-tetrahydropyran-3,4,5-triol, respectively.
Compound 12 produced its [M − H + HCOOH]− ion at m/z 583.20276 (C27H35O14, mass error = 1.077 ppm). As the ESI-MS2 base peak, the dominant characteristic ion was [M − H − Glc]− at m/z 375, corresponding to the cleavage from the dehydrated glucose. The major ions at m/z 568 and m/z 565 were generated by the neutral loss of CH3 and H2O, respectively. In addition, the molecular ion also produced ions at m/z 537 [M − H]−, m/z 522 [M − H − CH3]−, m/z 357 [M − H − Glc − H2O]− and m/z 327 [M − H − Glc − H2O − 2CH3]−. According to the literature data, it was identified as tanegoside [28].
Compounds 30 and 31 generated their [M + Na]+ ions at m/z 399.14093 (mass error = −1.238 ppm) and m/z 399.14133 (mass error = −0.236 ppm) (C20H24O7Na), respectively. The ESI-MS2 base peak ion at m/z 202 (C10H11O3Na) was produced by α-cleavage fragmentation from 8,12-position and 10,11-position of the B-ring. In addition, another major ion at m/z 219 (C10H12O4Na) was produced by α-cleavage fragmentation from 8,12-position and 9,10-position of the B-ring. Moreover, both of the molecular ions generated a serial of ions at m/z 381 [M + Na − H2O]+, m/z 368 [M + Na − CH2OH]+, m/z 384 [M + Na − CH3]+, m/z 369 [M + Na − 2CH3]+ and m/z 351 [M + Na − 2CH3 − H2O]+. According to the fragmentation pathways and the values of Clog P, compound 30 and 31 were tentatively determined as 8’-epitanegool and 4-[5-(4-hydroxy-3-methoxyphenyl)-4-hydroxymethyltetrahydrofuran-3-ylmethyl]-6-methoxybenzene-1,3-diol.
Compound 41 produced the [M + Na]+ ion at m/z 605.22021 (C28H38O13Na, mass error = −0.252 ppm). It generated its ESI-MS2 base peak ion at m/z 443 by loss of a dehydrated glucose, and further generated a serial of ions at m/z 425 [M + Na − H2O]+, m/z 413 [M + Na − 2CH3]+, m/z 412 [M + Na − OCH3]+ and m/z 395 [M + Na − H2O − 2CH3]+. In addition, the molecular ion also generated [M + Na − CH3]+, [M + Na − H2O]+ and [M + Na − OCH3]+ at m/z 590, m/z 578 and m/z 574, respectively. Combined with literature data, compound 41 was identified as lyoniresinol-2α-O-β-d-glucopyranoside [29].
Compounds 22 and 28 generated their [M − H]− ions at m/z 359.14923 (mass error = 0.315 ppm) and m/z 359.14935 (mass error = 0.435 ppm) (C20H23O6), respectively. Both of their deprotonated molecular ions produced [M − H − CH3]−, [M − H − H2O]−, [M − H − 2CH3]− and [M − H − 2CH3 − H2O]− at m/z 344, m/z 341, m/z 329 and m/z 311, respectively. In addition, compound 22 could generate the ion at m/z 205 (C12H13O3) by losing a neutral fragment (C8H10O3). However, compound 28 produced ions at m/z 343 [M − H − CH4]− and m/z 191 [M − H − C9H12O3]−. That indicated the presence of adjacent methoxyl groups of the C-ring. According to the fragmentation pathways and the values of Clog P, compound 22 and 28 were tentatively ascertained as 3(a,4-dihydroxy-3-methoxybenzyl)-4-(4-hydroxy-3-methoxybenzyl)tetrahydrofuran and 4-{4-[(3,4-dimethoxyphenyl)-hydroxymethyl]-tetrahydro-furan-3-ylmethyl}-benzene-1,2-diol.
2.1.3. Structural Characterization and Identification of Sesquiterpenoids
Compounds 35, 44 and 50 generated their [M + Na]+ ions at m/z 581.2564 (C27H42O12Na, mass error = −0.753 ppm), m/z 551.24579 (C26H40O11Na, mass error = −0.895 ppm) and m/z 419.20309 (C21H32O7Na, mass error = −2.229 ppm). That the deviation of compound 35 and 50 was 162 Da suggested compound 35 had one more glucose unit than compound 50. Similarly, that the deviation of compound 44 and 50 was 132 Da suggested compound 44 had one more apiose unit than compound 50. All of them displayed the same aglycone ions at m/z 257 (C15H22O2Na). In addition, compound 35 generated a serial of ions at m/z 563 [M + Na − H2O]+, m/z 551 [M + Na − 2CH3]+, m/z 533 [M + Na − 2CH3 − H2O]+, m/z 419 [M + Na − Glc]+, m/z 401 [M + Na − Glc − H2O]+, m/z 365 [M + Na − Glc − 3H2O]+ and m/z 347 [M + Na − Glc − 4H2O]+. Compound 44 generated a series of ions at m/z 533 [M + Na − H2O]+, m/z 521 [M + Na − 2CH3]+, m/z 503 [M + Na − 2CH3 − H2O]+, m/z 419 [M + Na − Api]+, m/z 387 [M + Na − Api − CH3OH]+, m/z 335 [M + Na − Api − C5H8O]+ and m/z 203 [M + Na − Api − Glc − C3H2O]+. Compared with the tR values and mass spectra with the reference standard, compound 50 was predicatively identified as tinocordiside [30]. Compound 35 and 44 were tentatively presumed to be 6-{1-[3,4-dihydroxy-6-hydroxymethyl-5-(3,4,5-trihydroxy-6-hydroxymethyltetrahydropyran-2-yloxy)-tetrahydropyran-2-yloxy]-1-methylethyl}-2,12-dimethyltricyclo[6.4.0.02,9]dodec-11-en-10-one and tinosinenside.
Compound 47 produced its [M − H + HCOOH]+ ion at m/z 543.20740 (C25H35O13, mass error = 0.336 ppm). It generated a series of ions at m/z 528 [M − H + HCOOH − CH3]−, m/z 525 [M − H + HCOOH − H2O]−, m/z 507 [M − H + HCOOH − 2H2O]−, m/z 497 [M − H + HCOOH − CH3 − CH2OH]− and m/z 411 [M − H + HCOOH − Xyl]−. Therefore, it was tentatively identified as 3,9-dihydorxymegastigmane-3-O-β-d-gluco-pyranosyl(6→1)-β-d-xylpyranoside.
Compounds 32 and 38 produced [M + Na]+ ions at m/z 435.19821 (C21H32O8Na, mass error = −1.675 ppm) and m/z 273.14627 (C15H22O3Na, mass error = 0.565 ppm). The [M + Na]+ ion of compound 32 produced the aglycone ion at m/z 273 in the MS2 spectrum, which originated from the neutral loss of an glucose moiety (162 Da). Compound 38 produced a serial of ions at m/z 255 [M + Na − H2O]+, m/z 245 [M + Na − CO]+, m/z 230 [M + Na − CO − CH3]+ and m/z 227 [M + Na − H2O − CO]+. Compound 32 generated [M + Na − Glc]+, [M + Na − Glc − CO]+, [M + Na − Glc − H2O]+, [M + Na − H2O]+ and [M + Na − CO]+ at m/z 273, m/z 245, m/z 255, m/z 417 and m/z 407, respectively. According to the literature data, compound 32 and 38 were tentatively determined as tinocordifolioside and tinocordifolin [31].
Compound 51 generated its [M + Na]+ ion at m/z 487.21439 (C21H36O11Na, mass error = −1.217 ppm). It produced the [M + Na − Api]+ and [M + Na − Api − Glc]+ ions at m/z 355 and m/z 193 due to the overall fracture of dehydrated apiose and glucose. In addition, the molecular ion also produced a serial of ions at m/z 469 [M + Na − H2O]+, m/z 472 [M + Na − CH3]+, m/z 457 [M + Na − 2CH3]+ and m/z 439 [M + Na − 2CH3 − H2O]+. Therefore, compound 51 was tentatively deduced as angelicoidenol-2-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside.
2.1.4. Structural Characterization and Identification of Other Compounds
Compound 60 showed [M − H + HCOOH]− ion at m/z 621.43665 (C36H61O8Na, mass error = 0.893 ppm). Its ESI-MS2 base peak ion at m/z 575 was generated by losing CH3 and CO. In addition, the major ions at m/z 606 and m/z 603 were produced by neutral loss of H2O and CH3, respectively. Moreover, the molecular ion also yielded ions at m/z 585 [M − H + HCOOH − 2H2O]− and m/z 560 [M − H + HCOOH − H2O − CO − CH3]−. By comparing with the literature data, compound 60 was tentatively identified as daucosterol [32].
Compound 58 and 59 produced their [M − H]− ions at m/z 455.35257 (mass error = 1.314 ppm) and m/z 455.35291 (mass error = 2.060 ppm) (C30H47O3). Both of the deprotonated molecular ions yield [M − H − H2O]−, [M − H − 2H2O]−, [M − H − H2O − CH2O]−, [M − H − COOH]− and [M − H − CO2]− at m/z 437, m/z 419, m/z 407, m/z 410 and m/z 411. According to the literature data and the values of Clog P, compounds 58 and 59 were tentatively deduced as ursolic acid and oleanic acid [33].
2.2. Isolated Compounds Identification
The raw spectral analysis data of three compounds, which were purified and identified from T. sinensis, are listed below.
Tinosinen (8): C22H32O13, white powder. ESI-MS m/z 572.2 [M + Na]+ and m/z 543.1 [M + K]+. 13C-NMR (CD3OD,150 MHz) δ 135.3 (C-1), 154.3 (C-2,6), 105.4 (C-3,5), 135.8 (C-4), 131.3 (C-7), 130.1 (C-8), 63.6 (C-9), 57.0 (OCH3), 105.1 (C-1′), 75.5 (C-2′), 85.2 (C-3′), 69.8 (C-4′), 78.1 (C-5′), 62.5 (C-6′), 111.4 (C-1″), 77.9 (C-2″), 80.6 (C-3″), 75.0 (C-4″), 65.2 (C-5″). 1H-NMR (CD3OD, 600 MHz) δ 6.74 (2H, s, H-3, H-5), 6.53 (1H, d, J = 15.9 Hz, H-7), 6.31 (1H, dt, J = 15.9, 5.6 Hz, H-8), 4.21 (2H, d, J = 5.6 Hz, H-9), 3.84 (6H, s, OCH3), 4.89 (1H, d, J = 7.7 Hz, H-1′), 3.59 (1H, dd, J = 8.9, 7.7 Hz, H-2′), 3.51 (1H, dd, J = 8.9, 8.8 Hz, H-3′), 3.44 (1H, dd, J = 8.8, 9.5 Hz, H-4′), 3.22 (1H, m, H-5′), 3.65 (1H, dd, J = 12.1, 5.0 Hz, H-6′a), 3.78 (1H, m, H-6′b), 5.30 (1H, d, J = 2.7 Hz, H-1″), 4.00 (1H, d, J = 2.7 Hz, H-2″), 3.77 (1H, m, H-4″a), 4.11 (1H, d, J = 9.3 Hz, H-4″b), 3.60 (2H, s, H-5″). Compared with the literature data, tinosinen was confirmed [28].
Syringaresinol-4-O-β-d-glucopyranoside (39): C28H36O13, white powder. ESI-MS m/z 603.2 [M + Na]+. 13C-NMR (CD3OD, 125 MHz) δ 139.6 (C-1), 105.4 (C-2, 6), 154.4 (C-3, 5), 135.8 (C-4), 87.2 (C-7), 55.5 (C-8), 73.0 (C-9), 133.2 (C-1′), 104.8 (C-2′, 6′), 149.4 (C-3′, 5′), 136.4 (C-4′), 87.6 (C-7′), 55.7 (C-8′), 72.9 (C-9′), 57.2 (3, 5-OCH3), 56.9 (3′, 5′-OCH3), 105.0 (C-1″), 75.8 (C-2″), 77.8 (C-3″), 71.4 (C-4″), 78.3 (C-5″), 62.7 (C-6″). 1H-NMR (CD3OD, 500 MHz) δ 6.71 (2H, s, H-2, H-6), 4.71 (1H, d, J = 4.4 Hz, H-7), 3.13 (1H, m, H-8), 3.91 (1H, dd, J = 9.5, 3.2 Hz, H-9a), 4.30~4.26 (1H, m, H-9b), 6.65 (2H, s, H-2′, H-6′), 4.76 (1H, d, J = 4.1 Hz, H-7′), 3.13 (1H, m, H-8′), 3.91 (1H, dd, J = 9.5, 3.2 Hz, H-9′a), 4.30~4.26 (1H, m, H-9′b), 3.85 (6H, s, 3, 5-OCH3), 3.84 (6H, s, 3′, 5′-OCH3), 4.84 (1H, d, J = 7.7 Hz, H-1″), 3.48 (1H, m, H-2″), 3.42 (1H, m, H-3″), 3.41 (1H, m, H-4″), 3.20 (1H, m, H-5″), 3.66 (1H, dd, J = 11.9, 5.2 Hz, H-6″a), 3.76 (1H, m, H-6″b). Compared with the literature data, syringaresinol-4-O-β-d-glucopyranoside was confirmed [28].
N-trans-Caffeoyltryramine (48): C17H17O4N, white powder. ESI-MS m/z 298.1 [M − H]−. 13C-NMR (CD3OD, 125 MHz) δ 126.9 (C-1), 113.6 (C-2), 145.3 (C-3), 147.3 (C-4), 115.0 (C-5), 120.6 (C-6), 140.7 (C-7), 116.9 (C-8), 167.8 (C-9), 129.9 (C-1′), 129.3 (C-2′, C-6′), 114.8 (C-3′, C-5′), 155.5 (C-4′), 34.4 (C-7′), 41.1 (C-8′). 1H-NMR (CD3OD, 500 MHz) δ 6.97 (1H, d, J = 1.8 Hz, H-2), 6.74 (1H, d, J = 8.1 Hz, H-5), 6.88 (1H, dd, J = 8.1, 1.8 Hz, H-6), 7.36 (1H, d, J = 15.6 Hz, H-7), 6.31 (1H, d, J = 15.7 Hz, H-8), 7.03 (2H, d, J = 8.2 Hz, H-2′, H-6′), 6.70 (2H, d, J = 8.2 Hz, H-3′, H-5′), 2.73 (2H, t, J = 7.4 Hz, H-7′), 3.43 (2H, t, J = 7.4 Hz, H-8′). Compared with the literature data, N-trans-caffeoyltryramine was confirmed [34].
3. Materials and Methods
3.1. Materials and Chemicals
T. Sinensis was purchased from Anguo Linshi Medicinal Materials Co., Ltd. (Anguo, Hebei, China) and then authenticated by Professor Chun-sheng Liu, Beijing University of Chinese Medicine. Reference compounds, including trans-syringin, tinocordiside, tinosposide A, N-trans-caffeoyltyramine were isolated from T. sinensis by the authors and their structures were fully characterized by chemical and spectroscopic methods (NMR and MS). N-trans-feruloyltyramine and berberine were purchased from Shanghai Tauto Biotech CO., Ltd. (Shanghai, China). Formic acid, methanol and acetonitrile (HPLC grade) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was purchased from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, Zhejiang, China).
3.2. Sample and Standards Preparation
The standard solutions of trans-syringin, tinocordiside, tinosposide A, N-trans-caffeoyltyramine, N-trans-feruloyltyramine and berberine were prepared in methanol at appropriate concentrations. Appropriate amounts of powdered dried alcoholic extracts of T. sinensis, which were refluxed with tenfold ethanol/water (70:30, v/v) for three times, were weighed precisely (0.13 g). The extracts were placed into 20 mL of methanol/water (70:30, v/v) and ultrasonically extracted at room temperature for 0.5 h, and then supplemented the loss with the same solvent. The mixture was filtrated and evaporated to nearly dry, and then washed it through Solid Phrase Extraction (SPE) C18 columns (J.T. Baker, Center Valley, PA, USA) with 4 mL distilled water and 4 mL methanol. Methanol eluent was filtered through a 0.22 µm membrane for analysis. All of the solutions were stored at 4 °C and brought to room temperature before analysis.
3.3. Extraction and Isolation
Ten kilogram of dried T. sinensis stems was extracted three times with reflux extraction method with 70% ethanol at 80 °C for 2 h. All extraction solutions were evaporated under reduced pressure to obtain the crude residue, which was suspended in pure water. Sequential liquid–liquid extraction for successive sample partition was performed by chloroform (CHCl3), ethyl acetate (EA), n-butanol (n-BuOH) and methyl alcohol (MeOH). The ethyl acetate (EA) extraction was subjected to silica gel columns with the step-gradient solvent system of petroleum ether (PE):ethyl acetate (EA) (12:1→0:1, v/v) and ethyl acetate (EA):methyl alcohol (MeOH) (6:1→0:1, v/v). And then Y90-101 was further isolated by multi silica gel columns, ODS column and Sephadex LH-20 column to yielded compound 48 (4.2 mg). The n-BuOH extraction was passed through an AB-8 macroporous resin column and then washed with H2O, 30% EtOH, 50% EtOH, 70% EtOH and 95% EtOH. The 30% EtOH fraction was further purified by Silica gel columns with elution of CHCl3–MeOH (15:1→0:1, v/v). Then, sample 11–23 was further isolated by Sephadex LH-20 column and multi silica gel columns to get compound 8 (3.2 mg) in CHCl3:MeOH:H2O (15:1:0.05). Sample 41–53 was further purified by multi silica gel columns to yield compound 39 (4.8 mg) in CHCl3:MeOH:H2O (15:1:0.05).
3.4. Instrumentation and Condition
HPLC analysis was performed on DIONEX Ultimate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a binary pump and an autosampler. Samples were separated on a Sunfire C18 column (250 × 4.6 mm i.d., 5 μm, Waters Corporation, Milford, MA, USA) at room temperature. The mobile phase consisted of 0.1% (v/v) formic acid and acetonitrile (B). A gradient program was adopted as follows: 0–5 min, 8–12% B; 5–25 min, 12–16% B, 25–45 min, 16–25% B; 45–75 min, 25–46% B; 75–80 min, 46–58% B; 80–95 min, 58–65% B; 95–105 min, 65–68% B; 105–130 min, 68–95% B; 130–140 min, 95–92% B. The flow rate was set as 1.0 mL/min.
The LTQ-Orbitrap XL mass spectrometer (Thermo Scientific (Bremen), Bremen, Germany) was connected to the HPLC system via an electrospray ionization (ESI) interface in a post-column splitting ratio of 1:4. The analysis was performed in both negative and positive ion mode with a mass range of m/z 100–1500. The optimized ESI parameters in negative ion mode were set as follows: capillary temperature of 350 °C; sheath gas (nitrogen) flow of 30 arb.; auxiliary gas (nitrogen) flow of 10 arb.; source voltage of 4.0 kV; capillary voltage of −35 V; tube lens voltage of −110 V. The capillary voltage was 25 V and tube lens voltage was 110 V in positive ion mode; and other parameters were same as those of negative ion mode. The resolution of the orbitrap mass analyzer was set at 30,000. The isolation width was 2 amu, and the normalized collision energy (CE) was set to 35%. Collision-induced dissociation (CID) was conducted in LTQ with an activation q of 0.25 and activation time of 30 ms. All instruments were controlled by the Xcalibur data system, and the data acquisition was carried out by analyst software Xcalibur (version 2.1) (Waltham, MA, USA) from Thermo Electron Corp.
1H (500 MHz), 13C (125 MHz) spectra were recorded on a Bruker AVANCE-500 NMR spectrometer (Bruker Daltonik GmbH, Rheinstetten, Karlsruhe, Germany). 1H (600 MHz), 13C (150 MHz) spectra were recorded on a Varian Inova 600 spectrometer (Varian Medical Systems, Palo Alto, CA, USA).
4. Conclusions
In this study, an effective and sensitive analytical method by HPLC-LTQ-Orbitrap-MSn was established for systematically characterizing non-diterpenoid constituents and guiding the extraction and isolation in T. sinensis extract. A total of 60 compounds attributed to four categories including 27 alkaloids, 23 phenylpropanoids, seven sesquiterpenoids and three other compounds were identified or tentatively characterized according to the tR and fragmentation pathways. In previous work, we have established a method for the content determination of total alkaloids in T. sinensis, and the results demonstrated that the alkaloid constituents were abundant in this herb, which were in accordance with this study [35]. Besides, 20 compounds were firstly characterized in the genus Tinospora and 13 of them were new compounds. Three natural compounds, including two phenylpropanoids and an alkaloid, were purified and identified from T. sinensis by systemic separation, which also demonstrated that the results of HPLC-LTQ-Orbitrap-MSn was reliable. The results serve well to illustrate the potential fragmentation pathways of non-deterpenoid constituents in T. sinensis, and the HPLC-LTQ-Orbitrap MSn platform was proved as an effective tool for rapid qualitative analysis of constituents. This study not only provides abundant information for better understanding of the chemical compounds in T. sinensis, but also benefits further quality control of this medicine.
Acknowledgments
The authors greatly appreciate the financial support from the graduate independent subject of Beijing University of Chinese Medicine (2017-JYB-XS-079).
Author Contributions
Bin Liu designed the experiments; Zi-Jian Wang, Qi-Shu Jiao and Lu-Lu Xu contribute to the data collection and analysis; Qi-Shu Jiao and Lu-Lu Xu contributed reagents/materials/analysis tools; Qi-Shu Jiao, Jia-Yu Zhang, Yan-Yan Jiang and Bin Liu wrote the paper.
Conflicts of Interest
All the authors declare that they have no conflict of interests.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Chinese Pharmacopeia Commission . Pharmacopeia of the People's Republic of China (Version 2015) China Medical Science Press; Beijing, China: 2015. [Google Scholar]
- 2.Wu F.R., Zeng C.Y., Dai W.B. Pharmacological Effects of Tinospora sinensis and Research Progress in its Clinical Application. China Licens. Pharm. 2014;11:37–40. [Google Scholar]
- 3.Chi S.S., She G.M., Han D., Wang W.H., Liu Z., Liu B. Genus Tinospora: Ethnopharmacology, Phytochemistry, and Pharmacology. Evid.-Based Complement. Altern. Med. 2016;2:1–32. doi: 10.1155/2016/9232593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai W.T., Xie Y.H., Wei Y.F., Huang L.P. Phytochemical and Pharmacological Research Advances of Kuan Jin Teng, a Tibetan Medicinal Plants. Mod. Chin. Med. 2016;18:1666–1674. [Google Scholar]
- 5.Maurya R., Gupta P., Chand K., Kumar M., Dixit P., Singh N., Dube A. Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res. 2009;23:1134–1143. doi: 10.1080/14786410802682239. [DOI] [PubMed] [Google Scholar]
- 6.Hegde S., Jayaraj M. A Review of the Medicinal Properties, Phytochemical and Biological Active Compounds of Tinospora sinensis (Lour.) Merr. J. Biol. Act. Prod. Nat. 2016;6:84–94. [Google Scholar]
- 7.Xu L.L., Guo F.X., Chi S.S., Wang Z.J., Jiang Y.Y., Liu B., Zhang J.Y. Rapid screening and identification of diterpenoids in Tinospora sinensis based on high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometry. Molecules. 2017;22:912. doi: 10.3390/molecules22060912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta P., Gupta P., Maurya U.R. A novel protein tyrosine phosphatase 1B inhibitor from Tinospora sinensis. Chron. Young Sci. 2012;3:199–203. doi: 10.4103/2229-5186.99569. [DOI] [Google Scholar]
- 9.Punitha D., Udhayasankar M.R., Danya U., Arumugasamy K., Shalimol A. Anti-inflammatory activity of characterized compound diosgenin isolated from Tinospora malabarica Miers in Ann. (Menispermaceae) in animal model. Int. J. Herb. Med. 2013;1:76–78. [Google Scholar]
- 10.Patel M.B., Mishra S.M. Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats. J. Funct. Foods. 2012;4:79–86. doi: 10.1016/j.jff.2011.08.002. [DOI] [Google Scholar]
- 11.Choi J., Shin K.M., Park H.J., Jung H.J., Kim H.J., Lee Y.S., Rew J.H., Lee K.T. Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med. 2004;70:1027–1032. doi: 10.1055/s-2004-832642. [DOI] [PubMed] [Google Scholar]
- 12.Xia N. Syringin exhibits anticancer effects in HeLa human cervical cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. Bangladesh J. Pharmacol. 2016;11:838–843. doi: 10.3329/bjp.v11i4.27755. [DOI] [Google Scholar]
- 13.Niu H.S., Liu I.M., Cheng J.T., Lin C.L., Hsu F.L. Hypoglycemic effect of syringin from Eleutherococcus senticosus in streptozotocin-induced diabetic rats. Planta Med. 2008;74:109–113. doi: 10.1055/s-2008-1034275. [DOI] [PubMed] [Google Scholar]
- 14.Sharma U., Bala M., Kumar N., Singh B., Munshi R.K., Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Xu X.J., Xu W., Huang J., Zhu D.Y., Qiu X.H. Rapid Characterization and Identification of Flavonoids in Radix Astragali by Ultra-High-Pressure Liquid Chromatography Coupled with Linear Ion Trap-Orbitrap Mass Spectrometry. J. Chromatogr. Sci. 2015;53:945–952. doi: 10.1093/chromsci/bmu155. [DOI] [PubMed] [Google Scholar]
- 16.Yu X.J., Zhang J.Y., Liu Q.Q., Wu Z.S., Liu S.S., Shi H., Ma Q. Chemical profiling of fifty constituents in Qi-gui-yin granule (QGY) by on-line high-performance liquid chromatography coupled with ESI-LTQ-Orbitrap mass spectrometer. Res. J. Chem. Environ. 2013;17:16–27. [Google Scholar]
- 17.Li Y., Liu Y., Liu R.R., Liu S.Y., Zhang X.P., Wang Z.J., Lu J.Q. HPLC-LTQ-orbitrap MSn profiling method to comprehensively characterize multiple chemical constituents in xiao-er-qing-jie granules. Anal. Methods. 2015;7:7511–7526. doi: 10.1039/C5AY00420A. [DOI] [Google Scholar]
- 18.Zhang Q.Q., Dong X., Liu X.G., Gao W., Li P., Yang H. Rapid separation and identification of multiple constituents in Danhong Injection by ultra-high performance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 2016;14:147–160. doi: 10.1016/S1875-5364(16)60008-0. [DOI] [PubMed] [Google Scholar]
- 19.Dunn W.B., Broadhurst D., Brown M., Baker P.N., Redman C.W.G., Kenny L.C., Kell D.B. Metabolic profiling of serum using Ultra Performance Liquid Chromatography and the LTQ-Orbitrap mass spectrometry system. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;871:288–298. doi: 10.1016/j.jchromb.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Zheng C.N., Hao H.P., Wang X., Wu X.L., Wang G.J., Sang G.W., Liang Y., Xie L., Xia C.H., Yao X.L. Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC-ESI-IT-TOF/MS: Shengmai injection as an example. J. Mass Spectrom. 2009;44:230–244. doi: 10.1002/jms.1502. [DOI] [PubMed] [Google Scholar]
- 21.Wu W., Song F., Yan C., Liu Z., Liu S. Structural analyses of protoberberine alkaloids in medicine herbs by using ESI-FT-ICR-MS and HPLC-ESI-MS(n) J. Pharm. Biomed. Anal. 2005;37:437–446. doi: 10.1016/j.jpba.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Xiang Q.Y., Hashi Y., Chen Z.L. Simultaneous detection of eight active components in Radix Tinosporae by ultra high performance liquid chromatography coupled with electrospray tandem mass spectrometry. J. Sep. Sci. 2016;39:2036–2042. doi: 10.1002/jssc.201600042. [DOI] [PubMed] [Google Scholar]
- 23.Qing Z.X., Cheng P., Zeng J.G. Research progress on mass spectral fragmentation behaviour of alkaloids in Macleaya cordata. Chin. Tradit. Herb. Drugs. 2013;44:2929–2939. [Google Scholar]
- 24.Lu X.R., Qin M.J., Xu D.R. Identification of Main Chemical Components of Tongguanwan Prescription by HPLC-ESI-MS/MS. Chin. J. Nat. Med. 2008;6:283–291. doi: 10.3724/SP.J.1009null. [DOI] [Google Scholar]
- 25.Bajpai V., Singh A., Chandra P., Negi M.P.S., Kumar N., Kumar B. Analysis of phytochemical variations in dioecious Tinospora cordifolia stems using HPLC/QTOF MS/MS and UPLC/QqQLIT-MS/MS. Phytochem. Anal. 2016;27:92–99. doi: 10.1002/pca.2601. [DOI] [PubMed] [Google Scholar]
- 26.Dong L.P., Chen C.X., Ni W., Xie B.B., Li J.Z., Liu H.Y. A new dinorclerone diterpenoid glycoside from Tinospora sinensis. Nat. Prod. Res. 2010;24:13–17. doi: 10.1080/14786410802253197. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y., Zhao H., Zou L.S., Liu X.H., Chai C., Wang S.N., Hua Y.J. Chemical constituents of eucommiae Cortex by LC-triple TOF MS/MS. J. Chin. Mass Spectrom. Soc. 2017;38:146–156. [Google Scholar]
- 28.Li W., Koike K., Liu L.J., Lin L.B., Fu X.W., Chen Y.J., Nikaido T. New lignan glucosides from the stems of Tinospora sinensis. Chem. Pharm. Bull. 2004;52:638–640. doi: 10.1248/cpb.52.638. [DOI] [PubMed] [Google Scholar]
- 29.Huang C., Li W., Ma F.H., Li Q., Asada Y., Koike K. Tinospinosides D, E, and tinospin E, further clerodane diterpenoids from Tinospora sagittata. Chem. Pharm. Bull. 2012;60:1324–1328. doi: 10.1248/cpb.c12-00482. [DOI] [PubMed] [Google Scholar]
- 30.Sabri G., Vishwakarma R.A. Tinocordiside, a New Rearranged Cadinane Sesquiterpene Glycoside from Tinospora cordifolia. J. Nat. Prod. 1997;60:839–841. [Google Scholar]
- 31.Maurya R., Handa S.S. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry. 1998;49:1343–1345. doi: 10.1016/S0031-9422(98)00093-4. [DOI] [Google Scholar]
- 32.Samita F., Ochieng C.O., Owuor P.O., Manguro L.A.O. New ceramide from the aerial part of Tinospora oblongifolia with cytotoxic activities. Nat. Prod. Res. 2014;28:661–666. doi: 10.1080/14786419.2014.895725. [DOI] [PubMed] [Google Scholar]
- 33.Lv Y., Xu S.W., Cai L., Zhou B., Chen L.S. Research on Isolation of 2α-Hydroxy-oleanolic Acid and 2α-Hydroxy-ursolic Acid and Their EI-MS(+) Differences. Chem. Ind. For. Prod. 2010;30:91–94. [Google Scholar]
- 34.Han S.H., Lee H.H., Lee I.S., Moon Y.H., Woo E.R. A new phenolic amide from lycium chinense miller. Arch. Pharm. Res. 2002;25:433–437. doi: 10.1007/BF02976596. [DOI] [PubMed] [Google Scholar]
- 35.Chi S.S., Han D., Liu B. Study on the determination method of total alkaloids in Tinospora sinensis caulis. Northwest Pharm. J. 2015;30:349–352. [Google Scholar]