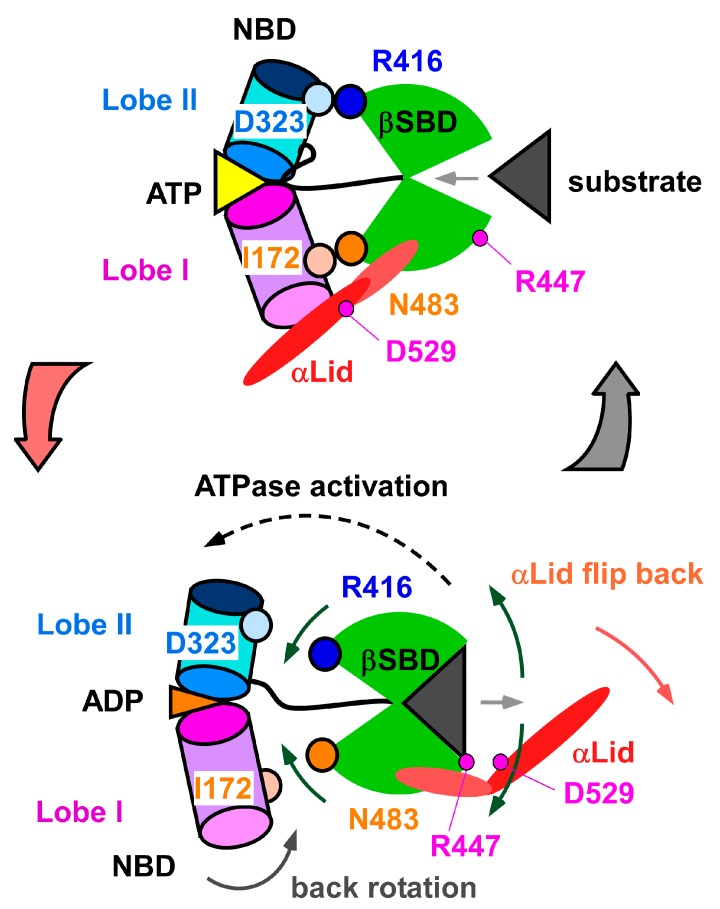
Figure 8.
Schematic showing interdomain communication with structural changes to both the SBD and NBD in association with client-peptide binding, and ATP hydrolysis and ADP turn over. The client-peptide (substrate) binding to SBD causes rearrangement of residues at the interdomain contact site to impair the interaction between SBD and NBD, which induces the back rotation of Lobe I in NBD to activate ATP hydrolysis. Substrate binding should also disrupt the inter-subdomain interaction that connects the αLid to the βSBD and is mediated by a salt bridge between R447 and D529. Such disruption of this interaction releases the αLid from βSBD and promotes the flip movement of the αLid to the NBD. The elevated αLid motion by disrupting the R447–D529 interaction also facilitates substrate release to promote progression of the chaperone cycle. The structural change in the lynchpin site for the inter-domain contact and also the intradomain allosteric structural change within SBD upon substrate binding are intimately related to peptide and nucleotide turnover, and their inter-relation between the structural change and turnover process promotes progression of the chaperone cycle of Hsp70.