Abstract
Rhizoma coptidis (RC) has been used as an herbal medicine in China for over one thousand years, and it was subjected to specific processing before use as materia medica. Processing is a pharmaceutical technique that aims to enhance the efficacy and/or reduce the toxicity of crude drugs according to traditional Chinese medicine theory. In this study, the chemical profiles of RC, ginger juice processed RC (GRC), and water processed RC (WRC) was determined to reveal the mechanism of processing of RC. UPLC-QTOF-MS analysis of methanol extract of RC, GRC, and WRC has been conducted to investigate the effect of processing on the composition of RC. HPLC-PDA was used to determine the variance of total alkaloids and seven alkaloids of RC during the processing. The volatiles of RC, GRC and ginger juice were separated by distillation, the change of volatiles content was recorded and analyzed, and the qualitative analysis of the volatiles was carried out using GC-MS. The microstructures of RC, GRC and WRC were observed using a light microscope. Results showed that ginger juice/water processing had limited influence on the composition of RC’s methanol extract, but significant influence on the content of some alkaloids in RC. Ginger juice processing significantly increased (p < 0.05) the volatiles content of RC and changed the volatiles composition obviously. Processing also had an influence on the microstructure of RC. This research comprehensively revealed the mechanism of ginger juice processing of RC.
Keywords: Rhizoma coptidis, processing, giner juice, alkaloid, volatiles
1. Introduction
Rhizoma coptidis (RC) is the dried rhizome of Coptis chinensis Franch, Coptis deltoidea C. Y. Cheng et Hsiao or Coptis teeta Wall (Fam. Ranunculaceae) [1], the most common used name is Coptis chinensis Franch. RC has been used in China for thousands of years and according to traditional Chinese medicine theory, RC can “clear the damp-heat, purge the fire and counteract the poison” [1,2]. Recent studies have indicated that RC possesses multispectral therapeutic activities, including antibacterial, antifungal, antiviral, antihyperglycemia, antihyperlipidemia, antihypertension, anti-inflammatory, and anti-oxidation effects [3,4].
Processing of Chinese Materia Medica (CMM) is a pharmaceutical technique to fulfill the different requirements of therapy, dispensing and making preparations according to traditional Chinese medicine theory. The aims of processing are to enhance the efficacy and/or reduce the toxicity of crude drugs [5]. Improper processing of drugs was one of the main reasons of occurrence and influencing factors of TCM-induced diseases [6].
RC was subjected to specific processing before it was used as materia medica in China. The purposes of processing RC were to reduce the side effects in the gastrointestinal tract and enhance its efficacy. The Chinese pharmacopoeia recorded three types of processed RC, RC processed with wine, RC processed with ginger juice, and RC processed with Fructus Evodiae [1]. Processed RC using different methods demonstrate different properties, and their pharmacological effects in the clinic were also different. RC processed with ginger juice was used for the treatment of stomach issues and it also has an anti-vomiting effect [7].
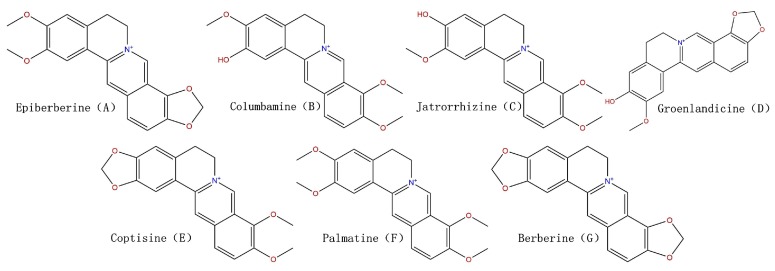
The major active constituents in Coptis chinensis Franch are alkaloids, including epiberberine, columbamine, jatrorrhizine, groenlandicine, coptisine, palmatine and berberine, shown in Figure 1. A number of studies on the determination of alkaloid content of RC have been reported [8,9,10]. However, little research on the difference of processed and unprocessed RC has been reported. Jiang et al. [7] identified 13 alkaloids in RC and processed RC by the UPLC-QTOF/MS method, and proved the chemical differences between crude and processed RC based on the PCA analysis. Huang et al. [11] established HPLC-PAD method for the determination of 11 Alkaloids of crude and wine-processed RC. Qian et al. [12] developed and validated a UHPLC-ESI-MS/MS method for simultaneous quantitative determination of ten alkaloids in rat plasma after administration of crude and wine-processed RC aqueous extracts.
Figure 1.
Chemical structure of the studied seven alkaloid in extracts of processed and unprocessed Rhizoma coptidis.
In addition, even though most studies of the processing of RC focused on alkaloids, the influence of processing on RC was not only the changing of alkaloids, and further study on the change of other constituents was needed. Ginger juice was a commonly-used adjuvant for CMM processing, which contains active ingredients such as gingerols, shogaols and volatiles [13,14]. The effect of ginger’s main component on RC’s processing has not been reported before, and the mechanism of processing on RC was also not fully illuminated.
In this study, the chemical profiles of unprocessed RC, ginger juice-processed Rhizoma coptidis (GRC) and water processed Rhizoma coptidis (WRC) were investigated (WRC was used as a control group). Both alkaloids and volatiles of RC and processed RC were determined. The aim of this study was to investigate the effect of processing on RC and to reveal the mechanism of ginger juice-processing working on RC.
2. Results
2.1. The Effect of Processing on the Composition of Methanol Extract of Rhizoma coptidis
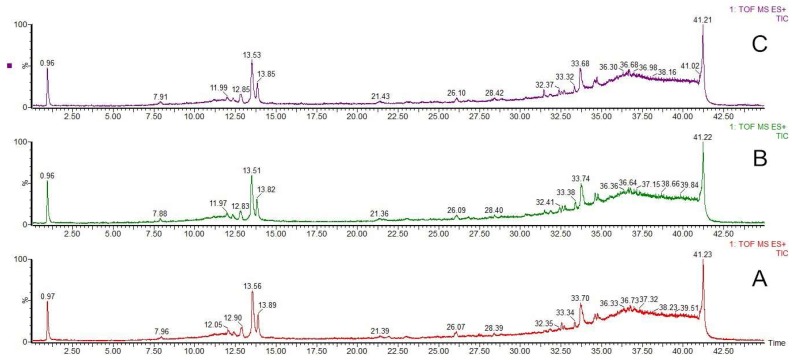
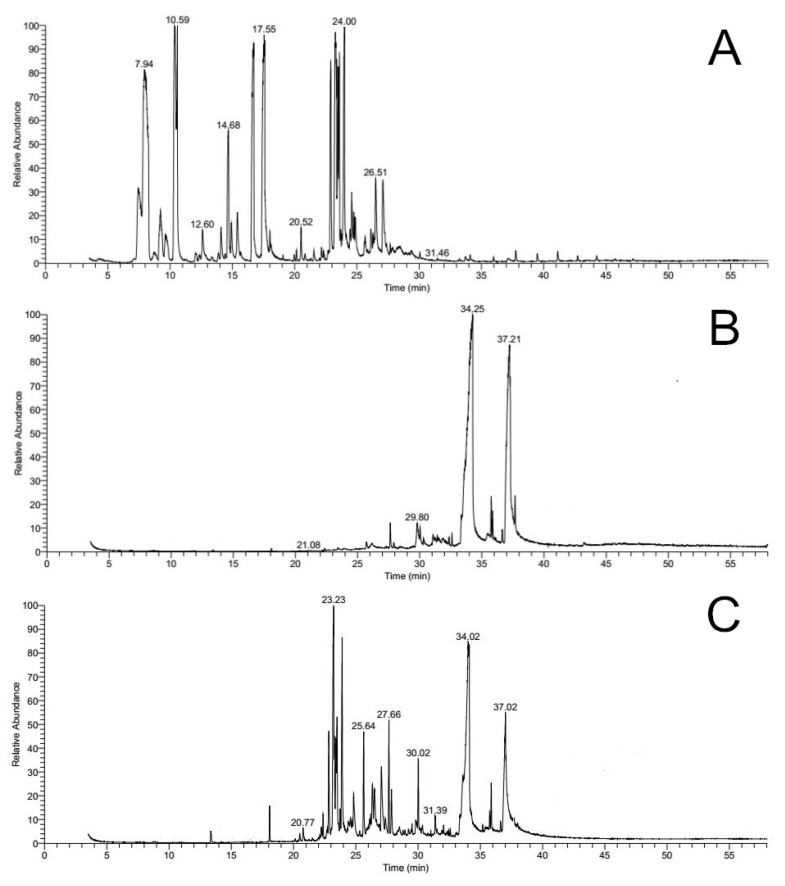
As shown in Figure 2, the chromatograms of RC, WRC and GRC are basically the same, and the results of the peak analysis revealed that no changes of the peaks were detected. It seems that ginger juice processing and water processing had no obvious influence on the composition of the methanol extract of RC. Wu et al. [15] reported that during the wine-processing of RC, a portion of berberubine was formed from berberine because of the high temperature. Jiang et al. [7] reported that the auxiliary material or heating process may enhance the transformation of dihydrochelerythrine from berberine during the processing of RC and the acidic condition in processing could enhance the reaction of protoberberine. However, in our research, no obvious chemical reactions were detected, which suggested that the reaction of berberine was not only influenced by temperature, but the auxiliary material (adjuvant for processing) also played an important role in the processing of RC.
Figure 2.
UPLC-QTOF-MS total ion current chromatogram of a methanol extract of Rhizoma coptidis (A), ginger juice-processed Rhizoma coptidis (B) and water-processed Rhizoma coptidis (C).
Ginger juice containing methanol-soluble gingerols, and one of the main active ingredients, 6-gingerol was anticipated to be transferred into RC during ginger processing before the experiment. However, no gingerol or shogaol were detected in the methanol extract of GRC. A reasonable explanation was that the amounts of gingerols and shogaol transferred into RC were too low to be detected. Above all, methanol-soluble compounds transferring from the adjuvant to the RC was not the main mechanism of ginger juice processing.
2.2. The Effect of Processing on Alkaloid Content of Rhizoma coptidis
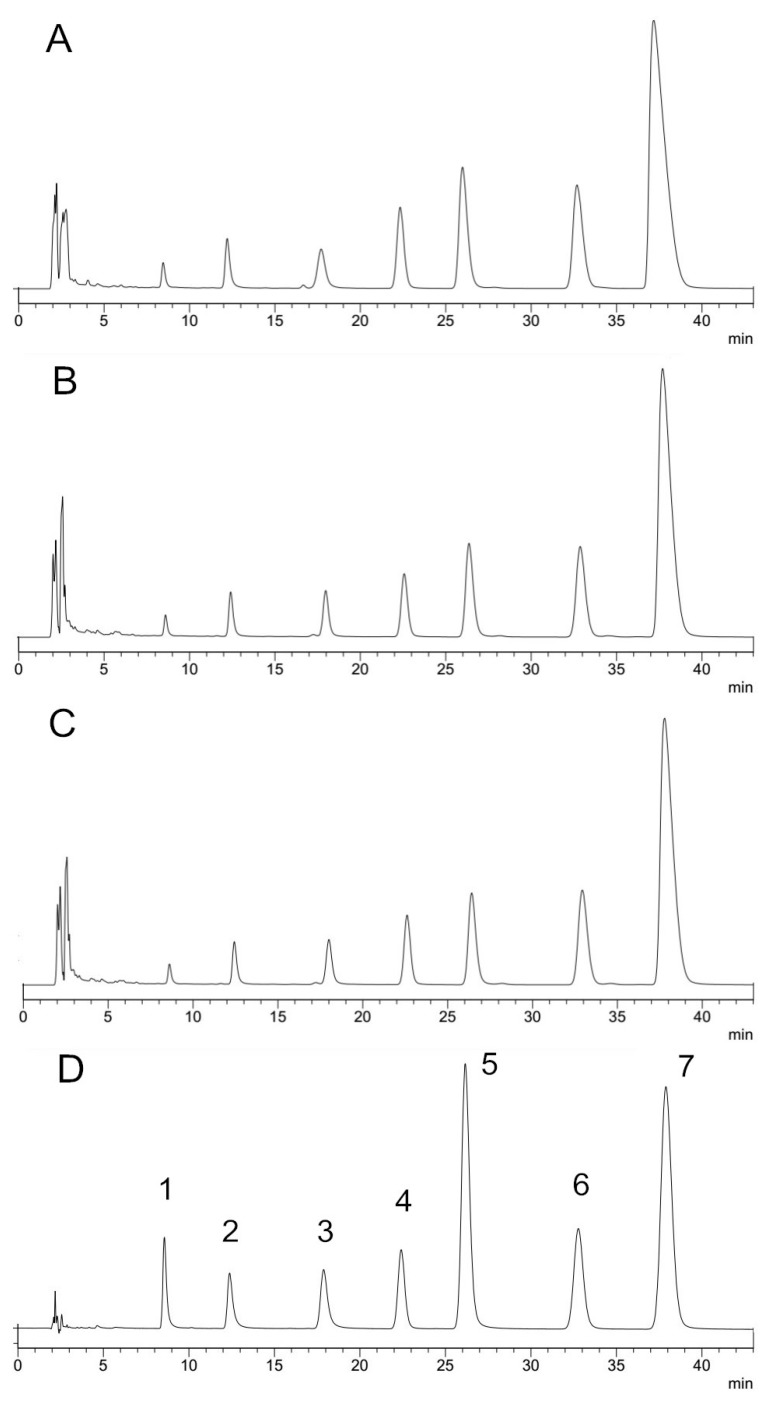
According to Wu et al. [15] processing may cause a quantitative change of alkaloid content. The main alkaloids of RC have been identified using reference substances by HPLC, the chromatograms are shown in Figure 3.
Figure 3.
HPLC chromatogram of methanol extract of Rhizoma coptidis (A), ginger juice-processed Rhizoma coptidis (B), water-processed Rhizoma coptidis (C), and the mixture of reference substances (D). 1. Groenlandicine 2. Jatrorrhizine 3. Columbamine 4. Epiberberine 5. Coptisine 6. Palmatine 7. Berberine.
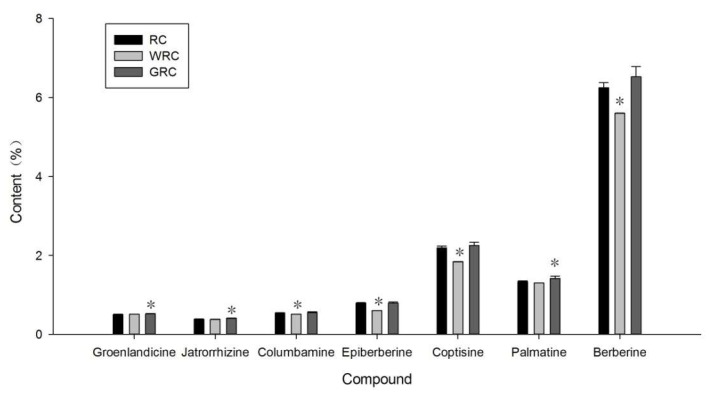
Quantitative study of total alkaloids and seven alkaloids of RC and processed RC (WRC, GRC) was conducted in this research. The results of the content determination of total alkaloids and seven alkaloids in RC, WRC, and GRC are listed in Table 1. Ginger juice processing increased the total alkaloid content (TAC) of RC from 11.99% ± 0.23% to 12.47% ± 0.46%, but the difference was not significant (p > 0.05). Water processing significantly (p < 0.05) decreased the total alkaloid content of RC to 10.73% ± 0.03%. As the RC specimens were subjected to covered moistening of equal time and drying at the same temperature during ginger juice processing and water processing, the different variation tendency of the TAC of RC processed by different methods suggested the chemical compositions of ginger juice was helpful to retain or increase the alkaloids of RC. Zhao et al. claim [5] that the wine could increase the dissolution of chemicals, and that vinegar may transfer the free alkaloids into soluble alkaloids, which would increase the alkaloid content. Ginger juice may also increase the alkaloid contents of RC by a similar mechanism.
Table 1.
Alkaloid content of unprocessed and processed Rhizoma coptidis. RC: Rhizoma coptidis, GRC: ginger processed Rhizoma coptidis, WRC:water processed Rhizoma coptidis. For each column, values followed by the same letter did not share significant differences at p < 0.05 (Duncan’s test).
| Content of alka%loids (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groenlandicine | Jatrorrhizine | Columbamine | Epiberberine | Coptisine | Palmatine | Berberine | Total | |
| RC | 0.50 ± 0.01a | 0.38 ± 0.01a | 0.54 ± 0.01a | 0.79 ± 0.01a | 2.19 ± 0.05a | 1.33 ± 0.03a | 6.25 ± 0.13a | 11.99 ± 0.23a |
| WRC | 0.51 ± 0.01a | 0.38 ± 0.00a | 0.51 ± 0.01b | 0.60 ± 0.01b | 1.84 ± 0.01b | 1.30 ± 0.00a | 5.6 ± 0.01b | 10.73 ± 0.03b |
| GRC | 0.52 ± 0.00b | 0.41 ± 0.01b | 0.55 ± 0.02a | 0.79 ± 0.03a | 2.25 ± 0.09a | 1.42 ± 0.05b | 6.53 ± 0.25a | 12.47 ± 0.46a |
As shown in Figure 4, ginger juice processing caused a significantly increase (p < 0.05) of groenlandicine, jatrorrhizine, and palmatine content of RC, while water processing led to a significant decrease (p < 0.05) of the columbamine, epiberberine, coptisine and berberine content of RC, while other compounds did not show significant difference. Previous studies showed that vinegar processing can improve the solubility of some alkaloids from Rhizoma corydalis and the mechanism was the formation of water-soluble salts [15]. The pH value of the ginger juice used in this study was determined to be about 6.5, and the weakly acidic condition was probably a factor that influenced the groenlandicine, jatrorrhizine, and palmatine content of RC.
Figure 4.
Seven alkaloids content of Rhizoma coptidis (RC), water-processed Rhizoma coptidis (WRC) and ginger juice-processed Rhizoma coptidis (GRC). Values are means ± SD, n = 3. * p < 0.05 of processed RC against RC.
The relative content of groenlandicine and jatrorrhizine in RC was not high, but the previous study showed groenlandicine and jatrorrhizine had a high number of pharmacological activities. Groenlandicine exhibited moderate inhibitory effects on anti-diabetic complications [16] and anti-Alzheimer effect [17], and it was also identified as an active principle with topoisomerase I-mediated DNA cleavage activity in vitro [18]. Jatrorrhizine exerted hepatoprotective activities [19], neuroprotective activity [20] and antihypercholesterolemic activity [21]. Palmatine had the third highest content of alkaloids in RC, and it was proven to have anti-bactericidal activity against H. pylori in vitro and in vivo [22], gastroprotective effect against gastric ulcers [23], heartprotective activity against acute myocardial injury [24] and memory-enhancing activity [25]. Jatrorhizine, groenlandicine, and palmatine exhibited significant peroxynitrite scavenging activities and ameliorated cell damage [17,26]. The increased of groenlandicine, jatrorrhizine, palmatine content in RC may relate to the change of RC pharmacologic activities.
2.3. The Effect of Ginger Juice Processing on Volatile Content of Rhizoma coptidis
The volatile content of RC and GRC was 0.003% ± 0.001% and 0.009% ± 0.001%, respectively. The volatile content of RC significantly increased (p < 0.05) after processing with ginger juice. Miyazawa et al. [27] reported dry coarsely-powdered RC (Coptis chinensis) hydro-distilled with a Likens–Nickerson-type apparatus using diethyl ether to yield 0.052% of yellowish green oil, but the author did not provide details of the separation method of the volatiles. The difference of the volatile content between previous research and our study may be attributed to the different method of volatiles extracting and the difference of the specimens. Additionally, no research on the quantitative change of volatiles of RC before and after ginger juice processing was reported before. The increase of volatile content of RC being processed with ginger juice was mainly caused by the transfer of volatiles from the ginger juice (see Section 2.4).
2.4. The Effect of Ginger Juice Processing on Volatiles Composition of Rhizoma coptidis
As shown in Figure 5, the volatiles of ginger juice were mainly compounds with relatively low boiling points (75 °C to 160 °C); the volatiles of RC and GRC were mainly compounds with the boiling point ranging from 180 °C to 235 °C and 135 °C to 235 °C, respectively. After ginger juice processing, the composition of the volatiles of RC obviously changed, and compounds with low boiling points (from the ginger juice) were transferred into RC (see Table 2). However, the volatiles of GRC were not all of the volatiles of RC and ginger juice. During the ginger juice processing, the heat volatilized most of the low boiling point volatiles (<135 °C) and part of the volatiles with low content in RC, while most volatiles with high boiling points (>135 °C) were retained.
Figure 5.
The total ion chromatogram of volatiles in ginger juice (A), Rhizoma coptidis (B), and ginger juice-processed Rhizoma coptidis (C) by GC-MS.
Table 2.
Volatiles compositions of RC, GRC and ginger juice.
| Peak Number | RT | RIa | RIb | Identified Compounds | RC | GRC | Ginger Juice |
|---|---|---|---|---|---|---|---|
| 1 | 4.28 | 800 | 800 [39] | Hexanal | - | - | 0.19% |
| 2 | 7.09 | 924 | 925 [40] | Tricyclene | - | - | 0.05% |
| 3 | 7.45 | 935 | 939 [39] | α-Pinene | - | - | 2.91% |
| 4 | 8.00 | 952 | 953 [39] | Camphene | - | - | 14.85% |
| 5 | 9.24 | 989 | 980 [39] | β-Pinene | - | - | 2.32% |
| 6 | 9.64 | 1001 | 1003 [39] | α-Phellandrene | - | - | 1.23% |
| 7 | 10.38 | 1025 | 1018 [39] | β-Phellandrene | - | - | 12.89% |
| 8 | 12.04 | 1079 | 1088 [39] | p-Mentha-1,4(8)-diene | - | - | 0.32% |
| 9 | 12.61 | 1097 | 1098 [39] | β-Linalool | - | - | 0.91% |
| 10 | 13.35 | 1122 | 1126 [41] | cis-p-Menth-2-en-1-ol | - | - | 0.15% |
| 11 | 14.09 | 1148 | 1148 [40] | Citronellal | - | - | 0.86% |
| 12 | 14.42 | 1159 | 1138 [42] | cis-Verbenol | - | - | 0.07% |
| 13 | 14.68 | 1168 | 1165 [39] | endo-Borneol | - | - | 3.75% |
| 14 | 14.92 | 1177 | 1183 [40] | Terpinen-4-ol | - | - | 0.89% |
| 15 | 15.40 | 1193 | 1189 [39] | α-Terpineol | - | - | 1.39% |
| 16 | 16.70 | 1241 | 1228 [40] | Neral | - | - | 8.87% |
| 17 | 17.55 | 1272 | 1270 [39] | Geranial | - | - | 10.31% |
| 18 | 18.01 | 1289 | 1291 [40] | 2-Undecanone | - | - | 0.30% |
| 19 | 19.06 | 1328 | 1337 [43] | δ-Elemene | - | - | 0.06% |
| 20 | 19.97 | 1362 | 1374 [40] | Cyclosativene | - | - | 0.10% |
| 21 | 20.23 | 1371 | 1377 [39] | α-Copaene | - | - | 0.16% |
| 22 | 20.52 | 1382 | 1391 [39] | β-Elemene | - | 0.34% | 0.30% |
| 23 | 21.29 | 1413 | 1418 [39] | Caryophyllene | - | - | 0.04% |
| 24 | 21.52 | 1424 | 1433 [43] | γ-Elemene | - | 0.16% | 0.22% |
| 25 | 22.15 | 1452 | 1458 [39] | β-Farnesene | - | - | 0.21% |
| 26 | 22.83 | 1483 | 1486 [40] | α-Curcumene | - | 4.27% | 4.32% |
| 27 | 23.22 | 1501 | 1502 [40] | Zingiberene | - | 15.73% | 8.40% |
| 28 | 23.41 | 1507 | 1509 [39] | α-Farnesene | - | 4.03% | 3.40% |
| 29 | 23.49 | 1510 | 1509 [39] | β-Bisabolene | - | 4.95% | 2.59% |
| 30 | 23.71 | 1518 | 1513 [39] | γ-Cadinene | - | 0.73% | - |
| 31 | 23.90 | 1525 | 1532 [40] | β-Sesquiphellandrene | - | 9.28% | 5.36% |
| 32 | 24.57 | 1548 | 1549 [43] | Elemol | - | 0.94% | 1.18% |
| 33 | 24.83 | 1557 | 1564 [39] | Nerolidol | - | 2.99% | 0.85% |
| 34 | 25.65 | 1586 | 1543 [44] | cis-Sesquisabinene hydrate | - | - | 0.56% |
| 35 | 25.86 | 1593 | 1586 [44] | trans-Sesquisabinene hydrate | - | - | 0.07% |
| 36 | 26.30 | 1609 | 1621 [41] | γ-Eudesmol | - | 2.52% | 0.19% |
| 37 | 26.51 | 1616 | 1613 [45] | Isoaromadendrene epoxide | - | - | 1.87% |
| 38 | 27.06 | 1655 | 1649 [39] | Eudesm-4(14)-en-11-ol | - | 4.93% | 2.45% |
| 39 | 27.37 | 1673 | 1681 [41] | β-Bisabolol | - | 1.01% | - |
| 40 | 28.44 | 1724 | 1715 [46] | Pentadecanal | 0.14% | - | - |
| 41 | 29.80 | 1782 | 1763 [47] | Tetradecanoic acid | 3.17% | 1.01% | - |
| 42 | 32.59 | 1921 | 1926 [47] | Hexadecanoic acid, methyl ester | 0.54% | 0.23% | - |
| 43 | 33.23 | 1947 | 1946 [48] | Geranyl-p-cymene | - | - | 0.05% |
| 44 | 33.56 | 1960 | 1951 [49] | Palmitoleic acid | 1.31% | 1.24% | - |
| 45 | 34.25 | 1987 | 1983 [47] | n-Hexadecanoic acid | 34.00% | 19.54% | |
| 46 | 35.48 | 2069 | - | cis-10-Heptadecenoic acid | 0.75% | - | - |
| 47 | 35.75 | 2090 | 2094 [47] | Linoleic acid, methyl ester | 2.02% | 0.69% | - |
| 48 | 37.21 | 2173 | 2159 [47] | cis-9,cis-12-Octadecadienoic acid | 40.80% | 11.70% | - |
| Total | 82.73% | 86.29% | 94.64% |
RIa: Retention index calculated from retention times relative to that of n-alkanes (C6–C26) on the the DB-5 column. RIb: literature retention index. RC: Rhizoma coptidis, GRC: ginger juice-processed Rhizoma coptidis. “-” means not detected or not available.
As shown in Table 2, the composition of volatiles of RC, GRC and ginger juice were analyzed. The numbers of identified peaks of RC, GRC and ginger juice were 8, 19, and 38, accounting for 82.73%, 86.29%, and 94.64% of their total volatile content, respectively. The main constituents (>3%) of volatiles of ginger juice included camphene (14.85%), β-phellandrene (12.89%), endo-borneol (3.75%), neral (8.87%), geranial (10.31%), α-curcumene (4.32%), zingiberene (8.40%), α-farnesene (3.40%) and β-sesquiphellandrene (5.36%), and that was in accord with previous study [28,29]. Miyazawa et al. [27] reported the main constituents of the essential oil of RC were found to be m-acetylanisole, 2-methylpropanoic acid, 3-hydroxy-2,4,4-trimethylpentyl ester, 2-methyl-propanoic acid, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester, and phenol. Gao et al. [30] claimed that monoterpenes and sesquiterpenes are major volatile compounds (volatiles were extracted using headspace solid-phase microextraction) in the coptis species. However, in our study, cis-9, cis-12-octadecadienoic acid (40.80%), n-hexadecanoic acid (34.00%) and tetradecanoic acid (3.17%) were determined to be the main constituents of RC volatiles. Different extracting methods of volatiles and different sources of RC specimens were the most likely reason causing the variance in the results. The volatile composition of RC obviously changed after processing with ginger juice, 13 new compounds were detected while two compounds (pentadecanal (0.14%), cis-10-heptadecenoic acid (0.75%)) were lost. The main constituents of GRC volatiles were α-curcumene (4.32%), zingiberene (15.73%), α-farnesene (4.03%), β-bisabolene (4.95%), β-sesquiphellandrene (9.28%), eudesm-4(14)-en-11-ol (4.93%), n-hexadecanoic acid (19.54%), and cis-9,cis-12-octadecadienoic acid (11.70%). Among them, n-hexadecanoic acid and cis-9,cis-12-octadecadienoic acid were the original compounds of RC, and the others were from ginger juice, it is worth mentioning that the relative content of n-hexadecanoic acid and cis-9,cis-12-octadecadienoic acid obviously increased after ginger processing(from 0.83:1 to 1.67:1). Additionally, nerolidol and β-bisabolol were detected in GRC volatiles but were detected in volatiles of RC or ginger juice. However, nerolidol and β-bisabolol were reported to be the constituent of ginger [29]. A reasonable explanation was the relative contents of nerolidol and β-bisabolol in volatiles of ginger juice were low and they were not identified, and that nerolidol and β-bisabolol in GRC were also from ginger juice.
The previous study showed ginger oil had strong antimicrobial activity, antioxidant activity, anti-inflammatory and antinociceptive properties, and the results of volatiles composition analysis indicated α-zingiberene, curcumene, sesquiphellandrene, citral, camphene, α-farnesene were the active ingredients [31,32,33,34]. Ginger oil also had antifungal activity [35], and ginger essential oil inhalation has implications for alleviating postoperative nausea and vomiting in abdominal surgery patients [36]. Additionally, zingiberene and sesquiphellandrene has anticancer potential [37,38]. Above all, the terpenes introduced from ginger juice in GRC during the processing may enhance the antimicrobial activity and antioxidant activity of RC and bring new pharmacological activities to RC.
2.5. The Effect of Processing on the Microstructure of Rhizoma coptidis
Zhao et al. [5] stated that sometimes the processing practice could increase the dissolving rate of chemicals which induces the change of chemical contents. In this research, the microstructures of RC and processed RC were observed using light microscope, and the authors attempted to reveal whether a relationship existed between the change of alkaloid content and the microstructure of RC during ginger juice processing.
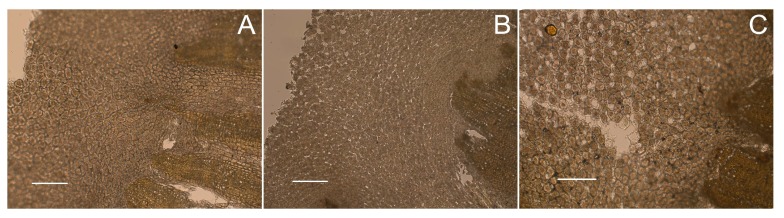
As shown in Figure 6, the pith texture of unprocessed RC was very tight, and no obvious intercellular space of parenchyma cells existed. After processing with ginger juice/water, the pith texture of RC became much looser. A large number of fine cracks were observed in the pith part of GRC, and irregular intercellular space formed potential paths to transfer compounds outwards, which may be one of the mechanisms by which ginger processing increased the alkaloid content of the methanol extract of RC. The irregular intercellular space of parenchyma cell was also observed in the pith part of WRC, which suggests water infiltration and thermal damage caused the change of the pith texture of RC. Since ginger juice processing and water processing changed the microstructure of RC in the same way, and both the two processing methods had a significant influence (p < 0.05) on some alkaloid content in RC, it was reasonable to deduce that the change of RC alkaloid content during processing may be related to the change in the microstructure of RC.
Figure 6.
The microscopic image of Rhizoma coptidis (A), ginger juice processed-Rhizoma coptidis (B) and water-processed Rhizoma coptidis (C) slices. Scale bar: 50 μm.
3. Materials and Methods
3.1. Material and Regents
Reference substances of groenlandicine, jatrorrhizine hydrochloride, columbamine, epiberberine, coptisine hydrochloride, palmatine hydrochloride and berberine hydrochloride were purchased from Desite Biotechnology Co., Ltd. (Chengdu, Sichuan, China). HPLC-grade acetonitrile and methanol were obtained from Fisher chemical (Shanghai, China). All other reagents were of analytical grade.
Rhizoma coptidis (dried rhizomes of the Coptis chinensis Franch) was purchased from Anguo Changda Medicinal Material Company (Baoding, Hebei, China) and authenticated by Professor Hui Cao in Jinan University, China. Ginger juice was prepared in the lab using fresh matured gingers (Zingiber officinale) purchased from a Sichuan local ginger market, and the ratio of ginger to ginger juice was 1:1 (m:v).
3.2. Processing of Rhizoma coptidis
The processing of ginger juice processed Rhizoma coptidis (GRC) was in accordance with Chinese pharmacopoeia; dried slices were mixed well with ginger juice, in a pot, heated with a gentle fire until the ginger juice is completely absorbed, and the drugs removed and allow to cool. The ratio of ginger to Rhizoma coptidis was 12.5:100. Processing Rhizoma coptidis was also conducted using water instead of ginger juice, labeled as WRC.
3.3. Preparation of Rhizoma coptidis Methanol Extracts
An accurately-weighted 0.2 g powder of crude RC, GRC, or WRC, was transferred into a stopper conical flask, and 25 mL of methanol: hydrochloride (100:1) was accurately added and weighed respectively. The extract was ultrasonicated for 30 min and cooled to room temperature, then methanol: hydrochloride (100:1) was added to compensate for the lost weight. The solution was filtered through a 0.45 μm membrane before injection.
3.4. Preparation of Standard Solution
The reference substances of groenlandicine, jatrorrhizine hydrochloride, columbamine, epiberberine, coptisine hydrochloride, palmatine hydrochloride and berberine hydrochloride were accurately weighed and dissolved in methanol: hydrochloride (100:1) to make up the stock solutions. The mixed standard solution was prepared by dilutions of the stock solution with methanol: hydrochloride (100:1) to obtain a final mixed standard solution containing 0.008 mg·mL−1 groenlandicine, 0.0065 mg·mL−1 jatrorrhizine, 0.012 mg·mL−1 columbamine, 0.013 mg·mL−1 epiberberine, 0.046 mg·mL−1 coptisine, 0.019 mg·mL−1 palmatine and 0.0535 mg·mL−1 berberine.
3.5. Qualitative Analysis of Rhizoma coptidis Extraction by UPLC-QTOF-MS
All chromatographic peaks were identified and confirmed using UPLC-QTOF-MS experiment using an ACQUITY UPLC system (Waters, Milford, MA, USA) coupled to a Xevo™ G2 Q-Tof triple quadrupole mass spectrometer (Waters, Milford, MA, USA). Chromatographic separation was conducted using an Agilent SB-C18 column (4.6 mm × 250 mm i.d., 5 μm. Agilent, Santa Clara, CA, USA) at a flow rate of 0.3 mL/min. The mobile phases were methanol (A) and 0.1% formic acid aqueous solution (B) with gradient elution program listed in Table 3. The injection volume was 10 µL and the column temperature was 40 °C. MS spectra were recorded in the range of m/z 50–1200 using electrospray ionization (ESI) as the ionization source in positive/negative ion-switching mode. The mass spectrometer settings used were: capillary voltage: 3 kV, source temperature: 100 °C, desolvation temperature: 300 °C, cone gas (nitrogen) flow: 50 L·h−1, desolvation gas (nitrogen) flow: 990 L·h−1. Instrument control and data acquisition and evaluation were performed with the Micromass MassLynx 4.1 software package provided by Waters.
Table 3.
Gradient table of the UPLC elution program.
| Time (Min) | Flow Rate | %A | %B | |
|---|---|---|---|---|
| 1 | Initial | 0.3 | 10 | 90 |
| 2 | 3 | 0.3 | 10 | 90 |
| 3 | 35 | 0.3 | 100 | 0 |
| 4 | 40 | 0.3 | 100 | 0 |
| 5 | 40.1 | 0.3 | 10 | 90 |
| 6 | 45 | 0.3 | 10 | 90 |
3.6. Quantitative Analysis of the Alkaloid Content of Rhizoma coptidis Extract by HPLC-PDA
The alkaloids of crude RC, WRC, and GRC were determined by the HPLC method [50]. A Shimadzu liquid chromatography system (LC-20AT, SPD-M20A, SIL-20A, CTO-10AS, Shimadzu Co., Kyoto, Japan) was employed to carry out the determination. Chromatographic separation was performed on an Agilent Zorbax Extend-C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase was prepared from acetonitrile (A) and a mix solution (0.25 mol·L−1 ammonium acetate and 8 mmol·L−1 lauryl sodium sulfate, adjusted to pH 9.3 before use) (B) at the ratio 36:64. The flow rate was 1.0 mL·min−1, the injection volume was 10 μL, and the column temperature was maintained at 35 °C. The UV absorbance between 200 and 400 nm was detected, and the absorbance at 270 nm was used for calculation. The alkaloid content for each alkaloid was calculated by comparing the peak area with the standard curve. The total alkaloid content was calculated as the sum of the content of the 7 individual alkaloids.
3.7. Qualitative and Quantitative Analysis of Volatiles of Processed and Unprocessed Rhizoma coptis by GC-MS
The volatiles of crude RC and GRC were extracted by the water distillation method. A total of 500 g of RC/GRC was transferred to a 10,000 mL flask, 10 times the amount of water was then added to the flask and mixed well. A volatile oil extractor was employed to extract the volatiles, extracted for five hours after boiling. The volume of volatiles was recorded. The volatiles were collected, and then dehydrated with a proper amount of anhydrous sodium sulfate. Twenty microliters of dissolved volatiles with 2 mL n-hexane was used as the sample for qualitative analysis.
The qualitative analysis of volatiles was conducted on a Trace GC ULTRA gas chromatograph (Thermo Scientific, Waltham, MA, USA) directly coupled to an ISQ Single Quadruple MS (Thermo Scientific). The volatiles were separated using an Agilent J&W DB-5 column (30 m × 0.25 mm × 0.25 μm). The oven temperature program was as follows: 50 °C (held for 3 min), then raised to 300 °C at a rate of 5 °C/min (held for 5 min). Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The MS fragmentation was performed by electronic impact (EI) at 70 eV, a source temperature of 300 °C, scanning times of 0.2 s, and a mass acquisition range of 35–800 amu. All compounds were also confirmed by the matching of their mass spectra with the NIST 2.0 mass spectral database (National Institute of Standards and Technology, Gaithersburg, MD, USA). Also, the retention indices were calculated for all volatile constituents using a homologous series of n-alkanes.
3.8. Observation of the Microstructure of Processed and Unprocessed Rhizoma coptidis
The crude RC, WRC, and GRC were soaked with 5% glycerin solution, then the microsection of crude RC, WRC, and GRC were prepared by freezing microtome (Leica CM1860, Nussloch, Germany). The microstructure of each microsection was observed using a light microscope (Axio Imager A2, Jena, Germany).
3.9. Statistical Analysis
The experiment was performed in triplicate. The experiment data was analyzed using SPSS 19.0 (Chicago, IL, USA) for analysis of variance (ANOVA) and Duncan’s test (p < 0.05). The data were reported as the mean ± standard deviation (SD).
4. Conclusions
Ginger juice processing is a very important technique to enhance the efficacy and reduce the toxicity of crude drugs. However, to date, the research on ginger juice processing has been limited, and the effect of ginger juice processing on crude drugs was still unclear. This research comprehensively revealed the effect of ginger juice processing on the chemical profiles of RC.
Generally, ginger juice/water processing did not obviously change the composition of the methanol extract of RC. Instead, ginger juice processing kept the TAC of RC with no significant change and significantly increased (p < 0.05) the content of three alkaloids; water processing significantly decreased (p < 0.05) the TAC and the content of four alkaloids in RC. As an adjuvant for processing, ginger juice helped to retain the main active ingredient of RC, alkaloids during the processing. The mechanism of ginger juice processing increased some alkaloid content of RC and may relate to the microstructure change caused by the processing, but further studies will be done to illuminate the relationship between microstructure change and alkaloid content change in RC.
In addition, ginger juice processing significantly increased (p < 0.05) the volatile content of RC; after ginger juice processing, the composition of RC volatiles also obviously changed, 13 new compounds were introduced, and two original compounds gone. The new compounds of RC volatiles were mainly from the ginger juice. In order to investigate the influence of the changing of RC volatiles on pharmacological activities, the difference of the effect on the gastrointestinal tract between RC and processed RC will be tested in the near future. Also, the relationship between the change of the chemical profile and the pharmacological activity of RC will be investigated.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author Contributions
The list authors contributed to this work as follows: C.Y. conceived and designed the experiments; C.Y., F.G. and C.Z. performed the research and analyzed the data; C.L., H.C. and B.Z. provide suggestions on the optimization of experiment design. C.Y. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors have declared no conflict of interest.
Footnotes
Sample Availability: Samples of the Rhizoma coptidis are available from the authors.
References
- 1.Commission C.P. Chinese Pharmacopoeia. 2015th ed. Volume 1 China Medical Science Press; Beijing, China: 2015. [Google Scholar]
- 2.Ho C.E., Goh Y.L., Zhang C. From prejudice to evidence: The case of Rhizoma coptidis in Singapore. Evid. Based Complement. Altern. Med. 2014;2014:871720. doi: 10.1155/2014/871720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang B., Yu X.T., Zhou Q., Zhao T.Y., Wang H., Gu C.J., Tong X.L. Effect of Rhizoma coptidis (Huang Lian) on Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2015;2015:921416. doi: 10.1155/2015/921416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan H.L., Chan K.G., Pusparajah P., Duangjai A., Saokaew S., Mehmood Khan T., Lee L.H., Goh B.H. Rhizoma coptidis: A Potential Cardiovascular Protective Agent. Front. Pharmacol. 2016;7:362. doi: 10.3389/fphar.2016.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Z., Liang Z., Chan K., Lu G., Lee E.L., Chen H., Li L. A unique issue in the standardization of Chinese materia medica: Processing. Planta Med. 2010;76:1975–1986. doi: 10.1055/s-0030-1250522. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Z.P., Jiang J.G. Analysis of the adverse reactions induced by natural product-derived drugs. Br. J. Pharmacol. 2010;159:1374–1391. doi: 10.1111/j.1476-5381.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X., Huang L.F., Wu L.B., Wang Z.H., Chen S.L. UPLC-QTOF/MS Analysis of Alkaloids in Traditional Processed Coptis chinensis Franch. Evid. Based Complement. Altern. Med. 2012;2012:942384. doi: 10.1155/2012/942384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F., Zhang T., Zhang R., Ito Y. Application of analytical and preparative high-speed counter-current chromatography for separation of alkaloids from Coptis chinensis Franch. J. Chromatogr. A. 1998;829:137–141. doi: 10.1016/S0021-9673(98)00776-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Wang F., Liu J., Lee F.S., Wang X., Yang H. Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections. Anal. Chim. Acta. 2008;613:184–195. doi: 10.1016/j.aca.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Wang Z.B., Song Y., Yang J., Wu L.J., Yang B.Y., Wang Q.H., Wang L.Q., Wang R.X., Yang C.J. Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch. Molecules (Basel, Switzerland) 2016;21:913. doi: 10.3390/molecules21070913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang P., Qian X., Li J., Cui X., Chen L., Cai B., Tan S. Simultaneous determination of 11 alkaloids in crude and wine-processed Rhizoma coptidis by HPLC-PAD. J. Chromatogr. Sci. 2015;53:73–78. doi: 10.1093/chromsci/bmu019. [DOI] [PubMed] [Google Scholar]
- 12.Qian X.C., Zhang L., Tao Y., Huang P., Li J.S., Chai C., Li W., Di L.Q., Cai B.C. Simultaneous determination of ten alkaloids of crude and wine-processed Rhizoma coptidis aqueous extracts in rat plasma by UHPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. J. Pharm. Biomed. Anal. 2015;105:64–73. doi: 10.1016/j.jpba.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L.Y., Su D., Zhu J., Deng Y.F. Comparison of Coptidis Rhizoma processed with different ginger juice based on metabolomics. Zhongguo Zhong Yao Za Zhi. 2016;41:2712–2719. doi: 10.4268/cjcmm20161424. [DOI] [PubMed] [Google Scholar]
- 15.Wu H., Waldbauer K., Tang L., Xie L., McKinnon R., Zehl M., Yang H., Xu H., Kopp B. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo) Molecules. 2014;19:11487–11504. doi: 10.3390/molecules190811487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H.A., Yoon N.Y., Bae H.J., Min B.S., Choi J.S. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch. Pharm. Res. 2008;31:1405–1412. doi: 10.1007/s12272-001-2124-z. [DOI] [PubMed] [Google Scholar]
- 17.Jung H.A., Min B.S., Yokozawa T., Lee J.H., Kim Y.S., Choi J.S. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009;32:1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi Y., Yamashita Y., Fujii N., Takaboshi K., Kawakami T., Kawamura M., Mizukami T., Nakano H. Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med. 1995;61:414–418. doi: 10.1055/s-2006-958127. [DOI] [PubMed] [Google Scholar]
- 19.Wang S., Li X., Niu Y., Liu Y., Zhu Y., Lu X., Fan X., Zhang X., Wang Y. Identification and screening of chemical constituents with hepatoprotective effects from three traditional Chinese medicines for treating jaundice. J. Sep. Sci. 2016;39:3690–3699. doi: 10.1002/jssc.201600437. [DOI] [PubMed] [Google Scholar]
- 20.Luo T., Zhang H., Zhang W.W., Huang J.T., Song E.L., Chen S.G., He F., Xu J., Wang H.Q. Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci. Lett. 2011;498:227–231. doi: 10.1016/j.neulet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Wu H., He K., Wang Y., Xue D., Ning N., Zou Z., Ye X., Li X., Wang D., Pang J. The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma coptidis. Phytomedicine. 2014;21:1373–1381. doi: 10.1016/j.phymed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Rong Q., Xu M., Dong Q., Zhang Y., Li Y., Ye G., Zhao L. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC Complement. Altern. Med. 2016;16:331. doi: 10.1186/s12906-016-1310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L., Wang X., Zhang S.L., Zhu X.M., Liu Y.Q., Song Z.J., Du W.J., Ji J., Cui C.L., He X., et al. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J. Nat. Med. 2017;71:257–264. doi: 10.1007/s11418-016-1057-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.M., Ha Y.M., Jin Y.C., Shi L.Y., Lee Y.S., Kim H.J., Seo H.G., Choi J.S., Kim Y.S., Kang S.S., et al. Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. Food Chem. Toxicol. 2009;47:2097–2102. doi: 10.1016/j.fct.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Dhingra D., Kumar V. Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze. Adv. Pharmacol. Sci. 2012;2012:357368. doi: 10.1155/2012/357368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokozawa T., Satoh A., Cho E.J., Kashiwada Y., Ikeshiro Y. Protective role of Coptidis Rhizoma alkaloids against peroxynitrite-induced damage to renal tubular epithelial cells. J. Pharm. Pharmacol. 2005;57:367–374. doi: 10.1211/0022357055470. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa M.K.J., Yamafuji C. Components of Essential Oil from Rootstock of Coptis chinensis. Nat. Med. 2004;58:222–225. [Google Scholar]
- 28.An K., Zhao D., Wang Z., Wu J., Xu Y., Xiao G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Pt BFood Chem. 2016;197:1292–1300. doi: 10.1016/j.foodchem.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Ding S.H., An K.J., Zhao C.P., Li Y., Guo Y.H., Wang Z.F. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe) Food Bioprod. Process. 2012;90:515–524. doi: 10.1016/j.fbp.2011.10.003. [DOI] [Google Scholar]
- 30.Gao X., Yang X., Mitrevski B.S., Marriott P.J. Headspace solid-phase microextraction combined with GCxGC-TOFMS for the analysis of volatile compounds of Coptis species rhizomes. J. Sep. Sci. 2011;34:1157–1166. doi: 10.1002/jssc.201100022. [DOI] [PubMed] [Google Scholar]
- 31.Jeena K., Liju V.B., Kuttan R. Antioxidant, anti-inflammatory and antinociceptive activities of essential oil from ginger. Indian J. Physiol. Pharmacol. 2013;57:51–62. [PubMed] [Google Scholar]
- 32.Ashraf S.A., Al-Shammari E., Hussain T., Tajuddin S., Panda B.P. In-vitro antimicrobial activity and identification of bioactive components using GC-MS of commercially available essential oils in Saudi Arabia. J. Food Sci. Technol. 2017;54:3948–3958. doi: 10.1007/s13197-017-2859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoferl M., Stoilova I., Wanner J., Schmidt E., Jirovetz L., Trifonova D., Stanchev V., Krastanov A. Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun. 2015;10:1085–1090. [PubMed] [Google Scholar]
- 34.Pandini J.A., Pinto F.G.S., Scur M.C., Santana C.B., Costa W.F., Temponi L.G. Chemical composition, antimicrobial and antioxidant potential of the essential oil of Guarea kunthiana A. Juss. Braz. J. Biol. 2017 doi: 10.1590/1519-6984.04116. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto-Ribeiro M.M., Grespan R., Kohiyama C.Y., Ferreira F.D., Mossini S.A., Silva E.L., Filho B.A., Mikcha J.M., Machinski M., Jr. Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013;141:3147–3152. doi: 10.1016/j.foodchem.2013.05.144. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.R., Shin H.S. Effectiveness of Ginger Essential Oil on Postoperative Nausea and Vomiting in Abdominal Surgery Patients. J. Altern. Complement. Med. 2017;23:196–200. doi: 10.1089/acm.2015.0328. [DOI] [PubMed] [Google Scholar]
- 37.Togar B., Turkez H., Tatar A., Hacimuftuoglu A., Geyikoglu F. Cytotoxicity and genotoxicity of zingiberene on different neuron cell lines in vitro. Cytotechnology. 2015;67:939–946. doi: 10.1007/s10616-014-9729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyagi A.K., Prasad S., Yuan W., Li S., Aggarwal B.B. Identification of a novel compound (beta-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs. 2015;33:1175–1186. doi: 10.1007/s10637-015-0296-5. [DOI] [PubMed] [Google Scholar]
- 39.Babushok V.I., Zenkevich I.G. Retention Indices for Most Frequently Reported Essential Oil Compounds in GC. Chromatographia. 2009;69:257–269. doi: 10.1365/s10337-008-0872-3. [DOI] [Google Scholar]
- 40.Huang B., Wang G., Chu Z., Qin L. Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS. Drying Technol. 2012;30:248–255. doi: 10.1080/07373937.2011.634976. [DOI] [Google Scholar]
- 41.Liu Z.L., Chu S.S., Liu Q.R. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils of Artemisia capillaris and Artemisia mongolica. Molecules. 2010;15:2600–2608. doi: 10.3390/molecules15042600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumen I., Hafizoglu H., Kilic A., Donmez I.E., Sivrikaya H., Reunanen M. Yields and constituents of essential oil from cones of Pinaceae spp. natively grown in Turkey. Molecules. 2010;15:5797–5806. doi: 10.3390/molecules15085797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riahi S., Pourbasheer E., Ganjali M.R., Norouzi P. Investigation of different linear and nonlinear chemometric methods for modeling of retention index of essential oil components: Concerns to support vector machine. J. Hazard. Mater. 2009;166:853–859. doi: 10.1016/j.jhazmat.2008.11.097. [DOI] [PubMed] [Google Scholar]
- 44.Varughese T., Unnikrishnan P.K., Deepak M., Balachandran I., Rema Shree A.B. Chemical Composition of the Essential Oils from Stem, Root, Fruit and Leaf of Piper longum Linn. J. Essent. Oil Bear. Plants. 2016;19:52–58. doi: 10.1080/0972060X.2015.1119065. [DOI] [Google Scholar]
- 45.Raal A., Orav A., Gretchushnikova T. Essential Oil Content and Composition in Tanacetum vulgare L. Herbs Growing Wild in Estonia. J. Essent. Oil Bear. Plants. 2014;17:670–675. doi: 10.1080/0972060X.2014.958554. [DOI] [Google Scholar]
- 46.Yaylı N., Yaşar A., Yaylı N., Albay C., Aşamaz Y., Coşkunçelebi K., Karaoğlu Ş. Chemical composition and antimicrobial activity of essential oils from Centaurea appendicigera and Centaurea helenioides. Pharm. Biol. 2009;47:7–12. doi: 10.1080/13880200802397970. [DOI] [Google Scholar]
- 47.Zhao C., Zeng Y., Wan M., Li R., Liang Y., Li C., Zeng Z., Chau F.T. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. J. Sep. Sci. 2009;32:660–670. doi: 10.1002/jssc.200800484. [DOI] [PubMed] [Google Scholar]
- 48.Sharopov F.S., Sulaimonova V.A., Setzer W.N. Composition of the Essential oil of Artemisia absinthium from Tajikistan. Rec. Nat. Prod. 2012;6:127–134. [Google Scholar]
- 49.Pozo-Bayón M.Á., Andújar-Ortiz I., Moreno-Arribas M.V. Volatile profile and potential of inactive dry yeast-based winemaking additives to modify the volatile composition of wines. J. Sci. Food Agric. 2009;89:1665–1673. doi: 10.1002/jsfa.3638. [DOI] [Google Scholar]
- 50.Yang Yong L.W., Sun J., Wang X., Hua L., Li L., Qin Y. Determination of Six Alkaloids in Crude and Processed Rhizoma coptidis by HPLC. World Sci. Technol./Mod. Tradit. Chin. Med. Mater. Med. 2015;17:596–602. [Google Scholar]